Abstract
Salinity is a widespread abiotic stress, which has strong adverse effects on plant growth and crop productivity. Exopolysaccharides (EPS) play a crucial role in plant growth‐promoting rhizobacteria (PGPR)‐mediated improvement of plant stress tolerance. This study aimed to assess whether Glutamicibacter sp. strain producing large amounts of EPS may promote tolerance of common reed, Phragmites australis (Cav.) Trin. ex Steud., towards salt stress. This halotolerant rizhobacterium showed tolerance to salinity (up to 1 M NaCl) when cultivated on Luria‐Bertani (LB) medium. Exposure to high salinity (300 mM NaCl) significantly impacted the plant growth parameters, but this adverse effect was mitigated following inoculation with Glutamicibacter sp., which triggered higher number of leaves and tillers, shoot fresh weight/dry weight, and root fresh weight as compared to non‐inoculated plants. Salt stress increased the accumulation of malondialdehyde (MDA), polyphenols, total soluble sugars (TSSs), and free proline in shoots. In comparison, the inoculation with Glutamicibacter sp. further increased shoot polyphenol content, while decreasing MDA and free proline contents. Besides, this bacterial strain increased tissue Ca+ and K+ content concomitant to lower shoot Na+ and root Cl− accumulation, thus further highlighting the beneficial effect of Glutamicibacter sp. strain on the plant behavior under salinity. As a whole, our study provides strong arguments for a potential utilization of EPS‐producing bacteria as a useful microbial inoculant to alleviate the deleterious effects of salinity on plants.
Keywords: exopolysaccharides, ion concentrations, osmolytes, PGPR, Phragmites australis , salinity stress
1. INTRODUCTION
Various environmental stresses such as flood, extreme temperatures, soil/water salinization, and drought impact the growth and development of plants and ultimately cause substantial decline in crop yield (Bano & Fatima, 2009; Jha et al., 2011). Under the ongoing climate changes, soil salinity is an expanding environmental issue, affecting millions of hectares of land around the world and resulting in enormous economic losses each year (Munns & Gilliham, 2015). According to Munns (2005), salinity affects plants' growth and development through two main physiological pathways (osmotic stress and ion toxicity). The first phase appears immediately upon salt stress exposure and is due to the osmotic stress caused by hypertonic conditions whereas the second phase occurs over several hours to days and weeks to develop and results from the toxic effects of salt ions (Na+ and Cl−) accumulating in the cells. Salt tolerance is known as a complex quantitative trait that is controlled by multiple genes and involves various physiological and biochemical mechanisms, which are also species specific (Chinnusamy et al., 2005; Flowers & Colmer, 2008; Shabala, 2013).
Common reed, Phragmites australis (Cav.) Trin. ex Steud., is a perennial reed grass considered as one of the most extensively distributed and productive fodder species in the world (Brix & Cizkova, 2001). P. australis is a species of major environmental significance due to its high intraspecific diversity, phenotypic plasticity, and evolutionary potential. P. australis also contributes to storm protection, sediment stabilization, and water filtration (Eller et al., 2017; Knight et al., 2018; Meyerson et al., 2009; Rodríguez & Brisson, 2015). In Tunisia, P. australis is frequent on coastal habitats, including shallow marshes, coastal mudflat areas, fringes of lagoons, and salt‐affected wetlands (Gorai et al., 2010), and it is often the single dominant and keystone species in its habitats (Liu et al., 2018). High salinity levels restrict biomass production of P. australis , thereby leading to reduced establishment capacity and vigor in brackish and salt marshes (Eller et al., 2017). Pagter et al. (2009) hypothesized that this may be due to both osmotic and ion‐specific effects. Therefore, it is essential to improve P. australis salt stress tolerance to minimize yield losses in a sustainable method.
Using salt‐tolerant microorganisms to boost the growth of salt‐stressed plants is a promising strategy to alleviate salinity (Dodd & Perez‐Alfocea, 2012; Egamberdieva et al., 2019). Furthermore, the application of halotolerant microorganisms for salinity stress management opens interesting challenges for using pyramiding strategies against salinity, as well as new biological methods to elucidate further mechanisms of action in plant stress tolerance (Dodd & Perez‐Alfocea, 2012; Kumar et al., 2018). Plant growth‐promoting rhizobacteria (PGPR), which colonize the rhizosphere of various plants, can promote plant growth and alleviate salt stress without harming the environment. In the last decade, a large number of reports documented that bacteria of various genera including Rhizobium, Bacillus, Pseudomonas, Azotobacter, Azospirillum, and Enterobacter conferred better behavior to their host plants against different abiotic stresses such as salinity (Egamberdieva et al., 2019; Etesami & Beattie, 2017; Etesami & Glick, 2020; Kumar et al., 2020; Sarkar et al., 2018). This PGPR capacity is notably ascribed to (i) the improvement of plant nutrition by increasing the solubilization of phosphorus and the potassium availability, biological nitrogen fixation, and iron sequestration, (ii) bacteria secretion of metabolic products (1‐aminocyclopropane‐1‐carboxylate [ACC] deaminase, which converts the plant ethylene substrate ACC to ammonia [NH3] and alpha‐ketobutyrate [C4H6O3], plant growth‐promoting hormones [mainly indole‐3‐acetic acid, IAA], exopolysaccharides [EPS], microorganism volatile organic compounds, and siderophores), and (iii) the activation of plant antioxidant defense mechanisms to alleviate oxidative damages in plants challenged with abiotic constraints (Bano & Fatima, 2009; Kohler et al., 2009; Kumar et al., 2018; Ullah et al., 2015).
High concentrations of Na+ and Cl− in the rhizosphere may inhibit nutrient‐related activities and change ion balance as Na+/Ca2+, Na+/K+, Na+/Mg2+, Cl−/NO3 −, and Cl−/H2PO4 ratios (Grattan & Grieve, 1998). Such phenomenon may cause an imbalance in the plant ionic composition, which would impact the physiological characteristics and growth of the plant (Munns et al., 2006). PGPRs alleviate the deleterious effects of salt stress by reducing the sodium absorption, as well as increasing the uptake of potassium in the leaves and roots, thereby maintaining an optimal K+/Na+ ratio for plant growth and induction of transcription factors under stress conditions (Karlidag et al., 2013). Nunkaew et al. (2015) indicated that exopolysaccharide producing halotolerant PGPRs can decrease plant sodium accumulation via binding Na+ in roots and preventing its translocation to leaves (Ashraf et al., 2004; Dodd & Perez‐Alfocea, 2012; Etesami & Beattie, 2017; Qin et al., 2016). The produced polysaccharides can also chelate free Na+ in the soil making it unavailable to plants (Liu et al., 2022). Zhang et al. (2022) reported that EPS production by rhizobacteria was induced by root exudates to support mature biofilm formation. EPS are involved in cell–cell aggregation, which is essential for bacteria anchoring and adhesion to plant roots (Bhagat et al., 2021). Therefore, EPS presence improves biofilm formation and root colonization by salt‐tolerant plant growth‐promoting rhizhospheric bacteria, which may impact salt resistance in plants (Bhagat et al., 2021). Sun et al. (2022) found that EPS production by bacteria around roots increases leaf water potential and improves plant nutrient uptake. Furthermore, EPS enhance soil physicochemical properties and promote its aggregate formation (Kumar et al., 2020). Thus, EPS‐producing PGPR can play a significant role in alleviating salinity in plants (Bhagat et al., 2021).
P. australis response to salinity is well documented, but the likely beneficial role of inoculation with halotolerant PGPR to improve this plant performance under saline conditions has still not been addressed. Therefore, the present study aims to (i) investigate the effectiveness of Glutamicibacter sp. to improve plant growth of P. australis under salt stress condition and (ii) identify some of the physiological and biochemical mechanisms modulated by this strain by emphasizing nutritional and biochemical traits (polyphenol, total soluble sugars [TSSs], proline, and markers of oxidative stress) of plants challenged or not with high salinity.
2. MATERIALS AND METHODS
2.1. Plant and microorganism sampling and characterization
Native P. australis rhizome sections were collected from reed populations in Borj Cedria (20 km to the east of Tunis, Tunisia). The PGPR strain Glutamicibacter sp. was isolated from rhizospheric soil from the salt‐affected area in Soliman Sebkha (36 42′35″N 10 26′08″E, 30 km south of Tunis, semi‐arid bioclimate) and was selected on the basis of its plant growth‐promoting attributes and its EPS, siderophore, and IAA production.
