Abstract
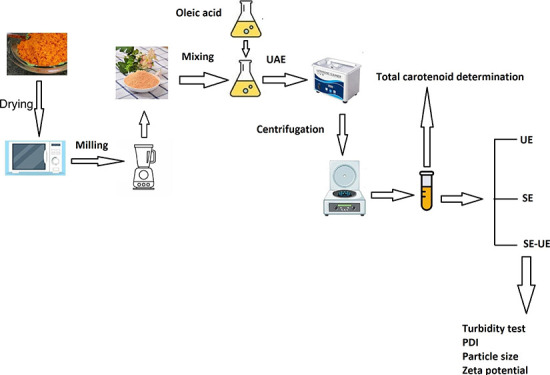
This study aimed to use oleic acid-based ultrasonic-assisted extraction (UAE) to recover carotenoids from carrot pomace and emulsify the enriched-carotenoid oleic acid using spontaneous and ultrasonic-assisted emulsification. The extraction performance of oleic acid was compared with traditional organic solvents, including hexane, acetone, and ethyl acetate. The one-factor experiments were employed to examine the impact of UAE conditions, including liquid-to-solid ratios, temperature, ultrasonic power, and time, on the extraction yield of carotenoids and to find the conditional ranges for the optimization process. The response surface methodology was employed to optimize the UAE process. The second-order extraction kinetic model was used to find the mechanism of oleic acid-based UAE. After that, the enriched-carotenoid oleic acid obtained at the optimal conditions of UAE was used to fabricate nanoemulsions using spontaneous emulsification (SE), ultrasonic-assisted emulsification (UE), and SE-UE. The effect of SE and UE conditions on the turbidity of nanoemulsion was determined. Then, the physiochemical attributes of the nanoemulsion from SE, UE, and spontaneous ultrasonic-assisted emulsification (SE-UE) were determined using the dynamic light scattering method. The extraction yield of carotenoids from carrot pomace by using sonication was the highest. The adjusted optimal conditions were 39 mL/g of LSR, 50 °C, 12.5 min, and 350 W of ultrasonic power. Under optimal conditions, the carotenoid content attained was approximately 163.43 ± 1.83 μg/g, with the anticipated value (166 μg/g). The particle sizes of nanoemulsion fabricated at the proper conditions of SE, UE, and SE-UE were 31.2 ± 0.83, 33.8 ± 0.52, and 109.7 ± 8.24 nm, respectively. The results showed that SE and UE are suitable methods for fabricating nanoemulsions. The research provided a green approach for extracting and emulsifying carotenoids from carrot pomace.
1. Introduction
The food industry generates an enormous quantity of waste and byproducts worldwide. Utilizing food waste and byproducts to produce bioactive natural components in this situation can further alleviate the environmental burden and promote the circular economy.1 The food processing wastes and byproducts from plants can be a potential source to produce the natural bioactive components compared to other sources, such as poultry, meat, and dairy.2,3 Using fruit/vegetable waste can address the escalating consumer demand for natural bioactive components and offers considerable potential for diverse applications within the food industry. Among these components, carotenoids are widely known for free radical quenching capacity and protecting organs from photooxidation.1 Furthermore, several clinical studies exhibit the positive correlation of high dietary intake of carotenoids with reduced incidents of various health conditions, such as cancers, chronic illnesses, and neurological and eye-related dysfunctions.4
Carrots are planted in various locations, such as America, China, Southwest Asia, and India.5,6 The processing of carrots before usage leads to enormous residues, encompassing peels and pomace. These residues are commonly used for animal feed or discarded into landfills.5 However, carrot pomace contains a variety of carotenoids such as α-carotene, β-carotene, and lutein. Therefore, developing a suitable extraction method that can effectively exploit carotenoids from carrot pomace is necessary.
Extraction is the engineering to separate bioactive components from the plant matrix. Conventional extraction techniques are commonly used to recover bioactive components from materials, such as wild thyme and watermelon rind.7,8 Nevertheless, the disadvantages of conventional methods are the overuse of solvents, leading to high energy usage and low extraction yield.9 The novel extraction approach, such as supercritical fluid extraction (SFE), employs the supercritical state of a substance to extract bioactive compounds from plants.10 Supercritical fluids possess liquid properties (solubilization ability, viscosity, and surface tension) and gas properties (diffusivity). In the SFE process, the cell membrane is partially disrupted as the solvents permeate the plant matrix. This phenomenon improves mass transfer, increasing the extraction efficiency of bioactive compounds.10 The SFE was applied to recover essential oil from Eryngium billardieri, oil from Ferulago angulata, Smyrnium cordifolium Boiss, Dracocephalum kotschyi seeds, Pistacia khinjuk, Portulaca oleracea seed, and omega-3 from D. kotschyi seeds.11−17 However, the drawback of this technique is high running and investment costs.18 The ultrasonic-assisted extraction (UAE) technique has been developed to address the drawbacks of conventional extraction techniques. UAE can improve extraction yield by generating the acoustic cavitation effect, destroying cell walls, and increasing solvent exposure to bioactive components.7 UAE is compatible with green chemistry standards due to reducing solvent usage, operation time, and environmental pollution.19 Therefore, UAE can be the appropriate method for the extraction of carotenoids from carrot pomaces.
Organic solvents, such as hexane, methanol, ethanol, ethers, and diethyl acetate, have been commonly used to extract carotenoids from plant materials for years. Nevertheless, organic solvents are toxic, volatile, and flammable, severely threatening the natural environment.20 Therefore, these problems strongly motivate the scientific community to discover green solvents as replacements for organic solvents for separating carotenoids. Oleic acid (cis-9-octadecenoic acid), a monounsaturated fatty acid, commonly occurs in plant seeds such as olives, sesames, and peanuts.21,22 Oleic acid is liquid at room temperature and has an extraction capacity for carotenoids due to their nonpolar properties.22 Oleic acid has distinctive attributes compared to organic solvents, such as high degradability, nonflammability, nontoxicity, and biocompatibility, which confirm the rules of green chemistry.20 Additionally, it can alleviate the risk of cardiovascular diseases, the oxidation of low-density lipoproteins, and insulin intolerance; thus, carotenoid-enriched extracts of oleic acid can directly be applied in food products and the pharmaceutical industry.21,23 Currently, numerous studies used plant oils combined with UAE to isolate carotenoids from plants, such as extracting carotenoids from Seabuckthorn pomace using corn and olive oils, carotenoids from carrot pomace using linseed oil, carotenoids from fresh carrots using sunflower oil, and carotenoids from Seabuckthorn using flaxseed oil.1,2,24−26 However, no study employed oleic acid as a solvent to obtain carotenoids from carrot pomace and investigated the extraction mechanism of oleic-based UAE.
Response surface methodology (RSM) is commonly employed to design experiments and find the formulation of food and pharmaceutical products.27 Using RSM, the influence of independent factors and the interaction among independent factors on dependent responses can be investigated by establishing the polynomial regression models.28 RSM with central Box-Behnken design (BBD) and composite design (CCD) models were commonly used to optimize the UAE process.29 The advantages of BBD compared to CCD do not possess extreme points; thus, it has fewer experimental trials than CCD.29 Therefore, the BBD model is selected in this study.
In recent years, there has been increasing interest in utilizing nanoemulsions to encapsulate lipophilic compounds, thereby expanding the potential applications of essential oils in food formulation.30 Nanoemulsions refer to emulsions with small droplets, typically below 200 nm. Compared to larger droplets, these nanoemulsions have enhanced kinetic stability against undesirable phenomena, such as flocculation, coalescence, and cream.31 There are two main categories of methods for fabricating a nanoemulsion system: high-energy methods (using ultrasound, microfluidizing, high-pressure homogenizing, rotor-stator mixing, and membranes) and low-energy methods [spontaneous emulsification (SE), phase inversion composition, and phase inversion temperature]. Ultrasonic-assisted emulsification (UE) and SE are common approaches to fabricating a nanoemulsion system. In SE, emulsification arises when two immiscible liquids contact each other under disequilibrium conditions, driven by distinction in chemical potential between the two phases.32 In UE, applying high-intensity ultrasonic waves in liquids induces the rapid oscillation and violent collapse of microbubbles, generating tremendous cavitation forces within their surrounding area.33 Several studies have utilized these techniques to fabricate carotenoid-loaded nanoemulsions. Surh et al. investigated the effect of SE conditions, such as oil type, surfactant type, and surfactant concentration, on the mean particle size and color of lutein-loaded nanoemulsion systems.34 Lad et al. fabricated oil-in-water emulsions using ultrasonic-assisted emulsification and coconut protein as an emulsifier.35 However, to the best of our knowledge, no study has been conducted in a transparent way from carotenoid recovery from carrot pomace to the direct generation of nanoemulsion systems from enriched-carotenoid oleic acid. Additionally, the effects of UE conditions on the turbidity, the correlation of nanoemulsion turbidity and mean particle sizes, and the comparison of UE, SE, and SE-UE were not elucidated. Furthermore, using oleic acid as an oil phase to encapsulate carotenoids from carrot pomace was not investigated.
