This randomized clinical trial investigates if paravertebral block (PVB) performed under video-assisted thoracoscopic surgery by a surgeon is noninferior to PVB performed by an anesthesiologist using an ultrasound-guided technique.
Key Points
Question
In minimally invasive thoracic surgery, is paravertebral block (PVB) undertaken by a surgeon under video-assisted thoracoscopic surgery (VATS) noninferior to PVB performed by an anesthesiologist using an ultrasound (US)–guided technique?
Findings
In this noninferiority randomized clinical trial, 196 patients were assigned to PVB-VATS or PVB-US. Results showed the noninferiority of PVB-VATS to PVB-US in the mean difference in total 48-hour opioid consumption.
Meanings
Results suggest that in cases of local resource or human limitations, an effective and easy postoperative analgesia can be provided by the surgeon under thoracoscopic vision.
Abstract
Importance
In minimally invasive thoracic surgery, paravertebral block (PVB) using ultrasound (US)–guided technique is an efficient postoperative analgesia. However, it is an operator-dependent process depending on experience and local resources. Because pain-control failure is highly detrimental, surgeons may consider other locoregional analgesic options.
Objective
To demonstrate the noninferiority of PVB performed by surgeons under video-assisted thoracoscopic surgery (VATS), hereafter referred to as PVB-VATS, as the experimental group compared with PVB performed by anesthesiologists using US-guided technique (PVB-US) as the control group.
Design, Setting, and Participants
In this single-center, noninferiority, patient-blinded, randomized clinical trial conducted from September 8, 2020, to December 8, 2021, patients older than 18 years who were undergoing a scheduled minimally invasive thoracic surgery with lung resection including video-assisted or robotic approaches were included. Exclusion criteria included scheduled open surgery, any antalgic World Health Organization level greater than 2 before surgery, or a medical history of homolateral thoracic surgery. Patients were randomly assigned (1:1) to an intervention group after general anesthesia. They received single-injection PVB before the first incision was made in the control group (PVB-US) or after 1 incision was made under thoracoscopic vision in the experimental group (PVB-VATS).
Interventions
PVB-VATS or PVB-US.
Main Outcomes and Measures
The primary end point was mean 48-hour post-PVB opioid consumption considering a noninferiority range of less than 7.5 mg of opioid consumption between groups. Secondary outcomes included time of anesthesia, surgery, and operating room occupancy; 48-hour pain visual analog scale score at rest and while coughing; and 30-day postoperative complications.
Results
A total of 196 patients were randomly assigned to intervention groups: 98 in the PVB-VATS group (mean [SD] age, 64.6 [9.5] years; 53 female [54.1%]) and 98 in the PVB-US group (mean [SD] age, 65.8 [11.5] years; 62 male [63.3%]). The mean (SD) of 48-hour opioid consumption in the PVB-VATS group (33.9 [19.8] mg; 95% CI, 30.0-37.9 mg) was noninferior to that measured in the PVB-US group (28.5 [18.2] mg; 95% CI, 24.8-32.2 mg; difference: −5.4 mg; 95% CI, −∞ to −0.93; noninferiority Welsh test, P ≤ .001). Pain score at rest and while coughing after surgery, overall time, and postoperative complications did not differ between groups.
Conclusions and Relevance
PVB placed by a surgeon during thoracoscopy was noninferior to PVB placed by an anesthesiologist using ultrasonography before incision in terms of opioid consumption during the first 48 hours.
Trial Registration
ClinicalTrials.gov Identifier: NCT04579276
Introduction
Paravertebral block (PVB) in thoracic surgery is routinely performed since the development of minimally invasive surgery.1,2 Historically, thoracic epidural analgesia was the criterion standard in open thoracic surgery to lessen the detrimental postoperative pain induced by rib spreading during thoracic surgical procedures; in addition, epidural analgesia conferred benefits such as decreased morphine consumption in patients and its related opioid-sparing effects.3,4,5,6 Video-assisted thoracic surgery (VATS) or robotic-assisted thoracic surgery (RATS), as part of the Enhanced Recovery After Surgery (ERAS) guidelines,7,8,9 are the standard of care in the management of early-stage lung cancer with the main benefit of preventing rib spreading, nerve stretching, rib fractures, and, therefore, inciting less postoperative pain. These approaches induce less inflammation, improved recovery, reduced duration of thoracic drainage, reduced in-hospital stay, and improved quality of life.10,11,12,13,14
However, even if ultrasound (US)–guided PVB is a reliable technique, pain-control failure is a routine issue due to technical problems, short duration, or insufficient staff training.15,16,17 Use of ultrasonography has the advantage of visualizing the paravertebral space, increasing the success of the procedure, and thus, reducing complications. Despite the use of ultrasonography, the procedure fails in 6% to 10% of cases.6,18,19 Thus, PVB under VATS may be an option with the advantages of a thoracoscopic direct visualization of the pleural space, assuring the right intercostal space and depth, especially in patients with overweight and resultant poor echogenic images on ultrasonography.
Because pain-control failure is highly detrimental for the short- and midterm postoperative outcomes, surgeons have to consider alternative locoregional analgesic options in situations with local resource limitations, surgery during evenings or weekends (during which time there are often staff shortages), failure of the current method, or an inexperienced anesthesiology team. The objective of this randomized clinical trial was to demonstrate that PVB undertaken by the surgeons under thoracoscopic vision (PVB-VATS) was noninferior to the standard of care based on US-guided technique (PVB-US) performed by the anesthesiologists.
