Key Points
Question
Does laparoscopic pancreaticoduodenectomy performed by experienced surgeons who surmounted the learning curve yield similar short-term outcomes to open pancreaticoduodenectomy in patients with pancreatic ductal adenocarcinoma?
Findings
In this randomized clinical trial that included 200 patients with pancreatic ductal adenocarcinoma, laparoscopic pancreaticoduodenectomy yielded similar short-term outcomes to open pancreaticoduodenectomy, with comparable postoperative length of stay, postoperative surgical complications, number of lymph nodes harvested, and 90-day mortality.
Meaning
For experienced pancreatic surgeons, performing laparoscopic pancreaticoduodenectomy in resectable pancreatic cancer was technically safe and did not increase the risk of intraoperative and postoperative complications in this study.
This randomized clinical trial compares short-term outcomes among patients undergoing laparoscopic vs open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma.
Abstract
Importance
The safety and efficacy of laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma remain controversial.
Objective
To compare laparoscopic and open pancreaticoduodenectomy performed by experienced surgeons in patients with pancreatic ductal adenocarcinoma.
Design, Setting, and Participants
This was a noninferiority, open-label randomized clinical trial between September 20, 2019 and March 20, 2022, at 10 hospitals in China. A total of 412 adult patients were assessed for eligibility; 200 patients with histologically confirmed or clinically diagnosed pancreatic ductal adenocarcinoma who were eligible to undergo pancreaticoduodenectomy were enrolled. Study recruitment is complete, and follow-up is ongoing. This article reports prespecified early safety results from the trial.
Interventions
Participants were randomized in a 1:1 ratio to undergo either laparoscopic or open pancreaticoduodenectomy, to be performed by experienced surgeons who had already performed at least 104 laparoscopic pancreaticoduodenectomy operations.
Main Outcomes and Measures
The primary end point is 5-year overall survival, but the data for this end point are not yet mature; thus, secondary short-term outcomes, including operative findings, complications, mortality, and oncological results are reported here. The outcomes were analyzed according to a modified intention-to-treat and per-protocol principle.
Results
Among 412 patients for eligibility, 200 patients were enrolled and randomly assigned 1:1 to have laparoscopic pancreaticoduodenectomy or open pancreaticoduodenectomy. The mean (SD) age was 61.3 (9.3) years, and 78 participants (39%) were female. Laparoscopic procedures had longer operative times (median [IQR], 330.0 [287.5-405.0] minutes vs 297.0 [245.0-340.0] minutes; P < .001). Patients in the laparoscopic group lost less blood than those in the open group (median [IQR], 145.0 [100.0-200.0] mL vs 200.0 [100.0-425.0] mL; P = .02). Ninety-day mortality occurred in 2 of 100 patients in the laparoscopic group and 0 of 100 patients in the open group. There was no difference in the rates of complications of the Clavien-Dindo grades III−IV (n = 17 [17.0%] vs n = 23 [23.0%]; P = .29), comprehensive complication index (median [IQR], 0.0 [0.0-22.6] vs 8.7 [0.0-26.2]; P = .79) or median (IQR) postoperative length of stay (14.0 [11.0-17.0] days vs 14.0 [12.0-18.5] days; P = .37) between the 2 groups.
Conclusions and Relevance
Laparoscopic pancreaticoduodenectomy performed by experienced surgeons in high-volume specialized institutions resulted in similar short-term outcomes compared with open pancreaticoduodenectomy among patients with pancreatic ductal adenocarcinoma.
Trial Registration
ClinicalTrials.gov Identifier: NCT03785743
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a highly fatal malignancy and is the third leading cause of death among adults, with an estimated 5-year survival rate of 11%.1,2 Pancreaticoduodenectomy (PD) was first developed for lesions of the head of the pancreas and has evolved over the last 100 years. With the development of minimally invasive surgery, laparoscopic PD (LPD), once known as the Mount Everest of pancreatic surgery, has undergone unprecedented development in recent years. Although various reports have shown that LPD is safe, there is a paucity of studies with sufficient numbers of patients that would allow laparoscopic surgery in PDAC to be clinically accepted.3,4,5
To comprehensively and systematically compare LPD and open PD (OPD), we conducted what is, to our knowledge, the largest randomized clinical trial (RCT) on LPD to date,6 which confirmed that LPD was safe compared to OPD but had marginal benefit.7 All participating surgeons and centers were experienced and constituted mature teams. The study participants had a wide range of diagnoses not limited to PDAC, including tumors in the distal common bile duct, duodenal papilla, and ampulla. These inconsistencies in pathological properties undoubtedly confounded and skewed the results, limiting our ability to assess the actual safety and efficacy of LPD in PDAC.
Against this background, the Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China’s International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM) designed and launched a multicenter RCT to compare short- and long-term outcomes of LPD vs OPD in resectable PDAC. The primary end point was the 5-year overall survival (OS) rate. The secondary end points included mortality and morbidity rates, length of stay, comprehensive complication index, and operative outcomes. In this article, we present the prespecified secondary (short-term) outcomes of this trial.
