Abstract
There is evidence indicating that the carbapenem antibiotic panipenem decreases plasma concentrations of valproic acid (VPA) in epileptic patients during VPA therapy. The mechanism for panipenem-induced changes in the pharmacokinetics of VPA was investigated in rats with and without bile duct cannulation. The effect of panipenem on the pharmacokinetics of diclofenac, which undergoes extensive enterohepatic recirculation, was also examined. VPA (50 mg/kg of body weight) or diclofenac (10 mg/kg of body weight) was administered intravenously under the steady-state plasma panipenem concentration of 4 μg/ml, which had been achieved by a constant infusion rate. Panipenem decreased the plasma VPA concentrations in rats without bile duct cannulation but did not change the volume of the initial space and protein binding of VPA. However, panipenem had no effect on the plasma VPA concentrations and the biliary excretion of VPA in rats with bile duct cannulation. The secondary increase in plasma diclofenac concentration observed in the absence of panipenem was diminished in the presence of panipenem. These findings suggest that panipenem decreases plasma VPA concentrations by suppressing its enterohepatic recirculation, probably due to a panipenem-induced decrease in the numbers of enteric bacteria.
The antiepileptic valproic acid (VPA) is widely used as a drug of first choice in the treatment of epileptic patients with generalized tonic-clonic and partial seizures. Its therapeutic range of 50 to 150 μg/ml is widely accepted (2, 3, 16, 21, 25). Since a concentration in plasma above 200 μg/ml appears to be commonly associated with adverse reactions, it is important in clinical therapy with VPA to maintain its therapeutic concentration range. VPA, therefore, is one of the drugs requiring the routine monitoring of concentrations in plasma in order to avoid subtherapeutic or toxic doses. It is well known that VPA is rapidly absorbed through the gastrointestinal tract, distributed throughout the body, and extensively metabolized in the liver, and it is primarily excreted into the bile in a glucuronide conjugate form (4, 16).
A large number of developed carbapenem antibiotics are widely used in the treatment of patients with various infectious diseases. One of these antibiotics is panipenem, which possesses potent activity against both gram-positive and gram-negative bacteria and is important for the effective treatment of patients afflicted with serious infections (7). There is a possibility that panipenem may be administered to epileptic patients undergoing VPA therapy when these patients present with serious bacterial infections. Indeed, it has recently been reported that seizures were induced when panipenem was administered to epileptic patients during chronic VPA therapy; these seizures were coincident with decreased concentrations of VPA in plasma, which continued to decrease with increasing duration of panipenem treatment (23a). To our knowledge, no data is available on the precise mechanism by which coadministration of panipenem produces a decreased concentration of VPA in plasma.
As part of a program for the development of guidelines for the safe use of antibiotics in patients receiving VPA treatment, the goal of the present study was first to clarify the possible mechanism of the pharmacokinetic interaction between VPA and panipenem by using rats. Secondly, the nonsteroidal anti-inflammatory agent diclofenac was studied as a model drug, since it is excreted into the bile in a primarily glucuronide conjugate form more often than other well-known drugs in rats (8, 11, 18). Thus, the effect of panipenem on the enterohepatic recirculation of diclofenac was also investigated.
MATERIALS AND METHODS
Chemicals.
Panipenem, which consists of N-benzoyl-β-alanine (betamipron), was used in this study in the form of a commercial preparation for injection (Carbenin; Sankyo Co. Ltd., Tokyo, Japan). VPA sodium salt, diclofenac sodium salt, and the internal standard, mefenamic acid, were purchased from Sigma Chemicals (St. Louis, Mo.). All other chemicals were commercially available and were of analytical grade. Panipenem, VPA sodium salt, and diclofenac sodium salt were dissolved in isotonic saline solution for intravenous administration.
Animals and experiments.
Eight- to 9-week-old male Wistar rats (Japan SLC, Hamamatsu, Japan) weighing 280 to 300 g were used for all experiments. The rats were housed under controlled environmental conditions (approximately 25°C) with a commercial food diet and water freely available.