Methods
Trial Design
We conducted a single-center, noninferiority, patient-blinded, randomized clinical trial comparing the PVB-VATS, defined as the experimental group, with the PVB-US defined as the control group. The study protocol received ethics committee approval from Ile de France IV institutional review board and agreement of the US Department of Health and Human Services on April 16, 2020. The trial protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. An oral and signed informed consent was collected from all participants. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Participants and Selection Criteria
Consecutive patients who were scheduled to undergo lung resection in the thoracic department of Marseille University Hospital were screened for enrollment in the study if they had the following inclusion criteria: (1) male or female sex 18 years or older, (2) VATS or RATS with lung resection (including wedge, segmentectomy, or lobectomy), and (3) signed informed consent. The exclusion criteria were as follows: (1) patient refusing to sign the consent form; (2) patients under any guardianship; (3) any surgery with pleura intervention such as talc, pleurectomy, or wall resection; (4) anesthesiologist untrained in the PVB-US technique; (5) presence of pain or daily use of analgesic before surgery (>level 2 of the World Health Organization analgesic ladder)20; (6) medical history of homolateral thoracic surgery; (7) patient refusing to submit to the method before the intervention; (8) conversion to thoracotomy during the intervention; (9) surgery not completed due to progression or medical cause; (10) patient extubated more than 2 hours after the end of the surgery; and (11) patient’s desire to exit the study. Participant race and ethnicity data were not gathered as this information is not used in French clinical trials.
Randomization
After a full preoperative workup according to European guidelines,21 patients who were eligible for the study were screened for the trial. Patients were included either at the first surgical consultation or the day before the surgery. Randomization was done the day of surgery by a third party not involved in the procedure, using the REDCap (Vanderbilt University) web application. Participants were randomly assigned in a 1:1 ratio to 1 of the 2 groups, and they were stratified to surgical approach VATS and RATS. The decision to stratify the patients’ cohort according to the surgical approach, ie, RATS or VATS, was made based on the findings of a previous prospective study,22 which showed that RATS was associated with increased use of morphine. The patients, the medical team in the unit, and the statistician were blinded to the group selected.
Protocol
The PVB-US was performed by an anesthesiologist in the operating room (OR) before surgical incision after the patient was induced and oriented in the decubitus lateral position. The PVB-VATS procedure was performed by the surgeon under thoracoscopic vision after a single surgical incision. The 2 techniques are described and photographed in the eMethods and eFigure 1 in Supplement 3 as the details of our ERAS protocol. In both groups, a standard concentration of 3 mg/kg of ropivacaine, 0.75%, was diluted in 40 mL of sodium chloride. Twenty milliliters were injected in 2 intercostal spaces corresponding to the incision points, which were from the fourth to the seventh intercostal spaces. A bolus of 3 mg of dexamethasone was administered intravenously, according to our protocols.23,24,25,26 Three trocars and 5 trocars were mostly used in VATS and RATS, respectively. A single chest tube (Ch 28) was used.
Outcomes
The primary outcome was opioid consumption within the first 48 hours after PVB. Secondary outcomes measured OR time occupancy, time of anesthesia, time of surgery, time spent in the postanesthesia care unit (PACU), pain through the visual analog scale (VAS) graded from 0 (no pain) to 100 (worst pain)27 both at rest and while coughing at different end points (4, 6, 12, 24, and 48 hours after PVB), postoperative complications during hospitalization and at 30 days (atelectasis, pneumonia, pleural effusion requiring drainage, atrial fibrillation, air leak over 5 days, pneumothorax), hospital readmissions at 30 days, the patient’s global satisfaction of pain management at 30 days (patient was asked if they were very satisfied, satisfied, unsatisfied, or very unsatisfied of the pain management during hospitalization), and existence of neuropathic pain at 30 days defined as skin insensitivity or electrical discharge located either at the surgical area or projected into the dermatome of a nerve.
Statistical Analysis
Sample Size Calculation
The sample size calculation was based on previous data in which the mean post-PVB opioid consumption within 48 hours was 30 mg.22,28,29 Noninferiority of PVB-VATS vs PVB-US would be concluded assuming a margin of error of less than 7.5 mg (ie, 25%) of opioid consumption within 48 hours. At a 5% significance level, 90% statistical power, and a 10% loss to follow-up, 166 patients were required (83 patients per group). The Power Analysis and Sample Size software program, version 2008 (NCSS), was used in the data analyses. Noninferiority tests were used for the difference between 2 means.30,31 No interim analysis was planned. No data imputation was performed.
Data Analyses
All data were collected in an electronic case report form developed on an open-source web application: REDCap. The methodology was based on the extension of the CONSORT statement for the reporting of noninferiority randomized clinical trials.32 For the primary outcome, the main analysis was performed on the per-protocol (PP) population and modified intention-to-treat (mITT) population.32,33,34 Noninferiority would be concluded if the lower limit for the between-group difference (opioid consumption within 48 hours) was inferior to the noninferiority margin for the 2 sets (Welsh test). Opioid consumption was expressed as mean and SD for each group and as the difference (PVB-VATS − PVB-US) and the 95% CI. Illustrations using violin plots were created. Analysis for the primary outcome was performed with adjustment for the type of surgery (segmentectomy-lobectomy and wedge resection), the indication for surgery (primary lung cancer and metastasis-benign lesions), the performance status (0-1 and ≥2), the American Society of Anesthesiology (ASA) score (1-2 and 3), and the pain levels at 4 hours after surgery, or H4 (<4 and ≥4). The result was presented as the estimate and the SE. Secondary outcomes were assessed for superiority. Secondary outcomes were compared between the 2 groups using the χ2 test or Fisher exact test for binary variables, and the t test or Mann-Whitney U test for continuous variables; risk ratios (RRs; for proportions) and mean differences (for continuous variables) were provided with their 95% CI. Pain levels were compared between groups at each time and globally (generalized linear models for repeated measures). All the outcomes were prespecified. All P values were 2-sided, and statistical significance was set at a P value < .05.
