Abstract
Background:
Obesity is highly prevalent and disabling, especially in individuals with severe mental illness including bipolar disorders (BD). The brain is a target organ for both obesity and BD. Yet, we do not understand how cortical brain alterations in BD and obesity interact.
Methods:
We obtained body mass index (BMI) and MRI-derived regional cortical thickness, surface area from 1231 BD and 1601 control individuals from 13 countries within the ENIGMA-BD Working Group. We jointly modeled the statistical effects of BD and BMI on brain structure using mixed effects and tested for interaction and mediation. We also investigated the impact of medications on the BMI-related associations.
Results:
BMI and BD additively impacted the structure of many of the same brain regions. Both BMI and BD were negatively associated with cortical thickness, but not surface area. In most regions the number of jointly used psychiatric medication classes remained associated with lower cortical thickness when controlling for BMI. In a single region, fusiform gyrus, about a third of the negative association between number of jointly used psychiatric medications and cortical thickness was mediated by association between the number of medications and higher BMI.
Conclusions:
We confirmed consistent associations between higher BMI and lower cortical thickness, but not surface area, across the cerebral mantle, in regions which were also associated with BD. Higher BMI in people with BD indicated more pronounced brain alterations. BMI is important for understanding the neuroanatomical changes in BD and the effects of psychiatric medications on the brain.
Key words: Body mass index, obesity, bipolar disorders, cortical thickness, surface area, heterogeneity, lithium, antipsychotics
Introduction
Obesity is the fifth leading cause of death globally and is one of the leading causes of disability (Di Angelantonio et al., 2016; GBD 2015 Obesity Collaborators et al., 2017; Nyberg et al., 2018). It is an even greater problem among people with severe mental illness (SMI) including bipolar disorders (BD). Based on meta-analysis of 120 studies, the pooled point prevalence of overweight and obesity in SMI was 60% and people with SMI had on average three times greater odds of obesity than the general population (Afzal et al., 2021). Aside from the impact of obesity on general health, obesity is commonly associated with structural brain alterations (Dekkers, Jansen, & Lamb, 2019; Fernández-Andújar, Morales-García, & García-Casares, 2021; García-García et al., 2019; Janowitz et al., 2015; Willette & Kapogiannis, 2015), and with an increased risk of cognitive impairment and dementia (Beydoun, Beydoun, & Wang, 2008; Pedditzi, Peters, & Beckett, 2016; Singh-Manoux et al., 2018; Tang et al., 2021). These issues may be particularly relevant in individuals who already have an increased risk of brain alterations (Hibar et al., 2018), cognitive impairment (Bora, Yucel, & Pantelis, 2009), and obesity (Vancampfort et al., 2015), such as people with BD. Individuals with BD and comorbid obesity face very specific challenges which require dedicated management and research efforts. However, very few studies up to date have investigated how the presence of obesity interacts with brain and cognitive changes in major psychiatric disorders.
Studying people with SMI and obesity could help identify preventable/treatable risk factors for neurostructural alterations, which may be associated with currently intractable psychiatric outcomes, including cognitive impairment. Indeed, we and others have shown that BD complicated by obesity-related metabolic alterations, specifically diabetes, is associated with lower psychosocial functioning (Hajek et al., 2005), higher rates of rapid cycling (Hajek et al., 2008), poor treatment response (Calkin et al., 2015), and worse psychiatric outcomes (Calkin et al., 2009). It could also explain why people with the same psychiatric diagnosis differ so markedly in their neurobiological/clinical outcomes, thus moving toward individualized medicine. Furthermore, weight gain is a common side effect of many psychiatric medications, which are also frequently associated with changes in brain structure. Therefore, we need to better understand the role obesity plays in the links between psychiatric disorders or medications and brain structure.
Previous studies in relatively small (76–112 participants) and highly selected groups [i.e. people with first episode of mania (Bond et al., 2014, 2011, 2019), adolescent BD participants (Islam, Metcalfe, MacIntosh, Korczak, & Goldstein, 2018), or offspring of people with BD (Mansur et al., 2018)] have suggested that obesity may be associated with brain alterations in BD, possibly with a stronger effect size or with some regional specificity compared to controls (Bond et al., 2014, 2011, 2019; Islam et al., 2018; Mansur et al., 2018). In a study including 2735 individuals (McWhinney et al., 2021a), we demonstrated that some of the most replicated subcortical brain alterations in BD, including larger ventricles, were to a large extent (up to 47%) mediated by obesity and that both BD and obesity were associated with similar subcortical alterations.
We need larger studies in more generalizable samples to better understand how the brain correlates of obesity map onto the cortical alterations in BD. To this end, we investigated the association between BD, medications, obesity, and neurostructural measures in a large, highly generalizable, multicenter sample from the ENIGMA-BD Working Group.
Methods
Participating sites
The ENIGMA-BD Working Group aims to improve replication and generalizability of neuroimaging studies of BD by combining existing, independently collected neuroimaging samples of BD from around the world (Ching et al., 2022; Hibar et al., 2018, 2016; McWhinney et al., 2021a, 2022a; Nunes et al., 2020). Seventeen independently collected ENIGMA-BD samples from 13 countries on six continents contributed individual-level structural MRI data, medication information, specifically medications used at the time of scanning for the following medication categories (lithium, first-, second-generation antipsychotics, anticonvulsants, antidepressants), and body mass index (BMI) values from a total of 1231 individuals with BD and 1601 healthy controls. Table 1 shows all participant characteristics.
Table 1.
Demographic, diagnostic, and treatment characteristics of sample
| Controls | Cases | Significance | |
|---|---|---|---|
| N | 1601 | 1231 | |
| Age, mean (s.d.) | 35.47 (12.63) | 42.12 (12.71) | t(2818) = 7.22, p < 0.001 |
| BMI, mean (s.d.) | 24.43 (4.12) | 26.78 (5.23) | t(2395)a = 11.48, p < 0.001 |
| Normal weight/overweight/obese, N (%) | 1014 (63.34)/437 (27.30)/150 (9.37) | 509 (41.3)/436 (35.40)/286 (23.20) | χ2 = 163.43, df = 2, p < 0.001 |
| Sex, N (%) female | 916 (57.21) | 743 (60.4) | χ2 = 2.71, p = 0.100 |
| Diagnosis, N (%) | N/A | ||
| BD-I | – | 904 (73.4) | |
| BD-II | – | 318 (25.8) | |
| BD-NOS | – | 9 (0.70) | |
| Treatment at time of scanning, N (%) / monotherapy N (%) | N/A | ||
| No treatment | – | 218 (17.7) | |
| Lithium | – | 556 (48.5) / 182 (46.7) | |
| Antiepileptic | – | 425 (39.2) / 80 (22.9) | |
| First-generation antipsychotic | – | 83 (7.6) / 5 (1.4) | |
| Second-generation antipsychotic | – | 371 (33.9) / 53 (14.9) | |
| Antidepressant | – | 431 (39.3) / 71 (19.9) | |
| Mood state, N (%) | N/A | ||
| Euthymic | – | 598 (58.5) | |
| Depressed | – | 365 (35.7) | |
| Manic | – | 43 (4.2) | |
| Hypomanic | – | 10 (1.0) | |
| Mixed | – | 6 (0.6) | |
| Age of onset, mean (s.d.) | – | 25.68 (10.94) | N/A |
| History of psychosis, N (%) | – | 457 (52.5) | N/A |
There were no missing age or BMI values. We used the Welch two-sample t test (unequal variance assumed), which relies on a Welch–Satterthwaite degrees of freedom adjustment, resulting in varying degrees of freedom.
