Abstract
Background
Negative symptoms of schizophrenia have recently been proposed to result from a decoupling of (intact) hedonic experience and (diminished) approach behavior. The current study challenged this view by exploring the hypothesis that negative symptoms are driven by a specific type of emotional experience abnormality, a reduction in the positivity offset (i.e. the tendency to experience greater levels of positive relative to negative emotion in low-arousal contexts), which limits the production of approach behaviors in neutral environments.
Methods
Participants included outpatients with SZ (n = 44) and healthy controls (CN: n = 48) who completed one week of active (ecological momentary assessment surveys of emotional experience and symptoms) and passive (geolocation, accelerometry) digital phenotyping. Mathematical modeling approaches from Cacioppo's Evaluative Space Model were used to quantify the positivity offset in daily life. Negative symptoms were assessed via standard clinical ratings, as well as active (EMA surveys) and passive (geolocation, accelerometry) digital phenotyping measures.
Results
Results indicated that the positivity offset was reduced in SZ and associated with more severe anhedonia and avolition measured via clinical interviews and active and passive digital phenotyping.
Conclusions
These findings suggest that current conceptual models of negative symptoms, which assume hedonic normality, may need to be revised to account for reductions in the positivity offset and its connection to diminished motivated behavior. Findings identify key real-world contexts where negative symptoms could be targeted using psychosocial treatments.
Key words: Ecological momentary assessment, emotional experience, hedonic capacity, negative symptoms, psychosis
Introduction
Deficits in motivation and pleasure have been considered core features of schizophrenia (SZ) since its initial conceptualization (Bleuler, 1911; Diefendorf & Kraepelin, 1907; Kraepelin, 1921). In psychiatrically healthy individuals, these processes are reciprocally connected, with motivational deficits leading to less frequent pleasurable experiences and hedonic deficits leading to reduced motivation for seeking out these experiences (Bradley & Lang, 2007). In contrast, emotional experience and behavior are decoupled in SZ, such that intact hedonic capacity fails to translate into volitional responding (Heerey & Gold, 2007). To explain this discrepancy, several conceptual models posit that negative symptoms result from dysfunctional cortico-striatal circuitry (Barch & Dowd, 2010; Kring & Barch, 2014; Strauss, Waltz, & Gold, 2013). Implicit among these models is the assumption that hedonic capacity is intact and deficits in multiple aspects of reward processing (e.g. reinforcement learning, effort-cost computation, value representation) that impact decision-making prevent intact hedonic responses from motivating approach behaviors. Although these models have been vital for our understanding of negative symptoms, they have not led to significant treatment breakthroughs, suggesting that our current mechanistic understanding is incomplete. One limitation of current models may be that the assumption of hedonic normality in SZ is premature, leading to a failure to adequately consider the role of emotional experience abnormalities in negative symptoms.
Caccioppo's seminal Evaluative Space Model (Cacioppo, 1999; Cacioppo & Berntson, 1994) presents a novel approach to understanding how emotional responses fail to generate motivated behavior in SZ. The ESM proposes that separate positive and negative evaluation systems evolved to guide motivated behavior. Both systems are characterized by an activation function representing the relationship between affective input into the system (i.e. arousal) and the resulting output (i.e. emotional response). Positive and negative activation functions are differentially calibrated to translate emotional responses from the affective system into adaptive motivated behaviors in specific contexts. The positive system is calibrated to respond with greater amounts of positive relative to negative emotion at lower levels of affective input, a function known as the ‘positivity offset.’ As affective input into the system increases, the negative system is calibrated to respond with greater levels of negative than positive emotion, a function termed the ‘negativity bias.’ (Cacioppo, 1999; Cacioppo & Berntson, 1994; Larsen, McGraw, & Cacioppo, 2001; Larsen, Norris, McGraw, Hawkley, & Cacioppo, 2009; Norris, Gollan, Berntson, & Cacioppo, 2010). Healthy individuals typically experience a greater balance of positive than negative emotion in most situations, which tend to be neutral and characterized by minimal affective input. The positivity offset is adaptive because it promotes exploratory behavior and approach of novel stimuli in neutral environments that allows for the acquisition of new rewards and resources. The negativity bias is adaptive because it leads to withdrawal behavior at high levels of arousal that are characteristic of highly negative or dangerous environments.
Using mathematical approaches from the ESM, Strauss, Visser, Lee, and Gold (2017) used a laboratory-based paradigm to compare the positivity offset and negativity bias in adults with SZ and controls (CN). Participants made unipolar reports of positivity, negativity, and arousal in response to pleasant, unpleasant, and neutral images from the International Affective Picture System (IAPS) (Lang, Bradley, & Cuthbert, 1997). Following methods from Ito and Cacioppo (2005), two separate regression equations were used to quantify parameters used to calculate the positivity offset and negativity bias. The predictor in these equations represents the affective input into the evaluative system (i.e. self-reported arousal). The dependent variable is the resulting output from the affective system (i.e. self-reported levels of positivity or negativity). From these equations, the intercepts and slopes for the positivity and negativity functions were used to calculate the positivity offset and negativity bias, where a greater intercept for positivity relative to negativity reflects the prototypical positivity offset and a greater slope for negativity reflects the negativity bias. Results indicated that although individuals with SZ displayed hedonic normality, as reported in dozens of other studies (i.e. comparable positive emotion and arousal to pleasant stimuli between SZ and CN) (Cohen & Minor, 2010; Llerena, Strauss, & Cohen, 2012), emotional experience abnormalities were present in SZ and associated with clinically rated anhedonia. Specifically, individuals with SZ displayed a reduction in the positivity offset compared to CN, as indicated by lower intercept values for positive emotion and reductions in the positive/negative intercept difference score. Lower difference scores also predicted higher ratings of anhedonia. Importantly, the diminished positivity offset existed in the presence of intact hedonic capacity, as indicated by greater slope for positive emotion in SZ than CN (i.e. at highest levels of arousal when stimuli are most motivationally significant, SZ produce comparably greater positive emotional responses than CN). Riehle, Pillny, and Lincoln (2022) replicated these findings in an online community sample of individuals varying in trait psychotic-like experiences and anhedonia. Collectively, findings from these laboratory-based studies provide a novel explanation for deficits in motivated behavior among individuals with SZ-spectrum symptoms despite intact hedonic capacity. However, the link between the diminished positivity offset and reductions in motivated behavior has only been inferred and not yet demonstrated empirically. To fully test the hypothesis that reductions in approach behaviors are associated with reductions in the positivity offset (despite intact hedonic capacity), it is necessary to examine emotional experience and motivated behavior during daily life.