Results
Study Population
The study was conducted from September 8, 2020, to December 8, 2021. During the study period, 199 patients were randomly assigned to intervention groups. The patient profile met the same variables as those of other studies establishing efficacity of the reference treatment. Among them, 3 patients were excluded because they did not meet inclusion criteria (Figure 1). The remaining 196 patients were included in the mITT analysis: 98 in the PVB-VATS group (mean [SD] age, 64.6 [9.5] years; 53 female [54.1%]; 45 male [45.9%]) and 98 in the PVB-US group (mean [SD] age, 65.8 [11.5] years; 36 female [36.5%]; 62 male [63.3%]). The baseline features are reported in Table 1. All the patients had operations. During the surgical procedure, there were 8 conversions (8%) to thoracotomy in the PVB-US group and 6 (6%) in the PVB-VATS group. One patient had a delayed tracheal extubation 2 hours after the end of surgery in the PBV-US group. There were no serious adverse events or unintended effects registered.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Flowchart.
ITT indicates intention to treat; PVB, paravertebral block.
Table 1. Demographic and Clinical Comparison Between the 2 Cohorts.
| Variable | PVB-US (n = 98) | PVB-VATS (n = 98) | RR or differencea (95% CI) |
|---|---|---|---|
| Sex, No. (%) | |||
| Male | 62 (63.3) | 45 (45.9) | 0.71 (0.53 to 0.94) |
| Female | 36 (36.7) | 53 (54.1) | 1.41 (1.06 to 1.87) |
| Age, mean (SD), y | 65.8 (11.5) | 63.6 (9.5) | 1.13 (−1.86 to 4.12) |
| BMI group, median (IQR), % | 25.1 (4.3) | 25.1 (4.4) | −0.04 (−1.28 to 1.19) |
| Smoking history, No. (%) | 73 (75) | 74 (76) | 0.95 (0.63 to 1.44) |
| Smoking index, mean (SD), pack-years | 35 (21) | 36 (23) | NA |
| FEV1, mean (SD), % of predicted | 90.9 (19.3) | 91.7 (20.3) | NA |
| DLCO, mean (SD), % | 81.5 (18.0) | 80.1 (21.4) | NA |
| FEV1/FVC ratio, mean (SD), % | 78.6 (12.5) | 78.5 (12.6) | NA |
| ASA score, No. (%) | |||
| 1 | 5 (5.1) | 9 (9.2) | NA |
| 2 | 69 (70.4) | 66 (67.3) | NA |
| 3 | 24 (24.5) | 23 (23.5) | NA |
| Performance status 0 or 1, No. (%) | 96 (98) | 94 (96) | 1.35 (0.75 to 2.42) |
| Performance status 2 and more, No. (%) | 2 (2) | 3 (3) | NA |
| Indications | |||
| Primary lung cancer | 70 (72) | 78 (79) | 0.79 (0.55 to 1.14) |
| Metastasis/benign lesions | 28 (28) | 20 (21) | NA |
| Surgical approach | |||
| VATS | 75 (76.5) | 73 (74.5) | 1.05 (0.76 to 1.44) |
| RATS | 23 (23.5) | 25 (25.5) | NA |
| Chest tubes, No. (%) | |||
| 1 | 96 (97) | 95 (96) | NA |
| ≥2 | 2 (3) | 3 (4) | 1.21 (0.58 to 2.50) |
| Ports, No. (%) | |||
| ≤3 | 70 (71) | 63 (64) | NA |
| >3 | 28 (28) | 35 (35) | 1.17 (0.88 to 1.56) |
| Lung resection, No. (%) | |||
| Lobectomy | 46 (46) | 45 (45) | NA |
| Segmentectomy | 27 (27.6) | 32 (32.7) | NA |
| Wedge resection | 25 (25.5) | 21 (21.4) | NA |
| Size of the tumor, mean (SD), mm | 18.4 (10.2) | 16.6 (10.9) | NA |
Abbreviations: ASA, American Society of Anesthesiology; BMI, body mass index; DLCO, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NA, not applicable; RATS, robotic-assisted thoracoscopic surgery; RR, risk ratio; US, ultrasound; VATS, video-assisted thoracoscopic surgery.
RRs are provided for proportions; the difference is provided for continuous variables.
Outcomes
The mean (SD) 48-hour opioid consumption in the PVB-VATS group (33.9 [19.8] mg; 95% CI, 30.0-37.9 mg) was noninferior to that of the PVB-US group in the PP and mITT populations (28.5 [18.2] mg; 95% CI, 24.8-32.2 mg; difference: −5.4 mg; 95% CI, −∞ to −0.93; noninferiority Welsh test, P ≤ .001) (Figure 2). The PVB-VATS group was noninferior to the PVB-US group after adjustment for the type of surgery, the indication of surgery, the ASA score, and the pain levels at H4 (estimate, −2.1; SE, 2.9; P < .001).
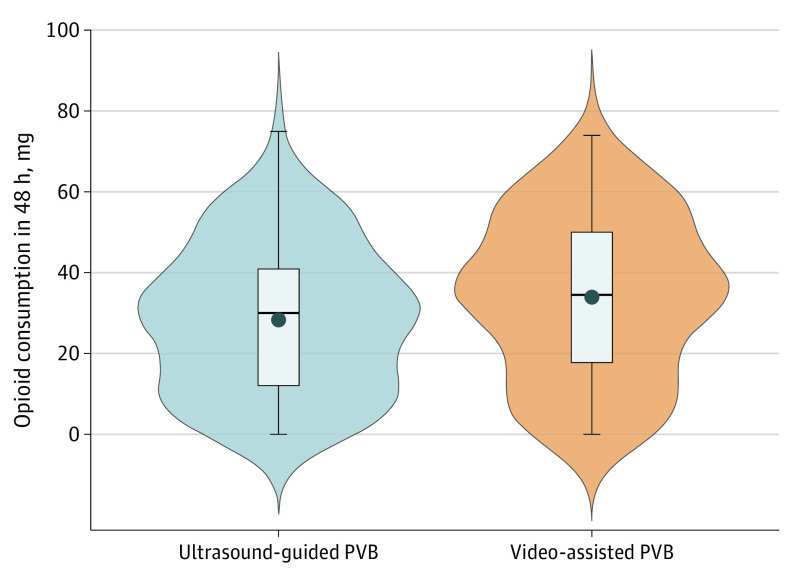
Figure 2. Primary Outcome: Opioid Consumption Within the First 48 Hours After Paravertebral Block (PVB).
