Abstract
Stimuli-responsive hydrogels are intriguing biomimetic materials. Previous efforts to develop mechano-responsive hydrogels have mostly relied on chemical modifications of the hydrogel structures. Here, we present a simple, generalizable strategy that confers mechano-responsive behavior on hydrogels. Our approach involves embedding hybrid vesicles, composed of phospholipids and amphiphilic block copolymers, within the hydrogel matrix to act as signal transducers. Under mechanical stress, these vesicles undergo deformation and rupture, releasing encapsulated compounds that can control the hydrogel network. To demonstrate this concept, we embedded vesicles containing ethylene glycol tetraacetic acid (EGTA), a calcium chelator, into a calcium-crosslinked alginate hydrogel. When compressed, the released EGTA sequesters calcium ions and degrades the hydrogel. This study provides a novel method for engineering mechano-responsive hydrogels that may be useful in various biomedical applications.
Keywords: Functional Material, Stimuli-Responsive Hydrogel, Stress-Induced Degradation, Vesicles, Block Copolymers
Graphical Abstract

We herein report a strategy that enables the development of a mechano-responsive hydrogel capable of on-demand degradation. Phospholipid-block copolymer hybrid vesicles embedded in a hydrogel matrix can act as signal transducers, releasing encapsulated compounds that control the hydrogel network when an external compressive stress is applied. This generalizable approach holds promise for the creation of diverse functional hydrogels.
Hydrogels have emerged as versatile materials with diverse applications in basic science research and applied healthcare, owing to their excellent water-retention ability, biomimetic structure, intrinsic flexibility, and unique mechanics.[1–4] Stimuli-responsive hydrogels, which can alter their mechanical, chemical, and/or structural properties in response to environmental cues, have gained considerable attention as soft actuators in many fields, including robotics and biomedicine.[5,6] Degradation is one of the important properties that stimuli-responsive hydrogels can exhibit, and this feature can be a critical design criterion for bioactive factor delivery systems and tissue engineering applications.[7–9] Various types of external stimuli, including light, pH, temperature, and enzymes, have been used as triggers to induce hydrogel degradation.[7,8] However, hydrogels that degrade in response to mechanical stress have not yet been reported.
Mechanosensation is universal to all living creatures, and mechanical forces govern various biological processes via mechanotransduction.[10–12] Existing mechano-responsive hydrogels can exhibit strain-stiffening,[13] self-healing,[14] and shear-thinning,[15] and these properties are usually endowed by specific modifications to the chemical nature of the hydrogel systems.[16] A current gap in technology is whether mechano-responsive degradable hydrogels can be developed, and if this capacity can be mediated by materials embedded within hydrogels, instead of the chemical modification of functional groups. Mechano-responsive degradable hydrogels have game-changing potential for biosensors, bioactive factor delivery, tissue engineering, and biomaterial science.[16,17] For example, such hydrogels could serve as biosensors and reporters for swelling and increased pressure and enable the release of pain relief and/or anti-inflammatory drugs under compressive stress in cartilage. Alternatively, hydrogel scaffolds replacing damaged tissue under mechanical load, such as bone, could degrade in response to compressive stress as the tissue regenerates.
Here, we present a mechano-responsive hydrogel system that utilizes vesicles, spherical enclosed membrane structures, as signal transducers capable of converting mechanical signals into chemical/biological signals that control the hydrogel network behavior. When the hydrogel is subjected to compressive stress, the vesicles embedded in the hydrogel release encapsulated compounds that subsequently regulate the hydrogel’s properties. As a demonstration, we embedded vesicles loaded with ethylene glycol tetraacetic acid (EGTA), a calcium chelator, into a calcium-crosslinked alginate hydrogel. We selected EGTA over EDTA due to its higher selectivity towards calcium ions. In response to compressive stress applied to the hydrogel, the incorporated vesicles release EGTA. As EGTA sequesters calcium ions from the calcium-crosslinked alginate hydrogel, it results in the degradation of the hydrogel. To the best of our knowledge, this is the first report of an on-demand hydrogel degradation strategy that directly responds to mechanical stress. This generalizable platform of incorporating vesicles can be easily applied to different types of hydrogels to introduce mechano-responsiveness without the need to modify their chemical structures. Hydrogels can be designed to respond and change in specific manners depending on the type of network-tuning compounds loaded in vesicles. This study provides valuable insights into the design of stimuli-responsive hydrogels that will benefit a wide range of biomedical applications, including tissue regeneration.
