Abstract
Suvorexant (SUV) is a new sedative/hypnotic medicine that is recommended to treat insomnia. It is an important medicine from a forensic point of view due to its sedative/hypnotic and depressant effects. To the best of our knowledge, high-performance thin-layer chromatography (HPTLC) bioanalytical methods have not been published to measure SUV in human urine and pharmaceutical samples. Accordingly, this study was designed and validated a sensitive and rapid bioanalytical HPTLC method to determine SUV in human urine samples for the very first time. The densitometric measurement of SUV and the internal standard (IS; sildenafil) was performed on glass-coated silica gel normal-phase-60F254S TLC plates using a mixture of chloroform and methanol (97.5:2.5 v/v) as the eluent system. Both the SUV and IS were detected at a wavelength of 254 nm. Both analytes were extracted using the protein precipitation technique utilizing methanol as the solvent. For the IS and SUV, the Rf values were 0.09 and 0.45, respectively. The proposed bioanalytical method for SUV was linear in the 50–1600 ng/band range. The current bioanalytical technique was linear, precise (% RSD = 3.28–4.20), accurate (% recovery = 97.58–103.80), robust (% recovery = 95.31–102.34 and % RSD = 2.81–3.15), rapid, and sensitive (LOD = 3.73 ng/band and LOQ = 11.20 ng/band). These findings suggested that the current bioanalytical method can be regularly used to determine SUV in wide varieties of urine samples.
1. Introduction
Sedative/hypnotic medicines belong to the central nervous system (CNS) class of medicines.1 Due to their frequent usage, ability to interact with other CNS depressants to produce additive effects, impairment-causing effects, and misuse potential, these medications are significant from a forensic perspective.2 Belsomra (Merck, Rahway, NJ) is the brand name for a relatively new class of sedative/hypnotic medications known as suvorexant (SUV).3,4 The molecular structure of SUV is shown in Figure 1. It is used to treat insomnia.5,6 According to reports, SUV is a highly effective dual orexin receptor (OX1R and OX2R) antagonist that causes a rapid onset of sleep by blocking the orexin neurons of the arousal system that promote wakefulness.3,6 Physicochemically, it has been found to be sparingly soluble in water and highly soluble in organic solvents.7 SUV had high oral bioavailability (82%) after oral administration.8,9 SUV has misuse potential, just like other sedative/hypnotic drugs, and was added to Schedule IV of the Federal Controlled Substances Act by the US Drug Enforcement Administration not long after receiving approval.10 The fact that SUV was effectively discovered in the post-mortem remains of three different autopsy cases suggested that the forensic toxicology community will encounter this medication more frequently.11 Because it is forensically important, its illegal use is highly expected, and hence, a rapid and sensitive bioanalytical method is necessary for its detection and quantitation in biological sample matrices. Due to its straightforward, affordable, and noninvasive collection method, urine is the most popular specimen for forensic investigation among various biological sample matrices.12
Figure 1.
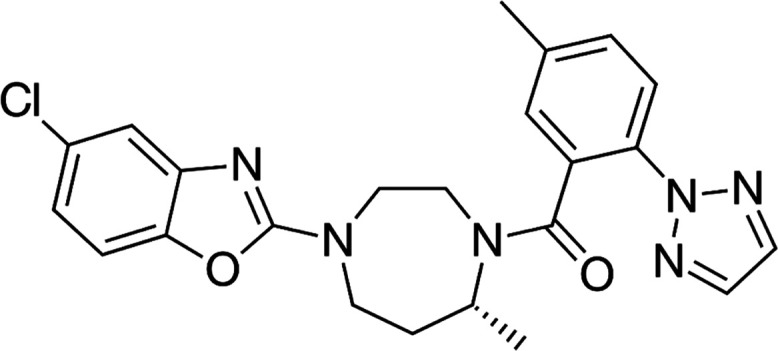
Molecular structure of suvorexant (SUV).