The current study used active and passive digital phenotyping to determine whether the positivity offset is reduced in daily life and associated with greater self-reported and objectively quantified measures of anhedonia and avolition. Active digital phenotyping refers to measurements collected via mobile devices in the real-world that are purposefully triggered by the participant (e.g. surveys) (Onnela & Rauch, 2016), whereas passive digital phenotyping involves unobtrusively collecting data via sensors within a mobile device (e.g. geolocation, accelerometry) (Onnela & Rauch, 2016). Preliminary psychometric studies support the reliability and validity of active and passive digital phenotyping measures of negative symptoms in SZ, as well as their feasibility and tolerability (Depp et al., 2019; Fulford et al., 2021; Granholm et al., 2019; Harvey et al., 2021; Miller, Raugh, Strauss, & Harvey, 2022; Narkhede et al., 2021; Raugh et al., 2020; Raugh et al., 2021; Strauss et al., 2022). When used in tandem, active and passive digital phenotyping methods offer promise for exploring questions regarding the nature of emotion-motivation interactions in SZ since the same computational approaches validated for the ESM can be used in conjunction with objectively measured and self-reported behaviors.
The following hypotheses were made: (1) Based on prior laboratory-based findings (Strauss et al., 2017), participants with SZ will demonstrate a reduced positivity offset compared to CN on measures of active digital phenotyping (EMA surveys) collected in daily life; (2) Hedonic capacity measured via active digital phenotyping will be intact or elevated in SZ based on prior evidence for an increased slope for positivity relative to CN (Strauss et al., 2017); (3) Consistent with findings from Strauss et al. (2017), the negativity bias, measured via active digital phenotyping, will be intact in SZ; (4) Reductions in the active digital phenotyping-derived positivity offset difference score, but not the negativity bias, will be significantly associated with anhedonia and avolition measured via clinical rating scales, active digital phenotyping measures of negative symptoms in daily life, active digital phenotyping measures of the frequency of positive emotional experiences, and passive digital phenotyping measures of behavior obtained via geolocation and accelerometry.
Method
Participants
Forty-six individuals with DSM-5 (American Psychiatric Association, 2013) diagnoses of schizophrenia or schizoaffective disorder (SZ) and 50 psychiatrically healthy controls (CN) participated in the study. Two SZ and 2 CN participants were excluded for not reaching a priori digital phenotyping compliance standards (i.e. responding to < 20% of momentary surveys), resulting in a final sample of 44 SZ (16 with schizophrenia and 28 with schizoaffective disorder) and 48 CN. Groups did not significantly differ on age, sex, ethnicity, or parental education. SZ had lower personal education and momentary survey adherence rates than CN. Moderately severe symptoms and a typical magnitude of cognitive impairment were observed in SZ (see Table 1).
Table 1.
Participant demographic and clinical characteristics
| SZ (n = 44) | CN (n = 48) | Test statistic | p value | |
|---|---|---|---|---|
| Age | 39.34 (12.02) | 38.56 (10.50) | F = 0.11 | 0.74 |
| Parental education | 13.98 (2.83) | 13.51 (2.86) | F = 0.58 | 0.40 |
| Personal education | 13.02 (2.27) | 15.73 (2.82) | F = 25.46 | < 0.001 |
| Female (%) | 63.60 | 72.90 | χ2 = 0.92 | 0.34 |
| Race (%) | χ2 = 7.19 | 0.21 | ||
| Black | 29.50 | 22.90 | ||
| Asian | 0.00 | 6.30 | ||
| Latinx | 4.50 | 12.50 | ||
| White | 59.10 | 47.90 | ||
| Multiracial | 6.80 | 6.30 | ||
| Other | 0.00 | 4.20 | ||
| MCCB | 41.91 (15.74) | 52.40 (10.19) | F = 13.90 | < 0.001 |
| BNSS total | 16.23 (13.66) | – | ||
| BNSS avolition | 2.10 (1.68) | – | ||
| BNSS anhedonia | 1.51 (1.51) | – | ||
| BNSS asociality | 1.50 (1.31) | – | ||
| BNSS alogia | 0.30 (0.91) | – | ||
| BNSS blunted affect | 1.16 (1.52) | – | ||
| PANSS positive | 2.57 (0.85) | – | ||
| PANSS negative | 1.89 (0.84) | – | ||
| PANSS general | 2.11 (0.51) | – | ||
| Survey adherence rate | 0.60 (0.23) | 0.71 (0.20) | F = 5.84 | 0.02 |
Note. SZ, schizophrenia group; CN, control group. MCCB, MATRICS Consensus Cognitive Battery; BNSS, Brief Negative Symptom Scale; PANSS, Positive and Negative Syndrome Scale. BNSS and PANSS domain scores reflect average of item scores within each domain.
Individuals with SZ were recruited from local community outpatient mental health centers and advertisements. Clinical diagnoses were determined via the SCID-5 (First, 2015). CN were recruited from the local community using advertisements. CN were free of current major psychiatric diagnoses as established via the SCID-5, current SZ-spectrum personality disorders as established via the SCID-PD (First, Williams, Benjamin, & Spitzer, 2015), family history of psychosis, and psychotropic medications. All participants denied lifetime neurological disease and did not meet criteria for a substance abuse disorder within the last 6 months (excluding nicotine use disorders). Participants received monetary compensation for their participation and provided written informed consent for a protocol approved by the University of Georgia Institutional Review Board. Participants were compensated $20 per hour for laboratory sessions, $1 per mobile survey completed, and $80 for returning the phone at the end of the study.
Procedure
The study consisted of three phases: (1) initial laboratory visit; (2) six consecutive days of digital phenotyping; and (3) final laboratory visit.
Phase 1: Initial laboratory visit
Clinical interviews were conducted to assess diagnoses and symptoms. Diagnostic and symptom interviews for SZ consisted of the SCID-5 (First, 2015) and Brief Negative Symptom Scale (BNSS; Kirkpatrick et al., 2011). CN interviews included the SCID-5 (First, 2015), and SCID-PD (First et al., 2015). All interviews were conducted by either Dr Strauss or lab personnel trained to reliability standards (inter-rater reliability of alpha > 0.80) who established consensus for diagnoses.