Methods
Study Design
An investigator-initiated, multicenter, parallel-group, open-label RCT comparing LPD and OPD was conducted at 10 centers of the MITG-P-CPAM group in 8 provinces of China. The trial was designed by the investigators, and an independent data and safety monitoring committee oversaw it and periodically assessed safety. The study protocol and all amendments were approved by the Tongji Hospital Ethics Committee and the institutional review board of each participating center. The trial protocol has been published previously and is available online8 and in Supplement 1. All patients provided written informed consent before enrollment. All authors had access to the study data and reviewed and approved the final manuscript. The trial was prepared, analyzed and reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.9
Patients
Between September 20, 2019, and March 20, 2022, we enrolled patients aged 18 to 75 years with histologically confirmed PDAC or clinically diagnosed PDAC identified by a multidisciplinary team without histopathologic evidence who were eligible to undergo PD.10 Patients were excluded if they had vascular invasion and required vascular resection as evaluated by the multidisciplinary team according to abdominal imaging data; evidence of distant metastases; lesions that required left, central, or total pancreatectomy or another form of palliative surgery; an American Society of Anesthesiologists score of 4 or higher; or a history of another malignant disease. Full details on eligibility criteria are included in the study protocol.8
Randomization and Masking
Block randomization was used in this study (block size of 4) with the investigators blinded to the block size. Eligible patients were enrolled by the treating investigators at each center and were randomly assigned in a 1:1 ratio by an interactive voice or web response system, without stratification, to undergo either LPD or OPD. The randomization list (random allocation sequence) was produced by an independent statistician using the proc plan procedure in SAS version 9.4 (SAS Institute). The independent statistician played no role in the clinical aspects of the study, ensuring random assignment of the patients’ identification numbers to randomization numbers after they were deemed eligible. Each number was linked to a treatment group and the external provider assigned them to the trial groups. The study was open label and patients and investigators were not blinded to treatment allocation. The data analysis process was blinded such that only group codes (A or B) were provided to the statistician during the statistical analysis. An independent contract research organization at the Department of Epidemiology and Biostatistics of Tongji Medical College, Huazhong University of Science and Technology, monitored this study and was responsible for the data management and statistical analyses.
Procedures
The techniques used for LPD and OPD have been previously described.11 The LPD group only included patients who intended to undergo total laparoscopic surgery. Patients who intended to undergo laparoscopic-assisted surgery were excluded. All participating surgeons were required to perform 50 pancreaticoduodenectomies annually, at least 20 of which should be laparoscopic and to have performed at least 104 LPDs. Detailed criteria are described in the study protocol and shown in Supplement 1. Surgical quality control was maintained by using mandatory intraoperative photographs or videos that identified specific surgical fields. Approximately 20% of LPD and OPD photographs or videos were randomly selected for evaluation. These photographs and videos were reviewed, and feedback on the operative quality was regularly provided to the investigators.
The lymph node clearance field was maintained as described previously.12 Pancreatic anastomosis procedures were not uniform in all study participants because each surgeon was allowed to follow their preference or the usual protocol of their center. Conversion was defined as when a skin incision was used for reasons other than trocar placement or specimen removal in any LPD group. Postoperative care was similar for both groups at all centers. After surgical resection, patients pathologically diagnosed with PDAC received adjuvant chemotherapy according to the National Comprehensive Cancer Network guideline.10 Different regimens recommended in the aforementioned guideline were permitted, and the treatment duration was left to the discretion of the responsible treating oncologist. Detailed information on adjuvant chemotherapy was recorded. Short-term follow-up was conducted at 1 day, 1 week, 1 month, and 3 months postoperatively on an in-hospital or outpatient base. Patients are being monitored for up to 5 years or until death.
Outcomes
The primary outcome was the 5-year OS rate, calculated from the surgery date to the date of death from any cause. Primary end point data are due to mature in March 2027, and will therefore be reported at a later date. The primary end point will be centrally reviewed by the quality control committee.
The prespecified secondary end points reported in this article included intraoperative indicators, mortality occurring within 90 days of surgery, and postoperative morbidity. Intraoperative indicators included estimated intraoperative blood loss, intraoperative transfusion (including transfusion of red blood cells and other blood products), and operation time. Mortality was defined as death from any cause within 90 days after surgery. Morbidity was defined as the occurrence of postoperative surgical complications related to PD, including major complications with a Clavien-Dindo score of 3 or higher,13 postoperative pancreatic fistula (POPF),14 postoperative bile leakage,15 postpancreatectomy hemorrhage (PPH),16 delayed gastric emptying,17 and chyle leakage,18 which were defined according to the system put forward by the International Study Group of Pancreatic Surgery. The comprehensive complication index19 was calculated as the sum of all scores for complications weighted for severity.20 Length of stay (LOS) was defined as the number of nights spent in the hospital from the end of the surgical procedure until discharge or death. The objective parameters for assessing the first off-bed activity included stable vital signs, no obvious tendency for bleeding, and a visual analogue scale pain score less than 5. The objective parameters for transfer out of the intensive care unit included stable vital signs and normal hematological or biochemical parameters. The nasogastric tube was removed if the daily output was less than 200 mL. Additionally, first oral intake was encouraged 1 day after nasogastric tube removal in the absence of vomiting or nausea. The surgeons were not involved in postoperative management and hence could not influence the postoperative care of the participants.
Sample Size Calculation
The sample size was calculated based on the primary end point and the noninferiority design of this trial. The details of the calculation were described in the protocol.8 Accordingly, 100 patients in each arm and 200 patients in total were included for randomization.
