Abstract
Arsenic is ubiquitous in soil and water environments and is consistently at the top of the Agency for Toxic Substances Disease Registry (ATSDR) substance priority list. It has been shown to induce toxicity even at low levels of exposure. One of the major routes of exposure to arsenic is through drinking water. This review presents current information related to the distribution of arsenic in the environment, the resultant impacts on human health, especially related to diabetes, which is one of the most prevalent chronic diseases, regulation of arsenic in drinking water, and approaches for treatment of arsenic in drinking water for both public utilities and private wells. Taken together, this information points out the existing challenges to understanding both the complex health impacts of arsenic and to implementing the treatment strategies needed to effectively reduce arsenic exposure at different scales.
Keywords: arsenic, diabetes, drinking water treatment, arsenic exposure
1. Introduction—Water Quality and Importance
Safe and affordable drinking water is a prerequisite for prosperity and sustainable development. The 2021 World Economic Forum report [1] lists natural resources crises, which includes water, as the fifth-highest existential threat globally. According to the 2017 WHO and UNICEF reports, more than 785 million people that year did not have access to basic water services [2]. While substantial progress has been made worldwide to provide access to clean drinking water, many regions have limited surface-water supplies and rely on groundwater resources. This has led to an increased risk of developing health issues in many parts of the world [3]. Metal(loid)s such as zinc (Zn), selenium (Se), copper (Cu), molybdenum (Mo), chromium (Cr), manganese (Mn), nickel (Ni), cobalt (Co), iron (Fe), magnesium (Mg), and arsenic (As) rank among the top priority metals that act as environmental toxicants in drinking water worldwide [4–10]. This review focuses in particular on As, a ubiquitous element that has been at the top of the Agency for Toxic Substances Disease Registry (ATSDR) substance priority list since 1997 [11], as it has been shown to induce toxicity even at low levels of exposure, thus representing a continuously growing public health concern.
Exacerbating the issue is the fact that exposure are not equal. Environmental racism and injustices result in people of color and low-income community members living in closer proximity to sources of environmental pollution (e.g., [12,13]). As a case in point, on 31 May 2022, the Biden–Harris Administration established a Department of Health and Human Services Office of Environmental Justice. As stated in the press release by HHS Secretary Xavier Becerra: “The blunt truth is that many communities across our nation—particularly low-income communities and communities of color—continue to bear the brunt of pollution from industrial development, poor land use decisions, transportation, and trade corridors” [14]. Social determinants of health (SDH), the nonmedical factors that influence health, are a primary indicator of one’s health and can account for 30–55% of health outcomes [15]. SDH factors can impact arsenic exposure, and in turn, influence the incidence of disease and morbidity. These factors are related to economic stability, education, health and health care, neighborhood and build environment, and social and community contexts and include, for example, access to healthy foods, quality of housing and infrastructure, environmental conditions, civic participation, and early childhood development [15,16]. These aforementioned SDH and others are influencing health disparities and inequities, thus creating vulnerabilities—the degree to which people and places can be harmed due to external stresses on human health (e.g., [17–20].
This review presents current information related to the distribution of arsenic in the environment, the resultant impacts on human health, especially related to diabetes, regulation of arsenic in drinking water, and approaches for treatment of arsenic in drinking water for both public utilities and private wells. Understanding these different perspectives is important for the prevention and mitigation of arsenic exposure. This review is presented in the context of diabetes—almost half a billion people worldwide live with this disease and the prevalence is projected to continue increasing [21]. The objective of this review is to delineate the existing challenges to understanding both the complex health impacts of arsenic and to implementing the treatment strategies needed to effectively reduce arsenic exposure at different scales.
2. Health Impacts of Arsenic in Drinking Water
Arsenic is a naturally occurring ubiquitous metalloid, the inorganic forms of which (iAs) are predominantly found in soil, sediment, and surface and groundwater reservoirs [22]. Depending on the pH, redox state, temperature, and solution composition, arsenic is generally soluble in groundwater [22,23]. Major sources of As contamination in drinking water include waste products from gold mining and mineral extraction, agricultural pesticides, and thermal springs, all of which contribute to As accumulation in groundwater [24]. While the gastrointestinal tract readily absorbs the inorganic forms of arsenic, resulting in their distribution throughout the body, they are mainly metabolized via methylation in the liver by arsenic methyltransferase (AS3MT) to their organic counterparts, namely, monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA), then excreted primarily in urine. More than 200 million people worldwide are exposed to iAs at concentrations above the EPA- and WHO-designated safe limit of 10 μg/L [25]. Based on data compiled from the mid- to late 1990s by the USGS from wells used throughout the US as public drinking water sources, it is estimated that 8% of the public drinking water supply may exceed 10 μg/L [26]. Importantly, consumption of arsenic-contaminated drinking water is associated with numerous disease states, including cancer, cardiovascular disease, skin lesions, nephrotoxicity, neurological disorders, and diabetes [8,27]. As such, investigations on how arsenic promotes disease progression, including diabetes, have garnered much attention over the past few decades, particularly because chronic exposure to arsenic in drinking water has been associated with an increased risk of type 2 diabetes in arsenic-rich areas worldwide [28].
Within the context of diabetes, understanding and mitigating the impact of SDH are priorities. For example, those of lower socioeconomic status are more likely to develop type 2 diabetes mellitus, experience more complications, and die sooner than those of higher socioeconomic status [29]. Furthermore, disadvantaged communities can experience several routes of arsenic exposure that are compounded by SDH factors [30–33]. For example, American Indians/Alaskan Natives (15.9%) and Hispanics (12.8%) have a greater prevalence of diabetes when compared to non-Hispanic whites (7.6%) across the US [34]. In addition to the years of life lost, $237 billion is spent in direct medical costs and $90 billion is lost in reduced productivity due to diabetes [35].
Finally, it is important to note that arsenic exposure can occur from food consumption as well as from drinking water. In general, food exposure is primarily from purchased foods, such as store-bought rice, cereals, and fruit juices. Meat, poultry, dairy products, cereals, and vegetables contain higher proportions of inorganic arsenic forms (e.g., [36–39], and food preparation practices can influence the concentration of arsenic in foods. For example, several studies have highlighted how cooking in arsenic-laden water, specifically boiling foods, such as maize grains, cereals (e.g., rice and quinoa), and vegetables that hold a noteworthy amount of water during boiling, can lead to arsenic exposure via the consumption of the cooked foods [40–43]. Since food type and preparation are tied to culture, place, geography, and race/ethnicity, it is critical to acknowledge how culturally relevant foods and cooking practices can influence individual/family/community arsenic exposure.
3. Arsenic Distribution in the Environment
Arsenic is naturally occurring and ubiquitous, distributed in the environment by both natural and anthropogenic processes [44]. It is present, at least in trace amounts, in nearly all crustal rocks and sediments. Arsenic is listed in 7133 minerals, inclusive of nonessential stoichiometries, and occurs as a principal structural constituent in 728 validated mineral species, including elemental arsenic (As0), arsenides (As3−), sulfides (As2+,3+,5+), oxides (As3+,5+), arsenites (As3+), and arsenates (As5+) [45]. While mineral specimens are rare in nature, arsenic occurs with ore minerals or alteration products, the most important being arsenian pyrite (Fe(S,As)2), arsenopyrite (FeAsS), and scorodite (FeAsO4·2H2O) [46,47]. Since the 1983 discovery of elevated dissolved arsenic in the Bangladeshi tube wells installed for pathogen-free drinking water, there has been widespread recognition of the large-scale global health problems resulting from chronic exposure, which has placed high priority on understanding the mobility, bioavailability, and toxicity of arsenic in the aqueous environment [48–51].
