Abstract
A clinical isolate of Pseudomonas aeruginosa, PAe191, was found to be highly resistant to all anti-Pseudomonas β-lactam antibiotics (except imipenem) and resistant also to aminoglycosides. It produced a β-lactamase (with an apparent pI of 7.6) which was not inhibited by clavulanic acid. Cloning and characterization of the β-lactamase gene showed that it coded for a novel extended-spectrum OXA-10 variant, called OXA-19, which differed from OXA-10 by nine amino acids and from OXA-13 by two, i.e., Asn in position 73 (Asn73) instead of Ser and Asp157 instead of Gly. Asparagine in position 157 is implicated in resistance to ceftazidime, while the amino acid in position 73, in this variant, seems to condition the level of resistance to penicillins. The oxa19 gene was found to be inserted, in a typical integron structure, immediately downstream from an aac(6′)-Ib gene coding for an aminoglycoside acetyltransferase variant, which was called AAC(6′)-Ib9.
A major mechanism of resistance of Pseudomonas aeruginosa to cephalosporins used in clinical practice, such as cefsulodin or ceftazidime, is the overproduction of its cephalosporinase, resulting from depression of the ampC transcription inhibition pathway (14). Alternatively, resistance to the broad-spectrum cephalosporins, including ceftazidime and cefepime, may result from the production of an extended-spectrum β-lactamase (ESBL). In P. aeruginosa, as opposed to members of the family Enterobacteriaceae, production of TEM- and SHV-type ESBLs (3) seems to be rare (22), and other extended-spectrum enzymes, such as PER-1 (23), OXA-2-, or OXA-10-derived ESBLs (8–10), and a clavulanic acid-susceptible class D enzyme, OXA-18 (24), have been described for clinical strains of this species. The enzymes of molecular class D (13) comprise those of group 2d of the functional classification scheme described by Bush et al. (3) and are characterized by high relative rates of oxacillin hydrolysis and generally low susceptibility to inhibition by clavulanic acid (3, 18). Like the other active-site serine β-lactamases, the class D enzymes have at least three conserved amino acid motifs, which are shown in Fig. 1 for the OXA-10 derivatives.
FIG. 1.
Amino acid differences between the β-lactamases of the OXA-10 group. Amino acid numbering is according to Huovinen et al. (13); the conserved motifs typical for class D enzymes are boxed. The amino acids shown in boldface contribute to the substrate profile, with asparagine in position 157 leading to extended-spectrum variants. The amino acids of OXA-10 that are not numbered are G20, S27, S50, D55, V89, T107, Y174, A197, E229, T230, S245, and E259; −, absence of an amino acid.
High-resolution crystallography studies of several class A penicillinases (34) have generated a basis for understanding the molecular mechanisms underlying the substrate profile extensions secondary to a large array of point mutations in TEM- or SHV-type ESBLs (16, 26). For the class D enzymes, no such studies have been reported, and only two amino acid changes have been implicated in the extension of the substrate profiles to ceftazidime, i.e., Gly157 to Asp in the OXA-10-derived OXA-11 (10) and OXA-14 (8) and Asp150 to Gly in the OXA-2-derived OXA-15 (9). These positions are, respectively, 14 and 2 amino acids downstream from the YGN triad in the OXA-10 and OXA-2 derivatives (6, 8–10, 13) (Fig. 1). Here, we describe a novel ESBL, OXA-19, from a clinical P. aeruginosa isolate, containing nine altered amino acids with respect to the non-ESBL OXA-10 (13) and two altered amino acids with respect to the non-ESBL OXA-13 (20), both of which appear to contribute to the level of resistance to anti-Pseudomonas penicillins and cephalosporins in Escherichia coli and P. aeruginosa.
