Abstract
The photosensitivity effect of lomefloxacin hydrochloride (LFLX) was investigated in terms of patient background factors (sex, age, underlying disease, complications, history, occupation, and residential condition, etc.), treatment factors (daily dosage of LFLX, duration of treatment, total dose, concomitant drugs, and previous medication, etc.), and correlations among them. In 100 institutions throughout Japan, 4,284 patients were enrolled over a period of 2 years, beginning in October 1991, avoiding the accumulation of patients in any specific season. Since 8 patients did not visit again after enrollment, the clinical records of 4,276 patients were analyzed. Photosensitivity in 44 patients was found (1.03%), but the symptoms in most patients were not severe and improved after discontinuation of LFLX treatment. The photosensitivity reaction was more prevalent in patients who were 60 years of age and older with concomitant diseases and complications, in patients treated with a total amount of 20 g and more of LFLX for 30 days or longer, and in patients with a history of previous treatment with quinolone drugs. Although the incidence and degree of the photosensitivity reaction vary significantly among new quinolone drugs, every quinolone drug is potentially phototoxic. In particular, long-term use of LFLX should be avoided, and patients taking LFLX should be advised to abstain from prolonged exposure to sunlight.
Lomefloxacin hydrochloride (LFLX) is a difluoroquinolone with two fluorine atoms at positions 6 and 8 on the quinolone ring. LFLX is characterized by a relatively long half-life of 6 to 8 h in circulation and good penetration into tissues after oral administration (17). During the clinical trials of LFLX before its commercial release, only one case of a photosensitivity reaction among 4,488 subjects was reported (24). After LFLX was first marketed in April 1990, Tozawa et al. (19) reported a frequent occurrence of photosensitivity reaction in patients on this drug. To determine the exact incidence of photosensitivity induced by LFLX, a nationwide, multicenter survey was performed during a period of 2 years. The present study presents the analysis of clinical records of 4,276 patients treated with LFLX.
MATERIALS AND METHODS
Patients.
One-hundred institutions in a nationwide distribution participated in this study. Patients with various infections were enrolled during the period from October 1991 to October 1993. The patients were informed of the potential of LFLX to lead to phototoxicity. Based on the assumption that the photosensitivity reaction occurs according to a Poisson distribution, with a true prevalence of 0.3%, approximately 4,000 patients were needed to record at least 10 events of photosensitivity reaction with a test power of 0.90. The present study was performed in eight divided districts in Japan (Kyushu-Okinawa, Chugoku-Shikoku, Kinki, Tokai-Hokuriku, South Kanto, North Kanto, Tohoku, and Hokkaido). The number of patients allotted to each district was based on the amount of LFLX sold in each district. The average number of patients enrolled in each institution was set at 60 throughout the study and at approximately 5 per month to avoid a seasonal bias. Patients with a history of allergy against LFLX and other quinolones; patients with severe cardiac, hepatic, or renal dysfunction; pregnant patients; pregnancy-expecting patients; lactating women; and children (patients who were less than 16 years old) were not enrolled. Main clinical indications for lomefloxacin included various cutaneous infections, acute and chronic respiratory tract infections, bile duct infections, bacterial enteritis, complicated and uncomplicated urinary tract infections, various oral cavity infections, various ocular infections, various gynecologic infections, otitis media, and others.
Drug and administration procedure.
Lomebact capsules containing 100 mg of LFLX (Shionogi & Co., Ltd., Osaka, Japan) were used. The drug was administered at a daily dose of 100 or 200 mg, two or three times daily. The patients were informed of the potential of LFLX to induce phototoxicity. The duration of LFLX treatment depended on the disease being treated and the judgment of the attending physicians. The period of follow-up after the end of the treatment was not regulated. The patients were advised to return to the prescribing physicians if they had a photosensitivity reaction.
Judgment.
At the start of administration, skin color (light, usual, or dark), hobby, occupation, and residential district of each patient were recorded. Usual skin color in Japanese means a color between white and dark, i.e., slightly yellowish to slightly brownish. When photosensitive skin lesions developed, severity, involved site of the body, season, situation of exposure to sunlight, relation to LFLX, treatment, and outcome were recorded. The case file, including photographs of the skin lesions, etc., of each patient was checked by the evaluation committee (members, Jirô Arata, Takeshi Horio, and Rinzo Soejima). The dermatologic diagnosis of photosensitivity reaction was based on the distribution of lesions (face, neck, arm, and upper chest, etc.) and the nature of the eruption. Photosensitivity reactions included unusually intense sunburn and various types of acute dermatitis on exposed areas.
