Abstract
Cannabis sativa L. produces a wide variety of volatile secondary metabolites that contribute to its unique aroma. The major volatile constituents include monoterpenes, sesquiterpenes, and their oxygenated derivates. In particular, the compounds ß-myrcene, D-(+)-limonene, ß-caryophyllene, and terpinolene are often found in greatest amounts, which has led to their use in chemotaxonomic classification schemes and legal Cannabis sativa L. product labeling. While these compounds contribute to the characteristic aroma of Cannabis sativa L. and may help differentiate varieties on a broad level, their importance in producing specific aromas is not well understood. Here, we show that across Cannabis sativa L. varieties with divergent aromas, terpene expression remains remarkably similar, indicating their benign contribution to these unique, specific scents. Instead, we found that many minor, nonterpenoid compounds correlate strongly with nonprototypical sweet or savory aromas produced by Cannabis sativa L. Coupling sensory studies to our chemical analysis, we derive correlations between groups of compounds, or in some cases, individual compounds, that produce many of these diverse scents. In particular, we identified a new class of volatile sulfur compounds (VSCs) containing the 3-mercaptohexyl functional group responsible for the distinct citrus aromas in certain varieties and skatole (3-methylindole) as the key source of the chemical aroma in others. Our results provide not only a rich understanding of the chemistry of Cannabis sativa L. but also highlight how the importance of terpenes in the context of the aroma of Cannabis sativa L. has been overemphasized.
Introduction
Cannabis sativa L. cultivation has increased substantially over the past decade resulting in multiple fast-growing industries.1 High-tetrahydrocannabinol (THC) varieties of Cannabis sativa L., often referred to as simply cannabis, are typically cultivated for consumption due to their intoxicating psychoactive effects.2−4 Low-THC Cannabis sativa L. (<0.3% THC), referred to as hemp, is primarily grown for the production of cannabidiol (CBD) and its utilization in textiles fibers.5 In particular, the high-THC cannabis industry has grown considerably over the past decade as legalization increases.6−10 During this time, consumer expectations have evolved concomitantly, with tetrahydrocannabinol (THC) content in particular proven to be a key driver in the industry, resulting in a race to continuously increase cannabinoid concentration.3,11,12 Nevertheless, consumer preference is also influenced by the aromatic qualities of a product.13,14 This has led to terpenes–a general term that when used within the industry describes a multitude of compounds that produce the aroma of cannabis – to emerge as secondary differentiators within the marketplace, leading to their routine testing at analytical laboratories.11,15−20 This phenomenon is partially in response to the commonly used, but inaccurate, classification of cannabis as either indica, sativa, or a hybrid of the two major species of Cannabaceae.11,13,21 These terms have historically been used to categorize cannabis based on their physical, aromatic, and psychoactive characteristics, sativa varieties being tall and narrow-leaved with energizing effects and indica varieties being short and bushlike with broad leaves and sedating effects.22,23 While this nomenclature is still commonly used, modern cannabis rarely fits into one of these two classifications and rather appear to be hybrids of the two, muddling their use in accurately differentiating cannabis varieties on a phenotypic, aromatic, or chemical level.11,24
To overcome the inaccuracies of the indica/sativa binary classification and better categorize cannabis varieties based on their psychoactive and aroma characteristics, terpenes have emerged as a prominent focus of research.17,19,25,26 Terpenes, diverse organic compounds found in various plants, including cannabis, contribute to the distinct aromas and flavors of different varieties.27 More importantly, terpenes are believed to play a significant role in the psychoactive and medicinal properties of cannabis, with specific terpenes potentially correlating with distinct psychoactive effects.22,28,29 In the context of Cannabis sativaL., the term terpenes typically refers to monoterpenes, monoterpenoids, sesquiterpenes, and sesquiterpenoids.15 These compounds are the major volatile constituents of the essential oils of cannabis and hemp, often constituting around 1 to 4% of the total mass of cured inflorescence. Their high concentrations have made them the primary focus of research in attempting to understand the complex aroma of cannabis.21 Terpenes are thus being investigated for their psychoactive modulatory effects, chemotaxonomic utility, and the primary chemical source of cannabis’ aroma.
Multiple cannabis classification schemes have been proposed based on key terpenes, including terpinolene, D-(+)-limonene, ß-caryophyllene, and ß-myrcene to help categorize cannabis more accurately.26,30 These results have been further reinforced by a recent study that found that cannabis grown across the United States falls into three major classes: terpinolene/ß-myrcene, D-(+)-limonene/ß-caryophyllene, or ß-myrcene/pinene dominant varieties.11 Interestingly, this study showed varieties with very different aroma characteristics are often found in the same cluster, which is contrary to the paradigm that these dominant terpenes dictate their aromatic character.11 For instance, Dogwalker OG, which possesses a skunky and woody aroma, was found in the same D-(+)-limonene/ß-caryophyllene cluster as Tropicana Cookies, which possesses an intense citrus and tropical aroma. Purple Punch, which possesses a sweet, grape-like scent, was also found in this cluster. This discrepancy suggests that while these classifications may be helpful for chemotaxonomic purposes, they lack the chemical information necessary to differentiate these varieties from an aroma perspective. This is reinforced by previous sensory studies finding limited evidence that specific cannabis aromas are correlated to terpene concentrations.13,21,31 Furthermore, a recent study indicates that the perceived subjective aroma of a cannabis product, rather than specific terpenes, correlates with user experience and preference.13 Taken together, these results strongly suggest that while aroma is a key property in differentiating cannabis varieties and user preferences, the importance of terpenes appears to be overstated.
Here, we show that these classifications and terpenes in general provide minimal information regarding the unique aromatic attributes of many cannabis varieties. By analyzing the volatile chemical profiles of 31 cannabis ice hash rosin extracts with a wide aromatic diversity using 2-Dimensional gas chromatography coupled with time-of-flight mass spectrometry and flame ionization detection, we identified a myriad of nonterpenoid compounds that strongly influence the unique aromatic properties of cannabis. These compounds are often found in similarly low concentrations (<1 μg/mg) to the recently discovered prenylated volatile sulfur compounds–the source of the skunky, gasoline-like odor of cannabis , yet can have a comparatively large odor impact.32,33 In particular, we identified a new class of tropical volatile sulfur compounds (VSCs) that are major contributors to certain varieties with a strong citrus or tropical fruit aroma, while skatole (3-methylindole), a highly pungent compound, was identified as a key aroma compound in savory/chemical varieties. Sensory experiments were then conducted and correlated to the chemical analysis, revealing that the major terpenes typically used for categorizing cannabis and tested for in analytical laboratories largely appear similar–even when the aromatic properties are drastically different–indicative of their benign nature toward the scent of many of these varieties. These results provide a greater understanding of the chemical composition of cannabis beyond terpenes and how these compounds contribute to the unique aromas that it produces. Furthermore, this catalog of compounds and their contributions to the scent of cannabis provides a new opportunity to classify varieties using key desirable aroma attributes.
Results
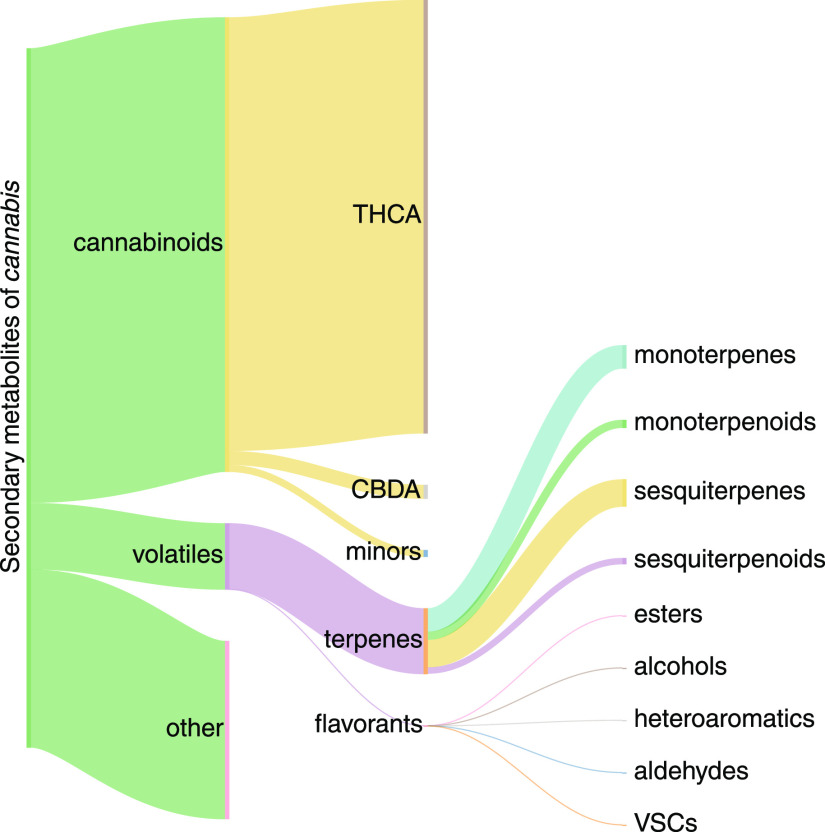
The phytochemical makeup of cannabis is rich in chemical diversity, as illustrated by the Sankey diagram in Figure 1 showing major classes of secondary metabolites identified in cannabis.15,17−19,27,32,34−37 While cannabinoids such as tetrahydrocannabinolic acid (THCA) – the precursor to THC – comprise a large fraction by weight, they do not contribute to the scent of cannabis due to their low volatility. Conversely, terpenes volatilize with ease under ambient conditions; hence, they are important as aroma compounds in nature. Other key classes of volatiles beyond terpenes exist in cannabis that we generally refer to as flavorants. These can be further classified by their chemical functionality, such as esters, alcohols, etc. While previous studies have detailed a number of these compounds, few have described them in the context of how they affect the aromatic properties of specific varieties.18,19
Figure 1.