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used are summarized in Table 1. P. aeruginosa PAe191 was isolated in 1991 at the Saint-Louis Hospital, Paris, France. This strain was highly resistant to ceftazidime (MIC, 512 μg/ml). Strain PAO38(pAZ310) was selected on ceftazidime (16 μg/ml) from strain PAO38(pAZ309), which was described previously (20). Strains were grown in Mueller-Hinton (MH) medium at 37°C.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype or description | Resistance phenotypea | Reference or source |
|---|---|---|---|
| Strain | |||
| PAe191 | Clinical strain of P. aeruginosa | Ti Ca Ak Gm Su Hg | This work |
| DH5α | E. coli; F′/endA1 hsdR17 (rk−mk+) supE44 thi-1 recA1 gyrA (Nalr) relA1 D(lacIZYA-argF)U169 deoR [f80dlacD(lacZ)M15] | Nal | New England Biolabs |
| PAO38Rif | Rifampin-resistant derivative of P. aeruginosa PAO38 | Ri (MIC, >500 μg/ml) | 20 |
| Plasmid | |||
| pHSS6 | 2.3-kb plasmid vector | Km | 32 |
| pK18 | 2.7-kb plasmid vector | Km | 25 |
| pNJR3-2 | 28-kb E. coli-P. aeruginosa shuttle vector | Tc | 28 |
| pAZ305 | 23-kb EcoRI fragment obtained from PAe191 total DNA, cloned into pHSS6; oxa19 | Km Ti Ca Gmb | This work |
| pAZ316 | 4.0-kb HindIII fragment from pAZ305 cloned into HindIII-digested pNJR3-2; oxa19 | Tc Ti Ca Gmb | This work |
| pAZ327 | 1,097-bp NarI-HindIII fragment from pAZ316 cloned into AccI-HindIII-digested pK18, oxa19 | Km Ti Ca | This work |
| pAZ309 | pNJR3-2 derivative encoding oxa13 | Tc Ti Ak | 20 |
| pAZ310 | In vitro-selected derivative of pAZ309 selected on ceftazidime; oxa13-1 | Tc Ti Ca Ak | This work |
| pAZ326 | 1,097-bp NarI-HindIII fragment from pAZ310 cloned into AccI-HindIII-digested pK18; oxa13-1 | Km Ti Ca | This work |
Ti, ticarcilline; Ca, ceftazidime; Km, kanamicin; Ak, amikacin; Gm, gentamicin; Nal, nalidixic acid; Ri, rifampin; Su, sulfamide; Tc, tetracycline; Hg, mercuric ions.
Gm phenotype conferred by AAC(6′)-Ib9 with Ser at position 119.
MIC determinations and antibiotics.
MICs on MH agar containing serially twofold-diluted antibiotics were determined. Plates inoculated with a Steers-type inoculator and ca. 104 CFU per spot were incubated at 37°C for 18 h. The MICs of β-lactams were determined alone or in combination with imipenem (0.25 μg/ml), clavulanic acid (2 μg/ml), or tazobactam (4 μg/ml). MIC determinations were repeated twice, with identical results. Antibiotics were provided from the following suppliers: ampicillin, aztreonam, and amikacin, Bristol Myers Squibb; ceftazidime, Glaxo Group Research, Ltd.; cefotaxime, Hoechst Roussel Pharmaceuticals Ltd.; piperacillin and tazobactam, Lederle Laboratories; imipenem, Merck Sharp and Dohme-Chibret; gentamicin, Schering Plough; clavulanic acid and ticarcillin, SmithKline Beecham; and cefsulodin, Takeda Laboratories.
β-Lactamase preparation.
Crude enzyme (S100) extracts were prepared as previously described (20). The supernatants were used immediately for the determination of kinetic parameters and isoelectric points. The protein concentrations were measured by the technique described by Bradford (2), and the hydrolysis rates in phosphate buffer (50 mM, pH 7.0) were determined spectrophotometrically at 30°C with a model 550S double-beam spectrophotometer (Perkin-Elmer), with ampicillin and ceftazidime as the substrates. One unit of β-lactamase was defined as the amount hydrolyzing 1 μmol of substrate per min.
IEF.