Participants were trained on digital phenotyping procedures and provided with a Blu Vivo 5R smartphone running Android operating system 7.0 programmed with the mEMA app from ilumivu to collect digital phenotyping data. Trained lab personnel instructed participants in the use of the phone and the mobile app, including a guided demonstration of survey notifications and a practice survey that provided an overview and explanation of the types of questions that would be asked. Participants were also trained on how to use and charge the Empatica wristband.
Phase 2: Digital phenotyping
Active Digital Phenotyping. Over the 6-day digital phenotyping phase, participants were prompted with eight momentary surveys per day that were quasi-randomly scheduled within 90-minute epochs between 9 AM and 9 PM. Surveys were scheduled between 18 min to 3 h apart from each other. Attempts to respond to the survey after a 15-min window were not permitted, but participants were allotted unlimited time to complete the questions. Surveys assessed the following:
Momentary Emotional Experience. Every survey probed current levels of positive and negative emotion using the modified Differential Emotions Scale (mDES; Fredrickson, Tugade, Waugh, and Larkin, 2003). Each prompt assessed five negative (anger, fear, sadness, shame, anxiety) and five positive emotions (amused, content, happy, love, pride) rated on a 0–100 sliding scale anchored between ‘Not at all’ and ‘Extremely.’ Participants also identified whether their current emotional context was positive, negative, neutral, or mixed. Context responses were used to determine the frequency of positive experiences endorsed over the digital phenotyping period.
Momentary Emotional Arousal. Every survey probed current emotional arousal by asking ‘How keyed-up or excited are you right now?’. Participants rated arousal on a 0–100 sliding scale anchored between ‘Not at all’ and ‘Extremely.’
Negative Symptoms. Momentary surveys probed for negative symptoms of anhedonia, avolition, and asociality. Anhedonia was measured by averaging across momentary responses for consummatory (i.e. ‘How much are you enjoying the activity?’ and ‘How much are you enjoying this social interaction?’) and anticipatory pleasure (i.e. ‘How much do you think you will enjoy that activity the next time you do it?’ and ‘How much do you think you will enjoy interacting with them next time?’). Avolition was measured by assessing participants' level of interest in a current activity (i.e. ‘How interested are you in the activity?’). If participants reported they were not engaged in an activity (i.e. doing ‘Nothing.’), avolition was measured via desire to engage in an activity (i.e. ‘How much do you want to be doing an activity right now?’). Lastly, asociality was measured by assessing participants' interest in a social interaction (i.e. ‘How interested are you in this social interaction?’). If participants denied interacting with anyone, asociality was measured via responses about their desire to interact with others (i.e. ‘How much do you want to be interacting with someone right now?’). All items were rated on a 0 (not at all) to 100 (extremely) scale. These items have shown convergent validity via associations with clinical ratings of the same domains on the BNSS; confirmatory factor analysis also indicates that the items constitute three separate factors: anhedonia, avolition, asociality (Raugh et al., 2020).
Infrequent Responding. To monitor infrequent responding, a question from the Chapman Anhedonia Scale (Eckblad, Chapman, Chapman, & Mishlove, 1982) was embedded within each momentary survey. Participants responded ‘True’ or ‘False’ to the items, which portrayed common, every day experiences (e.g. ‘Sometimes when walking down the sidewalk, I have seen children playing.’; ‘I cannot remember a time when I talked with someone who wore glasses.’). The rate of infrequent responding was low (< 7%) in both groups.
Passive Digital Phenotyping. Geolocation was passively measured throughout the digital phenotyping phase via the smartphone. Geolocation involves collecting GPS coordinates at predetermined intervals or every time the participant moves a certain radius in a space. Phone sensors were programmed to collect geolocation every 10 min or when participants moved more than 10 m. To index change in geolocation, distance from home and percentage of time at home were extracted as the primary variables of interest given face validity as measures of goal-directed exploratory behavior and the growing use of these variables in prior studies. Secondary geolocation variables were calculated for exploratory purposes (i.e. number of locations, location variance, time spent in different locations, number of flights, transition time) that were previously validated in relation to negative symptoms (Granholm et al., 2019; Raugh et al., 2020).
Using the mEMA application, sensors within the study phones were programmed to measure accelerometry with each change in XYZ coordinate motion (every change in accelerometry being logged as a single instance), with separate values output for X, Y, and Z movement axes. Participants wore an Empatica wristband that collected accelerometry as a gravitational force (g units) at 32 Hz between −16 g and 16 g. Accelerometry has shown convergent validity with clinically rated negative symptoms (Strauss et al., 2022). See Table 2 for information on geolocation and acceleration variables and Table 3 for descriptive statistics and group comparisons.
Table 2.
Geolocation and accelerometry variable definitions
| GPS or ACL | Variable | Abbreviation | Definition |
|---|---|---|---|
| GPS | Home time | Home | Amount of time at home |
| GPS | Distance change | Δd MPD Range |
Distance traveled in meters from previous sample Total meters traveled per day Range of distance per day |
| GPS | Distance from home | Δmh maxmh |
Meters from home for each sample Maximum distance from home per day |
| GPS | Stationary location clusters | nc | Number of meaningful locations |
| GPS | Location variance | lv | Variance within a specified timeframe: lv = log[(σ2 lat + 1) + (σ2 long + 1)] |
| GPS | Entropy and normalized entropy | ent nent |
Equity of time spent in different locations |
| GPS | Transition time | tt | Percentage of samples taken in transit |
| GPS | Flights | f.dur f.dist f.num |
Average duration of discrete trips per day Average distance of discrete trips per day Average number of discrete trips per day |
| ACL | ACL mean | ACLB.mean | Accelerometry band mean |
| ACL | ACL variance | ACLB.s.d. | Accelerometry band average standard deviation |
Note. GPS, geolocation; ACL, accelerometry.
Table 3.
