Abstract
The potential interaction between fluoxetine, a known inhibitor of cytochrome P-450 isoform 2D6 (CYP2D6), and ritonavir, a human immunodeficiency virus type 1 protease inhibitor, was evaluated in this open-label study. Sixteen male and female subjects ranging in age from 18 to 40 years completed the study. Subjects received single doses of 600 mg of ritonavir on days 1 and 10. On study days 3 to 10, all subjects received 30 mg of fluoxetine every 12 h for a total of 16 consecutive doses. Serial blood samples for determination of ritonavir concentrations in plasma were collected after the administration of ritonavir on days 1 and 10. A limited number of blood samples for determination of fluoxetine and norfluoxetine concentrations were collected after administration of the morning dose on day 10. A statistically significant increase (19%) in the ritonavir area under the concentration-time curve (AUC) was observed with concomitant fluoxetine administration, with individual changes ranging from −12 to +56%. The change in the ritonavir AUC with concomitant fluoxetine administration was positively correlated with the norfluoxetine 24-h AUC (AUC24) (r2 = 0.42), the norfluoxetine/fluoxetine AUC24 ratio (r2 = 0.53), and the fluoxetine elimination rate constant (r2 = 0.65), with larger increases in the ritonavir AUC tending to occur with higher norfluoxetine concentrations and higher fluoxetine elimination rate constants. The effect of fluoxetine appeared to be larger in subjects with the CYP2D6 wt/wt genotype. There was little or no effect on the time to maximum drug concentration (Cmax) in serum, Cmax, and the elimination rate constant of ritonavir with concomitant fluoxetine administration. Considering the magnitude of the change observed, no ritonavir dose adjustment is recommended during concomitant fluoxetine administration.
Human immunodeficiency virus (HIV) protease is a constitutive enzyme of HIV that processes the viral gag- and gag-pol-encoded polyproteins essential for the maturation of infectious virions; therefore, it represents a key target for intervention in the development of novel therapeutic agents for AIDS (20). Ritonavir (Norvir) is an HIV protease inhibitor that has been tested extensively for its ability to inhibit the HIV protease enzyme and HIV viral replication in cell culture. It has demonstrated activities against HIV types 1 and 2, including zidovudine-resistant HIV, in a variety of transformed and primary human cell lines (13). Ritonavir administered to HIV-positive patients showed potent antiviral activity (7, 16) and, as a result, was recently approved by the Food and Drug Administration for the treatment of HIV infection (1). Ritonavir undergoes extensive cytochrome P-450 (CYP450)-dependent biotransformation mediated primarily by CYP450 isoform 3A4 (CYP3A4) and, to a lesser extent, by CYP450 isoform 2D6 (CYP2D6) (14).
Fluoxetine (Prozac) is an antidepressant for oral administration that is effective through selective inhibition of serotonin reuptake. Fluoxetine is metabolized by N-demethylation to an active metabolite, norfluoxetine (2, 10). Fluoxetine is administered as the racemic mixture, and both S-fluoxetine and R-fluoxetine, as well as S-norfluoxetine, but not R-norfluoxetine, have been reported as pharmacologically active (3). Different CYP450 isoforms have been implicated in the conversion of R- and S-fluoxetine to R- and S-norfluoxetine in vitro, including, to a small extent, CYP2D6 (21, 23). The fluoxetine elimination half-life (t1/2) has been reported to range between 1 and 4 days, while that of norfluoxetine is longer, ranging from 7 to 15 days (2, 10). The terminal elimination t1/2 increases (1.9 to 5.7 days) and oral clearance decreases (35.5 to 10.8 liter/h) with multiple dosing, probably due to inhibition of its own metabolism. Fluoxetine and its metabolite have been shown to be potent inhibitors of CYP2D6, with R-fluoxetine and R-norfluoxetine being less potent than the S enantiomers (3, 5, 6). Significant in vivo interactions with tricyclic antidepressants, diazepam, alprazolam, carbamazepine, and antipsychotics have been reported (2, 10). Overall, these results suggest that the inhibition by fluoxetine and/or its major metabolite is not specific for the CYP2D6 isoform but may involve other CYP isoforms, including CYP3A.