EPS production by Glutamicibacter sp. was determined during the growth of this species when cultivated under increasing gradient of NaCl concentrations (0.1–1 M). Briefly, 150 mL flasks containing 50 mL of yeast extract mannitol (YEM) medium were inoculated with 0.1 mL of 108 colony‐forming unit (CFU) bacterial culture and incubated at 28°C with shaking (180 rpm) for 3 days (Dueñas et al., 2003). Another experiment was performed by cultivating the strain in YEM medium without NaCl (control). After incubation, EPS yield was determined using the phenol‐sulfuric acid method (Kazy et al., 2002). All experiments were performed in triplicate.
Bacterial strain was also assessed for its ability to survive under salt stress. The isolate was inoculated on Luria‐Bertani (LB) liquid medium supplemented with different NaCl concentrations (0.1–1 M) and incubated at 28 ± 2°C with shaking at 170 rpm for 48 h. Control medium was maintained with 0.1% NaCl (w/v). Turbidity was determined by measuring the optical density (OD) at 600 nm. All experiments were performed in triplicate.
Identification of the bacterial strain was performed by the isolation of genomic DNA as described by Pospiech and Neumann (1995) followed by polymerase chain reaction (PCR) amplification of 16S rRNA as described by Weisburg et al. (1991). Partial sequence obtained was matched against nucleotide sequence present in GenBank using the BLASTn program (http://www.ncbi.nlm.nih.gov) and deposited in the GenBank database under accession number MK847918.
2.2. Pot experiment and P. australis plant growth parameters
Several sections (approximately 5 g each) of P. australis rhizomes were inserted into 4‐L pots containing autoclaved limono‐sandy soil (pH 6.65; electrical conductivity: 0.05 dS m−1; 0.24 and 0.45 g kg−1 of dry soil of P2O5 and total N; 0.25, 0.95, 0.65, and 0.05 meq 100 g−1 of dry soil of Na+, K+, Ca2+, and Cl−, respectively). All rhizome sections had an apical bud. Pots were placed under daily irrigation with distilled water. After 70 days, sprouted P. australis rhizomes were transferred to 2‐L pots (two plants per pot) filled with sterilized soil. Plants were then grown for 40 days under greenhouse conditions. Twenty pots of similar P. australis plants were selected and assigned numbers. In the appropriate pots, plants were inoculated with 1 mL of the bacterial culture (107 CFU mL−1). Seven days after inoculation, plants were watered three times per week by a mixture of distilled water and a solution of 300 mM NaCl. NaCl was added gradually in four concentrations (50, 100, 200, and 300 mM) on alternative days to avoid an osmotic shock. Plants were maintained under these conditions for additional 20 days. The experiment consisted of a factorial design with two inoculation treatments: non‐inoculated (control) and inoculated plants with Glutamicibacter sp. and two saline treatments: plants treated with 0 mM NaCl and plants treated with 300 mM NaCl. Five replicates per treatment were used.
The fresh weight (FW) and dry weight (DW) of shoots and roots were determined after counting leaves and the tiller number. The DW of shoots and roots samples were determined after an oven‐drying of 1 week at 60°C.
2.3. Inorganic ion assay
Inorganic ions were extracted by mineralization of shoot and root dry powder (30 mg) in 30 mL nitric acid (0.1 N). Cations (Na+, K+, and Ca2+) were assayed by a Corning 480 flame photometer and chloride by coulometry using a Haake‐Buchler chloridometer.
2.4. Lipid peroxidation
The extent of lipid peroxidation was calculated by measuring the malondialdehyde (MDA) content formed through thiobarbituric acid (TBA) reaction following the method of Halliwell and Gutteridge (1989). Lipid peroxides were extracted by grinding 0.05 g of shoot with 2 mL of trichloroacetic acid (TCA) 0.1% (w/v). The homogenate was centrifuged at 12,000 g for 20 min, and then 1 mL sample of the supernatant was mixed with 1 mL of 0.5% (w/v) TBA in 20% (w/v) TCA. The mixture was incubated at 95°C for 30 min; the reaction was stopped by placing the reaction tubes in an ice water bath. Following cooling, the sample was centrifuged at 5000 g for 5 min, and the supernatant was read at 532 and 600 nm. The concentration of MDA was calculated from the extinction coefficient 155 mM−1 cm−1.
2.5. Determination of organic solutes in plant tissues
Shoot free proline content was spectrophotometrically quantified by the ninhydrin method (Bates et al., 1973). The plant material was homogenized in 3% aqueous sulfosalicylic acid and centrifuged at 20,000 g . Samples were incubated with 1 mL of ninhydrin acid and 1 mL glacial acetic acid and boiled at 100°C for 1 h. After termination of reaction in ice bath, the reaction mixture was extracted with 2 mL of toluene, and absorbance was read at 520 nm. The proline content in each sample was calculated from a standard curve prepared with L‐proline.
TSS content was estimated by the method of Yemm and Willis (1954). A sample of 25 mg of dry material was extracted in 80% ethanol solution. The extract was incubated for 30 min at 70°C and then centrifuged at 3000 g at 25°C for 30 min. TSS were analyzed by reacting 0.5 mL of the alcoholic extract with 5 mL freshly prepared anthrone and 2 mL ethanol (80%) and heated in a boiling water bath for 10 min. A standard curve was determined using glucose. After cooling, the absorbance was read at 640 nm with a spectrophotometer.
2.6. Estimation of total polyphenol content
Phenolic compounds were assayed using the Folin–Ciocalteu reagent, following the method of Singleton and Rosi (1965) slightly modified by Dewanto et al. (2002). Shoot extracts were obtained by stirring 1 g of dry powder with 10 mL of pure methanol for 30 min. Sample extract was added to deionized water and Folin–Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min before adding 7% sodium carbonate solution and adjusted with distilled water. After incubation in the dark for 90 min at ambient temperature, the absorbance was measured at 760 nm. Gallic acid was used to prepare standard curve, and results were expressed as mg gallic acid equivalent (GAE) per gram of DW (mg GAE g−1 DW).
2.7. Statistical analysis
Statistical analysis was performed using SPSS 20.0 statistical program (SPSS Inc., Chicago, IL, USA). The effects of the experimental factors, salt stress, and inoculation as well as the effect of their interactions on plant parameters were assessed by a two‐way analysis of variance (ANOVA) (Table 1), and means were compared according to Duncan's test at P < .05. Principal component analysis (PCA) and correlation analysis were performed using XLSTAT software v. 2014.
TABLE 1.
Two‐factor ANOVA (bacterial inoculation and saline stress) for all parameters studied of P. australis ; F‐values (P‐values).
| Bacterial inoculation (M) | Saline stress (S) | Interaction (B × S) | |
|---|---|---|---|
| SFW | 11.79 (.009) | 157.27 (<.001) | 0.413 (.538) |
| SDW | 7.345 (.024) | 29 (<.001) | 1.41 (.266) |
| RFW | 4.802 (.060) | 247.86 (<.001) | 8.043 (.022) |
| RDW | 2.958 (.124) | 33.11 (<.001) | 4.247 (.073) |
| TN | 6.658 (.024) | 11.836 (.005) | 2.959 (.111) |
| SN | 5.014 (.052) | 28.369 (<.001) | 1.044 (.334) |
| Polyphenols | 44.291 (<.001) | 66.028 (<.001) | 0 (.999) |
| Proline | 1.699 (.229) | 6027 (<.001) | 30.078 (.001) |
| TSS | 16.665 (.004) | 9.801 (.014) | 9.298 (.016) |
| MDA | 19.928 (.001) | 16.940 (.002) | 0.113 (.743) |
| S Na+ | 1.923 (.203) | 93.071 (<.001) | 20.116 (.002) |
| R Na+ | 1.413 (.265) | 1228 (<.001) | 3.630 (.089) |
| S K+ | 38.11 (<.001) | 0.783 (.402) | 0.084 (.780) |
| R K+ | 8.089 (.022) | 9.203 (<.016) | 8.067 (.022) |
| S K+/Na+ | 2.654 (.134) | 58.843 (<.001) | 14.047 (.004) |
| R K+/Na+ | 4.415 (.065) | 219.381 (<.001) | 6.910 (.027) |
| S Cl− | 11.174 (.007) | 436 (<.001) | 18.169 (.001) |
| R Cl− | 3.600 (.087) | 1590 (<.001) | 20.969 (.001) |
| S Ca2+ | 1.550 (.241) | 0.035 (.856) | 21.876 (.001) |
| R Ca2+ | 51.598 (<.001) | 15.935 (.003) | 1.506 (.248) |
Abbreviations: LN, leaves number; MDA, malondialdehyde; R Ca2+, root calcium content; R Cl−, root chloride content; RDW, root dry weight; RFW, root fresh weight; R K+, root potassium content; R Na+, root sodium content; S Ca2+, shoot calcium content; S Cl−, shoot chloride content; SDW, shoot dry weight; SFW, shoot fresh weight; S K+, shoot potassium content; S Na+, shoot sodium content; TN, tillers number; TSS, total soluble sugar.
