Abstract
Human immunodeficiency virus type 1 (HIV-1) strains resistant to nonnucleoside reverse transcriptase inhibitors (NNRTIs) may easily be selected for in vitro and in vivo under a suboptimal therapy regimen. Although cross-resistance is extensive within this class of compounds, newer NNRTIs were reported to retain activity against laboratory strains containing defined resistance-associated mutations. We have characterized HIV-1 resistance to loviride and the extent of cross-resistance to nevirapine, delavirdine, efavirenz, HBY-097, and tivirapine in a set of 24 clinical samples from patients treated with long-term loviride monotherapy by using a recombinant virus assay. Genotypic changes associated with resistance were analyzed by population sequencing. Overall, phenotypic resistance to loviride ranged from 0.04 to 3.47 log10-fold. Resistance was observed in samples from patients who had discontinued loviride for up to 27 months. Cross-resistance to the other compounds was extensive; however, fold resistance to efavirenz was significantly lower than fold resistance to nevirapine. No genotypic changes were detected in three samples; these were sensitive to all of the NNRTIs tested. The most common genotypic change was the K103N substitution. The range of phenotypic resistance in samples containing the K103N substitution could not be predicted from a genotypic analysis of known NNRTI resistance-associated mutations. The Y181C substitution was detected in one isolate which was resistant to loviride and delavirdine but sensitive to efavirenz, HBY-097, and tivirapine. Our data indicate that the available newer NNRTIs which retain activity against some HIV-1 strains selected by other compounds of this class in vitro may have compromised clinical efficacy in some patients pretreated with NNRTI.
Nonnucleoside reverse transcriptase (RT) inhibitors (NNRTIs) are potent inhibitors of and highly selective for human immunodeficiency virus type 1 (HIV-1) RT (28, 29). The first NNRTI compound to be described was a TIBO (tetra-hydroimidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-one and -thione) derivative (28). Although belonging to various structurally distinct chemical groups, they have the same mechanism of action in that they bind to the hydrophobic pocket close to the polymerase catalytic site of RT and slow the rate of polymerization catalyzed by the enzyme (39). Two NNRTIs—nevirapine and delavirdine—have been approved for clinical use in combination antiretroviral therapy (8, 25, 32). Efavirenz, a derivative of the newly developed benzoxazin-2-ones, is currently in phase III of clinical development (14, 36). Loviride, a member of the α-anilinophenylacetamide group, was tested in monotherapy and in double and triple drug combination trials (6, 34, 40, 41). Other NNRTIs that have been tested clinically include tivirapine, a derivative of the TIBO group of compounds previously known as 8-C1-TIBOs (5, 23, 30), and the quinoxaline compound HBY-097 (35, 38).
HIV-1 variants resistant to NNRTIs may easily be selected in vitro (12, 18, 31) and in vivo in a monotherapy antiretroviral regimen (10, 23, 37). Drug resistance has been shown to limit the antiviral efficacy of this class of drugs in clinical trials (24). Mutations resulting in resistance to this class of compound cluster in the hydrophobic pocket within the palm domain of the p66 RT subunit (42). Mutations commonly selected by NNRTIs occur at amino acid positions 98 to 108, 179 to 190, and 230 to 236. In in vitro selection experiments, individual NNRTIs may predominantly select one or two mutations, which may result in a disadvantage for the virus. For example, delavirdine selects for the P236L substitution in vitro, which confers increased sensitivity to other NNRTIs in vitro (12); HBY-097 was active against mutants selected by other NNRTIs and itself selects for the G190E substitution with severe impairment of RT activity in vitro (4, 18, 19). These considerations led to the proposal that it may be possible to use NNRTIs sequentially or that NNRTI combinations may be strategically useful; clinical trials testing the efficacy of NNRTI combinations are currently being developed. Evidence from clinical trials, however, indicates that clinical treatment results in the selection of a few main mutations. These include changes at amino acid positions 181 (Y181C) and 103 (K103N) with resulting broad cross-resistance to this class of compounds (10, 33, 35, 37). Newer NNRTIs, such as efavirenz, are active in vitro against virus strains with single mutations, such as those at position 98, 106, or 181, whereas higher-level resistance (an up to 1,500-fold increase in the 95% inhibitory concentration [IC95]) was observed against virus strains with double mutations (46). However, in patients treated with efavirenz, the K103N substitution was detected in the majority of patients with a rebound in plasma viral load (2). In in vitro site-directed mutagenesis experiments, the K103N substitution led to an 18-fold rise in the IC90, corresponding to a concentration of 64 nM, against HIV-1 strain NL4-3 (17). Based on pharmacokinetic data, calculation of ICs for virus strains harboring the K103N mutation with adjustment for protein binding showed that the achievable levels in plasma may possibly be sufficiently high to retain activity against virus strains with this substitution (1). Thus, it may be possible to use efavirenz or other new NNRTIs therapeutically in patients previously exposed to this class of drugs harboring HIV-1 strains with NNRTI resistance-associated mutations (1, 45, 46).
In this retrospective analysis, we investigated the development of resistance—at the phenotypic and genotypic levels—to loviride in patients treated with long-term loviride monotherapy within the INT-2 trial and its follow-up phases (40). The resistance analyses were based on a plasma-derived recombinant virus phenotypic assay. We investigated the levels of cross-resistance to five other NNRTIs: nevirapine, delavirdine, efavirenz, HBY-097, and the 8-C1-TIBO derivative tivirapine.
MATERIALS AND METHODS
Patient population.
Patients who had participated in the INT-2 trial (40) and had elected to continue taking loviride through the follow-up protocols were included in this study. Informed consent was obtained from all patients.
Sample preparation.
Plasma was prepared from whole blood collected in EDTA tubes and frozen at −70°C.