Schematic illustrating a common chemical profile of cannabis. Path size indicates the approximate relative amount of each compound class.
Many modern cannabis varieties are often described as exotic, which we define as varieties that are unusually sweet or savory. The former often have aroma descriptors such as sweet or fruity, while the latter include chemical or savory. This is in alignment with recent sensory studies that have developed a diverse lexicon to adequately describe the aroma of many cannabis varieties.14,21,31,38 To ensure that we captured many of the different aromas that cannabis produces, we procured 31 ice hash rosin samples of different varieties (Table S2) to build a robust catalog of compounds beyond terpenes.
Ice hash rosin is a type of cannabis exudate that separates and concentrates many of the secondary metabolites from plant tissue. This process begins by mechanically separating the trichomes of the plant from fresh-frozen cannabis – frozen plant inflorescence that is cut while the plant is still living (Figure 2). Trichomes are small, hairlike structures that appear on the surface of the plant and contain many of the desired secondary metabolites, including cannabinoids, terpenes, and flavorants. The isolated trichomes are then dried into a powder, transferred into micrometer-sized filter bags to separate any residual plant tissue, and then pressed under mild heat and pressure to partially decarboxylate THCA into THC, resulting in a thick, viscous oil known as ice hash rosin.
Figure 2.
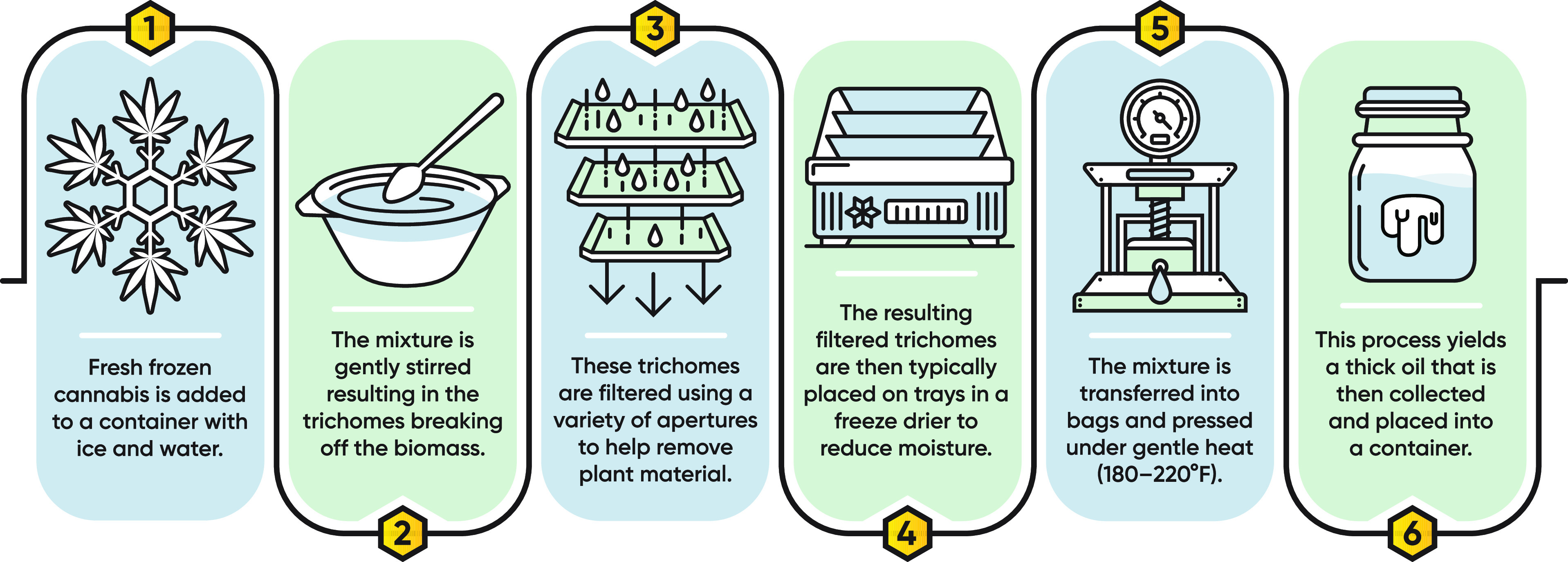
Workflow showing the cannabis ice hash rosin making process. The mild processing conditions result in a concentrated extract that preserves the aromatic properties of the inflorescence from which it is created, making it an ideal sample matrix for analysis of low concentration analytes.
These extracts were the sample matrix of choice for the following reasons: First, rosin is a concentrated form of cannabis, and thus, it has less plant tissue by weight compared to raw inflorescence, allowing for easier access to lower concentration compounds that are more difficult to detect. For instance, the average total analyte concentration of the samples used was ≈8% by mass. This contrasts with dried, cured inflorescence, which is typically at most 3–4%. Second, most rosin is produced using mild manufacturing conditions that help minimize loss of volatile compounds. Lastly, rosin extracts are produced from many plants, thus intrinsically creating an aggregate average of the secondary metabolite profile of a specific variety. This helps reduce sample inhomogeneity likely seen during the analysis of individual cannabis inflorescence samples.
Sensory Analysis of cannabis Rosin Extracts
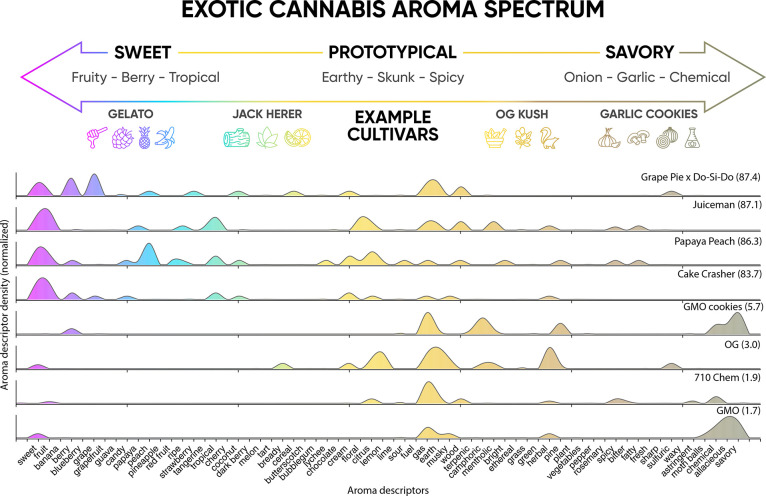
The aroma properties of each sample were determined by a sensory panel (see Methods). The tabulated results revealed a wide diversity of aroma characteristics that can be found in Tables S5–S9. The descriptors obtained were also refined and grouped into three classifications: sweet exotic, prototypical, and savory exotic (Table S3). This methodology allows for more general classifications that help establish trends between varieties. Each panelist recorded an exotic score, which we defined as a metric that quantifies the overall sweetness or fruitiness of a given variety, ranging from 0 (not sweet or fruity) to 100 (very sweet or fruity). Figure 3 illustrates the wide spectrum of different aromas that cannabis produces as defined by our sensory panel, with more prototypical aroma descriptors shown toward the middle and more exotic descriptors on the extreme ends. Shown below the aroma spectrum are the densities of reported aroma descriptors (i.e., the total number of instances a descriptor was assigned by the sensory panel) for the top-four and bottom-four ranked varieties by exotic score. A clear difference in sweet and savory descriptor densities is observed, with the four highest-ranked varieties having a much greater density of sweet descriptors, while the lowest four ranked have greater savory descriptor density. These data confirm that the samples analyzed have a wide aromatic diversity that is partitioned as expected when compared with their exotic scores.
Figure 3.
(Top) Schematic illustrating the spectrum of cannabis aromas reported from the sensory panel. (Bottom) Top four and bottom four ranked varieties by exotic score showing significant differences in reported sweet and savory descriptors from sensory experiments.