Vesicle membranes can exhibit pore-forming behavior and rupture when the membrane is stretched until critical lysis tension is exceeded.[18] The lysis threshold varies depending on membrane composition; for instance, the threshold is 5–10 mN/m for phosphatidylcholine membranes,[19] and higher values (>20 mN/m) are measured for polymer membranes.[20,21] Inspired by this, we selected vesicles as signal transducers capable of releasing molecules when the mechanical stimulus is applied to deform and rupture them. As phospholipid vesicles are known to exhibit low physical stability and a short lifetime,[22–24] we employed phospholipid-block copolymer hybrid vesicles for improved physical stability (Figure S1).
Figure 1A illustrates a process for producing a mechano-responsive degradable hydrogel. To begin with, vesicles containing a calcium chelator EGTA are mixed with an alginate macromer solution. As alginate can be crosslinked in the presence of divalent cations, calcium ions are added to form the hydrogel network. Upon compression of the hydrogel, the vesicles embedded within the hydrogel matrix undergo deformation, increasing membrane tension to form pores and potentially rupture, releasing the internal EGTA. Since EGTA has a much lower dissociation constant (Kd = 1.1 × 10−7 M)[25] than that of alginate macromers (Kd = 2 × 10−4 and 1 × 10−3 M for mannuronic acid and guluronic acid, respectively) for calcium,[26] the released EGTA reversibly and competitively binds to the calcium that crosslinked the hydrogel and ultimately degrades the hydrogel.
Figure 1.
(A) Schematic of preparation of a mechano-responsive degradable hydrogel. The vesicles containing EGTA, a calcium chelator, were incorporated into alginate macromer solution, and calcium ions were added to crosslink the hydrogel. Application of compressive stress to the hydrogel leads to the release of EGTA from the vesicles, resulting in the degradation of the hydrogel. (B) Schematic of generation of vesicles using the continuous droplet interface crossing encapsulation (cDICE) method. Water-in-oil droplets pass through an oil-water interface to form a complete bilayer structure. Note that the schematics are not drawn to scale.
Vesicles were generated using a modified continuous droplet interface crossing encapsulation (cDICE) method[27–29] (Figure 1B, see Supporting Information). In short, it is an emulsion-based method where monolayers in water-in-oil droplets acquire a second monolayer at the oil−water interface through centrifugal force by van der Waals attraction.[30] Poly(ethylene oxide)-b-polybutadiene (PEO14-b-PBD22, MW = 1.8 kDa, hereafter referred to as PEO-PBD) was selected as the block copolymer as it is a well-established amphiphilic polymer for hybrid vesicle formation. 1.8 kDa sized PEO-PBD was used, as its similar thickness to lipid membrane helps minimize the mismatch between lipid and polymer.[31] PEO-PBD was mixed with 1,2-dioleoyl-sn-glycero-3-phophocholine (DOPC) at 50/50 mol% to produce phospholipid-block copolymer hybrid vesicles. Generated vesicles had an average size of ~21.0 ± 8.5 μm (Figure S2) and had significantly better retention of encapsulated molecules when compared with DOPC phospholipid vesicles (Figure S1). The percentage of the vesicles embedded in the gel was 18.6 v/v%. The 0.8% alginate solution mixed with EGTA-loaded vesicles was crosslinked with 50 mM CaCl2 solution (see Supporting Information). Figure 2A shows that the vesicles were uniformly dispersed within the resulting hydrogel after crosslinking.
Figure 2.
(A) Brightfield and fluorescence images of the embedded vesicles and fluorescent beads within the hydrogel. (B) Monitoring of fluorescent dye (Cy-5)- in the same vesicle embedded in the hydrogel for 30 days. Consistent bead positions (white arrows) demonstrate the vesicle is maintaining its position within the hydrogel matrix. (C) Schematic illustration of the hydrogel compression setup. (D) Photographs of the hydrogels before and after application of external force using the setup in (C).