Various bioanalytical methods have been reported for the measurement and detection of SUV in numerous biological samples such as urine, plasma, and blood. A high-performance liquid chromatography (HPLC) bioanalytical method has been reported for the detection of SUV in rabbit plasma samples.13 A HPLC method has also been used to measure SUV in tablet dosage forms.14 A liquid chromatography–mass spectrometry (LC-MS)/MS (LC-MS/MS) bioanalytical method has also been reported to measure SUV in blood samples.15 A LC-MS/MS bioanalytical method has also been used to measure SUV along with other sedatives/hypnotics in the whole blood samples.16 There is also a bioanalytical method called LC-quadrupole/time-of-flight MS (LC-Q/TOF-MS) used to identify SUV in blood samples.17 A LC-MS/MS method was also used to measure SUV in human plasma samples using 96-well liquid–liquid extraction.18 In order to assess SUV in plasma samples, a number of ultraperformance LC-MS/MS (UPLC-MS/MS) bioanalytical methods were also applied.19−21 Gas chromatography MS (GC–MS), LC-Q/TOF-MS, and UPLC-MS/MS bioanalytical methods have been reported to measure SUV in urine samples.12,22,23
Most of the reported bioanalytical methods for the measurement of SUV in biological fluids are highly sensitive techniques, such as LC-MS/MS, GC-MS, and UPLC-MS/MS. These instruments are typically expensive to purchase and maintain, requiring highly specialized technical knowledge, making them difficult to obtain in most laboratories. A cost-effective, simple, and convenient bioanalytical test is therefore recommended in a resource-constrained situation, provided that its lower limit of quantification (LLOQ) is sufficient for regular estimation.24 Due to improvements in the stationary phases and the development of densitometers as detection tools, high-performance thin-layer chromatographic (HPTLC) bioanalytical methods attain precision and accuracy for the assessment of medicines in contrast to LC-based bioanalytical methods.25−27 However, HPTLC is only occasionally used to assess medications in biological samples.28−30
To the best of our knowledge, no single HPTLC method has been published for the determination of SUV in biological fluids or pharmaceutical dosage forms. Therefore, the goal of the current investigation was to design and verify a simple, economical, affordable, rapid, and sensitive HPTLC bioanalytical method for the first-ever assessment of SUV in spiked human urine samples. The present studies were performed on spiked urine samples only. The metabolism of SUV is possible after administration of the drug to humans and animals. The drug cannot be metabolized in spiked urine, so metabolism of drug was not taken into consideration. The proposed HPTLC bioanalytical methods was validated for numerous validation parameters following the Scientific Working Group for Forensic Toxicology (SWGTOX) guidelines.31
2. Results and Discussion
2.1. Development and Optimization of Analytical Conditions
By changing the makeup of the eluent systems, it is possible to vary how much SUV is measured in samples of human urine. In the beginning, various solvents were studied for this purpose, including acetone, cyclohexane, ethyl acetate, methanol, and chloroform. The retardation factor (Rf) values of SUV and IS using different eluent systems are included in Table 1. The findings indicated that when SUV was measured utilizing various eluent systems such as, acetone and cyclohexane, cyclohexane and ethyl acetate, and ethyl acetate and methanol, the Rf value was shifted toward the upper side (>0.85). Investigations into various chloroform and methanol ratios were also conducted. The SUV peak at Rf = 0.45 ± 0.01 was sharp and well-separated when the chloroform:methanol ratio was 97.5:2.5 (v/v).
Table 1. Rf Values of Suvorexant (SUV) and IS Were Recorded Using Different Eluent Systems (Mean ± SD; n = 3).
|
Rf |
||
|---|---|---|
| eluent system | IS | SUV |
| acetone/cyclohexane (90:10 v/v) | 0.18 ± 0.01 | 0.91 ± 0.03 |
| acetone/cyclohexane (97.5:2.5 v/v) | 0.17 ± 0.01 | 0.90 ± 0.03 |
| cyclohexane/ethyl acetate (90:10 v/v) | 0.15 ± 0.01 | 0.89 ± 0.02 |
| cyclohexane/ethyl acetate (97.5:2.5 v/v) | 0.14 ± 0.01 | 0.88 ± 0.02 |
| ethyl acetate/methanol (90:10 v/v) | 0.12 ± 0.01 | 0.87 ± 0.02 |
| ethyl acetate/methanol (97.5:2.5 v/v) | 0.11 ± 0.01 | 0.86 ± 0.02 |
| chloroform/methanol (90:10 v/v) | 0.10 ± 0.00 | 0.46 ± 0.01 |
| chloroform/methanol (97.5:2.5 v/v) | 0.09 ± 0.00 | 0.45 ± 0.01 |
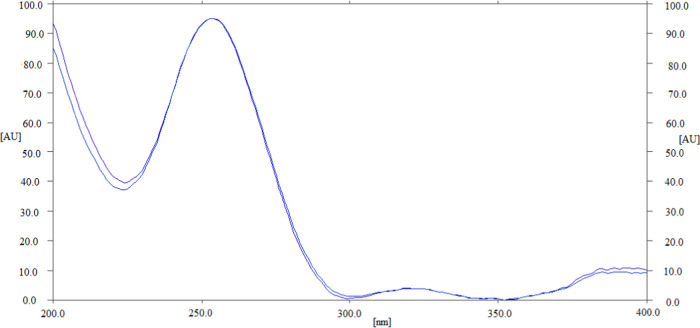
Various SUV solutions were created, and their UV absorption spectra were recorded in order to optimize the measurement wavelength for the study of SUV. The SUV presented maximal absorbance at 254 nm, which was chosen as the final wavelength for the complete analysis after evaluating their superimposed spectra (Figure 2).
Figure 2.
UV absorption spectra of SUV and internal standard (IS), superimposed.