To elucidate the pharmacokinetic interactions between panipenem and VPA or diclofenac, the rats were cannulated under light anesthesia with pentobarbital (25 mg/kg of body weight) with polyethylene tubes in the right jugular vein for drug administration and blood sampling and in the left femoral vein for infusion of panipenem (these rats are hereafter referred to as rats without bile duct cannulation). Panipenem was injected in a bolus loading dose of 250 μg/kg followed by a constant-rate infusion maintained at a dose of 8.3 μg/min through the femoral vein to achieve the desired steady-state concentration of 4 μg/ml, which was observed clinically. This dosage of panipenem was calculated by using pharmacokinetic parameters described previously (13). After 60 min of panipenem infusion, VPA sodium salt (50 mg/kg as VPA) or diclofenac sodium salt (10 mg/kg as diclofenac) was injected. To elucidate the effects of panipenem on the biliary excretion of VPA, the right jugular vein (for drug administration and blood sampling), the left femoral vein (for infusion of panipenem), and the bile duct (for bile collection) were cannulated (these rats are hereafter referred to as rats with bile duct cannulation). To investigate the effect of panipenem on the enterohepatic circulation of VPA, panipenem was directly injected into the duodenum. VPA (50 mg/kg) was administered intravenously 1 h after a single intraduodenal administration of panipenem (1.9 mg/kg) in rats without bile duct cannulation. The dose of panipenem is equivalent to that (loading dose plus maintenance dose administered for 1 h) used in experiments with panipenem infusion.
In all experiments, the rats were placed in plastic metabolic cages (Natsume, Tokyo, Japan) under anesthesia with pentobarbital. Body temperatures were maintained at 37°C throughout the experiments with the assistance of heat lamps. The control group was treated with isotonic saline in place of panipenem. Blood samples of approximately 0.25 ml were collected at appropriate intervals after the administration of VPA or diclofenac and were immediately centrifuged at 6,000 × g for 5 min to yield plasma samples. Bile samples were collected in preweighed tubes at three consecutive 30-min time points. The volume of bile was measured gravimetrically, with specific gravity assumed to be 1.0. Plasma and bile samples were stored at −40°C.
Protein binding experiments.
The effect of panipenem on the plasma protein binding of VPA was examined by the micropartition equilibrium dialysis method by using a cellulose membrane (Visking sheet; Samplatec Corp., Osaka, Japan) with a molecular cutoff of 10,000 to 20,000. Blood samples were obtained from the rats in the presence and absence of panipenem by exsanguination from the abdominal aorta under light ether anesthesia, and plasma samples were obtained. VPA-spiked isotonic phosphate buffer (pH 7.4) samples of various concentrations (25 and 50 μg/ml) were immediately dialyzed against an equal volume of plasma at 37°C for 6 h.
Drug analysis.
The concentration of VPA was measured by the fluorescence polarization immunoassay (FPIA) method with a TDX analyzer (Abbott Laboratories, North Chicago, Ill.). The concentration of VPA in plasma and on both sides of the dialysis membrane was measured directly by the FPIA method. The concentration of VPA in the bile was measured by appropriate dilution with phosphate buffer (pH 7.4). Free VPA, released by hydrolysis of conjugate in the bile, was measured according to the method described previously (4). Briefly, bile samples were hydrolyzed with 5N-NaOH for 30 min at 80°C to completely hydrolyze the conjugate. The solution was neutralized with 5N-HCl and was then diluted in phosphate buffer solution (pH 7.4). The resultant solution was mixed with normal plasma (1:1 [vol/vol]) for FPIA assay. The recovery rate of VPA from the bile was 98%. The intra- and interassay coefficients of variation ranged from 4 to 6% in plasma and bile samples with the desired concentrations of VPA added.