Violin plot showing probability density and indicating mean with SD. The mean (SD) of 48-hour opioid consumption in PVB–video-assisted thoracoscopic surgery (VATS) group (33.9 [19.8] mg; 95% CI, 30.0-37.9 mg) was noninferior to that measured in the PVB–ultrasound (US) group (28.5 [18.2] mg; 95% CI, 24.8-32.2 mg; difference, 5.4 mg; 95% CI, 0-0.93 mg; noninferiority Welsh test P ≤ .001).
The duration of OR time occupancy did not differ between groups (mean [SD], 217.9 [57.3] minutes in PVB-VATS and 232.1 [63.6] minutes in PVB-US; difference, 14.20 minutes; 95% CI, −2.86 to 31.26 minutes; P = .10). Duration of anesthesia was statistically shorter in the PVB-VATS group compared with the PVB-US group (mean [SD], 198.3 [56.6] minutes vs 218.6 [78.4] minutes; difference, 20.33 minutes; 95% CI, 0.88-39.77 minutes; P = .04). Operative duration and time spent in PACU did not differ between groups. Pain VAS scores at rest and while coughing at 4, 6, 12, and 48 hours after PVB were similar in the 2 groups (Figure 3). Pain VAS scores at rest and while coughing did not differ between groups, at each time (4, 6, 12, and 48 hours after PVB) (Figure 3) or globally. There were no differences in postoperative overall complications, occurring in 16 patients (16%) in the PVB-VATS group and 23 patients (23%) in the PVB-US group (RR, 0.98; 95% CI, 0.48-2.00; P = .20), in length of hospital stay (mean [SD], 5.2 [2.9] days in PVB-VATS and 5.4 [3.1] days in PVB-US; difference, 0.18 days; 95% CI, −0.67 to 1.02 days; P = .69), and in the rate of 30-day readmissions (8 patients [8%] in PVB-VATS and 5 patients (5%) in PVB-US; RR, 1.28; 95% CI, 0.81-2.02; P = .38) (Table 2 and eTables 1 and 2 in Supplement 3). The mean (SD) hospital opioid consumption in the PVB-VATS and PVB-US groups was 52.9 (55.9) mg and 59 (53.7) mg, respectively (P = .43). In 6 of 98 patients (6%) in the PVB-VATS group, the technique was considered a failure for the surgeon, and in 9 of 98 patients (10%) in the PVB-US group, the technique was considered a failure for the anesthesiologist.
Figure 3. Postoperative Pain Through the Visual Analog Scale (VAS) Score .

Postoperative pain through the VAS score at rest (A) and while coughing (B) at 4, 6, 12, 24, and 48 hours after video-assisted paravertebral block (PVB) vs ultrasound-guided PVB.
Table 2. Secondary Outcomes.
| Variable | PVB-US (n = 98) | PVB-VATS (n = 98) | RR or difference (95% CI)a | P valueb |
|---|---|---|---|---|
| OR time occupancy, mean (SD), min | 232.1 (63.6) | 217.9 (57.3) | 14.20 (−2.86 to 31.26) | .10 |
| Duration of anesthesia, mean (SD), min | 218.6 (78.4) | 198.3 (56.6) | 20.33 (0.88 to 39.77) | .04 |
| Time to extubation, mean (SD), min | 36.7 (43.5) | 33.5 (30.1) | 3.20 (−7.40 to 13.79) | .55 |
| Duration of surgery, mean (SD), min | 129.9 (61.7) | 130.4 (50.3) | −0.51 (−16.39 to 15.37) | .95 |
| Duration in PACU, mean (SD), min | 178.4 (116.6) | 179.4 (72.9) | −1.03 (−28.65 to 26.60) | .94 |
| Blood loss, mean (SD), mL | 106 (197) | 95 (159) | 10.63 (−40.27 to 61.52) | .68 |
| Conversion to thoracotomy, No. (%) | 8 (8) | 6 (6) | 0.85 (0.46 to 1.58) | .58 |
| Supplementary analgesia, No. (%) | ||||
| PVB catheter | 4 (4) | 4 (4) | 0.98 (0.48 to 2.00) | .79 |
| Epidural | 3 (3) | 3 (3) | 0.98 (0.44 to 2.22) | |
| Intravenous morphine | 3 (3) | 1 (1) | 0.49 (0.09 to 2.70) | |
| Postoperative complication, No. (%) | 23 (23) | 16 (16) | ||
| Atelectasis | 1 (1) | 2 (2) | 0.98 (0.48 to 2.00) | .20 |
| Pneumonia | 3 (3) | 2 (2) | ||
| Pleural effusion requiring drainage | 11 (11) | 6 (6) | ||
| Atrial fibrillation | 3 (3) | 3 (3) | ||
| Air leak >5 d | 7 (7) | 2 (2) | ||
| Pneumothorax | 11 (11) | 6 (6) | ||
| Length of hospital stay, mean (SD), d | 5.4 (3.1) | 5.2 (2.9) | 0.18 (−0.67 to 1.02) | .69 |
| Chest tube duration, mean (SD), d | 3.6 (4.8) | 2.9 (3.6) | 0.64 (−0.57 to 1.85) | .30 |
| ERAS protocol adhesion, No. (%) | 88 (90) | 94 (97) | 2.07 (0.77 to 5.56) | .07 |
| Readmission before day 30, No. (%) | 5 (5) | 8 (8) | 1.28 (0.81 to 2.02) | .38 |
| Global satisfaction of pain management, No. (%) | ||||
| Very satisfied | 44 (46) | 36 (37) | NA | .44 |
| Satisfied | 39 (41) | 42 (43) | NA | |
| Unsatisfied | 9 (9) | 11 (11) | NA | |
| Very unsatisfied | 3 (3) | 7 (7) | NA | |
| Neuropathic pain at day 30, No. (%) | 42 (43) | 43 (44) | 1.01 (0.76 to 1.34) | .94 |
| Antalgic consumption at day 30, No. (%) | 39 (40) | 38 (39) | 0.97 (0.73 to 1.29) | .84 |
Abbreviations: ERAS, Enhanced Recovery After Surgery; IV, intravenous; OR, operating room; PACU, postanesthesia care unit; PVB, paravertebral block, RR, risk ratio; US, ultrasound; VATS, video-assisted thoracoscopic surgery.