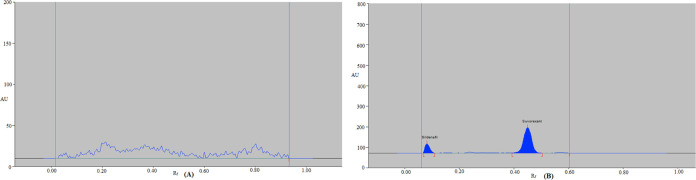
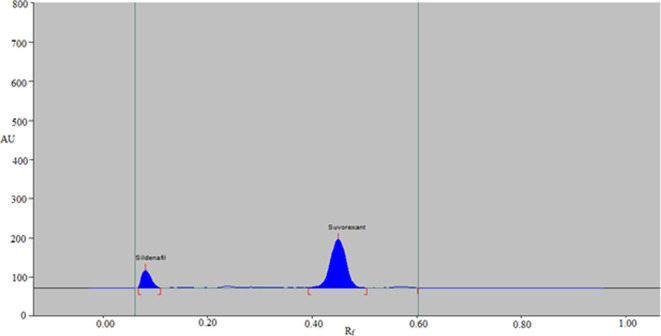
An internal standard (IS) is used in a popular bioanalytical method to control the severity of measurement errors when measuring medicines in biological samples, including blood, plasma, and urine. As a result, different medications were examined as the IS to determine which was better. The representative HPTLC chromatograms of the blank urine sample and urine sample spiked with SUV and IS are presented in Figure 3. The blank urine sample did not show any peak of the SUV and IS (Figure 3A). Based on the observations and findings, it was determined that sildenafil was the best option because its wavelengths were close to those of SUV. Additionally, using the suggested analytical technique, a good resolution between the SUV (0.45 ± 0.01) and IS (0.09 ± 0.00) was discovered (Figure 3B). Sildenafil was chosen as the IS for all of the following tests as a result.
Figure 3.
Typical HPTLC chromatograms of (A) blank urine sample and (B) urine sample spiked with SUV and IS.
2.2. Method Validation
The current bioanalytical method was validated according to SWGTOX guidelines for bioanalytical methods.31 The current bioanalytical method was validated for numerous parameters as described below:
2.2.1. Selectivity and Specificity
As described in the experimental section, the specificity and selectivity of the current bioanalytical method were determined. SUV and spiked urine spectra, peak areas, and Rf values showed a good correlation. With the use of chromatograms, it was discovered that the SUV was completely extracted from human urine samples without the development of any peaks for any urine constituents at the SUV and IS Rf values (Figure 3). These results demonstrated the specificity and selectivity of the presently used bioanalytical test.
2.2.2. Calibration Curves and Method Linearity
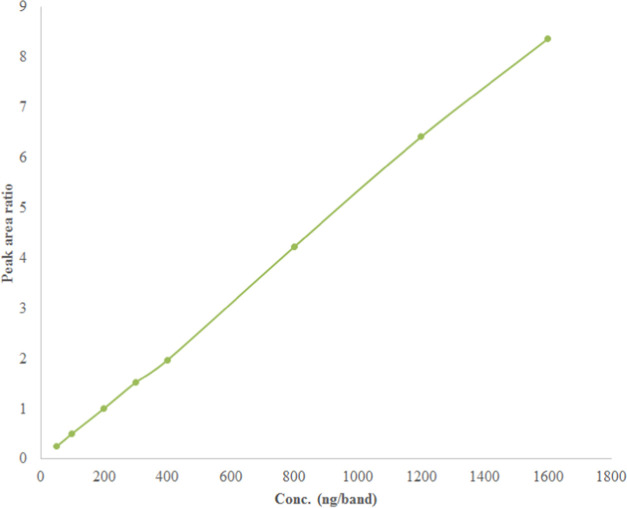
Eight unique SUV concentrations in human urine samples were plotted against the peak area ratio of SUV to the IS. The peak area ratio and concentration showed a good link in the results of the linear regression analysis. The representative linearity curve for SUV is presented in Figure 4. The regression coefficient (R) and determination coefficient (R2) values were found to be, respectively, 0.9997 and 0.9995. The current bioanalytical method’s linear range was determined to be 50–1600 ng/band. The y = 0.0053x – 0.0474, where y is the area ratio of SUV to the IS and x is the concentration of SUV, was obtained as the regression equation for the calibration curve of SUV in human urine samples. The findings of a linear regression study showed that the present bioanalytical method for measuring SUV in samples of human urine is linear and trustworthy.
Figure 4.
Representative linearity curve for SUV plotted between SUV concentrations and the area ratio of SUV to IS.
2.2.3. Sensitivity
Current bioanalytical test sensitivity was assessed in terms of “limit of detection (LOD) and limit of quantification (LOQ)”. Using a standard deviation technique, the “LOD and LOQ” values for the measurement of SUV were determined to be 3.73 and 11.20 ng/band, respectively. These findings suggested that the bioanalytical test currently used is sensitive enough to assess SUV in urine samples from humans.
2.2.4. Accuracy and Precision
By calculating the recovery and percent relative standard deviation (RSD) of SUV at three different quality control (QC) levels using six replications, the intra-assay accuracy and precision of the current bioanalytical method were evaluated. However, 18 replications were used to test the interassay accuracy and precision. Table 2 lists the results of the accuracy and precision tests. It was determined that the SUV’s intra-assay accuracy in terms of percent recovery was 96.89–101.64%. The interassay recovery rate for SUV was calculated to be between 97.42 and 103.80%, however. These findings showed that the present bioanalytical method for the extraction of SUV without pretreatment is highly effective. According to predictions, the SUV’s intra- and interassay precision in terms of percent RSD would be 3.28–3.67 and 3.55–4.20%, respectively. The current bioanalytical method’s precision was indicated by the low values of % RSD.