Statistical Analyses
Modified intention-to-treat (mITT) analyses included only those patients who underwent PD (excluding patients with intraoperatively detected nonresectable or metastatic disease who did not undergo PD). Data regarding patients who underwent laparoscopic surgery with conversion to open surgery were analyzed in the LPD group. Exploratory per-protocol (PP) analyses were performed, excluding those patients who had crossed over. Appropriate descriptive statistics were applied to all data and reported as medians with IQRs or means with SDs for continuous data and frequencies and percentages for categorical data. Surgical and disease-related characteristics and outcomes were compared between the 2 groups using t test for continuous data, Mann-Whitney U test for variables with nonparametric distributions, and χ2 or Fisher exact test for categorical data. The Newcombe method was used to calculate 95% CIs for between-group differences in intraoperative and postoperative complication rates.21 The mITT approach was used for all analyses, and the PP and as-treated populations were applied in the sensitivity analyses. Imputation of outcomes was not performed, given the low observed rate of missingness for the primary outcome. All statistical analyses were performed using SAS version 9.4 (SAS Institute), and analysis items with 2-sided P values <.05 were considered statistically significant.
Results
Patient Characteristics
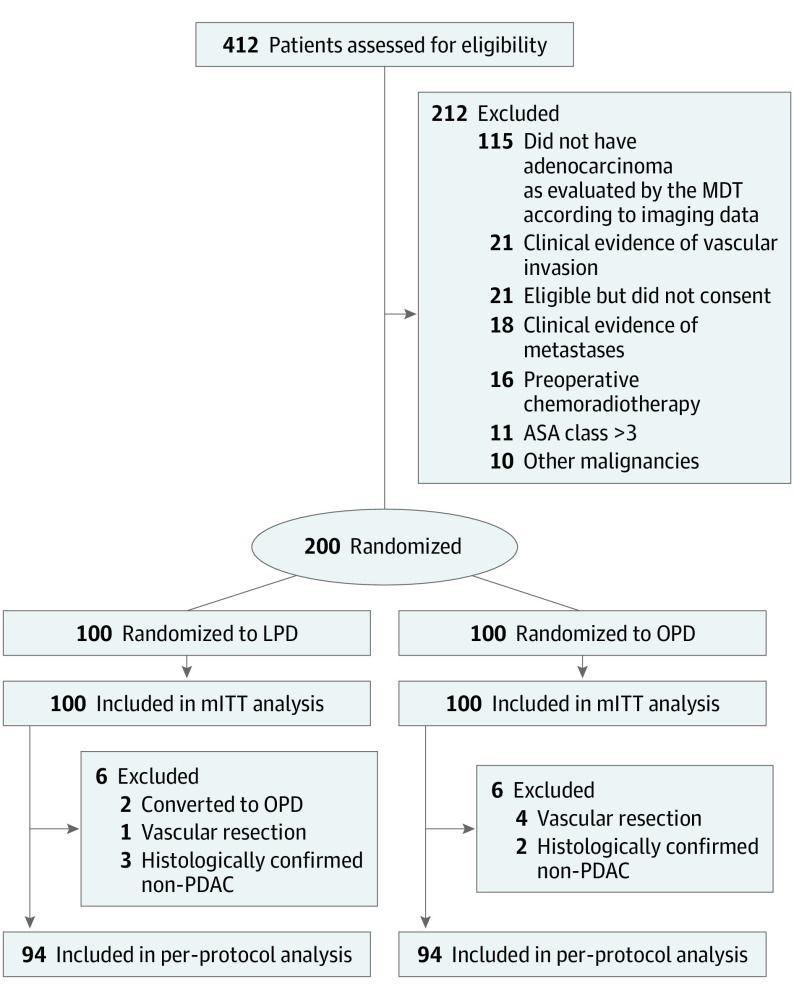
Between September 20, 2019, and March 20, 2022, of 412 patients initially assessed, 212 were excluded before enrollment, and 200 were randomly assigned to the LPD (n = 100) or OPD group (n = 100). The mean (SD) age was 61.3 (9.3) years, and 78 participants (39%) were female. Two patients moved from the LPD group to the OPD group because of unexpected intraoperative bleeding and vascular invasion. Five patients were excluded from the PP analysis, owing to vascular invasion and resection (1 in the LPD group and 4 in the OPD group). Five patients were histologically confirmed as non-PDAC (3 in the LPD and 2 in the OPD group) and were excluded from the PP analysis. Finally, 200 patients were included in the mITT analysis and 188 patients in the PP analysis. The flowchart of the study is shown in the Figure. The baseline clinical and pathologic characteristics of the patients were balanced between the 2 groups (Table 1).
Figure. Flow Diagram.
ASA indicates American Society of Anesthesiologists; LPD, laparoscopic pancreaticoduodenectomy; MDT, multidisciplinary team; mITT, modified intention-to-treat; OPD, open pancreaticoduodenectomy; PDAC, pancreatic ductal adenocarcinoma.