Online Supplementary Tables S1 and S2 list the demographic/clinical details for each cohort. Online Supplementary Table S3 provides the diagnostic instruments used to obtain diagnosis and clinical information. Online Supplementary Table S4 lists exclusion criteria for study enrolment. Briefly, all studies used standard diagnostic instruments, including Structured Clinical Interview for DSM Disorders (SCID; N = 12), Mini International Neuropsychiatric Interview (MINI; N = 2) and Diagnostic Interview for Genetic Studies (DIGS; N = 1). Most studies (N = 10) included both bipolar I (BDI) and bipolar II (BDII) disorders, six studies included only BDI and one study included only BDII participants. Substance abuse was an exclusion criterion in nine studies. Most studies did not exclude comorbidities, other than substance abuse. Consequently, the sample is a broad, ecologically valid, and generalizable representation of BD. All participating sites received approval from local ethics committees, and all participants provided written informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Data acquisition and parcellation
High-resolution T1-weighted brain anatomical MRI scans were acquired at each site; see online Supplementary Table S5 for details of scan acquisition. All groups used the same ENIGMA-standardized analytical protocol, including visual and statistical quality assessment, as documented at: http://enigma.ini.usc.edu/protocols/imaging-protocols/. These protocols are standardized across the consortium, are open-source, and freely available online, in order to foster open science/replication/reproducibility. They were applied in the previous publications by our group (Hibar et al., 2018; Nunes et al., 2020) and more broadly in large-scale ENIGMA studies of major depression, schizophrenia, attention deficit hyperactivity disorder (ADHD), obsessive compulsive disorder (OCD), post traumatic stress disorder (PTSD), epilepsy, and autism (Thompson et al., 2020).
Briefly, using the freely available and extensively validated FreeSurfer software, we performed parcellations of 34 cortical regions, per hemisphere (left and right), based on the Desikan–Killiany atlas. All segmented regions were used as target regions of interest (ROIs) for analysis. We also computed total intracranial volume (ICV) to standardize surface area estimates. Visual quality controls were performed on an ROI level aided by the ENIGMA-standardized visual inspection guide including pass/fail parcellation examples. In addition, we generated diagnostic histogram plots for each site and outliers which deviated from the site mean for each structure at >3 standard deviations were flagged for further review. All ROIs failing quality inspection were withheld from subsequent analyses, see online Supplementary Table S6. Previous analyses from the ENIGMA-BD Working Group showed that scanner field strength, voxel volume, and the version of FreeSurfer used for parcellation did not significantly influence the effect size estimates.
Statistical modeling
In this mega-analysis, we used linear mixed modeling (package nlme version 3.1-152 in R version 4.1.1) with individual subject cortical thickness or cortical surface area as dependent variables and with both BMI and group (participants with BD or healthy controls) as predictors. In each case, age, sex, and hemisphere (left or right) were also included as fixed predictors. Total ICV was included as a covariate in models of cortical surface area. Models also included a random effect of hemisphere within participants and a random effect of data collection site.
We created one model per region, with each model including both hemispheres and all of the covariates described above. We used BMI as a continuous variable, which captures more variability between participants, increases sensitivity, and was the preferred approach in most previous studies (Dekkers et al., 2019). BMI was normally distributed (online Supplementary Fig. S1). We checked the normality of model residuals using QQ plots and tested for multicollinearity using the variance inflation factor (VIF, shown in online Supplementary Table S7) of all predictor variables included in modeling. Variance in regional volumes was comparable between groups.
In post hoc analyses among individuals with BD, we separately explored the statistical effects of commonly prescribed medications. As the rates of monotherapy were low in this sample (see Table 1), we studied the association between number of jointly used medication classes (zero through three, including anticonvulsants, antipsychotics, and antidepressants) and BMI or cortical thickness or surface area. In the same model, we separately estimated the effects of current Li treatment. We used the same covariates and random-effect structure as described above. Interactions between BMI and either the number of medication classes or Li prescription were included where significant. The partial effect of the number of medication classes while adjusting for BMI was also compared with its effect without adjusting for BMI, but with all other covariates and random effects remaining. The a priori decision to analyze the effects of Li separately was motivated by the fact that statistical effects of Li on brain measures, which tend to be positive, may cancel the statistical effects of other medications such as antipsychotics, anticonvulsants, which tend to be negative (Hajek et al., 2012a; Hajek, Kopecek, Hoschl, & Alda, 2012b).
We adjusted all p values for multiple comparisons using false discovery rate (FDR), with adjusted p values reported, at α = 0.05. We calculated effect sizes for between-group differences (partial d), and associations between BMI and ROI volumes (partial r), together with their 95% confidence intervals (CIs), using model coefficients and their standard error (s.e.) (Nakagawa & Cuthill, 2007).
Mediation analysis
We tested whether the variance in regional thickness that was associated with the number of jointly used medication classes (zero through three, including anticonvulsants, antipsychotics, and antidepressants; direct path) remained significant after also accounting for variance associated with BMI (indirect path) in individuals with BD. The number of medication classes was modeled as the associated variable, BMI as the mediating variable, and cortical thickness was the dependent variable. We modeled the direct effect of the number of jointly used medication classes on thickness, in comparison with the indirect effect of this association through BMI as a mediator, corrected for age, sex, prescription of Li, and random effects. To test this, we built 5000 bootstrapped models using random selection with replacement. This method non-parametrically identified the 95% CI for effect sizes. The bootstrap CI, which did not include zero, indicated a significant indirect effect. Simulation research indicates that the bootstrap method is more robust to non-normality and has better type I error control than the Sobel test (Hayes, 2009). Nevertheless, for methodological consistency, we also applied the Sobel test to investigate whether accounting for BMI significantly mitigated group-related differences in thickness. All of these analyses were performed in R version (4.1.1).
These analyses were applied only to regions which met the criteria for mediation: (1) the number of medication classes was a significant predictor of the ROI thickness, and (2) the number of medication classes was a significant predictor of the mediator (BMI), and when modelled jointly, (3) BMI was a significant predictor of the thickness, and (4) the strength of the coefficient of the previously significant independent variable (number of medication classes) was reduced. These criteria applied to the fusiform gyrus.
Results
Regional morphometric differences by diagnosis and BMI
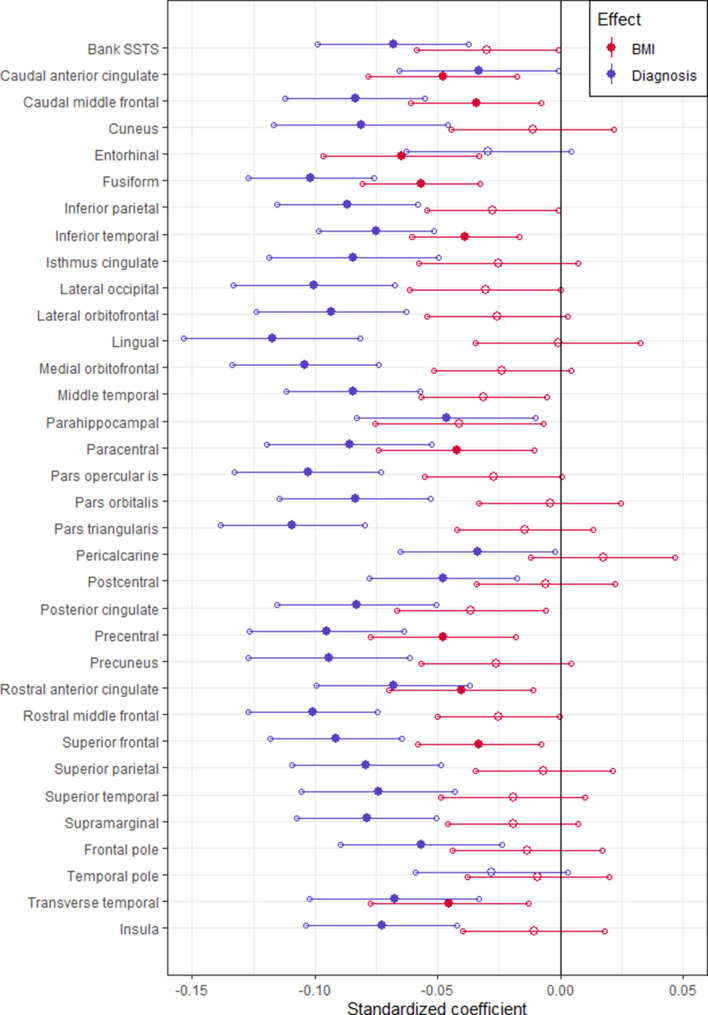
When modeled jointly, numerous regions showed significant partial effects of either BMI, diagnosis, or both (Fig. 1, Table 2). Participants with BD showed significantly thinner cortex relative to controls in all regions except for the entorhinal cortex and temporal pole. Higher BMI was associated with thinner cortex in nine of the same regions as BD, and it was uniquely associated with thinner entorhinal cortex. Surface area did not significantly differ between groups in any region, while higher BMI was associated with larger surface area in the isthmus of the cingulate gyrus (online Supplementary Table S8).