Since depression may occur in HIV-positive patients, concomitant use of ritonavir and fluoxetine is likely. The effect on ritonavir pharmacokinetics was evaluated in the present study, in which single doses of 600 mg of ritonavir were administered, alone and during fluoxetine dosing. Subjects were genotyped for CYP2D6. The recommended therapeutic dose of fluoxetine for the treatment of depression is 20 mg daily, and a steady state is achieved after 4 to 6 weeks of repeated administration. In the present study, 60 mg of fluoxetine was administered daily for 8 days prior to the second dose of ritonavir to obtain fluoxetine and norfluoxetine concentrations comparable to those observed at steady state with a daily dose of 20 mg. Since the parent and the metabolite have different inhibitory effects on CYP enzymes, this design assumes that the parent-to-metabolite ratios achieved with this dosing regimen and the regimen used clinically are similar. A similar study design has been used previously to assess the effect of fluoxetine on tricyclic antidepressants (4).
MATERIALS AND METHODS
Subjects.
Healthy males and females between the ages of 18 and 45 years, each with a body weight within the acceptable range for the subject’s height and gender, were eligible to participate in the study. Subjects had no recent history of drug or alcohol abuse and were negative for the hepatitis B virus. Only nonlactating females who were postmenopausal, surgically sterilized, practiced total abstinence or maintained a monogamous relationship with a vasectomized partner, and had a negative urine test for pregnancy were allowed to participate. Subjects were excluded from study participation if any of the following criteria applied: evidence of clinically significant cardiovascular, pulmonary, renal, hepatic, hematologic, metabolic, neurologic, psychiatric, gastrointestinal, immunologic, or endocrine disease, malignancy, or other abnormality; intake of an investigational drug within 4 weeks prior to the start of the study; intake of a monoamine oxidase inhibitor within 5 weeks prior to study start; use of any drugs, including over-the-counter medication, from 1 week prior to study start through study completion. All subjects gave written informed consent in compliance with Food and Drug Administration regulations, and Institutional Review Board approval was obtained.
Study design.
This was a phase I, open-label, single-center interaction study of healthy adult male and female volunteers. Ritonavir (600 mg) was administered as a liquid formulation (80-mg/ml solution) via an oral syringe at approximately 08:00 on days 1 and 10. The dose was given within 15 min after completion of a meal. Subjects received fluoxetine (30 mg) as the hydrochloride salt (Prozac pulvules; Dista Products Co.) as one 20-mg pulvule and one 10-mg pulvule every 12 h (q12h) at approximately 08:00 and 20:00 for 16 consecutive doses, from day 3 to day 10. All doses were administered with 200 ml of water. Subjects were confined and supervised for 11.5 days during the study, from day −1 (day prior to administration of the initial dose) through the 48-h blood collection on day 12. Subjects returned to the testing facility for the 60-, 72-, and 84-h blood collections on days 12, 13, and 14. Strenuous activity during confinement was not permitted. During confinement, subjects abstained from all food and beverages except for the scheduled meals and snacks provided in the study. Water was available ad libitum. All meals were standardized with regard to content during confinement. All meals served on day 1 were the same as those served on day 10. Grapefruit, grapefruit juice, and caffeine were not permitted during the study. Breakfast, lunch, and dinner were served at approximately 07:30, 13:30, and 19:30, and snacks were provided at approximately 22:00. Meals served on days 1 and 10 were consumed within 20 min and eaten at a reasonable pace. The sequence of starting the meals on days 1 and 10 was maintained to the minute such that the time intervals relative to dosing were the same among all subjects.
Blood collection and analysis.
Two 15-ml blood samples were obtained on day −1 for CYP2D6 genotype determination. CYP2D6 genotypes were determined at Georgetown University, Washington, D.C., by using standard PCR DNA amplification techniques. The genotypes in this study were identified with the two alleles labeled as normal, i.e., wild type (wt), or defective with the A (A) or B (B) mutation.
Blood samples (7 ml) were collected for determination of ritonavir concentrations in plasma at the following times relative to dose administration on days 1 and 10: prior to dosing (0 h) and at 1, 2, 3, 4, 6, 8, 10, 12, 18, 24, 30, 36, and 48 h postdosing. In addition, blood samples (7 ml) were collected at 60, 72, and 84 h after dosing on day 10. Ritonavir concentrations in plasma were determined by using a validated reverse-phase high-performance liquid chromatographic method with UV detection after extraction with ethyl acetate-hexane, followed by hexane washes of the reconstituted extract (17). Samples were assayed at Oneida Research Services, Inc., Whitesboro, N.Y. Standard curves ranged from 0.010 to 15.0 μg/ml, with a lower limit of quantitation of 0.010 μg/ml. Quality control samples (0.150, 7.50, and 12.0 μg/ml) had coefficients of variation of ≤7%.