Preparation of recombinant HIV-1 and drug susceptibility assay.
Drug resistance was tested by using the Antivirogram, a recombinant virus assay-based method, as previously described (13, 22). Loviride and tivirapine were produced by the Janssen Research Foundation. Delavirdine, efavirenz, and HBY-097 were kindly supplied by Pharmacia & Upjohn (Kalamazoo, Mich.), Dupont Merck Pharmaceutical Company (Wilmington, Del.), and Hoechst-Bayer (Frankfurt, Germany), respectively. Results are expressed as fold resistance (IC50 of recombinant/IC50 of wild type) or as log10 fold resistance (log10-R). The wild-type recombinant virus was based on pHXB2; a construct with RT deleted (pHIVΔRT) provided the background for the patient plasma-derived recombinant viruses (13, 22). Resistance was defined as an increase in the IC50 of ≥ fourfold (log10 0.60) compared to the wild type. The IC50s for the wild-type strain were 0.0165 to 0.065 μM loviride, 0.021 to 0.023 μM nevirapine, 1.32 to 1.61 μM delavirdine, 0.0021 to 0.0023 μM efavirenz, 0.0027 μM HBY-097, and 0.016 to 0.042 μM tivirapine. The high IC50 of delavirdine was due to instability of the compound in solution when stored at −20°C (product information from Pharmacia & Upjohn).
Sequence determination.
RT genotypes were determined by sequencing of the RT region from recombinant HIV-1, as well as directly from plasma-derived RT regions, as previously described (13).
RESULTS
Patient population.
The INT-2 trial (40), initiated in 1992, compared 100 mg of loviride given three times daily versus 400 mg of α-anilinophenylacetamide lead compound R18893 given three times daily versus placebo administration in a 6-month randomized trial involving patients with CD4 counts above 400 cells/mm3. Twenty-six patients who elected to go on to follow-up open-label protocols were available for resistance testing approximately 3 years afterward. The median time of loviride treatment was 28.5 months. Five patients had been exposed to R18893 during the randomized-treatment period. Three patients (B701, B710, and B720) had discontinued loviride for 8, 10, and 27 months, respectively, prior to resistance testing and had received no subsequent NNRTI therapy. Patient B701 withdrew consent, patient B710 switched to other antiretroviral treatment, and patient B720 discontinued antiretroviral treatment due to pregnancy. In terms of other RT inhibitor treatment, one patient had received zidovudine (ZDV) in combination with loviride for 9 months, and one patient had received ZDV plus lamivudine in combination with loviride for 3 months at the time of sampling. At the time of resistance testing, the median CD4 cell count was 428 (range, 168 to 818) cells/mm3 and the median viral load was 4.67 (range, 2.7 to 5.89) log10 HIV-1 RNA copies/ml. A summary of patient characteristics is provided in Table 1.
TABLE 1.
Patient characteristics
| Patient | Treatment duration (mo)
|
CD4 count (cells/mm3) | Viral load (log10 copies/ml) | ||
|---|---|---|---|---|---|
| R18893 | Loviride | Othera | |||
| B701 | None | 40 | None | 191 | 5.74 |
| B702 | 6 | 28 | None | 578 | 4.68 |
| B703 | None | 35 | None | 282 | 4.05 |
| B704 | None | 39 | None | 235 | 5.18 |
| B705 | None | 29 | None | 387 | 4.08 |
| B706 | None | 23 | None | 519 | 4.66 |
| B707 | None | 24 | None | 578 | 3.84 |
| B708 | None | 30 | None | 336 | 4.77 |
| B709 | 6 | 30 | None | 465 | 4.62 |
| B710 | None | 17 | ZDV, 9; Ind, 2 | 259 | 3.74 |
| B711 | None | 38 | None | 548 | 4.34 |
| B712 | None | 29 | None | 495 | 3.63 |
| B713 | None | 39 | None | 447 | 2.70 |
| B714 | 6 | 27 | None | 306 | 5.85 |
| B715 | None | 28 | None | 628 | 4.54 |
| B716 | None | 28 | ZDV, 3; 3TC, 3 | 271 | 4.90 |
| B717 | 2 | 5 | None | 492 | 5.36 |
| B718 | None | 39 | None | 598 | 5.71 |
| B719 | None | 42 | None | 818 | 5.25 |
| B720 | None | 8 | None | 727 | 2.70 |
| B721 | None | 21 | None | 168 | 5.44 |
| B722 | None | 28 | None | 409 | 3.04 |
| B723 | None | 29 | None | 294 | 4.71 |
| B724 | None | 31 | None | 460 | 3.28 |
| B725 | None | 26 | None | 297 | 5.89 |
| B726 | 2 | 28 | None | 376 | 4.92 |
| Median | 28.5 | 428 | 4.67 | ||
Ind, indinavir; 3TC, lamivudine.
Resistance to NRTIs.
By using the Antivirogram method, phenotypic resistance to all available RT inhibitors can be measured in one assay (13, 22). In two samples with low virus loads (B713 and B722), it was not possible to obtain an amplified product for recombination. Thus, a total of 24 samples were included in the resistance analysis.
All patient samples but the two originating from patients treated with nucleoside analogue RT inhibitors (Table 1) were fully sensitive to all five of the nucleoside RT inhibitors (NRTIs) tested (ZDV, zalcitabine, didanosine, stavudine, and lamivudine) (data not shown). Sample B710 had 14-fold resistance to ZDV; the M41L and T215Y ZDV resistance-associated mutations were detected in plasma- and recombinant-virus-derived RT sequences. Sample B716 was fully resistant to lamivudine (IC50, >100 μM) but sensitive to ZDV. The only genotypic change was the M184V mutation associated with lamivudine resistance (43).
Resistance to loviride.