3. RESULTS
3.1. Optimum NaCl tolerance of Glutamicibacter sp. and production of EPS in different NaCl concentrations
Glutamicibacter sp. showed the highest growth rate in the 100–200 mM NaCl salinity range, before progressively decreasing with increasing salt concentration (Figure 1a). EPS production by several halotolerant bacteria is frequently reported as a growth strategy that can form a protective biofilm around cells, which could protect them from oxidative damages under stress conditions. Therefore, we examined the potential role of EPS in salt tolerance of Glutamicibacter sp. As shown in Figure 1b, Glutamicibacter sp. produced EPS at various salt concentrations. EPS yield produced by Glutamicibacter sp. decreased at first with increasing salt concentration. This parameter showed a decrease of 14% and 36.5% at 200 and 400 mM NaCl compared to salt‐free conditions and then remained stable regardless of salt concentration.
FIGURE 1.
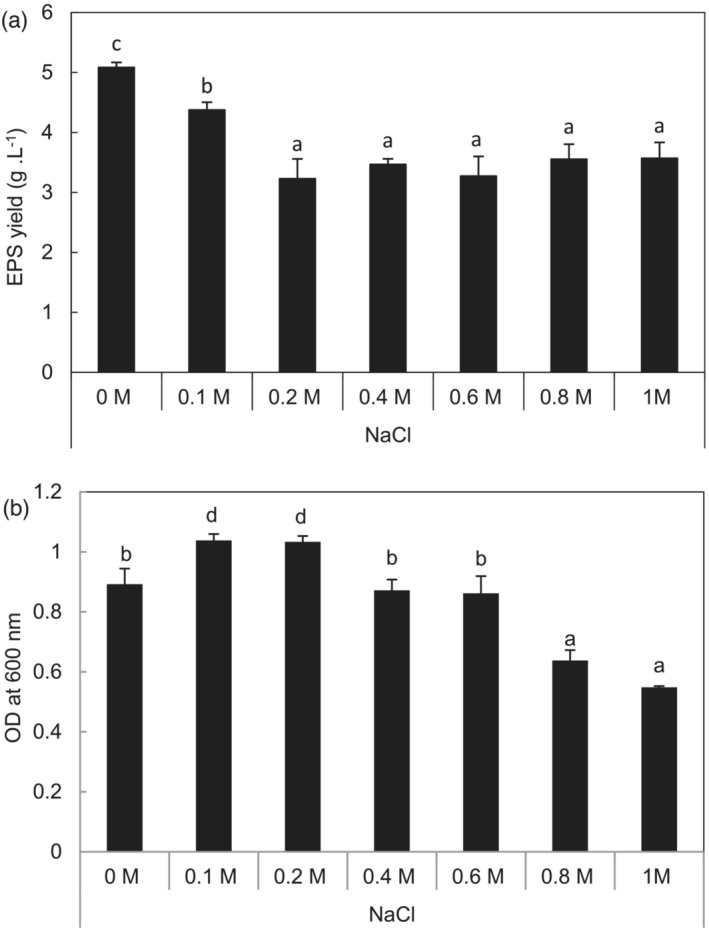
Determination of the growth rate and exopolysaccharides yield production of Glutamicibacter sp. in different saline levels (0.1–1 M NaCl). Different letters indicate statistically differences between salt concentrations (Duncan's least significant difference, P < .05, n = 3).
3.2. Inoculation effect on plant growth
P. australis plants showed significant reduction in growth traits and biomass in response to NaCl stress, but inoculation with Glutamicibacter sp. isolate significantly (at P < .05) improved plant growth compared to non‐inoculated stressed plants (Figure S1). P. australis inoculation with Glutamicibacter sp. strain in the presence of NaCl stimulated shoot FW and DW (+40% and +56%, respectively) (Figure 2a,b), root FW (+56%) (Figure 2c), and leaf and tiller numbers (+56% and +33%, respectively) (Figure 2e,f), when compared to non‐inoculated plants challenged with salinity. It is worth mentioning that under salt‐free conditions, the inoculation with Glutamicibacter sp did not significantly affect P. australis growth, except for root DW (approximately +17% as compared to non‐inoculated plants) (Figure 2d).
FIGURE 2.
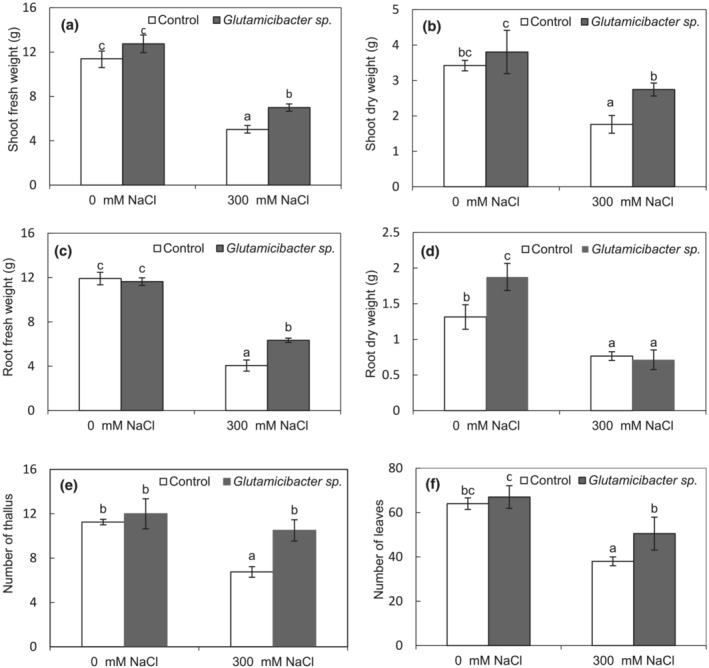
Effect of Glutamicibacter sp. on growth of P. australis under salt stress (300 mM NaCl): shoot fresh weight (a), root fresh weight (b), shoot dry weight (c), root dry weight (d), leaf number (e), and tiller number (f). Values are the means standard deviation of five replicates. Different letters indicate statistically differences between treatments (Duncan's least significant difference, P < .05, n = 5).
3.3. Inoculation effect on plant ion status
Under salt stress conditions, Na+ concentration in shoot and especially root of non‐inoculated plants increased by 90% and 270% as compared to the control plants, respectively (Table 2). This is consistent with the exclusive character of the plant when salt‐challenged. Glutamicibacter sp. inoculation improved plant capacity to avoid Na+ build‐up in shoots, as reflected by the significantly lower shoot Na+ concentration under salt stress (−20% compared to non‐inoculated stressed plants) (Table 2).
TABLE 2.
Effect of bacterial inoculation on nutrient accumulation in shoots and roots tissues of P. australis (mg/g DW): sodium (Na+), potassium (K+), chloride (Cl−), and calcium (Ca+) and K+/Na+ ratio in the absence (0 mM NaCl) and in presence of NaCl (300 mM NaCl).
| Treatments | Nutrients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Cl− | Ca2+ | K+/Na+ | ||||||
| Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | Root | |
| Control | 11.35 ± 0.20a | 12.8 ± 0.38a | 23.18 ± 0.81a | 13.11 ± 0.52a | 12.94 ± 0.72a | 12.37 ± 0.57a | 9.84 ± 0.17b | 22.5 ± 0.24a | 2.14 ± 0.03c | 0.91 ± 0.09b |
| Glutamicibacter sp. | 13.57 ± 0.22a | 13.47 ± 0.27a | 29.8 ± 2.59b | 13.11 ± 0.52a | 17.64 ± 0.50b | 15.25 ± 0.46a | 9.28 ± 0.10a | 30.06 ± 0.53b | 2 ± 0.19c | 0.81 ± 0.09b |
| 300 mM NaCl | 21.5 ± 1.92c | 47.4 ± 0.37b | 23.85 ± 0.93a | 13.25 ± 1.45a | 28.6 ± 0.54c | 60.01 ± 2.29c | 9.1 ± 0.23a | 25.98 ± 2.66a | 1.38 ± 0.07a | 0.36 ± 0.02a |
| Glutamicibacter sp. + 300 mM NaCl | 17.28 ± 0.64b | 44.51 ± 3.32b | 31.12 ± 1.31b | 17.25 ± 1.08b | 28.02 ± 0.58c | 53.08 ± 0.50b | 10.19 ± 0.12b | 36.73 ± 1.25c | 1.74 ± 0.05b | 0.37 ± 0.03a |
Note: Values are the means standard deviation of five replicates. Values sharing the different letters indicate significant differences between treatments at the 5% level (Duncan's t‐test).