The results of the sensory analysis showed clear differences among many varieties. Figure 4a shows the average exotic score of each variety as a function of rank (lowest ranking having the lowest exotic scores and vice versa), with the color of the markers indicating each variety’s highest concentration terpene. D-(+)-limonene-rich varieties are most common in the samples measured and correspond to seven of the highest and six of the lowest exotic scores. Figure 4b shows the sum of the instances of each descriptor classification as a function of the exotic score rank. Pearson correlation coefficients revealed a strong positive correlation between the sweet-exotic descriptor sums and exotic score (r = 0.77), indicating these descriptors trend strongly with increasing exotic scores. On the other hand, descriptors classified as savory-exotic had a moderate negative correlation (r = −0.47), which agrees with lower exotic scores having less sweet or fruity-like descriptors. Lastly, the descriptors within the prototypical descriptor classification showed less correlation (r = −0.39) than the other two. Many of these descriptors are highly representative of the terpene aroma characteristics commonly found in cannabis. For instance, ß-caryophyllene possesses a spicy, peppery aroma whereas D-(+)-limonene is orange and citrus. The descriptor gas, short for gasoline, in the context of cannabis refers to the pungent scent of fresh cannabis that is often synonymous with skunky. This aroma is derived primarily from prenylated VSCs.32 This descriptor was present in the sensory analysis of each sample except Trainwreck, indicating its pervasiveness across the samples measured. We note that the lack of the descriptor gas for Trainwreck, which is terpinolene-rich, is not unexpected as high terpinolene varieties have previously been shown to have lower concentrations of prenylated VSCs (or a complete lack thereof) than other dominant terpenes.32 As both terpenes and prenylated VSCs were identified in all varieties, the low correlation of the prototypical aromas they produce with the exotic score is not unexpected and suggests that they minimally contribute to the exotic notes in certain cannabis varieties.
Figure 4.
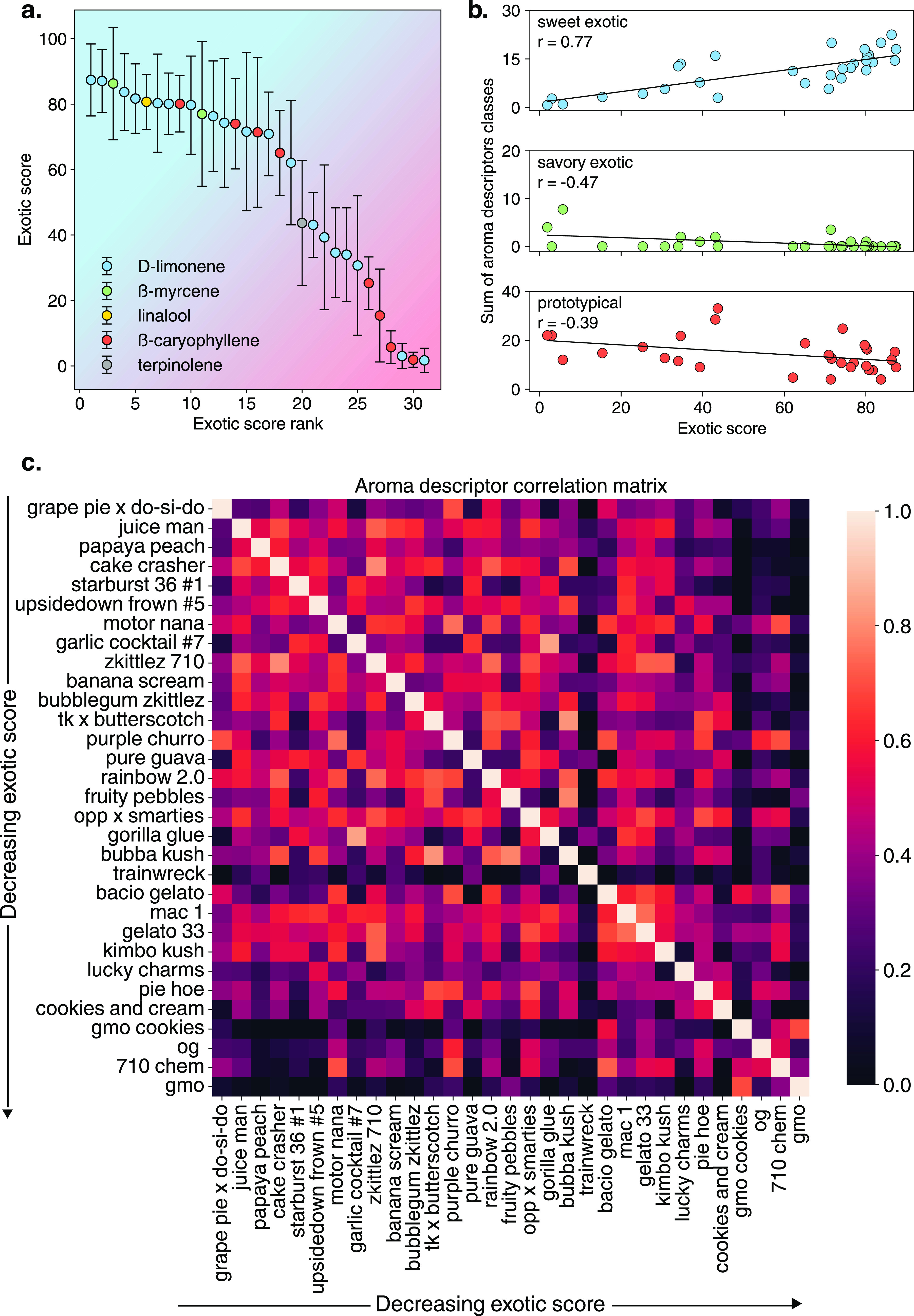
(a) Exotic scores for each variety are ranked from the highest to the lowest. Marker colors indicate the dominant terpene in each sample. (b) Correlation between aroma classifications shows sweet exotic descriptors correlating positively with increasing exotic score, savory exotic descriptors decreasing with exotic score, and minimal negative correlation between descriptors classified as prototypical and exotic score. (c) Correlation table showing similarities and dissimilarities between sensory descriptors used for each variety.
To understand the similarity of aromas based on sensory data, we calculated the Pearson correlation coefficients of the aroma descriptors for each variety, as shown in the correlation matrix in Figure 4c. This table allows for the inspection of the pairwise relationships between the sensory data of the 31 varieties, with higher values (lighter colors) indicating more similar aromas and lower values (darker colors) indicating less similar aromas (Table with coefficients shown can be found in Figure S1). As the varieties were ordered by the exotic score, greater similarities are expected for those higher on the y-axis than the lower. Indeed, lower-scoring varieties such as GMO, GMO Cookies, and 710 Chem have less correlation than those scoring higher. Concomitantly, for those ranked lowest, we see more similarities, indicating more similar aromas between those samples. These data provide us with the framework to relate the chemical composition of each variety to their aromatic properties.
Chemistry of cannabis Beyond Terpenes
To understand the chemical origins of the sensory analysis, we conducted two-dimensional gas chromatography coupled with mass spectrometry and flame ionization detection (GC × GC–TOF–MS/FID) to determine the volatile fingerprints of each sample. Our analysis found key compounds that contribute toward either sweet-exotic or savory-exotic descriptors as listed above. While a few compounds are omnipresent and found in all varieties, indicating less influence with respect to the unique aromas, many are only found in certain ones. Here, we describe specific classes of unique compounds that have rarely been described previously and their aromatic properties that help generate the diverse aroma of cannabis (Figure 5).
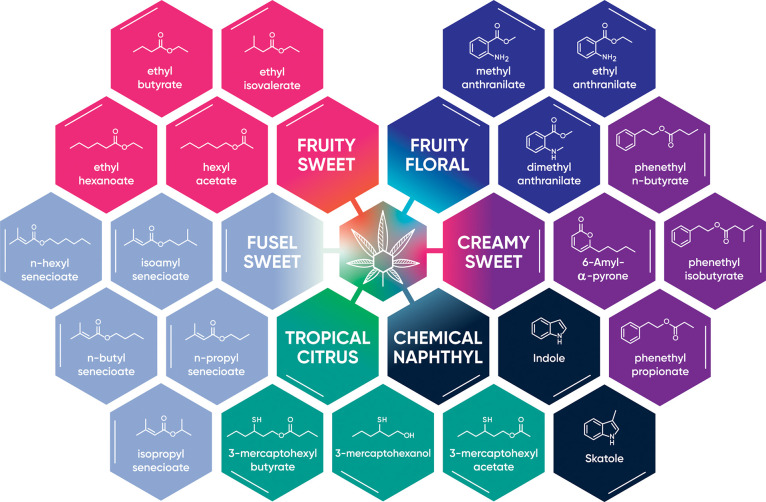
Figure 5.
Schematic highlighting key volatile compounds that produce specific aromas in cannabis.
Tropical Volatile Sulfur Compounds (VSCs)
Cannabis produces a family of prenylated volatile sulfur compounds that generate its skunky, gas-like aroma.32,33 Each of these compounds possesses strong, pungent, sulfuric aromas, with alliaceous notes produced by diprenyl disulfide and diprenyl sulfide and skunk-like notes by prenylthiol, prenylthioacetate, and prenylmethyl sulfide. We discovered that there exists another unique class of VSCs that produce tropical nuances–sulfur containing compounds that produce more citrus, fruity, sulfuric aromas–that includes 3-mercaptohexanol (3MH), 3-mercaptohexyl acetate (3MHA), and 3-mercaptohexyl butyrate (3MHB) (Figure 5). These compounds have extremely potent aromas, comparable in strength to prenylthiol and prenylthioacetate. All three are found in a multitude of tropical fruits such as passionfruit and grapefruit.39 3MH and 3MHA are also found in certain grapes and hops, which can translate to their presence in both wine and beer.40−43
We found that cannabis varieties containing these compounds take on a very strong, petroleum- or sulfuric citrus-like aroma, such as Garlic Cocktail #7 and Gorilla Glue. The importance of these compounds in dictating the perceived aroma is exemplified by these two varieties. Sensory analysis revealed similar aroma characteristics for these two varieties (r = 0.85), with citrus and tangerine descriptors used often by the panel. We note that Gorilla Glue has not traditionally been associated with this scent and rather should have a pungent, sulfuric, and spicy aroma. This suggests that the sample procured was labeled incorrectly and most likely is a different variety based on the presence of these compounds and resulting sensory analysis.