Therefore, the present research aims to assess the extraction capacity of oleic acid compared to acetone, hexane, and ethyl acetate from carrot pomace; then, this study examined the effect of UAE conditions, including liquid-to-solid ratios, ultrasonic power, temperature, and time, on the performance of carotenoid recovery. A BBD model was applied to optimize the UAE conditions for the carotenoid extraction. The UAE kinetics were also investigated using a second-order extraction model, and the antioxidant activities of the enriched-carotenoid oleic acid were evaluated. Then, enriched-carotenoid oleic acid was encapsulated to create nanoemulsions using SE, UE, and SE–UE. The effect of SE and UE on the turbidity of the nanoemulsions was investigated. After that, the physicochemical attributes (turbidity, particle size, polydispersity index (PDI), and zeta potential) of nanoemulsions from SE, UE, and SE–UE were compared.
2. Materials and Methods
2.1. Materials
Carrot pomace was obtained from Nam Viet Company, Di An, Binh Duong, Vietnam and then dried at 45 °C to achieve a moisture content of 7%. Dried carrot pomace was milled by a pulverizer (DP100-1, Qingdao Mars Labtech Co., Ltd.) to obtain carrot pomace powder (CPP). β-carotene (purity ≥95% HPLC), iron(II) sulfate heptahydrate (purity ≥99%), absolute ethanol (purity ≥99.8%), salicylic acid (purity ≥99%), hydrogen peroxide (purity 34.5–36.5%), 1,1-diphenyl-2-picrylhydrazyl (DPPH, purity ≥97%), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, purity 98%), and tween80 (99.7%) were obtained from Sigma-Aldrich Chemical Co., Ltd., Singapore. Oleic acid (purity ≥90%) was purchased from Xilong Scientific Co., Ltd., Guangdong, China.
2.2. Comparing the Extraction Performance of Oleic Acid to Other Solvents
CPP was accurately weighed at 2 ± 0.0010 g in an amber glass bottle, and 10 mL of solvents (oleic acid, hexane, ethyl acetate, and acetone) was poured into this bottle. Then, the mixture was put in an ultrasonic bath (model: RS22L, 40 kHz, Rama Viet Nam Joint Stock Company, District 9, Ho Chi Minh, Vietnam) at 300W of ultrasonic power and 30 °C for 15 min. The CPP in the mixture was separated using centrifugal machines at 1800 g at 30 °C for 30 min (DM0412, DLAB Scientific Co., Ltd., Shunyi, Beijing, China) before carotenoids, and antioxidant activities were determined.
2.3. Single-Factor Experiments
The quantified figure for CPP was placed in the amber glass jar, and 10 mL of oleic acid was added to this jar. The fixed parameters of UAE were 20 mL/g of LSR, 30 °C, 15 min, and 300 W. When one factor changed, the other was set at the above-mentioned conditions or the results of the previous section. The mixture was treated by ultrasound under different liquid-to-solid ratios (LSR at 5, 10, 20, 30, and 40 mL/g), ultrasonic power (0, 150, 300, 450, 600, and 750W), temperature (30–80 °C with the interval of 10 °C), and extraction time (5, 10, 15, 30, 50, 70, and 90 min). The carotenoid content (CC) in the extracts is used to evaluate the effect of ultrasonic conditions.
2.4. Experimental Design
BBD was chosen to optimize the extraction process, and the conditional ranges of factors (LSR, ultrasonic power, temperature, and extraction time) were selected from the results in Section 2.3. Three levels, +1, 0, and −1, were coded for upper, proper, and lower conditions, respectively. The highest extraction performance for each variable in Section 2.3 was proper conditions, while the upper and lower conditions were higher and lower values of proper conditions, respectively. A total of 29 experiments were performed; the relationships between factors and dependent variables were expressed by polynomial regression models, eq 1:
| 1 |
where A0,Ai, Aj, and Aij are the intercept, linear, and interaction coefficients, respectively, and n is the quantity of factors (n = 4). Y symbolizes the dependent variables, while X represents the factorial values. The four factors were LSR (20, 30, and 40 mL/g), temperature (40, 50, and 60 °C), time (10, 15, and 30 min), and ultrasonic power (300, 450, and 600 W). The dependent variable (Y) was the CC (microgram per gram of dried basis, μg/g).
2.5. Total Carotenoid Determination
The oleic acid extracts’ CCs were quantified by the Goula method using the spectrophotometer with slight modifications.36 Briefly, enrich-carotenoid oleic acid was shaken for 2 min, and the absorbance was measured by using a spectrophotometer (UV–vis spectrophotometer, Hach DR/2010, LabWrech, Midland, Ontario, Canada) at 450 nm by taking oleic acid as reference. CC was extrapolated byeq 2. The extinction coefficient of carotenoids in oleic acid was determined by the Karabagiaset method, in which β-carotene was used as a standard substance.37 The extinction coefficient of β-carotene (E) in oleic acid was determined as 1980 and that of other solvents was used in Chen et al.38
| 2 |
where K is the carotenoid contents in μg/g db; A is the absorbance of oleic acid extracts; d is the width of the cuvette (1 cm); and E is the extinction coefficient.
2.6. Extraction Kinetics
Extraction kinetic models founded on second-order rate rules were employed to describe the extraction process of carotenoids. For these models, the dissolution rate of carotenoids in the plant matrix to solvents can be expressed by eq 3:
| 3 |
where Ca is the concentration of carotenoids at the equilibrium point (μg/g), Cb is the concentration of carotenoids at any time (μg/g), k is the constant of second-order extraction rate (g/μg.min), and t is the extraction time (min).
Solving differential eq 2, we obtained
| 4 |
By reversing eq 4, a linear equation can be expressed as
| 5 |
where y, a, and b corresponded to t/Cb, 1/Cs, and 1/kCa.2 When t is equal to 0, the initial extraction rate, h (μg/g·min), became:
| 6 |
The constant of the second-order extraction rate is calculated by building t/Ca against t using eq 5. The activation energy (Ea, kJ/mol) and Arrhenius constant (Ae, g/μg·min) were calculated using eq 7:
| 7 |
2.7. Emulsification Procedures
2.7.1. Ultrasonic-Assisted Emulsification Procedure
UE was used for the production of nanoemulsions. At first, water and tween 80 were mixed by a magnetic stirrer for 5 min at 500 rpm, and then the oil phase (enriched-carotenoid oleic acid) was added slowly to the aqueous phase while mixing by a magnetic stirrer. The oil phase concentration was fixed for all nanoemulsions (10% volume/volume, v/v). The mixture was treated by ultrasonication under different surfactant-to-oil ratios (SOR, 1:1, 2:1, 3:1, 4:1, and 5:1), ultrasonic power (150, 300, 450, 600, 750, and 900W), temperature (30–60 °C with the interval of 10 °C), and time (5, 10, 15, 20, and 30 min). Then, the turbidity of the fabricated nanoemulsions is used to evaluate the effect of ultrasonic conditions.
2.7.2. SE Procedure
SE was used for the production of nanoemulsions. At first, enriched-carotenoid oleic acid and tween 80 were mixed by a magnetic stirrer for 5 min at 500 rpm, and then the water was added slowly for 30 min. The oil phase concentration was fixed for all nanoemulsions (10% v/v). The mixture was treated under different SORs (1:1, 2:1, 3:1, 4:1, 5:1, 6:1, and 7:1) and oil phase concentrations (10, 20, 30, and 40% v/v). Then, the turbidity of the fabricated nanoemulsions is used to evaluate the effect of SE conditions.
2.7.3. Combination of Spontaneous and Ultrasonic-Assisted Emulsification
A combination of SE and UE was used for the production of nanoemulsions. At first, enriched-carotenoid oleic acid and tween 80 were mixed by a magnetic stirrer for 5 min at 500 rpm, and then, the aqueous phase was added slowly to the mixed oil phase while mixing by magnetic stirrer for 30 min at 750 rpm. Then, the premixed emulsions were treated by UE with the best-suited oil phase concentration, SOR, ultrasonic power, temperature, and time from sectionSection 2.7.1. The most suitable oil phase concentration from Section 2.7.2 was fixed for all nanoemulsions. In the end, the turbidity of the fabricated nanoemulsions is used to evaluate the effect of SE-UE conditions.