Fig. 1.
Standardized coefficients for group differences (blue) and BMI effects (red) in predicting the cortical thickness of each region. Significant effects are shown using a filled marker (FDR-adjusted p < 0.05).
Table 2.
Results of multiple regression analysis in cortical thickness, including effect sizes for between-group differences (Cohen's d), 95% confidence interval, BMI effect sizes (part r), and their FDR-adjusted p values
| Diagnosis | BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | Controls | Patients | Effect (d) | 95% Low | 95% High | p | Part r | p |
| Bank SSTS | 1452 | 809 | 0.191 | 0.105 | 0.277 | <0.001* | −0.042 | 0.100 |
| Caudal anterior cingulate | 1568 | 833 | 0.086 | 0.002 | 0.170 | 0.048* | −0.063 | 0.014* |
| Caudal middle frontal | 1586 | 834 | 0.248 | 0.163 | 0.332 | <0.001* | −0.051 | 0.039* |
| Cuneus | 1519 | 820 | 0.196 | 0.111 | 0.281 | <0.001* | −0.014 | 0.596 |
| Entorhinal | 1393 | 811 | 0.075 | −0.011 | 0.162 | 0.089 | −0.085 | 0.001* |
| Fusiform | 1587 | 830 | 0.335 | 0.251 | 0.420 | <0.001* | −0.094 | 0.000* |
| Inferior parietal | 1565 | 832 | 0.256 | 0.172 | 0.340 | <0.001* | −0.041 | 0.100 |
| Inferior temporal | 1559 | 814 | 0.272 | 0.187 | 0.357 | <0.001* | −0.071 | 0.006* |
| Isthmus cingulate | 1579 | 830 | 0.206 | 0.121 | 0.290 | <0.001* | −0.031 | 0.199 |
| Lateral occipital | 1585 | 825 | 0.258 | 0.174 | 0.343 | <0.001* | −0.040 | 0.103 |
| Lateral orbitofrontal | 1592 | 833 | 0.257 | 0.173 | 0.341 | <0.001* | −0.036 | 0.140 |
| Lingual | 1576 | 827 | 0.276 | 0.192 | 0.361 | <0.001* | −0.001 | 0.957 |
| Medial orbitofrontal | 1570 | 832 | 0.294 | 0.210 | 0.379 | <0.001* | −0.034 | 0.160 |
| Middle temporal | 1508 | 817 | 0.264 | 0.178 | 0.349 | <0.001* | −0.050 | 0.050 |
| Parahippocampal | 1592 | 833 | 0.107 | 0.023 | 0.191 | 0.014* | −0.048 | 0.050 |
| Paracentral | 1591 | 834 | 0.214 | 0.130 | 0.298 | <0.001* | −0.053 | 0.037* |
| Pars opercularis | 1571 | 834 | 0.290 | 0.206 | 0.375 | <0.001* | −0.039 | 0.107 |
| Pars orbitalis | 1588 | 833 | 0.228 | 0.144 | 0.312 | <0.001* | −0.006 | 0.808 |
| Pars triangularis | 1573 | 833 | 0.312 | 0.227 | 0.396 | <0.001* | −0.021 | 0.408 |
| Pericalcarine | 1546 | 807 | 0.091 | 0.006 | 0.176 | 0.041* | 0.024 | 0.334 |
| Postcentral | 1554 | 832 | 0.134 | 0.050 | 0.219 | 0.002* | −0.009 | 0.717 |
| Posterior cingulate | 1588 | 831 | 0.215 | 0.131 | 0.299 | <0.001* | −0.048 | 0.050 |
| Precentral | 1568 | 832 | 0.254 | 0.170 | 0.338 | <0.001* | −0.065 | 0.013* |
| Precuneus | 1583 | 830 | 0.243 | 0.158 | 0.327 | <0.001* | −0.034 | 0.158 |
| Rostral anterior cingulate | 1536 | 830 | 0.185 | 0.100 | 0.269 | <0.001* | −0.056 | 0.034* |
| Rostral middle frontal | 1582 | 834 | 0.321 | 0.237 | 0.405 | <0.001* | −0.041 | 0.100 |
| Superior frontal | 1577 | 834 | 0.287 | 0.203 | 0.371 | <0.001* | −0.053 | 0.038* |
| Superior parietal | 1581 | 831 | 0.221 | 0.137 | 0.305 | <0.001* | −0.009 | 0.708 |
| Superior temporal | 1438 | 815 | 0.205 | 0.119 | 0.292 | <0.001* | −0.027 | 0.286 |
| Supramarginal | 1505 | 826 | 0.237 | 0.152 | 0.322 | <0.001* | −0.029 | 0.235 |
| Frontal pole | 1584 | 832 | 0.145 | 0.061 | 0.229 | 0.001* | −0.017 | 0.495 |
| Temporal pole | 1581 | 835 | 0.076 | −0.008 | 0.160 | 0.080 | −0.012 | 0.617 |
| Transverse temporal | 1595 | 833 | 0.165 | 0.081 | 0.248 | <0.001* | −0.056 | 0.034* |
| Insula | 1489 | 830 | 0.202 | 0.117 | 0.287 | <0.001* | −0.015 | 0.568 |
Significance is shown using asterisks (*p < 0.05)
BMI and group significantly interacted in lateral occipital cortical thickness (online Supplementary Fig. S2), with control participants showing a significant negative association between BMI and cortical thickness [t(2391) = −3.03, p = 0.002], while no significant association was seen in those with BD [t(2391) = 0.31, p = 0.757]. There was no interaction between BMI and sex for any of the regions or any of the measures. The full list of interactions is shown in online Supplementary Table S9.
Medications, clinical variables, BMI, and brain structure
In individuals with BD, higher BMI was associated with greater number of jointly used medication classes (i.e. anticonvulsant, antipsychotic, and/or antidepressant medications) per participant [t(1100) = 4.89, p < 0.001], but not with Li treatment [t(736) = −0.42, p = 0.676].