Blood samples (5 ml) were obtained for fluoxetine and norfluoxetine concentrations in plasma on day 10 at the following times relative to administration of the morning dose: prior to dosing (0 h) and at 6, 12 (prior to administration of the 20:00 dose), 18, 24, 48, and 72 h. Fluoxetine and norfluoxetine concentrations were determined by using a validated high-performance liquid chromatography procedure with fluorescence detection. Fluoxetine, norfluoxetine, and the internal standard were extracted from alkaline human plasma into a hexane-isoamyl alcohol mixture and back extracted into dilute acid. Sample analyses were conducted at Pharmaco LSR, Richmond, Va. The lower limit of quantitation was 2 ng/ml for both analytes, and standard curves ranged from 2.00 to 500 ng/ml (1-ml plasma volume). Quality control samples for both fluoxetine and norfluoxetine (5.00, 40.0, and 400 ng/ml) had coefficients of variation of ≤5%.
Pharmacokinetic and statistical methods.
Ritonavir pharmacokinetic parameters were estimated by using standard noncompartmental methods after dose administration on days 1 and 10. Maximal drug concentration in plasma (Cmax) and time to Cmax (Tmax) were obtained directly from individual concentration-time profiles. The area under the plasma concentration-time curve (AUC∞) was calculated as the sum of the AUC up to the last measurable concentration, computed by using the linear trapezoidal rule, and the extrapolation to infinity, calculated as the quotient of the last measurable concentration, and the terminal elimination rate constant (β). β was calculated as the negative of the slope of the regression of the logarithms of the drug concentrations in plasma versus time, from 18 h postdosing to 48 h postdosing. Samples were obtained at later time points on day 10 to ensure adequate characterization of the terminal elimination phase in the case of significant inhibition. However, to avoid any bias in the estimate of β due to differences in the sampling schedule, the same sampling times on days 1 and 10 were used to calculate β. The t1/2 of the terminal phase was obtained by dividing the natural logarithm of 2 by β. The apparent clearance was calculated as the dose/AUC∞ ratio.
Even though the sampling schedule for fluoxetine and its major metabolite was relatively sparse, fluoxetine and norfluoxetine AUCs were calculated by using the trapezoidal rule for the 0- to 24-h (AUC24) time interval on day 10. In addition, the fluoxetine apparent β was calculated by using concentrations measured from 24 to 72 h postdosing. The apparent elimination t1/2 was also calculated.
A paired t test was performed on the change in Tmax, Cmax, AUC∞, and β between day 1, when ritonavir was administered alone, and day 10, during concomitant administration of 30 mg of fluoxetine q12h. For both Cmax and AUC∞, a 95% confidence interval (CI) was obtained for the ratio of the mean on day 10, during administration of 30 mg of fluoxetine q12h, to the mean on day 1, when ritonavir was administered alone (9). The relationship between the change in the ritonavir AUC∞ and various fluoxetine or norfluoxetine pharmacokinetic parameters was explored by simple linear regression analysis. A two-way main-effects analysis of variance was performed to evaluate the effects of gender and genotype on ritonavir AUC∞, Cmax, and β and on the change in the ritonavir AUC from day 1 to day 10. The possibility of a gender-genotype interaction was ignored, since there were no female subjects with the B/wt genotype.
RESULTS
Subjects.
A total of 16 healthy male (n = 12) and female (n = 4) subjects were enrolled in and completed the study. Six subjects, all males, were identified as being heterozygous for the deactivating B CYP2D6 mutation (B/wt), while the remainder had the wt/wt genotype. The mean age of the volunteers ± the standard deviation (SD) was 29 ± 7 (range, 18 to 40 years). The mean weight and height ± SD were 79.4 ± 11.6 (range, 58.1 to 98.4) kg and 176 ± 8 (range, 161 to 188) cm, respectively.
Pharmacokinetics.