RRs are provided for proportions; the difference is provided for continuous variables.
Fisher exact test, χ2 test, and t test were used as appropriate.
Global satisfaction in pain management after surgery did not differ in both groups (Table 2 and eFigure 2 in Supplement 3). There was no loss of sight at 30 days. Neuropathic pain was present at 30 days in 43 patients (44%) in the PVB-VATS group and 42 patients (43%) in the PVB-US group (RR, 1.01; 95% CI, 0.76-1.34; P = .94). At 30 days, 38 patients (39%) in the PVB-VATS group and 39 patients (40%) in the PVB-US group were still under analgesic treatment (RR, 0.97; 95% CI, 0.73-1.29; P = .84).
Discussion
This randomized clinical trial showed that noninferiority was reached between PVB performed by surgeons during surgery and PVB performed by anesthesiologists using ultrasonography before the first incision in minimally invasive thoracic surgery using both VATS and RATS approaches. The mean 48-hour morphine consumption, pain VAS at rest and at coughing, global patient satisfaction, and 30-day evaluation were noninferior when PVB-VATS and PVB-US were compared. Our results also suggest that the PVB-VATS method reduced anesthesia duration without a significant effect on OR time occupancy. In addition, PVB-VATS did not compromise the implementation of ERAS protocol adoption and seemed to be equivalent in midterm outcomes at 30 days in residual pain or in the use of analgesia.
The rationale for this study was based on our clinical experience of pain failure after PVB-US due to technical problems or insufficient staff training. Our study supports the concept that reliable analgesia performed by a surgeon can be safely and correctly done in daily practice and should be systematically proposed in all situations where PVB-US is not performed or may be insufficient. Based on our results and even if all surgical scenarios are not fully explored by our study, we hypothesize that PVB-VATS can have real clinical benefits in other thoracic settings such as emergent situations or during evenings or weekends when the management of analgesia could be insufficient due to the availability of less experienced staff during those times.
The preferred and most studied regional block in minimally invasive thoracic surgery is PVB.35,36 Alternatives such as serratus block or intercostal block have been widely studied and seem to have similar results in postoperative pain care. Some studies found a better effect of PVB-US.37,38,39,40,41,42,43,44,45,46,47,48 In our study, we hypothesized that local analgesia administered by the surgeon would have been noninferior compared with PVB-US. Even if PVB-VATS seems to be effective, there are no sufficient data to definitively promote the technique.8 Kozanhan et al49 found a positive result for thoracoscopic vision regional block compared with no regional block after thoracotomy. Moreover, thoracoscopic-guided intercostal nerve block can be successfully performed for uniportal lobectomy.50 The main limit of the PVB-VATS is the problem of lung exclusion that is absolutely necessary or the existence of pleural adhesions resulting in problems with the ability to clearly identify the pleural space. In PVB-VATS, we encountered inefficient lung exclusion or pleural adhesions resulting in reduced or no visualization of the pleura. This occurred in 6 of 98 patients (6%) in the PVB-VATS group. In contrast, overweight was not an issue in the PVB-VATS group and should be seen as an advantage as compared with PVB-US, where there are poor echogenic image results in people with overweight. This occurred in 9 of 98 patients (10%) in the PVB-US group.
In response to ERAS guidelines, single injections were favored to continuous infusions in order to encourage patients to gain their autonomy as soon as possible. Infiltration of more than 1 intercostal space seems to result in more reliable radiographic and clinical distribution.51 PROSPECT guidelines studied 69 randomized clinical trials and found a global incidence of neuropathic pain after thoracic surgery to be 80% at 3 months and 75% at 6 months.52,53 VAS score higher than 40 during the early postoperative period has also proved to be a strong predictor for developing posttraumatic stress disorder.54
Early care of acute pain has therefore proved to minimize chronic pain in the long term, and serious consideration of perioperative pain care is essential for the long term.55,56,57,58 Patient satisfaction with pain management and overall hospital experience play a role in postoperative pain.59,60 In our study, the patient’s global satisfaction with pain control at 30 days was similar to that of previous studies.61,62 However, the numerical rating of neuropathic pain and the rate of patients requiring analgesia consumption at 30 days was surprisingly high. One explanation could be the strict definition of pain used in our study: skin insensitivity or electrical discharge. More critical is the very high proportion of patients still requiring analgesics 30 days after surgery, which suggests a failure to provide long-term analgesia.
Strengths and Limitations
To our knowledge, this study was the largest randomized clinical trial comparing 2 methods of PVB. We intentionally excluded all surgeries implicating the pleura or patients with traumatic injury with possible rib fractures due to a different management of the pain that is often more important and less protocolized.63
Some limitations to this study can be disclosed. First, patients were questioned only at postoperative day 30, and we have no data on 3- or 6-month pain assessment. Thus, chronic pain could not be adequately evaluated for assessing long-term outcome. Second, we acknowledge that we did not assess all nonopioid medication consumption during hospitalization and at day 30 in order to calculate morphine equivalents. Third, the study was not designed to compare VATS and RATS procedures. We were unable to assess the effect of both approaches on outcomes.
Conclusions
In this randomized clinical trial, PVB administered by a surgeon during thoracoscopy was found to be noninferior to PVB administered by an anesthesiologist using ultrasonography before making the initial incision in terms of opioid consumption during the first 48 hours. The choice of the type of procedure should probably rely on local hospital guidelines.