Table 1. Baseline Characteristics (mITT Analysis).
| Characteristic | No. (%) | |
|---|---|---|
| LPD group (n = 100) | OPD group (n = 100) | |
| Age, mean (SD), y | 61.9 (9.5) | 60.7 (9.0) |
| Sex | ||
| Male | 61 (61.0) | 61 (61.0) |
| Female | 39 (39.0) | 39 (39.0) |
| BMI, mean (SD) | 22.9 (2.9) | 22.3 (2.8) |
| ASA classification | ||
| I | 32 (32.0) | 22 (22.0) |
| II | 41 (41.0) | 54 (54.0) |
| III | 27 (27.0) | 24 (24.0) |
| ECOG scale score | ||
| 0-1 | 84 (84.0) | 85 (85.0) |
| 2 | 16 (16.0) | 15 (15.0) |
| Comorbidities | ||
| None | 59 (59.0) | 63 (63.0) |
| ≥1 | 41 (41.0) | 37 (37.0) |
| Previous surgery | 26 (26.0) | 32 (32.0) |
| Preoperative biliary drainage | 44 (44.0) | 37 (37.0) |
| Wirsung duct diameter, mm | ||
| <3 | 14 (14.0) | 17 (17.0) |
| ≥3 | 86 (86.0) | 83 (83.0) |
| Consistency of the pancreas | ||
| Soft/normal | 44 (44.0) | 39 (39.0) |
| Firm/hard | 56 (56.0) | 61 (61.0) |
| Mass size, mean (SD) mm | 2.9 (1.0) | 2.9 (1.0) |
| AJCC staging | ||
| IA | 17 (17.0) | 20 (19.2) |
| IB | 40 (40.0) | 32 (32.0) |
| IIA | 5 (5.0) | 3 (3.0) |
| IIB | 30 (30.0) | 36 (36.0) |
| III | 8 (8.0) | 9 (9.0) |
Abbreviations: ASA, American Society of Anesthesiologists; AJCC, American Joint Committee of Cancer; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECOG, Eastern Cooperative Oncology Group; LPD, laparoscopic pancreaticoduodenectomy; OPD, open pancreaticoduodenectomy; mITT, modified intention-to-treat.
Surgical Outcomes
Surgical outcomes are summarized in Table 2. The median operation time in the LPD group was 44.3 minutes longer than in the OPD group (median [IQR], 330.0 [287.5-405.0] minutes vs 297.0 [245.0-340.0] minutes; P < .001). The mean estimated volume of blood lost in the LPD group was 115.2 mL less than in the OPD group (median [IQR], 145.0 [100.0-200.0] mL vs 200.0 [100.0-425.0] mL; P = .02). Intraoperative transfusion was administered to 23 of 100 patients (23.0%) in the LPD group and 30 of 100 (30.0%) in the OPD group (P = .26). There were no significant differences between the 2 groups in the positive margin rate (3 [3.0%] vs 0; P = .25) or the number of lymph nodes retrieved (median [IQR], 17.0 [12.5-21.0] vs 16.0 [9.0-21.5]; P = .41); median (IQR) LOS (14.0 [11.0-17.0] days vs 14.0 [12.0-18.5] days, respectively; log-rank test P = .37); or the times for first off-bed activity, nasogastric tube removal, and first oral intake. There was no significant difference in time to adjuvant therapy (median [IQR], 41.5 [35.0-53.0] days vs 43.0 [32.0-57.0] days; P = .36) between the 2 groups (eTable 3 in Supplement 2).
Table 2. Surgical and Histopathological Outcomes (mITT and PP Analyses).
| Characteristic | mITT (n = 200), median (IQR) | Difference (95% CI)a | P valueb | PP (n = 188), median (IQR) | Difference (95% CI)a | P valueb | ||
|---|---|---|---|---|---|---|---|---|
| LPD group (n = 100) | OPD group (n = 100) | LPD group (n = 94) | OPD group (n = 94) | |||||
| Conversion from laparoscopy to open, No. (%) | 2 (2.0) | NA | NA | NA | NA | NA | NA | NA |
| Operative time, min | 330.0 (287.5 to 405.0) | 297.0 (245.0 to 340.0) | 44.3 (18.5 to 70.0) | <.001 | 330.0 (285.0 to 400.0) | 300.0 (240.0 to 340.0) | 40.8 (14.4 to 67.1) | .002 |
| EIBL, mL | 145.0 (100.0 to 200.0) | 200.0 (100.0 to 425.0) | −115.2 (−212.9 to −17.5) | .02 | 120.0 (100.0 to 200.0) | 200.0 (150.0 to 450.0) | −147.0 (−232.4 to −61.6) | <.001 |
| Intraoperative blood transfusion, No. (%) | 23 (23.0) | 30 (30.0) | −7.0 (−19.2 to 5.2) | .26 | 22 (23.4) | 28 (29.8) | −6.4 (−19.0 to 6.2) | .32 |
| Pancreatic reconstruction, No. (%) | ||||||||
| Duct to mucosa | 59 (59.0) | 63 (63.0) | NA | .56 | 55 (58.5) | 62 (66.0) | NA | .29 |
| Other | 41 (41.0) | 37 (37.0) | NA | 39 (41.5) | 32 (34.0) | NA | ||
| Vascular resection, No. (%) | 2 (2.0) | 4 (4.0) | −2.0 (−6.7 to 2.7) | .68c | 0 | 0 | NA | NA |
| Histopathological type | ||||||||
| PDAC, No. (%) | 97 (97.0) | 98 (98.0) | NA | .65c | 94 (100.0) | 94 (100.0) | NA | NA |