Table 2. Intra-assay and Interassay Precision and Accuracy of SUV for the Present HPTLC Method.
| intra-assay (n = 6) |
interassay (n = 18) |
|||||
|---|---|---|---|---|---|---|
| conc. added (ng/band) | conc. found (ng/band)a | precision (% RSD) | accuracy (% recovery) | conc. found (ng/band)a | precision (% RSD) | accuracy (% recovery) |
| 50 | 50.82 ± 1.87 | 3.67 | 101.64 | 48.71 ± 2.05 | 4.20 | 97.42 |
| 400 | 387.56 ± 13.56 | 3.49 | 96.89 | 415.23 ± 14.95 | 3.60 | 103.80 |
| 1600 | 1561.41 ± 51.31 | 3.28 | 97.58 | 1641.25 ± 58.41 | 3.55 | 102.57 |
Mean ± SD.
2.2.5. Robustness
Minor deliberate alterations in the composition of the eluent system were made in order to test the robustness of the current bioanalytical method. On recovery and RSD, the effect of the eluent system’s composition was observed. The percent recoveries for robustness were 95.31–102.34%, with percent RSD values of 2.81–3.15%. SUV’s Rf values were found to be between 0.44 and 0.46. These results demonstrated the robustness of the existing bioanalytical method.
2.2.6. Recovery
The recoveries from spiked urine samples were observed to range from 97.12 to 101.36% at three different QC levels. The recovery RSD values were found to be between 1.97 and 4.54%. These results suggested great efficiency for measuring SUV in human urine samples without the endogenous urine component interference.
2.2.7. Stability Studies
SUV stability assessments in human urine samples were done under a variety of circumstances. The stability investigations made use of low-quality control (LQC) and high-quality control (HQC) levels. Table 3 contains the results of the stability investigations. For stability investigations under varied storage circumstances, recoveries and precisions were determined to be 96.13–104.46 and 3.84–4.66%, respectively. The tested urine samples showed good stabilities in spiked urine samples both during sample preparation (benchtop stability) at 22 °C for at least 12 h and following storage in the refrigerator overnight below 8 °C. SUV was also determined to be suitably stable after three freeze–thaw cycles and 30 days of long-term storage at −80 °C. These results showed that the bioanalytical method used currently is stable.
Table 3. Stability Evaluation of SUV at Two Distinct Levels (LQC and HQC) (mean ± SD, n = 6).
| stability | nominal conc. (ng/band) | conc. found (ng/band) ± SD | precision (% RSD) | accuracy (% recovery) |
|---|---|---|---|---|
| benchtop (12 h) | 50 | 51.03 ± 2.23 | 4.36 | 102.06 |
| 1600 | 1545.21 ± 61.32 | 3.96 | 96.57 | |
| refrigeration (overnight) | 50 | 48.42 ± 2.03 | 4.19 | 96.84 |
| 1600 | 1671.41 ± 64.32 | 3.84 | 104.46 | |
| freeze thaw (three cycles) | 50 | 49.21 ± 2.14 | 4.34 | 98.42 |
| 1600 | 1538.23 ± 59.41 | 3.86 | 96.13 | |
| freezer at −80 °C (30 days) | 50 | 50.97 ± 2.38 | 4.66 | 101.94 |
| 1600 | 1574.31 ± 66.35 | 4.21 | 98.39 |
2.3. Literature Comparison
The reported bioanalytical methods and the current bioanalytical method for measuring SUV in human urine samples were compared. Table 4 lists the comparative validation settings. Numerous validation criteria of the new bioanalytical method, including linear range, accuracy, precision, LOD, and LOQ, were compared to previously published bioanalytical methods. The published GC-MS bioanalytical test was shown to be more sensitive and accurate than the current bioanalytical method for the measurement of SUV in urine samples with MS detection, although the current bioanalytical method’s precision was significantly higher than that of the stated GC-MS bioanalytical method.12 The sensitivity of the reported LC-Q/TOF-MS bioanalytical method for the measurement of SUV in urine samples with Q/TOF-MS detection was also better than that of the current bioanalytical method, but the accuracy and precision of this method were inferior to those of the current bioanalytical method.22 Similarly, the sensitivity of the reported UPLC-MS/MS bioanalytical method for the measurement of SUV in urine samples with MS/MS detection was also better than that of the current bioanalytical method, but the precision of this method was inferior to that of the current bioanalytical method.23 In comparison to previously published bioanalytical methods, the current HPTLC bioanalytical technique for the determination of SUV in human urine samples has been found to be simple, rapid, and cost-effective and have good accuracy and precision.12,22,23 Although the current HPTLC bioanalytical method had lower sensitivity than that of previously reported bioanalytical methods,12,22,23 its sensitivity was found to be sufficient for the measurement of SUV in human urine samples, and it overcomes the drawbacks of HPLC, LC-MS/MS, GC-MS, and UPLC-MS/MS methods such as “high cost and technical complications”. In addition, this method will offer a new densitometric measurement of SUV in human urine samples compared with the reported methods of SUV measurement. However, the main limitation of the present bioanalytical method is the development and validation of this method in spiked urine samples only. The proposed HPTLC bioanalytical method has not been applied to the determination of SUV in real samples. The application of the current HPTLC bioanalytical method in real samples can be considered for future investigations.
