Abstract
BACKGROUND
The incidence and mortality of liver cancer are among the highest of all malignant tumors in China. The high recurrence rate after conventional hepatectomy is worrying. There is a lack of effective prognostic indicators for liver cancer.
AIM
To explore the clinical significance of preoperative serum oxidative stress and serum uric acid (UA) levels in hepatitis B-related liver cancer.
METHODS
The medical records of 110 hepatitis B-related liver cancer patients who underwent hepatectomy in Gansu Provincial Hospital were retrospectively analyzed. Recurrence in patients within 3 years after surgery was determined. The logistic regression model and Pearson or Spearman correlation were used to analyze the correlation between oxidative stress level and UA, and the recurrence of hepatitis B-related liver cancer.
RESULTS
Compared with the non-recurrence group, the levels of superoxide dismutase (SOD) and glutathione (GSH) in the recurrence group were lower and the levels of malondialdehyde (MDA) and UA were higher (all P < 0.05). UA, SOD, MDA, and GSH were risk factors for postoperative recurrence in hepatitis B-related liver cancer patients (P < 0.05). UA was positively correlated with MDA (r = 0.395, P < 0.001) and negatively correlated with GSH (r = -0.204, P = 0.032). The area under the receiver operating characteristic curve (AUC) of SOD, MDA, GSH, and UA in predicting the prognosis was 0.276, 0.910, 0.199, and 0.784, respectively (all P < 0.001).
CONCLUSION
The preoperative serum SOD, GSH, MDA, and UA levels had significant predictive effects on postoperative recurrence of hepatitis B-related liver cancer.
Keywords: Hepatitis B, Liver cancer, Serum oxidative stress, Serum uric acid, Recurrence, Correlation
Core Tip: Hepatitis B-related liver cancer is characterized by high morbidity and mortality. Conventional surgery results in a poor prognosis and a high recurrence rate of liver cancer. In this study, we analyzed the clinical data of 110 patients with hepatitis B-related liver cancer who underwent hepatectomy and determined recurrence within three years after surgery. The correlation between preoperative serum oxidative stress level and serum uric acid, and recurrence of hepatitis B-related liver cancer was assessed. These findings provide a breakthrough in prognostic evaluation indicators of liver cancer.
INTRODUCTION
The incidence and mortality rate of liver cancer, also known as primary liver cancer, are among the highest of all malignant tumors in China, and is a serious threat to the health and life of our residents[1]. Hepatitis B virus (HBV) is the leading cause of hepatocellular carcinoma (HCC), which accounts for 90% of all liver cancers[2]. According to the data, more than 50% of HCCs worldwide are caused by HBV infection[3]. The HBV can change the genes in liver cells and cause liver lesions, thus inducing cirrhosis and even liver cancer (hepatitis B-related liver cancer)[4]. At present, hepatectomy is an important treatment for liver cancer, but the prognosis of patients after surgery is not ideal. The recurrence rate of HCC after surgery is as high as 70%[5]. Therefore, early improvement of the condition and prognosis of liver cancer is a hot research topic. Oxidative stress injury is involved in the process of liver fibrosis, thereby promoting disease progression[6]. Serum uric acid (UA) in critically ill patients is closely related to oxidative stress[7]. Thus, we speculate that there may be a relationship between preoperative oxidative stress and UA, and liver cancer prognosis, and could be used to assess the patient's condition and prognosis to guide clinical intervention. In addition, following a literature review, we found that there are few studies on the effects of oxidative stress and UA on the prognosis of HCC. Both these parameters may provide a breakthrough in the study of liver cancer prognosis evaluation indicators. Therefore, we analyzed oxidative stress, UA, and recurrence in hepatitis B-related liver cancer patients who underwent hepatectomy, to identify a simple and effective index for evaluation of the condition and recurrence of the disease, to improve the level of treatment.
MATERIALS AND METHODS
Materials
Hepatitis B-related liver cancer patients who underwent hepatectomy in Gansu Provincial Hospital from January 2016 to March 2019 were retrospectively analyzed. The inclusion criteria were: (1) Postoperative pathology confirmed HCC[8]; (2) Liver cancer in patients was caused by hepatitis B; and (3) The medical records, related indicators and follow-up data were complete. The exclusion criteria were: (1) Liver cancer combined with other tumors; (2) Patients who had received radiofrequency ablation, transcatheter arterial chemoembolization, molecularly targeted drugs, immune checkpoint inhibitors, and other anti-tumor treatment; and (3) Combined systemic infection.