Isoelectric focusing (IEF) of S100 extracts was for 2 h, with a mini-IEF cell 111 (Bio-Rad) and a gradient made up of two-thirds polyampholytes with a pH range of 3 to 9 and one-third of polyampholytes with a pH range of 2 to 11 (Serva). Extracts from OXA-10-, SHV-1-, and SHV-5-producing strains were used as standards for pIs of 6.1, 7.6, and 8.2, respectively. β-Lactamases were revealed by overlay with nitrocefin (1 mg/ml) in phosphate buffer (50 mM, pH 7.0).
Cloning experiments and DNA sequencing.
Standard DNA methodology (1) was used to clone two ca. 4.0-kb HindIII DNA fragments carrying either the in vitro-selected ESBL gene oxa13-1 or the in vivo-selected gene oxa19 (Table 1) (20) into the pNJR3-2 shuttle vector, allowing the expression of both genes in P. aeruginosa PAO38. These fragments were also cloned into M13mp18 and M13mp19 phage vectors and were partially sequenced as described previously (20). The sequenced stretches which have been determined for oxa19 and oxa13-1 and their adjacent integron regions are represented schematically in Fig. 1. The −35 sequences of the promoter of the aac(6′)-Ib, oxa13-1 cistron were taken to be identical with that of the original oxa13-containing integron (20). For expression of the two ESBL genes in an isogenic E. coli background, both genes were cloned on a NarI-HindIII fragment into the vector pK18.
Nucleotide sequence accession numbers.
The nucleotide sequences for oxa19 [including aac(6′)-Ib] and oxa13-1 have been deposited in GenBank under accession numbers AF043381 and AF043558, respectively.
RESULTS AND DISCUSSION
Resistance phenotypes of PAe191 and PAO38(pAZ316).
PAe191 was resistant to all anti-Pseudomonas β-lactam antibiotics tested, except imipenem, and also to aminoglycosides, sulfonamides, and mercuric ions. Ticarcillin, piperacillin, and ceftazidime were not protected when associated with a β-lactamase inhibitor, such as clavulananic acid or tazobactam. However, the high-level resistance of PAe191 to ceftazidime was reversed by imipenem (Table 2). The protective effect of imipenem on otherwise hydrolyzed β-lactam compounds in P. aeruginosa has been previously observed in the presence of OXA-10-related enzymes (20). In order to characterize the β-lactamase responsible for this phenotype and the nucleotide sequences controlling its production, a ca. 4.0-kb HindIII fragment from PAe191 DNA was cloned into pNJR3-2, yielding pAZ316 (Table 1). PAO38(pAZ316) showed the same β-lactam resistance pattern as that of PAe191, and a MIC of gentamicin greater than that of amikacin was observed. Both PAe191 (see Fig. 3, lane 3) and PAO38(pAZ316) (data not shown) produced only one β-lactamase, with an apparent pI (pIapp) of 7.6, which was then likely to be an ESBL. No plasmid was found in the clinical strain PAe191 despite the use of techniques (15, 35) allowing the extraction of large plasmids, such as the OXA-1-encoding plasmid RGN238 (12).
TABLE 2.