Table 4. Comparison of the Current Bioanalytical HPTLC Method with Literature Methods for the Measurement of SUV in Urine Samplesa.
| analytical method | linear range | extraction method | detection method | accuracy (% recovery) | precision (% RSD) | LOD | LOQ | refs |
|---|---|---|---|---|---|---|---|---|
| GC-MS | 10–1000 (ng/mL) | LLE | MS | 98–101 | <11 | 10 (ng/mL) | 10 (ng/mL) | (12) |
| LC-Q/TOF-MS | 2–250 (ng/mL) | LLE | Q/TOF-MS | 98–104 | 3–8 | 0.5 (ng/mL) | 5 (ng/mL) | (22) |
| UPLC-MS/MS | 0.27–1000 (ng/mL) | DLLME | MS/MS | - | 4.79–12.08 | 0.1 (ng/mL) | 0.27 (ng/mL) | (23) |
| HPTLC | 50–1600 (ng/band) | PP | UV | 96.89–103.80 | 3.28–4.20 | 3.73 (ng/band) | 11.20 (ng/band) | present work |
LLE: liquid–liquid extraction; DLLME: dispersive liquid–liquid microextraction; PP: protein precipitation; LOD: limit of detection; LOQ: limit of quantification.
3. Conclusions
For the first time, a simple, rapid, perceptive, and accurate HPTLC bioanalytical method has been created to quantify SUV in human urine samples. The current bioanalytical method introduces a novel concept that allows for the measurement of SUV without the need for a previous pretreatment with urine. The chromatograms that were recorded showed that there was no interference from the urine samples that had a high SUV throughput. Therefore, it would be quite valuable when used to assess SUV’s pharmacokinetics. The findings add credence to the idea that assessing nonpolar and moderately polar medicines in urine samples will be made possible by the polar stationary phase of HPTLC plates. The new bioanalytical technique is more cost-effective, simple, accurate, and precise than the majority of previously published bioanalytical methods for measuring SUV in samples of human urine. The current HPTLC bioanalytical test is effective for measuring SUV over a range of urine sample types.
4. Materials and Methods
4.1. Materials
SUV (purity: 99.2%) was procured from “Beijing Mesochem Technology Co. Ltd. (Beijing, China)”. The IS (sildenafil) (purity: 99.0%) was provided by “Astra Zeneca Pharmaceuticals (Wilmington, U.K.)”. HPLC-grade solvents, such as methanol and chloroform, were provided by “E-Merck (Darmstadt, Germany)”. Urine samples were collected from healthy human volunteers and refrigerated until further use.
4.2. Instrumentation and Analytical Conditions
Both the analyte and IS in human urine samples were measured using the “HPTLC CAMAG TLC system (CAMAG, Muttenz, Switzerland)”. A “CAMAG Automatic TLC Sampler 4 (ATS4) sample applicator (CAMAG, Geneva, Switzerland)” was utilized to apply samples as 6 mm bands.24 The “normal-phase-60F254S TLC plates (E-Merck, Darmstadt, Germany)” were employed as the stationary phase in order to identify SUV and the IS. The sample applicator was equipped with a “CAMAG microliter syringe (Hamilton, Bonaduz, Switzerland)”. For all of the measurements, the application rate for the detection of SUV and IS was fixed at 150 nL/s. The TLC plates were positioned inside a “CAMAG automated development chamber 2 (ADC2) (CAMAG, Muttenz, Switzerland)” with 80 mm distance. The combination of chloroform/methanol (97.5:2.5 v/v) was employed as the eluent system. The development chamber was completely saturated with the mobile phase vapors for half an hour at 22 °C. The measurement of USV and the IS was carried out at 254 nm. The slit size was fixed at 4 × 0.45 mm2, and the scan speed was adjusted to 20 mm/s. Six replications were used for each analysis. The data were deciphered utilizing the “WinCAT (version 1.4.3.6336, CAMAG, Muttenz, Switzerland)” program.
4.3. Sample Preparation
For their stock solutions, which had a concentration of 200 μg/mL, the working standards of the SUV and IS were carefully weighed out and dispensed into the eluent system. In order to obtain the working standard for calibration curves and QC samples, the stock SUV solution was further diluted by using an eluent system. Then, to obtain the calibration curve range of 50–1600 ng/band and QC samples, the working standards of calibration curves and QC samples were spiked to 200 μL of drug-free human urine samples. To obtain the working standard of a 200 ng/band IS solution, the stock solution of the IS was also diluted using an eluent system. Then, 10 μL of IS (200 ng/band) was transferred into each sample except blank urine samples. The samples were vortexed for 20 s, and then, 400 μL of methanol was transferred into each sample tube for protein precipitation. The samples were vortexed gently for 1 min followed by cold centrifugation at 10,500 rpm maintained at 4 °C for 8 min. After centrifugation, the upper layer was filtered with microsyringe filters (size: 0.22 μm), and then, a constant amount of 10 μL of each sample was transferred into the TLC plate for densitometric analysis. While spiked urine samples were kept in a deep freezer at −80 °C for further analysis, all working solutions were kept in the refrigerator.23
4.4. Sample Extraction
Prior to sample processing procedures, all urine samples (calibration curves, QCs, and validation solutions) maintained at −80 °C were vortexed for approximately 30 s and brought to room temperature for 2 h at 22 °C for defrosting. Each sample of urine, including the blank samples, was divided into 200 μL aliquots and put into a brand-new 2.0 mL centrifuge tube. After that, 10 μL of IS (200 ng/band) was added. The samples were vortexed once more for 20 s, and the proteins were then precipitated with 400 μL of methanol. The samples were once more gently vortexed for a minute. The samples were centrifuged for 8 min at 4 °C at 10,500 rpm. 10 μL of the sample was placed on normal-phase TLC plates for the measurement of the SUV and IS after centrifugation.23
4.5. Method Validation
According to SWGTOX’s guidelines for validating bioanalytical methods, the current HPTLC method was validated.31 Numerous parameters of the proposed bioanalytical method were validated. Usually, the FDA bioanalytical method guidelines are followed for the analysis of drugs in biological fluids.32 However, the studied analyte, i.e., SUV, is a drug of abuse and important in the case of forensic/toxic analysis.2 In the case of forensic/toxic analysis, SWGTOX guidelines are preferred over the FDA guidelines.32,33 As a result, SWGTOX guidelines were followed in these studies.31 The present studies were performed on spiked human urine samples, and human volunteers were not enrolled in this study. As a result, ethical approval is not required for these studies.