Data collection
The clinicopathological features included age, gender, hepatitis B surface antigen, TNM stage, tumor diameter, tumor differentiation, lymph node metastasis, tumor number, and alpha-fetoprotein. Serum oxidative stress indices, superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GSH) were determined in addition to UA level.
Surgery and detection methods
The patients were treated with hepatectomy under general anesthesia. The size and volume of the liver were determined according to preoperative imaging data. According to the primary site of HCC, the tumor, and the surrounding blood vessels, the patients were reasonably selected for local hepatectomy, segmental hepatectomy, lobectomy, hemihepatectomy, and other surgical treatment. According to the intraoperative situation, the Pringle method was used to block the hepatic portal system, 5 min each time.
SOD, MDA, and GSH levels were detected by chemical colorimetry, and UA level was detected by the uricase method.
Follow-up indicators
The recurrence data in the outpatient or inpatient system were reviewed. The last visit record or telephone follow-up record was used as the follow-up result to collect information on tumor recurrence within 3 years after surgery. Patients lost to follow-up or death were defined as censored.
Statistical analysis
SPSS 17.0 was used to process the data. The data were described by mean ± SD, cases or percentages (%), and the differences between groups were tested by the t-test or chi-square test. Multiple factors were analyzed with a logistic regression model, and Pearson or Spearman correlation analysis was used for bivariate correlation analysis. The predictive ability was analyzed by the receiver operating characteristic (ROC) curve. A P value > 0.05 was considered statistically significant.
RESULTS
Clinicopathological features of the recurrence group and non-recurrence group
In total, 110 patients were enrolled, including 69 recurrent patients (recurrence group) and 41 non-recurrent patients (non-recurrence group). In comparison with the non-recurrence group, the proportion of patients with TNM stage III-IV (59.42% vs 26.83%), high tumor differentiation (56.52% vs 31.71%), and lymph node metastasis (43.48% vs 21.95%) was high in the recurrence group (P < 0.05) (Table 1).
Table 1.
Comparison of clinicopathological features between the recurrence group and non-recurrence group, n (%)
|
Groups
|
Recurrence group (n = 69)
|
Non-recurrence group (n = 41)
|
t value
|
P value
|
| Age (mean ± SD, yr) | 54.63 ± 15.58 | 55.10 ± 15.29 | ||
| Gender | 3.497 | 0.061 | ||
| Men | 40 (57.97) | 31 (75.61) | ||
| Female | 29 (42.03) | 10 (24.39) | ||
| HbsAg | 0.313 | 0.576 | ||
| Negative | 22 (31.88) | 11 (26.83) | ||
| Positive | 47 (68.12) | 30 (73.17) | ||
| TNM staging | 10.959 | 0.001 | ||
| Stage I-II | 28 (40.58) | 30 (73.17) | ||
| Stage III-IV | 41 (59.42) | 11 (26.83) | ||
| Tumor diameter | 0.979 | 0.323 | ||
| < 5 cm | 27 (39.13) | 20 (48.78) | ||
| ≥ 5 cm | 42 (60.87) | 21 (51.22) | ||
| Degree of tumor differentiation | 6.353 | 0.012 | ||
| Low differentiation | 30 (43.48) | 28 (68.29) | ||
| High differentiation | 39 (56.52) | 13 (31.71) | ||
| Lymph node metastasis | 5.208 | 0.022 | ||
| Yes | 30 (43.48) | 9 (21.95) | ||
| No | 39 (56.52) | 32 (78.05) | ||
| Number of tumors | 0.298 | 0.585 | ||
| Multiple | 44 (63.77) | 24 (58.54) | ||
| Single | 25 (36.23) | 17 (41.46) | ||
| Alpha-fetoprotein (μg/L) | 3.137 | 0.077 | ||
| < 200 | 37 (53.62) | 29 (70.73) | ||
| ≥ 200 | 32 (46.38) | 12 (29.27) |
Oxidative stress level and UA level between the two groups
Compared with the non-recurrence group, the levels of SOD (41.26 kU/L ± 7.01 kU/L vs 46.82 kU/L ± 6.12 kU/L) and GSH (29.40 kU/L ± 7.92 kU/L vs 39.44 kU/L ± 8.90 kU/L) were lower in the recurrence group, and the levels of MDA (5.78 nmol/L ± 0.92 nmol/L vs 4.18 nmol/L ± 0.82 μmol/L) and UA (376.27 μmol/L ± 82.90 μmol/L vs 281.36 μmol/L ± 84.86 μmol/L) were higher (P < 0.05) (Table 2).