A vesicle-embedded hydrogel system was first used to investigate two aspects: i) the ability of vesicles to retain small molecule compounds while embedded in a hydrogel matrix, and ii) the ability of vesicles to release compounds in response to applied compressive stress. Figure 2B demonstrates how vesicles can stably retain loaded compounds within the hydrogel (Supplementary Video 1). Over a period of one month, the fluorescence signal of dye (Cy-5, MW = 681 g/mol) inside the same vesicle remained stable. Additionally, embedded fluorescent beads remained at the same locations around the vesicle, indicating that the vesicle maintained its position within the matrix (Figure S3). To assess the compound-releasing behavior of the vesicles under compression, the embedded vesicles and their internal fluorescent dye signal were monitored while gradually increasing the applied compressive stress to the hydrogel using the setup shown in Figure S4A. The shape of the vesicles became distorted as we increased the pressure from 2.9 to 12.8 kPa and about 33% of the monitored (n = 12) vesicles lost their internal fluorescence signal with flattened membrane shape, indicating vesicle rupture (Figure S4B). These results confirmed that the vesicle-containing hydrogel can be used as an on-demand mechano-responsive system that stably retains and releases the loaded compounds inside the vesicles in the absence and presence, respectively, of external mechanical stress.
A compression-responsive degradable hydrogel was then generated by incorporating EGTA-loaded vesicles into the alginate hydrogel. The EGTA concentration for the vesicle encapsulation was 880 mM. This concentration was chosen based on a simple experiment to check the required EGTA concentration for degrading the entire gel within a short amount of time (~20 min) (see Supporting Information, Figure S5). Following encapsulation of EGTA in vesicles using cDICE, the final EGTA concentration within the vesicles was examined, as it is common that the inner solution of the droplets can be diluted by the outer aqueous solution during the bilayer formation.[27] The EGTA concentration within the vesicles was found to be 844 mM, indicating an encapsulation efficiency of ~96% (Figure S6).
To characterize the degradation behavior of the hydrogel under compression, three different approaches were used: i) analysis of Brownian motion of fluorescent beads embedded in the alginate hydrogel, ii) measurement of the gel weight after applying compression, and iii) mechanical property measurement of hydrogel with and without compressive stress. Figure 2C illustrates how compressive stress was applied to the hydrogel. A hydrogel was placed in a 96-well plate, and a 3D-printed support was placed on top of the hydrogel followed by a weight. The compressive stress applied to the hydrogel was calculated by dividing the force imparted by the weight and the 3D-printed support by the contact area of the gel, assuming negligible frictional forces between the wall and the support (see Supporting Information). During each experiment, 33 or 55 kPa of compressive stress was applied for 20 min. Figure 2D shows photographs of 0.8% hydrogel samples before and after applying 55 kPa compressive stress using the described setup. 0.1 μm size fluorescent beads were also embedded in all sets of hydrogels to examine their Brownian motion as their diffusivity reflects the gel or solution state of the hydrogel (Figure S7).