The number of medication classes was significantly associated with smaller cortical thickness in 22 of 34 regions (64.7%), and in nearly all instances, these associations remained significant when controlling for the effects of BMI (online Supplementary Table S10). Exceptions included the pars opercularis, superior temporal gyrus, and supramarginal gyrus. There was a significant interaction between BMI and the number of medication classes in the isthmus cingulate [t(1858) = −4.34, p = 0.001], with progressively steeper associations between BMI and thickness in those with more medications (see online Supplementary Fig. S3).
There was an interaction between current Li-use and BMI in 13 of the ROIs (38.2%), including caudal and rostral anterior cingulate, medial orbitofrontal gyrus, postcentral gyrus, pars opercularis, pars triangularis, rostral and caudal middle frontal, superior frontal, superior temporal, supramarginal, frontal pole, and insula, such that people who were prescribed Li at the time of scanning showed stronger negative association between BMI and cortical thickness than individuals with BD who were not treated with Li (online Supplementary Table S10 and Fig. S4). Amongst the remaining regions, which did not show interaction between BMI and Li, Li was positively associated with cortical thickness even when controlling for the negative effect of BMI in nine regions, including cuneus, precuneus, inferior parietal, lateral occipital, lingual, paracentral, precentral, pericalcarine, and superior parietal gyri.
BMI, Li treatment, and the number of medication classes showed negligible multicollinearity (VIF < 1.004). BMI was not significantly associated with illness duration, history of psychotic symptoms, diagnostic subtype, or mood state (online Supplementary Table S11).
Mediating effect of medications
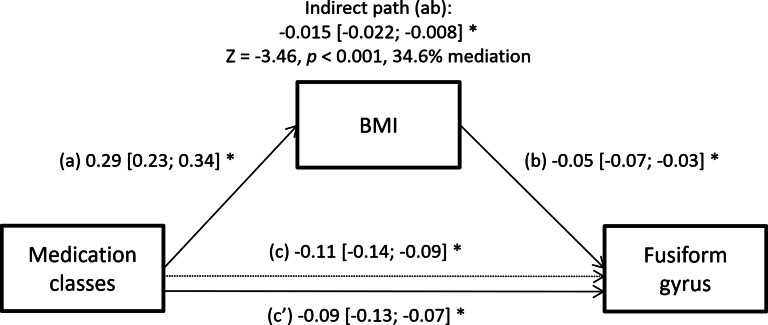
Only the fusiform gyrus met the criteria for investigating whether BMI mediates the relationship between the number of medication classes and cortical thickness (online Supplementary Table S10). Specifically, there was a significant indirect effect of the number of medication classes on lower fusiform gyrus thickness through BMI (Est = −0.015, 95% CI −0.022 to −0.008), with 34.6% mediation (Z = 3.46, p < 0.001, see Fig. 2).
Fig. 2.
The effect of medication classes and BMI on cortical thickness. Path (c) represents the direct effect, while (a) through (b) represent the indirect path through BMI, and (c′) represents the adjusted direct effect after accounting for BMI. We show standardized coefficients along with their 95% CI derived from bootstrapping. Significant effects (95% CI that excludes zero) are marked by asterisks. In all models, we controlled for the covariates age, sex, Li treatment, and data collection site, while paths b, c, and c’ were additionally adjusted for a random effect of hemisphere.
Discussion
In this study of 2832 individuals, we found substantial overlap between regions associated with BMI and BD. Specifically, with exception of a single ROI (entorhinal cortex), all of the regions which were negatively associated with BMI were also negatively associated with the diagnosis of BD. In contrast, only a single ROI (isthmus of the cingulate gyrus) showed association between BMI and surface area, which was positive. Importantly, about a third of the negative association between number of psychiatric medications and cortical thickness in the fusiform gyrus was partially mediated by the association between number of medications and higher BMI. Furthermore, the association between BMI and cortical thickness in isthmus cingulate became steeper with higher number of medication classes used jointly. In contrast, significant negative associations between BMI and cortical thickness in isthmus cingulate and rostral anterior cingulate became non-significant when modelled jointly with a significant positive effect of Li on cortical thickness. There was an interaction between Li and BMI, such that the brain correlates of BMI were more pronounced in individuals with v. without current Li treatment in a number of frontal regions, as well as anterior cingulate. The statistical effect of BMI on brain structure was linear in all regions, thus it would be most pronounced in people with obesity, but also manifest in overweight individuals.
The cortical correlates of obesity in this study closely replicated findings from previous large-scale studies, which also reported negative associations between obesity and cortical thickness or volume, especially in caudal and rostral anterior cingulate, entorhinal cortex, and several frontal lobe regions (Janowitz et al., 2015; McWhinney et al., 2022b; Opel et al., 2021), but with much fewer correlates in surface area (McWhinney et al., 2022b; Opel et al., 2021). These are some of the same regions which are consistently associated with BD, but also with other major psychiatric disorders (Hibar et al., 2018; van Erp et al., 2018). Interestingly, in all of these regions both BMI and BD showed partial association with cortical thickness, when controlling for the other factor. In other words, brain changes in cingulate and frontal regions will be greater in people with both obesity and BD than in those with either condition alone.
It is unclear whether these regions are particularly susceptible to obesity, or whether their changes predispose individuals to obesity. The mechanisms through which brain structure could predispose to obesity involve alterations in the reward system (Opel et al., 2015), cue triggered learning and Pavlovian conditioning to hedonic food (Meyer, Risbrough, Liang, & Boutelle, 2015), and in appetitive behavior (Löscher, Brandt, & Ebert, 2003; Malkova, Mishkin, Suomi, & Bachevalier, 2010). On the other hand, obesity could affect brain structure through a range of mechanisms, including among others systemic inflammation, oxidative stress, insulin resistance/diabetes, hypertension or dyslipidemia (Cox et al., 2019; Goldstein et al., 2020; Hajek et al., 2014; Parimisetty et al., 2016; Van Gaal, Mertens, & De Block, 2006; Willette & Kapogiannis, 2015; Wisse, 2004). Speculating about how the regions described above could predispose to obesity would be post-hoc and inconclusive. However, previous studies investigating associations between genetic risk for obesity and brain structure reported involvement of a much smaller set of regions, i.e. surface area of lateral occipital lobe (Opel et al., 2021) or medial prefrontal volume (Opel et al., 2017) than those reported here and in other previous studies of association between obesity and brain structure (Janowitz et al., 2015; McWhinney et al., 2022b; Opel et al., 2021). Therefore, brain correlates of obesity most likely include both causes and consequences of obesity. Considering the greater extent of associations between obesity and brain structure relative to the cih the much smaller extent of associations between genetic risk for obesity and brain structure, most brain changes likely represent consequences of obesity.
An especially interesting and relevant question is the role of obesity in brain effects of psychiatric medications. In keeping with other studies, we found that antipsychotics and anticonvulsants were negatively associated with brain structure (Andreasen, Liu, Ziebell, Vora, & Ho, 2013; Fleisher et al., 2011; Fusar-Poli et al., 2013; Hibar et al., 2016, 2018; Tariot et al., 2011; Van Gestel et al., 2019) and positively with BMI (Mitchell et al., 2013; Tek et al., 2016). Considering the negative association between obesity and brain structure, perhaps the negative associations between medications and brain structure could be mediated by obesity (Joober, Schmitz, Malla, Sengupta, & Karma, 2006; McWhinney et al., 2021a, 2021b). Across most regions, the association between number of medications and cortical thickness remained significant when we controlled for BMI, which is in keeping with another study (Jorgensen et al., 2017). In a single region, the fusiform gyrus, about a third of the association between medications and cortical thickness was related to the indirect association between medications and BMI and between BMI and brain structure. In a single region, isthmus cingulate, the association between BMI and cortical thickness became steeper with growing numbers of medications used jointly. So, while the obesitogenic effects of medications may mediate or moderate the negative association between medications and brain structure in some regions, for the most part, the negative statistical effect of medications on brain structure was independent from their obesitogenic effects.