We were able to test phenotypic resistance to loviride in 24 of the 26 samples. The results are listed in Table 2. Overall, resistance ranged from 0.04 to 3.47 log10-R. These values correspond to IC50s ranging from 0.0187 to 49.225 μM.
TABLE 2.
NNRTI resistance-associated mutations and loviride resistance
| Sample | Mutation(s) in RT sequences from:
|
IC50 (μM) | Log10 fold resistance | |
|---|---|---|---|---|
| Plasma | Recombinant virus | |||
| B701 | K103N | NAa | 0.475 | 1.12 |
| B702 | K103N | K103N | 1.6506 | 2 |
| B703 | None | None | 0.0187 | 0.04 |
| B704 | K103N | K103N | 1.7026 | 2.01 |
| B705 | K103N, K238T | K103N, K238T | 49.225 | 3.47 |
| B706 | K103N | K103N, P225P/L | 2.09 | 2.1 |
| B707 | K103N | NA | 1.3 | 1.9 |
| B708 | K103S | K103S | 2.419 | 2.17 |
| B709 | K103N | NA | 1.599 | 1.4 |
| B710 | None | None | 0.0412 | 0.4 |
| B711 | V108I | NA | 0.134 | 0.3 |
| B712 | K103N | K103N | 3.7342 | 2.35 |
| B714 | K103S | K103N | 0.9554 | 1.76 |
| B715 | None | None | 0.1427 | 0.57 |
| B716 | K103N | K103N | 0.9494 | 1.76 |
| B717 | K103N | K013N | 1.6452 | 2 |
| B718 | K101E, K103K/N, E138A | K101E, K103K/N, E138A | 8.996 | 2.74 |
| B719 | A98G | A98X, K238T | 0.42 | 1.4 |
| B720 | K103N | NA | 0.4352 | 1.05 |
| B721 | K103N | K103N | 1.24 | 1.88 |
| B723 | Y181C | Y181C | 9.2802 | 2.75 |
| B724 | K101Q | K101Q | 0.29 | 1.26 |
| B725 | K103N | K103N, I108I/V | 0.9846 | 1.78 |
| B726 | K103N | K103N | 2.8346 | 2.24 |
NA, not applicable.
Interestingly, resistance was detected in two of the samples originating from patients who had discontinued loviride for 8 and 27 months: B701 and B720 (13-fold and 11-fold, respectively).
The NNRTI resistance-associated mutations that were detected are presented in Table 2. No mutations were detected in plasma-derived or recombinant-virus-derived RT sequences in three samples (B703, B710, and B715); these were loviride sensitive (Table 2). Repeat analysis of new samples from these patients yielded similar results (data not shown). The most common mutation was at amino acid position 103. The K103N change was found in 14 samples as the sole mutation and in 2 samples in combination with other mutations in an analysis of plasma-derived or recombinant-virus-derived RT sequences; in one sample, the change was K103S. All samples with genotypic changes at position 103 were resistant to loviride (Table 2). The median log10-R value for samples containing K103N was 1.89 (range, 1.05 to 2.24). Genotypic changes at position 103 also occurred in combination with changes at position 238 and in combination with changes at positions 101 and 138. The highest level of resistance to loviride (2,983-fold; IC50, 49.23 μM) was seen in a sample with the K103N mutation in combination with K238T. Y181C was detected in one sample with 562-fold resistance to loviride. Other NNRTI resistance-associated changes observed in plasma-derived RT sequences were V108I, A98G, and K101Q.
In most cases in which sequences from plasma-derived and recombinant-virus-derived RT regions were available, these were concordant. Exceptions were observed in samples B714 (K103S versus K103N), B719, and B725, the latter showing double mutations in the recombinant-virus-derived sequence.
Resistance to other NNRTIs.
The recombinant HIV isolates were tested for susceptibility to nevirapine, delavirdine, efavirenz, HBY-097, and tivirapine. In general, a high degree of cross-reactivity was observed. All loviride-sensitive samples were sensitive to the other NNRTIs. The degree of cross-reactivity to other NNRTIs in loviride-resistant samples varied within samples and within compounds.