Geological processes concentrate arsenic in the Earth’s crust through magmatic and hydrothermal processes, becoming enriched most commonly in chalcophile metallic ore deposits [52]. In depositional systems, arsenic accumulates in aquifer sediments comprising geologically young (Cenozoic) alluvium, commonly hydrologically down-gradient of geothermally and magmatically enriched zones [53]. Leaching of arsenic into drinking water sources results in serious and extensive human and ecosystem health risks. Natural processes that mobilize arsenic to contaminate ground and surface waters from its primary geogenic sources include (i) redox-driven weathering, principally oxidative weathering of (arsenian) sulfides and reductive dissolution (arsenic sorbed) ferric hydroxides, (ii) volcanism, and (iii) biological activity. Elevated concentrations of arsenic in groundwater aquifers have been observed along the Pacific Ring of Fire [54] and reported in hot spots with arsenic at levels problematic to health in the Bengal delta [55,56], Red River delta (China) [57], Mekong delta (Vietnam) [58], Indus delta (Pakistan) [59], Taiwan [48,60], the western United States [61], Canada [62], and Argentina [63–65]. Anthropogenic activities also mobilize arsenic into the environment from extraction and beneficiation of ore, fossil fuel combustion, and the application of arsenic-containing pesticides, herbicides, and fertilizers [66–68].
Arsenic is unlike many other inorganic contaminants in that processes of environmental biogeochemical cycling in the range of pH and Eh common to the shallow subsurface can alter its speciation, which in turn affect its solid–aqueous phase partitioning [68,69]. That elevated levels of arsenic in groundwater threaten human health in widespread areas is known; however, dissolved arsenic concentrations are commonly spatially unpredictable [56]. The variable character of dissolved arsenic has been attributed to its redoximorphic speciation, electronic structure, and bonding properties, which result in dynamic transformation of its chemical form and phase stability [70]. The processes governing arsenic mobility in aquifers and through sediments are sorption, precipitation, and dissolution. These sequestration and release mechanisms are affected by pH, Eh, and concentrations of competing ions and are generally tied directly to coupled environmental redox reactions with iron and sulfur [69–72]. Arsenic is removed from the aqueous phase by two primary mechanisms—methylation and subsequent volatilization—and sequestration to the solid phase by (i) sorption at mineral surface sites [73–76], (ii) (co)precipitation with metal (hydr)oxides [69,77,78], or (iii) precipitation as arsenic sulfide under sulfur-reducing conditions [69,79,80]. The reverse reaction of arsenic mobilization is controlled by dissolution of host sulfides or metal hydroxide sorption sites, driven by geochemical redox [81,82]. Recently, nearly 80 studies of arsenic in groundwater around the world, aggregating over 200,000 measurements, were evaluated with machine learning to build a predictive model of arsenic exposure risk [83]. The authors examined 52 environmental variables and found that texture (clay and sand content), pH, and climate showed the greatest statistical importance for predicting elevated dissolved arsenic in aquifers. Model results indicate that 94 to 220 million people are potentially exposed to high levels of arsenic in groundwater, with 85–90% in South Asia.
The solid and aqueous speciation of arsenic directly affects its solubility, mobility, and possibly toxicity [84–86]. In the absence of high sulfide activity, dissolved arsenic in interstitial and surface water is generally present in two oxidation states: arsenite (the trivalent species, HxAsO3x−3) under suboxic environments, or arsenate (the pentavalent species HxAsO4x−2) in oxic zones (Figure 1). To a lesser extent, arsenic is found as aqueous organic metabolites [87,88]. Arsenate has dissociation constants of pKa1 of 2.2, pKa2 of 7.0, and a pKa3 of 11.5 [89], and in aerobic waters it is generally found as a combination of the mono- and divalent oxyanions H2AsO4− and HAsO4−2. Under reducing conditions, dissolved arsenic is present as arsenite with pKa1 = 9.2 and pKa2 = 13.4 (Figure 1).
Figure 1.
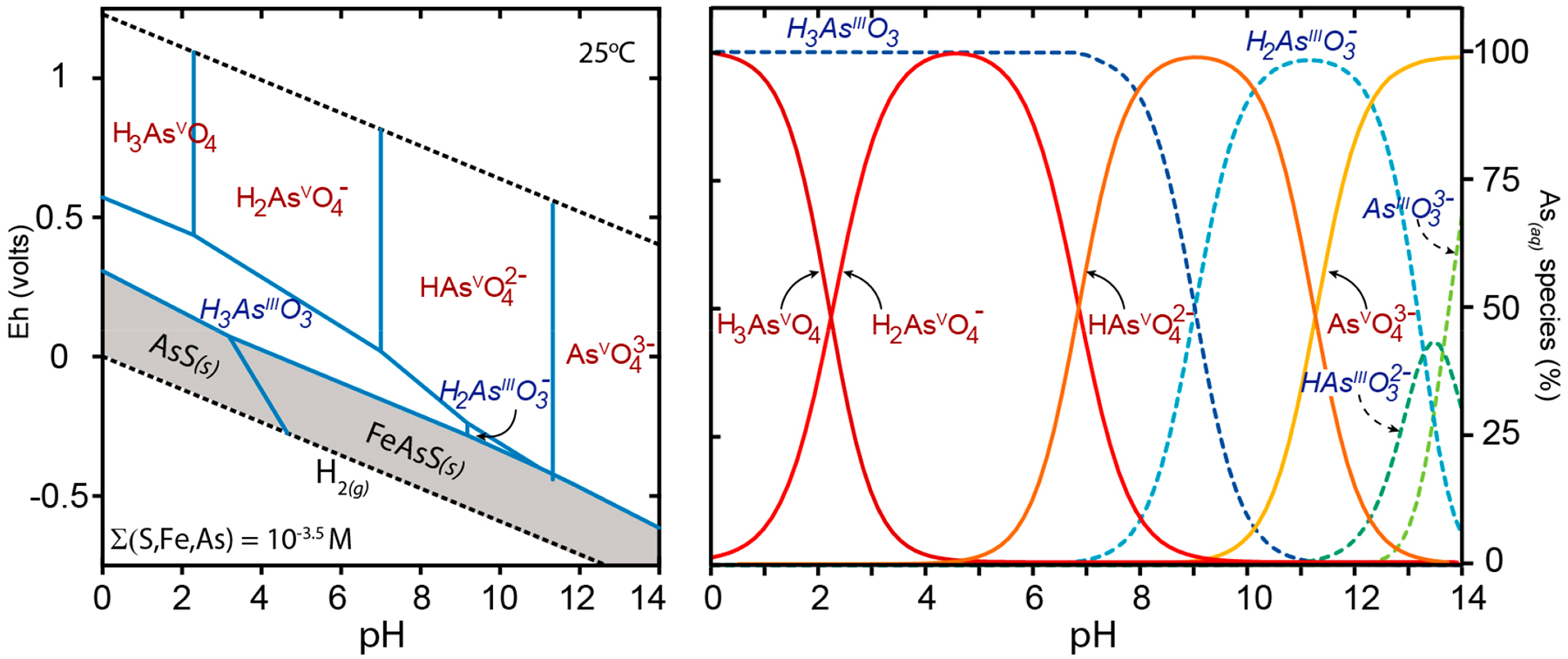
Eh-pH activity diagram of arsenic species at 25 °C, 1 bar, (S, Fe, As) = 10−3 M (left). Dashed lines bound the stability field of water, arsenate (AsV) species are shown in red, arsenite (AsIII) species are shown in blue italics, solid phases are shown with a darkened background. The distribution of pH-dependent dissolved arsenic species are shown (right) with arsenate as red solid lines and red text and arsenite in dashed blue lines with blue italic text. Dissolved arsenic species become protonated at low pH and the charge on the oxyanion decreases. Under environmental conditions (pH ≈ 5–9), arsenate generally exists as H2AsO4− and HAsO42−, while arsenite is the uncharged molecule H3AsO30. Under highly reducing conditions and in the presence of high sulfur activity, solid-phase arsenic sulfides (e.g., AsS) are stable.