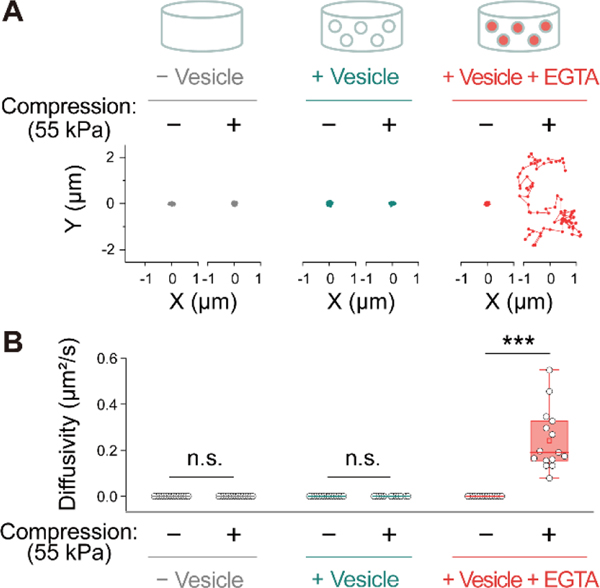
The Brownian motion of the fluorescent beads embedded in hydrogels was first analyzed (Figure 3 and S8). Videos recording the motion of the fluorescent beads in the samples were acquired for 80 frames at ~286 ms intervals, using 488 nm laser excitation. After applying 55 kPa of compressive stress, the hydrogels that contained EGTA-loaded vesicles showed increased movements of the fluorescent beads, with a significant increase of diffusivity from 0 μm2/s to 0.24 μm2/s (Figure 3, S8, and Supplementary Video 2). The increased diffusivity indicates that the beads had an increased range of movement within the hydrogel network, implying the dissociation of the calcium crosslinks in alginate hydrogel due to the released EGTA. In two control groups, a hydrogel without vesicles and a hydrogel with vesicles without EGTA, the motion of the beads did not change after stress was applied. These results demonstrate that alginate hydrogels containing EGTA-loaded vesicles can be successfully degraded under an external mechanical stress.
Figure 3.
Characterization of hydrogel degradation through Brownian motion analysis of fluorescent beads in compressed and uncompressed hydrogels. (A) Representative examples demonstrating the Brownian motion of the fluorescent beads. (B) Diffusion coefficient of the fluorescent beads (n = 15). Statistical significance was determined using a two-tailed Student’s t-test: n.s. (not significant); *** p < 0.001.
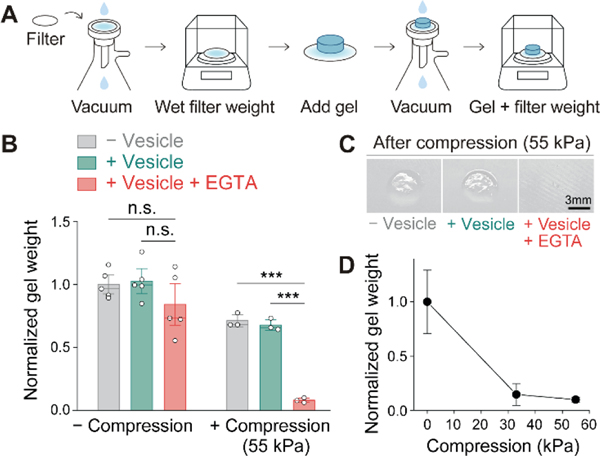
After confirming hydrogel degradation at a microscopic level by analyzing fluorescent bead movements, the overall hydrogel degradation was evaluated by measuring the remained gel weight after stress application. To remove the degraded portion from a hydrogel after compression, the gel was rinsed on a wetted hydrophilic nylon filter membrane with a vacuum apparatus, and the residual hydrogel weight was measured (Figure 4A, S9, and Supporting Information). As expected, the hydrogels that contained EGTA-loaded vesicles showed significant weight reduction (90%) after the compression was applied (Figure 4B). The difference is clearly shown in the photographs in Figure 4C. A lower stress of 33 kPa was also found to trigger hydrogel degradation at a slightly lower degree compared to 55 kPa (Figure 4D).
Figure 4.
Characterization of hydrogel degradation through residual hydrogel weight measurement after compression. (A) Illustration showing the process used to measure the weight of the residual hydrogel. (B) Comparison of the normalized hydrogel weight from different conditions with and without applied stress (55 kPa). Statistical significance was determined by ANOVA: n.s. (not significant); *** p < 0.001. (C) Photographs showing the residual hydrogels on the membrane filter after stress application (55 kPa). (D) Normalized weight of hydrogels containing EGTA-loaded vesicles under different stress conditions (0, 33, 55 kPa). Error bars represent standard deviations (n = 3–5).