| Other, No. (%) | 3 (3.0) | 2 (2.0) | NA | 0 | 0 | NA | ||
| Histologic grade, No. (%)d | ||||||||
| Well | 7 (7.0) | 8 (8.0) | NA | .69 | 7 (7.5) | 6 (6.4) | NA | .65 |
| Moderately | 62 (62.0) | 56 (56.0) | NA | 58 (61.7) | 53 (56.4) | NA | ||
| Poorly | 31 (31.0) | 36 (36.0) | NA | 29 (30.9) | 35 (37.2) | NA | ||
| Lymph node countd | 17.0 (12.5 to 21.0) | 16.0 (9.0 to 21.5) | 0.8 (−1.1 to 2.7) | .41 | 17.0 (13.0 to 21.0) | 16.0 (9.0 to 21.0) | 1.0 (−1.0 to 2.9) | .21 |
| Tumor-positive nodesd,c | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.5) | −0.4 (−1.0 to 0.2) | .15e | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.0) | −0.3 (−0.8 to 0.3) | .28 |
| Positive resection margind | 3 (3.0) | 0 | 3.0 (−0.3 to 6.3) | .25c | 3 (3.2) | 0 | 3.2 (−0.4 to 6.7) | .08c |
| Time to first off-bed activity, d | 3.0 (2.0 to 5.0) | 3.0 (2.0 to 4.0) | −0.3 (−0.9 to 0.3) | .33e | 3.0 (2.0 to 5.0) | 3.0 (2.0 to 4.0) | −0.1 (−0.6 to 0.4) | .61 |
| Time to first oral food intake, d | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 0.1 (−0.9 to 1.2) | .84e | 4.0 (3.0 to 5.0) | 4.0 (3.0 to 5.0) | 0.2 (−0.9 to 1.3) | .59 |
| Time to postoperative nasogastric tube removal, d | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 5.0) | −0.3 (−1.7 to 1.0) | .60e | 3.0 (2.0 to 4.0) | 3.0 (2.0 to 5.0) | −0.4 (−1.8 to 1.0) | .35 |
| Length of ICU stay, d | 3.0 (3.0 to 6.0) | 3.0 (3.0 to 4.0) | 0.1 (−0.8 to 0.8) | .92e | 3.0 (3.0 to 5.0) | 3.00 (3.0 to 4.0) | 0.2 (−0.4 to 0.9) | .54 |
| Length of stay, d | 14.0 (11.0 to 17.0) | 14.0 (12.0 to 18.5) | −0.8 (−2.9 to 1.2) | .37 | 14.0 (11.0 to 17.0) | 14.0 (12.0 to 18.0) | −0.6 (−2.8 to 1.6) | .43 |
Abbreviations: EIBL, estimated intraoperative blood loss; ICU, intensive care unit; LPD, laparoscopic pancreaticoduodenectomy; mITT, modified intention-to-treat; NA, not applicable; OPD, open pancreaticoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; PP, per-protocol.
Categorical variables were described as risk difference (95% CI), continuous variables as mean difference (95% CI).
Except where otherwise noted, P values are derived from t test for continuous data and χ2 test for categorical data.
The mean number of tumor-positive lymph nodes was 0.86 in the LPD group vs 1.02 in the OPD group in the mITT analysis, whereas in the PP analysis, these values were 0.88 vs 0.98, respectively.
For pathological stage of malignancy.
Wilcoxon rank sum test.
Postoperative Complications
The distributions of mortality and morbidities are shown in Table 3. Within 90 days of operation, there were 2 (2.0%) deaths in the LPD and none in the OPD group (P = .50). Complication-related death during hospitalization in the LPD group was caused by PPH in 1 patient (the patient died on postoperative day 6); the other death was caused by liver metastasis in the third month after operation. The overall postoperative morbidity rate was 46.0% in the LPD group and 54.0% in the OPD group (P = .26). The comprehensive complication index scores were similar between the 2 groups (median [IQR], 0.0 [0.0-22.6] vs 8.7 [0.0-26.2]; P = .79). Moreover, severe complications (Clavien-Dindo grade III or greater) occurred in 17 of 100 patients (17.0%) after LPD and 23 of 100 (23.0%) after OPD (P = .29). There were no significant differences in the overall POPF or clinically significant POPF (grade B/C) rates between the 2 groups (P = .62 and P = .62, respectively). PPH (n = 7 [7.0%] vs n = 5 [5.0%]; P = .55) was comparable between groups. There were also no significant differences in the incidence of other surgery-related morbidities, such as delayed gastric emptying, biliary leakage, or intra-abdominal infection, between groups. Furthermore, the reoperation rates (n = 3 [3.0%] after LPD and n = 2 [2.0%] after OPD, P = .50) were similar. There was no significant difference in the 90-day readmission rate between the 2 groups (n = 0 [0.0%] after LPD compared to n = 2 [2.0%] after OPD, P = .16). The reasons for readmission were abdominal pain and vomiting (1 patient each).