Arsenate has been shown to strongly adsorb to positively charged surface sites of metals (oxy)hydroxides and phyllosilicates [73,77,90–92]. Iron, Earth’s most abundant redox active element, is commonly found as solid-phase ferric (oxy)hydroxide, which is insoluble under all but very low pH and Eh ranges and exerts strong control over arsenic cycling in the environment. Positively charged ferric surface coatings in sediments or suspended colloidal particles act as excellent sorbents of oxyanion arsenic (e.g., [75,93]). Therefore, arsenate is significantly immobilized in well-oxygenated sediments rich in iron. When organic matter is broken down through a series of electron transfer reactions in flooded sediments, oxygen is depleted, conditions become suboxic, and redox conditions favor the dissolution of ferric solids [81,94]. This reduction of iron (and arsenic) in suboxic environments is recognized as a primary mechanism of arsenic contamination of groundwater, especially sedimentary aquifers [95].
Groundwater flow, coupled with spatially variant gradients of redox potential and iron and sulfur activities, moves arsenic into and out of solution and thereby through pore spaces in aquifer sediments. Arsenic lability is a function of speciation and the biogeochemical redox characteristics of the subsurface environment controlled by molecular-scale interactions of arsenic at the sediment–water interface. In conditions where microbial activity, including metabolic and detoxification mechanisms, promote a transition from aerobic to anoxic porewaters, arsenate can be reduced to arsenite [88,96]. At the pH of most natural waters, arsenite does not dissociate, is neutral in solution, and the uncharged dissolved species is not as readily adsorbed at metal hydroxide surface sites. Therefore, arsenic phase partitioning in aquifer sediments is generally a function of redox potential and pH [66,71,88,97].
4. Diabetes and Arsenic
4.1. Diabetes Types and Risk Factors
Not only epidemiological but also a large body of experimental evidence supports the potential role of arsenic in promoting the development of diabetes mellitus (DM). Diabetes mellitus, a metabolic disorder characterized by hyperglycemia and dyslipidemia, is classified into insulin-dependent diabetes mellitus (type 1 diabetes, T1D) and non-insulin-dependent diabetes mellitus (type 2 diabetes, T2D) [98]. T2D, which makes up 90% of all diabetes cases, involves disruptions in whole-body glucose homeostasis due to resistance of peripheral tissue to insulin and decreased insulin production by pancreatic β-cells [99]. In T1D, the immune system destroys the pancreatic β-cells, leading to insulin deficiency [100]. Several toxic metals, such as cadmium, chromium, zinc, mercury, nickel, and arsenic, are known to adversely affect key metabolic pathways, which ultimately plays a role in promoting the development of metabolic disorders, including T1D and T2D [8]. Pathologically, these toxic metals accumulate in the liver, kidney, and pancreas to alter or impair the activity of critical enzymes, organelles, and signaling pathways, leading to adverse effects on metabolism. Critically, these pathological metabolic shifts result in significant increases in blood glucose levels, dyslipidemia, and eventually impaired organ function as a result of constant disruption of physiological homeostasis [101]. While genetics, diet, and lifestyle are established risk factors for developing DM, there is an increased interest in understanding the role of environmental exposure, including arsenic, as a causative factor in driving the diabetes epidemic.
4.2. Epidemiological Link between iAs Exposure and Diabetes
The 2011 National Toxicology Program workshop to assess the link between diabetes and the environment found an association between iAs exposure in drinking water and enhanced risk of developing DM, at least at concentrations ≥150 μg/L [102]. Epidemiologically, there are several indicators that exposure to iAs in drinking water causes diabetogenic effects. For example, a positive correlation between urinary iAs and its methylated metabolite DMA and increased fasting blood glucose, glycated hemoglobin, and fasting plasma insulin levels, was identified in a patient cohort from northern Mexico. Interestingly, insulin resistance was negatively correlated with iAs exposure in this same cohort, which may shed light on the differential regulation of T2D depending on other confounding variables (i.e., climate, diet, genetic predispositions) [103]. Assessment of the relationship between ingestion of iAs and prevalence of DM in 891 adults in southern Taiwan also showed a positive correlation between iAs exposure and increased blood glucose levels [104]. Another study conducted in four townships in Taiwan where people consumed iAs-containing well water between the 1900s and 1970s indicated an increase in mortality as a result of diabetes [105]. Reports have also indicated a significant increase in the number of individuals with elevated cholesterol and triglyceride levels in areas with higher iAs concentrations in the drinking water (56 μg/L) compared to an unexposed population (2 μg/L) in Serbia [106]. Furthermore, there was a 9% increase in blood glucose levels (>130 mg/dL) in individuals who consumed iAs-contaminated drinking water for a period of 6 months in Bangladesh [107]. Reports also indicated that a mean iAs concentration of 11 μg/L caused an elevated standardized mortality rate due to diabetic kidney disease and cerebrovascular disease in southeastern Michigan [108].
The number of epidemiological studies examining the relationship between diabetes and arsenic in drinking water has risen in recent years. These studies, which also include follow-up studies, have consistently found evidence linking arsenic in drinking water to diabetes [109–112]. In addition, more recent studies have utilized larger sample sizes, refined measures of exposure and outcome, and advanced statistical techniques, while also adjusting for potential confounding factors including, but not limited to, age, sex and lifestyle. These and other examples of the epidemiological evidence supporting arsenic promotion of diabetes are summarized in Table 1. Despite the wealth of epidemiological evidence, additional research is still needed to elucidate the underlying biological mechanisms by which arsenic exposure might contribute to the onset and progression of diabetes, which is discussed in more detail below.
Table 1.
Epidemiological evidence supporting arsenic promotion of diabetes.