As a final method of characterizing hydrogel degradation, the local elasticity of hydrogels with and without the applied force was measured using a nanoindenter (Hysitron TI-950 Triboindenter). A significant reduction (p < 0.01) of Young’s modulus was observed when 55 kPa compressive stress was applied to the hydrogels containing EGTA-loaded vesicles, consistent with the previous results that showed a gel-to-solution transition (Figure 5). In contrast, when compressive stress was applied to the hydrogels that did not include vesicles or that included vesicles without EGTA, Young’s modulus did not decrease. It was also observed that without the compression, the Young’s modulus of the hydrogels that contained vesicles (with or without EGTA) was lower than that of the hydrogel without vesicles. This is probably because vesicle-embedded hydrogels contain approximately 18.6% of their volume with liquid-loaded vesicles, whereas hydrogels without vesicles possess alginate-crosslinked networks in these regions.
Figure 5.
Mechanical characterization of the hydrogel conditions (without vesicles, vesicles without EGTA, vesicles with EGTA) with and without the stress application (55 kPa) for 20 minutes. Statistical significance was determined by ANOVA: n.s. (not significant); * p < 0.05; ** p < 0.01. Error bars represent standard deviations (n = 3).
In summary, we have developed a hydrogel system that can degrade in response to external compressive stress. This generalizable approach, embedding mechanosensitive vesicles, may enable creation of different functional hydrogels that can dynamically change their network by responding to external stress, depending on the type of hydrogel and the compound loaded in vesicles. Moreover, this system offers many adjustable parameters, including vesicle size, density, and membrane composition, which allow tuning the responsiveness for specific stress intensity. Note that while vesicle size can be tuned, having a size variation within a hydrogel might provide an advantage of enabling gradual compound release in response to a designed range of stress intensity, as the amount of stress required to reach the lytic membrane tension for different sizes will vary. Additionally, the functionality of the system can be further expanded when vesicles are used as nanoreactors or cell-like assemblies by encapsulating protein expression systems.[32] We also envision incorporating stem cells into the mechano-responsive hydrogel so that differentiation behavior can be tuned in response to changes in hydrogel crosslink density[33] or from the released chemicals when external compressive stress is applied. This simple approach of introducing mechano-responsiveness in hydrogels will find numerous applications in mechanobiology, tissue engineering, and regenerative medicine.
Supplementary Material
Acknowledgements
We thank the Liu lab members for the helpful discussions. Support is acknowledged from National Institutes of Health (R01 EB030031 (A.P.L.) and R21 AR080363 (A.P.L., E.A)), Office of Naval Research (N000141812876 and N00014-20-1-2479 (N.A.K.)), Newton Award (HQ00342010033 (N.A.K.)), and Kwanjeong Educational Foundation (C.-M.L). We thank Samuel Chen for his help with 3D printing the cylindrical supports.
Footnotes
Institute and/or researcher Twitter usernames: @LiuLab2012
Supporting information for this article is given via a link at the end of the document.
References
- [1].Ali F, Khan I, Chen J, Akhtar K, Bakhsh EM, Khan SB, Gels 2022, 8, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou Y, Damasceno PF, Somashekar BS, Engel M, Tian F, Zhu J, Huang R, Johnson K, McIntyre C, Sun K, Yang M, Green PF, Ramamoorthy A, Glotzer SC, Kotov NA, Nat Commun 2018, 9, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abalymov A, Van Der Meeren L, Saveleva M, Prikhozhdenko E, Dewettinck K, Parakhonskiy B, Skirtach AG, ACS Biomater Sci Eng 2020, 6, 3933–3944. [DOI] [PubMed] [Google Scholar]