One-way ANOVAs comparing passive digital phenotyping variables between groups
| Variable | Abbreviation | SZ | CN | Test statistic |
|---|---|---|---|---|
| Home time | Home | 0.60 (0.29) | 0.45 (0.22) |
F(1, 76) = 5.97, p = 0.02,  = 0.07 = 0.07 |
| Distance change | Δd MPD Range |
67.74 (76.44) 9033.09 (9543.91) 1202.92 (11 822.85) |
68.93 (92.87) 9092.60 (9486.33) 28 934.47 (64 540.62) |
F(1, 77) = 0.004, p = 0.95,  = 0 = 0 F(1, 71) = 0.001, p = 0.98, 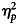 = 0 = 0 F(1, 75) = 2.40, p = 0.13,  = 0.03 = 0.03 |
| Distance from home | Δmh maxmh |
6177.27 (11 581.12) 13 876.34 (19 382.35) |
12 785.67 (15 852.94) 20 339.53 (21 115.99) |
F(1, 76) = 4.30, p = 0.04,  = 0.05 = 0.05 F(1, 74) = 1.89, p = 0.17,  = 0.03 = 0.03 |
| Stationary location clusters | nc | 3.36 (1.15) | 4.33 (1.95) |
F(1, 74) = 6.76, p = 0.01,  = 0.09 = 0.09 |
| Location variance | lv | 0.70 (0.01) | 0.72 (0.15) |
F(1, 73) = 1.12, p = 0.30,  = 0.02 = 0.02 |
| Entropy and normalized entropy | ent nent |
0.21 (0.12) 0.18 (0.07) |
0.23 (0.15) 0.16 (0.07) |
F(1, 74) = 0.51, p = 0.48,  = 0.01 = 0.01 F(1, 74) = 1.34, p = 0.25,  = 0.02 = 0.02 |
| Transition time | tt | 0.18 (0.14) | 0.21 (0.12) |
F(1, 75) = 1.04, p = 0.31,  = 0.01 = 0.01 |
| Flights | f.dur f.dist f.num |
10.23 (14.85) 3267.51 (4176.62) 0.91 (0.79) |
14.40 (16.66) 3991.39 (5636.92) 1.23 (0.65) |
F(1, 75) = 1.31, p = 0.26,  = 0.02 = 0.02 F(1, 75) = 0.40, p = 0.53,  = 0.01 = 0.01 F(1, 75) = 3.97, p = 0.05, 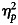 = 0.05 = 0.05 |
| ACL mean | ACLB.mean | 0.91 (0.06) | 0.93 (0.11) |
F(1, 52) = 0.45, p = 0.50,  = 0.01 = 0.01 |
| ACL variance | ACLB.s.d. | 0.14 (0.02) | 0.22 (0.49) |
F(1, 52) = 0.62, p = 0.44,  = 0.01 = 0.01 |
Note. SZ = schizophrenia group; CN = control group. Values reflect mean (s.d.) unless otherwise indicated. Refer to Table 2 for variable definitions.
Phase 3: Final laboratory visit
The final laboratory visit occurred one week after the initial laboratory visit, at the end of the digital phenotyping protocol. Participants returned the phone to the lab, completed the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008), and other procedures not reported in the current study.
Data analysis
All analyses were conducted using SPSS v.27 except for multi-level supplemental analyses performed in R. Methods for calculating the positivity offset and negativity bias are based on Ito and Cacioppo (2005). The positivity offset and negativity bias are characterized by regression parameters where the positivity offset is represented as the intercept for positivity (the output at zero input) and the negativity bias represented as the slope for negativity (greater rate of change in output per unit of input). Positivity offset and negativity bias were assessed across participants within each group. Two regression analyses were conducted for each subject using the equation E = Ax + b, where E is either unipolar positivity or negativity ratings and A is the mean arousal rating. To model the positive motivational system, the intercept value derived from the equation represents the strength of the positivity offset, where positivity offset scores (i.e. the positive – negative intercept difference score) were calculated from multiple regression conducted on each participant and used to obtain the intercept score for positive and negative emotion for each day. To model the negative motivational system, the slope derived from the equation represents the strength of the negativity bias, which reflects the magnitude of increase in negative emotion output per unit of increase in affective input. The negativity bias difference score was calculated as the difference between the negativity and positivity slopes (i.e. negativity slope – positivity slope). The average intercept and slope difference scores were then calculated across all days and summarized at the level of the week to obtain the most reliable estimate. These difference scores then served as the dependent variables for primary analyses used to examine group differences and correlations. See Table 4 for a summary of the ESM variables.
Table 4.
Evaluative space model definitions and formulas
| Variable | Definition | Equation |
|---|---|---|
| Positivity intercept | Positive affective output when affective input is absent | b in the equation E = Ax + b where E = unipolar positivity rating and A = mean arousal rating |
| Positivity slope | Change in positive affective output per 1 unit change in arousal | x in the equation E = Ax + b where E = unipolar positivity rating and A = mean arousal rating |
| Negativity intercept | Negative affective output when affective input is absent | b in the equation E = Ax + b where E = unipolar negativity rating and A = mean arousal rating |
| Negativity slope | Change in negative affective output per 1 unit change in arousal | x in the equation E = Ax + b where E = unipolar negativity rating and A = mean arousal rating |
| Positivity offset | Greater positive affective output than negative affective output when affective input is absent leading to approach motivation | Positivity intercept – Negativity intercept |
| Negativity bias | Greater gain in negative affective output than positive affective output with increasing levels of affective input leading to withdrawal motivation | Negativity slope – Positivity slope |
Note. Adapted from Cacioppo, Berntson, Norris, & Gollan (2012).
Preliminary analyses of standard comparisons of valence and arousal, including effects of emotional context, were conducted as described in Strauss et al. (2017) and displayed in online Supplemental Materials. For primary analyses, within-group paired sample t tests were conducted comparing positive and negative intercepts and slopes to determine if each group demonstrated the prototypical positivity offset and negativity bias. Separate one-way ANOVAs were conducted on the 1-week average positivity offset intercept difference score, the negativity bias difference score, and the raw positivity and negativity intercept and slopes to assess group differences in positivity and negativity parameters. Raw intercept comparisons reflect group differences in how the positivity and negativity systems are calibrated to respond when affective input is absent, and the slope comparisons group differences in hedonic capacity. Pearson correlations were used to examine the relationship between positivity and negativity parameters with accelerometry and geolocation as measures of avolition in the SZ group (see Table 2 for digital phenotyping variables included in correlational analyses), as well as avolition and anhedonia measured via the BNSS and digital phenotyping. Additionally, the association between the number of positive experiences participants endorsed over the digital phenotyping period and the positivity offset difference score was examined using bivariate correlations.