A separate question is the interplay between the putative neuroprotective effects of Li and its impact on weight. Interestingly, in this study, Li remained positively associated with cortical thickness across numerous regions, even when we controlled for negative associations between BMI and regional thickness. Conversely, a significant negative association between BMI and cortical thickness became non-significant in the isthmus cingulate and rostral anterior cingulate when modelled jointly with the significant positive effect of Li on cortical thickness. In other words, BMI did not cancel the positive association between Li and cortical thickness, while Li did cancel the negative association between BMI and cortical thickness for some regions.
Interestingly, in some regions, people who were using Li at the time of scanning showed stronger negative association between BMI and cortical thickness than BD individuals not treated with Li. These findings were quite robust and replicated across approximately one-third of regions. It is possible that regions which are negatively associated with BD will not show negative effects of additional variables, such as BMI, unless the impact of the illness is mitigated by, for example, Li. Indeed, we found this interaction mostly in regions which were more strongly associated with diagnosis than with BMI, including medial orbitofrontal gyrus as well as pars opercularis and triangularis of the inferior frontal gyrus. In addition, this interaction among individuals with BD could have decreased the apparent effect of BMI in these regions. Indeed, in the whole group, BMI was not associated with thickness of these regions. This is also in keeping with greater negative association between BMI and cortical thickness in controls than in people with BD.
Since we do not understand the origins of brain alterations in BD, it is highly relevant to study variables which could be associated with the structure of the brain, such as BMI. On the theoretical level, such studies could help explain the differences in brain measures among people with the same diagnosis, as they may differ in BMI. They could also explain similarities in brain measures across people with BD or schizophrenia, as they share a high risk of obesity. On the clinical level, comorbid obesity in people with major psychiatric disorders is associated with poor functioning, greater risk of chronicity, disability and suicide, poor treatment response, and functional deterioration (Bora, Akdede, & Alptekin, 2017; Calkin et al., 2015, 2009; Hajek et al., 2008, 2005; McIntyre et al., 2010; Salvi et al., 2020). The associations between obesity and brain structure might help explain these links and provide new treatment options for some of these currently difficult to treat outcomes. Lastly, similar studies could help identify risk factors for neuroimaging outcomes, which may provide new opportunities for prevention or treatment of brain alterations with dietary/lifestyle medication or surgical interventions focused on weight management (Mansur et al., 2017; Mueller et al., 2015; Shan et al., 2019; Tuulari et al., 2016).
This study benefits from several unique advantages. The large sample size (2832 individuals) allowed us to test for interactions among relevant factors, which could not be conclusively studied in smaller, less powered studies. The multi-site nature of this study, with data from 17 sites in 13 countries, ensured highly generalizable representation of BD from around the world. We focused on overweight/obesity, which is a highly prevalent, but understudied factor in relation to brain structure in BD and which could also provide important insights into negative associations between obesitogenic medications and brain structure. In addition to novel findings on the interplay between BMI, BD, or medications and brain structure, we provide several replications of previous findings of associations between obesity or BD and specific regions of interest.
This study has the following limitations. The cross-sectional nature of our study does not allow us to discern the direction of the association, as brain alterations may predate or result from obesity. More detailed markers beyond BMI were not broadly available throughout the ENIGMA-BD Working Group. At the same time, BMI is much easier to acquire and is by far the most frequently used measure (García-García et al., 2019; Willette & Kapogiannis, 2015), thus allowing for a more direct comparison with previous studies. Due to confidentiality reasons related to legacy datasets, we could not access raw, whole-brain data and could not use methods, such as voxel-based morphometry. Aside from using ENIGMA-standardized processing methods, we also addressed any differences between scanners statistically by using mixed models and including site as a random factor in all analyses. While there are other approaches, this is still by far the most utilized and accepted method for dealing with site effects (McWhinney et al., 2021a; Thompson et al., 2020). Information about medications was limited to current usage at the time of scan. The study was not designed to comprehensively test the effects of medication, which would require a randomized controlled design. Therefore, the medication findings should be interpreted with caution, as medication prescriptions in clinical practice are not random. Also, medication details were limited to current prescription, without any measures of duration, dosage, compliance, previous medication exposure, treatment response or symptom levels at the time of prescription, so we cannot address the effects of these factors. The basic ENIGMA covariates, which are available across sites, did not contain any measures of cognitive/psychosocial functioning. Therefore, we could not evaluate the structure/function links. Fat content near the MRI coil may lead to slight signal intensity changes, but the vast majority of individuals in this study were normal weight to overweight. Psychiatric and other medical comorbidities, which might not be available for all the patients enrolled, may influence the interplay between BMI, BD, and neuroimaging findings. Finally, using other neuroimaging modalities could provide further insights into the mechanisms of the BMI effect. Last but not least, caution is needed when interpreting mediation analyses in observational studies.
To conclude, we confirmed consistent associations between higher BMI and lower cortical thickness across the cerebral mantle, in regions which were also associated with BD. There were few or no correlates of either condition with cortical surface area. In most regions number of medications remained associated with lower cortical thickness regardless of BMI, but there were also instances of mediation and moderation of associations between number of medication classes and cortical thickness by BMI. In terms of Li treatment, either the positive association between Li and cortical thickness was present regardless of BMI or people treated with Li showed steeper association between BMI and cortical thickness. All in all, BMI is important for understanding the neuroanatomical alterations in BD and the neurostructural correlates of psychiatric medications. We need prospective studies to investigate whether obesity is a modifiable/preventable risk factor for brain alterations in BD and whether the obesity-related negative psychiatric outcomes are related to obesity-related brain alterations.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291723000223.