Data analysis.
Case records were analyzed by the Drug Information Department and Data Analysis Center of Shionogi & Co., Ltd., under the supervision of the evaluation committee. Factors correlating with a photosensitivity reaction were divided into patient background factors (sex, age, distinction between in- and outpatient, infectious disease being treated, other concomitant disease, complication, history, skin color, hobby, occupation, and residential district, etc.) and treatment factors (daily dosage of LFLX, daily frequency of administration, duration of administration, total dose, previous medication, concomitant drugs, and previous quinolone administration) and were statistically analyzed.
On the basis of the contingency table categorizing presence or absence of a photosensitivity reaction, patient background factors, and treatment factors, the hypothesis that patient background factors or treatment factors are independent of the development of a photosensitivity reaction was tested for each factor.
When a factor had a natural order categorically, the Wilcoxon rank sum test was used. When a factor was not categorized by order, the chi-square test or the direct-probability calculation method was used. To visually evaluate and interpret the relationship among factors affecting the development of a photosensitivity reaction, the chi-square automatic interaction detection method (CHAID) (5) was used. Specifically, analysis was performed by using the presence or absence of a photosensitivity reaction as the response and patient background factors or treatment factors as the explanatory variables. The results were summarized with P values. In the interpretation of P values, the significance level was set at less than 0.05%.
RESULTS
At the outset of the study, 4,284 patients were enrolled. The numbers of patients recruited by each center varied from 2 to 120. Because 8 patients did not visit again, 4,276 patients were evaluated. The numbers of patients varied from 127 to 1,145, according to districts. A histogram of enrollment by month is shown in Fig. 1. The patient background factors are presented in Table 1. The dosages and durations of LFLX treatment are shown in Table 2.
FIG. 1.
Histogram of patient enrollment by month.
TABLE 1.
Background of patients
| Factor | No. of patients
|
||
|---|---|---|---|
| Male | Female | Total (%) | |
| Age (yr) | |||
| 6–19 | 67 | 81 | 148 (3.5) |
| 20–39 | 423 | 530 | 953 (22.3) |
| 40–59 | 624 | 738 | 1,362 (31.9) |
| 60–79 | 906 | 686 | 1,592 (37.2) |
| 80–97 | 153 | 68 | 221 (5.2) |
| Treatment category | |||
| Urology | 1,211 | 1,225 | 2,436 (57.0) |
| Internal medicine/diseases of respiratory system | 409 | 363 | 772 (18.1) |
| Dermatology | 142 | 136 | 278 (6.5) |
| Otolaryngology | 115 | 106 | 221 (5.2) |
| Orthopedics | 90 | 76 | 166 (3.9) |
| Other | 206 | 197 | 403 (9.4) |
| Concomitant disease | |||
| Absent | 868 | 1,267 | 2,135 (49.9) |
| Present | 1,305 | 836 | 2,141 (50.1) |
| Outpatient | 1,841 | 1,908 | 3,749 (87.7) |
| Inpatient | 159 | 117 | 276 (6.5) |
| In-out | 173 | 78 | 251 (5.9) |
| Total | 2,173 | 2,103 | 4,276 (100) |
TABLE 2.