Generally, no significant difference was found with respect to shoot and root K+ concentration between non‐inoculated control plants and non‐inoculated stressed plants (Table 2). Yet, Glutamicibacter sp. significantly increased shoot K+ concentration under non‐salt stress as compared with control and salt‐stressed plants (~30% on average). Under stress condition, Glutamicibacter sp. significantly enhanced K+ concentration in root as compared to control (Table 2). Besides, the K+/Na+ ratio was enhanced by Glutamicibacter sp. in shoot of salt challenged plants (Table 2). Glutamicibacter sp. inoculation significantly decreased root Cl− content by 12% under salt condition, as compared to salt‐stressed plants only (Table 2). Calcium accumulation was higher in root than in shoot in inoculated and non‐inoculated plants (Table 2). Root Ca2+ concentration was significantly higher in inoculated plants compared to non‐inoculated plants under both saline and non‐saline conditions (Table 2).
3.4. Inoculation effect on lipid peroxidation and total polyphenol content
Lipid peroxidation level of P. australis plants were significantly increased by rising NaCl concentration. Glutamicibacter sp. significantly reduced this parameter under both non‐saline and salt stress conditions; the decrease amounted to ~17% in both conditions (Figure 3a). Leaf total polyphenol content was higher with increasing concentration of salt (36% compared to the control) (Figure 3b). Interestingly, inoculated plants showed higher leaf polyphenol content than non‐inoculated plants irrespective of salinity level (Figure 3b).
FIGURE 3.
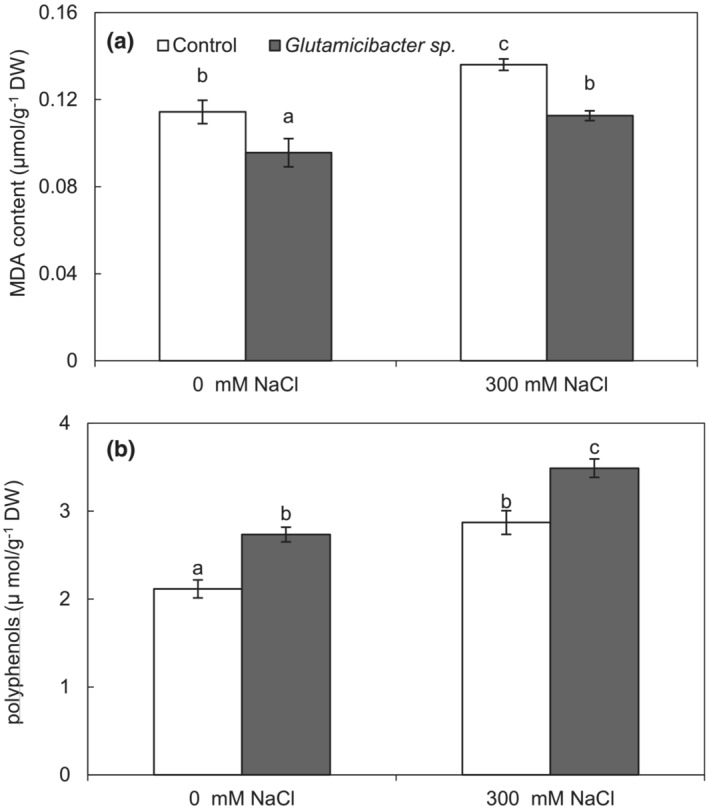
Effect of Glutamicibacter sp. on lipid peroxidation (a) and polyphenols content (b) in P. australis leaves under salt stress (300 mM NaCl). Values are the means standard deviation of five replicates. Different letters indicate statistically differences between treatments (Duncan's least significant difference, P < .05, n = 5).
3.5. Effect of Glutamicibacter sp. inoculation on free proline and TSSs content
Under salt‐free conditions, no significant difference of leaf TSSs and proline content was observed between control and Glutamicibacter sp. treatment (Figure 4a,b). In non‐inoculated plants, the TSSs and proline contents were increased by salt as compared to the control (Figure 4a,b). However, inoculation with Glutamicibacter sp. could significantly offset this effect by maintaining the TSS content in salt‐treated plants and decreasing proline content by 30% as compared to salt‐stressed plants only (Figure 4b).
FIGURE 4.
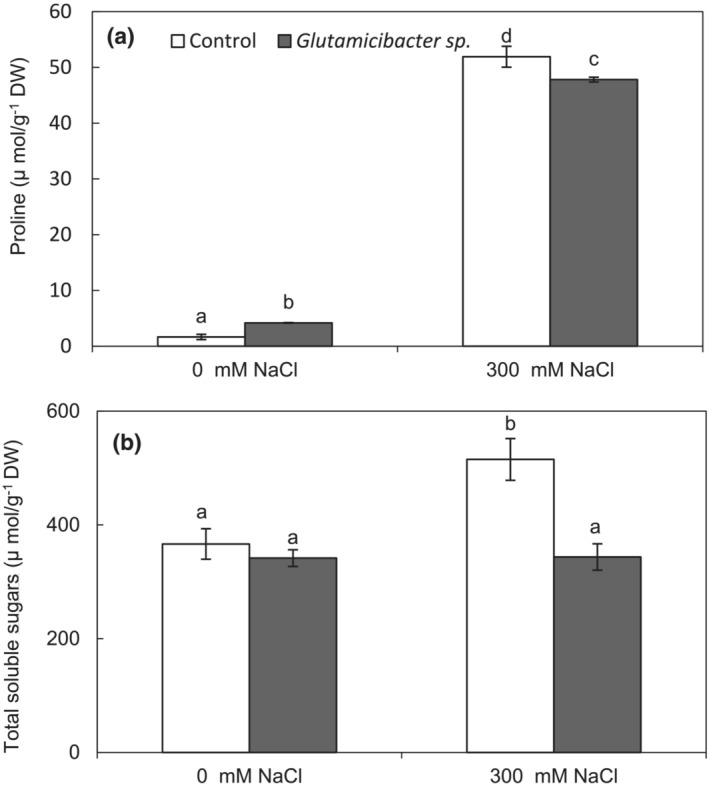
Effect of Glutamicibacter sp. on free proline (a) and total soluble sugars (b) in P. australis under salt stress (300 mM NaCl). Values are the means standard deviation of five replicates. Different letters indicate statistically differences between treatments (Duncan's least significant difference, P < .05, n = 5).
3.6. Correlation analysis and PCA
For a more accurate interpretation of our data, a PCA (Figure 5) and correlation analysis (Table 3) were performed, taking into account all determined parameters characterizing plants under salt stress and bacterial inoculation. Globally, these analyses confirmed the observed positive effect of Glutamicibacter sp. inoculation, enabling P. australis to successfully cope with salt stress.
FIGURE 5.
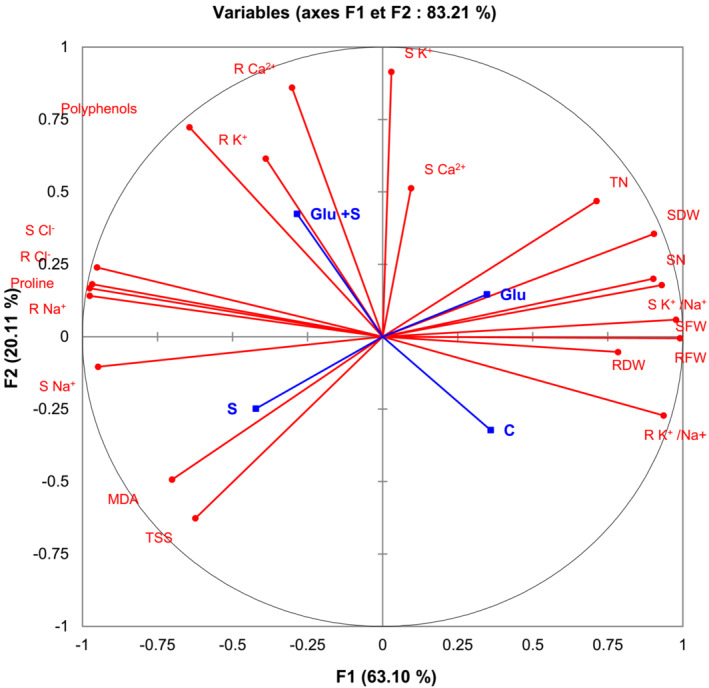
Principal component analysis (PCA). Circles (•) represent different analysis parameters. Squares (■) represent different treatments (C, Glu, S, and S + Glu). All studied parameters and the different treatments are projected onto the F1–F2 principal factorial plane that explains 83.21% of the variation. LN, leaves number; MDA, malondialdehyde; R Ca2+, root calcium content; R Cl−, root chloride content; RDW, root dry weight; RFW, root fresh weight; R K+, root potassium content; R Na+, root sodium content; S Ca2+, shoot calcium content; S Cl−, shoot chloride content; SDW, shoot dry weight; SFW, shoot fresh weight; S K+, shoot potassium content; S Na+, shoot sodium content; TN, tillers number; TSS, total soluble sugar.