MICs of different β-lactams for PAe191 and OXA13-1, OXA-19- and OXA-13-producing transformants
| Antibiotic + inhibitora | MIC (μg/ml) for the following strains (plasmid/enzyme):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| PAe191 | PAO38 (pAZ310/OXA13-1) | PAO38 (pAZ316/OXA-19) | PAO38 (pAZ309/OXA-13)b | PAO38 (pNJR-32) | DH5α (pAZ326/OXA13-1) | DH5α (pAZ327/OXA19) | DH5α (pK18) | |
| Ampicillin | >512 | >512 | >512 | —c | >512 | 8 | 64 | 2 |
| Ticarcillin | 512 | 64 | 256 | 256 | 16 | 8 | 64 | 1 |
| + CLA | 512 | 64 | 256 | 256 | 16 | 8 | 32 | 1 |
| Piperacilin | 128 | 32 | 64 | 32 | 2 | 2 | 8 | 0.5 |
| + TAZ | 128 | 16 | 64 | 32 | 2 | 0.5 | 1 | 0.5 |
| Cefotaxime | 128 | 32 | 32 | 16 | 16 | — | — | — |
| Ceftazidime | 512 | 256 | 256 | 2 | 2 | 8 | 16 | 0.12 |
| + CLA | 256 | 128 | 128 | 2 | 16 | 2 | 2 | 0.06 |
| + IMI | 32 | 16 | 32 | 16 | 16 | — | — | — |
| Aztreonam | 64 | 8 | 16 | 8 | 2 | — | — | — |
| Cefsulodin | 64 | 64 | 64 | 32 | 2 | — | — | — |
| Imipenem | 1 | 1 | 1 | 1 | 1 | — | — | — |
| Amikacin | 32 | 16 | 8 | 16 | 4 | — | — | — |
| Gentamicin | 256 | 4 | 64 | — | 4 | — | — | — |
CLA, clavulanic acid (2 μg/ml); TAZ, tazobactam (4 μg/ml); IMI, imipenem (0.25 μg/ml).
Data are from reference 20.
—, not determined.
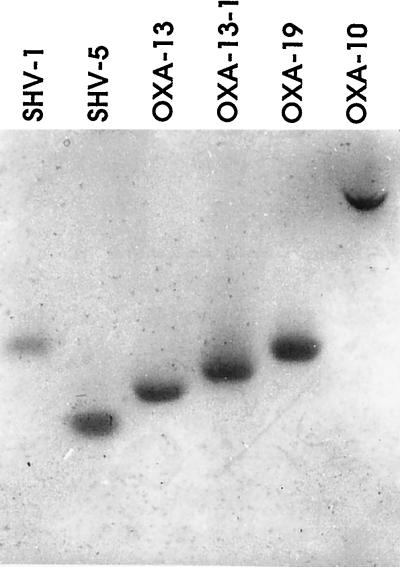
FIG. 3.
Isoelectric focusing of extended-spectrum OXA-10-related β-lactamases. Enzymes SHV-1 (pIapp, 7.6), SHV-5 (pIapp, 8.2), OXA-13 (pIapp, 8 [18]), and OXA-10 (pIapp, 6.1) were used as pI standards. The two novel β-lactamases, OXA-13-1 and OXA-19, had estimated pIapps of 7.8 and 7.6, respectively. β-Lactamase activity was revealed by overlaying the gel with nitrocefin.
Nucleotide sequence of the gene cluster encoding aminoglycoside and β-lactam resistance in PAe191.
Sequence analysis of a ca. 2-kb fragment from pAZ316 revealed the presence of two resistance genes, each flanked by a recombinational element at its 3′ extremity, which is typical of those found in the resistance gene cassettes in integrons (11, 30, 33). Only four mutations (Fig. 1) were observed in comparison with the sequence of a very similar integron-borne cluster described previously (20). The first was in the −35 sequence of the promoter P1, located within the integrase gene intI1, which controls the expression of the resistance genes (33). The three other mutations were in the resistance genes themselves. The gene immediately downstream from intI1 encoded an AAC(6′)-Ib variant, called AAC(6′)-Ib9, with a Leu-to-Ser change at position 119 with respect to the otherwise identical AAC(6′)-Ib protein encoded by pAZ301 (20) and to other typically amikacin resistance-conferring variants (36). This amino acid change has been associated previously with a shift from amikacin to gentamicin resistance in vitro after site-directed mutagenesis (27) and in clinical isolates of Pseudomonas fluorescens (17) and Enterobacteriaceae (4). The second resistance gene had two nucleotide differences accounting for two amino acid differences in comparison with OXA-13 (Fig. 1 and 2) (20), with Asn73 instead of Ser and Asp157 instead of Gly. This novel variant was named OXA-19. As already mentioned, Asp157 has been associated previously with the extension of the β-lactam resistance spectrum to ceftazidime (8, 10, 21).