- [4].Yu P, Li Y, Sun H, Ke X, Xing J, Zhao Y, Xu X, Qin M, Xie J, Li J, ACS Appl Mater Interfaces 2022, 14, 27360–27370. [DOI] [PubMed] [Google Scholar]
- [5].Lavrentieva A, Pepelanova I, Seliktar D, Tunable Hydrogels: Smart Materials for Biomedical Applications, Springer Nature, 2021. [DOI] [PubMed] [Google Scholar]
- [6].Ding A, Jeon O, Tang R, Bin Lee Y, Lee SJ, Alsberg E, Advanced Science 2021, 8, 2004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kloxin AM, Kasko AM, Salinas CN, Anseth KS, Science 2009, 324, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Patterson J, Hubbell JA, Biomaterials 2010, 31, 7836–7845. [DOI] [PubMed] [Google Scholar]
- [9].Rial R, Liu Z, Ruso JM, Nanomaterials 2020, 10, 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ingber DE, Ann Med 2003, 35, 564–577. [DOI] [PubMed] [Google Scholar]
- [11].Liu AP, Chaudhuri O, Parekh SH, Integrative Biology (United Kingdom) 2017, 9, 383–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang N, Tytell JD, Ingber DE, Nat Rev Mol Cell Biol 2009, 10, 75–82. [DOI] [PubMed] [Google Scholar]
- [13].Kouwer PHJ, Koepf M, Le Sage VAA, Jaspers M, Van Buul AM, Eksteen-Akeroyd ZH, Woltinge T, Schwartz E, Kitto HJ, Hoogenboom R, Picken SJ, Nolte RJM, Mendes E, Rowan AE, Nature 2013, 493, 651–655. [DOI] [PubMed] [Google Scholar]
- [14].Li L, Yan B, Yang J, Chen L, Zeng H, Advanced Materials 2015, 27, 1294–1299. [DOI] [PubMed] [Google Scholar]
- [15].Highley CB, Rodell CB, Burdick JA, Advanced Materials 2015, 27, 5075–5079. [DOI] [PubMed] [Google Scholar]
- [16].Chen J, Peng Q, Peng X, Han L, Wang X, Wang J, Zeng H, ACS Appl Polym Mater 2020, 2, 1092–1107. [Google Scholar]
- [17].Roy A, Manna K, Pal S, Mater Chem Front 2022, 6, 2338–2385. [Google Scholar]
- [18].Go YK, Leal C, Chem Rev 2021, 121, 13996–14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olbrich K, Rawicz W, Needham D, Evans E, Biophys J 2000, 79, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K, Chem Soc Rev 2018, 47, 8572–8610. [DOI] [PubMed] [Google Scholar]
- [21].Discher DE, Ahmed F, Annu Rev Biomed Eng 2006, 8, 323–341. [DOI] [PubMed] [Google Scholar]
- [22].Yan X, Scherphof GL, Kamps JAAM, J Liposome Res 2005, 15, 109–139. [DOI] [PubMed] [Google Scholar]
- [23].De Leo V, Milano F, Agostiano A, Catucci L, Polymers (Basel) 2021, 13, 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brodszkij E, Westensee IN, Holleufer SF, Ade C, Andres PDD, Pedersen JS, Städler B, Appl Mater Today 2022, 29, 101549. [Google Scholar]
- [25].Tsien RY, Biochemistry 1980, 19, 2396–2404. [DOI] [PubMed] [Google Scholar]
- [26].Steginsky CA, Beale JM, Floss HG, Mayer RM, Carbohydr Res 1992, 225, 11–26. [DOI] [PubMed] [Google Scholar]
- [27].Van De Cauter L, Fanalista F, Van Buren L, De Franceschi N, Godino E, Bouw S, Danelon C, Dekker C, Koenderink GH, Ganzinger KA, ACS Synth Biol 2021, 10, 1690–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bashirzadeh Y, Wubshet N, Litschel T, Schwille P, Liu AP, Journal of Visualized Experiments 2021, e63332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wubshet NH, Wu B, Veerapaneni S, Liu AP, Biophys J 2022, 122, 2068–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abkarian M, Loiseau E, Massiera G, Soft Matter 2011, 7, 4610–4614. [Google Scholar]
- [31].Jacobs ML, Boyd MA, Kamat NP, Proc Natl Acad Sci U S A 2019, 116, 4031–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu AP, Appel EA, Ashby PD, Baker BM, Franco E, Gu L, Haynes K, Joshi NS, Kloxin AM, Kouwer PHJ, Mittal J, Morsut L, Noireaux V, Parekh S, Schulman R, Tang SKY, Valentine MT, Vega SL, Weber W, Stephanopoulos N, Chaudhuri O, Nat Mater 2022, 21, 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jeon O, Kim TH, Alsberg E, Acta Biomater 2021, 136, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.