2.7.4. Turbidity, Particle Size, PDI, and Zeta Potential Determination
The turbidity measurement followed the method of Sin Neen Liew et al. with slight modification,39 where the turbidity of all the formulated nanoemulsions was analyzed using a UV–vis spectrophotometer. The absorbance of the undiluted nanoemulsions was measured at a wavelength of 600 nm. Distilled water was used as a blank.
The dynamic light scattering (DLS) approach was employed to measure the samples’ particle size, PDI, and zeta potential. The samples’ physicochemical characteristics were determined using a HORIBA Nanoparticle Analyzer nanoPartica SZ-100 (HORIBA Scientific, Japan). The samples were diluted 100 times with distilled water. The measurements were conducted at 25 °C under low light conditions. When a light beam passes through nanoemulsions, some of the light is scattered in all directions by the particles and an angle is measured to determine the particle size and PDI. The nanoparticle analyzer calculates the zeta potential by determining the electrophoretic mobility and then using the Henry equation.
2.8. Antioxidant Activity Determination
The antioxidant activities of the enriched-carotenoid oleic acid were measured using the colorimetric method, and Trolox was employed to build the standard curves. DPPH radical quenching capacity (DPPH) was quantified by the Minaxi Shama method1 using a DPPH substance in ethanol. The hydroxyl radical quenching capacity (OH) was determined by the Yuan method,40 employing salicylic acid as a color reagent. DPPH and OH were presented as micromoles of Trolox equivalent per gram of dried basis (μM TE/g db).
2.9. Statistical Analysis
Minitab (version 19.1, Minitab Inc., Pennsylvania, USA) was employed to conduct statistical analysis in which the chosen confidence value is 95%. The experiments were repeated threefold, and the experimental findings were presented as mean ± standard deviation. The graphs were constructed using Origin Pro 2022 (OriginLab, Northampton, Massachusetts, United States), while Design Expert (v13, Stat-Ease Inc., Minneapolis, Minnesota, United States) performed the optimization process.
3. Results and Discussions
3.1. Single-Factor Experiment
3.1.1. Solvent Comparison
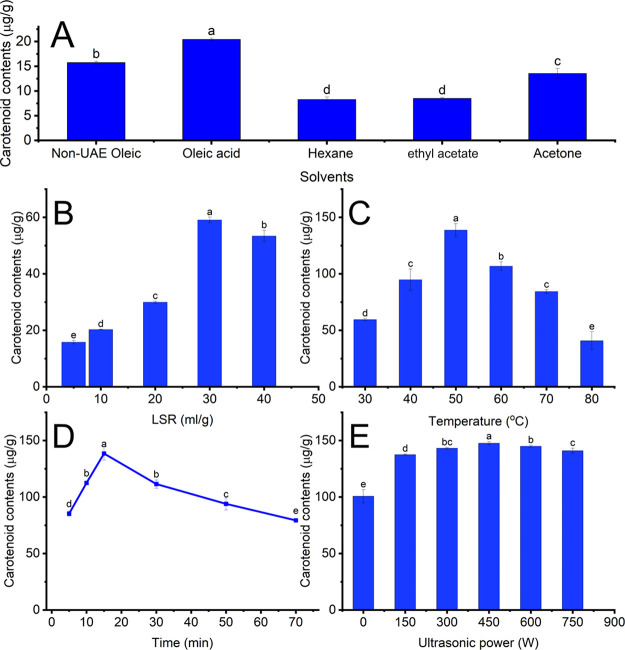
The UAE conditions and solvents profoundly influence the extraction yield of carotenoids from carrot pomace, which is closely associated with the destructive effect of ultrasound and the polarity of oleic acid. This study examined the influence of oleic-based-UAE conditions, including LSR, ultrasonic power, temperature, and time, on the extraction yield of carotenoids, and the results are presented in Figure 1A–E. Such an examination would be useful for designing a novel and green procedure and selecting suitable parameters to extract carotenoids from carrot pomace. Figure 1A compares the extraction performance of oleic acid with those of other conventional organic solvents. Oleic-acid-based UAE had the highest extraction yield of carotenoid from CPP, followed by oleic-acid-based non-UAE and acetone-based UAE. Cis-double bonds in oleic acid molecules can generate π–π interaction with the double bonds of carotenes in CPP, enhancing the extraction yield of carotenoids from carrot pomace.41−43 This trend achieved a consensus with Raviadaran et al., who created nanoemulsions using sonication. Revathi Raviadaran suggested that polyglycerol polyricinoleate can generate a stable water-in-oil emulsion with plant oils because it can bind with fatty acids in plant oils.41 The extraction yield of oleic-acid-based UAE was higher than that of non-UAE, which can be attributed to the acoustic cavitation effect. The cavitation effect creates a shockwave and facilitates particle collision, which triggers sonoporation and fragmentation of the material. This phenomenon improves the solubility of carotenoids in oleic acid due to a reduction in the particle size of materials.44 Therefore, oleic acid-based UAE was suitable for extracting carotenoids from CPP.
Figure 1.
Impact of solvents and UAE conditions on carotenoid contents. (A) Impact of solvents. (B) Impact of LSR. (C) Impact of temperature. (D) Impact of time. (E) Impact of ultrasonic power. Different letters demonstrate significant differences (p < 0.05).
3.1.2. Effect of Solid-to-Liquid Ratios
The effect of different LSR on the oleic acid-based UAE of carotenoids from CPP was determined using oleic acid as a green solvent. As presented in Figure 1B, the extraction yield of carotenoids considerably rose with increasing LSR (from 5 to 30 mL/g). The high LSR can restrict the generation of small equilibriums, enhancing the diffusion rate of oleic acid into CPP. Additionally, the high LSR can absorb more ultrasonic energy and impose more cavitation effects on the CPP surface, creating small pores and fragmentation in the cellular structure. The combination of these effects can improve the extraction efficiency of carotenoids from CPP.45 Nevertheless, the CC decreased as the LSR increased to 40 mg/g. The excessive absorption of ultrasonic energy at higher LSR can degrade carotenoids, shrinking extraction efficiency.46 This trend was consensus with Marana and Priya, who recovered pectin from sisal waste using UAE. In this research, the highest recovery yield of pectin from sisal waste rose as the LSR experienced an increase from 20 to 30 mL/g, followed by a further rise in LSR to 40 mL/g.47 As a result, the most suitable range of LSR for recovering carotenoids from CPP using oleic acid was 20–40 mL/g.
3.1.3. Effect of Temperature
The influence of temperature on the extraction of carotenoids was investigated in the range of 30 to 70 °C, and the results are depicted in Figure 1C. The CC increased as the temperature rose from 30 to 50 °C. The high extraction yield of carotenoids at moderate temperatures can be attributed to vicious reduction and solvent solubility increment, which promotes the cavitation effect and the mass transfer of carotenoids into oleic acid.44 On the other hand, the reverse trend was true when the temperature rose to 80 °C. The high temperature can destroy carotenoids, decreasing extraction efficiency.44 These results agreed with Andi Suo, who obtained carotenoids from apricot flesh using corn oil-based UAE. In this study, the CC increased as temperature rose and peaked at 41.53 °C. However, when the temperature increased over 41.53 °C, the recovery yield of carotenoids from apricot flesh decreased.48 Therefore, 40–60 °C was the most proper range for obtaining carotenoids from CPP.
3.1.4. Effect of Time
The effect of time on the extraction efficiency of carotenoids was determined from 5 to 70 min, and the results are shown in Figure 1D. The recovery of carotenoids was high at the initial stage and significantly increased to 15 min. The high extraction efficiency at the initial stage can be ascribed to the large differences in the CC between CPP and oleic acid.44 By contrast, extending the extraction time from 15 to 70 min can reduce the recovery of CCs, which can be accounted for the destruction of carotenoids by long-time exposure to ultrasonic waves.46 Chutia and Mahanta also reported similar results while extracting carotenoids from passion fruit peels using olive oil. In their study, the extraction yield of carotenoids improved as sonication time increased between 10 and 40 min, followed by a moderate reduction with a continuous rise in time.49 Therefore, 10 to 30 min was the most suitable range for extracting carotenoids from CPP.