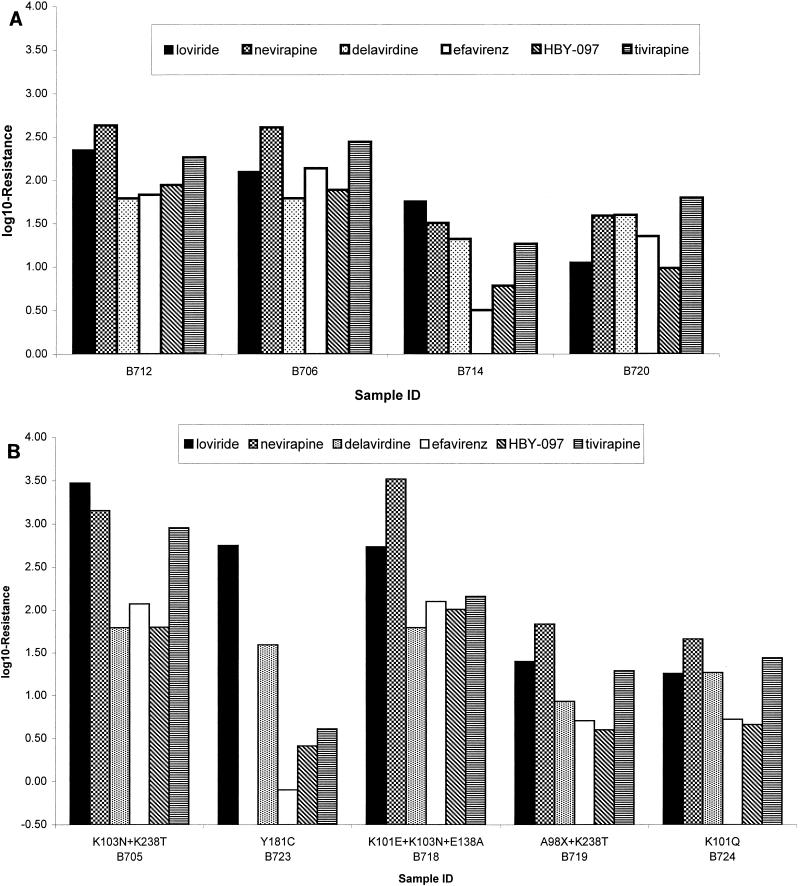
All samples with K103N showed decreased susceptibility to nevirapine and delavirdine. The median log10-R value for nevirapine was 2.14 (range, 0.92 to 2.64). Due to the high IC50 of delavirdine for the wild type, yielding >62.2-fold-resistance in most cases, a median could not be calculated. The median log10-R values were 1.53 (range, 0.51 to 2.14) for efavirenz, 1.36 (range, 0.79 to 1.95) for HBY-097, and 1.80 (range, 0.45 to 2.45) for tivirapine. Four representative samples with genotypic changes at amino acid position 103 are shown in Fig. 1A. The fold resistance to efavirenz was low in some cases (e.g., 3.2-fold in B714, corresponding to an IC50 of 0.0067 μM), as shown in Fig. 1A. In this same sample, resistance to HBY-097 was also low (6.1-fold; IC50, 0.0165 μM). In other cases, however, resistance to efavirenz in samples containing the K103N mutation was high, e.g., sample B706, with an efavirenz resistance value of 130-fold and an efavirenz IC50 of 0.29 μM. Sample B720 originated from a patient who had discontinued loviride for 27 months; in this case, resistance to all compounds, including efavirenz, was >10-fold.
FIG. 1.
Phenotypic resistance to loviride, nevirapine, delavirdine, efavirenz, HBY-097, and tivirapine in representative samples containing the K103N mutation (A) or other NNRTI resistance-associated mutations (B). Due to the high delavirdine IC50 for the wild-type strain, the maximum resistance measurable was 62.2-fold (1.79 log10-R). ID, identification.
Figure 1B shows the fold resistance values for samples with mutations other than the K103N. Interestingly, sample B723, containing the Y181C mutation, while displaying high resistance to loviride (560-fold; IC50, 9.28 μM) and delavirdine (39.2-fold; IC50, 62.9 μM) retained full sensitivity to efavirenz (0- to 8-fold; IC50, 0.0016 μM) and HBY-097 (2.6-fold; IC50, 0.0071 μM). A nevirapine resistance measurement was not available for this sample. Resistance to tivirapine was low (fourfold; IC50, 0.133 μM). Samples containing changes at position 101 (B724) or double mutations involving positions 98 and 238 (B719) were also less than 10-fold resistant to efavirenz.
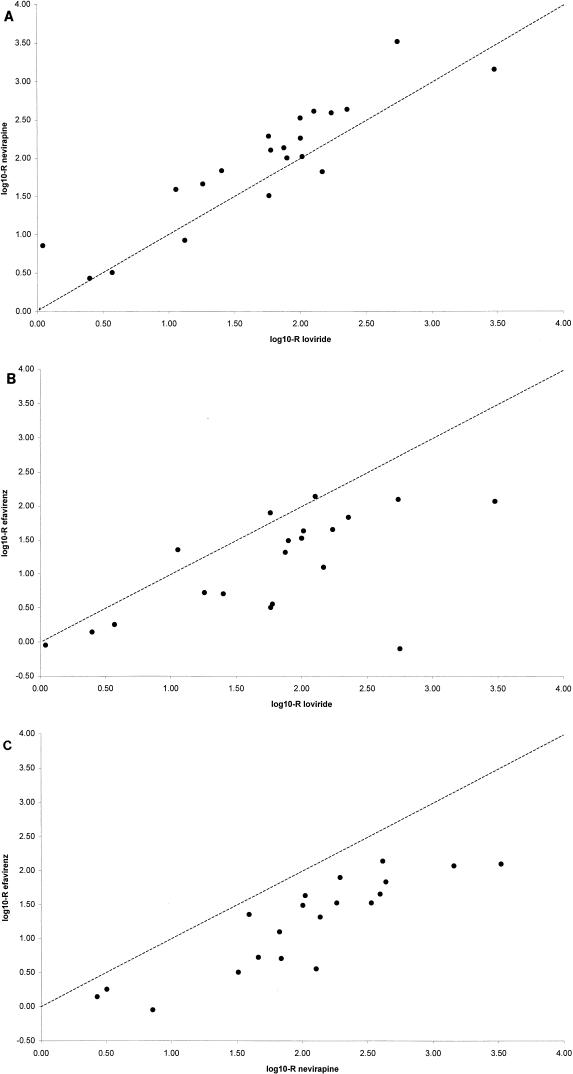
In general, the log10-R values for nevirapine were higher than those for loviride. In contrast, the log10-R values for efavirenz, HBY-097, and tivirapine were lower than those for loviride. Because of the high delavirdine IC50s for the wild-type strain, yielding a maximum measurable resistance level of 62-fold, this type of comparison was not possible for this compound. Figure 2 illustrates the comparison of resistances to loviride, nevirapine, and efavirenz. Nevirapine resistance (Fig. 2A) was higher than loviride resistance in the majority of samples (P = 0.0046), whereas efavirenz resistance was lower in most samples, with a P value of 0.0004 (Fig. 2B). Resistance to efavirenz was lower than resistance to nevirapine in all samples (Fig. 2C). The difference in the fold resistance values for efavirenz and nevirapine was highly significant, with a P value of <0.0001.