TABLE 3.
Pearson's correlation matrix analyzing C, Glu, S, and S + Glu treatments and different studied parameters.
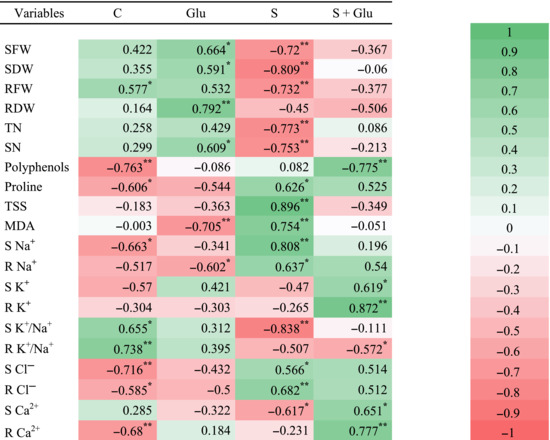
|
Abbreviations: LN, leaves number; MDA, malondialdehyde; R Ca2+, root calcium content; R Cl−, root chloride content; RDW, root dry weight; RFW, root fresh weight; R K+, root potassium content; R Na+, root sodium content; S Ca2+, shoot calcium content; S Cl−, shoot chloride content; SDW, shoot dry weight; SFW, shoot fresh weight; S K+, shoot potassium content; S Na+, shoot sodium content; TN, tillers number; TSS, total soluble sugar.
Statistically significant correlation at 0.05.
Statistically significant correlation at 0.01.
Statistically significant correlation at 0.001.
Positive correlations were found under Glutamicibacter sp. inoculation for shoot and root DWs and shoot FW. By contrast, this treatment was found to be negatively correlated with MDA content and root Na+ content (Figure 5 and Table 3). A high salinity condition was negatively correlated with plant growth‐related traits (shoot DW/FW and root FW, leaves number, and tillers number) (Figure 5 and Table 3). Besides, salt treatment was positively correlated with proline, MDA, TSS, Na+, and Cl− contents. Positive correlations were found under Glutamicibacter sp. inoculation under salt stress for polyphenols and shoot/root K+ and Ca2+ contents. Finally, the results obtained by PCA and correlation analysis showed a perfect match with our trait‐by‐trait analyses.
4. DISCUSSION
Due to their sessile state, plants are unavoidably affected by and respond to external biotic and abiotic stresses. So far, plants have to confront the stresses and develop diverse mechanisms of adaptation to avoid or tolerate their adverse effects so as to endure and to survive (Li et al., 2021). Soil salinization is one of the major environmental stressors that adversely impact plant growth and development in affected regions globally (Sunita et al., 2020). In order to adapt plant growth and salt stress tolerance, it is urgent to adopt new sustainable and environmental‐friendly agricultural practices. The application of halotolerant PGPRs is widely recognized as an effective biological strategy for mitigating the harmful effects of high salinity in plants. It is well documented that some salt‐tolerant bacteria and their extracellular polymers such as EPS can alleviate the adverse effects of salinity stress in plants by regulating plant cell physiological conditions (Sharma et al., 2016; Sun et al., 2022).
In the present study, we screened halotolerant rizhobacterium that produced a large amount of EPS, which were helpful to tolerate abiotic stress. EPS production is an important criterion for the classification of stress‐tolerant microbes. Bhagat et al. (2021) indicated that EPS promotes bacterial survival under stress conditions by providing carbon source and enhancing water retention. Therefore, EPS helps in the establishment of plant–bacteria interactions by providing a micro‐environment in which bacteria can survive under stress conditions (Bhagat et al., 2021). In the last few years, research showed that EPS production is highly beneficial for plants subjected to salt stress (Liu et al., 2022; Sun et al., 2022; Talebi Atouei et al., 2019). Microbial EPS act as a physical barrier in the soil protecting roots and promoting plant growth under high salinity conditions (Bhagat et al., 2021; Talebi Atouei et al., 2019). Besides, EPS production by bacteria in high salinity soil improves the quality, fertility, and stability of soil (Costa et al., 2018).
This study indicates that salinity significantly reduced shoot and root DWs of P. australis , which agrees with previously reported findings (Pagter et al., 2009). However, plants inoculated with Glutamicibacter sp. showed improved growth and hence better response than non‐inoculated plants under salt stress conditions. Similar results were reported for salt tolerance in various plants induced by EPS‐producing PGPR (Etesami & Beattie, 2017; Singh & Jha, 2017). Glutamicibacter species has been reported as an endophyte by various studies on tomato, potato, and maize (Afzal et al., 2019) and on halophytic species including the coastal halophytes Limonium sinense, Cakile maritima , Matthiola tricuspidata, and Crithmum maritimum (Christakis et al., 2021; Presta et al., 2016; Xiong et al., 2019). In contrast, several studies showed a co‐occurrence of Glutamicibacter species as endophytic and rhizospheric strain (Khan et al., 2022). This could be due to the possible colonization of endophytes originating from the rhizospheric soil and later the colonization of roots and aerial organs of the plants.
Our findings indicate that salinity increases the plant uptake of Na+ and Cl−. According to previous literature, the strong accumulation of toxic ions (Na+ and Cl−) disturbs water balance and ion homeostasis, which then impacts other major physiological processes, such as hormonal status, transpiration, photosynthesis, translocation of nutrients, and other metabolic processes (Munns, 2002; Sharma et al., 2016; Santander et al., 2019).
By contrast, maintenance of intracellular ion homeostasis, particularly K+ and Na+ homeostasis, is one of the most powerful mechanisms involved by PGPRs for plants adapting to saline conditions (Munns & Tester, 2008). Indeed, PGPRs contribute to restricting the uptake and transport of Na+ and Cl− from the root to shoot and maintain plant mineral status. The K+/Na+ ratio clearly decreased in salt‐treated plants due to the antagonism of Na+ and K+ at uptake sites in roots and the effect of Na+ on K+ transport into the xylem or inhibition of uptake processes (Hu & Schmidhalter, 2005). This is in agreement with a previous study pointing that inoculation with Glutamicibacter sp. may help plants to maintain ion homeostasis and high K+/Na+ ratio in shoots by reducing the uptake and/or transport of Na+ and Cl− to shoots and boosting the activity of high affinity K+ transporters (Xiong et al., 2019). Reduced Na+ concentration in P. australis stressed plants, due to Glutamicibacter sp. inoculation, may have also contributed to prevent accumulation of cellular Na+ to toxic levels. There are several reports on lower Na+ concentrations in plants inoculated with PGPR under salinity stress (Radhakrishnan & Baek, 2017; Sharma et al., 2016; Shukla et al., 2012; Singh & Jha, 2017; Upadhyay et al., 2011). In the present study, one may hypothesize that the excess of Na+ resulting from the increasing levels of salinity might have been retained in the soil of inoculated plants, likely in relationship with Glutamicibacter sp.‐produced EPS that bind cations, including Na+, thus decreasing the content of Na+ available for plant (Radhakrishnan & Baek, 2017). Hence, the reduction of Na+ concentration in the leaves of inoculated P. australis could be due to a lower percentage of exchangeable Na+ in the soil cultivated with such plants.
Inoculation with Glutamicibacter sp. showed a higher level of K+ accumulation in P. australis plants might be the additional reason in the alleviation of salt stress. Glutamicibacter sp. increased plant Ca+ uptake under saline conditions. Ca + is an essential second messenger that plays a vital role in salt signal transductions (Kader & Lindberg, 2010). Maintenance of high Ca+ levels is a potential mechanism to alleviate the damages caused by the high concentrations of salts in soil (Yang et al., 2016).
Salinity impairs leaf net CO2 assimilation rate by limiting photosynthesis, causing an over‐depletion of photosynthetic electron chain, and redirecting the photon energy into processes that favor the production of reactive oxygen species (ROS) (Hichem et al., 2009; Johnson et al., 2003), which are considered as the key inducers of the programmed cell death in plants (De Pinto et al., 2012). Previous studies showed that oxidative damages and ROS generation caused by soil salinity were reflected in MDA content of plant leaves (Yazici et al., 2007). Therefore, peroxidation of membrane lipids is a useful indicator of salt‐induced oxidative damages in membranes (Li et al., 2012). The present study revealed that growth reduction under saline condition in P. australis is associated with increased lipid peroxidation levels. However, there was a substantial reduction of the MDA content after plant inoculation with Glutamicibacter sp. suggesting that bacteria protects the plants from the imposed salt stress. In relationship to our findings, the lower level of lipid peroxidation is important in terms of salt tolerance as showed in different studies (de Azevedo Neto et al., 2006; Koca et al., 2007).