Table 2.
Comparison of preoperative serum oxidative stress level and serum uric acid between the two groups (mean ± SD)
| Groups |
Oxidative stress
|
Serum UA (μmol/L) | ||
|
SOD (kU/L)
|
MDA (nmol/L)
|
GSH (kU/L)
|
||
| Recurrence group (n = 69) | 41.26 ± 7.01 | 5.78 ± 0.92 | 29.40 ± 7.92 | 376.27 ± 82.90 |
| Non-recurrence group (n = 41) | 46.82 ± 6.12 | 4.18 ± 0.82 | 39.44 ± 8.90 | 281.36 ± 84.86 |
| t value | 4.212 | 9.176 | 6.137 | 5.755 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
SOD: Superoxide dismutase; MDA: Malondialdehyde; GSH: Glutathione; UA: Uric acid.
Analysis of recurrence risk factors
The clinicopathological features (including TNM stage, tumor differentiation, lymph node metastasis), serum oxidative stress level, and UA level as the independent variables and recurrence (0 = no recurrence, 1 = recurrence) as the dependent variable were incorporated into the logistic regression model. It was shown that UA (Exp (B) = 5.899, P = 0.019], SOD [Exp (B) = 0.844, P = 0.043], MDA [Exp (B) = 11.465, P = 11.465], and GSH [Exp (B) = 0.889, P = 0.029] were risk factors for postoperative recurrence (P < 0.05) (Table 3).
Table 3.
Multivariate logistic regression analysis of the prognosis of patients with hepatitis B-related liver cancer
|
Independent variable
|
B
|
S. E
|
Wals
|
P value
|
Exp (B)
|
95%CI
|
|
|
Lower limit
|
Upper limit
|
||||||
| TNM staging | 1.026 | 0.879 | 1.361 | 0.243 | 2.789 | 0.498 | 15.628 |
| Degree of tumor differentiation | 1.775 | 0.914 | 3.774 | 0.052 | 5.899 | 0.984 | 35.355 |
| Lymph node metastasis | -1.183 | 1.005 | 1.387 | 0.239 | 0.306 | 0.043 | 2.194 |
| SOD | -0.17 | 0.084 | 4.098 | 0.043 | 0.844 | 0.716 | 0.995 |
| MDA | 2.439 | 0.714 | 11.676 | 0.001 | 11.465 | 2.83 | 46.454 |
| GSH | -0.117 | 0.054 | 4.74 | 0.029 | 0.889 | 0.8 | 0.988 |
| Serum UA | 0.013 | 0.005 | 5.541 | 0.019 | 1.013 | 1.002 | 1.024 |
95%CI: 95% confidence interval; SOD: Superoxide dismutase; MDA: Malondialdehyde; GSH: Glutathione; UA: Uric acid.
Analysis of the relationship between the risk factors
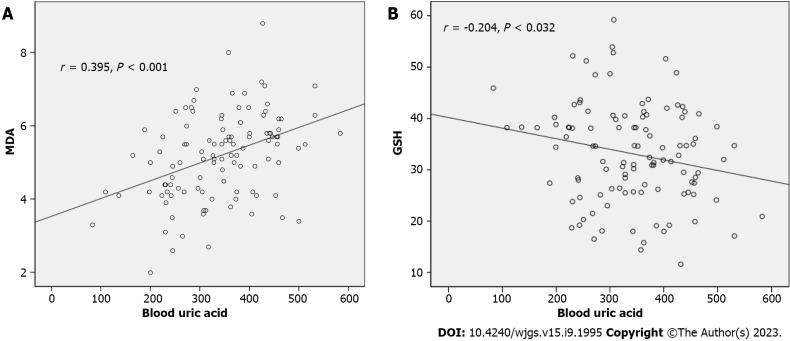
SOD was negatively correlated with the TNM stage and lymph node metastasis (r = -0.203, -0.219; P = 0.033, 0.021). MDA was positively correlated with the TNM stage and lymph node metastasis (r = 0.275, 0.216; P = 0.004, 0.024). GSH was negatively correlated with lymph node metastasis (r = -269; P = 0.004). UA showed no correlation with SOD (r = -0.185, P = 0.053). UA was positively correlated with MDA (r = 0.395, P < 0.001) (Figure 1A), and negatively correlated with GSH (r = -0.204, P = 0.032) (Figure 1B).