Table 3. Postoperative Complications (mITT and PP Analyses).
| Characteristic | mITT (n = 200), No. (%) | Risk difference (95% CI) | P valuea | PP (n = 188), No. (%) | Risk difference (95% CI) | P valuea | ||
|---|---|---|---|---|---|---|---|---|
| LPD group (n = 100) | OPD group (n = 100) | LPD group (n = 94) | OPD group (n = 94) | |||||
| Overall complications | 46 (46.0) | 54 (54.0) | −8.0 (−0.2 to 5.8) | .26 | 44 (46.8) | 50 (53.2) | −6.5 (−20.75 to 7.95) | .38 |
| Clavien-Dindo classification ≥III | 17 (17.0) | 23 (23.0) | −6.0 (−17.1 to 5.1) | .29 | 16 (17.0) | 23 (24.5) | −7.5 (−19.1 to 4.15) | .21 |
| CCI score, median (IQR) | 0.0 (0.0 to 22.6) | 8.7 (0.0 to 26.2) | −0.6 (−5.0 to 3.8) | .79b | 0.0 (0.0 to 22.6) | 8.7 (0.0 to 26.2) | −0.3 (−4.9 to 4.4) | .56 |
| Postoperative pancreatic fistula | 23 (23.0) | 26 (26.0) | −3.0 (−14.9 to 8.9) | .62 | 22 (23.4) | 24 (25.5) | −2.15 (−14.4 5 to 10.25) | .73 |
| Grade B/C | 8 (8.0) | 10 (10.0) | −2.0 (−9.9 to 5.9) | .62 | 7 (7.5) | 9 (9.6) | −2.15 (−10.15 to 5.85) | .60 |
| Postoperative bile leakage | 14 (14.0) | 13 (13.0) | 1.0 (−8.5 to 10.5) | .84 | 13 (13.8) | 13 (13.8) | 0.05 (−9.95 to 9.95) | >.99 |
| Grade B/C | 4 (4.0) | 5 (5.0) | −1.0 (−6.7 to 4.7) | .73 | 4 (4.3) | 5 (5.3) | −1.15 (−7.25 to 5.05) | .73 |
| Delayed gastric emptying | 26 (26.0) | 31 (31.0) | −5.0 (−17.5 to 7.5) | .43 | 26 (27.7) | 30 (31.9) | −4.35 (−17.35 to 8.85) | .52 |
| Grade B/C | 9 (9.0) | 10 (10.0) | −1.0 (−9.1 to 7.1) | .81 | 9 (9.6) | 10 (10.6) | −1.15 (−9.75 to 7.65) | .81 |
| Postpancreatectomy hemorrhage | 7 (7.0) | 5 (5.0) | 2.0 (−4.6 to 8.6) | .55 | 7 (7.5) | 5 (5.3) | 2.1 5 (−4.95 to 9.15) | .55 |
| Grade B/C | 7 (7.0) | 5 (5.0) | 2.0 (−4.6 to 8.6) | .55 | 7 (7.5) | 5 (5.3) | 2.35 (−4.95 to 9.15) | .55 |
| Intra-abdominal infection | 16 (16.0) | 15 (15.0) | 1.0 (−9.0 to 11.0) | .85 | 16 (17.0) | 15 (16.0) | 1.15 (−9.55 to 11.75) | .84 |
| Reoperation | 3 (3.0) | 2 (2.0) | 1.0 (−3.3 to 5.3) | .50c | 3 (3.2) | 2 (2.1) | 1.15 (−3.55 to 5.75) | >.99c |
| Hospital readmission within 90 d | 0 | 2 (2.0) | −2.0 (−4.7 to 0.7) | .16c | 0 | 2 (2.0) | −2.15 (−5.05 to 0.85) | .50c |
| Mortality within 30 d | 1 (1.0) | 0 | 1.0 (−1.0 to 3.0) | >.99c | 1 (1.1) | 0 | 1.15 (−1.0 5 to 3.15) | >.99c |
| Mortality within 90 d | 2 (2.0) | 0 | 2.0 (−0.7 to 4.7) | .50c | 1 (1.1) | 0 | 1.15 (−1.0 5 to 3.15) | >.99c |
Abbreviations: CCI, comprehensive complication index; LPD, laparoscopic pancreaticoduodenectomy; mITT, modified intention-to-treat; NA, not applicable; OPD, open pancreaticoduodenectomy; PP, per-protocol.
Except where otherwise noted, P values are derived from t test for continuous data and χ2 test for categorical data.
Wilcoxon rank sum test.
Fisher exact test.
PP and AT Populations
Sensitivity analysis demonstrated that results from the PP analysis and AT population were consistent with the mITT analysis. Details are shown in (Tables 2 and 3 and in eTable 1 in Supplement 2.
Discussion
The short-term outcomes of this trial show that mortality rates, postoperative complication rates, and oncological outcomes were not significantly different between the LPD and OPD groups for patients with PDAC. Moreover, LOS and function recovery details such as first time off-bed and time of first oral intake did not favor LPD as expected. The primary end point of the 5-year OS is scheduled to be analyzed in 2027.
Four RCTs comparing LPD with OPD for pancreatic or periampullary tumors have been published to date.3,4,5,6 Three trials (Pancreatic Head and Periampullary Cancer Laparoscopic Versus Open Surgical Treatment [PLOT],4 Comparison of Perioperative Outcomes Between Laparoscopic and Open Approach for Pancreatoduodenectomy [PADULAP],5 and Total Laparoscopic Pancreaticoduodenectomy Versus Open Pancreaticoduodenectomy [TJDBPS01]6) showed that LPD was associated with a shorter length of stay than OPD, with no significant difference in overall complication and mortality rates; the Laparoscopic Versus Open Pancreatoduodenectomy for Pancreatic or Periampullary Tumours (LEOPARD-2) trial3 found no difference in functional recovery but higher 90-day mortality in the LPD group.3 However, these RCTs enrolled patients with various diagnoses and only reported short-term outcomes. In addition, the learning curve was still a great controversy. To our knowledge, no published RCTs on LPD have addressed this concern, perhaps contributing to the current dispute and confusion. This is the first RCT, to our knowledge, to assess the long-term oncological and short-term surgical outcomes of LPD and OPD for PDAC treatment among experienced surgeons.