Indole Derivatives
Another key class of compounds that we identified was the heterocyclic compounds indole (1H-indole) and skatole (3-methyl-1H-indole). While previously detected in the smoke residue of cannabis, they have not been reported in cannabis inflorescence or extracts.44 Indole, the core structure of many biologically important compounds, including tryptophan and melatonin, was identified in many varieties. Although in low concentrations, its ubiquity across samples suggests that this compound most likely contributes to the general, prototypical aroma of cannabis, rather than influencing specific exotic scents. It possesses a floral, mothball-like scent characteristic of many indole-derived compounds.
While indole is common across the samples measured, skatole trends strongly with savory exotic varieties such as GMO, Garlic Cookies, and 710 chem. The aroma of this compound is complex and changes drastically at different concentrations and in the presence of other aroma compounds. It is most well known as a key contributor to the odor of mammalian and bird feces, resulting from the decomposition of tryptophan in the digestive tract.45 Nonetheless, it is also used in many fragrance-based applications, as well as can be found in certain food products.46 As an isolated compound, it possesses a strong ammoniacal scent, reminiscent of mothballs. This aroma persists in cannabis, as the majority of varieties that had skatole present also had strong savory sensory descriptions. We also note that the two high-ranked varieties Fruity Pebbles and Garlic Cocktail #7 contained skatole (71.4 and 80.1 exotic scores, respectively). The former had sensory notes of chemicals, suggesting that although it may have a sweet scent derived from other flavorants, skatole can still be detectable. The latter had a significant amount of tropical VSCs that dominated the sensory results of this variety, indicating that these compounds can mask the characteristic scent of skatole.
Many of the varieties containing skatole that were described as savory or chemical also have key esters that generate fruity or sweet aromas. This suggests that the aroma potency of skatole, referred to as substantivity or tenaciousness in the perfuming industry, may overpower the aroma produced by esters but not necessarily the potent VSCs, as is the case with Garlic Cocktail #7. Thus, how skatole modulates the overall aroma of a given variety is dependent on the other compounds present and their pungency.
Senecioates: Ubiquitous Esters in Exotic cannabis
One observation we made during cataloging of flavorants was the presence of a family of compounds containing the 3-methyl-2-butenoate (senecioate) group. This is congruent with another recent report of ethyl senecioate in cannabis and its possible link to certain varieties.47 The senecioate functional group can be considered an oxidized prenyl group, wherein the C1-positioned carbon contains a carboxyl group. As was noted previously,47 this functional group is similar to that of recently discovered prenylated VSCs,32 further showing that cannabis has a propensity to generate secondary metabolites related to this key functional group.
The isopropyl, n-propyl, n-butyl, isoamyl, and n-hexyl analogues were not available commercially and were thus synthesized following a known procedure (see methods). Sensory analysis revealed that each possesses a similar, fruity fusel base note with different nuances. For instance, the isopropyl and n-propyl senecioates were found to be fusel and sweet, whereas the n-butyl and isoamyl senecioates contained notes of banana. The n-hexyl analogue was found to have a green apple top note, which correlates with other compounds containing hexyl chains, such as hexyl acetate. These compounds are found in many varieties ranked highly by their exotic score, such as Starburst 36 #1 and Motornana, with ethyl and isopropyl senecioate typically found in the highest concentrations.
Esters: Flavor of Fruits
Esters are found in nearly every fruit and other plants and contribute strongly to their aromas, flavors, and appeal.39 Likewise, we identified more than 30 esters in cannabis alone. While not traditionally known for sweet or fruity aromas, our sensory panel results show certain varieties can indeed possess a wide range of sweet- or fruit-related aromas. Our data show that cannabis produces a wide range of esters with different aromatic characteristics, even within a single variety. For instance, Banana Scream has over 15 different esters present that we detected, each with different aroma descriptors ranging from fruity, pineapple, or banana. Two compounds of note include ethyl hexanoate and n-propyl hexanoate: The former possesses a fruity, apple-like aroma, and the latter possesses a blackberry, pineapple scent. The concentrations of these compounds correlate with an increasing exotic score, indicating their importance toward producing more sweet or fruity aromas.
On the other hand, n-hexyl n-butyrate and n-hexyl hexanoate were found in all samples, both of which are described in the literature as having green and fruity notes. As these compounds are found in each variety and often in higher concentration than other flavorants, they most likely have less influence on the unique aromatic properties of each specific variety. Interestingly, Gorilla Glue has a significantly higher concentration of n-hexyl n-butyrate than other varieties yet was only modestly ranked by the exotic score (65.1). This further suggests that this compound minimally influences the exotic aroma of cannabis.
Structurally Unique Compounds
Compounds with even more diverse functionalities were identified during our analysis. The compounds methyl, dimethyl, and ethyl anthranilate were detected in many varieties, with the latter two found in more sweet exotic varieties. While methyl anthranilate has been detected in cannabis previously,18 the dimethyl and ethyl analogues have not. These compounds each produce slightly different grapelike aromas, with methyl anthranilate possessing a strong concord grapelike scent and flavor. It is commonly used as a flavorant in different food and beverage products and is found naturally in many plants and fruits.48
Another set of related compounds with unique structural features is phenethyl n-butyrate, isobutyrate, and n-propanoate. These compounds were only identified in a select few high exotic score varieties, including Papaya Peach and Juiceman. These compounds possess mild, sweet, honeylike aromas. The few varieties these compounds were identified in are more recently bred, which may suggest that this class of compounds is only expressed in more modern varieties. As such, they may present a unique chemical fingerprint for identifying genetic divergence in these varieties.
We last highlight 6-amyl-α-pyrone, a lactone with a creamy, coconut aroma. This compound was identified only in high exotic score varieties, indicating its possible implication in these aromas detected by the sensory panel. It has been detected in peaches and is produced by Trichoderma, a fungus commonly found in soil.49
Importance of Terpenes and Flavorants in Exotic cannabis
To understand the chemical relationships between varieties, we calculated Pearson correlation coefficients between their volatile chemical fingerprints, as shown in the correlation matrices in Figure 6. These data provide a convenient way to compare the similarity between each variety on a chemical level, where a higher correlation indicates more similar chemical profiles. We did this by measuring the correlation of all analytes in each data set and decomposed into key compound classes, namely, monoterpenes and monoterpenoids, sesquiterpenes and sesquiterpenoids, and flavorants.
Figure 6.
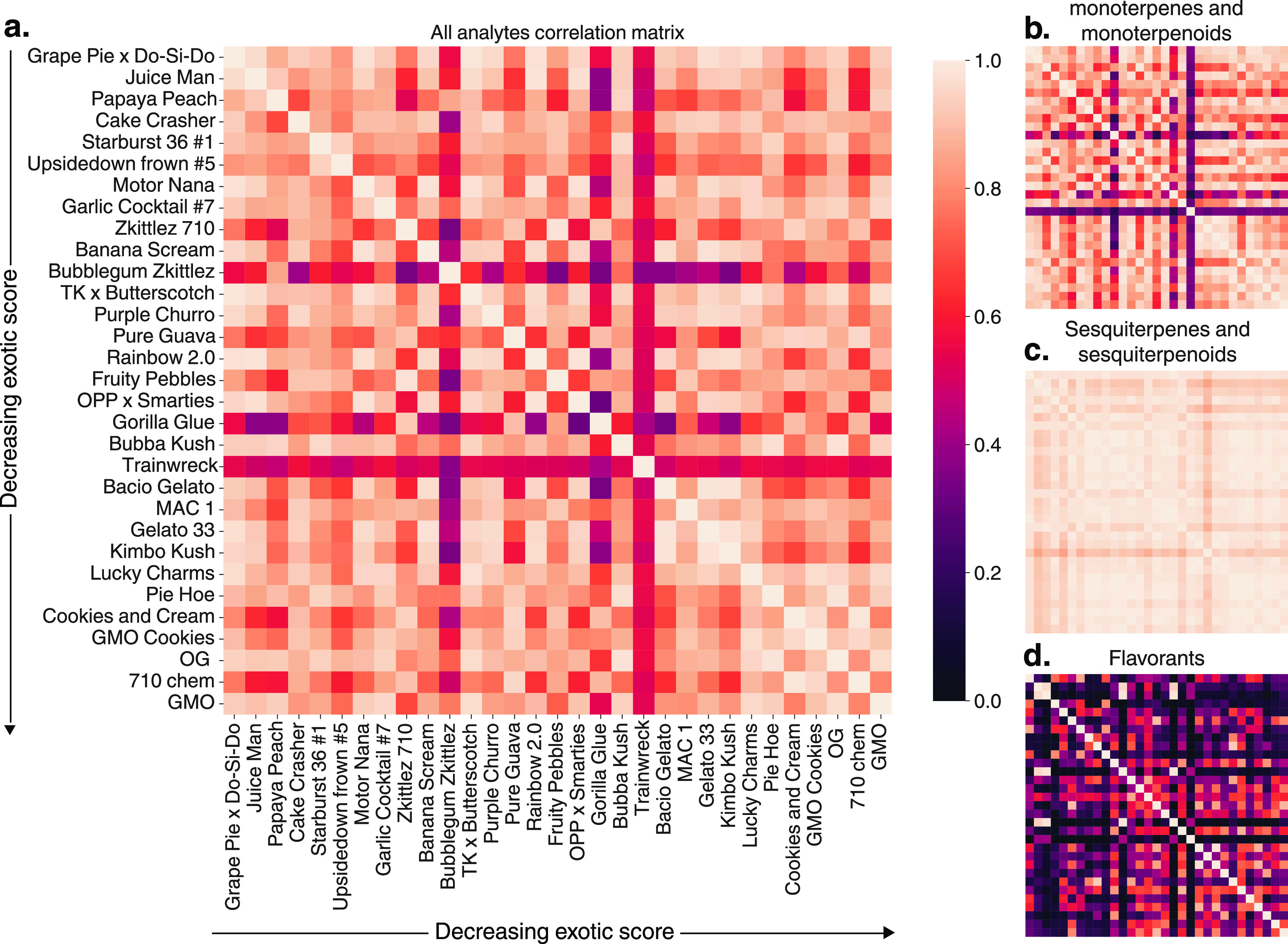
(a) Correlation matrix considering all analytes showing no trend between the exotic score and the chemical similarities of varieties. (b) Correlation table of monoterpenes and monoterpenoids. (c) Correlation table of sesquiterpenes and sesquiterpenoids. (d) Correlation table of flavorants. These data reveal that flavorants show divergent chemical compositions that other classes do not.