Additional exploratory analyses examining the effects of sex, diagnosis (i.e. schizoaffective disorder v. schizophrenia), and associations with medication status, cognition, positive symptoms, and depressive symptoms are reported in online Supplemental Materials.
Results
Group comparisons of positivity and negativity parameters
Both SZ (t = 2.13, p = 0.04) and CN (t = 9.61, p < 0.001) demonstrated the positivity offset, with significantly higher intercepts for positivity than negativity. Slopes for the positivity function did not significantly differ between groups. As hypothesized, the positivity offset intercept difference score was significantly reduced in SZ compared to CN (F(1, 92) = 10.86, p = 0.001,  = 0.11). Neither group demonstrated the negativity bias, evidenced by nonsignificant differences between the slope for positivity and negativity in SZ (t = −1.69, p = 0.10) and CN (t = 0.52, p = 0.61). Groups did not significantly differ on the negativity bias slope difference score (see Table 5 and Figure 1).
= 0.11). Neither group demonstrated the negativity bias, evidenced by nonsignificant differences between the slope for positivity and negativity in SZ (t = −1.69, p = 0.10) and CN (t = 0.52, p = 0.61). Groups did not significantly differ on the negativity bias slope difference score (see Table 5 and Figure 1).
Table 5.
One-way ANOVAs comparing positivity and negativity parameters in schizophrenia and control groups
| SZ | CN | Test statistic | |
|---|---|---|---|
| Positivity intercept | 45.38 (24.86) | 52.91 (21.06) |
F(1, 92) = 2.47, p = 0.12,  = 0.03 = 0.03 |
| Negativity intercept | 30.41 (30.25) | 11.19 (12.60) |
F(1, 92) = 16.30, p < 0.001,  = 0.15 = 0.15 |
| Positivity slope | −0.15 (1.06) | 0.06 (0.26) |
F(1, 92) = 1.72, p = 0.19,  = 0.02 = 0.02 |
| Negativity slope | 0.29 (1.38) | 0.03 (0.19) |
F(1, 92) = 1.73, p = 0.19,  = 0.02 = 0.02 |
| Positivity offset Difference score | 14.97 (46.66) | 41.72 (30.07) |
F(1, 92) = 10.86, p = 0.001,  = 0.11 = 0.11 |
| Negativity bias difference score | 0.44 (1.73) | −0.03 (0.40) |
F(1, 92) = 3.37, p = 0.07,  = 0.04 = 0.04 |
Note. SZ, schizophrenia group; CN, control group. Positivity Offset Difference Score = Positivity Intercept – Negativity Intercept. Negativity Bias Difference Score = Negativity slope –positivity slope. Values reflect mean (s.d.) unless otherwise indicated.
Fig. 1.
Positivity and negativity functions.
Note. CN, control group; SZ, schizophrenia group. Affective input (i.e. arousal) is depicted on the x-axis and affective output (i.e. positivity or negativity) is depicted on the y-axis. The output when arousal = 0 represents the intercept of the positivity and negativity functions (i.e. the response of the affective system when input is absent). A greater intercept for positivity than negativity reflects the positivity offset, which activates approach motivation. The slopes of the lines represent the gain in positivity or negativity with increasing levels of arousal. A greater slope for negativity than positivity reflects the negativity bias, which activates withdrawal motivation.
Correlations between positivity and negativity parameters and negative symptoms
In SZ, greater reductions in positivity offset were associated with reduced vigor of movement (i.e. ACLB.mean) (r = 0.53, p = 0.02) and greater variability in movement (i.e. ACLB.s.d.) (r = −0.52, p = 0.02) measured by the wristband (see Table 2 for digital phenotyping variable definitions). Greater variability in movement is more common among sedentary compared to active individuals, with the latter demonstrating steady fluctuations in movement and speed throughout the day. Past research suggests that individuals with SZ spend more time in sedentary than active contexts compared to CN (Strassnig et al., 2021), suggesting that this finding reflects the relationship between deficient activity and approach motivation in daily life. Lower positivity offset scores were associated with more severe avolition and anhedonia measured via the BNSS (avolition: r = −0.34, p = 0.03; anhedonia: r = −0.43, p < 0.01) and active digital phenotyping (avolition: r = −0.57, p < 0.001; anhedonia: r = −0.58, p < 0.001) in SZ. Lastly, greater reductions in the positivity offset were associated with a lower frequency of positive events in SZ (r = 0.34, p = 0.03). Correlations between the negativity bias score and clinically rated and active and passive digital phenotyping measures of avolition and anhedonia were all nonsignificant (p's all > 0.05).
In SZ, lower raw positivity intercepts were significantly associated with more severe anhedonia (r = −0.36, p = 0.02) and avolition (r = −0.32, p = 0.03) measured via the BNSS and active digital phenotyping (anhedonia: r = −0.63, p < 0.001; avolition: r = −0.57, p < 0.001). Greater reductions in the positivity slope in SZ were significantly correlated with more severe clinically-rated anhedonia (r = −0.36, p = 0.02). In SZ, higher intercepts for the negativity function were associated with more severe anhedonia (r = 0.34, p = 0.02) and avolition (r = 0.41, p = 0.01) measured via active digital phenotyping, as well as greater variability in movement measured by the wristband (i.e. ACLB.s.d.) (r = 0.54, p = 0.01). Correlations with secondary passive digital phenotyping variables used for exploratory purposes were all nonsignificant.