FIG. 2.
Structure of the integron encoding the β-lactamases OXA-19 and OXA-13-1. Nucleotide sequences are identical in both clusters, except for three differences in the −35 sequences of the promoter and in the aac(6′)-Ib and the oxa genes. Amino acid numbering is from Tran Van Nhieu and Collatz (36) and Huovinen et al. (13), respectively. Arrows represent the following open reading frames: intI1, for integrase; aac(6′)-Ib, for the aminoglycoside acetyltransferases genes aac(6′)-Ib10 on pAZ310 (20) and aac(6′)-Ib9 on pAZ316; oxa, for the OXA variants indicated on the left; and qacEΔ1, for the typically truncated quaternary ammonium resistance-conferring gene associated with the 3′ conserved segment of integrons. The intI1 and qacEΔ1 genes have been only partially sequenced here (indicated in boldface). Data for pAZ309 are from reference 20, and data for pAZ310 and pAZ316 are from the present study. Boldface, differences with respect to the OXA-13-encoding plasmid pAZ309; dashed line, the oxa13-1 region that has been sequenced.
Selection in vitro and characterization of an ESBL variant of OXA-13.
To determine whether a derivative of OXA-13 conferring the resistance phenotype of OXA-19 in P. aeruginosa could be generated by a point mutation, a spontaneous mutant of PAO38(pAZ309), which was called PAO38(pAZ310), was selected on ceftazidime (Table 1). An OXA variant of pIapp 7.8 was obtained (Fig. 3). It differed from OXA-13 by only a Gly157-to-Asp change and was named OXA-13-1. Although the production of this enzyme in PAO38(pAZ310) led to a resistance pattern similar to that of OXA-19 in PAO38(pAZ316) (Table 2), the level of resistance of PAO38(pAZ316) to some of the β-lactams tested, especially ticarcillin and piperacillin, appeared to be higher than that of PAO38(pAZ310). In repeated assays, the MIC of ampicillin for a pAZ316-containing E. coli strain was also found to be four times as high as that for the same strain containing pAZ310 (data not shown). These observations could be related to a ca. 30-times-higher specific activity of OXA-19 against ampicillin, in comparison with OXA-13-1 (Table 3), while the specific activities of both enzymes against ceftazidime were similar.
TABLE 3.
Specific activities of OXA-13-1 and OXA-19 from P. aeruginosa and E. coli producers
| Substrate (50 mM) | Sp act (mU/mg)
|
|||
|---|---|---|---|---|
| PAO38
|
DH5α
|
|||
| pAZ316/ OXA-19 | pAZ310/ OXA-13-1 | pAZ327/ OXA-19 | pAZ326/ OXA-13-1 | |
| Ampicillin | 999 | 28 | 826 | 30 |
| Ceftazidime | 0.9 | 1.0 | 1.5 | 1.8 |
Effect of Asn or Ser in position 73 on the level of resistance to penicillins in OXA-19 and OXA-13-1 producers.
Two of the three nucleotide differences that distinguish the aac(6′)-Ib, oxa clusters of pAZ316 and pAZ310 (Fig. 2) could be involved in modulating the levels of penicillin resistance, the difference in the −35 promoter sequence and that concerning amino acid position 73. Cloning of the NarI-HindIII fragments from both plasmids carrying oxa13-1 or oxa19 into pK18 yielded pAZ326 and pAZ327, respectively, leaving only the difference in position 73 (Fig. 2). It eliminated the likely difference in promoter strength (the −35 sequence TTGACA [Fig. 2] being known to occur in promoters stronger than TGGACA in E. coli [19]) and the possible influence of secondary-structure variations in the so-called 59-bp element (5) on oxa expression. Table 2 shows the β-lactam resistance patterns of E. coli DH5α harboring pAZ326 or pAZ327. In these constructs, the higher MICs of penicillins for the OXA-19-producing strain correlated with a higher specific activity of OXA-19 against ampicillin in comparison with that of OXA-13-1 (Table 3). Thus, it is conceivable that Asn in position 73 conditions the level of resistance to β-lactams, and to penicillins in particular, also for P. aeruginosa PAO38(pAZ316) and, hence, the clinical strain PAe191.