Dosages of lomefloxacin
| Dosage factor | No. of patients
|
||
|---|---|---|---|
| Male | Female | Total (%) | |
| Daily dose (mg) | |||
| 100 | 14 | 15 | 29 (0.7) |
| 200 | 242 | 251 | 493 (11.5) |
| 300 | 950 | 800 | 1,750 (40.9) |
| 400 | 635 | 705 | 1,340 (31.3) |
| 600 | 324 | 325 | 649 (15.2) |
| 800 | 8 | 7 | 15 (0.4) |
| Dosage design (times/day) | |||
| 1 | 21 | 20 | 41 (1.0) |
| 2 | 874 | 951 | 1,825 (42.7) |
| 3 | 1,273 | 1,126 | 2,399 (56.1) |
| 4 | 5 | 6 | 11 (0.3) |
| Therapy period (days) | |||
| 5 | 3 | 8 (0.19) | |
| 2–4 | 210 | 240 | 450 (10.5) |
| 5–7 | 636 | 991 | 1,627 (38.1) |
| 8–14 | 716 | 599 | 1,315 (30.8) |
| 15–29 | 312 | 144 | 456 (10.7) |
| 30–59 | 178 | 65 | 243 (5.7) |
| 60–89 | 53 | 30 | 83 (1.9) |
| 90–140 | 63 | 31 | 94 (2.2) |
| Total dose (g) | |||
| 0.2–1 | 89 | 96 | 185 (4.3) |
| 1.1–3.0 | 778 | 1,110 | 1,888 (44.2) |
| 3.1–5.0 | 460 | 423 | 883 (20.7) |
| 5.1–10.0 | 499 | 325 | 824 (19.3) |
| 10.1–15.0 | 158 | 68 | 226 (5.3) |
| 15.1–20.0 | 77 | 41 | 118 (2.8) |
| 20.1–42.0 | 112 | 40 | 152 (3.6) |
| Total | 2,173 | 2,103 | 4,276 (100) |
A photosensitivity reaction was found in 44 patients (1.03%). The severity was mild in 17 patients (38.6%), moderate in 25 patients (50.8%), and severe in one patient. The eruption disappeared after discontinuation of LFLX in 41 patients. One patient developed a postinflammatory pigmentation. One was lost to follow-up.
Photosensitivity reactions in patients at 22 of 100 participating institutions were observed. The 22 institutions were widely located from Hokkaido to Okinawa. The numbers of patients with photosensitivity reactions among the 22 institutions were 1 each in 8 institutions, 2 each in 10 institutions, 3 each in 3 institutions, and 7 in 1 institution. During the 2 years of this survey, two to six cases of photosensitivity were observed monthly. The incidences were 1.34% (28 of 2,086 cases) in April through September and 0.73% (16 of 2,190 cases) in October through March. The incidence was slightly higher in spring and summer (P = 0.0498).
The incidence of photosensitivity reactions according to the category of patient background factors is shown in Table 3. Photosensitivity occurred in 31 males (1.4%) and in 13 females (0.6%), the incidence being significantly higher in males (P = 0.0096). Age distribution was as follows: 3 (0.3%) patients younger than 40 years of age, 3 (0.2%) patients from 40 to 59 years old, and 38 (2.1%) patients 60 years and older. The incidence was significantly higher in patients who were 60 years of age and older (P = 0.00005). Seven (0.3%) patients had no concomitant disease, and 37 (1.7%) patients had some concomitant systemic disease. The incidence was significantly higher in patients with some concomitant systemic disease (P = 0.00005). By occupation, photosensitivity was observed in 9 (5.8%) of 156 agricultural workers, in 5 (1.5%) of 338 other outdoor workers, in 25 (1.2%) of 2,171 patients without jobs, and in 5 (0.4%) of 1,329 indoor workers. The incidence among agricultural workers was significantly higher (P < 0.00005). The incidence of photosensitivity reaction classified by treatment factors is shown in Table 5. Photosensitivity was found in 21 (0.7%) of the patients without any previous medication and in 23 (2.1%) of the patients with previous medication. The incidence in patients with some previous medication was significantly higher (P = 0.0001). A history of previous quinolone administration was found in 8 (3.6%) of 44 patients who developed photosensitivity. The incidence was significantly higher in patients with a history of previous quinolone administration (P = 0.0001).
TABLE 3.
Incidence of photosensitivity classified by background factors of patients
| Classification | No. of patients
|
Photosensitivity incidence (%) | |
|---|---|---|---|
| Enrolled | With photosensitivity | ||
| Sex | |||
| Male | 2,173 | 31 | 1.4 |
| Female | 2,103 | 13 | 0.6 |
| Age (yr) | |||
| 6–39 | 1,101 | 3 | 0.3 |
| 40–59 | 1,362 | 3 | 0.2 |
| 60–97 | 1,813 | 38 | 2.1 |
| Concomitant systemic disease | |||
| Absent | 2,135 | 7 | 0.3 |
| Present | 2,141 | 37 | 1.7 |
| Skin color | |||
| White | 591 | 5 | 0.8 |
| Usual | 3,161 | 31 | 1.0 |
| Dark | 518 | 8 | 1.5 |
| Unknown | 6 | 0 | |
| Occupation | |||
| No | 2,171 | 25 | 1.2 |
| Indoor job | 1,329 | 5 | 0.4 |
| Outdoor joba | 338 | 5 | 1.5 |
| Agriculture | 156 | 9 | 5.8 |
| Others | 282 | 0 | |
| Total | 4,276 | 44 | 1.0 |
Excluding agriculture.