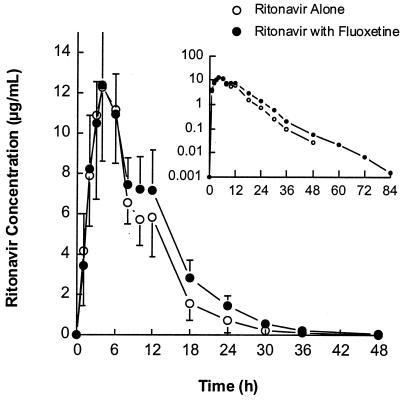
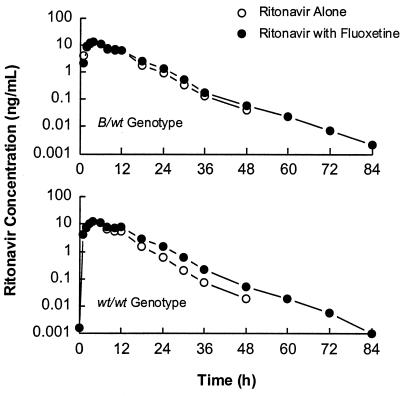
Ritonavir concentrations peaked approximately 4 h after dosing (range of 3 to 6 h) and decreased thereafter with a harmonic mean t1/2 of approximately 5 h both before and after fluoxetine administration (Table 1). Mean plasma ritonavir concentration-versus-time profiles after administration of 600 mg of ritonavir alone and with fluoxetine are illustrated for all subjects in Fig. 1 and separately for subjects with the CYP2D6 B/wt and wt/wt genotypes in Fig. 2. A statistically significant 19% increase in the ritonavir mean AUC∞ was observed with concomitant fluoxetine administration, with individual changes ranging from −12 to +56%. Tmax, Cmax, and β were similar after administration of ritonavir alone and with fluoxetine, and no statistically significant differences were noted in any of these parameters. Individual ritonavir pharmacokinetic profiles were characterized with the presence of a double or secondary peak (or shoulder) at approximately 10 to 12 h postdosing. The increase in the ritonavir AUC∞ during concomitant fluoxetine dosing was more apparent starting at approximately 10 h postdosing. Although the difference in the fluoxetine effect on the ritonavir AUC∞ between CYP2D6 genotypes was not statistically significant (P = 0.116), the increase may be more pronounced in subjects with the wt/wt genotype. The magnitude of the increase in the mean AUC∞ was 27% in subjects with the wt/wt genotype (n = 10) and 7% in subjects with the B/wt genotype (n = 6). A marginally significant CYP2D6 genotype effect (P = 0.083) was observed on the day 1 ritonavir AUC∞, with the least-squares mean being 25% larger in subjects with the B/wt genotype. No statistically significant differences were noted between genotypes in Cmax and β.
TABLE 1.
Ritonavir pharmacokinetic parameters after administration of ritonavir alone or with fluoxetinea
| Ritonavir administration | Tmax (h) | Cmax (μg/ml)b | AUC∞ (μg · h/ml)c | β (h−)d | t1/2 (h)e |
|---|---|---|---|---|---|
| Alone | 4.4 ± 1.2 | 13.12 ± 3.39 | 128.1 ± 34.1 | 0.145 ± 0.026 | 4.78 |
| With fluoxetine | 4.2 ± 0.8 | 12.53 ± 2.85 | 152.3 ± 33.0 | 0.137 ± 0.014 | 5.07 |
The values shown are means ± SD (n = 16).
Ratio of means, 0.955; 95% CI for the ratio of means, 0.850 to 1.076.
Ratio of means, 1.190; 95% CI for the ratio of means, 1.065 to 1.338.
Calculated by using ritonavir concentrations in plasma from 18 to 24 h postdosing.
Reported as a harmonic mean.
FIG. 1.
Mean ritonavir concentrations in plasma ± SD after administration of 600 mg of ritonavir alone or with fluoxetine in all subjects.
FIG. 2.
Mean ritonavir concentrations ± SD in plasma after administration of 600 mg of ritonavir alone or with fluoxetine in subjects with the CYP2D6 B/wt and wt/wt genotypes.
A statistically significant gender effect was observed in the day 1 ritonavir AUC∞ (P = 0.029) and Cmax (P = 0.008), in addition to a marginally significant effect (P = 0.084) in β, with larger values of all three parameters for females—39, 46, and 19% higher least-squares means, respectively—relative to those for male subjects. A statistically significant gender effect was observed in AUC∞ (P = 0.014) on day 10 as well, while no statistically significant differences were observed in Cmax and β on that day.