An asparagine is generally found at position 73 (numbering of Huovinen et al [13]) in OXA variants of the OXA-10 branch (13, 29), whether they are penicillinases, such as OXA-5 (5) or OXA-7 (31), or of the extended spectrum, such as OXA-11 (10), OXA-14 (8), or OXA-16 (7). Thus, OXA-13 seems to be a rather rare variant with respect to the amino acid in position 73. Although the effect of a serine at this position is not known in OXA-10 derivatives with a glycine in position 157 which have a penicillinase profile, its association with aspartate at position 157 in the ESBL OXA-13-1 seems to result in a relatively low level of resistance to penicillins and a relatively low level of penicillinase activity in the producing strains.
ACKNOWLEDGMENT
This work was supported by a grant from the Institut National de la Santé et de la Recherche Médicale, Paris, France (CRI 95061).
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). Current protocols in molecular biology. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing a principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Bush K, Jacoby G A, Medeiros A A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casin I, Bordon F, Bertin P, Coutrot A, Podglajen I, Brasseur R, Collatz E. Aminoglycoside 6′-N-acetyltransferase variants of the Ib type with altered substrate profiles in clinical isolates of Enterobacter cloacae and Citrobacter freundii. Antimicrob Agents Chemother. 1998;42:209–215. doi: 10.1128/aac.42.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collis C M, Hall R M. Expression of antibiotic resistance genes in the integrated cassettes of integrons. Antimicrob Agents Chemother. 1995;39:155–162. doi: 10.1128/aac.39.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couture F, Lachapelle J, Levesque R C. Phylogeny of LCR-1 and OXA-5 with class A and class D β-lactamases. Mol Microbiol. 1992;6:1693–1705. doi: 10.1111/j.1365-2958.1992.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 7.Danel F, Hall L M C, Livermore D M. Program and Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Laboratory mutants of OXA-10 β-lactamase giving ceftazidime resistance in Pseudomonas aeruginosa, abstr. no. C191. [Google Scholar]
- 8.Danel F, Hall L M C, Gur D, Livermore D M. OXA-14, another extended-spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1881–1884. doi: 10.1128/aac.39.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danel F, Hall L M C, Gur D, Livermore D M. OXA-15, an extended-spectrum variant of OXA-2 β-lactamase, isolated from a Pseudomonas aeruginosa strain. Antimicrob Agents Chemother. 1997;41:785–790. doi: 10.1128/aac.41.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall L M, Livermore D M, Gur D, Akova M, Akalin H E. OXA-11, an extended spectrum variant of OXA-10 (PSE-2) β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993;37:1637–1644. doi: 10.1128/aac.37.8.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall R M, Collis C M. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. doi: 10.1111/j.1365-2958.1995.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 12.Hedges R W, Datta N, Kontomichalou P, Smith J T. Molecular specificities of R factor-determined β-lactamase: correlation with plasmid compatibility. J Bacteriol. 1974;117:56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huovinen P, Huovinen S, Jacoby G A. Sequence of PSE-2 β-lactamase. Antimicrob Agents Chemother. 1988;32:134–136. doi: 10.1128/aac.32.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs C, Huang L J, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 1994;13:1684–1694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knox J R. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert T, Ploy M C, Courvalin P. A spontaneous point mutation in the aac(6′)-Ib′ gene results in altered substrate specificity of aminoglycoside 6′-N-acetyltransferase of a Pseudomonas fluorescens strain. FEMS Microbiol Lett. 1994;115:297–304. doi: 10.1111/j.1574-6968.1994.tb06654.x. [DOI] [PubMed] [Google Scholar]