Discussion
The current study aimed to determine if the positivity offset is reduced in the daily lives of people with SZ and associated with negative symptoms measured via clinical interviews and digital phenotyping. Consistent with several past laboratory-based and experience sampling studies, digital phenotyping results indicated that hedonic capacity is intact in SZ across positive, negative, and neutral contexts (Cohen & Minor, 2010; Gard & Kring, 2009; Gard, Kring, Gard, Horan, & Green, 2007; Gold, Waltz, Prentice, Morris, & Heerey, 2008; Kring & Moran, 2008). Conversely, emotional experience abnormalities emerged when the ESM was applied. Specifically, the positivity offset measured via active digital phenotyping was significantly reduced in SZ compared to CN, suggesting that patients experience reduced levels of positive relative to negative emotion at low levels of arousal. The nonsignificant group differences in the slope for the positivity function indicates that patients' positive emotions increase as arousal increases, suggesting that hedonic capacity is intact in SZ and hedonic deficits are only present when affective input is low. This extends laboratory-based evidence for the positivity offset reduction in SZ (Strauss et al., 2017) by demonstrating that it also occurs in the context of daily life, outside of a controlled laboratory setting with controlled emotional stimuli. Also extending past findings (Strauss et al., 2017), greater reductions in the positivity offset were associated with increased avolition and anhedonia measured via the BNSS and active and passive digital phenotyping. The reduced positivity offset was also associated with the frequency of positive experiences measured via the active digital phenotyping emotion context item (i.e. the behavioral component of anhedonia). Together, these findings extend past laboratory-based studies (Riehle et al., 2022; Strauss et al., 2017), providing a direct link between the positivity offset reduction and real-world behavior. The association between the positivity offset reduction and digital phenotyping measures of negative symptoms indicate that it may be a relevant target for improving deficits in approach behavior in SZ. Further, results indicated that the negativity bias is intact in SZ during everyday activities and unrelated to any measures of avolition or anhedonia. These findings were also consistent with prior laboratory evidence (Strauss et al., 2017), suggesting that within the ESM, negative symptoms were associated with the positivity offset rather than the negativity bias.
The current findings should be considered in the context of certain limitations. First, the positivity offset and negativity bias were calculated based on subjective reports of arousal and emotion. Future laboratory-based and digital phenotyping studies should incorporate physiological measures of arousal and emotional responding (e.g. heart rate variability, skin conductance, pupil dilation) to further understand abnormalities within the context of the ESM, including those at the neural level. Research in psychiatrically healthy individuals indicates that genetic polymorphisms involved in the serotonin system and other neuromodulatory genes may influence the magnitude of the positivity offset (Ashare, Norris, Wileyto, Cacioppo, & Strasser, 2013; Norris, Larsen, Crawford, & Cacioppo, 2011). fMRI studies are also necessary for identifying brain regions implicated in the diminished positivity offset in SZ, such as abnormalities within the limbic system and reward circuitry (Costa, Lang, Sabatinelli, Versace, & Bradley, 2010; Norris et al., 2011). Second, the accelerometry and geolocation variables included in the study belong to the ‘third generation’ of negative symptom assessments that are still being validated. Additional work is needed to extend findings from preliminary validation studies (Narkhede et al., 2021; Raugh et al., 2020; Strauss et al., 2022) and identify which are the strongest, most reliable measures of negativity symptoms. Third, the current sample included adult outpatients with chronic, stable SZ. Thus, it is unclear if the results would extend to earlier stages of illness and those with greater symptom severity. Finally, a clinical comparison group was not included. Past laboratory-based behavioral findings indicate that the positivity offset is reduced in individuals with depression (Gollan et al., 2016), who also commonly experience avolition and anhedonia. This may suggest that the positivity offset is not specific to SZ and could be a transdiagnostic mechanism underlying avolition and anhedonia. Further, given that the association between the positivity offset and depression was also significant (see online Supplemental Materials), future studies should examine the role of both primary and secondary negative symptom factors.
Findings have important clinical implications. Interventions like behavioral activation and activity scheduling may effectively increase the positivity offset and improve avolition or anhedonia. These approaches are main components of Negative Symptom Focused Cognitive Behavior Therapy (Perivoliotis, Grant, & Beck, 2010), and past studies indicated that they are feasible and effective at reducing negative symptoms in SZ (Choi, Jaekal, & Lee, 2016; Grant, Huh, Perivoliotis, Stolar, & Beck, 2012; Lee et al., 2018; Mairs, Lovell, Campbell, & Keeley, 2011); however, no study to date has examined the relationship between behavioral activation, the positivity offset, and deficits in motivation and pleasure in SZ. Mobile health (mHealth) interventions may provide an alternative way to target real-world impairments in hedonic and motivational processes from the perspective of the ESM. For example, mHealth apps could be programmed to assist in activity scheduling, including sending reminders for activities, and to notify patients to become behaviorally activated in neutral contexts in a way that will provide opportunities to enhance positive emotion. Passive digital phenotyping could be directly incorporated into treatment, such as sending notifications to become behaviorally activated when objective behavioral markers (e.g. speed of movement, activity index) fall below a relevant threshold. Emotion regulation interventions delivered via in-person therapy and/or mHealth may also help patients to more effectively increase positive emotion and decrease negative emotion as a means of normalizing the positivity offset to facilitate motivated behavior. Emotion regulation strategies like savoring and reappraisal may be particularly beneficial for increasing positive emotion and decreasing negative emotion (Favrod et al., 2019).
In conclusion, the present findings support the hypothesis that the diminished positivity offset is associated with negative symptoms in SZ. These results refute prior assumptions of hedonic normality and affect-behavior decoupling. Instead, deficits in motivated behavior appear to be driven by an imbalance in positive relative to negative affect in low arousal contexts. The specificity of when affective abnormalities drive motivated behavior deficits, at low levels of arousal, should be used to personalize novel treatment approaches to everyday contexts where avolition and anhedonia are most relevant. Pending replication and extension, conceptual models of negative symptoms should incorporate affective abnormalities like the positivity offset reduction, in addition to dysfunctional reward processing, as an important process leading to negative symptoms.
Acknowledgments
The authors would like to thank the participants who dedicated their time to participating in this study, as well as staff in Dr Strauss' lab who facilitated study coordination.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291722003774.
click here to view supplementary material
Financial support
This work was supported by the National Institute of Mental Health (grant number R21 MH 112925).