Trial Protocol
Statistical Analysis Plan
eMethods. Paravertebral Block Under Thoracoscopic Vision or Ultrasound-Guided and ERAS Protocol
eFigure 1. PVB-US and PVB-VATS
eTable 1. Readmissions Causes (Cumulative)
eTable 2. Clinical Outcomes at 4, 6, 12, 24, 48 Hours After PVB and on the Day of Discharge
eFigure 2. Number of Patients Who Said Were Feeling Pain At 4, 6, 12, 24, 48 Hours After PVB and on the Day of Discharge
Data Sharing Statement
References
- 1.D’Ercole F, Arora H, Kumar PA. Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth. 2018;32(2):915-927. doi: 10.1053/j.jvca.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 2.Eason MJ, Wyatt R. Paravertebral thoracic block—a reappraisal. Anaesthesia. 1979;34(7):638-642. doi: 10.1111/j.1365-2044.1979.tb06363.x [DOI] [PubMed] [Google Scholar]
- 3.Ng A, Swanevelder J. Pain relief after thoracotomy: is epidural analgesia the optimal technique? Br J Anaesth. 2007;98(2):159-162. doi: 10.1093/bja/ael360 [DOI] [PubMed] [Google Scholar]
- 4.Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103(5):1079-1088. doi: 10.1097/00000542-200511000-00023 [DOI] [PubMed] [Google Scholar]
- 5.Razi SS, Stephens-McDonnough JA, Haq S, et al. Significant reduction of postoperative pain and opioid analgesics requirement with an enhanced recovery after thoracic surgery protocol. J Thorac Cardiovasc Surg. 2021;161(5):1689-1701. doi: 10.1016/j.jtcvs.2019.12.137 [DOI] [PubMed] [Google Scholar]
- 6.Bialka S, Copik M, Daszkiewicz A, et al. Comparison of different methods of postoperative analgesia after thoracotomy—a randomized controlled trial. J Thorac Dis. 2018;10(8):4874-4882. doi: 10.21037/jtd.2018.07.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berna P, Quesnel C, Assouad J, et al. Guidelines on enhanced recovery after pulmonary lobectomy. Anaesth Crit Care Pain Med. 2021;40(1):100791. doi: 10.1016/j.accpm.2020.100791 [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91-115. doi: 10.1093/ejcts/ezy301 [DOI] [PubMed] [Google Scholar]
- 9.Umari M, Carpanese V, Moro V, et al. Postoperative analgesia after pulmonary resection with a focus on video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg. 2018;53(5):932-938. doi: 10.1093/ejcts/ezx413 [DOI] [PubMed] [Google Scholar]
- 10.Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836-844. doi: 10.1016/S1470-2045(16)00173-X [DOI] [PubMed] [Google Scholar]
- 11.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86(6):2008-2016. doi: 10.1016/j.athoracsur.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 12.Jones NL, Edmonds L, Ghosh S, Klein AA. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia. 2013;68(2):179-189. doi: 10.1111/anae.12067 [DOI] [PubMed] [Google Scholar]
- 13.Flores RM, Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg. 2008;85(2):S710-S715. doi: 10.1016/j.athoracsur.2007.09.055 [DOI] [PubMed] [Google Scholar]
- 14.NEJM Evidence. Video-assisted thoracoscopic or open lobectomy in early-stage lung cancer. Accessed November 28, 2022. https://evidence.nejm.org/doi/full/10.1056/EVIDoa2100016 [DOI] [PubMed]
- 15.Yeung JH, Gates S, Naidu BV, Wilson MJ, Gao Smith F. Paravertebral block vs thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016;2(2):CD009121. doi: 10.1002/14651858.CD009121.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosiński S, Fryźlewicz E, Wiłkojć M, Ćmiel A, Zieliński M. Comparison of continuous epidural block and continuous paravertebral block in postoperative analgesia after video-assisted thoracoscopic surgery lobectomy: a randomised, noninferiority trial. Anaesthesiol Intensive Ther. 2016;48(5):280-287. doi: 10.5603/AIT.2016.0059 [DOI] [PubMed] [Google Scholar]
- 17.Pace MM, Sharma B, Anderson-Dam J, Fleischmann K, Warren L, Stefanovich P. Ultrasound-guided thoracic paravertebral blockade: a retrospective study of the incidence of complications. Anesth Analg. 2016;122(4):1186-1191. doi: 10.1213/ANE.0000000000001117 [DOI] [PubMed] [Google Scholar]
- 18.Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade: failure rate and complications. Anaesthesia. 1995;50(9):813-815. doi: 10.1111/j.1365-2044.1995.tb06148.x [DOI] [PubMed] [Google Scholar]
- 19.Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade: incidence of failed block and complications. Anaesthesia. 2001;56(12):1184-1188. doi: 10.1046/j.1365-2044.2001.02084-2.x [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . Cancer Pain Relief. WHO Publications Center USA; 1986. [Google Scholar]
- 21.Brunelli A, Charloux A, Bolliger CT, et al. ; European Respiratory Society; European Society of Thoracic Surgeons Joint Task Force on Fitness For Radical Therapy . The European Respiratory Society and European Society of Thoracic Surgeons clinical guidelines for evaluating fitness for radical treatment (surgery and chemoradiotherapy) in patients with lung cancer. Eur J Cardiothorac Surg. 2009;36(1):181-184. doi: 10.1016/j.ejcts.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 22.Duclos G, Charvet A, Resseguier N, et al. Postoperative morphine consumption and anaesthetic management of patients undergoing video-assisted or robotic-assisted lung resection: a prospective, propensity score-matched study. J Thorac Dis. 2018;10(6):3558-3567. doi: 10.21037/jtd.2018.05.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25(5):403-409. doi: 10.1017/S0265021507003079 [DOI] [PubMed] [Google Scholar]