FIG. 2.
Resistance comparisons of nevirapine versus loviride (A), efavirenz versus loviride (B), and efavirenz versus nevirapine (C). The dashed line represents a 1:1 ratio. P values (paired t test): A, 0.0046; B, 0.0004; C, <0.0001.
DISCUSSION
This is the first report of resistance and cross-resistance to loviride, nevirapine, delavirdine, efavirenz, HBY-097, and the 8-C1-TIBO derivative tivirapine in a set of clinical HIV-1 isolates. In this analysis of recombinant HIV-1 isolates from patients given long-term treatment with loviride, we have shown that cross-resistance within this class of drugs is extensive and may be expressed toward newer NNRTIs such as HBY-097 and efavirenz. Although the increase in resistance to nevirapine was significantly greater than the increase in resistance to efavirenz, the changes in the IC50s of the latter may be large in some cases, leading to a prediction of suboptimal virological responses to efavirenz in some patients. However, the clinical impact of these changes cannot be assessed without clinical trials. As in other reported analyses, the major mutation detected in the HIV-1 RT was K103N appearing as the sole mutation or in combination with other NNRTI resistance-associated changes. However, not all samples contained K103N. In addition, other NNRTI resistance-associated changes were selected for.
For the NNRTIs, in contrast to NRTIs, the achievable levels of non-protein-bound drug in plasma correlate directly with the potential for in vivo antiviral activity. Efavirenz and HBY-097, with an IC90 or IC95 of 3 to 7 nM (18, 46), are more potent compounds than the first-generation NNRTIs nevirapine (IC90, 710 nM) (20, 21) and delavirdine (IC90, 45 to 100 nM) (12). In vitro studies with efavirenz have demonstrated an 18-fold loss of activity due to the K103N mutation in laboratory strains of HIV-1; however, it was calculated that the achievable levels of the non-protein-bound drug in plasma may be sufficient to inhibit K103N strains in vivo (1, 46). The implications of our findings of resistance in clinical samples are that not all NNRTI-pretreated patients with HIV-1 strains containing the K103N mutation would be expected to benefit from subsequent efavirenz or HBY-097 treatment. In addition, our data indicate that it may be difficult to deduce potential efavirenz or HBY-097 activity from the viral genotype. For example, resistance in samples containing the K103N substitution ranged from 11- to 226-fold for loviride and from 3.2- to 139-fold for efavirenz. It is possible that background polymorphisms may contribute to the variation in the level of resistance observed in K103N-containing HIV-1 strains. However, a direct comparison with the patients’ baseline isolates was not possible in this study and the fold resistance values reported describe the change in IC50 compared to the wild-type virus. The amount of variation that would be expected in a calculation of the fold increase in the IC50 compared to the IC50 for a baseline isolate is not known. In the one sample containing the Y181C substitution, full sensitivity to efavirenz and HBY-097 was retained, thus confirming previous reports based on laboratory HIV-1 constructs with defined mutations with a clinical isolate (17, 46).
The mechanisms for the differences in activity between the second-generation NNRTI and the first-generation compounds—lower IC50s and activity against some first-generation drug-resistant strains—are not completely understood. These could be related to differences in drug-enzyme interactions at the molecular level (42). For example, the Y181C and Y188L substitutions may eliminate favorable contacts between the enzyme and the inhibitor, as shown for TIBO compounds and for HBY-097 (7, 15). The K103N mutation, on the other hand, confers resistance by reducing the rate of NNRTI binding (15). It will be of interest to investigate the molecular interactions of wild-type and mutant RTs with second-generation compounds such as efavirenz.
In a study of resistance in patients experiencing a viral rebound while being treated with efavirenz (2), the most common mutations were K103N, V108I, and P225H. Combination of K103N with V108I or P225H was frequent. In our study, we observed the K103N-plus-V108I combination in one sample; we did not observe any P225H substitutions, either alone or in combination with K103N. Thus, other polymorphisms are likely involved in the level of resistance observed. The clinical significance of the differences in the level of resistance is not clear.
Mutations leading to NNRTI resistance in vivo are stable and do not appear to represent a fitness disadvantage, in contrast to some NNRTI resistance-associated mutations selected for in vitro (18, 19) or resistance-associated mutations selected in vivo by other drug classes (27, 44, 47). NNRTI resistance-associated genotypes have been shown to occur naturally in HIV-1 type O strains, HIV-2, and in a significant proportion of NNRTI-naive, HIV-1 type B-infected individuals (3, 9, 11, 21, 26). Recently, the horizontal transmission of a nevirapine-resistant virus was reported (16). Nevirapine resistance associated with Y181C and A98G genotypic changes was found to be stable over a period of 2 years in the absence of nevirapine treatment (16). In our study, K103N-associated resistance to loviride and other NNRTIs remained stable in patients having discontinued NNRTI treatment for periods of 8 to 27 months. These considerations, in view of the potential for cross-resistance, have implications for treatment strategies. NNRTI combined with NRTIs or with protease inhibitors have shown good clinical efficacy in first-line treatment trials (24, 36). However, if NNRTI resistance exists due to prior NNRTI treatment failure, the options for subsequent treatment with this class of drugs may be severely limited. Future research with this class of compounds should include the development of compounds selected for activity against NNRTI-resistant HIV-1 and the clinical testing of NNRTI combinations.
ACKNOWLEDGMENTS
This work was supported by a grant from the Janssen Research Foundation.
We thank L. T. Bacheler and J. P. Kleim for providing efavirenz and HBY-097, respectively, and for critically reviewing the manuscript.