| Country | Study Population | Age | Adjustments | Duration | As Concentration (In ppb or ppm) | Diabetic Assessment/Methods of Detection | Ref. |
|---|---|---|---|---|---|---|---|
| Bangladesh | 140 diabetic vs. 180 non-diabetic controls recruited with HbAlc level > 7% | ≥20 years | Age, sex, family history of diabetes, smoking habit, betel nut chewing, education | 2010 | 69.3–100.9 ppm in drinking water for 9.8–13.6 years | FBG ≥ 200mg/dL | [113] |
| 115 exposed subjects diagnosed as arsenicosis patients (>50 μg/L As water consumption and skin lesions) and 120 unexposed volunteers | 14–85 years | Age, height and body weight | 2001–2003 | drinking water (0.218 ppm) and spot urine (20.235 ppm) | FBG ≥ 140 mg/dL | [114] | |
| 163 subjects with keratosis exposed to arsenic and 854 unexposed individuals | >30 years | Age, sex and body mass index | NR | 0.01–2.1 ppm in drinking water | history of symptoms: previously diagnosed diabetes, glycosuria and blood sugar level after glucose intake (OGTT) | [115] | |
| 1595 subjects depending on drinking water from wells: 1841 drank arsenic-contaminated drinking water but 114 had not | ≥30 years | Age, sex and body mass index | NR | well water > 0.05 ppm | Glycosuria | [116] | |
| 40 workers occupationally exposed to arsenic, 26 without any known As exposure and 6 who directly handle As containing products | 20–60 years | Sex, occupation, age, smoking habit | NR | 22.3–294.5 nmol per mmol of creatinine in urine sample of the exposed group | glycosylated hemoglobin (HbA1c) 5.4% compared to reference group 4.4% | [117] | |
| Chile | population based cancer case-control study of 1301 participants in Northern Chile | ≥25 years | Age, sex, race, hypertension, cancer, socioeconomic status, smoking status | 2007–2010 | >0.8 ppm arsenic water concentration | physician diagnosed diabetes or oral hypoglycemic medication use | [118] |
| China | 2090 women with singleton pregnancy from the Tongji Maternal and Child Health Cohort (TMCHC) | ≥25 years | Pregnancy, education, income, ethnicity, fetal sex | 2013 | 0.3 ppb | Urine samples and oral glucose tolerance test, FBG ≥ 92 mg/dL | [119] |
| 335 gestational diabetes mellitus and 343 controls without GDM based on a prospective cohort established in Beijing, China | <35–≥35 years | Age, ethnicity, education, occupation, | 2017–2018 | 220 ppm | FBG ≥ 5.1 mmol/L, maternal hair samples | [120] | |
| 1527 pregnant women drawn from Mother and Child Microbiome Cohort (MCMC) study | <30–≥30 years | Education, BMI | 2017–2018 | 0.83 ppb | 75-g oral glucose tolerance test (OGTT), FBG ≥ 5.1 mmol/L, 1 h postprandial ≥ 10.0 mmol/L, or 2 h postprandial glucose ≥ 8.5 mmol/L | [121] | |
| 3474 women who were part of the Ma’anshan Birth Cohort (MABC) Study conducted from the City of Ma’anshan, Anhui Province of China | ≤ 24 years, 25–29 years, ≥ 30 years | Maternal age, BMI, gravidity, parity, income, education | 2013–2014 | 0.0047 ppb | FBG ≥ 5.1 mmol/L; 1 h, ≥10.0 mmol/L; or 2 h, ≥8.5 mmol/L | [122] | |
| Croatia | 202 adult urban participants from the city of Osijek in eastern Croatia and city of Zagreb in western Croatia | ≥45 years | Age, gender, education, smoking, family history if diabetes, physical activity, dietary consumption, origin of water used for drinking | 2018 | 0.5–361 ppb total urine As | FBG ≥ 3.5 mmol/L, HbA1c ≥ 37 mmol/L, insulin ≥ 15 pmol/L | [123] |
| India | Natives to Nallampatti, an agricultural village in south India and part of the KMCH-NNCD cross-sectional study | ≥20 and ≤85 years | Age, sex, alcohol intake, smoking, tobacco use, BMI, education, occupation, familial diabetic history | 2015 | 4.10–63.30 ppm creatinine units of arsenic | blood investigation included a random glucose, HbA1c, cystatin-c, non-fasting lipid profile, uric acid and hemoglobin | [124] |
| Italy | 3390 art glass workers employed in 17 industrial facilities for at least 1 year | <40, 40–65 and >65 years | Age, sex, history of disease/mortality | 1950–1985 | 3.26 ppb in glassworks (>10 μg/m3 in glassworks) | All causes of death coded according to the 8th revision of the ICD | [125] |
| 258 subjectswith a minimum of two-year residency in the regions and without occupational exposure to As | ≥5 years | Age, sex, source of drinking water | 1993–2008 | 3–215 ppb iAs in drinking water, 2.3–233.7 ng/mL tAs in Urine | FBG ≥ 126 mg/dL, OGTT ≥ 200 mg/dL, HbA1c levels > 7%, self-reported diagnosis, or medication | [103] | |
| 200 diabetic cases and 200 controls | ≥30 years | Age, height, weight, body mass index, smoking habit, family history of diabetes, employment, location | 1960 | intermediate total As concentration in urine (63.5–104 μg/g creatinine) | FBG ≥ 126 mg/100 mL (> or =7.0 mmol/l) or a history of diabetes treated with insulin or oral hypoglycemic agents | [126] | |
| 1160 adults with a minimum 5 year residency in study area | ≥18 years | Age, gender, ethnicity, education/occupation, smoking status, alcohol consumption, recent seafood intake, drinking water sources (well, treatment plant or other) and use and medical history | 2008–2013 | <0.01–419.8 ppb As in drinking water, tAs 0.52–491.5 ppb in urinary As. | FBG ≥ 126 mg/dL, 2HPG ≥ 200 mg/dL, self-reported diagnosis, or medication | [127] | |
| 49 healthy individuals and 77 patients | NR | Age, sex, geographical location history of disease | NR | 0.32–9.82 ppb As in diabetic patients, mean As 3.44 ppb | Urine samples of diabetic patients to test As concentration | [128] | |
| 1451 randomly selected participants from Spain (representative sample of a general population) | ≥20 years | Age, sex, somking status, education, seafood consumption | 2001–2003 | 3.8 ppb of total plasma As, 106,000 ppb of total urine As, 14,900 ppb μg/g of iAs and 66,500 ppb of Asb in participants with diabetes | FBG ≥ 126 mg/dL and glycosylated hemoglobin (HbA1c) level > 6.5% or physician diagnosis or glucose lowering medication use | [129] | |
| Sweden | 43 smelter workers exposed to iAs dust for 13–45 years | 44–70 years | age, height, smoking habit, alcohol consumption | 1987 | 1.6–63 ppb As in work-room air at the smelter | self-reported type 2 diabetes | [130] |
| 12 cases with DM on death certificate and 31 controls employed in a Swedish copper smelter | 30–74 years | Age, history of diseas/death | 1960–1976 | <0.5–>0.5 ppb As | death certificate, medical record | [131] | |
| 5498 art glass workers in southeastern Sweden | ≥45 years | Age, occupation (glassworkers vs. glassblowers, other foundry workers and unspecified glass workers) | 1950–1982 | <1.9 ppb As in Swedish glassworks; <6 μg/m3 As in Swedish glassworks | All causes of death coded according to the 8th revision of the ICD | [132] | |
| Taiwan | 891 adults in southern Taiwan village where arseniasis if hyperendemic | ≥30 years | Age, sex, body mass index, activity level at work | 1960–1970 | 0.1–15 ppm-year or higher | oral glucose tolerance test (OGTT) or self-reported history of diabetes treated with sulfonylurea or insulin | [104] |
| Cancer and noncancer diseases | All age group | Sex, Age | 1971–1994 | 0.25–1.14 ppm As in artesian well water | All causes of death coded according to the 8th or 9th revision of the ICD | [105] | |
| 446 nondiabetic residents in a village in Taiwan | ≥30 years | Age, body mass index and cumulative arsenic exposure | 1988–1989 | median As of artesian well water from 0.7 to 0.93 ppm | FBG ≥ 7.8 mmol/L and/or a 2 h post-load glucose level > or = 11.1 mmol/L. | [133] | |