Figure 1.
Relationship between serum uric acid and malondialdehyde levels and glutathione levels. A: Malondialdehyde levels; B: Glutathione levels; MDA: Malondialdehyde; GSH: Glutathione.
AUC evaluated predictive power
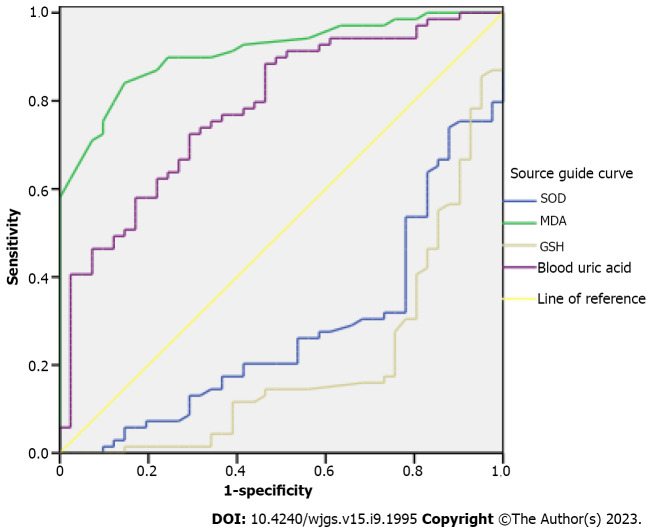
AUC of SOD, MDA, GSH, and UA in predicting postoperative recurrence was 0.276 [95% confidence interval (95%CI): 0.179-0.373], 0.910 (95%CI: 0.858-0.963), 0.199 (95%CI: 0.110-0.288), and 0.784 (95%CI: 0.697-0.871), respectively, all P < 0.001 (Table 4, Figure 2).
Table 4.
Area under the receiver operating characteristic curve of preoperative serum oxidative stress and serum uric acid levels in predicting prognosis of patients with hepatitis B-related liver cancer
| Variable | AUC | Standard error | P value |
95%CI
|
|
|
Lower limit
|
Upper limit
|
||||
| SOD | 0.276 | 0.050 | < 0.001 | 0.179 | 0.373 |
| MDA | 0.910 | 0.027 | < 0.001 | 0.858 | 0.963 |
| GSH | 0.199 | 0.045 | < 0.001 | 0.110 | 0.288 |
| Serum UA | 0.784 | 0.044 | < 0.001 | 0.697 | 0.871 |
95%CI: 95% confidence interval; SOD: Superoxide dismutase; MDA: Malondialdehyde; GSH: Glutathione; UA: Uric acid; AUC: Area under the receiver operating characteristic curve.
Figure 2.
Area under the receiver operating characteristic curve of preoperative serum oxidative stress and serum uric acid levels in predicting prognosis of patients with hepatitis B-related liver cancer. SOD: Superoxide dismutase; MDA: Malondialdehyde; GSH: Glutathione.
DISCUSSION
Chronic HBV infection is closely related to HCC, and deterioration of chronic HBV infection can lead to liver cancer[9]. Early effective diagnosis is of great significance for improving prognosis and reducing recurrence. Clinically, an ultrasound examination is used for early diagnosis. However, abdominal B-ultrasound requires a high level of operation and is highly subjective[10]. Therefore, the identification of effective diagnostic indicators to improve the diagnosis of HBV-related liver cancer is urgently needed.