First, the result of analysis of the influence of the factors on the incidence of photosensitivity and of the interaction among the factors when patient background factors were used as explanatory variables is shown in Fig. 2. Among background factors, the age factor most strongly affected the development of photosensitivity, and the incidence in subjects who were 60 years of age and older was high. Among patients who were 60 years of age and older, the incidence of a photosensitivity reaction was influenced by occupation and underlying disease, but these factors did not affect the incidence in patients who were less than 60 years of age. In consideration of these interactions, a profile based on background factors was prepared, and an image of patients who are more disposed to develop photosensitivity under LFLX treatment was determined. The profiles and their incidence based on the background factors in patients with a high incidence included patients 60 years of age and older and outdoor workers in 13 cases (6.2%); and patients 60 years of age and older, patients who were indoor workers or not working, patients with concomitant diseases, and those living in hilly districts in 15 cases (3.8%).
FIG. 2.
Influence of background factors of patients on incidence of photosensitivity to lomefloxacin. ∗, incidence (number of photosensitivity cases/number of evaluated cases).
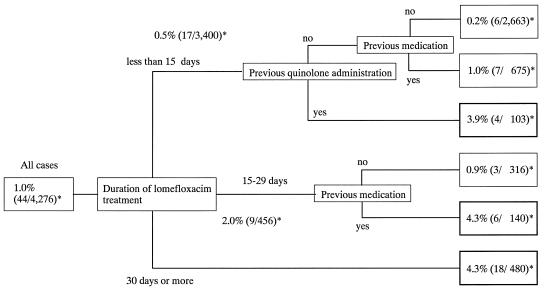
The influence of treatment factors was analyzed (Fig. 3). Among treatment factors, the duration of treatment was most often associated with the occurrence of a photosensitivity reaction. The incidence was high when patients were treated with NFLX for 30 days or more. In patients who were treated for fewer than 15 days or for 15 to 29 days, the incidence of photosensitivity was related to previous quinolone medication. This relationship was not found, however, in patients who were treated for 30 days or more.
FIG. 3.
Influence of treatment factors on incidence of photosensitivity to lomefloxacin. ∗, incidence (number of photosensitivity cases/number of evaluated cases).
Because the age of patients and the duration of treatment were most associated with the incidence of a photosensitivity reaction, the incidence was assessed by combining the following factors: a contingency table dividing the patients by age into 60 years and older and into less than 60 years and by treatment duration into the groups 30 days and more and fewer than 30 days was prepared, and the incidence of a photosensitivity reaction in each cell was calculated (Table 4). The incidence in patients who were 60 years of age and older and took LFLX for more than 30 days was the highest, i.e., 6.43% (18 of 280). The incidence in patients who were 60 years of age and older but took LFLX for fewer than 30 days was 1.30% (20 of 1,533), and that in patients who were aged fewer than 60 years who took LFLX for 30 days and more was 0% (0 of 140).
TABLE 4.
Incidence of photosensitivity reactions classified by treatment
| Treatment | No. of patients
|
Photosensitivity incidence (%) | |
|---|---|---|---|
| Enrolled | With photosensitivity | ||
| Lomefloxacin daily dose (mg/day) | |||
| 100 | 29 | 0 | |
| 200 | 493 | 5 | 1.0 |
| 300 | 1,750 | 22 | 1.3 |
| 400 | 1,340 | 14 | 1.0 |
| 600 | 649 | 3 | 0.5 |
| 800 | 15 | 0 | |
| Dosage design (times/day) | |||
| 1 | 41 | 1 | 2.4 |
| 2 | 1,825 | 18 | 1.0 |
| 3 | 2,399 | 25 | 1.0 |
| 4 | 11 | 0 | |
| Concomitant drug | |||
| No | 1,405 | 10 | 0.7 |
| Yes | 2,871 | 34 | 1.2 |
| Previous medicationa | |||
| No | 3,174 | 21 | 0.7 |
| Yes | 1,073 | 23 | 2.1 |
| Unknown | 29 | 0 | |
| Previous quinolone administration | |||
| No | 4,054 | 36 | 0.9 |
| Yes | 222 | 8 | 3.6 |
| Total | 4,276 | 44 | 1.0 |
Any medications other than quinolones and unidentified medications given during a month prior to the present LFLX treatment.