The resulting correlation matrix measuring all analytes (Figure 6a) is remarkably similar to the monoterpene and monoterpenoids matrix (Figure 6b). Sesquiterpene and sesquiterpenoid correlations are even stronger among all samples (Figure 6c). These data show that there are minimal differences between varieties in these two key classes, even when the aromatic properties are widely divergent. Conversely, the flavorant correlation between varieties is widely disparate (Figure 6d).
These results reveal a few important features. First, monoterpenes and monoterpenoids, which are often found in high concentrations, have strong chemical similarities between varieties despite their widely divergent aromas, suggesting that they are not the origin of the unique scents assigned to each variety. For example, GMO and Grape Pie × Do-Si-Do have the lowest and highest exotic scores, respectively, yet have a similar monoterpene and monoterpenoid profile (r = 0.91). This high chemical similarity but minimal aroma similarity suggest that these compounds do not contribute strongly to the aroma differences of these varieties. Sesquiterpenes and sesquiterpenoids appear even more similar across varieties, with the lowest Pearson coefficient of r = 0.86 found between samples.
Conversely, flavorants have wide ranging correlations depending on the varieties measured. This is especially evident when n-hexyl hexanoate is omitted due to its high concentration across all samples. The correlation coefficients range from near unity positively, as is the case for Papaya Peach and Juice Man (r = 0.99), to minimally correlated, such as between OG and Bacio Gelato (r = −0.16). The average flavorant correlation between samples was only r = 0.37, whereas monoterpene/monoterpenoid and sesquiterpene/sesquiterpenoid correlations were r = 0.65 and r = 0.84, respectively. The weak flavorant relationships between many of these varieties suggest that the aromatic differences perceived during sensory analysis may arise from this class of compounds rather than the dominant terpene classes.
Correlating the Volatile Chemical Profile of cannabis to Aroma
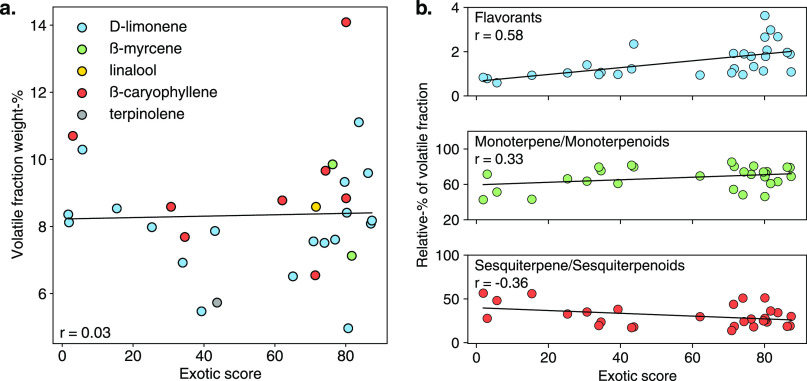
The revelation that the aroma of exotic cannabis is not necessarily driven by terpenes as traditionally thought led us to further analyze total amounts of volatile compounds (Figure 7a) and relative amounts of key classes of compounds as a function of the exotic score (Figure 7b). The total volatile concentration was not correlated to the exotic score (r = 0.03), indicating that the higher concentration of these compounds does not increase the perceived exotic nature of the aromas. These data further validate that terpenes, which typically account for over 95% of this concentration, minimally influence the perceived exotic nature of the samples, as an increase in their quantities would then lead to higher exotic scores, which was not observed. Further, when analyzing the relative amounts of monoterpene and sesquiterpene classes (Figure 7b), we also find a low correlation to exotic score. On the other hand, we saw a much more prominent correlation between flavorant relative wt % and exotic score (r = 0.58), further suggesting that flavorants drive the unique aroma properties of these varieties. We note that Gorilla Glue was omitted during this analysis for each class of compounds due to its outlier status in each.
Figure 7.
(a) Weight-% of volatile fraction color coded by dominant terpene for each sample as a function of the exotic score shows no correlation. These data confirm that the total concentration of volatiles did not influence the perceived exotic nature of the samples. (b) Relative percentages of different classes within the volatile fraction of each sample. Strong correlations were observed for flavorants, confirming their importance in producing exotic aromas. Monoterpene and monoterpenoid and sesquiterpene and sesquiterpenoid classes are divergent but with a lower correlation to the exotic score, indicating their lower importance in producing these aromas. Gorilla Glue data point was omitted due to its outlier status.
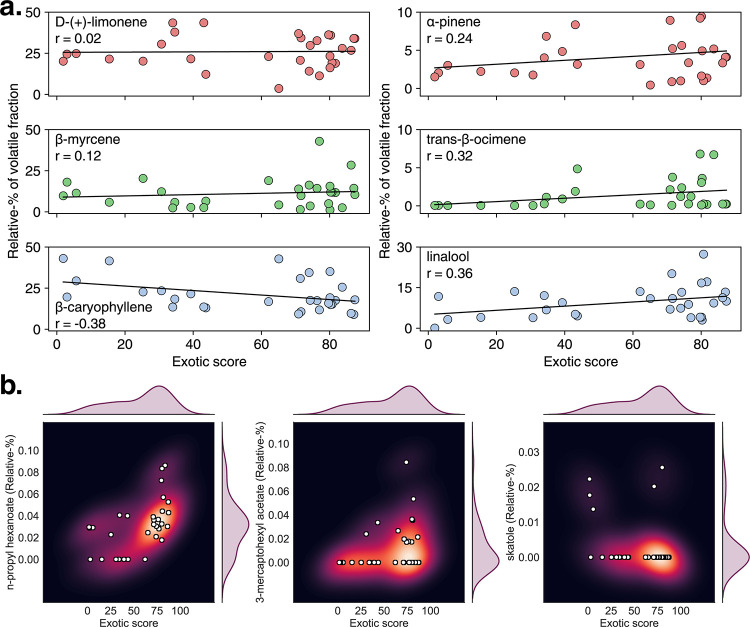
Individual compounds were then analyzed to determine how they may contribute to the exotic aroma of cannabis, with example compounds from this analysis shown in Figure 8a. We found that two of the most often referenced terpenes, D-(+)-limonene and ß-myrcene, have a minimal correlation to the perceived exotic qualities of the samples (r = 0.02, r = 0.12, respectively). ß-caryophyllene was found to have modest negative correlation (r = −0.38), indicating that this compound tends to be present in lower relative amounts in more sweet-exotic varieties. On the other hand, α-pinene, trans-ß-ocimene, and linalool were identified as having a modest correlation with exotic scores (r = 0.24, r = 0.32, r = 0.36, respectively). The latter two are not unsurprising as linalool has a light, floral aroma and trans-ß-ocimene has a sweet, floral scent. These data show that while overall terpene concentrations may not correlate strongly with the exotic nature of these varieties, certain compounds may still contribute in some capacity.
Figure 8.
(a) Correlation between major aroma compounds and the exotic score. D-(+)-limonene and ß-myrcene show minimal correlation, whereas ß-caryophyllene is modestly negatively correlated. α-pinene, trans-ß-ocimene, and linalool show positive correlation at higher exotic scores. (b) Key flavorant compounds showing clusters at a higher exotic score. N-propyl hexanoate and 3-mercaptohexyl acetate show clustering at higher exotic scores, whereas skatole shows minor clustering at lower exotic scores, with two outliers with higher scores (Garlic Cocktail #7 and Fruity Pebbles).