3.1.5. Effect of Ultrasonic Power
The effect of ultrasonic power on the extraction of carotenoids was examined from 150 to 750 W. As shown in Figure 1E, the CC peaked at 450 W, followed by a moderate reduction. Increasing CC with a rise in ultrasonic power can be attributed to the enhancement of sonoporation and shear forces on the material surface, enhancing the diffusivity of oleic acid into CPP. This phenomenon can facilitate the solubility of intracellular substances in the solvents, improving the extraction yield. However, high ultrasonic power can trigger the degradation of carotenoids, decreasing extraction yield.46 This trend reached a consensus with Al-Dhabi et al., who found the effect of ultrasonic power on the recovery yield of phenolics from spent coffee grounds. Al-Dhabi reported that as ultrasonic power increased from 100 to 200 W, the extraction yield of phenolics increased, followed by a decrease with a continuous rise in power to 250 W.50 Therefore, the ultrasonic power from 300 to 600 W was the most suitable range for attaining carotenoids from CPP.
3.2. Optimization of the Oleic-Acid-Based UAE Process for Carotenoid Recovery
The experimental results of the RSM with BBD models and regression coefficients are demonstrated in Table 1. The ANOVA and polynomial regression models were built using Design Expert 13. A polynomial model (eq 8), which is presented below, is used to express a relationship between factors and dependent responses:
| 8 |
Table 1. Experimental Values of the UAE Process and the Regression Coefficient of the Polynomial Regressiona.
| carotenoid
contents (μg/g) |
ANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| no | LSR | temperature | time | ultrasonic power | predicted values | experimental values | regression coefficients | carotenoid | |
| 1 | 0 | 1 | 1 | 0 | 65.7 | 66.0 ± 3.0 | intercept | A0 | 143.2* |
| 2 | 1 | –1 | 0 | 0 | 104.0 | 98.9 ± 1.5 | linear | A1 | 22.95* |
| 3 | 1 | 0 | 1 | 0 | 85.9 | 89.6 ± 4.6 | A2 | –0.45 | |
| 4 | –1 | 0 | 0 | –1 | 38.4 | 35.9 ± 0.9 | A3 | –8.00* | |
| 5 | 0 | 0 | –1 | –1 | 106.2 | 106.0 ± 2.7 | A4 | –8.86* | |
| 6 | 1 | 1 | 0 | 0 | 91.6 | 85.3 ± 4.4 | interaction | A12 | –5.77* |
| 7 | 0 | 0 | 1 | –1 | 98.0 | 94.2 ± 6.3 | A13 | –18.61* | |
| 8 | 1 | 0 | –1 | 0 | 139.2 | 132.0 ± 2.0 | A14 | –37.46* | |
| 9 | 1 | 0 | 0 | 1 | 66.6 | 65.3 ± 3.4 | A23 | –3.91* | |
| 10 | 0 | –1 | 0 | 1 | 63.4 | 72.4 ± 4.1 | A24 | 6.62* | |
| 11 | –1 | 0 | –1 | 0 | 56.0 | 69.9 ± 0.8 | A34 | –3.88 | |
| 12 | 0 | 0 | –1 | 1 | 96.2 | 85.9 ± 1.5 | quadratic | A11 | –28.43* |
| 13 | –1 | 1 | 0 | 0 | 57.2 | 58.3 ± 0.1 | A22 | –39.94* | |
| 14 | 0 | –1 | 1 | 0 | 74.4 | 76.4 ± 2.3 | A33 | –25.18* | |
| 15 | 0 | –1 | –1 | 0 | 82.6 | 78.4 ± 10.7 | A44 | –24.81* | |
| 16 | 0 | 1 | 0 | –1 | 80.2 | 79.2 ± 2.6 | R2 | 0.9881 | |
| 17 | 0 | 1 | 0 | 1 | 75.8 | 77.0 ± 3.4 | adjusted R2 | 0.9762 | |
| 18 | –1 | –1 | 0 | 0 | 46.6 | 48.2 ± 4.2 | predicted R2 | 0.9314 | |
| 19 | 0 | 1 | –1 | 0 | 89.5 | 93.7 ± 4.2 | F values | 83.02 | |
| 20 | 1 | 0 | 0 | –1 | 159.2 | 155.6 ± 3.5 | RMSE | 3.44 | |
| 21 | –1 | 0 | 1 | 0 | 77.3 | 73.2 ± 2.9 | |||
| 22 | –1 | 0 | 0 | 1 | 95.6 | 85.6 ± 1.3 | residual | 24.48 | |
| 23 | 0 | –1 | 0 | –1 | 94.4 | 101.0 ± 2.9 | lack of fit | 34.27 | |
| 24 | 0 | 0 | 1 | 1 | 72.5 | 84.0 ± 1.2 | pure error | <0.0001 | |
| 25 | 0 | 0 | 0 | 0 | 143.2 | 143.2 ± 0.7 | |||
| 26 | 0 | 0 | 0 | 0 | 143.2 | 143.2 ± 0.7 | |||
| 27 | 0 | 0 | 0 | 0 | 143.2 | 143.2 ± 0.7 | |||
| 28 | 0 | 0 | 0 | 0 | 143.2 | 143.2 ± 0.7 | |||
| 29 | 0 | 0 | 0 | 0 | 143.2 | 143.2 ± 0.7 | |||
*: significant statistic (p < 0.05); RMSE: root-mean-square error.
The ANOVA can illustrate the reliability of the polynomial models. As shown in Table 1, the polynomial model was significant for quantifying CCs (p < 0.05). The F-value of lack of fit was found by the mean square of lack of fit dividing that of pure error. The large F-value of lack of fit resulted in the large p-value, demonstrating that the polynomial regression model could sufficiently explain the correlation between factors and the dependent response. The determination and adjusted determination coefficients (R2 and adjusted R2) were higher than 0.93 for CCs, which is suitable for expressing the relationship between factors and dependent responses. The ANOVA confirmed that the polynomial regression models could fit the experimental values and were proper for predicting independent responses. LSR, ultrasonic power, temperature, and time posed interactions on CCs (p < 0.05). Regarding linear effect, LSR had a positive and the most profound impact on CCs due to having the highest value of regression coefficients, while others negatively affected. Almost all factors mutually influenced CCs, except the time and ultrasonic power, which did not have an interactive effect.
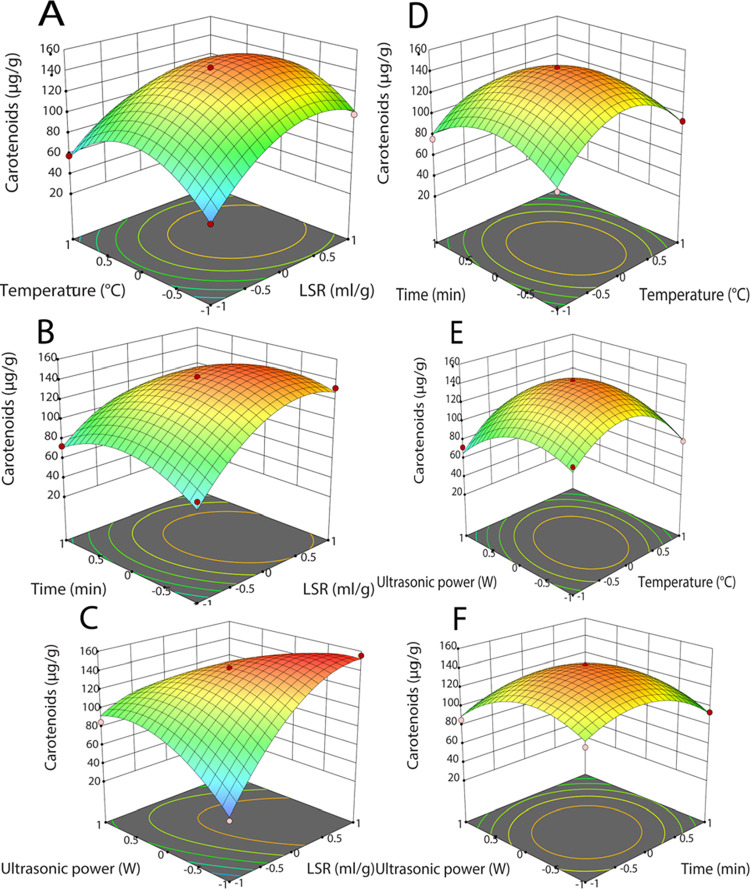
The 3D response surface plots (Figure 2A–F) were constructed from the regression model to visualize the interaction of the factors and the dependent response. The ultrasonic power and time positively influenced CCs, while other mutual interactions were negative. As ultrasonic power and LSR increased, the CCs reached the highest point, followed by a significant drop. Increasing extraction efficiency can result from the degradation of plant cell walls, the turbulent effect generated by ultrasound, and the high solubility of solvent at high temperatures. However, excessive temperature and ultrasonic power can deteriorate carotenoids, declining extraction yield.49 The extraction efficiency of carotenoids peaked, followed by a considerable decrease when the time and temperature increased. The high gradient solvent at the beginning phase, high diffusivity, and high solubility can synergize to increase the extraction yield. Contrastingly, prolonged exposure to high temperatures can destroy carotenoids, dropping extraction yield.49 These results agreed with Andi Suo, who attained carotenoids from apricot flesh using corn oil-based UAE.48 According to the regression models, the optimized conditions for oleic-acid-based UAE were 38.7 mL/g of LSR, 50.61 °C, 12.57 min, and 346.35 W of ultrasonic power to obtain the CCs at 166 μg/g.