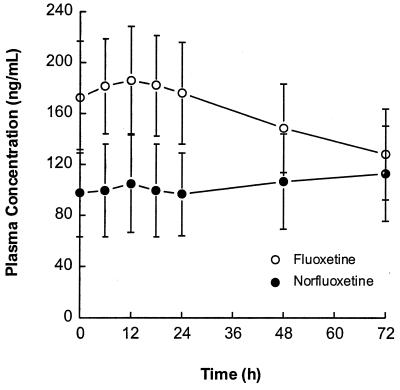
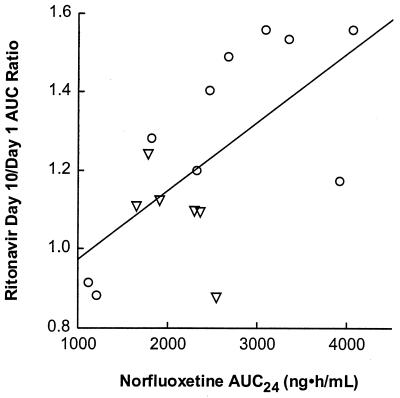
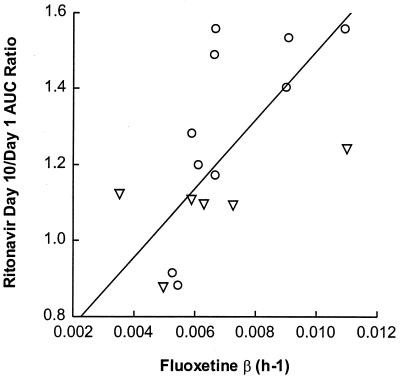
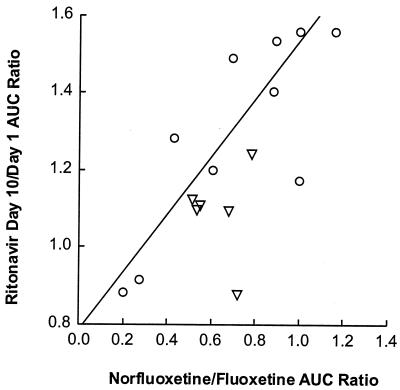
Fluoxetine and norfluoxetine concentration-versus-time profiles on day 10 are illustrated in Fig. 3. Individual fluoxetine apparent elimination t1/2 values ranged between 2.6 and 8.2 days, with a harmonic mean of 4.2 days (Table 2). Metabolite-to-parent AUC ratios for the 0- to 24-h time interval varied between 0.18 and 0.99, with a mean of 0.58. Little or no relationship was observed between the ratio of day 10 to day 1 ritonavir AUC∞ and fluoxetine AUC24 (r2 = 0.16, P = 0.13) or the total AUC of fluoxetine and norfluoxetine (r2 = 0.02, P = 0.57). However, statistically significant relationships were observed between the ritonavir AUC∞ ratio (day 10/day 1) and the norfluoxetine AUC24 (r2 = 0.42, P = 0.007), the norfluoxetine/fluoxetine AUC24 ratio (r2 = 0.53, P = 0.002), and the apparent fluoxetine β (r2 = 0.65, P = 0.0002), with larger increases in ritonavir AUC∞ tending to occur with higher norfluoxetine concentrations and higher fluoxetine β values. These relationships are illustrated in Fig. 4 to 6.
FIG. 3.
Mean plasma fluoxetine and norfluoxetine concentration-versus-time profiles ± SD on day 10.
TABLE 2.
Fluoxetine and norfluoxetine pharmacokinetic parameters on day 10a
| Drug | AUC24 (ng · h/ml) | β (h−1) | t1/2 (days)b | Metabolite/ parent ratio |
|---|---|---|---|---|
| Fluoxetine | 4,342 ± 946 | 0.00692 ± 0.00209 | 4.18 | 0.582 ± 0.226 |
| Norfluoxetine | 2,408 ± 863 |
The data shown are means ± SD (n = 16).
Reported as the harmonic mean.
FIG. 4.
Ratio of ritonavir AUC∞ (day 10/day 1) versus norfluoxetine AUC24. Circles represent subjects with the CYP2D6 wt/wt genotype; triangles represent subjects with the CYP2D6 B/wt genotype. Symbols represent individual data; the line represents the results of the regression analysis. The parameters of the regression line were as follows: intercept, 0.800 ± 0.141; slope, 0.000175 ± 0.00006; P = 0.007; r2 = 0.416.