- 18.Ledent P, Raquet X, Joris B, van Beeumen J, Frère J M. A comparative study of class D β-lactamases. Biochem J. 1993;292:555–562. doi: 10.1042/bj2920555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lévesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 20.Mugnier P, Podglajen I, Goldstein F W, Collatz E. Carbapenems as inhibitors of OXA-13, a novel, integron-encoded β-lactamase in Pseudomonas aeruginosa. Microbiology. 1998;144:1021–1031. doi: 10.1099/00221287-144-4-1021. [DOI] [PubMed] [Google Scholar]
- 21.Mugnier P, Podglajen I, Gutmann L, Collatz E. Program and Abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Cloning and nucleotide sequence analysis of two Pseudomonas aeruginosa genes encoding the novel OXA-type β-lactamase variants OXA-12 and OXA-13, susceptible to inhibition by imipenem, abstr. no. C98. [Google Scholar]
- 22.Mugnier P, Dubrous P, Casin I, Arlet G, Collatz E. A TEM-derived extended-spectrum β-lactamase in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1996;40:2488–2493. doi: 10.1128/aac.40.11.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann P, Naas T. Sequence analysis of PER-1 extended-spectrum β-lactamase from Pseudomonas aeruginosa and comparison with class A β-lactamases. Antimicrob Agents Chemother. 1994;38:104–114. doi: 10.1128/aac.38.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philippon L N, Naas T, Bouthors A-T, Barakett V, Nordmann P. OXA-18, a class D clavulanic-acid inhibited extended spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2188–2195. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pridmore R D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56:309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- 26.Raquet X, Lamotte-Brasseur J, Fonzé E, Goussard S, Courvalin P, Frère J M. TEM β-lactamase mutants hydrolysing third-generation cephalosporins. J Mol Biol. 1994;244:625–639. doi: 10.1006/jmbi.1994.1756. [DOI] [PubMed] [Google Scholar]
- 27.Rather P N, Munayyer H, Mann P A, Hare R S, Miller G H, Shaw K J. Genetic analysis of bacterial acetyltransferases: identification of amino acids determining the specificities of the aminoglycoside 6′-N-acetyltransferase Ib and IIa proteins. J Bacteriol. 1992;174:3196–3203. doi: 10.1128/jb.174.10.3196-3203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robillard N J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990;34:1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanschagrin F, Couture F, Levesque R C. Primary structure of OXA-3 and phylogeny of oxacillin-hydrolyzing class D β-lactamases. Antimicrob Agents Chemother. 1995;39:887–893. doi: 10.1128/aac.39.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt F R J, Nücken E J, Henschke R B. Structure and function of hot spots providing signals for site-directed specific recombination and gene expression in Tn21 transposons. Mol Microbiol. 1989;3:1545–1555. doi: 10.1111/j.1365-2958.1989.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 31.Scoulica E, Aransay A, Tselentis Y. Molecular characterization of the OXA-7 β-lactamase gene. Antimicrob Agents Chemother. 1995;39:1379–1382. doi: 10.1128/aac.39.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seifert S H, So M, Heffron F. Shuttle mutagenesis: a method of introducing transposons into transformable organisms. In: Setlow J K, Hollander A, editors. Genetic engineering, principles and methods. Vol. 8. New York, N.Y: Plenum; 1986. pp. 128–134. [Google Scholar]
- 33.Stokes H W, Hall R M. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 34.Strynadka N C J, Adachi H, Jensen S E, Johns K, Sielecki A, Betzel C, Sutoh K, James M N G. Molecular structure of the acyl-enzyme intermediate in β-lactam hydrolysis at 1.7 Å resolution. Nature (London) 1992;359:700–705. doi: 10.1038/359700a0. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984;20:608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tran Van Nhieu G, Collatz E. Primary structure of an aminoglycoside 6′-N-acetyltransferase, AAC(6′)-4, fused in vivo with the signal peptide of the Tn3-encoded β-lactamase. J Bacteriol. 1987;169:5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]