Analysis of the relative amounts of flavorants revealed important relationships between sensory and chemical data. Unlike the dominant terpenes, many of these compounds were found only on a sample-by-sample basis. We find that the majority of esters and tropical VSCs are present in high-ranking exotic varieties but lower relative amounts in low scoring varieties. In particular, we highlight n-propyl hexanoate and 3-mercaptohexyl acetate. Clear clustering at higher concentrations is found in high-rank varieties (Figure 8b) for these compounds. On the other hand, skatole, which possesses a strong chemical aroma, has greater clustering at lower exotic scores. We note that Fruity Pebbles and Garlic Cocktail #7 also had detectable skatole concentrations but higher exotic score (71.4 and 80.1 respectively). These varieties, unlike the other high skatole varieties GMO, 710Chem, and GMO Cookies (exotic scores of 1.7, 1.9, and 5.7, respectively), had higher concentrations of flavorants and tropical VSCs, which may explain why they were so highly ranked. Nonetheless, we note that Fruity Pebbles was still identified as having a slight chemical note by a sensory panelist, indicating that while sweet exotic descriptors were most prominent, the specific aroma produced by skatole was still detectable.
Certain specific sweet exotic descriptors were used during sensory analysis that do not currently have clear chemical origins. The sensory panel reported a wide range of various fruit-associated descriptors, such as apple, grape, banana, and multiple types of berry-related descriptors. This problem is compounded by the subjective nature of the human sense of smell, making it difficult with the current data set to establish clear trends between these specific aromas. Future studies that expand the sensory panel to a larger group with improved training will help derive these relationships. Additionally, the analysis of more varieties with similar exotic aromas may help further elucidate these chemical-sensory relationships. For instance, procuring multiple apple-smelling varieties, such as Apple Kush, Sour Apple, and Apple Jack, may help derive relationships between specific compounds and this aroma. This approach may help define specific cannabis classes based on both key aromatic characteristics and their underlying chemistry.
Discussion
While terpenes do not show clear trends related to the exotic aroma of cannabis, we hypothesize that terpene profiles may still produce subclasses of aromas that are characteristic of one another. For instance, ß-caryophyllene-rich varieties with high flavorant concentrations may generally lead to similar aromatic qualities that are different from D-(+)-limonene-rich varieties. As ß-caryophyllene has a milder, more spicy scent than D-(+)-limonene, it is reasonable to expect varieties with the former profile to have a characteristic aroma that is different than the latter profile. Nonetheless, our data show that the presence of key, pungent low concentration compounds such as tropical VSCs or skatole may still drive varieties with divergent terpene profiles to have similar aroma characteristics. Conducting a wider analysis on varieties with more diverse terpene profiles will help understand the interplay between terpene and flavorant profiles.
We note that our analysis only included one terpinolene-rich variety, Trainwreck, due to their rarity in ice hash rosin form. This is not unexpected as a previous survey of cannabis across the United States showed terpinolene rich varieties to be less common than the other dominant terpenes ß-caryophyllene, limonene, and ß-myrcene.11 These varieties represent the single terpene class that appears chemically distinct from the others in multiple chemotaxonomic studies and are also generally considered to produce the most consistently energizing psychoactive effects.11,25,26,30 As these classification schemes rely solely on terpenes, it is plausible that this class is indeed aromatically unique compared to the others due to its terpene profile rather than flavorants. Indeed, Trainwreck had a low sensory similarity to other varieties as well as the lowest chemical similarity to other varieties, validating this distinct chemotaxonomic class. While ranked modestly by the sensory panel (exotic score = 43.7), it lacked many of the key flavorants that were present in higher ranking varieties such as VSCs or n-propyl hexanoate but had unusually high concentrations of cherry propanol. While this compound is found in low concentrations in the other samples (average concentration of 0.016 μg/mg), it was found at 0.739 μg/mg in Trainwreck, nearly 18 times higher. Thus, we hypothesize that the combination of the unique terpene profile in this sample and the surprisingly high cherry propanol levels may account for the perceived mild sweetness or fruitiness reported by the sensory panel. Studies focused on other terpinolene varieties are ongoing to determine how other flavorants may combine with this terpene profile to produce unique scents.
We hypothesize that some of these newly reported compounds, like terpenes, may possess biological activity that could influence the psychoactive effects brought on by THC. Indole and skatole in particular may harbor functionality due to their similarity to other compounds in nature with similar chemical structure.50 For instance, the compound 3–3′-diindolylmethane (DIM), which can be considered two skatole molecules fused together through the 3-positioned methyl group, has shown promising anticancer effects, as well as been found to be a CB2 receptor agonist.51,52 Future investigations into the possible biological activity of these compounds and others reported here may further shed light on how they may influence the properties of specific varieties.
The identification of flavorants and their correlation to the various sweet or savory aromas of cannabis has implications within the regulated cannabis industry. Prior to this work, terpenes have largely been the presumed source of nearly all cannabis scents and flavors, thus leading to their use within regulatory testing and packaging to describe the flavor and aroma of cannabis products. However, our results show that terpenes contribute primarily only to the characteristic aroma of cannabis rather than the more unique and desirable attributes of many varieties. Cannabis labeling or certificates of analysis (COAs) that contain only terpene information thus may mislead consumers or producers into believing that these compounds are the sole source of a given product aroma or that a product will possess certain aroma attributes that are rather produced by entirely different compounds. Our results help rectify this misconception and provide the necessary chemical understanding to more adequately describe the chemical origins of the many exotic aromas produced by cannabis. Lastly, flavorants, along with terpenes, may find use in the development of new “freshness” indicators for products. For instance, studies investigating the rates of volatilization or degradation of these key compounds over time may help guide consumers in purchasing products.
Conclusions
We conducted coupled sensory and chemical analyses on 31 different ice hash rosin cannabis extracts to determine how different chemical classes affect the aroma of each. Our sensory analysis revealed highly divergent aroma characteristics for many samples that we broke down into three primary classes: Sweet exotic, prototypical, and savory exotic. We found that varieties across both sweet and savory exotic classes often have very similar terpene profiles, indicating that they are not the driving force behind the unique aromatic differences. Detailed chemical analysis using two-dimensional gas chromatography revealed that minor, nonterpenoid compounds are responsible for this discrepancy. While found in low concentration, often accounting for less than 0.05% of the mass of the samples, their odor impact can be substantial. In particular, we identified key classes of compounds that correlated with specific aromas: tropical volatile sulfur compounds (VSCs) containing the 3-mercaptohexyl functional group were found in a subset of varieties that produce a strong, sulfuric, petroleum-citrus aroma that was easily identified during sensory analysis. Conversely, varieties described as savory or chemical were found to contain skatole, a compound with an extremely pungent chemical aroma. Our results yield a more complete understanding of the unique aromas that cannabis produces and help establish these nonterpenoid compounds as an important part of the phytochemistry of cannabis. Furthermore, the discovery that terpenes have less influence on the differentiating characteristics of the aroma of cannabis than traditionally thought may have important ramifications for the legal cannabis industry related to product labeling and marketing, laboratory testing, and quality indicators for end consumers and producers alike.
Experimental Section
Sample Procurement and Preparation
Ice hash rosin cannabis samples of different varietals were procured from various dispensaries around the Los Angeles, CA area. The samples were chosen to maximize the aromatic diversity of samples based on their known aroma attributes and the sensory panel’s experience or product description. Samples that are currently popular based on educational Web sites including Leafly (leafly.com) and Weedmaps (weedmaps.com) were given priority to emulate the current state of the cannabis marketplace. Samples were stored in an −8 °C freezer until measured to retain their volatile chemical profile. Approximately 100 ± 5 mg of the samples were transferred into a 20 mL scintillation vial followed by addition of 2 mL of hexanes. The resulting solution was agitated for 5 min to fully dissolve the matrix. The resulting solution was then transferred into a 2 mL sample vial by using a filtered syringe. Each sample was collected in triplicate.
Analytical Standards
Several different reference materials were acquired for the purpose of compound quantitation and confirmation. A 35-compound terpene analytical standard (LGC Standards) was used to quantify the major components in the samples. A custom 17 compound flavorant standard (FLV-1) was supplied from LGC Standards prepared in triacetin that was further diluted in ethanol. Multiple custom flavorant standards were then created in-house using analytical grade standards when available. Standards were purchased from different sources, including Sigma-Aldrich, Vigon International, and Penta International. Prenylthiol (Penta International, 95% in 1% triacetin) was prepared in ethanol. 3-Mercaptohexanol, 3-mercaptohexyl acetate, and 3-mercaptohexyl butyrate (Excellentia, >97%) were prepared in hexanes. Senecioates were synthesized in-house (>97%) and prepared in hexanes. Table S1 shows the complete list of standards used and their calibration statistics. Five or 6-point Calibration curves were used to quantify the compounds. Figures S15–S70 show mass spectra of flavorant analytes in select varieties along with NIST v17 mass spectral database data. Additionally, each analyte reported was structurally validated by confirming similar elution times and mass spectra of standards.
Synthesis of Senecioates
In each esterification, 3-methyl-2-butenoic acid (1 equiv) was dissolved in dichloromethane at room temperature. 4-Dimethylaminopyridine (0.085 equiv) and 4 equiv of a respective alcohol (n-propanol, isopropanol, n-butanol, isoamyl alcohol, or n-hexanol) were then added. The reaction solution was cooled in an ice bath before adding N, N′-dicyclohexylcarbodiimide (1.2 equiv). This addition of the coupling reagent led to a precipitate forming as the reaction progressed. After 24 h, the reaction mixture was filtered and the organic layer was washed with aqueous 0.5 N HCl, saturated sodium bicarbonate, and dried over magnesium sulfate. The organic layer was filtered and concentrated. The crude material was purified by column chromatography using silica gel and eluted with a mixture of 90:10 hexane–diethyl ether. After the purified material was dried under high-vacuum pressure, the senecioates were analyzed by GC, FTIR, 1H NMR, and 13C NMR spectroscopy. Each of the FTIR traces indicated an ester formed by the carbonyl stretch at ∼1700 cm–1. The ester resonance was also confirmed in the 13C NMR spectrum of each target compound at ∼166 ppm. Lastly, the aliphatic chains of the respective alcohols in the esterification coupling were easily identified in both the 1H and 13C spectra. Complete experimental conditions and spectral data can be found in the Supporting Information.