4.5.1. Selectivity and Specificity
By contrasting the spectra, peak area ratio, and Rf values of spiked urine sample bands with those of SUV and IS, we evaluated the specificity of the current bioanalytical technique. By contrasting the area response in the blank urine matrix at the Rf values of SUV and IS with urine spiked with LLOQ (i.e., 50 ng/band), we evaluated the selectivity of the current bioanalytical method. Blank urine samples were spiked six times with LLOQ concentrations of SUV and the IS (200 ng/band). Using a protein precipitation procedure, the analyte and IS were extracted, and their quantities were determined using the current HPTLC bioanalytical technique.31
4.5.2. Calibration Curves and Linearity
The calibration curves were produced in spiked urine samples in six replications at eight different concentrations (50, 100, 200, 300, 400, 800, 1200, and 1600 ng/band) in the range of 50–1600 ng/band. Plotting the calibration curve between the peak SUV to IS area ratios and SUV concentrations allowed determination of the linear range. The analysis of least-squares linear regression was used to forecast the calibration curves data. Eight different SUV levels (50, 100, 200, 300, 400, 800, 1200, and 1600 ng/band) were used to evaluate the calibration curve’s accuracy and precision in order to measure the calibration curve’s reliability.31
4.5.3. Sensitivity
By using the standard deviation approach, the sensitivity of the current bioanalytical test was evaluated in terms of the “LOD and LOQ”. The “LOD and LOQ” values were determined using eqs 1 and 2
| 1 |
| 2 |
where σ is the standard deviation of intercept and S is the calibration curve’s slope in this instance.34
4.5.4. Precision and Accuracy
Urine samples were examined for precision and accuracy for the current bioanalytical method at three distinct QC levels [LQC = 50 ng/band, middle QC (MQC) = 400 ng/band, and HQC = 1600 ng/band]. Six replications (n = 6) on the same day were used to measure the intra-assay precision and accuracy. However, utilizing 18 replications (n = 18) over three consecutive days, the interday precision and accuracy were calculated. According to SWGTOX, the acceptable limits of precision uncertainty are 15% for the other QC levels and 20% for the LQC level.31 However, the accuracy’s uncertainties must not exceed 20% for the LQC level and 15% for the remaining QC levels.31
4.5.5. Robustness
In order to identify minor intentional modifications in the chromatographic settings, the robustness of the current bioanalytical methods was assessed. For this, a little intentional modification to the eluent system was made, and the MQC (400 ng/band) level was examined. After making minor intentional adjustments to the eluent system’s composition for the examination of robustness, recovery and precision were estimated.29
4.5.6. Recovery Studies
Three distinct quality control levels (LQC, MQC, and HQC) were used to evaluate the recovery of this bioanalytical test in urine samples. The peak area ratio of urine spiked with SUV before extraction (A) and urine spiked with SUV after extraction (B) was determined. The percent recovery was then calculated using eq 3(31)
| 3 |
4.5.7. Stability Study
Six replications of LQC and HQC were measured in human urine samples to assess the stability of SUV under varied storage conditions. Utilizing a newly made calibration curve of SUV, the full stability metrics were measured. The short-term stability or benchtop stability of SUV was determined by processing and measuring LQC and HQC samples after 12 h of storage at 22 °C. The freeze–thaw stability of the spiked LQC and HQC urine samples was assessed after they were frozen at −80 °C and thawed at 22 °C. To test freeze–thaw stability, three different freeze–thaw cycles were performed. The long-term stability was assessed by measuring the spiked LQC and HQC urine samples stored at −80 °C for 30 days. The stability of the working and standard SUV and IS solutions was also examined during the course of 12 h at 22 °C and an overnight period at refrigerator temperature (below 8 °C). If the uncertainties in the mean levels of the LQC and HQC levels were achieved within the specified thresholds of accuracy (±15%) and precision (≤15%), the samples were deemed to be stable.31
Acknowledgments
The authors are thankful to the Researchers Supporting Project number (RSPD2023R1040), King Saud University, Riyadh, Saudi Arabia, for supporting this work. The authors are also thankful to Prince Sattam bin Abdulaziz University for supporting this work via project number PSAU/2023/R/1444. The authors are also thankful to AlMaarefa University for their generous support.
The authors declare no competing financial interest.