This study found that compared with patients without recurrence, the levels of SOD and GSH in patients with recurrence were lower, and the levels of MDA and UA were higher. SOD, GSH, MDA, and UA were closely related to postoperative recurrence, which was similar to the results of related studies[11]. It is suggested that the antioxidant capacity of patients with postoperative recurrence is low, and the oxidative stress response of tissues and organs is strong. Oxidative stress occurs throughout the process of liver fibrosis. Oxidative stress is considered to be the most critical factor in the transition from simple fatty liver to nonalcoholic steatohepatitis[12]. SOD and GSH are important antioxidants and oxygen-free radical scavengers. MDA, GSH, and SOD are indicators that are usually used to assess the body's ability to produce and save oxygen-free radicals[13]. SOD is a natural superoxide radical. Other enzymes in the body will immediately decompose hydrogen peroxide into harmless water[14]. Therefore, SOD can specifically remove harmful free radicals in the body, in order to remove the damage caused by free radical oxidation of some components in the body. It can be seen that the lower the SOD level, the weaker the body's antioxidant capacity, and the more difficult it is to protect liver cells from oxidative stress injury. The synthesis of GSH can enable cells to escape the damage caused by oxidative stress, so that the cells are in a state of redox balance, thereby preventing cell death induced by lipid peroxidation[15]. The decrease in GSH level in patients with recurrence suggests lipid peroxidation damage, which eventually leads to hepatocyte necrosis[16,17]. MDA is formed by lipid peroxidation of the membrane, which causes serious damage to the membrane[18]. The more MDA, the more intense the membrane lipid peroxidation. SOD is negatively correlated with MDA, usually after oxidative stress stimulation, SOD decreases and MDA increases[19]. The increase in serum MDA in relapsed patients can indicate that the oxidative stress state of cells is at a higher level, and the decrease in antioxidant capacity of cells indicates a higher possibility of liver injury. UA is mainly a product formed by hydrolysis and oxidation of purine nucleotides. Human purines are mainly derived from liver synthesis or nucleotide degradation (endogenous), the part of purine involved in the formation of UA accounted for 80% of all UA[20]. Several studies have proposed a correlation between UA and primary liver cancer. UA may be used as an auxiliary serological diagnostic indicator and a nutritional assessment indicator for patients with liver cancer[21]. Current conclusions on the relationship between UA and the degree of liver function damage are inconsistent. Our study showed that higher UA is more likely to cause recurrence, similar to the results of related studies[22]. SOD, MDA, GSH, and UA have clinical significance in predicting recurrence in patients. The AUC of MDA and UA was 0.910 and 0.784, respectively. This also shows that preoperative serum oxidative stress levels as well as UA are closely related to the prognosis of hepatitis B-related liver cancer.
There were limitations to this study. We only analyzed the clinicopathological features, oxidative stress level, and blood UA level of patients with and without recurrence of HBP-related liver cancer after surgery and did not analyze other factors, such as the expression levels of WNT1 and WNT2 in cancerous tissues and adjacent tissues, and serum enzymes before surgery. These confounding factors may affect the study results, and further research is required in the future. In addition, this is a retrospective study and selective bias, information bias, and confounding bias may exist. Therefore, prospective randomized controlled trials are needed to verify the findings of this study.
CONCLUSION
The preoperative serum levels of SOD, GSH, MDA, and UA in patients with postoperative recurrence of hepatitis B-related liver cancer were lower, and the preoperative serum levels of SOD, GSH, MDA, and UA were higher. The preoperative serum levels of SOD, GSH, MDA, and UA had a higher predictive effect on postoperative recurrence. However, the small sample size in this study may have led to bias in the results. Future research should be undertaken to explore the optimal prediction thresholds of SOD, MDA, GSH, and UA to further improve the prediction efficiency of postoperative recurrence.
ARTICLE HIGHLIGHTS
Research background
Liver cancer is one of the most common malignant tumors in China and is associated with high morbidity and mortality rates, which seriously threaten the health and life of Chinese residents. The prognosis following conventional hepatectomy is not ideal, with a recurrence rate of up to 70%.
Research motivation
The purpose of this study was to analyze the correlation between preoperative serum oxidative stress level and serum uric acid (UA), and prognosis in patients with hepatitis B-related liver cancer. This relationship was determined to identify simple and effective evaluation indicators for the assessment of disease condition and prognosis, and to provide data support for clinical improvement of treatment.