REFERENCES
- 1.Bacheler L T, Anton E, Baker D, Cordova B, Fiske W, Garber S, Logue K, Rizzo C, Tritch R, Erickson-Viitanen S. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Impact of mutation, plasma protein binding and pharmacokinetics on clinical efficacy of the HIV-1 nonnucleoside reverse transcriptase inhibitor DMP 266, abstr. I-115; p. 38. [Google Scholar]
- 2.Bacheler L T, George H, Hollis G, Abremski K The SUSTIVA Resistance Study Team. Program and abstracts of the 5th Conference on Retroviruses and Opportunistic Infections. 1998. Resistance to Efavirenz (SUSTIVA) in vivo, abstr. 703; p. 56. [Google Scholar]
- 3.Bacolla A, Shih C K, Rose J M, Piras G, Warren T C, Grygon C A, Ingraham R H, Cousins R C, Greenwood D J, Richman D, Cheng Y C, Griffin J A. Amino acid substitutions in HIV-1 reverse transcriptase with corresponding residues from HIV-2. J Biol Chem. 1993;268:16571–16577. [PubMed] [Google Scholar]
- 4.Boyer P L, Gao H Q, Hughes S H. A mutation at position 190 of human immunodeficiency virus type 1 reverse transcriptase interacts with mutations at positions 74 and 75 via the template primer. Antimicrob Agents Chemother. 1998;42:447–452. doi: 10.1128/aac.42.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckheit R W, Germany-Decker J, Hollingshead M G, Allen L B, Shannon W M, Janssen P A J, Chirigos M A. Differential antiviral activity of two TIBO derivatives against the human immunodeficiency and murine leukemia viruses alone and in combination with other anti-HIV agents. AIDS Res Hum Retroviruses. 1993;9:1097–1106. doi: 10.1089/aid.1993.9.1097. [DOI] [PubMed] [Google Scholar]
- 6.CAESAR Coordinating Committee. Randomized trial of addition of lamivudine or lamivudine plus loviride to zidovudine-containing regimens for patients with HIV-1 infection: the CAESAR trial. Lancet. 1997;349:1413–1421. [PubMed] [Google Scholar]
- 7.Das K, Ding J, Hsiou Y, Clark A D, Jr, Moereels H, Koymans L, Andries K, Pauwels R, Janssen P A, Boyer P L, Clark P, Smith R H, Jr, Kroeger Smith M B, Michejda C J, Hughes S H, Arnold E. Crystal structures of 8-C1 and 9-C1 TIBO complexed with wild-type HIV-1 RT and 8-C1 TIBO complexed with the Tyr181Cys HIV-1 RT drug-resistant mutant. J Mol Biol. 1996;264:1085–1100. doi: 10.1006/jmbi.1996.0698. [DOI] [PubMed] [Google Scholar]
- 8.Davey R T, Chiatt D G, Reed G F, Freimuth W W, Herpin B R, Metcalf J A, Eastman P S, Falloon J, Kovacs J A, Polis M A, Walker R E, Masur H, Boyle J, Coleman S, Cox S R, Wathen L, Daenzer C L, Lane H C. Randomized, controlled phase I/II trial of combination therapy with delavirdine and conventional nucleosides in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1996;40:1657–1664. doi: 10.1128/aac.40.7.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jong M D, Shuurman D R, Lange J M A, Boucher C A B. Replication of a pre-existing resistant HIV-1 subpopulation in vivo after introduction of a strong selective pressure. Antiviral Ther. 1996;1:33–41. [PubMed] [Google Scholar]
- 10.Demeter L M, Meehan P M, Morse G, Gerondelis P, Dexter A, Berrios L, Cox S, Freimuth W, Reichman R C. HIV-1 drug susceptibilities and reverse transcriptase mutations in patients receiving combination therapy with didanosine and delavirdine. J Acquired Immune Defic Syndr Hum Retrovirol. 1997;14:136–144. doi: 10.1097/00042560-199702010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Descamps D, Loussert-Ajaka I, Collin G, Candotti D, Bouchaud O, Sargosti S, Simon F, Brun-Vézinet F. Program and abstracts of the 5th International Workshop on HIV Drug Resistance. 1996. Susceptibility of HIV-1 group O to antiretroviral agents, abstr. 13; p. 7. [Google Scholar]
- 12.Dueweke T J, Pushkarskaya T, Poppe S M, Swaney S M, Zhao J Q, Chen I S Y, Stevenson M, Tarpley W G. A mutation in reverse transcriptase of bis(heteroaryl)piperazine-resistance human immunodeficiency virus type 1 that confers increased sensitivity to other nonnucleoside inhibitors. Proc Natl Acad Sci USA. 1993;90:4713–4717. doi: 10.1073/pnas.90.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertogs K, De Bèthune M P, Miller V, Ivens T, Schel P, Van Cauwenberge A, Van Den Eynde C, Van Gerwen V, Azijn H, Van Houtte M, Peeters F, Staszewski S, Conant M, Bloor S, Kemp S, Larder B, Pauwels R. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob Agents Chemother. 1998;42:269–276. doi: 10.1128/aac.42.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicks C, Haas D, Seekins D, Cooper R, Gallant J, Mileno M, Ruiz N M, Manion D J, Ploughman L M, Labriola D F The Dupont Merck Pharmaceutical Company Clinical Development Team. Abstracts—Latebreaker Program of the 6th European Conference on Clinical Aspects and Treatment of HIV-Infection. 1997. A phase II, double-blind, placebo-controlled, dose-ranging study to assess the antiretroviral activity and safety of efavirenz (DMP 266) in combination with open-label zidovudine, abstr. 920; p. 5. [Google Scholar]
- 15.Hsiou Y, Das K, Ding J, Clark A D, Jr, Boyer P L, Janssen P A J, Kleim J P, Rösner M, Hughes S H, Arnold E. Programs and abstracts of the 2nd International Workshop on HIV Drug resistance and Treatment Strategies. 1998. Crystal structures of wild-type and mutant HIV-1 reverse transcriptase and nonnucleoside inhibitors: implications for drug resistance mechanisms, abstr. 21; p. 17. [Google Scholar]