Figure 2.
3D response graphics: (A–F) Interactive effect of UAE parameters on carotenoid contents.
The verification of regression models was conducted under adjusted optimized conditions to demonstrate their fit. The adjusted optimized conditions were 39 mL/g of LSR, 50 °C, 12.5 min, and 350 W of ultrasonic power. Under these parameters, the obtained CC was 163.43 ± 1.83 μg/g (data not shown), which was close to the predicted value (166 μg/g). Therefore, the regression model was proper for anticipating carotenoid extraction using oleic-based UAE. The antioxidant activity of enriched-carotenoid oleic acid was investigated under optimal UAE conditions. The DDPH and OH of enriched-carotenoid extracts were 11.21 ± 1.07 and 117.2 ± 1.2 μM TE/g (data not shown), respectively.
3.3. Extraction Kinetics
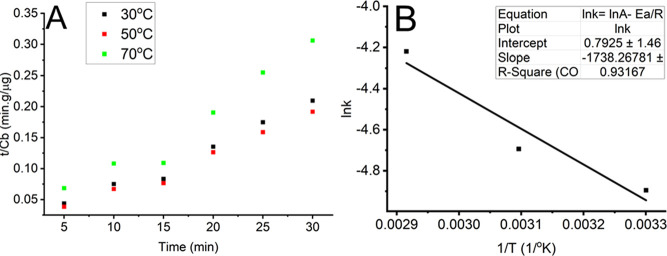
The second-order kinetic model was employed to depict the kinetics of carotenoid extraction from CPP. As presented in Figure 3A,B, graphs of t/Cb versus t were built, and the k, h, and determination coefficient (R2) values for extraction kinetic models were also calculated. The k was quantified at 0.007, 0.009, and 0.015 (data not shown) at 30, 50, and 70 °C, respectively, expressing their rising trend with increasing temperature. The h values were 166.67, 238.10, and 156.25 μg/g (data not shown), and the R2 of extraction kinetic models was 0.9995, 0.9975, and 0.9828 (data not shown) at 30, 50, and 70 °C, respectively. The h value peaked at 50 °C followed by a significant reduction. The result showed the interaction between time and temperature, which agreed with the regression model. Ea, which was used to determine the extraction mechanism of oleic-based UAE, was determined from kinetic extraction models. If Ea is >40 kJ/mol, the mechanism of the extraction process is solubilization. If the Ea is smaller than 20 kJ/mol, the extraction process is managed by a diffusion mechanism. If Ea is 20–40 kJ/mol, diffusion and solubilization mechanisms control the extraction process. The Ea value of oleic-acid-based UAE was 14.72 (kJ/mol, data not shown), which indicated that the diffusion mechanism managed the extraction of carotenoids from CPP. Hobbi et al. constructed the extraction kinetics to determine the mechanism of the solid-to-liquid extraction process of phenolics from apple pomace using water, ethanol, and acetone solution as solvents. This research reported that the extraction mechanism of phenolics using three solvents was diffusion.51
Figure 3.
Second-order kinetic models (A) of oleic-based UAE for extracting carotenoid contents from CPP and thermodynamic analysis of the process (B).
3.4. Nanoemulsification of Enriched-Carotenoid Oleic Acid
3.4.1. Effect of SE Conditions
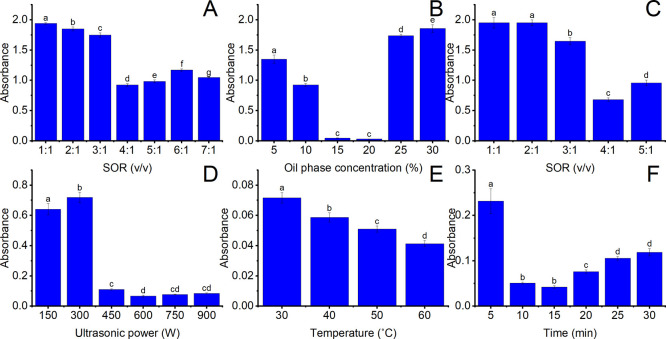
Figure 4A–F presents the effect of SE and UE conditions on the turbidity of nanoemulsion. Figure 4A represents the effect of SOR on the turbidity of enriched-carotenoid oleic acid nanoemulsions using SE. The experimental condition was fixed at 10% v/v of the oil phase. The impact of different SORs (ranging from 1:1 to 7:1) on the turbidity of enriched-carotenoid oleic acid nanoemulsions using SE was investigated. As shown in Figure 4A, the absorbance reached the lowest at an SOR of 4:1. The addition of a greater surfactant amount, which may decline the interfacial tension between the oil and water phases, results in the reduction of turbidity and promote the formation of smaller droplet sizes.52 However, excessive SOR (above 4:1) led to an increase in absorbance. A higher surfactant concentration can elevate viscosity, which may enhance the interfacial tension between two phases, thereby fostering larger particle size formation.53 The results agreed with those of Jose et al., who investigated the impact of surfactant in the emulsification of noni fruit extract using coconut oil. This study showed that particle size diminished as the surfactant concentration increased from 5 to 10 weight/weight (wt %).54 Therefore, it could be concluded that the 4:1 SOR was the most suitable for fabricating nanoemulsions.
Figure 4.
Effect of SE and UE conditions on the turbidity of nanoemulsions. (A) Effect of SOR in SE on the turbidity of nanoemulsions. (B) Effect of oil phase concentration in SE on the turbidity of nanoemulsions. (C) Effect of SOR in UE on the turbidity of nanoemulsions. (D) Effect of ultrasonic power on the turbidity of nanoemulsions. (E) Effect of temperature in UE on the turbidity of nanoemulsions. (F) Effect of time in UE on the turbidity of nanoemulsions. The same character shows insignificant statistical differences (p < 0.05).
Figure 4B represents the effect of oil phase concentration on the absorbance of enriched-carotenoid oleic acid nanoemulsions using SE. The experimental condition was fixed at a SOR 4:1. The effect of different oil phase concentrations (5–30%) on the turbidity of enriched-carotenoid oleic acid nanoemulsions using SE was investigated. As presented in Figure 4B, the absorbance decreased with a rise in the oil phase volume and reached its lowest value at 20% of the oil phase. It can be explained that the surfactant concentration rises with the increased oil phase, leading to the greater diffusion of a surfactant into water. This phenomenon enhances the entropy of the system, promoting the formation of smaller droplet sizes.53 Nevertheless, the absorbance increased when the concentration of the oil phase rose. It can be attributed to the fact that oil phase concentration did not form a bicontinuous microemulsion, thus impeding the generation of small oil droplets during the mixing process.55 In addition, the lack of surfactant quantities may inadequately cover the oil droplets, intensifying coalescence tendencies and leading to augmented particle sizes and absorbance of nanoemulsion systems.56 This trend concurred with that of Chuesiang et al., who discovered the formation of cinnamon oil nanoemulsions. This research achieved the smallest mean droplet diameter (101 nm) by using a total lipid phase comprising 40:60 wt % of cinnamon oil and medium chain triglyceride. This article indicated that mean droplet diameters of 107.30 and 100.76 nm were obtained at intermediate cinnamon oil levels of 30 and 40 wt %, respectively. In contrast, low (0–20 wt %) and high (60–100 wt %) cinnamon oil levels resulted in larger droplets. Therefore, an oil phase concentration of 20% was the most suitable for fabricating nanoemulsions.