Controversy exists regarding the safety and efficacy of LPD in treating PDAC. Croome et al22 reported no significant differences in mortality and morbidity rates between OPD and LPD. Shorter median LOS and longer progression-free survival were seen in the LPD group compared with the OPD group. In the subgroup analysis of our previous TJDBPS01 trial,6 LPD had a shorter LOS, longer operative time, and better functional recovery than OPD in treating PDAC. No significant differences were observed in mortality and morbidity. In a meta-analysis, Feng et al23 showed no significant differences in OS, mortality, and morbidity between LPD and OPD groups, but compared with OPD, LPD resulted in a significantly higher rate of R0 resection and longer operative time.
The LEOPARD-2 trial3 was discontinued early because of mortality in the LPD group (5 of 50 patients [10%] in the LPD group and 1 of 49 [2%] in the OPD group). The authors suggested that the main reason for this relatively high mortality rate was that surgeons had not surmounted the learning curve. Our TJDPBS01 trial6 showed mortality rates of 1.7% and 2.0% following LPD and OPD, respectively, by surgeons who had surmounted the learning curve. This was in the range of 0 to 7.2% reported in previous studies comparing LPD and OPD in PDAC.23 In the present trial, the mortality rate was 2.0% and 0.0% in the LPD and OPD groups, respectively (P = .50). Although previous studies have shown that LPD achieved a noninferiority outcome in terms of mortality in PDAC compared with OPD, both LEOPARD-2 and this trial exhibited a higher absolute percentage of mortality in the LPD group. In an earlier retrospective study with the largest data set on LPD, the mortality rate following LPD increased from 4.4% to 6.4% and decreased to 0.83% as the learning curve progressed. During learning curve progression, surgeons should be vigilant when treating PDAC with LPD.
Most of the existing evidence suggests that there is no difference in overall complications between LPD and OPD in the treatment of pancreatic cancer. In this trial, the rate of complications of Clavien-Dindo grade III or greater in the LPD and OPD groups was 17.0% and 23.0%, respectively. Major complications after LPD were POPF, PPH, and delayed gastric emptying. In this study, the POPF rate was 21.0% vs 14.0% in the LPD and OPD groups, respectively. Moreover, in our previous trial, the POPF rates were 22.0% vs 26.5% in the 2 groups of patients with PDAC and 30.6% vs 29.3% for those without PDAC.6 A lower POPF rate may have been observed among patients with PDAC because they were likely to have a harder pancreatic texture.6 In this trial, PPH occurred in 7 (7.0%) and 5 (5.0%) patients in the LPD and OPD groups, respectively. One death in the LPD group was caused by PPH following POPF. The 3 deaths (60.0%) in the LPD group of LEOPARD-2 were also caused by PPH following POPF.3 Meanwhile, TJDBPS01 subgroup analyses showed that once patients had POPF, the benefit of LPD for functional recovery was negligible.6 These results suggested that once POPF and PPH occur, they may have more serious outcomes in LPD, possibly due to the less or later formation of abdominal adhesion after laparoscopic surgery. Thus, it is more difficult to limit the leakage of digestive juice or abdominal infection, leading to worse consequences.
Minimal invasive surgery is considered a benefit for postoperative recovery. Some outcomes include shortening LOS, earlier off-bed activity, earlier oral intake, and a fast approach to adjuvant therapy. Nickel et al24 summarized the existing RCT and concluded that there was neither a significant difference in the initial LOS nor in the total LOS between LPD and OPD groups. Meanwhile, in our previous trial,6 the median LOS was 1 day shorter in patients undergoing LPD compared to those undergoing OPD. Based on PDAC, Feng et al23 reported that LPD has a shorter LOS compared with OPD (mean deviation, −2.73; 95% CI, −4.44 to −1.03; P = .002). In the present trial, no LOS benefit was seen between the 2 groups. The reason for this phenomenon may be that the sample size was small, and the differences were not enough to reach statistically significant levels. It is also possible that minimally invasive surgery for PDAC, like PD, cannot reduce LOS. The days for off-bed activity, oral intake, and entering adjuvant therapy were comparable in the groups. These results suggest that the laparoscopic surgery technique might, at best, become equivalent to the open approach but may not necessarily be better in terms of postoperative recovery according to the current evidence.
The special magnifying effect of the laparoscopic equipment is considered advantageous for reducing intraoperative blood loss and lymph node dissection. In this trial, the mean estimated volume of blood lost in the LPD group was 115.2 mL less than in the OPD group. Such results were in accordance with our previous trial and other studies. Stauffer et al25 reported that LPD was associated with greater lymph node harvest. The magnified views and flexible angles provided by laparoscopy can facilitate lymph node dissection. In our trial, the median number of lymph nodes harvested was 17 in the LPD and 16 in the OPD group. This was because, in this trial, all the patients recruited had resectable tumors. Meanwhile, in contribution to our strategy of strict qualification and standardization, there was no significant difference in the number of lymph node dissections between laparotomy and laparoscopy. The results of this study showed that the two surgical methods had the same effect on the number of lymph node dissections, suggesting that LPD and OPD have the same tumor radical effect. The impact of this on the long-term survival of patients remains to be seen.