click here to view supplementary material
Financial support
We gratefully acknowledge the following contributions and research funding sources that made this study possible: PT and CRKC of the Marina del Rey studies were supported by NIH grant U54 EB020403 from the Big Data to Knowledge (BD2K) Program; CRKC also acknowledges NIA T32AG058507 and partial research support from Biogen, Inc. (Boston, USA) for work unrelated to the topic of this manuscript. The St. Göran study was supported by grants from the Swedish Research Council (2018-02653), the Swedish foundation for Strategic Research (KF10-0039), the Swedish Brain foundation, and the Swedish Federal Government under the LUA/ALF agreement (ALF 20170019, ALFGBG-716801). This work is also part of the German multicenter consortium ‘Neurobiology of Affective Disorders. A translational perspective on brain structure and function’, funded by the German Research Foundation (Deutsche Forschungsgemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). Principal investigators (PIs) with respective areas of responsibility in the FOR2107 consortium are: Work Package WP1, FOR2107cohort and brain imaging: TK (speaker FOR2107; DFG grant numbers KI 588/14-1, KI 588/14-2), UD (co-speaker FOR2107; DA 1151/5-1, DA 1151/5-2), AK (KR 3822/5-1, KR 3822/7-2), IN (NE 2254/1-1 and NE 2254/2-1), CK (KO 4291/3-1). Further support from the German sites were provided by MNC and FOR2107-Muenster: This work was funded by the German Research Foundation (SFB-TRR58, Project C09 to UD) and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant Dan3/012/17 to UD and grant SEED11/18 to NO); FOR2107-Muenster: This work was supported by grants from the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant MzH 3/020/20 to TH) and the German Research Foundation (DFG grants HA7070/2-2, HA7070/3, HA7070/4 to TH). The NUIG sample was supported by the Health Research Board (HRA_POR/2011/100). The Medellin studies (GIPSI) were supported by the PRISMA UNION TEMPORAL (UNIVERSIDAD DE ANTIOQUIA / HOSPITAL SAN VICENTE FUNDACIÓN), Colciencias-INVITACIÓN 990 de 3 de agosto de 2017, Codigo 99059634. The San Raffaele site was supported by the Italian Ministry of Health RF-2011-02350980 project. This research was also supported by the Irish Research Council (IRC) Postgraduate Scholarship, Ireland awarded to LN and to GM, and by the Health Research Board (HRA-POR-324) awarded to DMC. We thank the participants and the support of the Welcome-Trust HRB Clinical Research Facility and the Centre for Advanced Medical Imaging, St. James Hospital, Dublin, Ireland. The NUIG sample was supported by the Health Research Board (HRA_POR/2011/100). JS and RTK received support from the William K. Warren Foundation National Institute of Mental Health (R21MH113871); JS also acknowledges the National Institute of General Medical Sciences (P20GM121312). This study was also funded by EU-FP7-HEALTH-222963 ‘MOODIN- FLAME’ and EU-FP7-PEOPLE-286334 ‘PSYCHAID’. The Barcelona group would like to thank CIBERSAM (EPC) and the Instituto de Salud Carlos III (PI18/00877 and PI19/00394) for their support. This work was supported by the Singapore Bioimaging Consortium (RP C009/2006) research grant awarded to KS. The CIAM group (FMH – PI) was supported by the University Research Committee, University of Cape Town, and South African funding bodies National Research Foundation and Medical Research Council; DJS from CIAM was supported by the SAMRC. The Sydney studies were supported by the Australian National Health and Medical Research Council (NHMRC) Program Grant 1037196, Project Grants 1063960 and 1066177, the Lansdowne Foundation, Good Talk, and Keith Pettigrew Family; as well as the Janette Mary O'Neil Research Fellowship to JMF. The study was also supported by NIMH grant number: R01 MH090553(to RAO). Funding for the Oslo-Malt cohort was provided by the South Eastern Norway Regional Health Authority (2015-078), the Ebbe Frøland foundation, and a research grant from Throne-Holst. EV acknowledges the support of the Spanish Ministry of Science and Innovation (PI15/00283, PI18/00805) integrated into the Plan Nacional de I + D + I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER), the Instituto de Salud Carlos III, the CIBER of Mental Health (CIBERSAM), the Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2017 SGR 1365), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357. Lastly, this study was supported by the Canadian Institutes of Health Research (103703, 106469, 142255, 180449, and 487045), Nova Scotia Health Research Foundation, Dalhousie Clinical Research Scholarship to TH, Brain & Behavior Research Foundation (formerly NARSAD), and 2007 Young Investigator and 2015 Independent Investigator Awards to TH. PMT and CRKC received a grant from Biogen, Inc., for research unrelated to this manuscript. DJS has received research grants and/or consultancy honoraria from Lundbeck and Sun. LNY has received speaking/consulting fees and/or research grants from Abbvie, Alkermes, Allergan, AstraZeneca, CANMAT, CIHR, Dainippon Sumitomo Pharma, Janssen, Lundbeck, Otsuka, Sunovion, and Teva. TE received speaker's honoraria from Lundbeck and Janssen Cilag. EV has received grants and served as consultant, advisor, or CME speaker for the following entities (unrelated to the present work): AB-Biotics, Abbott, Allergan, Angelini, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, Janssen, Lundbeck, Otsuka, Sage, Sanofi-Aventis, and Takeda. The authors declare no conflicts of interest.
References
- GBD 2015 Obesity Collaborators, Afshin, A., Forouzanfar, M. H., Reitsma, M. B., Sur, P., Estep, K., … Murray, C. J. L. (2017). Health effects of overweight and obesity in 195 countries over 25 years. The New England Journal of Medicine, 377(1), 13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal, M., Siddiqi, N., Ahmad, B., Afsheen, N., Aslam, F., Ali, A., … Zavala, G. A. (2021). Prevalence of overweight and obesity in people with severe mental illness: Systematic review and meta-analysis. Frontiers in Endocrinology, 12, 769309. 10.3389/fendo.2021.769309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen, N. C., Liu, D., Ziebell, S., Vora, A., & Ho, B. C. (2013). Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: A prospective longitudinal MRI study. American Journal of Psychiatry, 170(1535-7228 (Electronic)), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun, M. A., Beydoun, H. A., & Wang, Y. (2008). Obesity and central obesity as risk factors for incident dementia and its subtypes: A systematic review and meta-analysis. Obesity Reviews, 9(3), 204–218. 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond, D. J., Ha, T. H., Lang, D. J., Su, W., Torres, I. J., Honer, W. G., … Yatham, L. N. (2014). Body mass index-related regional gray and white matter volume reductions in first-episode mania patients. Biological Psychiatry, 76(1873-2402 (Electronic)), 138–145. [DOI] [PubMed] [Google Scholar]
- Bond, D. J., Lang, D. J., Noronha, M. M., Kunz, M., Torres, I. J., Su, W., … Yatham, L. N. (2011). The association of elevated body mass index with reduced brain volumes in first-episode mania. Biological Psychiatry, 70(1873-2402 (Electronic)), 381–387. [DOI] [PubMed] [Google Scholar]
- Bond, D. J., Su, W., Honer, W. G., Dhanoa, T., Batres, Y. C., Lee, S. S., … Yatham, L. N. (2019). Weight gain as a predictor of frontal and temporal lobe volume loss in bipolar disorder: A prospective MRI study. Bipolar Disorders, 21(1), 50–60. [DOI] [PubMed] [Google Scholar]
- Bora, E., Akdede, B. B., & Alptekin, K. (2017). The relationship between cognitive impairment in schizophrenia and metabolic syndrome: A systematic review and meta-analysis. Psychological Medicine, 47(1469-8978 (Electronic)), 1030–1040. [DOI] [PubMed] [Google Scholar]
- Bora, E., Yucel, M., & Pantelis, C. (2009). Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of Affective Disorders, 113(0165-0327 (Print)), 1–20. [DOI] [PubMed] [Google Scholar]