The concentration of diclofenac in plasma was measured by high-performance liquid chromatography (HPLC), with a minor modification of the method described previously (5). Briefly, the HPLC apparatus was a Shimadzu (Kyoto, Japan) LC-6A system consisting of an LC-6A liquid pump, an SPD-6A UV spectrophotometric detector, and an SIL-6A autoinjector. The UV detector was set at 279 nm, and a Cosmosil 5C18 column (Nacalai Tesque, Kyoto, Japan) was used with a column oven (OTC-6A) heated to 40°C. The mobile phase consisted of 1/15 M acetic acid solution–acetonitrile (1:1 [vol/vol]), and the flow rate was 1.5 ml/min. One hundred fifty microliters of acetonitrile containing mefenamic acid (1 μg/ml) as an internal standard was added to 50 μl of plasma sample and vortexed. After centrifugation at 6,000 × g for 5 min, 150 μl of the supernatant was evaporated to dryness under a nitrogen gas stream at 40°C. The residue was reconstituted with 200 μl of the mobile phase and injected into the HPLC system. The assay was shown to be linear for the concentrations studied, with a correlation coefficient of 0.999. The within-day and between-day coefficients of variation for the assay were less than 7%.
Data analysis.
The data concerning concentrations of VPA in plasma over time were analyzed individually for each rat by noncompartmental methods. The area under the plasma VPA concentration-time curve (AUC) was calculated by the trapezoidal rule method. The volume of the initial distribution space (VC) was calculated by dividing the dose by the concentration in plasma at zero time. The biliary clearance (CLBILE) was calculated by dividing the total amount of VPA excreted into the bile within the collection period by the corresponding AUC. All computer analyses were performed by the nonlinear least-squares regression program MULTI, written by Yamaoka et al. (24).
Statistical analysis.
Results are expressed as the means ± standard deviations for the indicated numbers of experiments. Statistical analysis was performed by analysis of variance, with a P value of <0.05 taken as the limit of statistical significance.
RESULTS
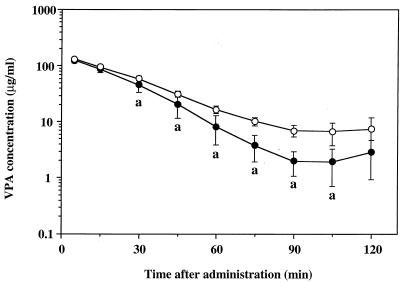
The present study focused on whether panipenem influences the protein binding, biliary excretion, and enterohepatic circulation of VPA in rats. First, the effect of panipenem on concentrations of VPA in plasma was examined in rats without bile duct cannulation. Mean semilogarithmic plasma VPA concentration-time curves after a single intravenous administration at a dose of 50 mg/kg in the presence or absence of panipenem are illustrated in Fig. 1. Concentrations of VPA in plasma after 30 min of the single injection were significantly lower in the presence of panipenem than in the absence of panipenem. The estimated model-independent pharmacokinetic parameters of VPA can be summarized as follows: AUC (up to the last measured concentration of VPA), 4.51 ± 0.33 and 3.62 ± 0.66 (significantly different [P < 0.05]) mg · min/ml in the absence and presence of panipenem, respectively; and VC, 0.333 ± 0.023 and 0.325 ± 0.035 liters/kg in the absence and presence of panipenem, respectively (each value represents the mean ± standard deviation [n = 6]). In parallel with these changes, the AUC up to the last measured concentration of VPA in plasma was decreased by 20% in the presence of panipenem compared with that in the absence of panipenem whereas no significant differences between the Vcs of the two groups were observed.
FIG. 1.
Semilogarithmic plots of plasma VPA concentration-time data after a single intravenous administration in the presence (solid circles) or absence (open circles) of panipenem in rats without bile duct cannulation. Each plot represents the mean ± standard deviation (n = 6). a, significantly different from the concentration in the absence of panipenem (P < 0.05).
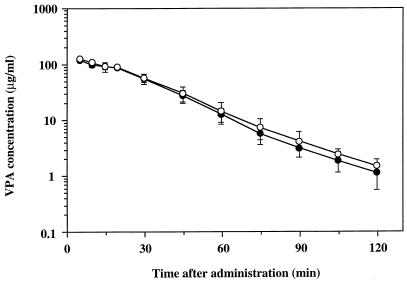
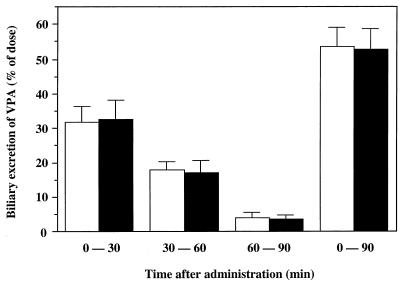
In the second set of experiments, the effect of panipenem on the biliary excretion of VPA was investigated by using rats with bile duct cannulation. There were no significant differences between the plasma VPA concentration-time curves in the presence and absence of panipenem (Fig. 2). As summarized in Table 1, no significant panipenem-dependent differences between any pharmacokinetic parameters in the two groups were observed. There was also no significant difference in the percentage of VPA (unchanged plus conjugated) recovered from bile over the 90-min experimental period with or without panipenem (Fig. 3).