Polyphenol synthesis by plants is one of the adaptive mechanisms for reducing oxidative damages (Hichem et al., 2009; Nautiyal et al., 2008). In the present study, salinity significantly increased polyphenols' content in P. australis shoots, and bacterial inoculation also improved the amount of polyphenols. Polyphenols may eliminate radical species, thus preventing the propagation of oxidative chain reactions (Rice‐Evans et al., 1997). Other studies also reported that inoculation with bacteria increased the content of polyphenols in maize leaves regardless of salt concentration (Rojas‐Tapias et al., 2012). This strongly suggests that Glutamicibacter sp. improved P. australis tolerance through protecting plants from Na+ toxicity and oxidative stress induced by salinity. Potassium also plays a major role in plant salt stress tolerance, notably to minimize oxidative cell damages, at least in part by reducing ROS formation during photosynthesis and inhibiting activation of O2 −‐generating NADPH oxidase (Cakmak, 2005; Shen et al., 2000).
High salt concentrations in soil and water create a low osmotic potential, which reduces the availability of water to plants (Pérez‐Romero et al., 2020). Osmotic adjustment by accumulation of solutes in plant cells in response to decreasing water potential in their environment partly contributes to maintain turgor pressure under these challenging conditions (Blum et al., 1996). Proline and TSSs are useful biochemical indicators of salinity tolerance in plants (Ashraf & Harris, 2004). Our results show that leaf concentrations of proline and TSSs increased with application of salt stress. It is reported that proline protects higher plants against salt/osmotic stresses, not only by adjusting osmotic pressure but also by stabilizing the structure of proteins and scavenging ROS under salt stress (Ashraf & Foolad, 2007). Under salt stress, PGPR‐treated plants showed low proline content, but the level was higher than the basal level of proline in the control. This suggests that PGPR‐treated plants do not face much salt stress; therefore, the proline accumulation is less. Nadeem et al. (2007) and Rojas‐Tapias et al. (2012) similarly showed that plant proline contents are increased by saline stress, but decreased by inoculation with PGPR. Soluble sugars act as important osmolytes to maintain the cell homeostasis, where they constitute about 50% of the total osmotic potential in plant cells during salt stress (Gupta & Kaur, 2005); thus, increasing the TSS content could decrease osmotic potential in cells and maintain normal physiological function of plant cells in abiotic stress conditions (Bohnert & Shen, 1998). Similar to proline, the TSSs in inoculated plants are lower than in non‐inoculated P. australis plants.
In conclusion, our data further confirm that PGPR can play a pivotal role in conferring salt tolerance in plants. EPS produced by Glutamicibacter sp. under saline condition plays a significant role in alleviating salinity stress because it can chelate free Na+ from the soil and restrict Na+ entry into P. australis plants. Glutamicibacter sp. clearly improved plant salt tolerance through increasing plant antioxidative capacity and maintaining ion homeostasis, which strongly suggests that it may be useful to improve the growth of plants grown in saline alkaline soil.
AUTHOR CONTRIBUTIONS
Rabaa Hidri performed the experiments, analyzed data, and wrote the manuscript. Ouissal Metoui‐Ben Mahmoud, Chedly Abdelly, and Rozario Azcon conceived and designed the study. Walid Zorrig contributed in data analysis. Ahmed Debez revised the manuscript. All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
PEER REVIEW
The peer review history for this article is available in the Supporting Information for this article.
Supporting information
Figure S1. Morphological aspect of Phragmites australis plants, at the age of 127 days, exposed during the last 20 days of culture to two concentrations of NaCl (0 and 300 mM NaCl) (with and without bacterial inoculation).
Data S1. Peer Review.
ACKNOWLEDGMENTS
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR10CBBC02).
Hidri, R. , Metoui‐Ben Mahmoud, O. , Zorrig, W. , Azcon, R. , Abdelly, C. , & Debez, A. (2023). The halotolerant rizhobacterium Glutamicibacter sp. alleviates salt impact on Phragmites australis by producing exopolysaccharides and limiting plant sodium uptake. Plant Direct, 7(10), e535. 10.1002/pld3.535
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/supporting information; further inquiries can be directed to the corresponding author.
REFERENCES
- Afzal, I. , Shinwari, Z. K. , Sikandar, S. , & Shahzad, S. (2019). Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiological Research, 221, 36–49. 10.1016/j.micres.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Ashraf, M. , & Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59, 206–216. 10.1016/j.envexpbot.2005.12.006 [DOI] [Google Scholar]
- Ashraf, M. , & Harris, P. J. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Science, 166, 3–16. 10.1016/j.plantsci.2003.10.024 [DOI] [Google Scholar]
- Ashraf, M. , Hasnain, S. , Berge, O. , & Mahmood, T. (2004). Inoculating wheat seedlings with exopolysaccharide‐ producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biology and Fertility of Soils, 40, 157–162. [Google Scholar]
- Bano, A. , & Fatima, M. (2009). Salt tolerance in Zea mays (L.) following inoculation with rhizobium and pseudomonas . Biology and Fertility of Soils, 45, 405–413. 10.1007/s00374-008-0344-9 [DOI] [Google Scholar]
- Bates, L. S. , Waldren, R. P. , & Teare, I. D. (1973). Rapid determination of free proline for water‐stress studies. Plant and Soil, 39, 205–207. 10.1007/BF00018060 [DOI] [Google Scholar]
- Bhagat, N. , Raghav, M. , Dubey, S. , & Bedi, N. (2021). Bacterial exopolysaccharides: Insight into their role in plant abiotic stress tolerance. Journal of Microbiology and Biotechnology, 31(8), 1045–1059. 10.4014/jmb.2105.05009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum, A. , Munns, R. , Passioura, J. B. , & Turner, N. C. (1996). Genetically engineered plants resistant to soil dry and salt stress: How to interpret osmotic relations? Plant Physiology, 110, 1050–1053. 10.1104/pp.110.4.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert, H. J. , & Shen, B. (1998). Transformation and compatible solutes. Scientia Horticulturae, 78, 237–260. 10.1016/S0304-4238(98)00195-2 [DOI] [Google Scholar]
- Brix, H. , & Cizkova, H. (2001). Introduction: Phragmites‐dominated wetlands, their functions and sustainable use. Aquatic Botany, 2(69), 87–88. 10.1016/S0304-3770(01)00131-0 [DOI] [Google Scholar]
- Cakmak, I. (2005). The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science, 168(4), 521–530. 10.1002/jpln.200420485 [DOI] [Google Scholar]
- Chinnusamy, V. , Jagendorf, A. , & Zhu, J. K. (2005). Understanding and improving salt tolerance in plants. Crop Science, 45(2), 437–448. 10.2135/cropsci2005.0437 [DOI] [Google Scholar]
- Christakis, C. A. , Daskalogiannis, G. , Chatzaki, A. , Markakis, E. A. , Mermigka, G. , Sagia, A. , & Sarris, P. F. (2021). Endophytic bacterial isolates from halophytes demonstrate phytopathogen biocontrol and plant growth promotion under high salinity. Frontiers in Microbiology, 1001, 681567. 10.3389/fmicb.2021.681567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, O. Y. , Raaijmakers, J. M. , & Kuramae, E. E. (2018). Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Frontiers in Microbiology, 9, 1636. 10.3389/fmicb.2018.01636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azevedo Neto, A. D. , Prisco, J. T. , Enéas‐Filho, J. , de Abreu, C. E. B. , & Gomes‐Filho, E. (2006). Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt‐tolerant and salt‐sensitive maize genotypes. Environmental and Experimental Botany, 56(1), 87–94. 10.1016/j.envexpbot.2005.01.008 [DOI] [Google Scholar]
- De Pinto, M. C. , Locato, V. , & De Gara, L. (2012). Redox regulation in plant programmed cell death. Plant, Cell & Environment, 35, 234–244. 10.1111/j.1365-3040.2011.02387.x [DOI] [PubMed] [Google Scholar]
- Dewanto, V. , Wu, X. , Adom, K. K. , & Liu, R. H. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry, 50(10), 3010–3014. 10.1021/jf0115589 [DOI] [PubMed] [Google Scholar]