- 24.Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth. 2013;110(2):191-200. doi: 10.1093/bja/aes431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohta M, Kalra B, Sethi AK, Kaur N. Efficacy of dexmedetomidine as an adjuvant in paravertebral block in breast cancer surgery. J Anesth. 2016;30(2):252-260. doi: 10.1007/s00540-015-2123-8 [DOI] [PubMed] [Google Scholar]
- 26.Ding W, Chen Y, Li D, et al. Investigation of single-dose thoracic paravertebral analgesia for postoperative pain control after thoracoscopic lobectomy—a randomized controlled trial. Int J Surg. 2018;57:8-14. doi: 10.1016/j.ijsu.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 27.Bodian CA, Freedman G, Hossain S, Eisenkraft JB, Beilin Y. The visual analog scale for pain: clinical significance in postoperative patients. Anesthesiology. 2001;95(6):1356-1361. doi: 10.1097/00000542-200112000-00013 [DOI] [PubMed] [Google Scholar]
- 28.Duclos G, Resseguier N, Ronfle R, Thomas PA, Leone M. Complemental analysis about postoperative opioid consumption between video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) for early-stage lung cancer. J Thorac Dis. 2018;10(10):E764-E765. doi: 10.21037/jtd.2018.09.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayman EO, Brennan TJ. Video-assisted thoracoscopic surgery vs robotic-assisted thoracoscopic surgery and postoperative opioid consumption. J Thorac Dis. 2018;10(suppl 26):S3222-S3223. doi: 10.21037/jtd.2018.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turhan Ö, Sivrikoz N, Sungur Z, Duman S, Özkan B, Şentürk M. Thoracic paravertebral block achieves better pain control than erector spinae plane block and intercostal nerve block in thoracoscopic surgery: a randomized study. J Cardiothorac Vasc Anesth. 2021;35(10):2920-2927. doi: 10.1053/j.jvca.2020.11.034 [DOI] [PubMed] [Google Scholar]
- 31.Sertcakacilar G, Pektas Y, Yildiz GO, Isgorucu O, Kose S. Efficacy of ultrasound-guided erector spinae plane block versus paravertebral block for postoperative analgesia in single-port video-assisted thoracoscopic surgery: a retrospective study. Ann Palliat Med. 2022;11(6):1981-1989. doi: 10.21037/apm-22-75 [DOI] [PubMed] [Google Scholar]
- 32.Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB Sr, Massaro JM, Sullivan LM. Noninferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med. 2003;22(2):169-186. doi: 10.1002/sim.1425 [DOI] [PubMed] [Google Scholar]
- 34.Matilde Sanchez M, Chen X. Choosing the analysis population in noninferiority studies: per protocol or intent to treat. Stat Med. 2006;25(7):1169-1181. doi: 10.1002/sim.2244 [DOI] [PubMed] [Google Scholar]
- 35.Chu L, Zhang X, Lu Y, et al. Improved analgesic effect of paravertebral blocks before and after video-assisted thoracic surgery: a prospective, double-blinded, randomized controlled trial. Pain Res Manag. 2019;2019:9158653. doi: 10.1155/2019/9158653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinthorsdottir KJ, Wildgaard L, Hansen HJ, Petersen RH, Wildgaard K. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg. 2014;45(6):959-966. doi: 10.1093/ejcts/ezt525 [DOI] [PubMed] [Google Scholar]
- 37.Pai P, Hong J, Phillips A, Lin HM, Lai YH. Serratus anterior plane block vs intercostal block with incision infiltration in robotic-assisted thoracoscopic surgery: a randomized controlled pilot trial. J Cardiothorac Vasc Anesth. 2022;36(8 Pt A):2287-2294. doi: 10.1053/j.jvca.2021.10.022 [DOI] [PubMed] [Google Scholar]
- 38.Zengin M, Sazak H, Baldemir R, Ulger G, Alagoz A. The effect of erector spinae plane block and combined deep and superficial serratus anterior plane block on acute pain after video-assisted thoracoscopic surgery: a randomized controlled study. J Cardiothorac Vasc Anesth. 2022;36(8 Pt B):2991-2999. doi: 10.1053/j.jvca.2022.01.048 [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Wu J, Huang Q, et al. Acute pain after serratus anterior plane or thoracic paravertebral blocks for video-assisted thoracoscopic surgery: a noninferiority randomised trial. Eur J Anaesthesiol. 2021;38(suppl 2):S97-S105. doi: 10.1097/EJA.0000000000001450 [DOI] [PubMed] [Google Scholar]
- 40.Rice DC, Cata JP, Mena GE, Rodriguez-Restrepo A, Correa AM, Mehran RJ. Posterior intercostal nerve block with liposomal bupivacaine: an alternative to thoracic epidural analgesia. Ann Thorac Surg. 2015;99(6):1953-1960. doi: 10.1016/j.athoracsur.2015.02.074 [DOI] [PubMed] [Google Scholar]
- 41.Elsabeeny WY, Ibrahim MA, Shehab NN, Mohamed A, Wadod MA. Serratus anterior plane block and erector spinae plane block vs thoracic epidural analgesia for perioperative thoracotomy pain control: a randomized controlled study. J Cardiothorac Vasc Anesth. 2021;35(10):2928-2936. doi: 10.1053/j.jvca.2020.12.047 [DOI] [PubMed] [Google Scholar]
- 42.Baldinelli F, Capozzoli G, Pedrazzoli R, Feil B, Pipitone M, Zaraca F. Are thoracic wall blocks efficient after video-assisted thoracoscopy surgery-lobectomy pain? a comparison between serratus anterior plane block and intercostal nerve block. J Cardiothorac Vasc Anesth. 2021;35(8):2297-2302. doi: 10.1053/j.jvca.2020.09.102 [DOI] [PubMed] [Google Scholar]