- 16.Imrie A, Beveridge A, Genn W, Vizzard J, Cooper D A The Sydney Primary HIV Infection Study Group. Transmission of human immunodeficiency virus type 1 resistant to nevirapine and zidovudine. J Infect Dis. 1997;175:1502–1506. doi: 10.1086/516487. [DOI] [PubMed] [Google Scholar]
- 17.Jeffrey S, Baker D, Tritch R, Rizzo C, Logue K, Bacheler L. 5th Conference on Retroviruses and Opportunistic Infections. 1998. A resistance and cross resistance profile for SUSTIVA (efavirenz, DMP 266), abstract 702. [Google Scholar]
- 18.Kleim J P, Bender R, Kirsch R, Meichsner C, Paessens A, Rösner M, Rübsamen-Waigmann H, Kaiser R, Wichers M, Schneweis K E, Winkler I, Reiss G. Preclinical evaluation of HBY 097, a new nonnucleoside reverse transcriptase inhibitor of human immunodeficiency virus type 1 replication. Antimicrob Agents Chemother. 1995;39:2253–2257. doi: 10.1128/aac.39.10.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleim J P, Bender R, Kirsch R, et al. Mutational analysis of residue 190 of HIV-1 reverse transcriptase. Virology. 1994;200:696–701. doi: 10.1006/viro.1994.1233. [DOI] [PubMed] [Google Scholar]
- 20.Koup R A, Merluzzi V J, Hargrave K D, et al. Inhibition of HIV-1 replication by the dipyridodiazepinone BI-RG-587. J Infect Dis. 1991;163:966–970. doi: 10.1093/infdis/163.5.966. [DOI] [PubMed] [Google Scholar]
- 21.Larder B A, Kohli A, Bloor S, Kemp S D, Harrigan P R, Schooley R T, Lange J M A, Pennington K N, St. Clair M H The Protocol 34,225-02 Collaborative Group. Human immunodeficiency virus type 1 drug susceptibility during zidovudine (AZT) monotherapy compared with AZT plus 2′,3′-dideoxyinosine or AZT plus 2′,3′-dideoxycytidine combination therapy. J Virol. 1996;70:5922–5929. doi: 10.1128/jvi.70.9.5922-5929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller V, Phillips A, Rottmann C, Staszewski S, Pauwels R, Hertogs K, De Bèthune M P, Kemp S D, Bloor S, Harrigan P R, Larder B A. Dual resistance to zidovudine (ZDV) and lamivudine (3TC) in patients treated with ZDV/3TC combination therapy: association with therapy failure. J Infect Dis. 1998;177:1521–1532. doi: 10.1086/515304. [DOI] [PubMed] [Google Scholar]
- 23.Moeremans M, De Raeymaeker M, Van den Broeck R, Stoffels P, De Brabander M, De Cree J, Hertogs K, Pauwels R, Staszewski S, Andries K. Programs and abstracts of the 4th International Workshop on HIV Drug Resistance. 1995. Virological analysis of HIV-1 isolates in patients treated with the non-nucleoside reverse transcriptase inhibitor R091767, (−)-(S)-8-chloro-4,5,6,7-tetrahydro-5-methyl-6-(3-methyl-2-butenyl)imidazo[4,5,1-jk] [1,4]benzodiazepine-2(1H)-thione monohydrochloride (8-chloro-TIBO), abstr. 38; p. 33. [Google Scholar]
- 24.Montaner J S G, Reiss P, Cooper D, Vella S, Harris M, Conway B, Wainberg M A, Smith D, Robinson P, Hall D, Myers M, Lang J M A for The INCAS Study Group. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients. JAMA. 1998;279:930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 25.Murphy R L, Montaner J. Nevirapine: a review of its development, pharmacological profile and potential for clinical use. Exp Opin Invest Drugs. 1996;5:1183–1199. [Google Scholar]
- 26.Nájera I, Holguín Á, Quinones-Mateu M E, Muñoz-Fernández M, Nájera R, López-Galíndez C, Domingo E. pol gene quasispecies of human immunodeficiency virus mutations associated with drug resistance in virus from patients undergoing no drug therapy. J Virol. 1995;69:23–31. doi: 10.1128/jvi.69.1.23-31.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijhuis M, Back N, de Jong D, Keulen W, Schuurman R, Berkhout B, Boucher C. Program and abstracts of the 5th International Workshop on HIV Drug Resistance. 1996. Host cell-dependent replication efficacy of 3TC-resistant HIV-1 variants, abstr. 86; p. 54. [Google Scholar]
- 28.Pauwels R, Andries K, Desmyter J, et al. Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature. 1990;343:470–473. doi: 10.1038/343470a0. [DOI] [PubMed] [Google Scholar]
- 29.Pauwels R, Andries K, Debyser Z, et al. Potent and highly selective human immunodeficiency virus type 1 inhibition by a series of α-anilinophenylacetamide derivatives targeted at HIV-1 reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:1711–1715. doi: 10.1073/pnas.90.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauwels R, Andries K, Debyser Z, Kukla M J, Schols D, Breslin H J, Woestenborghs R, Desmyter J, Janssen M A C, De Clercq E, Janssen P A J. New tetrahydroimidazo[4,5,1-jk][1,4]-benzodiazepin-2(1H)-one and -thione derivatives are potent inhibitors of human immunodeficiency virus type 1 replication and are synergistic with 2′,3′-dideoxynucleoside analogs. Antimicrob Agents Chemother. 1994;38:2863–2870. doi: 10.1128/aac.38.12.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richman D, Shih C K, Lowy I, et al. HIV-1 mutants resistant to nonnucleoside reverse transcriptase inhibitors arise in tissue culture. Proc Natl Acad Sci USA. 1991;88:11241–11245. doi: 10.1073/pnas.88.24.