References
- Azevedo K.; Johnson M.; Wassermann M.; Evans-Wall J. Drugs of abuse-opioids, sedatives, hypnotics. Crit. Care Clin. 2021, 37, 501–516. 10.1016/j.ccc.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Silva-Bessa A.; Forbes S. L.; Ferreira M. T.; Dinis-Oliveira R. J. Toxicological analysis of drugs in human mummified bodies and proposed guidelines. Curr. Drug Res. Rev. 2023, 15, 62–72. 10.2174/2589977514666220914084543. [DOI] [PubMed] [Google Scholar]
- Bennett T.; Bray D.; Neville M. W. Suvorexant, a dual orexin receptor antagonist for the management of insomnia. Pharm. Ther. 2014, 39, 264–266. [PMC free article] [PubMed] [Google Scholar]
- Yang L. P. H. Suvorexant: first global approval. Drugs 2014, 74, 1817–1822. 10.1007/s40265-014-0294-5. [DOI] [PubMed] [Google Scholar]
- Ahmed Z.; Ahmad A.; Khan S. A.; Husain A. Pharmacological, pharmaceutical and safety profile of suvorexant: a dual orexin receptors antagonist for treatment of insomnia. Int. Educ. Sci. Res. J. 2015, 1, 26–30. [Google Scholar]
- Patel K. V.; Aspesi A. V.; Evoy K. E. Suvorexant: a dual orexin receptor antagonist for the treatment of sleep onset and sleep maintenance insomnia. Ann. Pharmacother. 2015, 49, 477–483. 10.1177/1060028015570467. [DOI] [PubMed] [Google Scholar]
- Gundlapalli S.; Devarapalli R.; Mudda R. R.; Chennuru R.; Rupakula R. Novel solid forms of insomnia drug suvorexant with improved solubility and dissolution: accessing salts from a salt solvate route. CrystEngComm 2023, 21, 7739–7749. 10.1039/d1ce01269j. [DOI] [Google Scholar]
- Cui D.; Cabalu T.; Yee K. L.; Small J.; Li X.; Liu B.; Maciolek C.; Smith S.; Liu W.; McCrea J. B.; Prueksaritanont T. In vitro and in vivo characterisation of the metabolism and disposition of suvorexant in humans. Xenobiotica 2016, 46, 882–895. 10.3109/00498254.2015.1129565. [DOI] [PubMed] [Google Scholar]
- Dubey A. K.; Handu S. S.; Mediratta P. K. Suvorexant: the first orexin receptor antagonist to treat insomnia. J. Pharmacol. Pharmacother. 2015, 6, 118–121. 10.4103/0976-500X.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Drug Enforcement Administration. Schedules of controlled substances: placement of suvorexant into Schedule IV. Final rule. Fed. Regist. 2014, 79, 51243–51247. [PubMed]
- Waters B.; Hara K.; Ikematsu N.; Takayama M.; Matsusue A.; Kashiwagi M.; Kubo S. I. Tissue distribution of suvorexant in three forensic autopsy cases. J. Anal. Toxicol. 2018, 42, 276–283. 10.1093/jat/bkx110. [DOI] [PubMed] [Google Scholar]
- Carson M.; Kerrigan S. J. Quantification of suvorexant in urine using gas chromatography/mass spectrometry. J. Chromatogr. B 2017, 1040, 289–294. 10.1016/j.jchromb.2016.10.042. [DOI] [PubMed] [Google Scholar]
- Siddhartha S.; Ratna J. V.; Tata S. K. Development and validation of high performance liquid chromatographic method for the determination of suvorexant in rabbit plasma by HPLC-UV detection. J. Emerging Technol. Innov. Res. 2019, 6, 194–203. [Google Scholar]
- Siddhartha S.; Ratna J. V.; Santosh T. Development and validation of HPLC method for the determination of suvorexant in pharmaceutical dosage forms. Int. J. Pharm. Drug Anal. 2018, 6, 425–434. [Google Scholar]
- Skillman B.; Kerrigan S. Identification of suvorexant in blood using LC-MS-MS: Important considerations for matrix effects and quantitative interferences in targeted assays. J. Anal. Toxicol. 2020, 44, 245–255. 10.1093/jat/bkz083. [DOI] [PubMed] [Google Scholar]
- Garcia L.; Tiscione N. B.; Yeatman D. T.; Richards-Waugh L. Novel and Nonroutine benzodiazepines and suvorexant by LC-MS-MS. J. Anal. Toxicol. 2021, 45, 462–474. 10.1093/jat/bkaa109. [DOI] [PubMed] [Google Scholar]
- Skillman B.; Kerrigan S. Quantification of suvorexant in blood using liquid chromatography-quadrupole/time of flight (LC-Q/TOF) mass spectrometry. J. Chromatogr. B 2018, 1091, 87–95. 10.1016/j.jchromb.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Breidinger S. A.; Simpson R. C.; Mangin E.; Woolf E. J. Determination of suvorexant in human plasma using 96-well liquid-liquid extraction and HPLC with tandem mass spectrometric detection. J. Chromatogr. B 2015, 1002, 254–259. 10.1016/j.jchromb.2015.07.056. [DOI] [PubMed] [Google Scholar]
- Xu R.-A.; Chen K.-L.; Jiao Y.; Huo X.-L.; Zhang Y. Quick method for the determination of suvorexant in plasma. Lat. Am. J. Pharm. 2017, 36, 2185–2189. [Google Scholar]