| 66,667 residents living in endemic areas and 639,667 in nonendemic areas | ≥25 years | Age, sex | 1999–2000 | artesian well water > 0.35 ppm | All causes of death coded according to the 9th revision of the ICD (ICD-9 code 250 and A181) | [134] | |
| 4 townships in southwestern Taiwan where blackfoot disease is endemic | NR | Age, Sex | 1971–2000 | arsenic concentration of artesian well water ranged from 0.35 to 1.14 ppm with a median of 0.78 ppm | All causes of death coded according to the 8th or 9th revision of the ICD (ICD-9 code 250). | [135] | |
| 1297 subjects from an arsenicosis endemic area in southwestern Taiwan | ≥40 years | Age, sex, smoking status, education, exercise, alcohol consumption, betel nut intake | 1990, 2002–2003 | 0.7–0.93 ppm As in well water | FBG, cholesterol, triglycerides, low and high density lipoproteins, urine acid and urine creatinine levels, arsenic methylation patterns and GSTO1 genotypes linked to metabolic syndrome as an early factor for diabetes | [136] | |
| UK | 32 insulin treated (ITDM), 55 non-insulin treated (NITDM) diabetic patients and 30 nondiabetic individuals (C-DNM) from Oxford, England | 18–78 years | Age, body mass index, glucose, insulin | NR | 0.018–0.2 ppm As | Glucose levels and insulin treatment | [137] |
| USA | 4549 American Indian participants | 45–75 years | Age, sociodemographic, smoking and alcohol status, height, weight, blood pressure | 1989–1991, 1998–1999 | 5.9–14 ppm iAs 14.3 ppb in Arizona, 11.9 ppb in Dakota, 7 ppb in Oklahoma | FBG ≥ 126 mg = dL, 2HPG ≥ 200 mg = dL, self-reported diagnosis, or medication | [138] |
| 1393 smelter workers | <20–40+ | Age, sex, race, occupation | 1946–1977 | 0.5–5 ppb As of air concentration in the insecticide building | All causes of death coded according to ICD | [139] | |
| 8014 copper smelter workers in Montana | <20–≥30 | Sex, Race | <1957,1938–1989 | 0.29–11.3 ppb of airborne As | All causes of death coded according to the 8th or 9th revision of the ICD (ICD-8 codes 460–519) | [140] | |
| 1827 boys and 1305 girls | 2–14 years | Age, sex | 1907–1932 | 140–1600 ppm soil As concentration | All causes of death coded according to death records from the National Death Index, ≥47 and from Washington State (1900–1990), Oregon State (1971–1979), and California State (1960–1990), to locate deaths of cohort members | [141] | |
| Historical ward membership records of the Church of Jesus Christ of Latter-day Saints (LDS) (also known as the Mormons) | <50–80+ | Age, sex | 1977 | mean As 150 ppb, median As 14 to 166 ppb | Death certificate, mortality from hypertensive heart disease | [142] | |
| 1185 respondents from 19 townships in arsenic contaminated area | ≥35 years | Age | 1992–1993 | 2–>10 ppb As, with a median of 2 ppb As | Self reported | [143] | |
| 788 adults aged 20 years or older who participated in the 2003–2004 National Health and Nutrition Examination Survey (NHANES) and had urine arsenic determinations | ≥20 years | Age, sex, race, ethnicity; educational, smoking and alcohol consumption status; and dietary recall | 2003–2004 | 7.1 ppb total As, 3 ppb dmAs, 0.9 ppb arsenobetaine | FBG ≥ 126 mg/dL, self-reported physical diagnosis or use of insulin/oral hypoglycemic medication | [144] | |
| 3925 people on tribal tolls in 13 American Indian communities | <55-≥65 | Age, sex, education, body mass index, smoking status, alcohol consumption | 1989–1991 | 7.9–24.2 ppb urine As, median urine As 14.1 ppb | Glycated hemoglobin and insulin resistance, fasting glucose level of 126 mg/dL or higher, 2 h glucose levels of 200 mg/dL or higher, hemoglobin A1c (HbA1c) of 6.5% or higher, or diabetes treatment | [145] | |
| cohort of American Indians in Arizona, Oklahoma, North Dakota and South Dakota | ≥30 years | Age, ancestry, family relationships | 1998–1999, 2001–2003, 2005–2006, 2014–2015 | median exposure of 5.93 ppb | FBG ≥ 126 mg/dL, or use of insulin or oral hypoglycemic medications | [109] | |
| non-institutionalized civilian resident population from NHANES | ≥20 years | Body mass index, age, gender, race/ethnicity, education, income, cigarette use, alcohol intake and physical activity | 2011–2014 | 246–260.6 ng/h | Spot urine samples, FBG ≥ 100 mg/dL or use of medication to treat hyperglycemia | [146] | |
| 4549 members of 13 tribes based in Arizona, Oklahoma, North Dakota and South Dakota | 45–75 years | Age, sex, study region, medical history, smoking status | 1989–ongoing | 10.2–11.2 nmol per mmol of creatinine in urine sample of the exposed group | Urinary arsenic species measured using HPLC to identify differentially methylated position | [110] | |
| 2919 participants recruited by Strong Heart Family Study | ≥25 years | Age, sex, education, smoking history, alcohol use, medical history | 1998–1999, 2001–2003 | median 0.52 ppb | Urine arsenic, FBG ≥ 126 mg/dL, self-reported physician diagnosis or self-reported use of insulin or oral diabetes treatment | [111] | |
| Pregnant women with and without GDM who received prenatal care at the University of Oklahoma Health Sciences Center (OUHSC) Women’s Clinic and High Risk Pregnancy Clinic | ≥18 years | Maternal age, race/ethnicity, education, income, history of GDM diagnosis | 2009–2010 | 1.25 ppb total arsenic | BG ≥ 135 mg/dL | [147] | |
| 688 participants including type 1, type 2 and control participants from SEARCH, a study being conducted in South Carolina, Colorado and Columbia | 10–22 years | Age, sex, race, education, height, weight | 2003–2006 | 0.0429–0.0502 ppb iAs | Clinical diabetes assigned by the health provider | [148] | |
| 5114 African-American and white men and women who are part of the CRADIA study living in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA | ≥25 years | Age, gender, race, education, smoking status, alcohol consumption, physical activity, BMI, dietary intake | 1987–88; 2015–2016 | <0.0593–≥0.1692 ppm toenail arsenic level | fasting glucose ≥ 126 mg/dL, non-fasting glucose ≥ 200 mg/dL, 2 h postchallenge glucose ≥ 200 mg/dL, hemoglobin A1c ≥ 6.5%, or use of glucose-lowering medications. | [112] |
4.3. Mechanisms Associated with iAs-Induced Diabetogenesis
Epidemiological studies have revealed a greater incidence of diabetes among residents in areas highly contaminated with iAs, including Bangladesh [107], Taiwan [133], and Mexico [103]. These epidemiological studies in iAs-exposed populations clearly demonstrate an association between iAs and the pathological progression of DM. Along with the epidemiological evidence, laboratory studies have also shown that exposure to iAs can produce effects that correspond to diabetic phenotypes.
Despite a vast wealth of epidemiological correlations, as well as in vivo and in vitro experimental determinations of iAs-promoted diabetic phenotypes, mechanistic insight has remained limited. A variety of mechanisms for arsenic’s diabetogenic effects have been proposed and demonstrated across a variety of tissue types and diabetic contexts. However, the exact mechanism for iAs-induced diabetic effects is still a matter of debate. Studies conducted thus far have implicated inhibition of insulin-dependent glucose uptake, pancreatic β-cell damage and/or dysfunction, and stimulation of hepatic gluconeogenesis as some of the major mechanisms involved in iAs-induced diabetes [125]. At the transcriptional level, other potential mechanisms of iAs-induced dysfunction include modulation of expression of genes involved in insulin signaling [149,150], as well as influencing adipocyte differentiation [151,152] (Figure 2). Thus, arsenic exerts its pro-diabetogenic effects by affecting multiple organ systems, diminishing their function over time.
Figure 2.