- Dodd, I. C. , & Perez‐Alfocea, F. (2012). Microbial amelioration of crop salinity stress. Journal of Experimental Botany, 63, 3415–3428. 10.1093/jxb/ers033 [DOI] [PubMed] [Google Scholar]
- Dueñas, M. , Munduate, A. , Perea, A. , & Irastorza, A. (2003). Exopolysaccharide production by Pediococcus damnosus 2.6 in a semidefined medium under different growth conditions. International Journal of Food Microbiology, 87(1–2), 113–120. 10.1016/S0168-1605(03)00060-6 [DOI] [PubMed] [Google Scholar]
- Egamberdieva, D. , Wirth, S. , Bellingrath‐Kimura, S. D. , Mishra, J. , & Arora, N. K. (2019). Salt‐tolerant plant growth promoting rhizobacteria for enhancing crop productivity of saline soils. Frontiers in Microbiology, 10, 2791. 10.3389/fmicb.2019.02791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller, F. , Skálová, H. , Caplan, J. S. , Bhattarai, G. P. , Burger, M. K. , Cronin, J. T. , & Brix, H. (2017). Cosmopolitan species as models for ecophysiological responses to global change: The common reed Phragmites australis . Frontiers in Plant Science, 8, 1833. 10.3389/fpls.2017.01833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etesami, H. , & Beattie, G. A. (2017). Plant‐microbe interactions in adaptation of agricultural crops to abiotic stress conditions. In Probiotics and plant health (pp. 163–200). Springer. 10.1007/978-981-10-3473-2_7 [DOI] [Google Scholar]
- Etesami, H. , & Glick, B. R. (2020). Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environmental and Experimental Botany, 178, 104–124. [Google Scholar]
- Flowers, T. J. , & Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytologist, 179, 945–963. 10.1111/j.1469-8137.2008.02531.x [DOI] [PubMed] [Google Scholar]
- Gorai, M. , Ennajeh, M. , Khemira, H. , & Neffati, M. (2010). Combined effect of NaCl‐salinity and hypoxia on growth, photosynthesis, water relations and solute accumulation in Phragmites australis plants. Flora, 205(7), 462–470. 10.1016/j.flora.2009.12.021 [DOI] [Google Scholar]
- Grattan, S. R. , & Grieve, C. M. (1998). Salinity–mineral nutrient relations in horticultural crops. Scientia Horticulturae, 78(1–4), 127–157. 10.1016/S0304-4238(98)00192-7 [DOI] [Google Scholar]
- Gupta, A. K. , & Kaur, N. (2005). Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. The Journal of Biosciences, 30(5), 761–776. 10.1007/BF02703574 [DOI] [PubMed] [Google Scholar]
- Halliwell, B. , & Gutteridge, J. (1989). Free radicals in biology and medicine (2nd ed.). Clarendon Press. [Google Scholar]
- Hichem, H. , Mounir, D. , & Naceur, E. A. (2009). Differential responses of two maize (Zea mays L) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Industrial Crops and Products, 30, 144–151. 10.1016/j.indcrop.2009.03.003 [DOI] [Google Scholar]
- Hu, Y. , & Schmidhalter, U. (2005). Drought and salinity: A comparison of their effects on mineral nutrition of plants. Journal of Plant Nutrition and Soil Science, 168, 541–549. 10.1002/jpln.200420516 [DOI] [Google Scholar]
- Jha, Y. , Subramanian, R. B. , & Patel, S. (2011). Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiologiae Plantarum, 33, 797–802. 10.1007/s11738-010-0604-9 [DOI] [Google Scholar]
- Johnson, S. M. , Doherty, S. J. , & Croy, R. R. D. (2003). Biphasic superoxide generation in potato tubers a self‐amplifying response to stress. Plant Physiology, 131, 1440–1449. 10.1104/pp.013300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader, M. A. , & Lindberg, S. (2010). Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signaling & Behavior, 5(3), 233–238. 10.4161/psb.5.3.10740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlidag, H. , Yildirim, E. , Turan, M. , Pehluvan, M. , & Donmez, F. (2013). Plant growth promoting rhizobacteria alleviate deleterious effects of salt stress on strawberry plants (Fragaria ananassa). Horticultural Science, 48(5), 563–567. [Google Scholar]
- Kazy, S. K. , Sar, P. , Singh, S. P. , Sen, A. K. , & Souza, S. F. D. (2002). Extracellular polysaccharides of a copper‐sensitive and a copper‐resistant Pseudomonas aeruginosa strain: Synthesis, chemical nature and copper binding. World Journal of Microbiology and Biotechnology, 18, 583–588. 10.1023/A:1016354713289 [DOI] [Google Scholar]
- Khan, T. , Alzahrani, O. M. , Sohail, M. , Hasan, K. A. , Gulzar, S. , Rehman, A. U. , Mahmoud, S. F. , Alswat, A. S. , & Abdel‐Gawad, S. A. (2022). Enzyme profiling and identification of endophytic and rhizospheric bacteria isolated from Arthrocnemum macrostachyum . Microorganisms, 10(11), 2112. 10.3390/microorganisms10112112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, I. A. , Wilson, B. E. , Gill, M. , Aviles, L. , Cronin, J. T. , Nyman, J. A. , & Diaz, R. (2018). Invasion of Nipponaclerda biwakoensis (Hemiptera: Aclerdidae) and Phragmites australis die‐back in southern Louisiana, USA. Biological Invasions, 20(10), 2739–2744. 10.1007/s10530-018-1749-5 [DOI] [Google Scholar]
- Koca, H. , Bor, M. , Özdemir, F. , & Türkan, İ. (2007). The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environmental and Experimental Botany, 60(3), 344–351. 10.1016/j.envexpbot.2006.12.005 [DOI] [Google Scholar]
- Kohler, J. , Hernández, J. A. , Caravaca, F. , & Roldán, A. (2009). Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environmental and Experimental Botany, 65, 245–252. 10.1016/j.envexpbot.2008.09.008 [DOI] [Google Scholar]
- Kumar, A. , Singh, S. , Gaurav, A. K. , Srivastava, S. , & Verma, J. P. (2020). Plant growth‐promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Frontiers in Microbiology, 11, 1216. 10.3389/fmicb.2020.01216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A. , Singh, V. K. , Tripathi, V. , Singh, P. P. , & Singh, A. K. (2018). Plant growth‐promoting rhizobacteria (PGPR): Perspective in agriculture under biotic and abiotic stress. Crop Improvement through Microbial Biotechnology, 333–342. 10.1016/B978-0-444-63987-5.00016-5 [DOI] [Google Scholar]
- Li, L. , Sinkko, H. , Montonen, L. , Wei, G. , Lindström, K. , & Räsänen, L. A. (2012). Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiology Immunology, 79, 46–68. 10.1111/j.1574-6941.2011.01198.x [DOI] [PubMed] [Google Scholar]
- Li, P. S. , Kong, W. L. , & Wu, X. Q. (2021). Salt tolerance mechanism of the rhizosphere bacterium JZ‐GX1 and its effects on tomato seed germination and seedling growth. Frontiers in Microbiology, 12, 1342. 10.3389/fmicb.2021.657238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Pei, C. , Liu, S. , Guo, X. , Du, N. , & Guo, W. (2018). Genetic and epigenetic changes during the invasion of a cosmopolitan species (Phragmites australis). Ecology and Evolution, 8(13), 6615–6624. 10.1002/ece3.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Chai, J. , Zhang, Y. , Zhang, C. , Lei, Y. , Li, Q. , & Yao, T. (2022). Halotolerant rhizobacteria mitigate the effects of salinity stress on maize growth by secreting exopolysaccharides. Environmental and Experimental Botany, 204, 105098. 10.1016/j.envexpbot.2022.105098 [DOI] [Google Scholar]
- Meyerson, L. A. , Saltonstall, K. , Chambers, R. M. , Silliman, B. R. , Bertness, M. D. , & Strong, D. (2009). Phragmites australis in eastern North America: A historical and ecological perspective. Salt Marshes under Global Sieg, 57–82. [Google Scholar]
- Munns, R. (2002). Comparative physiology of salt and water stress. Plant, Cell & Environment, 25(2), 239–250. 10.1046/j.0016-8025.2001.00808.x [DOI] [PubMed] [Google Scholar]
- Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytologist, 167, 645–663. 10.1111/j.1469-8137.2005.01487.x [DOI] [PubMed] [Google Scholar]
- Munns, R. , & Gilliham, M. (2015). Salinity tolerance of crops—What is the cost? New Phytologist, 208(3), 668–673. 10.1111/nph.13519 [DOI] [PubMed] [Google Scholar]
- Munns, R. , James, R. A. , & Läuchli, A. (2006). Approaches to increasing the salt tolerance of wheat and other cereals. Journal of Experimental Botany, 57(5), 1025–1043. 10.1093/jxb/erj100 [DOI] [PubMed] [Google Scholar]
- Munns, R. , & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Nadeem, S. M. , Ahmad, Z. Z. , Naveed, M. , & Arshad, M. (2007). Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Canadian Journal of Microbiology, 53, 1141–1149. 10.1139/W07-081 [DOI] [PubMed] [Google Scholar]