Conflict of interest
Dr Strauss is one of the original developers of the Brief Negative Symptom Scale (BNSS) and receives royalties and consultation fees from ProPhase LLC in connection with commercial use of the BNSS and other professional activities; these fees are donated to the Brain and Behavior Research Foundation. Dr Strauss has received honoraria and travel support from ProPhase LLC for training pharmaceutical company raters on the BNSS. In the past two years, Dr Strauss has consulted for and/or been on the speaker bureau for Minerva Neurosciences, Acadia, and Lundbeck pharmaceutical companies. All other authors have no conflicts to report.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- American Psychiatric Association, D.-T. F. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). Arlington, VA, USA: American Psychiatric Publishing, Inc. [Google Scholar]
- Ashare, R. L., Norris, C. J., Wileyto, E. P., Cacioppo, J. T., & Strasser, A. A. (2013). Individual differences in positivity offset and negativity bias: Gender-specific associations with two serotonin receptor genes. Personality and Individual Differences, 55(5), 469–473. doi: 10.1016/j.paid.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch, D. M., & Dowd, E. C. (2010). Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophrenia Bulletin, 36(5), 919–934. doi: 10.1093/schbul/sbq068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler, E. (1911). Dementia Praecox or the group of schizophrenias. New York: International Universities Press Inc. [Google Scholar]
- Bradley, M. M., & Lang, P. J. (2007). Emotion and motivation. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (3rd ed., pp. 581–607). New York, NY, USA: Cambridge University Press. [Google Scholar]
- Cacioppo, J. T. (1999). The affect system Has parallel and integrative processing components form follows function. Journal of Personality and Social Psychology, 76(5), 839–855. Retrieved from https://psycnet.apa.org/record/1999-13561-011. [Google Scholar]
- Cacioppo, J. T., & Berntson, G. G. (1994). Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin, 115(3), 401–423. doi: 10.1037/0033-2909.115.3.401 [DOI] [Google Scholar]
- Choi, K.-H., Jaekal, E., & Lee, G.-Y. (2016). Motivational and behavioral activation as an adjunct to psychiatric rehabilitation for mild to moderate negative symptoms in individuals with schizophrenia: A proof-of-concept pilot study. Frontiers in Psychology, 7, 1759. doi: 10.3389/fpsyg.2016.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A. S., & Minor, K. S. (2010). Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin, 36(1), 143–150. doi: 10.1093/schbul/sbn061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp, C. A., Bashem, J., Moore, R. C., Holden, J. L., Mikhael, T., Swendsen, J., … Granholm, E. L. (2019). GPS Mobility as a digital biomarker of negative symptoms in schizophrenia: A case control study. npj Digital Medicine, 2(1), 108. doi: 10.1038/s41746-019-0182-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefendorf, A. R., & Kraepelin, E. (1907). Clinical psychiatry: A textbook for students and physicians, abstracted and adapted from the 7th German edition of Kraepelin's Lehrbuch der Psychiatrie. New York, NY, USA: MacMillan Co. [Google Scholar]
- Favrod, J., Nguyen, A., Chaix, J., Pellet, J., Frobert, L., Fankhauser, C., … Bonsack, C. (2019). Improving pleasure and motivation in schizophrenia: A randomized controlled clinical trial. Psychotherapy and Psychosomatics, 88(2), 84–95. doi: 10.1159/000496479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First, M. B. (2015). Structured Clinical Interview for the DSM (SCID). In R. L. Cautin & S.O. Lilienfeld (Eds.), The encyclopedia of clinical psychology. [Google Scholar]
- First, M. B., Williams, J. B. W., Benjamin, L. S., & Spitzer, R. L. (2015). User's guide for the SCID-5-PD (Structured Clinical Interview for DSM-5 Personality Disorder). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Fredrickson, B. L., Tugade, M. M., Waugh, C. E., & Larkin, G. R. (2003). What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology, 84(2), 365–376. doi: 10.1037//0022-3514.84.2.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford, D., Mote, J., Gonzalez, R., Abplanalp, S., Zhang, Y., Luckenbaugh, J., Onnela, … Gard, D. E. (2021). Smartphone sensing of social interactions in people with and without schizophrenia. Journal of Psychiatric Research, 137, 613–620. doi: 10.1016/j.jpsychires.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard, D. E., & Kring, A. M. (2009). Emotion in the daily lives of schizophrenia patients: Context matters. Schizophrenia Research, 115(2–3), 379–380. doi: 10.1016/j.schres.2009.07.017 [DOI] [PubMed] [Google Scholar]
- Gard, D. E., Kring, A. M., Gard, M. G., Horan, W. P., & Green, M. F. (2007). Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research, 93, 253–260. doi: 10.1016/j.schres.2007.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, J. M., Waltz, J. A., Prentice, K. J., Morris, S. E., & Heerey, E. A. (2008). Reward processing in schizophrenia: A deficit in the representation of value. Schizophrenia Bulletin, 34(5), 835–847. doi: 10.1093/schbul/sbn068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan, J. K., Hoxha, D., Hunnicutt-Ferguson, K., Norris, C. J., Rosebrock, L., Sankin, L., & Cacioppo, J. (2016). Twice the negativity bias and half the positivity offset: Evaluative responses to emotional information in depression. Journal of Behavior Therapy and Experimental Psychiatry, 52, 166–170. doi: 10.1016/j.jbtep.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm, E., Holden, J. L., Mikhael, T., Link, P. C., Swendsen, J., Depp, … Harvey, P. D. (2019). What do people with schizophrenia do all day? Ecological momentary assessment of real-world functioning in schizophrenia. Schizophrenia Bulletin, 46(2), 242–251. doi: 10.1093/schbul/sbz070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, P. M., Huh, G. A., Perivoliotis, D., Stolar, N. M., & Beck, A. T. (2012). Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Archives of General Psychiatry, 69(2), 121–127. doi: 10.1001/archgenpsychiatry.2011.129 [DOI] [PubMed] [Google Scholar]
- Harvey, P. D., Miller, M. L., Moore, R. C., Depp, C. A., Parrish, E. M., & Pinkham, A. E. (2021). Capturing clinical symptoms with ecological momentary assessment: Convergence of momentary reports of psychotic and mood symptoms with diagnoses and standard clinical assessments. Innovations in Clinical Neuroscience, 18(1–3), 24–30. Retrieved from https://pubmed.ncbi.nlm.nih.gov/34150360/. [PMC free article] [PubMed] [Google Scholar]