Limitations
Our study had several limitations. First, the patients included in this trial were strictly qualified and did not represent the general population with PDAC presenting at the participating hospitals. Many patients with PDAC would undergo neoadjuvant therapy before surgery. Hence, the most precise statement supported by the results of this trial is that laparoscopic PDAC surgery is comparable to open surgery when performed by experienced surgeons in selected patients. Second, all participating patients in this study were in China, and since the characteristics of Asian populations differ from those in other countries or races, particularly concerning body mass index, the results may not apply to patients generally. Third, we have not presented long-term outcomes as the primary end point of this trial. Although short-term outcomes were comparable between the 2 groups, long-term outcomes would confirm the final impact of LPD among patients with PDAC.
Conclusions
The findings in this study indicate that, in selected patients treated by skilled surgeons, LPD for PDAC provided similar short-term safety outcomes as OPD. The in-hospital recovery after laparoscopic surgery was noninferior to that after open surgery. Long-term follow-up to assess survival is necessary to ascertain the oncological safety of laparoscopic resection in patients with PDAC.
Trial protocol
eTable 1. Postoperative Complications among as-treat analysis
eTable 2. Information of participating surgeons
eTable 3. Outcomes of adjuvant therapy (mITT and PP Analysis)
Data sharing statement
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi: 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- 2.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049-1057. doi: 10.1016/S0140-6736(04)15841-8 [DOI] [PubMed] [Google Scholar]
- 3.van Hilst J, de Rooij T, Bosscha K, et al. ; Dutch Pancreatic Cancer Group . Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4(3):199-207. doi: 10.1016/S2468-1253(19)30004-4 [DOI] [PubMed] [Google Scholar]
- 4.Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017;104(11):1443-1450. doi: 10.1002/bjs.10662 [DOI] [PubMed] [Google Scholar]
- 5.Poves I, Burdío F, Morató O, et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann Surg. 2018;268(5):731-739. doi: 10.1097/SLA.0000000000002893 [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Li D, Chen R, et al. ; Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China’s International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM) . Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6(6):438-447. doi: 10.1016/S2468-1253(21)00054-6 [DOI] [PubMed] [Google Scholar]
- 7.Schneider M, Büchler M. Laparoscopic pancreatoduodenectomy: extensive learning curve, marginal benefits. Lancet Gastroenterol Hepatol. 2021;6(6):413-414. doi: 10.1016/S2468-1253(21)00059-5 [DOI] [PubMed] [Google Scholar]
- 8.Pan S, Qin T, Yin T, et al. ; Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China’s International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM) . Laparoscopic versus open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: study protocol for a multicentre randomised controlled trial. BMJ Open. 2022;12(4):e057128. doi: 10.1136/bmjopen-2021-057128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439-457. doi: 10.6004/jnccn.2021.0017 [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Feng Y, Zhao J, et al. Total laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy (TJDBPS01): study protocol for a multicentre, randomised controlled clinical trial. BMJ Open. 2020;10(2):e033490. doi: 10.1136/bmjopen-2019-033490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Zhang H, Zhu F, et al. Pancreaticoduodenectomy for borderline resectable pancreatic head cancer with a modified artery-first approach technique. Hepatobiliary Pancreat Dis Int. 2017;16(2):215-221. doi: 10.1016/S1499-3872(16)60171-6 [DOI] [PubMed] [Google Scholar]
- 13.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 14.Bassi C, Marchegiani G, Dervenis C, et al. ; International Study Group on Pancreatic Surgery (ISGPS) . The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161(3):584-591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 15.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680-688. doi: 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142(1):20-25. doi: 10.1016/j.surg.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 17.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761-768. doi: 10.1016/j.surg.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 18.Besselink MG, van Rijssen LB, Bassi C, et al. ; International Study Group on Pancreatic Surgery . Definition and classification of chyle leak after pancreatic operation: a consensus statement by the International Study Group on Pancreatic Surgery. Surgery. 2017;161(2):365-372. doi: 10.1016/j.surg.2016.06.058 [DOI] [PubMed] [Google Scholar]
- 19.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1-7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 20.Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1-7. doi: 10.1007/s00464-016-5222-1 [DOI] [PubMed] [Google Scholar]
- 21.Campbell M. Confidence intervals and Newcombe’s method—what are they? Midwifery. 2005;21(1):80-83. doi: 10.1016/j.midw.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 22.Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260(4):633-638. doi: 10.1097/SLA.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 23.Feng Q, Liao W, Xin Z, et al. Laparoscopic pancreaticoduodenectomy versus conventional open approach for patients with pancreatic duct adenocarcinoma: an up-to-date systematic review and meta-analysis. Front Oncol. 2021;11:749140. doi: 10.3389/fonc.2021.749140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic versus open pancreaticoduodenectomy: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2020;271(1):54-66. doi: 10.1097/SLA.0000000000003309 [DOI] [PubMed] [Google Scholar]
- 25.Stauffer JA, Coppola A, Villacreses D, et al. Laparoscopic versus open pancreaticoduodenectomy for pancreatic adenocarcinoma: long-term results at a single institution. Surg Endosc. 2017;31(5):2233-2241. doi: 10.1007/s00464-016-5222-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Postoperative Complications among as-treat analysis
eTable 2. Information of participating surgeons
eTable 3. Outcomes of adjuvant therapy (mITT and PP Analysis)
Data sharing statement