FIG. 6.
Ratio of ritonavir AUC∞ (day 10/day 1) versus fluoxetine β. Circles represent subjects with the CYP2D6 wt/wt genotype; triangles represent subjects with the CYP2D6 B/wt genotype. Symbols represent individual data; the line represents the results of the regression analysis. The parameters of the regression line were as follows: intercept, 0.597 ± 0.127; slope, 90.4 ± 17.6; P = 0.0002; r2 = 0.653.
The regimens were well tolerated, and all of the adverse events reported in this study were rated mild or moderate in severity.
DISCUSSION
Increases in ritonavir AUC∞ were observed with concomitant fluoxetine administration, with the magnitude of the increase apparently related to norfluoxetine concentrations rather than fluoxetine concentrations. Statistically significant correlations were observed between the change in ritonavir AUC∞ and the norfluoxetine AUC, the norfluoxetine/fluoxetine AUC24 ratio, and the fluoxetine apparent β. Further investigation of the fluoxetine β revealed a statistically significant relationship with the norfluoxetine AUC (r2 = 0.43, P = 0.006) but not the fluoxetine AUC (r2 = 0.12, P = 0.20).
Differences in inhibition potency against various CYP450 isoforms have been reported for fluoxetine and norfluoxetine. While the magnitudes of in vitro inhibition of CYP2D6 by fluoxetine and its metabolite are similar, in vitro inhibition of CYP3A by norfluoxetine has been reported to be three- to sevenfold more potent than that of fluoxetine (18, 19, 22). The Ki values for the inhibition of sparteine metabolism by CYP2D6 were reported to be 0.60 and 0.43 μM for fluoxetine and norfluoxetine, respectively (6). Inhibition of CYP3A activity has been assessed by using the 6β-hydroxylation of cortisol and testosterone, the formation of nortriptyline from amitriptyline, and the 4- and α-hydroxylation of alprazolam, with respective Ki values of 60, 75, 44, 83, and 47 μM for fluoxetine and 19, 11, 12, 11, and 9 μM for norfluoxetine. In vivo inhibitory effects of fluoxetine have been consistent with the in vitro findings, with substantially larger effects being observed with CYP2D6 substrates (4) than with CYP3A substrates (11).
Ritonavir conversion to its metabolites M-1 and M-11 is mediated predominantly by CYP3A4, while both CYP3A4 and CYP2D6 contribute to the formation of the major metabolite M-2 (14). From the in vitro experiments, the dominant isoform in the overall metabolism of ritonavir appeared to be CYP3A. This was reflected by lower Km values for CYP3A than for CYP2D6 (0.7 versus 10 μM for M-2 formation) and strong inhibition of M-2 formation by anti-CYP3A4 immunoglobulin. In this regard, it should be appreciated that the fraction of total P-450 in human liver is much higher for CYP3A (28.8%) than for CYP2D6 (1.8%) (22). The present study served as a confirmation of expectations based on the in vitro data. Although there were no subjects with the CYP2D6 poor-metabolizer genotype in the present study, the difference in day 1 ritonavir apparent clearance between the B/wt and wt/wt genotypes was not large (least-square means AUC∞ values, 160 versus 128 μg · h/ml), and the effect of fluoxetine was much smaller than expected if CYP2D6 were dominant.