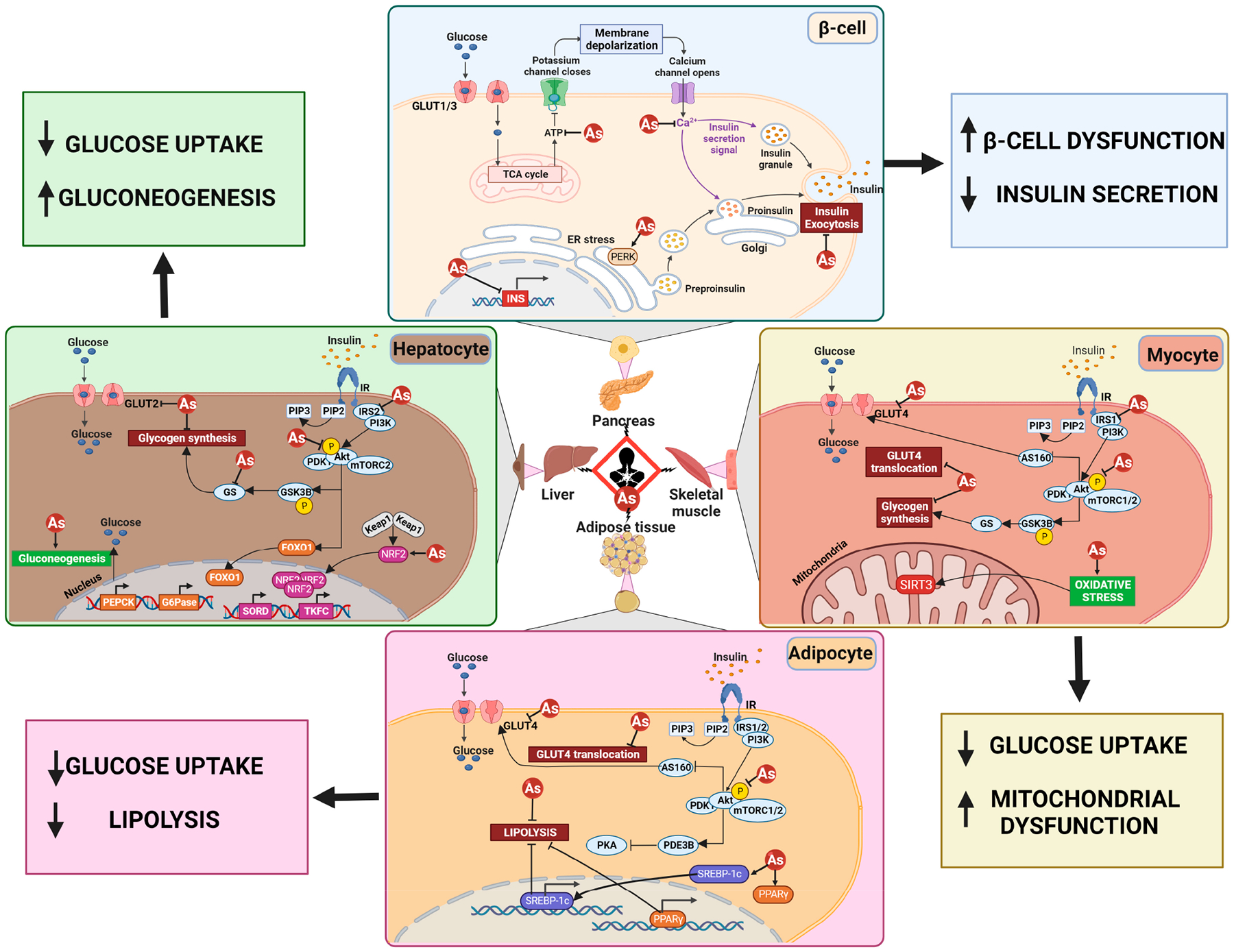
A variety of mechanisms have been proposed for the diabetogenic effects of arsenic. Shown here are (top left) stimulation of hepatic gluconeogenesis; (top right) a decrease in insulin secretion from beta cells; (bottom left) decreased glucose uptake and lipolysis in adipocytes; and (bottom right) decreased glucose uptake due to increased mitochondrial dysfunction.
Specifically, in vitro and in vivo studies have shown iAs-dependent inhibition of glucose transporter 4 (GLUT4) recruitment to the plasma membrane either directly or through inhibition of Akt, a key signaling enzyme required for GLUT4 translocation [153,154]. Arsenic can also play a role in decreasing the phosphorylation of mechanistic target of mTOR and p70, key regulators of insulin-stimulated glucose uptake [155]. In addition to inhibiting insulin signaling, iAs also stimulates hepatic gluconeogenesis by inducing the increased expression of phosphoenolpyruvate carboxykinase (PEPCK), a rate-limiting enzyme in gluconeogenesis, thus resulting in hyperglycemia even under fasted conditions [156,157]. Studies have also linked chronic iAs exposure to impaired pancreatic β-cell function, as higher blood glucose levels result in an increased demand on β-cells to produce more insulin, leading to their dysfunction over time [158]. Arsenic exerts its diabetogenic effects on skeletal muscle function through induction of oxidative stress and disruption of calcium homeostasis [159,160]. Arsenic induces oxidative stress in skeletal muscle by inhibiting enzymes involved in oxidative phosphorylation, resulting in decreased ATP production and increased oxidative stress [161]. This increases production of reactive species inhibits GLUT4 translocation, and interferes with the Akt pathway, leading to decreased glucose uptake in skeletal muscle [162]. In adipose tissue, chronic iAs exposure is also known to contribute to the development of obesity and other metabolic disorders through induction of oxidative stress, as well as disruption of adipokine signaling, and dysregulation of lipid metabolism [163]. Arsenic exposure can also decrease PDE3b (phosphodiesterase 3b) expression and activity, an enzyme that regulates lipolysis and glucose uptake in adipocytes, resulting in hyperglycemia and insulin resistance [164]. In addition, SREBP (a transcription factor that regulates lipid metabolism) and PPARg (a nuclear receptor that regulates adipogenesis and glucose metabolism) have both been shown to be activated by iAs in adipocytes, resulting in increased expression of lipogenic genes and adipogenesis, eventually leading to the development of obesity, insulin resistance, and hyperglycemia [164–166].
In the liver, iAs exposure can have harmful effects on hepatocytes by altering hepatic gene expression and signaling pathways involved in liver metabolism. For example, prolonged, non-canonical activation of the transcription factor NRF2 (nuclear factor erythroid 2-related factor 2), which results from autophagy inhibition, and p62-dependent sequestration of Keap1, the negative regulator of NRF2, has been shown to mediate insulin resistance and glucose intolerance in wild-type mice exposed to 25 ppm iAs for 20 weeks [167]. Besides NRF2, iAs has also been shown to influence the expression of other transcription factors that may be related to enhanced diabetes risk [168,169]. Chronic iAs exposure increased the gene expression of PEPCK (phosphoenolpyruvate carboxykinase) and G6PC1 (glucose-6-phosphatase), two key gluconeogenic enzymes that promote hepatic glucose synthesis and thus contribute to hyperglycemia, via prolonged activation of the transcription factor FOXO1 (forkhead box O1) [170]. Exposure to iAs also increased SORD (sorbitol dehydrogenase), TKFC (transketolase-like protein 1), and KHK (ketohexokinase) expression in the liver, leading to increased hepatic glucose production via the polyol pathway, ultimately contributing to hyperglycemia in mice [167]. Overall, iAs exposure has been shown to induce diabetogenesis through multiple tissue-specific mechanisms.