FIG. 2.
Semilogarithmic plots of plasma VPA concentration-time data after a single intravenous administration in the presence (solid circles) or absence (open circles) of panipenem in rats with bile duct cannulation. Each plot represents the mean ± standard deviation (n = 5 to 6).
TABLE 1.
Pharmacokinetic parameters of VPA after a single intravenous administration in the presence or absence of panipenem in rats with bile duct cannulationa
| Panipenem | AUC (mg · min/ml)b | VC (liters/kg) | CLBILE (liters/h/kg) |
|---|---|---|---|
| Absent | 4.13 ± 0.50 | 0.341 ± 0.026 | 6.16 ± 0.74 |
| Present | 3.86 ± 0.50 | 0.384 ± 0.023 | 6.66 ± 0.62 |
Each value represents the mean ± standard deviation (n = 3 to 4). No significant difference was noted between the parameters in the presence and absence of panipenem.
Up to the last measured concentration of VPA.
FIG. 3.
Biliary excretion of VPA after a single intravenous administration in the presence (solid bars) or absence (open bars) of panipenem in rats with bile duct cannulation. Each bar represents the mean ± standard deviation (n = 6 to 7).
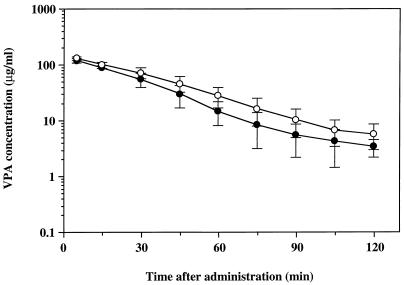
As it is thought that panipenem may influence the numbers of enterohepatic bacteria, the effect of panipenem on the enterohepatic circulation of VPA was also investigated in rats pretreated with a single intraduodenal administration of panipenem. As shown in Fig. 4, concentrations of VPA in plasma were decreased by a single intraduodenal administration of panipenem, and the corresponding AUC was also decreased by about 20% (4.11 ± 0.81 versus 5.31 ± 1.03 mg · min/ml) but did not reach statistical significance.
FIG. 4.
Semilogarithmic plots of plasma VPA concentration-time data after a single intravenous administration in rats pretreated with intraduodenal administration of saline (open circles) or panipenem (solid circles). Each plot represents the mean ± standard deviation (n = 3).
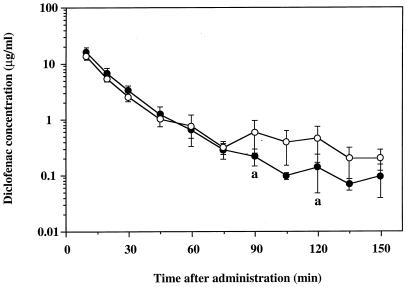
In the third set of experiments, the effect of panipenem on the enterohepatic recirculation of diclofenac, used as a model drug, was further investigated. Figure 5 shows that concentrations of diclofenac in plasma at 90 and 120 min after a single intravenous administration were significantly lower in the presence of panipenem than in the absence of panipenem and that the secondary increase in the concentration of diclofenac in plasma, which represents enterohepatic recirculation, was diminished by panipenem. Also, there were no significant differences between the Vcs of diclofenac in the presence (0.324 ± 0.075 liters/kg) and absence (0.335 ± 0.064 liters/kg) of panipenem, as was seen with VPA.
FIG. 5.
Semilogarithmic plots of plasma diclofenac concentration-time data after a single intravenous administration in the presence (solid circles) or absence (open circles) of panipenem in rats without bile duct cannulation. Each plot represents the mean ± standard deviation (n = 6). a, significantly different from the concentration in the absence of panipenem (P < 0.05).