DISCUSSION
Since norfloxacin was first marketed in 1984, several new quinolones have been developed and are widely used. Their safety, however, has not yet been fully established. Quinolone-specific adverse reactions have become common, such as toxicity in young animals, dizziness and insomnia, and convulsion when administered concomitantly with nonsteroidal antiinflammatory drugs. A photosensitivity reaction caused by quinolones was first reported for nalidixic acid (2, 3, 11). As new quinolones have become widely used, photosensitivity due to new quinolones has become prevalent. However, the actual incidence has not yet been established.
During the clinical trials of lomefoxacin before its commercial release, only one case of a photosensitivity reaction among 4,488 subjects (0.02%) was reported (24). In this case, a patient on lomefloxacin developed a severe sunburn after ocean fishing. After this drug had been marketed, however, photosensitivity reactions were reported from several institutions (6, 25, 26). Tozawa et al. (19), Department of Urology, Anjo Kosei Hospital, reported a high incidence, i.e., 5.6% (19 of 338 patients). Therefore, in addition to the follow-up investigation at this institution, a nationwide survey of the incidence of photosensitivity due to lomefloxacin was performed.
Among a total of 56,285 patients treated with lomefloxacin during development of this drug and during a period of 3 years in a postmarketing survey, photosensitivity was found in only 20 patients (0.04%) (15). Later, Tozawa et al. (20) reported that when the dosage of LFLX was limited to 400 mg daily in two divided doses for fewer than 14 days, a photosensitivity reaction developed in only 1 of 324 patients (0.3%).
There are many reports of photosensitivity due to new quinolones. Using mice in vivo, Wagai et al. (22) reported phototoxicity of nalidixic acid, enoxacin, ofloxacin, lomefloxacin, and levofloxacin; Maruya et al. (8) reported phototoxicity of ciprofloxacin, lomefloxacin, ofloxacin, enoxacin, and norfloxacin; and Sanchez et al. (14) reported phototoxicity of pefloxacin, norfloxacin, and ciprofloxacin. Using rats, Masuoka et al. (10) found phototoxicity of sparfloxacin and nalidixic acid. Aoki et al. (1) also observed phototoxicity of lomefloxacin, enoxacin, ofloxacin, norfloxacin, ciprofloxacin, tosufloxacin, nalidixic acid, sparfloxacin, and fleroxacin in guinea pigs.
Wagai et al. (21), Maruya et al. (8), and Robertson et al. (13) reported independently that active oxygens are produced upon UVA irradiation of quinolones and that these active oxygens, but not photodegradation products, are involved in phototoxicity. In photoallergy due to quinolone antibacterial drugs, Yamada et al. (23) demonstrated that ENX can be photoallergic by the adjuvant and strip method. Horio et al. (4) developed a photoallergic reaction to lomefloxacin and nalidixic acid in guinea pigs under maximizing conditions with cyclophosphamide pretreatment.
Clinically, photosensitivity from all new quinolones has been reported, although the incidence is markedly different among drugs. In the postmarketing survey of recently sold sparfloxacin and fleroxacin, photosensitivity was found in 53 of 10,024 patients (0.53%) taking sparfloxacin and in 94 patients taking fleroxacin, although the total number of patients treated with fleroxacin is not known. For both drugs, the incidence in patients who were 60 years of age and older and treated for 2 or more weeks was high (for fleroxacin, Megalocin report issued by Kyorin Pharmaceutical Co., Ltd. in 1995; for sparfloxacin, internal postmarketing survey document by Dainippon Pharmaceutical Co., Ltd.). Photosensitivity was observed in 4 of 190 patients (2.1%) treated with Y-26611 (16), a drug for which development for clinical use was discontinued during clinical trials. The U.S. product labeling (Maxaquin) for lomefloxacin reports a photosensitivity rate of 2.4%. This incidence is higher than that from our present study. This difference may be attributed to different skin types and different life styles. Matsumoto et al. (9) and Marutani et al. (7) reported that photostability was greatly improved and that phototoxicity was reduced by introducing a methoxy group at position 8 of the quinolone ring. Cytotoxicity of the hydrogen and halogen also decreased. On the basis of these results, it is possible that the photosensitivity reaction is easily caused by new quinolone drugs such as lomefloxacin, sparfloxacin, fleroxacin, and Y-26611 that have a fluorine atom at position 8 of the quinolone ring.