In terms of acute arsenic iAs toxicity, including its effects on glucose metabolism, the binding of iAs to thiol (SH) groups has been shown. The reactivity of iAs on sulfhydryl groups can inactivate over 200 enzymes, and thus could be responsible, at least in part, for the widespread pathogenic effects of iAs on different organ systems [171,172]. Arsenic, in its trivalent form (As3+), is also known to inhibit pyruvate and α-ketoglutarate dehydrogenase during acute poisoning, both of which are essential enzymes for gluconeogenesis and glycolysis [171]. In its pentavalent form (As5+), it can substitute for phosphate, disrupting protein phosphorylation and oxidative phosphorylation [173]. However, whether this occurs in a chronic exposure context, as well as at more physiologically relevant concentrations, has yet to be determined.
Increasing iAs levels in the blood correlated with increasing levels of ROS and decreased antioxidant capacity in the plasma of iAs-exposed individuals in Taiwan, suggesting the influence of the iAs-ROS axis on promoting diabetes [174]. Oxidative stress, inflammation, and apoptosis have all been implicated as pathways that could converge to link iAs exposure with DM onset and progression [175]. These mechanisms fit arsenic’s effects on systemic metabolism, as in normal mice, iAs exposure has been shown to result in prediabetic effects via alterations to lipid metabolism, gluconeogenesis, and insulin secretion, while also worsening diabetic outcomes in a diabetic mouse model [156]. Thus, the ability of arsenic to dysregulate these processes involved in both early and later outcomes associated with DM, establishes iAs as a relevant diabetogen.
Finally, iAs is also known to impact various components of the epigenetic machinery. Exposure to iAs has been linked to varied gene expression of AS3MT [153], CAPN10 [158], GSTO1 [136] and NOTCH2 [176]. Differences in genotype, as well as single-nucleotide polymorphisms (SNPs) in any of these critical genes, can dictate the risk of developing DM, as the iAs metabolite profile, as well as glucose metabolism, can vary greatly. Supporting this notion, studies in both human cohorts and in vivo experimental models have shown iAs-induced changes in epigenetic regulation of glucose homeostasis, specifically DNA methylation and miRNA suppression of DM-related genes involved in glycemic regulation [177,178]. Furthermore, iAs-associated changes in DNA methylation of DM-related genes were observed in the peripheral blood leukocytes of individuals consuming high levels of iAs in the drinking water in Mexico [179]. At the miRNA level, a study examining newborn umbilical cord blood samples for miRNA expression following in utero iAs exposure indicated altered expression of miR-107 and miR-20b, both of which have been associated with DM [180]. Similar in vivo studies in the liver tissue of mice exposed to various concentrations of sodium arsenite also revealed altered miRNA expression profiles [181].
4.4. Future Research Needs
Based on the evidence described above, it is clear that arsenic toxicity is dependent on exposure dose, frequency, duration, and species involved, as well as the age, gender, and individual genetic susceptibilities of the exposed individual, amongst many other variables [182]. Several of these parameters should be further explored in future studies to determine the association between iAs toxicity in drinking water and the progression of DM. Specifically, the epigenetic aspect of iAs-controlled diabetes induction remains understudied, which could provide key insight into understanding this aspect of iAs promotion of diabetes, particularly when changes in diabetes-relevant gene expression are observed. Dietary influences and genetic polymorphisms in response to iAs exposure should also be further studied, as they could provide key insight into how different regional populations are affected during exposure. Investigating the role of iAs in dictating diabetogenic changes at the cellular level also requires more experimental evidence using consistent and exposure-relevant doses of iAs. Improved consistency and dose relevance, coupled with the identification of appropriate biomarkers for iAs-induced DM, will allow for a better comparison amongst exposed and unexposed groups.
Another important issue moving forward is that the conditions of exposure to iAs in humans overall need to be more fully characterized so that better biomarkers can be developed and the separation of relevant forms of iAs and their level of toxicity at the tissue vs. systemic level can be better defined. The bioaccumulation of various forms of iAs in cellular versus animal models and their relevance to human physiological settings is also therefore considered an important area for further research. Gender and age differences in susceptibility to iAs and their relation to development of diabetes are also poorly defined. In addition, metal–metal interactions should also be studied to define the consequences of iAs interaction with other harmful metals, as arsenic is not the only toxic component present during exposure. Altogether, while much is known epidemiologically regarding the increased risk of diabetes associated with chronic arsenic exposure, a great deal still needs to be done at the experimental level to increase our understanding of arsenic’s diabetogenic effects and generate relevant therapies for this subset of diabetic patients.
5. Regulation of Arsenic in Drinking Water
Disrupting exposure to arsenic requires understanding of water sources that may contain elevated levels, whether naturally occurring or as a result of contamination. Monitoring arsenic in drinking water is critical, especially for those who are on private wells. Private well-water quality outreach and sampling campaigns have been conducted across the country to protect human health and address arsenic exposure. For example, the collaborative public health project “All About Arsenic,” was initiated in 2015 by researchers at Mount Desert Island Biological Laboratory and Dartmouth College’s Toxic Metals Superfund Research Program to “to expand private well water testing for arsenic and other elements and to build data literacy among students and the wider public” [183,184]. A key factor to ensure the success of these monitoring and educational programs is public participation, research conducted with nonprofessionals, who may contribute to the research question, generation of theory or hypothesis, data collection, data analysis, data interpretation, and/or translating research to action (e.g., [185]). Public participation in research is a valuable model for investigations across disciplines and can connect science and practice to people and policy (e.g., [186]). Practices led by institutions only have been critiqued for their lack of accessibility, diversity, justice, equity, and inclusion [187]. Community-based participatory research and community science efforts that champion placed-based topics and local experts and address community questions are strongly recommended and can increase the rigor and relevance of the effort (e.g., [187–189]). For example, Gardenroots [31,190–193], established in 2010, revealed that in one community, the local water utility was serving water that exceeded the arsenic drinking water standard (0.010 mg L−1) [190]. Gardenroots participants worked together to identify and notify additional households that were connected to the public water supply. They also reported their test results to USEPA and Arizona Department of Environmental Quality, advocating that this issue needed to be addressed (Gardenroots also notified and sent the results to the USEPA). As a result, the municipal water suppler was issued seven notices of violation by the ADEQ, one for exceeding the arsenic drinking water standard. Additionally, arsenic concentrations in private well water exceeded the drinking water standard for several participants who relied solely on this water source. University of Arizona researchers worked closely with those households to provide information regarding water treatment technologies that could be implemented to reduce their arsenic concentrations [190].
6. Approaches to Removal of Arsenic from Drinking Water
The present USEPA national primary drinking water regulations (NPDWS) limit for arsenic is 10 μg/L. This is a compromise between consumer health protection and water treatment costs, as the USEPA has set a public health goal of arsenic in drinking water at “zero.” Arsenic is found in drinking water in two forms of inorganic arsenic, although organic forms of arsenic also exist and can be found in aquatic environments such as benthic sediments. The common forms of inorganic arsenic include arsenate (As+5) and arsenite (As+3) (Figure 1). The more oxidized arsenate ions predominate in moderately to well-aerated water sources, whereas arsenite forms predominate in organic matter-rich, oxygen-limited waters. A 2014 study of 65 drinking water wells from 28 states in the US [194] showed that either arsenate or arsenite predominated in 91% of the wells, while the remaining wells had a combination of the two arsenic forms. The 91% of wells with a dominant arsenic form were distributed approximately evenly between arsenic and arsenate.