- Iqbal M.; Ezzeldin E.; Khalil N. Y.; Al-Rashood S. T. A.; Al-Rashood K. A. Simple and highly sensitive UPLC-ESI-MS/MS assay for rapid determination of suvorexant in plasma. J. Anal. Toxicol. 2017, 41, 114–120. 10.1093/jat/bkw111. [DOI] [PubMed] [Google Scholar]
- Iqbal M.; Khalil N. Y.; Ezzeldin E.; Al-Rashood K. A. Simultaneous detection and quantification of three novel prescription drugs of abuse (suvorexant, lorcaserin and brivaracetam) in human plasma by UPLC-MS-MS. J. Anal. Toxicol. 2019, 43, 203–211. 10.1093/jat/bky078. [DOI] [PubMed] [Google Scholar]
- Sullinger S.; Bryand K.; Kerrigan S. Identification of suvorexant in urine using liquid chromatography-quadrupole/time-of-flight mass spectrometry (LC-Q/TOF-MS). J. Anal. Toxicol. 2017, 41, 224–229. [DOI] [PubMed] [Google Scholar]
- Iqbal M.; Ezzeldin E.; Khalil N. Y.; Alam P.; Al-Rashood K. A. UPLC-MS/MS determination of suvorexant in urine by a simplified dispersive liquid-liquid micro-extraction followed by ultrasound assisted back extraction from solidified floating organic droplets. J. Pharm. Biomed. Anal. 2019, 164, 1–8. 10.1016/j.jpba.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Alam P.; Iqbal M.; Ezzeldin E.; Khalil N. Y.; Foudah A. I.; Alqarni M. H.; Shakeel F. Simple and accurate HPTLC-densitometric method for quantification of delafloxacin (a novel fluoroquinolone antibiotic) in plasma samples: application to pharmacokinetic study in rats. Antibiotics 2020, 9, 134 10.3390/antibiotics9030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Yazbi F. A.; Amin O. A.; El-Kimary E. I.; Khamis E. F.; Younis S. E. High-performance thin-layer chromatographic methods for the determination of febuxostat and febuxostat/diclofenac combination in human plasma. J. Chromatogr. B 2018, 1086, 89–96. 10.1016/j.jchromb.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Bhatt N. M.; Chavada V. D.; Sanyal M.; Shrivastav P. S. Densitometry and indirect normal-phase HPTLC-ESI-MS for separation and quantification of drugs and their glucuronide metabolites from plasma. Biomed. Chromatogr. 2019, 33, E4602 10.1002/bmc.4602. [DOI] [PubMed] [Google Scholar]
- Alam P.; Salem-Bekhit M. M.; Al-Joufi F. A.; Alqarni M. H.; Shakeel F. Quantitative analysis of cabozantinib in pharmaceutical dosage forms using green RP-HPTLC and green NP-HPTLC methods: A comparative evaluation. Sustainable Chem. Pharm. 2021, 21, 100413 10.1016/j.scp.2021.100413. [DOI] [Google Scholar]
- Abo-Zeid M. N.; El-Gizawy S. M.; Atia N. N.; El-Shaboury S. R. Efficient HPTLC-dual wavelength spectrodensitometric method for simultaneous determination of sofosbuvir and daclatasvir: Biological and pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 156, 358–365. 10.1016/j.jpba.2018.04.049. [DOI] [PubMed] [Google Scholar]
- El-Gizawy S. M.; El-Shaboury S. R.; Atia N. N.; Abo-Zeid M. N. New, simple and sensitive HPTLC method for simultaneous determination of anti-hepatitis C sofosbuvir and ledipasvir in rabbit plasma. J. Chromatogr. B 2018, 1092, 432–439. 10.1016/j.jchromb.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Alam P.; Iqbal M.; Foudah A. I.; Alqarni M. H.; Shakeel F. Quantitative determination of canagliflozin in human plasma samples using a validated HPTLC method and its application to a pharmacokinetic study in rats. Biomed. Chromatogr. 2020, 34, E4929 10.1002/bmc.4929. [DOI] [PubMed] [Google Scholar]
- Scientific working group for forensic toxicology (SWGTOX) standard practices for method validation in forensic toxicology J. Anal. Toxicol. 2013, 37, 452–474.. [DOI] [PubMed]
- Yakimavets V.; Qiu T.; Panuwet P.; D’Souza P. E.; Brennan P. A.; Dunlop A. L.; Ryan P. B.; Barr D. B. Simultaneous quantification of urinary tobacco and marijuana metabolites using solid-supported liquid-liquid extraction coupled with liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2022, 1208, 123378 10.1016/j.jchromb.2022.123378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal M.; Alshememry A.; Imam F.; Kalam M. A.; Akhtar A.; Ali E. A. UPLC-MS/MS based identification and quantification of a novel dual orexin receptor antagonist in plasma samples by validated SWGTOX guidelines. Toxics 2023, 11, 109 10.3390/toxics11020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran N. A.; Kumar L.; Reddy M. S.; Pai G. K. An analytical “quality by design” approach in RP-HPLC method development and validation for reliable and rapid estimation of irinotecan in an injectable formulation. Acta Pharm. 2021, 71, 57–79. 10.2478/acph-2021-0008. [DOI] [PubMed] [Google Scholar]