- Calkin, C., Ruzickova, M., Uher, R., Hajek, T., Slaney, C. M., Garnham, J. S., … Alda, M. (2015). Insulin resistance and outcome in bipolar disorder. The British Journal of Psychiatry, 206(1472-1465 (Electronic)), 52–57. [DOI] [PubMed] [Google Scholar]
- Calkin, C., van de Velde, C, Ruzickova, M., Slaney, C., Garnham, J., Hajek, T., … Alda, M. (2009). Can body mass index help predict outcome in patients with bipolar disorder? Bipolar Disorders, 11(1399-5618 (Electronic)), 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching, C. R. K., Hibar, D. P., Gurholt, T. P., Nunes, A., Thomopoulos, S. I., Abé, C., … ENIGMA Bipolar Disorder Working Group. (2022). What we learn about bipolar disorder from large-scale neuroimaging: Findings and future directions from the ENIGMA Bipolar Disorder Working Group. Human Brain Mapping, 43(1), 56–82. 10.1002/hbm.25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, S. R., Lyall, D. M., Ritchie, S. J., Bastin, M. E., Harris, M. A., Buchanan, C. R., … Deary, I. J. (2019). Associations between vascular risk factors and brain MRI indices in UK Biobank. European Heart Journal, 40(28), 2290–2300. 10.1093/eurheartj/ehz100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers, I. A., Jansen, P. R., & Lamb, H. J. (2019). Obesity, brain volume, and white matter microstructure at MRI: A cross-sectional UK biobank study. Radiology, 291(3), 763–771. 10.1148/radiol.2019181012 [DOI] [PubMed] [Google Scholar]
- Di Angelantonio, E., Bhupathiraju, S. N., Wormser, D., Gao, P., Kaptoge, S., de Gonzalez, A. B., … Hu, F. B. (2016). Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet, 388(10046), 776–786. 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Andújar, M., Morales-García, E., & García-Casares, N. (2021). Obesity and gray matter volume assessed by neuroimaging: A systematic review. Brain Sciences, 11(8), 999. 10.3390/brainsci11080999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher, A. S., Truran, D., Mai, J. T., Langbaum, J. B., Aisen, P. S., Cummings, J. L., … Tariot, P. N. (2011). Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology, 77(1526-632X (Electronic)), 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli, P., Smieskova, R., Kempton, M. J., Ho, B. C., Andreasen, N. C., & Borgwardt, S. (2013). Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neuroscience & Behavioral Reviews, 37(1873-7528 (Electronic)), 1680–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-García, I., Michaud, A., Dadar, M., Zeighami, Y., Neseliler, S., Collins, D. L., … Dagher, A. (2019). Neuroanatomical differences in obesity: Meta-analytic findings and their validation in an independent dataset. International Journal of Obesity, 43(5), 943–951. 10.1038/s41366-018-0164-4 [DOI] [PubMed] [Google Scholar]
- Goldstein, B. I., Baune, B. T., Bond, D. J., Chen, P., Eyler, L., Fagiolini, A., … Fiedorowicz, J. G. (2020). Call to action regarding the vascular-bipolar link: A report from the vascular task force of the international society for bipolar disorders. Bipolar Disorders, 22(5), 440–460. 10.1111/bdi.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T., Calkin, C., Blagdon, R., Slaney, C., Uher, R., & Alda, M. (2014). Insulin resistance, diabetes mellitus, and brain structure in bipolar disorders. Neuropsychopharmacology, 39(1740-634X (Electronic)), 2910–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T., Cullis, J., Novak, T., Kopecek, M., Hoschl, C., Blagdon, R., … Alda, M. (2012a). Hippocampal volumes in bipolar disorders: Opposing effects of illness burden and lithium treatment. Bipolar Disorders, 14(1399-5618 (Electronic)), 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T., Hahn, M., Slaney, C., Garnham, J., Green, J., Ruzickova, M., … Alda, M. (2008). Rapid cycling bipolar disorders in primary and tertiary care treated patients. Bipolar Disorders, 10(1399-5618 (Electronic)), 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T., Kopecek, M., Hoschl, C., & Alda, M. (2012b). Reduced hippocampal volumes in bipolar disorders are masked by exposure to lithium – Meta-analysis. Journal of Psychiatry and Neuroscience, 37(5), 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek, T., Slaney, C., Garnham, J., Ruzickova, M., Passmore, M., & Alda, M. (2005). Clinical correlates of current level of functioning in primary care-treated bipolar patients. Bipolar Disorders, 7(1398-5647), 286–291. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hibar, D. P., Westlye, L. T., Doan, N. T., Jahanshad, N., Cheung, J. W., Ching, C. R. K., … Andreassen, O. A. (2018). Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Molecular Psychiatry, 23(4), 932–942. 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar, D. P., Westlye, L. T., van Erp, T. G., Rasmussen, J., Leonardo, C. D., Faskowitz, J., … Andreassen, O. A. (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(1476-5578 (Electronic)), 1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. H., Metcalfe, A. W. S., MacIntosh, B. J., Korczak, D. J., & Goldstein, B. I. (2018). Greater body mass index is associated with reduced frontal cortical volumes among adolescents with bipolar disorder. Journal of Psychiatry & Neuroscience, 43(2), 120–130. 10.1503/jpn.170041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz, D., Wittfeld, K., Terock, J., Freyberger, H. J., Hegenscheid, K., Volzke, H., … Grabe, H. J. (2015). Association between waist circumference and gray matter volume in 2344 individuals from two adult community-based samples. Neuroimage, 122(1095-9572 (Electronic)), 149–157. [DOI] [PubMed] [Google Scholar]
- Joober, R., Schmitz, N., Malla, A., Sengupta, S., & Karma, S. (2006). Is olanzapine a brain-sparing medication? Archives of General Psychiatry, 63(11), 1292. 10.1001/archpsyc.63.11.1292 [DOI] [PubMed] [Google Scholar]
- Jorgensen, K. N., Nesvag, R., Nerland, S., Morch-Johnsen, L., Westlye, L. T., Lange, E. H., … Agartz, I. (2017). Brain volume change in first-episode psychosis: An effect of antipsychotic medication independent of BMI change. Acta Psychiatrica Scandinavica, 135(1600-0447 (Electronic)), 117–126. [DOI] [PubMed] [Google Scholar]
- Löscher, W., Brandt, C., & Ebert, U. (2003). Excessive weight gain in rats over extended kindling of the basolateral amygdala. Neuroreport, 14(14), 1829–1832. 10.1097/00001756-200310060-00014 [DOI] [PubMed] [Google Scholar]
- Malkova, L., Mishkin, M., Suomi, S. J., & Bachevalier, J. (2010). Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta). Behavioral Neuroscience, 124(6), 742–760. 10.1037/a0021622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur, R. B., McIntyre, R. S., Cao, B., Lee, Y., Japiassú, L., Chen, K., … Lin, K. (2018). Obesity and frontal-striatal brain structures in offspring of individuals with bipolar disorder: Results from the global mood and brain science initiative. Bipolar Disorders, 20(1), 42–48. 10.1111/bdi.12559 [DOI] [PubMed] [Google Scholar]
- Mansur, R. B., Zugman, A., Ahmed, J., Cha, D. S., Subramaniapillai, M., Lee, Y., … McIntyre, R. S. (2017). Treatment with a GLP-1R agonist over four weeks promotes weight loss-moderated changes in frontal-striatal brain structures in individuals with mood disorders. European Neuropsychopharmacology, 27(11), 1153–1162. 10.1016/j.euroneuro.2017.08.433 [DOI] [PubMed] [Google Scholar]
- McIntyre, R. S., Danilewitz, M., Liauw, S. S., Kemp, D. E., Nguyen, H. T., Kahn, L. S., … Taylor, V. H. (2010). Bipolar disorder and metabolic syndrome: An international perspective. Journal of Affective Disorders, 126(1573-2517 (Electronic)), 366–387. [DOI] [PubMed] [Google Scholar]