DISCUSSION
It is generally accepted that plasma protein binding is the limiting factor in drug disposition, since only the unbound portion of a drug is capable of diffusing across various biological membranes to be distributed in the body and therefore subject to metabolism and renal excretion. Indeed, the pharmacological activity of a drug is closely related to the concentration in the blood of the drug which is unbound to protein. The protein binding potency of VPA in rats is moderate (approximately 60%), whereas panipenem consists of the organic transport inhibitor N-benzoyl-β-alanine (betamipron), which exhibits much stronger protein binding than panipenem (13, 23). Previous studies in our laboratory have demonstrated that the presence of high concentrations of betamipron (400 μg/ml) inhibits the protein binding of enprofylline, which is highly bound to plasma proteins (>90%) and increases the volume of distribution (23). Therefore, the effect of panipenem and/or betamipron on the protein binding of VPA was examined. The estimated steady-state plasma betamipron concentration in this study was very low (<1 μg/ml) compared with that in our previous studies (23), and indeed, the present study confirmed that there were also no significant differences in the protein binding of VPA after panipenem treatment (data not shown). These results suggest that panipenem-induced decreases in plasma VPA concentrations are not caused by changes in the protein binding behavior of VPA.
Several studies have shown that about 50% of the administered dose of VPA is glucuronidated and the glucuronide is excreted in the bile, undergoing enterohepatic recirculation in rats (4, 12, 14, 15). The percentages of VPA and its conjugated form excreted in the bile (approximately 2 and 55% of the VPA dose, respectively) are in agreement with the results of Dickinson and coworkers (4). These results suggest that panipenem had no effect on the biliary excretion and metabolism of VPA.
In general, carbapenem antibiotics cannot be absorbed from the gastrointestinal tract. A minor part of the panipenem administered is excreted into the bile (20), whereas the majority is excreted into the urine by glomerular filtration and an active tubular-secretion mechanism (13). A number of strains of anaerobic genera, including Clostridium sp. and Bacteroides sp., and aerobic genera, including Enterococcus faecalis and Staphylococcus epidermidis, are well known to be able to deconjugate bile salts. On the basis of these findings and the evidence that the main metabolite excreted into the bile is valproate glucuronide conjugate (4, 9, 15), we propose that panipenem may suppress the enterohepatic recirculation of VPA.
It is well known that diclofenac is primarily excreted into the bile and that the major portion of diclofenac administered is converted to its glucuronide conjugate in the liver and excreted into the bile duct; diclofenac deconjugated by enteric bacteria is reabsorbed from the gut (17, 19). In the present study, panipenem tended to decrease the AUC of diclofenac from 60 to 150 min (AUC60–150), although the difference found between these measures under the panipenem (22.8 ± 11.9 mg · min/ml) and control (50.8 ± 21.3 mg · min/ml) conditions was not statistically significant. This lack of statistical significance is probably due to interindividual differences in enterohepatic recirculation. The lack of significant differences between the VCs of diclofenac in the presence and absence of panipenem suggests that panipenem did not change the protein binding behavior of diclofenac, which is highly bound to plasma protein (6, 10, 11). The results reported here are thus consistent with the suggestion that the enterohepatic recirculation of VPA and diclofenac is suppressed by panipenem-induced decreases in the numbers of enteric bacteria, which are closely related to the production of β-glucuronidase. This suggestion could be confirmed in future experiments measuring β-glucuronidase activity in the small intestines of rats.
In conclusion, panipenem decreased the plasma concentrations of both VPA and diclofenac after a single intravenous administration in rats without bile duct cannulation but did not change these measures in rats with bile duct cannulation. The possible mechanism responsible for panipenem-induced pharmacokinetic changes may involve the suppression of the enterohepatic recirculation of VPA. On the basis of these observations, further studies are needed to examine the possibility that VPA interacts with the other carbapenems, which possess potent activity against both gram-positive and gram-negative bacteria. It should be noted that whereas VPA has been shown to undergo extensive hepatic metabolism and enterohepatic recirculation in rats, this is not the case in humans, due to species differences in hepatic metabolism (4, 9, 22). Thus, although the data obtained in this study cannot be extrapolated directly to humans, the results of the current study should nonetheless provide useful information for designing drug regimens in epileptic patients during VPA therapy. This study demonstrates that care should be taken in prescribing potent antibiotics to patients receiving chronic VPA therapy.