3.4.2. Effect of Ultrasonic-Assisted Emulsification Conditions
Figure 4C represents the effect of SOR (ranging from 1:1 to 5:1) on the absorbance of enriched-carotenoid oleic acid nanoemulsions using UE. The experimental condition was fixed at 10% v/v of the oil phase, ultrasonic power of 300 W, and 30 °C for 10 min. When one factor changed, the remaining were fixed at the mentioned experimental conditions or the most suitable conditions of the previous sections. Absorbance decreased with increasing surfactant concentration and reached the lowest at SOR of 4:1. The increase in surfactant concentration reduces the surface tension in the oil–water interface, which can facilitate the disruption of oil droplets during sonication, decreasing the particle size of nanoemulsions. However, the absorbance rose when higher SOR was incorporated. A higher surfactant concentration can increase viscosity, which may increase the cavitation threshold. The cavitation threshold defines a minimum acoustic pressure requisite to initiate cavity formation during the expansion cycle in the liquid environment.57 This phenomenon reduces the impact of the cavitation effect on the oil/water interface, leading to declining emulsifying effectiveness. Therefore, it could be concluded that SOR 4:1 was the most suitable for fabricating nanoemulsions.
Figure 4D represents the effect of ultrasonic power (150–900 W) on the absorbance of enriched-carotenoid oleic acid nanoemulsions using UE. As shown in Figure 4D, the absorbance decreased from 0.64 to 0.07 when higher power was applied. In addition, there was no significant difference among the three power levels: 600, 750, and 900 W. The decrease in absorbance with the increased acoustic amplitude can be attributed to the increased number of acoustic bubbles; then, the implosion of these bubbles intensifies the level of cavitation activity.58 The amplified cavitation intensity leads to greater fragmentation of droplets, improving emulsifying yield.41 This trend agrees with the findings of Raviadaran et al., who investigated the impact of polyglycerol polyricinoleate and NaCl on ultrasound-assisted water-in-palm oil nanoemulsion stability. In this article, the droplet size decreased when the ultrasonic amplitude increased.41 Therefore, it could be concluded that 600 W was the most effective for fabricating nanoemulsions.
Figure 4E represents the effect of the temperature (ranging from 30 to 60 °C) on the absorbance of enriched-carotenoid oleic acid nanoemulsions using UE. The absorbance decreased when a higher temperature was applied. Since oil droplets are liquefied at high temperatures, the acoustic cavitation has contributed to the gradual reduction of absorbance at 60 °C compared to 30 °C. This phenomenon may also be due to the decline in the interfacial tension, which plays a crucial role in forming smaller droplet sizes, leading to lower absorbance.52 Carotenoid is thermolabile and easily degradable at higher temperatures (over 50 °C), so the temperature of the emulsifying process should be kept at 50 °C or below. This trend agrees with Adel Mirmajidi Hashtjin and Soleiman Abbasi, who explored the optimal conditions for ultrasonic emulsification in orange peel essential oil nanoemulsions production.59 In this research, the particle size increased when the temperature rose to 25 °C. However, it reduced when higher temperatures were applied. Therefore, it could be concluded that 50 °C was the most effective for fabricating nanoemulsions.
Figure 4F represents the effect of time (ranging from 5 to 30 min) on the absorbance of enriched-carotenoid oleic acid nanoemulsions using UE. The absorbance reached the lowest at 10 and 15 min, as they showed no significant difference. When a longer time is applied, it can encourage the formation of smaller droplets due to the micro shearing and turbulence effect. Additionally, irradiation time profoundly impacts the adsorption rate of emulsifiers to the O/W surface and the size distribution of newly created droplets in an oil-in-water (O/W) nanoemulsion system.60 However, when the time exceeded 15 min, the absorbance level rose again. The applied ultrasonic energy cannot reduce the particle size of nanoemulsions due to an insufficient applied force to break tiny particles continuously. The turbulent effect of sonication can increase the contact between nanoparticles, leading to their coalescence. This coalescence can raise the diameter of the nanoparticles and cause a higher absorbance level. This trend agreed with Hashtjin and Abbas.59 Therefore, it could be concluded that 10 min was the most effective time for fabricating nanoemulsions.
3.5. Comparing Spontaneous, Ultrasonic-Assisted, and Spontaneous-Ultrasonic-Assisted Emulsification
Table 2 compares the physicochemical properties of three approaches (SE, UE, and SE–UE). SE was conducted at SOR 4:1 and 20 wt % oil phase concentration. UE was conducted at SOR 4:1, ultrasonic power of 600 W, and 50 °C for 10 min. SE–UE was conducted under the most effective conditions obtained from Section 3.4. Particle size is an important attribute since it influences the appearance, bioavailability, optical properties, and rheological properties, of nanoemulsions. There are various methods to determine the particle size of nanoemulsions, such as microscopy, electric pulse counting, sedimentation, ultrasonic spectrometry, nuclear magnetic resonance, static light, and DLS. Among these methods, DLS is commonly employed to determine the particle size of nanoemulsions due to simple procedure, short determination time, and high precision.61 PDI describes the particle size distribution range and represents the homogeneity of the nanoemulsion systems. The low PDI indicates a limited droplet size distribution, while the high PDI shows the heterogeneous properties of nanoemulsion systems. In addition to particle size, the PDI is an indicator of the stability of nanoemulsion systems. Zeta potential characterizes the electric attributes of the droplet in nanoemulsion systems. When the absolute zeta potential values are higher, nanoemulsion systems are more stable.61 As can be seen, the largest particle size and absorbance were observed in SE–UE nanoemulsions. It can be attributed to the coalescence of droplets caused by sonication. The applied ultrasonic energy cannot reduce the particle size of SE-based nanoemulsions, and sonication’s turbulent effect can increase contact between nanoparticles, leading to their recoalescence and raising droplet sizes.41 Meanwhile, the particle size of the UE-based nanoemulsion was larger than that of SE. SE occurred based on thermodynamics, while ultrasonic vibration fabricated UE-based nanoemulsion. These results may indicate that the thermodynamic degree is more efficient than ultrasonic vibration in breaking droplets. On the other hand, PDI was the lowest when using SE–UE, which can be attributed to the fragmentation of larger droplets by sonication, improving the homogeneity of droplet size. All zeta potentials were relatively negative, which represents a good nanosystem. In conclusion, SE and UE are recommended in the fabrication of enriched-carotenoid oleic acid nanoemulsions.
Table 2. Physicochemical Attributes of Nanoemulsions from SE, UE, and SE–UEa.
| SE | UE | SE–UE | |
|---|---|---|---|
| absorbance | 0.030 ± 0.002a | 0.051 ± 0.002b | 0.082 ± 0.004c |
| particle size (nm) | 31.2 ± 0.83a | 33.8 ± 0.52b | 109.7 ± 8.24c |
| PDI | 0.398 | 0.293 | 0.251 |
| zeta potential (mV) | –2.0 ± 0.5a | –3.6 ± 0.7b | –1.9 ± 0.3c |
Different characters show significant statistical distinctions (p < 0.05).
4. Conclusions
Carotenoids were extracted with the assistance of ultrasonication from carrot pomace, using oleic acid as a solvent. Then, the extracted carotenoid was fabricated by three approaches (SE, UE, and SE–UE). The optimized conditions for oleic acid-based UAE were 38.7 mL/g of LSR, 50.61 °C, 12.57 min, and 346.35 W of ultrasonic power. The diffusion mechanism managed the extraction of carotenoids from CPP. SE was conducted at SOR 4:1 and 20 wt % oil phase concentration. UE was performed at SOR 4:1, ultrasonic power of 600 W, and 50 °C for 10 min. SE–UE was conducted at the most effective conditions obtained from Section 3.4. SE and UE were the effective emulsification methods, with particle sizes of 31.2 and 33.8 nm. This study developed an environmentally friendly and sustainable approach to extracting carrot pomace carotenoids. Furthermore, this research also found two effective methods for fabricating nanoemulsions of the extract, thereby extending their shelf life. The prepared enriched-carotenoid oleic acid-based nanoemulsions can be applied to food products as color ingredients and antibrowning agents in meat, vegetables, confectionary products, and dairy products.
Acknowledgments
We acknowledge Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for supporting this study.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Author Contributions
T.P.V.: conceptualization, methodology, investigation, software, visualization, formal analysis, data curation, writing-original draft, writing-review & editing. H.K.L.T.: formal analysis, investigation, writing- original draft. T.M.N.T.: investigation. H.T.V.N.: investigation. T.H.P.: investigation. T.H.P.N.: investigation. V.K.N.: investigation. T.C.T.D.: investigation. L.G.K.N.: investigation. T.Q.C.: investigation. D.Q.N.: visualization, supervision, writing—review & editing.