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richman D, Rosenthal A S, Skoog M, Eckner R J, Chou T-C, Sabo J P, Merlazzio V. BI-RG-587 is active against zidovudine-resistant human immunodeficiency virus type 1 and synergistic with zidovudine. Antimicrob Agents Chemother. 1991;35:305–308. doi: 10.1128/aac.35.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richman D D, Havlir D, Corbeil J, Looney D, Ignacio C, Spector S A, Sullivan J, Cheeseman S, Barringer K, Panletti D, Shih C-K, Myers M, Griffin J. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68:1660–1666. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozenbaum W The AVANTI Study Group. Program and abstracts of the 4th Conference on Retroviruses and Opportunistic Infections. 1997. AVANTI 1. A randomized, double-blind, comparative trial to evaluate the efficacy, safety and tolerance of combination antiretroviral regimens for the treatment of HIV-1 infection: AZT/3TC versus AZT/3TC/loviride in antiretroviral naive patients, abstr. 368; p. 132. [Google Scholar]
- 35.Rübsamen-Waigmann H, Waigmann M A, Huquenel E, Shah A, Paessens A, Kleim J P, Rosner M. XI International Conference on AIDS. 1996. Antiviral profile of HBY-097, a nonnucleoside inhibitor of HIV-1 RT in a phase I study, abstr. Mo. A. 1102. [Google Scholar]
- 36.Ruiz N M, Manion D J, Labriola D F, Friedman P A, Gorelick K J, Faulkner E B, Goldberg A P The DMP 266 Development Team. Abstracts—Latebreaker Program of the 6th European Conference on Clinical Aspects and Treatment of HIV-Infection. 1997. HIV-1 suppression to “<1 copy/mL” (OD = background) by Amplicor assay in patients receiving indinavir +/− DMP 266 (efavirenz). Results of DMP 266-003, cohort IV, abstr. 921; p. 5. [Google Scholar]
- 37.Salzman N K, Lane H C, Chappey C, Imamichi H, Zhang Y M. Program and abstracts of the 5th International Workshop on HIV Drug Resistance. 1996. Patterns of HIV drug resistance during combined or monotherapy with delavirdine, abstr. 51; p. 32. [Google Scholar]
- 38.Shah A, Kumor K, Sullivan J, Amand R, Cole S, Agarwal V, Krol G, Huguenel E, Suarez J R, Heller A H. XI International Conference on AIDS. 1996. Safety, tolerability and pharmacokinetics of HBY-097 in asymptomatic and mildly symptomatic HIV positive patients, abstr. Mo. B. 1326. [Google Scholar]
- 39.Spence R A, Kati W M, Anderson K S, Johnson K A. Mechanism of inhibition of HIV-1 reverse transcriptase by nonnucleoside inhibitors. Science. 1995;267:988–993. doi: 10.1126/science.7532321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staszewski S, Miller V, Kober A, Colebunders R, Vandercam B, Delescluse J, Clumeck N, Van Wanzeele F, De Brabander M, De Creè J, Moeremans M, Andries K, Boucher C, Stoffels P, Janssen P A J members of The Loviride Collaborative Study Group. Evaluation of the efficacy and tolerance of R 018893, R 089439 (loviride) and placebo in asymptomatic HIV-1-infected patients. Antiviral Ther. 1996;1:42–50. [PubMed] [Google Scholar]
- 41.Staszewski S, Miller V, Rehmet S, Stark T, De Creè J, De Brabander M, Peeters M, Andries K, Moeremans M, De Raeymaeker M, Pearce G, Van Den Broeck R, Verbiest W, Stoffels P. Virological and immunological analysis of a triple combination pilot study with loviride, lamivudine and zidovudine in HIV-1-infected patients. AIDS. 1996;10:F1–F7. doi: 10.1097/00002030-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Tantillo C, Ding J, Jacobo-Molina A, et al. Locations of anti-AIDS drug binding sites and resistance mutations in the three-dimensional structure of HIV-1 reverse transcriptase inhibitors. J Mol Biol. 1994;243:369–387. doi: 10.1006/jmbi.1994.1665. [DOI] [PubMed] [Google Scholar]
- 43.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakefield J K, Jablonski S A, Morrow C D. In vitro enzymatic activity of human immunodeficiency virus type 1 reverse transcriptase mutants in the highly conserved YMDD amino acid motif correlates with the infectious potential of the proviral genome. J Virol. 1992;66:6806–6812. doi: 10.1128/jvi.66.11.6806-6812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winslow D L, Garber S, Reid C, Scarnati H, Baker D, Rayner M M, Anton E D. Selection conditions affect the evolution of specific mutations in the reverse transcriptase gene associated with resistance to DMP 266. AIDS. 1996;10:1205–1209. doi: 10.1097/00002030-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Young S D, Britcher S F, Tran L O, Payne L S, Lumma W C, Lyle T A, Huff J R, Anderson P S, Olsen D B, Carroll S S, Pettibone D J, O’Brien J A, Ball R G, Balani S K, Lin J H, Chen I-W, Schleif W A, Sardana V V, Long W J, Byrnes V W, Emini E A. L-743,726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1995;39:2602–2605. doi: 10.1128/aac.39.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zennou V, Mammano F, Paulous S, Mathez D, Clavel F. Loss of viral fitness associated with multiple Gag and Gag-Pol processing defects in human immunodeficiency virus type 1 variants selected for resistance to protease inhibitors in vivo. J Virol. 1998;72:3300–3306. doi: 10.1128/jvi.72.4.3300-3306.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]