The reasons for the higher incidence in patients who were 60 years of age and older are not clear. Because blood levels of ofloxacin, lomefloxacin, enoxacin, and fleroxacin reportedly increase with age (12, 18), a higher concentration in the skin due to this increase might partially explain a higher incidence of photosensitivity in elderly persons. Elderly persons may stay longer in the sunshine while walking slowly or sitting on benches. Longer treatment may increase the probability of sun exposure. Because quinolones are potentially phototoxic substances, intense exposure to UV light could induce phototoxic skin reactions in patients taking these agents. Patients with a history of receiving a new quinolone may have acquired photoallergy to the new quinolone. In the present study, photosensitivity was more associated with patients with a history of previous quinolone treatment when the duration of the present treatment with lomefloxacin was shorter than 30 days in comparison to when it was 30 days or longer. It is difficult to determine, however, whether the photosensitivity observed in patients taking lomefloxacin was photoallergic or phototoxic. Patients taking lomefloxacin should be advised to avoid excessive exposure to sunlight. Moreover, the duration of lomefloxacin treatment should be short.
ACKNOWLEDGMENT
We thank the physicians in the 100 participating institutions for their kind cooperation in this survey.
REFERENCES
- 1.Aoki K, Yoshida H, Yamazaki M, Harada M, Horio T. Proceedings of the 39th General Meeting of West-Japan Branch of the Japan Society of Chemotherapy, Oita, Japan. 1991. Basic studies on photosensitivity caused by quinolone antibacterial agents. [Google Scholar]
- 2.Baes H. Photosensitivity caused by nalidixic acid. Dermatologica. 1968;136:61–64. [Google Scholar]
- 3.Braunes G T. Bullous photoreactions due to nalidixic acid. Am J Med. 1975;58:576–580. doi: 10.1016/0002-9343(75)90134-5. [DOI] [PubMed] [Google Scholar]
- 4.Horio T, Miyachi H, Asada Y, Aoki Y, Harada M. Phototoxicity and photoallergenicity of quinolones in guinea pigs. J Dermatol Sci. 1994;7:130–135. doi: 10.1016/0923-1811(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 5.Kass G V. An exploratory technique for investigating large quantities of categorical data. Appl Statist. 1980;29:119–127. [Google Scholar]
- 6.Kurumaji H, Shouo M. Proceedings of the 15th Meeting of the Japanese Society of Contact Dermatitis, Nagoya, Japan. 1990. Two cases of photosensitivity caused by lomefloxacin. [Google Scholar]
- 7.Marutani K, Matsumoto M, Otabe Y, Nagamuta M, Tanaka K, Miyoshi A, Hasegawa T, Nagano H, Matsubara S, Kamide R, Yokota T, Matsumoto F, Ueda Y. Reduced phototoxicity of a fluoroquinolone antibacterial agent with a methoxy group at the 8 position in mice irradiated with long-wavelength UV light. Antimicrob Agents Chemother. 1993;37:2217–2223. doi: 10.1128/aac.37.10.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruya K, Matsumoto M, Otabe Y, Nagamuta M, Tanaka K, Matsubara H, Yokota K, Matsumoto F. Proceedings of the 41st General Meeting of the Japan Society of Chemotherapy, Tokyo, Japan. 1993. Phototoxicity of quinolone antibacterial agents having substitution at position 8 caused by irradiation with long-wave UV (UVA). III. Phototoxicity in mice. [Google Scholar]
- 9.Matsumoto M, Kojima K, Nagano H, Matsubara S, Yokota T. Photostability and biological activity of fluoroquinolones substituted at the position 8 after UV irradiation. Antimicrob Agents Chemother. 1992;36:1715–1719. doi: 10.1128/aac.36.8.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka N, Ueda Y, Kudo Y, Ohnishi K. Photosensitivity study on sparfloxacin (2)—oral phototoxicity test in rats. Jpn Pharmacol Ther. 1991;19:1309–1316. [Google Scholar]