REFERENCES
- 1.Booth C L, Pollack G M, Brouwer K L R. Hepatobiliary disposition of valproic acid and valproate glucuronide: use of a pharmacokinetic model to examine the rate-limiting steps and potential sites of drug interactions. Hepatology. 1996;23:771–780. doi: 10.1002/hep.510230418. [DOI] [PubMed] [Google Scholar]
- 2.Bruni J, Wilder B J, Willmore L J, Perchalski R J, Villarreal H J. Steady-state kinetics of valproic acid in epileptic patients. Clin Pharmacol Ther, 1978;24:324–332. doi: 10.1002/cpt1978243324. [DOI] [PubMed] [Google Scholar]
- 3.Covanis A, Gupta A K, Jeavons P M. Valproate: monotherapy and polytherapy. Epilepsia. 1982;23:693–720. doi: 10.1111/j.1528-1157.1982.tb05085.x. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson R G, Harland R C, Llias A M, Rodgers P M, Kaufman S N, Lynn R K, Gerber N. Disposition of valproic acid in the rat: dose-dependent metabolism, distribution, enterohepatic recirculation and choleretic effect. J Pharmacol Exp Ther, 1979;211:583–595. [PubMed] [Google Scholar]
- 5.El-Sayed Y M, Abdel-Haameed M E, Suleiman M S, Najib N M. A rapid and sensitive high-performance liquid chromatographic method for the determination of diclofenac sodium in serum and its use in pharmacokinetic studies. J Pharm Pharmacol. 1988;40:727–729. doi: 10.1111/j.2042-7158.1988.tb07005.x. [DOI] [PubMed] [Google Scholar]
- 6.Evans A M, Hussein Z, Rowland M. Influence of albumin on the distribution and elimination kinetics of diclofenac in the isolated perfused rat liver: analysis by the impulse-response technique and the dispersion model. J Pharm Sci, 1993;82:421–428. doi: 10.1002/jps.2600820417. [DOI] [PubMed] [Google Scholar]
- 7.Fukuoka Y, Ikeda Y, Yamashiro Y, Takahata M, Todo Y, Narita H. In vitro and in vivo antibacterial activities of T-3761, a new quinolone derivative. Antimicrob Agents Chemother. 1993;37:384–392. doi: 10.1128/aac.37.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuyama T, Yamaoka K, Ohata Y, Nakagawa T. A new analysis method for disposition kinetics of enterohepatic circulation of diclofenac in rats. Drug Metab Dispos. 1994;22:479–485. [PubMed] [Google Scholar]
- 9.Gugler R, von Unruh G E. Clinical pharmacokinetics of valproic acid. Clin Pharmacokinet. 1980;5:67–83. doi: 10.2165/00003088-198005010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Hussein Z, Evans A M, Rowland M. Physiologic models of hepatic drug clearance: influence of altered protein binding on the elimination of diclofenac in the isolated perfused rat liver. J Pharm Sci. 1993;82:880–885. doi: 10.1002/jps.2600820904. [DOI] [PubMed] [Google Scholar]
- 11.Insel P A. Analgesic-antipyretics and antiinflammatory agents: drugs employed in the treatment of rheumatoid arthritis and gout. In: Gilman A G, Rall T W, Nies A S, Taylor P, editors. The pharmacological basis of therapeutics. 8th ed. New York, N.Y: McGraw-Hill, Inc.; 1990. pp. 638–681. [Google Scholar]
- 12.Liu M J, Pollack G M. Pharmacokinetics and pharmacodynamics of valproate analogs in rats. II. Pharmacokinetics of octanoic acid, cyclohexanecarboxylic acid, and 1-methyl-1-cyclohexane carboxylic acid. Biopharm Drug Dispos. 1993;14:325–339. doi: 10.1002/bdd.2510140406. [DOI] [PubMed] [Google Scholar]
- 13.Naganuma H, Hirouchi Y, Kawahara Y, Inui K, Tanigawara Y, Hori R, Kuwahara S. Nephroprotective effect and its mechanism of betamipron (2)—relationships of renal excretion. Chemotherapy (Tokyo) 1991;39:178–189. [Google Scholar]