- McWhinney, S., Abé, C., Alda, M., Benedetti, F., Bøen, E., Del Mar Bonnin, C., … ENIGMA Bipolar Disorders Working Group. (2021a). Association between body mass index and subcortical brain volumes in bipolar disorders-ENIGMA study in 2735 individuals. Molecular Psychiatry, 26(11), 6806–6819. 10.1038/s41380-021-01098-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney, S., Abé, C., Alda, M., Benedetti, F., Bøen, E., Del Mar Bonnin, C., … ENIGMA Bipolar Disorders Working Group. (2022a). Diagnosis of bipolar disorders and body mass index predict clustering based on similarities in cortical thickness-ENIGMA study in 2436 individuals. Bipolar Disorders, 24(5), 509–520. 10.1111/bdi.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney, S., Brosch, K., Calhoun, V. D., Crespo-Facorro, B., Crossley, N. A., Dannlowski, U., … Hajek, T. (2022b). Obesity and brain structure in schizophrenia – ENIGMA study in 3021 individuals. Molecular Psychiatry, 27(9), 3731–3737. 10.1038/s41380-022-01616-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney, S., Kolenic, M., Franke, K., Fialova, M., Knytl, P., Matejka, M., … Hajek, T. (2021b). Obesity as a risk factor for accelerated brain ageing in first-episode psychosis – a longitudinal study. Schizophrenia Bulletin, 47(6), 1772–1781. 10.1093/schbul/sbab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, M. D., Risbrough, V. B., Liang, J., & Boutelle, K. N. (2015). Pavlovian conditioning to hedonic food cues in overweight and lean individuals. Appetite, 87, 56–61. 10.1016/j.appet.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Mitchell, A. J., Vancampfort, D., Sweers, K., van Winkel, R., Yu, W., & De Hert, M. (2013). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders – A systematic review and meta-analysis. Schizophrenia Bulletin, 39(1745-1701 (Electronic)), 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, K., Möller, H. E., Horstmann, A., Busse, F., Lepsien, J., Blüher, M., … Pleger, B. (2015). Physical exercise in overweight to obese individuals induces metabolic- and neurotrophic-related structural brain plasticity. Frontiers in Human Neuroscience, 9, 372. 10.3389/fnhum.2015.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa, S., & Cuthill, I. C. (2007). Effect size, confidence interval and statistical significance: A practical guide for biologists. Biological Reviews, 82(4), 591–605. 10.1111/j.1469-185X.2007.00027.x [DOI] [PubMed] [Google Scholar]
- Nunes, A., Schnack, H. G., Ching, C. R. K., Agartz, I., Akudjedu, T. N., Alda, M., … ENIGMA Bipolar Disorders Working Group. (2020). Using structural MRI to identify bipolar disorders – 13 site machine learning study in 3020 individuals from the ENIGMA Bipolar Disorders Working Group. Molecular Psychiatry, 25(9), 2130–2143. 10.1038/s41380-018-0228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg, S. T., Batty, G. D., Pentti, J., Virtanen, M., Alfredsson, L., Fransson, E. I., … Kivimäki, M. (2018). Obesity and loss of disease-free years owing to major non-communicable diseases: A multicohort study. The Lancet Public Health, 3(10), e490–e497. 10.1016/S2468-2667(18)30139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel, N., Redlich, R., Grotegerd, D., Dohm, K., Haupenthal, C., Heindel, W., … Dannlowski, U. (2015). Enhanced neural responsiveness to reward associated with obesity in the absence of food-related stimuli: Obesity and neural responsiveness to reward. Human Brain Mapping, 36(6), 2330–2337. 10.1002/hbm.22773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel, N., Redlich, R., Kaehler, C., Grotegerd, D., Dohm, K., Heindel, W., … Dannlowski, U. (2017). Prefrontal gray matter volume mediates genetic risks for obesity. Molecular Psychiatry, 22(1476-5578 (Electronic)), 703–710. [DOI] [PubMed] [Google Scholar]
- Opel, N., Thalamuthu, A., Milaneschi, Y., Grotegerd, D., Flint, C., Leenings, R., … Dannlowski, U. (2021). Brain structural abnormalities in obesity: Relation to age, genetic risk, and common psychiatric disorders: Evidence through univariate and multivariate mega-analysis including 6420 participants from the ENIGMA MDD working group. Molecular Psychiatry, 26(9), 4839–4852. 10.1038/s41380-020-0774-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parimisetty, A., Dorsemans, A.-C., Awada, R., Ravanan, P., Diotel, N., & Lefebvre d'Hellencourt, C. (2016). Secret talk between adipose tissue and central nervous system via secreted factors – An emerging frontier in the neurodegenerative research. Journal of Neuroinflammation, 13(1), 67. 10.1186/s12974-016-0530-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedditzi, E., Peters, R., & Beckett, N. (2016). The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age and Ageing, 45(1), 14–21. 10.1093/ageing/afv151 [DOI] [PubMed] [Google Scholar]
- Salvi, V., Salvo, G. D., Korčáková, J., Torriero, S., Aragno, E., Kolenič, M., … Hajek, T. (2020). Insulin resistance is associated with verbal memory impairment in bipolar disorders. Journal of Affective Disorders, 266, 610–614. 10.1016/j.jad.2020.01.145 [DOI] [PubMed] [Google Scholar]
- Shan, H., Li, P., Liu, H., Nie, B., Yin, X., Zhang, T., … Shan, B. (2019). Gray matter reduction related to decreased serum creatinine and increased triglyceride, hemoglobin A1C, and low-density lipoprotein in subjects with obesity. Neuroradiology, 61(6), 703–710. 10.1007/s00234-019-02202-3 [DOI] [PubMed] [Google Scholar]
- Singh-Manoux, A., Dugravot, A., Shipley, M., Brunner, E. J., Elbaz, A., Sabia, S., & Kivimaki, M. (2018). Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimer's & Dementia, 14(2), 178–186. 10.1016/j.jalz.2017.06.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X., Zhao, W., Lu, M., Zhang, X., Zhang, P., Xin, Z., … Stehouwer, C. D. A. (2021). Relationship between central obesity and the incidence of cognitive impairment and dementia from cohort studies involving 5,060,687 participants. Neuroscience and Biobehavioral Reviews, 130, 301–313. 10.1016/j.neubiorev.2021.08.028 [DOI] [PubMed] [Google Scholar]
- Tariot, P. N., Schneider, L. S., Cummings, J., Thomas, R. G., Raman, R., Jakimovich, L. J., … Aisen, P. S. (2011). Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Archives of General Psychiatry, 68(1538-3636 (Electronic)), 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek, C., Kucukgoncu, S., Guloksuz, S., Woods, S. W., Srihari, V. H., & Annamalai, A. (2016). Antipsychotic-induced weight gain in first-episode psychosis patients: A meta-analysis of differential effects of antipsychotic medications. Early Intervention in Psychiatry, 10(1751-7893 (Electronic)), 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, P. M., Jahanshad, N., Ching, C. R. K., Salminen, L. E., Thomopoulos, S. I., Bright, J., … ENIGMA Consortium. (2020). ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Translational Psychiatry, 10(1), 100. 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari, J. J., Karlsson, H. K., Antikainen, O., Hirvonen, J., Pham, T., Salminen, P., … Nummenmaa, L. (2016). Bariatric surgery induces white and grey matter density recovery in the morbidly obese: A voxel-based morphometric study. Human Brain Mapping, 37(11), 3745–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort, D., Stubbs, B., Mitchell, A. J., De Hert, M., Wampers, M., Ward, P. B., … Correll, C. U. (2015). Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psychiatry, 14(3), 339–347. 10.1002/wps.20252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp, T. G. M., Walton, E., Hibar, D. P., Schmaal, L., Jiang, W., Glahn, D. C., … Turner, J. A. (2018). Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biological Psychiatry, 84(9), 644–654. 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gaal, L. F., Mertens, I. L., & De Block, C. E. (2006). Mechanisms linking obesity with cardiovascular disease. Nature, 444(7121), 875–880. 10.1038/nature05487 [DOI] [PubMed] [Google Scholar]
- Van Gestel, H., Franke, K., Petite, J., Slaney, C., Garnham, J., Helmick, C., … Hajek, T. (2019). Brain age in bipolar disorders: Effects of lithium treatment. The Australian and New Zealand Journal of Psychiatry, 53(12), 1179–1188. 10.1177/0004867419857814 [DOI] [PubMed] [Google Scholar]
- Willette, A. A., & Kapogiannis, D. (2015). Does the brain shrink as the waist expands? Ageing Research Reviews, 20(1872-9649 (Electronic)), 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse, B. E. (2004). The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. Journal of the American Society of Nephrology: JASN, 15(11), 2792–2800. 10.1097/01.ASN.0000141966.69934.21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291723000223.
click here to view supplementary material