- Nautiyal, C. S. , Govindarajan, R. , Lavania, M. , & Pushpangadan, P. (2008). Novel mechanism of modulating natural antioxidants in functional foods: Involvement of plant growth promoting rhizobacteria NRRL B‐30488. Journal of Agricultural and Food Chemistry, 56, 4474–4481. 10.1021/jf073258i [DOI] [PubMed] [Google Scholar]
- Nunkaew, T. , Kantachote, D. , Nitoda, T. , Kanzaki, H. , & Ritchie, R. J. (2015). Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydrate Polymers, 15, 334–341. 10.1016/j.carbpol.2014.08.099 [DOI] [PubMed] [Google Scholar]
- Pagter, M. , Bragato, C. , Malagoli, M. , & Brix, H. (2009). Osmotic and ionic effects of NaCl and Na2SO4 salinity on Phragmites australis . Aquatic Botany, 90(1), 43–51. 10.1016/j.aquabot.2008.05.005 [DOI] [Google Scholar]
- Pérez‐Romero, J. A. , Mateos‐Naranjo, E. , López‐Jurado, J. , Redondo‐Gómez, S. , & Torres‐Ruiz, J. M. (2020). Importance of physiological traits vulnerability in determine halophytes tolerance to salinity excess: A comparative assessment in Atriplex halimus . Plants, 9(6), 690. 10.3390/plants9060690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospiech, A. , & Neumann, B. (1995). A versatile quick‐prep of genomic DNA from gram‐positive bacteria. Trends in Genetics, 11, 217–218. 10.1016/S0168-9525(00)89052-6 [DOI] [PubMed] [Google Scholar]
- Presta, L. , Fondi, M. , Perrin, E. , Maida, I. , Miceli, E. , Chiellini, C. , Maggini, V. , Bogani, P. , Di Pilato, V. , Rossolini, G. M. , Mengoni, A. , & Fani, R. (2016). Arthrobacter sp. EpRS66 and Arthrobacter sp. EpRS71: Draft genome sequences from two bacteria isolated from Echinacea purpurea rhizospheric soil. Frontiers in Microbiology, 7, 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin, Y. , Druzhinina, I. S. , Pan, X. , & Yuan, Z. (2016). Microbially mediated plant salt tolerance and microbiome‐based solutions for saline agriculture. Biotechnology Advances, 34, 1245–1259. 10.1016/j.biotechadv.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan, R. , & Baek, K. H. (2017). Physiological and biochemical perspectives of non‐salt tolerant plants during bacterial interaction against soil salinity. Plant Physiology and Biochemistry, 116, 116–126. 10.1016/j.plaphy.2017.05.009 [DOI] [PubMed] [Google Scholar]
- Rice‐Evans, C. , Miller, N. , & Paganga, G. (1997). Antioxidant properties of phenolic compounds. Trends in Plant Science, 2, 152–159. 10.1016/S1360-1385(97)01018-2 [DOI] [Google Scholar]
- Rodríguez, M. , & Brisson, J. (2015). Pollutant removal efficiency of native versus exotic common reed (Phragmites australis) in North American treatment wetlands. Ecological Engineering, 74, 364–370. 10.1016/j.ecoleng.2014.11.005 [DOI] [Google Scholar]
- Rojas‐Tapias, D. , Moreno‐Galván, A. , Pardo‐Díaz, S. , Obando, M. , Rivera, D. , & Bonilla, R. (2012). Effect of inoculation with plant growth‐promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Applied Soil Ecology, 61, 264–272. 10.1016/j.apsoil.2012.01.006 [DOI] [Google Scholar]
- Santander, C. , Sanhueza, M. , Olave, J. , Borie, F. , Valentine, A. , & Cornejo, P. (2019). Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. Journal of Plant Nutrition and Soil Science, 19, 321–331. 10.1007/s42729-019-00032-z [DOI] [Google Scholar]
- Sarkar, A. , Ghosh, P. K. , Pramanik, K. , Mitra, S. , Soren, T. , Pandey, S. , Mondal, M. H. , & Maiti, T. K. (2018). A halotolerant Enterobacter sp displaying ACC deaminase activity promotes rice seedling growth under salt stress. Research in Microbiology, 169, 20–32. 10.1016/j.resmic.2017.08.005 [DOI] [PubMed] [Google Scholar]
- Shabala, S. (2013). Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany, 112, 1209–1221. 10.1093/aob/mct205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Kulkarni, J. , & Jha, B. (2016). Halotolerant rhizobacteria promote growth and enhance salinity tolerance in peanut. Frontiers in Microbiology, 7, 1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, W. Y. , Nada, K. , & Tachibana, S. (2000). Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiology, 124, 431–439. 10.1104/pp.124.1.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, P. S. , Agarwal, P. K. , & Jha, B. (2012). Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant‐growth‐promoting rhizobacteria. Journal of Plant Growth Regulation, 31, 195–206. 10.1007/s00344-011-9231-y [DOI] [Google Scholar]
- Singh, R. P. , & Jha, P. N. (2017). The PGPR Stenotrophomonas maltophilia SBP‐9 augments resistance against biotic and abiotic stress in wheat plants. Frontiers in Microbiology, 8, 1945. 10.3389/fmicb.2017.01945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, V. L. , & Rosi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158. 10.5344/ajev.1965.16.3.144 [DOI] [Google Scholar]
- Sun, L. , Cheng, L. , Ma, Y. , Lei, P. , Wang, R. , Gu, Y. , Li, S. , Zhang, F. , & Xu, H. (2022). Exopolysaccharides from Pantoea alhagi NX‐11 specifically improve its root colonization and rice salt resistance. International Journal of Biological Macromolecules, 209, 396–404. 10.1016/j.ijbiomac.2022.04.015 [DOI] [PubMed] [Google Scholar]
- Sunita, K. , Mishra, I. , Mishra, J. , Prakash, J. , & Arora, N. K. (2020). Secondary metabolites from halotolerant plant growth promoting rhizobacteria for ameliorating salinity stress in plants. Frontiers in Microbiology, 11, 567768. 10.3389/fmicb.2020.567768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi Atouei, M. , Pourbabaee, A. A. , & Shorafa, M. (2019). Alleviation of salinity stress on some growth parameters of wheat by exopolysaccharide‐producing bacteria. Iranian Journal of Science and Technology, Transactions a: Science, 43, 2725–2733. 10.1007/s40995-019-00753-x [DOI] [Google Scholar]
- Ullah, A. , Heng, S. , Munis, M. F. H. , Fahad, S. , & Yang, X. (2015). Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria. Environmental and Experimental Botany, 117, 28–40. 10.1016/j.envexpbot.2015.05.001 [DOI] [Google Scholar]
- Upadhyay, S. K. , Singh, J. S. , & Singh, D. P. (2011). Exopolysaccharide‐producing plant growth‐promoting rhizobacteria under salinity condition. Pedosphere, 21, 214–222. 10.1016/S1002-0160(11)60120-3 [DOI] [Google Scholar]
- Weisburg, W. G. , Barns, S. M. , Pelletier, D. A. , & Lane, D. J. (1991). 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 173, 697–703. 10.1128/jb.173.2.697-703.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Y. W. , Gong, Y. , Li, X. W. , Chen, P. , Ju, X. Y. , Zhang, C. M. , Yuan, B. , Lv, Z. P. , Xing, K. , & Qin, S. (2019). Enhancement of growth and salt tolerance of tomato seedlings by a natural halotolerant actinobacterium Glutamicibacter halophytocola KLBMP 5180 isolated from a coastal halophyte. Plant and Soil, 445, 307–322. 10.1007/s11104-019-04310-8 [DOI] [Google Scholar]
- Yang, H. , Hu, J. , Long, X. , Liu, Z. , & Rengel, Z. (2016). Salinity altered root distribution and increased diversity of bacterial communities in the rhizosphere soil of Jerusalem artichoke . Scientific Reports, 6(1), 1–10. 10.1038/srep20687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici, I. , Türkan, I. , Sekmen, A. H. , & Demiral, T. (2007). Salinity tolerance of purslane (Portulaca oleracea L) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environmental and Experimental Botany, 61, 49–57. 10.1016/j.envexpbot.2007.02.010 [DOI] [Google Scholar]
- Yemm, E. W. , & Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. Journal of Biochemistry, 57, 508–514. 10.1042/bj0570508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Qian, Y. , Fan, D. , Tian, Y. , & Huang, X. (2022). Biofilm formed by Hansschlegelia zhihuaiae S113 on root surface mitigates the toxicity of bensulfuron‐methyl residues to maize. Environmental Pollution, 292, 118366. 10.1016/j.envpol.2021.118366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Morphological aspect of Phragmites australis plants, at the age of 127 days, exposed during the last 20 days of culture to two concentrations of NaCl (0 and 300 mM NaCl) (with and without bacterial inoculation).
Data S1. Peer Review.
Data Availability Statement
The original contributions presented in the study are included in the article/supporting information; further inquiries can be directed to the corresponding author.