- 14.Ogiso T, Ito Y, Iwaki M, Yamahata T. Disposition and pharmacokinetics of valproic acid in rats. Chem Pharm Bull. 1986;34:2950–2956. doi: 10.1248/cpb.34.2950. [DOI] [PubMed] [Google Scholar]
- 15.Pollack G M, Brouwer K L. Physiologic and metabolic influences on enterohepatic recirculation: simulations based upon the disposition of valproic acid in the rat. J Pharmacokinet Biopharm. 1991;19:189–225. doi: 10.1007/BF01073869. [DOI] [PubMed] [Google Scholar]
- 16.Rall T W, Schleifer L S. Drugs effective in the therapy of the epilepsies. In: Gilman A G, Rall T W, Nies A S, Taylor P, editors. The pharmacological basis of therapeutics. 8th ed. New York, N.Y: McGraw-Hill, Inc.; 1990. pp. 450–453. [Google Scholar]
- 17.Reuter B K, Davies N M, Wallace J L. Nonsteroidal anti-inflammatory drug enteropathy in rats: role of permeability, bacteria, and enterohepatic circulation. Gastroenterology. 1997;112:109–117. doi: 10.1016/s0016-5085(97)70225-7. [DOI] [PubMed] [Google Scholar]
- 18.Stierlin H, Faigle J W, Sallmann A, King W, Richter W J, Kriemier H P, Alt K O, Winkler T. Biotransformation of diclofenac sodium (Voltaren R) in animals and in man. 1. Isolation and identification of principal metabolites. Xenobiotica. 1983;9:601–610. doi: 10.3109/00498257909042327. [DOI] [PubMed] [Google Scholar]
- 19.Tabata K, Yamaoka K, Fukuyama T, Nakagawa T. Evaluation of intestinal absorption into the portal system in enterohepatic circulation by measuring the difference in portal-venous blood concentrations of diclofenac. Pharm Res. 1995;12:880–883. doi: 10.1023/a:1016217221977. [DOI] [PubMed] [Google Scholar]
- 20.Tanimura H, Ochiai M, Sugimoto Y, Aoki Y, Nakamura M, Nakatsuka H, Ichimiya G, Kobayashi Y, et al. Tissue concentrations and clinical efficacy of panipenem/betamipron in surgical infections. Chemotherapy (Tokyo) 1991;39:585–595. [Google Scholar]
- 21.Vajda F J E, Drummer O H, Morris P M, McNeil J J, Blandin P F. GAS chromatographic measurement of plasma levels of sodium valproate: tentative therapeutic range of a new anticonvulsant in the treatment of refractory epileptics. Clin Exp Pharmacol Physiol. 1978;5:67–73. doi: 10.1111/j.1440-1681.1978.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 22.Vree T B, van der Klejin E. Pharmacokinetics and renal excretion of 2-n-propyl pentanoate in man, dog and rhesus monkey. Pharm Weekbl. 1977;112:290–292. [Google Scholar]
- 23.Wang L, Hasegawa T, Nadai M, Nabeshima T. The effects of N-benzoyl-β-alanine, a new nephroprotective drug, on the distribution and renal excretion of enprofylline in rats. J Pharm Pharmacol. 1993;45:622–626. doi: 10.1111/j.2042-7158.1993.tb05665.x. [DOI] [PubMed] [Google Scholar]
- 23a.Welfare Ministry of Japan. Adverse reaction publication 137. Tokyo: Welfare Ministry of Japan; 1996. [Google Scholar]
- 24.Yamaoka K, Tanigawara Y, Nakagawa T, Uno T. A pharmacokinetic analysis program (MULTI) for microcomputer. J Pharmacobio-Dyn. 1981;4:879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]
- 25.Yu H Y. Clinical implication of serum protein binding in epileptic children during sodium valproate maintenance therapy. Ther Drug Monit. 1985;23:243–251. doi: 10.1097/00007691-198412000-00006. [DOI] [PubMed] [Google Scholar]