Comprehensive Two-Dimensional Gas Chromatography
GC × GC analysis was performed using the INSIGHT reverse fill flush flow modulator (SepSolve Analytical). This was coupled for data generation to an Agilent 7890B GC equipped with a BPX5 (20 m × 0.18 mm ID × 0.18 μm film thickness) first dimension column and Mega Wax (4.8 m × 0.32 mm ID × 0.15 μm film thickness) second dimension column and BenchTOF Select Time of flight mass spectrometer (Markes International). Time-of-flight mass spectrometry (TOF-MS) was used to identify compounds. Quantification of compounds was performed using a flame ionization detector (FID). Sample introduction was done using direct injection with an Agilent 7693 Injector Tower (G4513A). The needle was washed 3 times with isopropanol and hexanes before and after injection. The injection volume used was 5 μL. The inlet split flow and temperature were 20:1 and 280 °C, respectively. The TOF-MS detector source was held at 280 °C and a transfer line temperature of 260 °C. A solvent delay of 6 min was used and had an acquisition rate of 60 Hz.
The GC × GC column configuration was an apolar to polar setup. The GC oven ramp rates used were as follows: The oven was initially set to 45 °C and held for 3 min. The oven was then ramped at a rate of 3 °C per minute to 98 °C, followed by a 6 °C per minute ramp rate to 140 °C, followed by a 8.5 °C per minute ramp rate to 170 °C followed by a 2 °C per minute ramp rate to 190 °C, followed last by a 15 °C per minute ramp to 260 °C and held for 13 min to remove any remaining compounds from the column. The modulation period set for the flow modulator was 6.0 s. Data were collected, integrated, and analyzed using the ChromSpace software platform (Sepsolve Analytical). Statistical analysis and data transformations were performed using Terplytics. Figures S6–S13 showing GC × GC–FID chromatograms have been realigned to account for void time (2.5 s) in the second dimension. Analyte concentrations can be found in Tables S10–S14. Relative amounts of the volatile fraction for each analyte can be found in Tables S15–S19. Correlation matrices for sensory analysis and chemical analysis with Pearson correlation coefficients can be found in Figures S1–S5.
Sensory Analysis
A panel of seven cannabis users was constructed to determine the subjective sensory properties of each varietal. Each panelist reviewed the products independent of one another to prevent any panelist interference. The panelists were not trained in an effort to emulate a typical cannabis consumer sensory experience. Between each sample, the users were asked to refresh their palettes by smelling a neutral base, such as the back of their hand to prevent any sensory fatigue between reviewing samples. Approximately 500 mg of each sample was stored in 20 mL scintillation vials and warmed to room temperature to ensure the rosin matrix provided minimal interference. Given the viscous matrix of cannabis extracts, it is important that the products are evaluated at room temperature to obtain an accurate representation of the aroma characteristics. The users measured each sample and were asked to describe the aromatic qualities. Intensity descriptors were allowed and used as multipliers depending on the term used; these descriptors can be found in Table S4. The users were then asked to rate how sweet or fruity each sample was on a scale of 0–100, where 0 represented aromas considered more prototypical or savory and 100 represented the sweetest. No formal lexicon was used to prevent the panel from using terminology they found useful. The raw user input was then refined and classified based on the sensory panels interpretation of the descriptors into either sweet exotic, prototypical, or savory exotic as seen in Table S3. The results of the sensory panel were then tabulated as summarized in Tables S5–S9.
Acknowledgments
We thank Abstrax Tech Inc. for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c04496.
Calibration data of analytes; tabulated sensory panel data; GCxGC analyte concentration table; GCxGC analyte relative-% table; GCxGC chromatograms of all samples; mass spectra of analytes; and experimental synthesis details (PDF)
Author Contributions
I.W.H.O. conceived the study, analyzed the results, and wrote the manuscript. T.R.P. conducted GC × GC experiments and edited the manuscript. M.E.S. collected GC × GC data and synthesized and characterized senecioates. M.A.O. analyzed GC × GC data and edited the manuscript. M.R.A. provided guidance on sensory studies and extraction processes. J.J.G. and N.S.S. helped develop the GC × GC methodology and edited the manuscript. J.R.P. and K.N. edited the manuscript and conducted sensory experiments. B.G.M. and I.S. provided samples, guidance on extraction processes, and edited the manuscript. K.A.K. managed the project and edited the manuscript. M.F.Z.P. managed the project, provided guidance on synthesis experiments, and edited the manuscript. T.J.M. managed the project, provided guidance on sensory experiments, and wrote and edited the manuscript.
The authors declare the following competing financial interest(s): I.W.H.O., T. R. P., M.A.O., T.J.M., K. N., M. E. S., M. F. Z. P., and K.A.K. have filed patents related to the results described herein.
Special Issue
Published as part of the ACS Omegavirtual special issue “Phytochemistry”.
Supplementary Material
References
- ElSohly M. A.; Chandra S.; Radwan M.; Majumdar C. G.; Church J. C. A Comprehensive Review of Cannabis Potency in the United States in the Last Decade. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6 (6), 603–606. 10.1016/j.bpsc.2020.12.016. [DOI] [PubMed] [Google Scholar]
- de Ferreyro Monticelli D.; Bhandari S.; Eykelbosh A.; Henderson S. B.; Giang A.; Zimmerman N. Cannabis Cultivation Facilities: A Review of Their Air Quality Impacts from the Occupational to Community Scale. Environ. Sci. Technol. 2022, 56 (5), 2880–2896. 10.1021/acs.est.1c06372. [DOI] [PubMed] [Google Scholar]
- Jikomes N.; Zoorob M. The Cannabinoid Content of Legal Cannabis in Washington State Varies Systematically Across Testing Facilities and Popular Consumer Products. Sci. Rep. 2018, 8 (1), 4519. 10.1038/s41598-018-22755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A.; Albayram O.; Draffehn A.; Michel K.; Piyanova A.; Oppenheimer H.; Dvir-Ginzberg M.; Rácz I.; Ulas T.; Imbeault S.; Bab I.; Schultze J. L.; Zimmer A. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017, 23 (6), 782–787. 10.1038/nm.4311. [DOI] [PubMed] [Google Scholar]
- Ahmed A. T. M. F.; Islam M. Z.; Mahmud M. S.; Sarker M. E.; Islam M. R. Hemp as a potential raw material toward a sustainable world: A review. Heliyon 2022, 8 (1), 9733. 10.1016/j.heliyon.2022.e08753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D.; Goodman S.; Wadsworth E.; Rynard V.; Boudreau C.; Hall W. Evaluating the impacts of cannabis legalization: The International Cannabis Policy Study. Int. J. Drug Policy 2020, 77, 102698 10.1016/j.drugpo.2020.102698. [DOI] [PubMed] [Google Scholar]
- Smart R.; Pacula R. L. Early evidence of the impact of cannabis legalization on cannabis use, cannabis use disorder, and the use of other substances: Findings from state policy evaluations. Am. J. Drug Alcohol Abuse 2019, 45 (6), 644–663. 10.1080/00952990.2019.1669626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulkins J. P.; Kilborn M. L. Cannabis legalization, regulation, & control: a review of key challenges for local, state, and provincial officials. Am. J. Drug Alcohol Abuse 2019, 45 (6), 689–697. 10.1080/00952990.2019.1611840. [DOI] [PubMed] [Google Scholar]
- Adinoff B.; Cooper Z. D. Cannabis legalization: progress in harm reduction approaches for substance use and misuse. Am. J. Drug Alcohol Abuse 2019, 45 (6), 707–712. 10.1080/00952990.2019.1680683. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. T.; Yarnell S.; Radhakrishnan R.; Ball S. A.; D’Souza D. C. Marijuana Legalization: Impact on Physicians and Public Health. Annu. Rev. Med. 2016, 67 (1), 453–466. 10.1146/annurev-med-050214-013454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J.; Vergara D.; Keegan B.; Jikomes N.; Shahid M. Q. The phytochemical diversity of commercial Cannabis in the United States. PLoS One 2022, 17 (5), e0267498 10.1371/journal.pone.0267498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe A. L.; Johnson V.; Harrelson J.; McGlaughlin M. E.; Walker L. A. Uncomfortably high: Testing reveals inflated THC potency on retail Cannabis labels. PLoS One 2023, 18 (4), e0282396 10.1371/journal.pone.0282396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb J.; Demirel S.; Sackett J. L.; Russo E. B.; Wilson-Poe A. R. The Nose Knows: Aroma, but Not THC Mediates the Subjective Effects of Smoked and Vaporized Cannabis Flower. Psychoactives 2022, 70–86. 10.1093/ckj/sfad070. [DOI] [Google Scholar]