- 11.Moore D E, Hemmens V J, Yip H. Photosensitization by drugs: nalidixic and oxolinic acids. Photochem Photobiol. 1984;39:57–61. doi: 10.1111/j.1751-1097.1984.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 12.Morita M, Suzuki K. Accumulation of new-quinolones in the blood of elderly patients. Chemotherapy. 1993;41:1272–1276. doi: 10.1177/030006059302100604. [DOI] [PubMed] [Google Scholar]
- 13.Robertson D G, Epling G A, Kiely J S, Bailey D L, Song B. Mechanistic studies on the phototoxic potential of PD11756, a quinolone antibacterial compound. Toxicol Appl Pharmacol. 1991;111:221–232. doi: 10.1016/0041-008x(91)90026-b. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez J P, Bridges A J, Bucsh R, Domagala J M, Gogliotti R D, Hagen S E, Heifetz C L, Joannides E T, Sesnie J C, Shapiro M A, Szotek D L. New 8-(trifluoromethyl)-substituted quinolones. The benefits of the 8-fluoro group with reduced phototoxic risk. J Med Chem. 1992;35:361–367. doi: 10.1021/jm00080a023. [DOI] [PubMed] [Google Scholar]
- 15.Shionogi and Co., Ltd. Results of post-marketing surveillance of Lomebact. 3rd annual report (internal document). Osaka, Japan: Shionogi and Co.; 1993. [Google Scholar]
- 16.Soejima R, Kobayashi H, Kumazawa J, Takeuchi M. Laboratory and clinical investigations on a new-quinolone, Y-26611—problems in extrapolating data to clinical studies. Chemotherapy. 1993;41:9–23. [Google Scholar]
- 17.Soejima R, Matsumoto F. Lomefloxacin. Jpn J Antibiot. 1991;44:1045–1061. [PubMed] [Google Scholar]
- 18.Soejima R, Yokoyama H, Endo K. Proceedings of the 17th International Congress of Chemotherapy. 1992. Age-related pharmacokinetics of fleroxacin; pp. 476–477. [Google Scholar]
- 19.Tozawa K, Washida H, Noguti K, Honma H, Yamada Y, Kang K. Proceedings of the 39th General Meeting of West-Japan Branch of the Japan Society of Chemotherapy, Oita, Japan. 1991. Clinical studies on photosensitivity caused by lomefloxacin. [Google Scholar]
- 20.Tozawa K, Washida H, Honma H, Yamada Y, Kang K. Proceedings of the 41st General Meeting of West-Japan Branch of the Japan Society of Chemotherapy, Kobe, Japan. 1993. Clinical studies on photosensitivity reaction caused by lomefloxacin (2) [Google Scholar]
- 21.Wagai N, Tawara K. Possible direct role of reactive oxygens in the cause of cutaneous phototoxicity induced by five quinolones in mice. Arch Toxicol. 1992;66:392–397. doi: 10.1007/BF02035128. [DOI] [PubMed] [Google Scholar]
- 22.Wagai N, Yamaguchi F, Sekiguchi M, Tawara K. Phototoxic potential of quinolone antibacterial agents in Balb/c mice. Toxicol Lett. 1990;54:299–388. doi: 10.1016/0378-4274(90)90197-t. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Saito K, Kurosaka R, Uchiyama M, Ito M. A case of photosensitive disease induced by enoxacin—case report and animal experiment. Skin Res. 1989;31:77–81. [Google Scholar]
- 24.Yamamoto Y, Tamaki H, Ikeda M, Arata J. Basic and clinical studies on NY-198 in dermatology. Chemotherapy. 1988;36:1266–1269. [Google Scholar]
- 25.Yasuda M. Proceedings of the Local Meeting of Dermatologists, Aichi Prefecture. 1990. A case of photosensitivity caused by lomefloxacin. [Google Scholar]
- 26.Yoshizawa M, Hashimoto A, Asai T. Proceedings of the Meeting of Fukuoka Branch. Japanese Dermatological Association; 1990. Solar exanthem-type drug rush caused by lomefloxacin. [Google Scholar]