No funding was received for this manuscript.
The authors declare no competing financial interest.
References
- Sharma M.; Hussain S.; Shalima T.; Aav R.; Bhat R. Valorization of seabuckthorn pomace to obtain bioactive carotenoids: An innovative approach of using green extraction techniques (ultrasonic and microwave-assisted extractions) synergized with green solvents (edible oils). Ind. Crops Prod. 2022, 175, 114257 10.1016/j.indcrop.2021.114257. [DOI] [Google Scholar]
- Bhimjiyani V. H.; Borugadda V. B.; Naik S.; Dalai A. K. Enrichment of flaxseed (Linum usitatissimum) oil with carotenoids of sea buckthorn pomace via ultrasound-assisted extraction technique: Enrichment of flaxseed oil with sea buckthorn. Curr.t Res. Food Sci. 2021, 4, 478–488. 10.1016/j.crfs.2021.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M.; Bhat R. Extraction of Carotenoids from Pumpkin Peel and Pulp: Comparison between Innovative Green Extraction Technologies (Ultrasonic and Microwave-Assisted Extractions Using Corn Oil). In Foods 2021, 10, 787. 10.3390/foods10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedor J.; Burda K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranvarachat B.; Devahastin S. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. J. Food Eng. 2014, 126, 17–26. 10.1016/j.jfoodeng.2013.10.024. [DOI] [Google Scholar]
- Sharma K. D.; Karki S.; Thakur N. S.; Attri S. Chemical composition, functional properties and processing of carrot—a review. J. Food Sci. Technol. 2012, 49 (1), 22–32. 10.1007/s13197-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babotă M.; Frumuzachi O.; Gâvan A.; Iacoviţă C.; Pinela J.; Barros L.; Ferreira I. C. F. R.; Zhang L.; Lucini L.; Rocchetti G.; et al. Optimized ultrasound-assisted extraction of phenolic compounds from Thymus comosus Heuff. ex Griseb. et Schenk (wild thyme) and their bioactive potential. Ultrason. Sonochem. 2022, 84, 105954 10.1016/j.ultsonch.2022.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Zhao Y.; Yu S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. 10.1016/j.foodchem.2014.09.110. [DOI] [PubMed] [Google Scholar]
- Sharmila G.; Nikitha V. S.; Ilaiyarasi S.; Dhivya K.; Rajasekar V.; Kumar N. M.; Muthukumaran K.; Muthukumaran C. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind.l Crops Prod. 2016, 84, 13–21. 10.1016/j.indcrop.2016.01.010. [DOI] [Google Scholar]
- Sodeifian G.; Usefi M. M. B. Solubility, Extraction, and Nanoparticles Production in Supercritical Carbon Dioxide: A Mini-Review. ChemBioEng Rev. 2023, 10 (2), 133–166. 10.1002/cben.202200020. [DOI] [Google Scholar]; (acccessed 2023/08/25)
- Sodeifian G.; Sajadian S. A.; Saadati Ardestani N. Experimental optimization and mathematical modeling of the supercritical fluid extraction of essential oil from Eryngium billardieri: Application of simulated annealing (SA) algorithm. J. Supercrit. Fluids 2017, 127, 146–157. 10.1016/j.supflu.2017.04.007. [DOI] [Google Scholar]
- Sodeifian G.; Ansari K. Optimization of Ferulago Angulata oil extraction with supercritical carbon dioxide. J. Supercrit. Fluids 2011, 57 (1), 38–43. 10.1016/j.supflu.2011.02.002. [DOI] [Google Scholar]
- Sodeifian G.; Azizi J.; Ghoreishi S. M. Response surface optimization of Smyrnium cordifolium Boiss (SCB) oil extraction via supercritical carbon dioxide. J. Supercrit. Fluids 2014, 95, 1–7. 10.1016/j.supflu.2014.07.023. [DOI] [Google Scholar]
- Sodeifian G.; Sajadian S. A.; Honarvar B. Mathematical modelling for extraction of oil from Dracocephalum kotschyi seeds in supercritical carbon dioxide. Nat. Prod. Res. 2018, 32 (7), 795–803. 10.1080/14786419.2017.1361954. [DOI] [PubMed] [Google Scholar]
- Sodeifian G.; Ghorbandoost S.; Sajadian S. A.; Saadati Ardestani N. Extraction of oil from Pistacia khinjuk using supercritical carbon dioxide: Experimental and modeling. J. Supercrit. Fluids 2016, 110, 265–274. 10.1016/j.supflu.2015.12.004. [DOI] [Google Scholar]
- Sodeifian G.; Ardestani N. S.; Sajadian S. A.; Moghadamian K. Properties of Portulaca oleracea seed oil via supercritical fluid extraction: Experimental and optimization. J. Supercrit. Fluids 2018, 135, 34–44. 10.1016/j.supflu.2017.12.026. [DOI] [Google Scholar]
- Sodeifian G.; Sajadian S. A.; Saadati Ardestani N. Supercritical fluid extraction of omega-3 from Dracocephalum kotschyi seed oil: Process optimization and oil properties. J. Supercrit. Fluids 2017, 119, 139–149. 10.1016/j.supflu.2016.08.019. [DOI] [Google Scholar]
- More P. R.; Jambrak A. R.; Arya S. S. Green, environment-friendly and sustainable techniques for extraction of food bioactive compounds and waste valorization. Trends Food Sci. Technol. 2022, 128, 296–315. 10.1016/j.tifs.2022.08.016. [DOI] [Google Scholar]
- Wang X.; Liu X.; Shi N.; Zhang Z.; Chen Y.; Yan M.; Li Y. Response surface methodology optimization and HPLC-ESI-QTOF-MS/MS analysis on ultrasonic-assisted extraction of phenolic compounds from okra (Abelmoschus esculentus) and their antioxidant activity. Food Chem. 2023, 405, 134966 10.1016/j.foodchem.2022.134966. [DOI] [PubMed] [Google Scholar]
- Chen X.-Q.; Li Z.-H.; Liu L.-L.; Wang H.; Yang S.-H.; Zhang J.-S.; Zhang Y. Green extraction using deep eutectic solvents and antioxidant activities of flavonoids from two fruits of Rubia species. LWT 2021, 148, 111708 10.1016/j.lwt.2021.111708. [DOI] [Google Scholar]
- Pastor R.; Bouzas C.; Tur J. A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radical Biol. Med. 2021, 172, 372–385. 10.1016/j.freeradbiomed.2021.06.017. [DOI] [PubMed] [Google Scholar]
- Mander L.; Liu H. -W.. Comprehensive natural products II: chemistry and biology; Elsevier, 2010. [Google Scholar]
- Lopez-Huertas E. Health effects of oleic acid and long chain omega-3 fatty acids (EPA and DHA) enriched milks. A review of intervention studies. Pharmacol. Res. 2010, 61 (3), 200–207. 10.1016/j.phrs.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Sachindra N. M.; Mahendrakar N. S. Process optimization for extraction of carotenoids from shrimp waste with vegetable oils. Bioresour. Technol. 2005, 96 (10), 1195–1200. 10.1016/j.biortech.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Tiwari S.; Upadhyay N.; Singh A. K.; Meena G. S.; Arora S. Organic solvent-free extraction of carotenoids from carrot bio-waste and its physico-chemical properties. J. Food Sci. Technol. 2019, 56 (10), 4678–4687. 10.1007/s13197-019-03920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Fabiano-Tixier A. S.; Tomao V.; Cravotto G.; Chemat F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20 (1), 12–18. 10.1016/j.ultsonch.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Sodeifian G.; Sajadian S. A.; Saadati Ardestani N. Evaluation of the response surface and hybrid artificial neural network-genetic algorithm methodologies to determine extraction yield of Ferulago angulata through supercritical fluid. J. Taiwan Inst. Chem. Eng. 2016, 60, 165–173. 10.1016/j.jtice.2015.11.003. [DOI] [Google Scholar]
- Sodeifian G.; Saadati Ardestani N.; Sajadian S. A. Extraction of seed oil from Diospyros lotus optimized using response surface methodology. J. Forest. Res. 2019, 30 (2), 709–719. 10.1007/s11676-018-0631-8. [DOI] [Google Scholar]
- Boateng I. D.; Kuehnel L.; Daubert C. R.; Agliata J.; Zhang W.; Kumar R.; Flint-Garcia S.; Azlin M.; Somavat P.; Wan C. Updating the status quo on the extraction of bioactive compounds in agro-products using a two-pot multivariate design. A comprehensive review. Food Funct. 2023, 14 (2), 569–601. 10.1039/D2FO02520E. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L.; Rojas-Graü M.; Soliva-Fortuny R.; Martin-Belloso O. Physicochemical characterization and antimicrobial activity of foodgrade emulsions and nanoemulsions incorporating essential oils. Food Hydrocolloids 2014, 43, 547–556. 10.1016/j.foodhyd.2014.07.012. [DOI] [Google Scholar]
- Wilson R. J.; Li Y.; Yang G.; Zhao C.-X. Nanoemulsions for drug delivery. Particuology 2022, 64, 85–97. 10.1016/j.partic.2021.05.009. [DOI] [Google Scholar]
- Solans C.; Morales D.; Homs M. Spontaneous emulsification. Curr. Opin. Colloid Interface Sci. 2016, 22, 88–93. 10.1016/j.cocis.2016.03.002. [DOI] [Google Scholar]
- Jafari S.; McClements D. J.. Nanoemulsions: Formulation, Applications, and Characterization; 2018.