- Wise K.; Phan N.; Selby-Pham J.; Simovich T.; Gill H.; Mastinu A. Utilisation of QSPR ODT modelling and odour vector modelling to predict Cannabis sativa odour. PLoS One 2023, 18 (4), e0284842 10.1371/journal.pone.0284842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira A.; Berman P.; Futoran K.; Guberman O.; Meiri D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019, 91 (17), 11425–11432. 10.1021/acs.analchem.9b02844. [DOI] [PubMed] [Google Scholar]
- Booth J. K.; Page J. E.; Bohlmann J.; Hamberger B. Terpene synthases from Cannabis sativa. PLoS One 2017, 12 (3), e0173911 10.1371/journal.pone.0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizpurua-Olaizola O.; Soydaner U.; Óztúrk E.; Schibano D.; Simsir Y.; Navarro P.; Etxebarria N.; Usobiaga A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79 (2), 324–331. 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed] [Google Scholar]
- Rice S.; Koziel J. A.; Glendinning J. I. Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis. PLoS One 2015, 10 (12), e0144160 10.1371/journal.pone.0144160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini M.; Charvoz C.; Dujourdy L.; Baldovini N.; Filippi J.-J. Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L. J. Chromatogr. A 2014, 1370, 200–215. 10.1016/j.chroma.2014.10.045. [DOI] [PubMed] [Google Scholar]
- LaVigne J. E.; Hecksel R.; Keresztes A.; Streicher J. M. Cannabis sativa terpenes are cannabimimetic and selectively enhance cannabinoid activity. Sci. Rep. 2021, 11 (1), 8232. 10.1038/s41598-021-87740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe A. L.; Naibauer S. K.; McGlaughlin M. E.; Gilbert A. N. Human olfactory discrimination of genetic variation within Cannabis strains. Front. Psychol. 2022, 13, 942694 10.3389/fpsyg.2022.942694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain,” No Gain. Front. Plant Sci. 2019, 9, 1969. 10.3389/fpls.2018.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E. B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4 (8), 1614–1648. 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- Watts S.; McElroy M.; Migicovsky Z.; Maassen H.; van Velzen R.; Myles S. Cannabis labelling is associated with genetic variation in terpene synthase genes. Nat. Plants 2021, 7 (10), 1330–1334. 10.1038/s41477-021-01003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischedick J. T. Identification of Terpenoid Chemotypes Among High (−)-trans-Δ(9)- Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars. Cannabis Cannabinoid Res. 2017, 2 (1), 34–47. 10.1089/can.2016.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischedick J. T.; Hazekamp A.; Erkelens T.; Choi Y. H.; Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71 (17), 2058–2073. 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Sommano S. R.; Chittasupho C.; Ruksiriwanich W.; Jantrawut P. The Cannabis Terpenes. Molecules 2020, 25 (24), 5792. 10.3390/molecules25245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N.; Eyal A. M.; Zeitouni D. B.; Hen-Shoval D.; Davidson E. M.; Danieli A.; Tauber M.; Ben-Chaim Y. Selected cannabis terpenes synergize with THC to produce increased CB1 receptor activation. Biochem. Pharmacol. 2023, 212, 115548 10.1016/j.bcp.2023.115548. [DOI] [PubMed] [Google Scholar]
- Hanuš L. O.; Hod Y. Terpenes/Terpenoids in Cannabis: Are They Important?. Med. Cannabis Cannabinoids 2020, 3 (1), 25–60. 10.1159/000509733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. A.; Russo E. B.; Smith K. M. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2018, 84 (04), 225–233. 10.1055/s-0043-122240. [DOI] [PubMed] [Google Scholar]
- Gilbert A. N.; DiVerdi J. A.; Glendinning J. I. Consumer perceptions of strain differences in Cannabis aroma. PLoS One 2018, 13 (2), e0192247 10.1371/journal.pone.0192247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald I. W. H.; Ojeda M. A.; Pobanz R. J.; Koby K. A.; Buchanan A. J.; Del Rosso J.; Guzman M. A.; Martin T. J. Identification of a New Family of Prenylated Volatile Sulfur Compounds in Cannabis Revealed by Comprehensive Two-Dimensional Gas Chromatography. ACS Omega 2021, 6 (47), 31667–31676. 10.1021/acsomega.1c04196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel J. A.; Guenther A.; Vizuete W.; Wright D. W.; Iwasinska A. Skunky” Cannabis: Environmental Odor Troubleshooting and the “Need-for-Speed. ACS Omega 2022, 7 (23), 19043–19047. 10.1021/acsomega.2c00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.; Dai K.; Xie Z.; Chen J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10 (1), 3309. 10.1038/s41598-020-60172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C. M.; Hausman J.-F.; Guerriero G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sanchez I. J.; Verpoorte R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7 (3), 615–639. 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- ElSohly M. A.; Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78 (5), 539–548. 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Gilbert A. N.; DiVerdi J. A. Use of rating scales versus check-all-that-apply ballots in quantifying strain-specific Cannabis aroma. J. Sens. Stud. 2019, 34 (4), e12499 10.1111/joss.12499. [DOI] [Google Scholar]
- El Hadi M. A.; Zhang F.-J.; Wu F.-F.; Zhou C.-H.; Tao J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T.; Morimoto M.; Kobayashi M.; Yako N.; Wanikawa A. Behaviors of 3-Mercaptohexan-1-ol and 3-Mercaptohexyl Acetate during Brewing Processes. J. Am. Soc. Brew. Chem. 2008, 66 (3), 192–196. 10.1094/ASBCJ-2008-0702-01. [DOI] [Google Scholar]
- Sonia C.; Catherine V. Combinatorial Synthesis and Screening of Novel Odorants Such as Polyfunctional Thiols. Comb. Chem. High Throughput Screen. 2006, 9 (8), 583–590. [DOI] [PubMed] [Google Scholar]
- Lermusieau G.; Collin S. Volatile Sulfur Compounds in Hops and Residual Concentrations in Beer—A Review. J. Am. Soc. Brew. Chem. 2003, 61 (3), 109–113. 10.1094/ASBCJ-61-0109. [DOI] [Google Scholar]
- Tominaga T.; Niclass Y.; Frérot E.; Dubourdieu D. Stereoisomeric Distribution of 3-Mercaptohexan-1-ol and 3-Mercaptohexyl Acetate in Dry and Sweet White Wines Made from Vitis vinifera (Var. Sauvignon Blanc and Semillon). J. Agric. Food Chem. 2006, 54 (19), 7251–7255. 10.1021/jf061566v. [DOI] [PubMed] [Google Scholar]
- Haq M. Z.-u.; Rose S. J.; Deiderich L. R.; Patel A. R. Identification and quantitative measurement of some N-heterocyclics in marijuana smoke condensates. Anal. Chem. 1974, 46 (12), 1781–1785. 10.1021/ac60348a057. [DOI] [PubMed] [Google Scholar]
- Yokoyama M. T.; Carlson J. R. Microbial metabolites of tryptophan in the intestinal tract with special reference to skatole. Am. J. Clin. Nutr. 1979, 32 (1), 173–178. 10.1093/ajcn/32.1.173. [DOI] [PubMed] [Google Scholar]
- Zgarbová E.; Vrzal R. Skatole: A thin red line between its benefits and toxicity. Biochimie 2023, 208, 1–12. 10.1016/j.biochi.2022.12.014. [DOI] [PubMed] [Google Scholar]
- Anterola A. M. L.; Abrams J.. Beyond terpenes: An untargeted approach for characterizing and categorizing cannabis cultivars to derive a robust chemotaxonomy. In ACS Fall National Meeting 2022; Chicago, IL, 2022; 3752003. [Google Scholar]
- Wang J.; Luca V. D. The biosynthesis and regulation of biosynthesis of Concord grape fruit esters, including ‘foxy’ methylanthranilate. Plant J. 2005, 44 (4), 606–619. 10.1111/j.1365-313X.2005.02552.x. [DOI] [PubMed] [Google Scholar]
- Kalyani A.; Prapulla S. G.; Karanth N. G. Study on the production of 6-pentyl-α-pyrone using two methods of fermentation. Appl. Microbiol. Biotechnol. 2000, 53 (5), 610–612. 10.1007/s002530051665. [DOI] [PubMed] [Google Scholar]
- Umer S. M.; Solangi M.; Khan K. M.; Saleem R. S. Indole-Containing Natural Products 2019–2022: Isolations, Reappraisals Syntheses, and Biological Activities. Molecules 2022, 27 (21), 7586. 10.3390/molecules27217586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahardhika A. B.; Ressemann A.; Kremers S. E.; Gregório Castanheira M. S.; Schoeder C. T.; Müller C. E.; Pillaiyar T. Design, synthesis, and structure–activity relationships of diindolylmethane derivatives as cannabinoid CB2 receptor agonists. Arch. Pharm. 2023, 356 (3), 2200493 10.1002/ardp.202200493. [DOI] [PubMed] [Google Scholar]
- Tucci P.; Brown I.; Bewick G. S.; Pertwee R. G.; Marini P. The Plant Derived 3–3′-Diindolylmethane (DIM) Behaves as CB2 Receptor Agonist in Prostate Cancer Cellular Models. Int. J. Mol. Sci. 2023, 24, 3620. 10.3390/ijms24043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.