Research objectives
To explore the correlation between serum oxidative stress level and serum UA, and prognosis before hepatitis B-related liver cancer recurrence. It was found that serum oxidative stress level and serum UA before hepatitis B-related liver cancer were closely related to prognosis, which is helpful for clinicians to more effectively evaluate prognosis, recurrence and to guide treatment decision-making.
Research methods
The analysis methods used in this study involved a logistic regression model, Pearson analysis, Spearman analysis, and a receiver operating characteristic (ROC) curve, and the analysis target was the correlation between serum oxidative stress level, serum UA, and recurrence of hepatitis B-related liver cancer. The analysis of different research data layer by layer was rigorous and scientific.
Research results
This study found that superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), and UA were all risk factors for postoperative recurrence in patients with hepatitis B-related liver cancer. Serum UA was positively correlated with MDA and negatively correlated with GSH. MDA and UA can predict the prognosis of patients with hepatitis B-related liver cancer. However, we could not determine the specific mechanism of the effect of these four indicators on postoperative recurrence in patients with hepatitis B-related liver cancer.
Research conclusions
This study found that SOD, GSH, MDA, and UA were all risk factors for postoperative recurrence in patients with hepatitis B-related liver cancer. Furthermore, ROC curve analysis showed that only MDA and UA predicted an AUC above 0.5, which was different to previous studies.
Research perspectives
Future research should include a larger sample and prospectively focus on the specific mechanism of oxidative stress level and UA level on the prognosis of hepatitis B-related liver cancer.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Gansu Provincial Hospital (Approval No. 2023-288).
Informed consent statement: All study participants or their legal guardian provided informed written consent regarding personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: The authors declare no conflicts of interest for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: June 6, 2023
First decision: June 21, 2023
Article in press: July 26, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Napolitano M, Italy; Thakur U, India S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
Contributor Information
Jin-Xia Hou, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Yu-Bin Wang, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Jing Wu, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Guo-sheng Ding, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Yang Wu, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Lian-Hua Wei, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Fang Wang, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China.
Zhe-Mei Zhang, Department of Clinical Laboratory, Gansu Provincial Hospital, Gansu Provincial Clinical Research Center for Laboratory Medicine, Lanzhou 730000, Gansu Province, China. zhangzm1122@126.com.
Data sharing statement
Data for this study can be obtained from the corresponding author.
References
- 1.Sohn W, Lee HW, Lee S, Lim JH, Lee MW, Park CH, Yoon SK. Obesity and the risk of primary liver cancer: A systematic review and meta-analysis. Clin Mol Hepatol. 2021;27:157–174. doi: 10.3350/cmh.2020.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu ZP, Wang MD, Chen ZY, Yang T. Primary prevention of hepatitis B-related liver cancer. J Hepatobiliary Surg . 2021;29:341–344. [Google Scholar]
- 3.Bian J, Zhang PH, Li JJ. The Relationship Between EGF Gene Polymorphism and the Sensitivity of HBV-related Primary Liver Cancer to Cisplatin. Pract J Cancer. 2022:37: 404–407. [Google Scholar]
- 4.Yang Q, Wei WH, Chen LL, Zhong QY, Wang YY. Value of microRNA in differential diagnosis of chronic HBV infection and HBV-related liver cancer. Chin J Nosocomiol . 2022;32:21–25. [Google Scholar]