- Surh J.; Decker E. A.; McClements D. J. Utilisation of spontaneous emulsification to fabricate lutein-loaded nanoemulsion-based delivery systems: factors influencing particle size and colour. Int. J. Food Sci. Technol. 2017, 52 (6), 1408–1416. 10.1111/ijfs.13395. [DOI] [Google Scholar]; (accessed August 24, 2023)
- Lad V. N.; Murthy Z. V. P. Enhancing the Stability of Oil-in-Water Emulsions Emulsified by Coconut Milk Protein with the Application of Acoustic Cavitation. Ind. Eng. Chem. Res. 2012, 51 (11), 4222–4229. 10.1021/ie202764f. [DOI] [Google Scholar]
- Goula A. M.; Ververi M.; Adamopoulou A.; Kaderides K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason. Sonochem. 2017, 34, 821–830. 10.1016/j.ultsonch.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Karabagias I.; Michos C.; Badeka A.; Kontakos S.; Stratis I.; Kontominas M. G. Classification of Western Greek virgin olive oils according to geographical origin based on chromatographic, spectroscopic, conventional and chemometric analyses. Food Res. Int. 2013, 54 (2), 1950–1958. 10.1016/j.foodres.2013.09.023. [DOI] [Google Scholar]
- Chen H.-M.; Meyers S. P. A rapid quantitative method for determination of astaxanthin pigment concentration in oil extracts. J. Am. Oil Chem. Soc. 1984, 61 (6), 1045–1047. 10.1007/BF02636215. [DOI] [Google Scholar]; (acccessed 2022/11/18)
- Liew S. N.; Utra U.; Alias A. K.; Tan T. B.; Tan C. P.; Yussof N. S. Physical, morphological and antibacterial properties of lime essential oil nanoemulsions prepared via spontaneous emulsification method. LWT 2020, 128, 109388 10.1016/j.lwt.2020.109388. [DOI] [Google Scholar]
- Yuan H.; Dong L.; Zhang Z.; He Y.; Ma X. Production, structure, and bioactivity of polysaccharide isolated from Tremella fuciformis. Food Sci. Hum. Wellness 2022, 11 (4), 1010–1017. 10.1016/j.fshw.2022.03.030. [DOI] [Google Scholar]
- Raviadaran R.; Ng M. H.; Manickam S.; Chandran D. Ultrasound-assisted water-in-palm oil nano-emulsion: Influence of polyglycerol polyricinoleate and NaCl on its stability. Ultrason. Sonochem. 2019, 52, 353–363. 10.1016/j.ultsonch.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Grumezescu A. M.; Holban A. M.. Ingredients extraction by physicochemical methods in food; Academic Press, 2017. [Google Scholar]
- Aldred E. M.; Buck C.; Vall K.. Chapter 22 - Terpenes. In Pharmacology, Aldred E. M.; Buck C.; Vall K., Eds.; Churchill Livingstone, 2009; pp 167–174. [Google Scholar]
- Kumar K.; Srivastav S.; Sharanagat V. S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahindrakar K. V.; Rathod V. K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. 2020, 149, 107841 10.1016/j.cep.2020.107841. [DOI] [Google Scholar]
- Rao M. V.; Sengar A. S. C. K. S.; Rawson A. Ultrasonication - green technology extraction technique for spices: A review. Trends Food Sci. Technol. 2021, 116, 975–991. 10.1016/j.tifs.2021.09.006. [DOI] [Google Scholar]
- Maran J. P.; Priya B. Ultrasound-assisted extraction of pectin from sisal waste. Carbohydr. Polym. 2015, 115, 732–738. 10.1016/j.carbpol.2014.07.058. [DOI] [PubMed] [Google Scholar]
- Suo A.; Fan G.; Wu C.; Li T.; Cong K. Green extraction of carotenoids from apricot flesh by ultrasound assisted corn oil extraction: Optimization, identification, and application. Food Chem. 2023, 420, 136096 10.1016/j.foodchem.2023.136096. [DOI] [PubMed] [Google Scholar]
- Chutia H.; Mahanta C. L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547 10.1016/j.ifset.2020.102547. [DOI] [Google Scholar]
- Al-Dhabi N. A.; Ponmurugan K.; Maran Jeganathan P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. 10.1016/j.ultsonch.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Hobbi P.; Okoro O. V.; Delporte C.; Alimoradi H.; Podstawczyk D.; Nie L.; Bernaerts K. V.; Shavandi A. Kinetic modelling of the solid–liquid extraction process of polyphenolic compounds from apple pomace: influence of solvent composition and temperature. Bioresour. Bioprocess. 2021, 8 (1), 114. 10.1186/s40643-021-00465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M.; Losada-Barreiro S.; Bravo-Díaz C.; Vicente A. A.; Monteiro L. S.; Paiva-Martins F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates in fish oil-in-water emulsified systems: Emulsion and nanoemulsion comparison. Food Chem. 2020, 310, 125716 10.1016/j.foodchem.2019.125716. [DOI] [PubMed] [Google Scholar]
- Phase Behaviour of Surfactant Systems. In Applied Surfactants, 2005; pp 53–72.
- Jose D.; Muenmuang C.; Kitiborwornkul N.; Yasurin P.; Asavasanti S.; Tantayotai P.; Sriariyanun M. Effect of surfactants and Co-surfactants in formulation of noni fruit extract in virgin coconut oil-based emulsion. J. Ind. Chem. Soc. 2022, 99 (10), 100729 10.1016/j.jics.2022.100729. [DOI] [Google Scholar]
- Chuesiang P.; Siripatrawan U.; Sanguandeekul R.; McLandsborough L.; Julian McClements D. Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: Impact of oil phase composition and surfactant concentration. J. Colloid Interface Sci. 2018, 514, 208–216. 10.1016/j.jcis.2017.11.084. [DOI] [PubMed] [Google Scholar]
- Lago A. M. T.; Neves I. C. O.; Oliveira N. L.; Botrel D. A.; Minim L. A.; de Resende J. V. Ultrasound-assisted oil-in-water nanoemulsion produced from Pereskia aculeata Miller mucilage. Ultrason. Sonochem. 2019, 50, 339–353. 10.1016/j.ultsonch.2018.09.036. [DOI] [PubMed] [Google Scholar]
- Patil S. S.; Pathak A.; Rathod V. K. Optimization and kinetic study of ultrasound assisted deep eutectic solvent based extraction: A greener route for extraction of curcuminoids from Curcuma longa. Ultrason. Sonochem. 2021, 70, 105267 10.1016/j.ultsonch.2020.105267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry K. S.; Micheli S.; McClements D. J.; McLandsborough L. Effectiveness of a spontaneous carvacrol nanoemulsion against Salmonella enterica Enteritidis and Escherichia coli O157:H7 on contaminated broccoli and radish seeds. Food Microbiol. 2015, 51, 10–17. 10.1016/j.fm.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Hashtjin A. M.; Abbasi S. Optimization of ultrasonic emulsification conditions for the production of orange peel essential oil nanoemulsions. J. Food Sci. Technol. 2015, 52 (5), 2679–2689. 10.1007/s13197-014-1322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.-H.; Chiang B.-H. Process optimization and stability of d-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason. Sonochem. 2012, 19 (1), 192–197. 10.1016/j.ultsonch.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Jafari S. M.; McClements D. J.. Nanoemulsions: formulation, applications, and characterization; Academic Press, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.