- 43.Guerra-Londono CE, Privorotskiy A, Cozowicz C, et al. Assessment of intercostal nerve block analgesia for thoracic surgery: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(11):e2133394. doi: 10.1001/jamanetworkopen.2021.33394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiyama T, Kataoka Y, Shindo K, et al. Retrolaminar block vs paravertebral block for pain relief after less-invasive lung surgery: a randomized, noninferiority controlled trial. Cureus. 2021;13(2):e13597. doi: 10.7759/cureus.13597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Y, Li XK, Zhou H, et al. Paravertebral block with modified catheter under surgeon’s direct vision after video-assisted thoracoscopic lobectomy. J Thorac Dis. 2020;12(8):4115-4125. doi: 10.21037/jtd-20-1068B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel A, Kumar V, Garg R, et al. Comparison of analgesic efficacy of ultrasound-guided thoracic paravertebral block vs surgeon-guided serratus anterior plane block for acute postoperative pain in patients undergoing thoracotomy for lung surgery—a prospective randomized study. Saudi J Anaesth. 2020;14(4):423-430. doi: 10.4103/sja.SJA_143_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jessula S, Atkinson L, Casey P, et al. Surgically positioned paravertebral catheters and postoperative analgesia after open abdominal aortic aneurysm repair. J Vasc Surg. 2019;70(5):1479-1487. doi: 10.1016/j.jvs.2019.02.037 [DOI] [PubMed] [Google Scholar]
- 48.Helms O, Mariano J, Hentz JG, et al. Intraoperative paravertebral block for postoperative analgesia in thoracotomy patients: a randomized, double-blind, placebo-controlled study. Eur J Cardiothorac Surg. 2011;40(4):902-906. doi: 10.1016/j.ejcts.2011.01.067 [DOI] [PubMed] [Google Scholar]
- 49.Kozanhan B, Semerkant T, Esme H, Canitez A, İyisoy MS. Evaluation of rhomboid intercostal and subserratus plane block under direct vision for postoperative analgesia in thoracic surgeries: a prospective, randomized controlled trial. Eur J Cardiothorac Surg. 2022;62(6):ezac498. doi: 10.1093/ejcts/ezac498 [DOI] [PubMed] [Google Scholar]
- 50.Kang DK, Kang MK, Woon H, Hwang YH. The feasibility of thoracoscopic-guided intercostal nerve block during uniportal video-assisted thoracoscopic lobectomy of the lung. J Minim Access Surg. 2022;18(4):567-570. doi: 10.4103/jmas.jmas_261_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naja ZM, El-Rajab M, Al-Tannir MA, et al. Thoracic paravertebral block: influence of the number of injections. Reg Anesth Pain Med. 2006;31(3):196-201. doi: 10.1097/00115550-200605000-00003 [DOI] [PubMed] [Google Scholar]
- 52.Chow TKF. PROSPECT guidelines no longer recommend thoracic epidural analgesia for video-assisted thoracoscopic surgery. Anaesthesia. 2022;77(8):937. doi: 10.1111/anae.15722 [DOI] [PubMed] [Google Scholar]
- 53.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand. 1999;43(5):563-567. doi: 10.1034/j.1399-6576.1999.430513.x [DOI] [PubMed] [Google Scholar]
- 54.Jeantieu M, Gaillat F, Antonini F, et al. Postoperative pain and subsequent PTSD-related symptoms in patients undergoing lung resection for suspected cancer. J Thorac Oncol. 2014;9(3):362-369. doi: 10.1097/JTO.0000000000000084 [DOI] [PubMed] [Google Scholar]
- 55.Elmore B, Nguyen V, Blank R, Yount K, Lau C. Pain management following thoracic surgery. Thorac Surg Clin. 2015;25(4):393-409. doi: 10.1016/j.thorsurg.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 56.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618-1625. doi: 10.1016/S0140-6736(06)68700-X [DOI] [PubMed] [Google Scholar]
- 57.Ren P, Du Y, He G, Jiang D. Efficacy and safety of general anesthesia combined with paravertebral blockade on postoperative recovery in patients undergoing pulmonary surgery: a systematic review and meta-analysis. J Thorac Dis. 2022;14(2):431-442. doi: 10.21037/jtd-22-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Homma T, Doki Y, Yamamoto Y, et al. Risk factors of neuropathic pain after thoracic surgery. J Thorac Dis. 2018;10(5):2898-2907. doi: 10.21037/jtd.2018.05.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feray S, Lubach J, Joshi GP, Bonnet F, Van de Velde M; PROSPECT Working Group of the European Society of Regional Anaesthesia and Pain Therapy . PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2022;77(3):311-325. doi: 10.1111/anae.15609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maher DP, Wong W, Woo P, et al. Perioperative factors associated with HCAHPS responses of 2758 surgical patients. Pain Med. 2015;16(4):791-801. doi: 10.1111/pme.12651 [DOI] [PubMed] [Google Scholar]
- 61.Siu E, Quick JS, Xu X, Correll DJ. Evaluation of the determinants of satisfaction with postoperative pain control after thoracoscopic surgery: a single-center, survey-based study. Anesth Analg. 2019;128(3):555-562. doi: 10.1213/ANE.0000000000003756 [DOI] [PubMed] [Google Scholar]
- 62.Cairns A, Battleday FM, Velikova G, et al. General patient satisfaction after elective and acute thoracic surgery is associated with postoperative complications. J Thorac Dis. 2020;12(5):2088-2095. doi: 10.21037/jtd-19-3345b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komatsu T, Sowa T, Kino A, Fujinaga T. The importance of pleural integrity for effective and safe thoracic paravertebral block: a retrospective comparative study on postoperative pain control by paravertebral block. Interact Cardiovasc Thorac Surg. 2015;20(3):296-299. doi: 10.1093/icvts/ivu395 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods. Paravertebral Block Under Thoracoscopic Vision or Ultrasound-Guided and ERAS Protocol
eFigure 1. PVB-US and PVB-VATS
eTable 1. Readmissions Causes (Cumulative)
eTable 2. Clinical Outcomes at 4, 6, 12, 24, 48 Hours After PVB and on the Day of Discharge
eFigure 2. Number of Patients Who Said Were Feeling Pain At 4, 6, 12, 24, 48 Hours After PVB and on the Day of Discharge
Data Sharing Statement