The effect of fluoxetine on ritonavir clearance appears to be largely mediated through inhibition of CYP2D6, although minor effects at CYP3A cannot be excluded. The observation that greater inhibitory effects were observed in subjects with the CYP2D6 wt/wt genotype than in those with the B/wt genotype indicates that the partial inhibitory effect at CYP2D6 was much greater than that at CYP3A, particularly when one considers that CYP2D6 accounts for only a small fraction of ritonavir total clearance. The observation that a larger norfluoxetine AUC was associated with greater inhibitory effects might indicate that part of the observed effect is associated with CYP3A inhibition, since the metabolite is a substantially better inhibitor of this isoform than is the parent drug. However, it must be appreciated that norfluoxetine is a relatively weak inhibitor of CYP3A, and this metabolite is a slightly more potent inhibitor at CYP2D6 than is fluoxetine. More importantly, it should be noted that ritonavir has very high affinity for CYP3A, with Km values for the formation of its metabolites M-2, M-1, and M-11 ranging from 0.08 to 0.71 μM (14). Norfluoxetine’s Ki values at this isoform are typically greater than 10 μM, indicating lower binding affinity for the enzyme than that of ritonavir. From the low Km values for ritonavir, it would be expected that it should be a potent inhibitor of CYP3A, and this indeed has been observed in vitro (50% inhibitory concentration for nifedipine oxidation, 0.07 μM). In contrast, the concentration of ritonavir required for 50% inhibition of CYP2D6-mediated O-demethylation of dextromethorphan was 2.5 μM. Thus, from mass action considerations and the various in vitro data, it appears that the fluoxetine effect on ritonavir pharmacokinetics was probably CYP2D6 mediated. Based on this premise, it would be expected that the effect of fluoxetine on steady-state pharmacokinetics of ritonavir would be smaller than that observed in the present study, since autoinduction of CYP3A occurs with multiple dosing (12) and since CYP2D6 is not known to be inducible.
The effect of ritonavir on the metabolism of fluoxetine was not investigated in the present study. The fluoxetine apparent clearance was calculated to be 13.8 liters/h based on the mean AUC24 of fluoxetine of 4,342 ng · h/ml. This clearance is probably an overestimate of the true value, since a steady state may not have been obtained, but the value is nonetheless comparable to the 10 liters/h reported in the literature. The harmonic mean fluoxetine t1/2 of 4.2 days is within the range of values reported in the literature. Regardless, it should be noted that the standard initial dosing regimen for fluoxetine is 20 mg/day; thus, the exposure attained in the present study at day 10 with 60 mg daily meets or exceeds that normally attained at steady state in patients treated for depression. Differences in fluoxetine AUC values between subjects with poor and extensive drug-metabolizing CYP2D6 enzymes have been reported (8, 15), leading to the inference that CYP2D6 is responsible for most of the clearance. Since fluoxetine binds avidly to CYP2D6, substantial competitive inhibition by ritonavir at this isoform is not expected.
In summary, therapeutic concentrations of fluoxetine and norfluoxetine produced minor but statistically significant effects on the apparent clearance of ritonavir. The 95% CIs for the ratio of ritonavir AUC∞ means and the ratio of Cmax means are reasonably narrow and do not extend to a great distance from unity. This indicates that the data of this study do indeed support the inference that the interaction effect is limited in magnitude. The mechanism of the effect is not precisely known, but it is believed to be due in part to postabsorption inhibition of ritonavir elimination, with greater effects observed in subjects with higher norfluoxetine concentrations and possibly a greater effect with the CYP2D6 wt/wt genotype. No ritonavir dose adjustment is recommended during concomitant fluoxetine administration.
FIG. 5.
Ratio of ritonavir AUC∞ (day 10/day 1) versus norfluoxetine/fluoxetine AUC24 ratio. Circles represent subjects with the CYP2D6 wt/wt genotype; triangles represent subjects with the CYP2D6 B/wt genotype. Symbols represent individual data; the line represents the results of the regression analysis. The parameters of the regression line were as follows: intercept, 0.785 ± 0.118; slope, 0.751 ± 0.190; P = 0.002; r2 = 0.527.