- Heerey, E. A., & Gold, J. M. (2007). Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology, 116(2), 268–278. doi: 10.1037/0021-843x.116.2.268 [DOI] [PubMed] [Google Scholar]
- Ito, T. A., & Cacioppo, J. T. (2005). Variations on a human universal: Individual differences in positivity offset and negativity bias. Cognition and Emotion, 19(1), 1–26. doi: 10.1080/02699930441000120 [DOI] [Google Scholar]
- Kirkpatrick, B., Strauss, G. P., Nguyen, L., Fischer, B. A., Daniel, D. G., Cienfuegos, A., & Marder, S. R. (2011). The brief negative symptom scale: Psychometric properties. Schizophrenia Bulletin, 37(2), 300–305. doi: 10.1093/schbul/sbq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin, E. (1921). Dementia praecox and paraphrenia. The Journal of Nervous and Mental Disease, 54(4), 384. [Google Scholar]
- Kring, A. M., & Barch, D. M. (2014). The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. European Neuropsychopharmacology, 24(5), 725–736. doi: 10.1016/j.euroneuro.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring, A. M., & Moran, E. K. (2008). Emotional response deficits in schizophrenia: Insights from affective science. Schizophrenia Bulletin, 34(5), 819–834. doi: 10.1093/schbul/sbn071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, P. J., Bradley, M. M., & Cuthbert, B. N. (1997). International affective picture system (IAPS): Technical manual and affective ratings. 1(39–58), 3. doi: 10.1007/978-3-319-28099-8_42-1 [DOI] [Google Scholar]
- Larsen, J. T., McGraw, A. P., & Cacioppo, J. T. (2001). Can people feel happy and sad at the same time? Journal of Personality and Social Psychology, 81(4), 684–696. Retrieved from https://pubmed.ncbi.nlm.nih.gov/11642354/. [PubMed] [Google Scholar]
- Larsen, J. T., Norris, C. J., McGraw, A. P., Hawkley, L. C., & Cacioppo, J. T. (2009). The evaluative space grid: A single-item measure of positivity and negativity. Cognition and Emotion, 23(3), 453–480. doi: 10.1080/02699930801994054 [DOI] [Google Scholar]
- Lee, E., Cha, Y.-J., Oh, J.-H., Hwang, N.-R., Choi, K.-H., & Seo, H.-J. (2018). F58. Community-based multi-site randomized controlled trial of behavioral activation for negative symptoms of individuals with chronic schizophrenia. Schizophrenia Bulletin, 44(suppl_1), S242–S242. doi: 10.1093/schbul/sby017.589 [DOI] [Google Scholar]
- Llerena, K., Strauss, G. P., & Cohen, A. S. (2012). Looking at the other side of the coin: A meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophrenia Research, 142(1), 65–70. doi: 10.1016/j.schres.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairs, H., Lovell, K., Campbell, M., & Keeley, P. (2011). Development and pilot investigation of behavioral activation for negative symptoms. Behavior Modification, 35(5), 486–506. doi: 10.1177/0145445511411706 [DOI] [PubMed] [Google Scholar]
- Miller, M. L., Raugh, I. M., Strauss, G. P., & Harvey, P. D. (2022). Remote digital phenotyping in serious mental illness: Focus on negative symptoms, mood symptoms, and self-awareness. Biomarkers in Neuropsychiatry, 6, 100047. doi: 10.1016/j.bionps.2022.100047 [DOI] [Google Scholar]
- Narkhede, S. M., Luther, L., Raugh, I. M., Knippenberg, A. R., Esfahlani, F. Z., Sayama, H., … Strauss, G. P. (2021). Machine learning identifies digital phenotyping measures most relevant to negative symptoms in psychotic disorders: Implications for clinical trials. Schizophrenia Bulletin, 48(2), 425–436. doi: 10.1093/schbul/sbab134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, C. J., Gollan, J., Berntson, G. G., & Cacioppo, J. T. (2010). The current status of research on the structure of evaluative space. Biological Psychology, 84(3), 422–436. doi: 10.1016/j.biopsycho.2010.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, C. J., Larsen, J. T., Crawford, L. E., & Cacioppo, J. T. (2011). Better (or worse) for some than others: Individual differences in the positivity offset and negativity bias. Journal of Research in Personality, 45(1), 100–111. doi: 10.1016/j.jrp.2010.12.001 [DOI] [Google Scholar]
- Nuechterlein, K. H., Green, M. F., Kern, R. S., Baade, L. E., Barch, D. M., Cohen, J. D., … Marder, S. R. (2008). The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. American Journal of Psychiatry, 165(2), 203–213. doi: 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- Onnela, J. P., & Rauch, S. L. (2016). Harnessing smartphone-based digital phenotyping to enhance behavioral and mental health. Neuropsychopharmacology, 41(7), 1691–1696. doi: 10.1038/npp.2016.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perivoliotis, D., Grant, P. M., & Beck, A. T. (2010). Negative symptom focused cognitive behavior therapy: A comprehensive treatment manual. Philadelphia, PA: The University of Pennsylvania. [Google Scholar]
- Raugh, I., Howie, S., Gonzalez, C., Chapman, H., Cohen, A., Kirkpatrick, B., … Strauss, G. (2020). Geolocation as a digital phenotyping measure of negative symptoms and functional outcome. Schizophrenia Bulletin, 46, 1596–1607. doi: 10.1093/schbul/sbaa121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raugh, I. M., James, S. H., Gonzalez, C. M., Chapman, H. C., Cohen, A. S., Kirkpatrick, B., & Strauss, G. P. (2021). Digital phenotyping adherence, feasibility, and tolerability in outpatients with schizophrenia. Journal of Psychiatric Research, 138, 436–443. doi: 10.1016/j.jpsychires.2021.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle, M., Pillny, M., & Lincoln, T. M. (2022). Expanding the positivity offset theory of anhedonia to the psychosis continuum. npj Schizophrenia, 8(1), 47. doi: 10.1038/s41537-022-00251-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassnig, M., Miller, M., Moore, R., Depp, C., Pinkham, A., & Harvey, P. (2021). Assessing avolition with ecological momentary assessment. Biological Psychiatry, 89, S140–S141. doi: 10.1016/j.biopsych.2021.02.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, G. P., Raugh, I. M., Zhang, L., Luther, L., Chapman, H. C., Allen, D. N., … Cohen, A. S. (2022). Validation of accelerometry as a digital phenotyping measure of negative symptoms in schizophrenia. npj Schizophrenia, 8(1), 37. doi: 10.1038/s41537-022-00241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, G. P., Visser, K. H., Lee, B. G., & Gold, J. M. (2017). The positivity offset theory of anhedonia in schizophrenia. Clinical Psychological Science, 5(2), 226–238. doi: 10.1177/2167702616674989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss, G. P., Waltz, J. A., & Gold, J. M. (2013). A review of reward processing and motivational impairment in schizophrenia. Schizophrenia Bulletin, 40(Suppl_2), S107–S116. doi: 10.1093/schbul/sbt197 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291722003774.
click here to view supplementary material