- 5.Hou YF, Guo HY, Li F. Relationship between preoperative serum high-sensitivity C-reactive protein and prognosis of hepatitis B-related hepatocellular carcinoma. Chin J Curr Adv Gen Surg . 2021;24:548–552. [Google Scholar]
- 6.Chen Y, Yuan LP, Wang BY, Hao FF, Yang YS, Xiao PY. Periplaneta Americana extract plays an anti-fibrosis role in inhibiting oxidative stress via Nrf2/HO-1 pathway. Chin J Hosp Pharm. 2022;42:367–372. [Google Scholar]
- 7.Luo D, Wu JX, Qin GY, Liao XW. Correlation analysis of serum uric acid with early-onset epilepsy and oxidative stress levels in patients with acute cerebral in-farction. Trauma Crit Care Med. 2021;9:206–210. [Google Scholar]
- 8.Pinato DJ, Cortellini A, Sukumaran A, Cole T, Pai M, Habib N, Spalding D, Sodergren MH, Martinez M, Dhillon T, Tait P, Thomas R, Ward C, Kocher H, Yip V, Slater S, Sharma R. PRIME-HCC: phase Ib study of neoadjuvant ipilimumab and nivolumab prior to liver resection for hepatocellular carcinoma. BMC Cancer. 2021;21:301. doi: 10.1186/s12885-021-08033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Yao L, Qiu BD, Wang XY. Serum GP73 and hepcidin levels are corelated with the prognosis of hepatitis B-related liver cancer patients. Chin Hepatol. 2021;26:1007–1010. [Google Scholar]
- 10.Zeng SL, Zhu W, Fang CH, He SS, Zhang P, Wen S, Zhang K. Three-dimensional visualization evaluation and VR study of giant liver cancer with blood vessels as the axis. Chin J Gen Surg . 2019;34:323–327. [Google Scholar]
- 11.Yu M, Zhang C, Tang H, Xiao C. Correlation between Serum Oxidative Stress Level and Serum Uric Acid and Prognosis in Patients with Hepatitis B-Related Liver Cancer before Operation. J Healthc Eng. 2022;2022:1964866. doi: 10.1155/2022/1964866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi XL, Ye ML. Effects of gypenoside capsule on glycolipid metabolism, oxidative stress and liver fibrosis in patients with nonalcoholic fatty liver disease. Anhui Yiyao Zazhi. 2022;26:824–828. [Google Scholar]
- 13.Zhou SF, Zhan W, Bian CF. Effect of dexmedetomidine on oxidative stress, liver function and expression of Toll-like receptor 2 and Toll-like receptor 4 in peripheral blood monouclear cells in patients with primary liver cancer undergoing hepalobectomy. Xinxiang Med Univ. 2020;37:869–872. [Google Scholar]
- 14.Mao LY, Lu SZ, Wang WQ, Zhang CB, Zhang LN. Correlation between CXCL3 and cellular oxidative stress in the liver cancer cell SMMC-7721. Linchuang Zhongliuxue Jichu Zazhi. 2022;35:93–99. [Google Scholar]
- 15.Zhao YF, Tao Y. Emerging insights into the functional role of the SLC7A11 gene in malignant neoplasms. Chin J Clin Oncol. 2019;46:795–799. [Google Scholar]
- 16.Huang MQ, Hu LX, Chu XF. Effect of simultaneous chemotherapy of systemic chemotherapy with glutathione on hepatic function in patients with metastatic liver cancer and its safety analysis. Anhui Yiyao Zazhi. 2019;23:2279–2282. [Google Scholar]
- 17.Qiu XY, Jia LQ, Song L, Wang Q. Study on Effect and Mechanism of Huayu Qutan Fomula on Mice with High-Fat and Liver Cancer Based on Ferroptosis Related Protein. Zhongguo Jianzhu Chuantong Yixue. 2021;39:137–141. [Google Scholar]
- 18.Jin FD, Zhang T, Zhang Z, Yin XZ, Quan JS. Protective effect of rutin on oxidative stress injury of HepG2 cells and its mechanism. J Jilin Univ (Med Ed) 2020;46:1117–1123. [Google Scholar]
- 19.Liu HY. The therapeutic effect of fluorouracil combined with radiotherapy on patients with primary liver cancer and its effect on serum LPO and MDA levels. Gonggong Weisheng Yixue Zazhi. 2020;31:105–108. [Google Scholar]
- 20.Cui HT, Zhao HM, Wu YP, Dong J, Zhang X. Correlation analysis of serum UA, Alb and sFas levels with primary liver cancer. Chin J Clin Ration Drug Use. 2015;22:136–137. [Google Scholar]
- 21.Liu Y, Ou X, Yan YR, Lu XC, Li M, Zhou MJ. Serum uric acid and albumin levels and their correlation analysis in 144 patients with liver cancer. J Pract Med. 2014;16:2652–2654. [Google Scholar]
- 22.Wang D, Zhu JY, Li GM, Leng XS. Results of long-time follow up of patients who survived more than 5 years after liver transplantation: A single center experience. J Peking Univ Health Sci . 2011;43:612–615. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study can be obtained from the corresponding author.