REFERENCES
- 1.Abbott Laboratories. Norvir package insert. Abbott Park, Ill: Abbott Laboratories; 1996. [Google Scholar]
- 2.Altamura A C, Moro A R, Percudani M. Clinical pharmacokinetics of fluoxetine. Clin Pharmacokinet. 1994;26:201–214. doi: 10.2165/00003088-199426030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Baumann, P., and B. Rochat. 1995. Comparative pharmacokinetics of selective serotonin reuptake inhibitors: a look behind the mirror. Int. Clin. Psychopharmacol. 10(Suppl. 1):15–21. [DOI] [PubMed]
- 4.Bergstrom R F, Peyton A L, Lemberger L. Quantification and mechanism of the fluoxetine and tricyclic antidepressant interaction. Clin Pharmacol Ther. 1992;51:239–248. doi: 10.1038/clpt.1992.18. [DOI] [PubMed] [Google Scholar]
- 5.Brøsen K, Skjelbo E. Fluoxetine and norfluoxetine are potent inhibitors of P450IID6—the source of the sparteine/debrisoquine oxidation polymorphism. Br J Clin Pharmacol. 1991;32:136–137. doi: 10.1111/j.1365-2125.1991.tb05630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crewe H K, Lennard M S, Tucker G T, Woods F R, Haddock R E. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34:262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, Aguado A G, De Lomas J G, Delgado R, Borleffs J C C, Hsu A, Valdes J M, Boucher C A B, Cooper D A. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 8.Dista Products Co.; Eli Lilly & Co. Prozac package insert. Indianapolis, Ind: Dista Products Co. and Eli Lilly & Co.; 1996. [Google Scholar]
- 9.Fieller E. Some problems in interval estimation. J R Statistical Soc B. 1954;16:175–185. [Google Scholar]
- 10.Fulton B, McTavish D. Fluoxetine—an overview of its pharmacodynamics and pharmacokinetic properties and review of its therapeutic efficacy in obsessive-compulsive disorder. Cent Nerv Syst Drugs. 1995;3(4):305–322. [Google Scholar]
- 11.Greenblatt D J, Preskorn S H, Cotreau M M, Horst W D, Harmatz J S. Fluoxetine impairs clearance of alprazolam but not of clonazepam. Clin Pharmacol Ther. 1995;52(5):479–486. doi: 10.1038/clpt.1992.175. [DOI] [PubMed] [Google Scholar]
- 12.Hsu A, Granneman G R, Witt G, Locke C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Liu P, Decourt J P, Fourtillan J B, Girault J, Leonard J M. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempf D J, Marsh K C, Denissen J F, McDonald E, Vasavanonda S, Flentge C A, Green B E, Fino L, Park C H, Kong X-P, Wideburg N E, Saldivar A, Ruiz L, Kati W M, Sham H L, Robins T, Stewart K D, Hsu A, Plattner J J, Leonard J M, Norbeck D W. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci USA. 1995;92:2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar G N, Rodrigues A D, Buko A M, Denissen J F. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;227:423–431. [PubMed] [Google Scholar]
- 15.Lebel, M., J. Turgeon, F. Vallée, P.-M. Bélanger, F. Paquet, and B. A. Hamelin. 1995. Genetic determinant of sertraline (S) and fluoxetine (F) disposition in 20 healthy volunteers. Pharm. Res. 12(Suppl.):S-374.
- 16.Markowitz M, Saag M, Powderly W G, Hurley A M, Hsu A, Valdes J M, Henry D, Sattler F, La Marca A, Leonard J M, Ho D D. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 17.Marsh K C, Eiden E, McDonald E. Determination of ritonavir, a new HIV protease inhibitor, in biological samples using reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;704:307–313. doi: 10.1016/s0378-4347(97)00454-4. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen B B, Mäenpää J, Pelkonen O, Loft S, Poulsen H E, Lykkesfeldt J, Brøsen K. Selective serotonin reuptake inhibitors and theophylline metabolism in human liver microsomes: potent inhibition by fluvoxamine. Br J Clin Pharmacol. 1995;39:151–159. doi: 10.1111/j.1365-2125.1995.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robins T, Plattner J. HIV protease inhibitors: their anti-HIV activity and potential role in treatment. J Acquired Immune Defic Syndr. 1993;6:162–170. [PubMed] [Google Scholar]
- 20.Schmider J, Greenblatt D J, Von Molke L L, Harmatz J S, Shader R I. N-demethylation of amitriptyline in vitro: role of cytochrome P-450 3A (CYP3A) isoforms and effect of metabolic inhibitors. J Pharmacol Exp Ther. 1995;275:592–597. [PubMed] [Google Scholar]
- 21.Stevens J C, Wrighton S A. Interaction of the enantiomers of fluoxetine and norfluoxetine with human liver cytochromes P450. J Pharmacol Exp Ther. 1993;266(2):964–971. [PubMed] [Google Scholar]
- 22.Von Moltke, L. L., D. J. Greenblatt, J. Schmider, J. S. Harmatz, and R. I. Shader. 1995. Metabolism of drugs by cytochrome P450 3A isoforms: implications for drug interactions in psychopharmacology. Clin. Pharmacokinet. 29(Suppl. 1):33–44. [DOI] [PubMed]
- 23.Wang J-P, Unadkat J D. ISSX Proceedings, 4th International ISSX Meeting. 1995. Human P450 isoforms involved in the formation of the active metabolites of (R)- and (S)-fluoxetine; p. 385. [Google Scholar]