Although arsenate and arsenite are known to have different toxicities, the USEPA only monitors and regulates arsenic cumulatively in its elemental form (As). The amount of arsenic in surface and groundwater depends primarily on the surrounding geology, as well as industrial activity, including, among others, mining and oil extraction. Arsenic is commonly associated with pyritic (iron- and sulfur-containing) minerals, which when exposed to oxidizing–acidic conditions release arsenic in the water environment as arsenate and/or arsenite ions. A common arsenic mineral is arsenopyrite, often found with other pyritic minerals rich in copper, lead, cadmium and other metals.
Lowering the levels of arsenic in drinking water is difficult due to the complex chemistry of this element. Although the arsenic in arsenic-rich minerals is relatively insoluble in natural waters (except in extreme redox and pH conditions), areas with high amounts of arsenic-containing minerals often have naturally high levels of dissolved arsenic in groundwater. Lowering the levels of this element to drinking water standards can be difficult and expensive due to its shifting chemical forms. For example, changing the water redox potential or pH conditions can lead to the precipitation of arsenate and arsenite with iron, calcium and other cations leading to the formation of secondary arsenic-rich minerals. The pH range of most potable water sources is 6 to 9, which when combined varying oxygen levels can lead to the presence of arsenate or arsenite as previously described. These arsenic species have difference sizes, charge (−), and reactivity, complicating their removal from water using precipitation, absorption, ion exchange, and nanofiltration processes used in today’s best available water treatment technologies. The next sections present a summary of treatment technologies that can be used to lower arsenic levels for both public utilities and home water treatment systems.
The USEPA has guidelines and recommendations for the selection of best-available technology (BAT) to mitigate arsenic in water, given variables such as the number of connections (consumers), water quality, levels of arsenic in water, location, infrastructure, etc. [195]. Public water utilities should follow these guidelines in the selection and testing of the BAT or BATs to ensure consistent compliance to the arsenic standard at the lowest cost to the consumer. There is sometimes tension between the choice of arsenic levels and cost of treatment [194].
6.1. Technologies for Public Water Utilities
6.1.1. Blending
Mixing two water sources to produce water with arsenic levels below the NPDWS is an acceptable technology available to water utilities with diverse sources of potable water such as surface water and groundwater [196].
6.1.2. Coagulation/Filtration
The addition of iron salts such as ferric chloride or sulfate to well-aerated water leads to the formation of insoluble amorphous ferric hydroxides that adsorb preferably arsenate anions entrapping them into a coagulant that can settle and be filtered out of the water. The efficiency of the treatment process can be optimized up to 95% by adjusting the pH with the proper selection of iron salts and the addition of oxidizing agents such as chlorine and permanganate to oxidize arsenite to arsenate. This treatment technology produces significant amounts of potentially hazardous arsenic-contaminated residues that must be disposed of (usually landfilled) following federal and state guidelines [195].
6.1.3. Oxidation/Filtration
Oxygen-free groundwater may have significant amounts of soluble iron and/or manganese present, often accompanied by soluble arsenite. In this case, water aeration or the addition of an oxidizing chemical leads directly to the formation of both arsenate and insoluble ferric hydroxides that can sorb the arsenate. This is followed by filtration to remove iron–manganese–arsenic particles. The efficacy of this approach depends on the initial ratio of iron to arsenic present in the water. This technology also requires the proper disposal of arsenic-contaminated residues [195].
6.1.4. Metal Oxides
Since arsenic anions have a high affinity for positively charged metal oxides, adsorptive materials composed of solid porous media such as aluminum oxides (activated alumina) and many types of ferric hydroxy-oxides (GFH) (alone or coated onto inert solid media) are options for closed water treatment systems. These approaches can efficiently filter out arsenate with up to 95% removal, provided that all forms of arsenic are present as arsenate. This again may require the conversion of arsenite, if present, to arsenate with the addition of oxidants as well as pH adjustment to optimize arsenic removal efficiencies. Since there are many manufacturers of these materials and varying costs, pilot studies are usually required to test materials and determine best pre- and posttreatment(s) needed to optimize arsenic removal and lower costs. Importantly, knowledge of the water chemistry (e.g., salinity, pH, alkalinity, redox potential, and the concentrations of other potentially competing ions) is also needed [195]. The presence of other ions such as fluoride, silica, and sulfate can also interfere with the adsorption of arsenate. Once spent, these porous media must be disposed of as potentially hazardous arsenic contaminated residues.
Innovative particle coatings and nanoparticles made of and with carbon, alumina, iron, titanium, zirconium and other elements are being explored for As removal from water [197] with varying degrees of success, higher costs and remaining challenges associated with the disposal of spent materials.
6.1.5. Anion Exchange Resins
Porous synthetic organic polymer beats populated with positively charged sites saturated with a common anion such as chloride (Cl−) can be used to efficiently remove arsenate ions from water. These resins can be manufactured to preferentially remove arsenate anions over other common anions as mentioned previously. Ion exchange resins are more expensive than inorganic porous media but have the advantage that they can be reused after regeneration with alkali solutions. Resin regenerant wastes containing arsenic must be disposed of as hazardous waste. As with other treatment technologies, pre- and posttreatment(s) may be necessary to oxidize any reduced arsenic forms to arsenate [195].
6.1.6. Enhanced Lime Softening
The addition of lime [Ca(OH)2] to water is commonly used to reduce hardness through precipitation of calcium and magnesium. This technology can also be used to remove arsenic [198,199]. Lime is added to bring the pH of the system to higher than 10.5. This results in precipitation of carbonates (CaCO3) and hydroxides [Mg(OH)2], and when arsenate is present, it too will precipitate. Magnesium additions may be needed if not present in the water and posttreatment is required for pH adjustment. This process is quite efficient for arsenate, but less efficient for arsenite. The technology requires large amounts of lime, which in turn generates large amounts of waste sludge. In addition, the high operating pH can be problematic and the treated water needs pH adjustment following treatment [195].
6.1.7. Nanofiltration and Reverse Osmosis
These similar types of membrane filtration (adsorption of ions onto a semiporous membrane) processes are best suited and most cost-effective for treatment of water with total dissolved solids (TDS) greater than 500 mg/L and when other ions besides arsenic must be lowered to meet drinking water standards. A complete analysis of all major and minor water quality parameters must be performed for the initial evaluation and testing of these processes, since implementing either technology just to reduce arsenic levels in water would not be cost-effective. Note that reverse osmosis (RO) systems are more expensive to operate than nanofiltration, but are more efficient at lowering arsenate. Pressures from 50 to over 200 psi may be used to force influent water through a semiporous membrane, which produces scaling and fouling requiring periodic flushing. This membrane cleaning step can produce significant volumes of brackish water that must be disposed appropriately. Up to 70% of the influent water may be lost during the membrane cleaning cycle, depending on the levels of particulates, bacteria and salts present in the water [197]. For example, very hard water can increase membrane scaling significantly. As with previous technologies, the arsenate forms are preferentially adsorbed (RO > 95%). Therefore, if arsenite is present in the influent, it must be converted to arsenate with pretreatment oxidation [195].
6.2. Home Treatments
Point of use (POU) devices are typically used by homeowners that have elevated levels of arsenic in their well water [200]. Today, homeowners have an increasing array of point of entry (POE) water systems to lower arsenic and other contaminants in water including water softeners, alkali, permanganate, chlorination, activated carbon, GFH, and RO systems, costing thousands of dollars to install and maintain. In the next sections we will summarize three low cost POU systems available to homeowners to lower arsenic levels in water.
6.2.1. Distillation
This process is straightforward, requiring the use of a steam-distilling unit that generates steam that when condensed produces contaminant-free, disinfected water. Tabletop steam-distillation units are slow and energy-intensive, producing enough water for daily drinking and cooking. During the distillation process, all arsenic forms and other ions present in water concentrate and precipitate, forming a scale in the distillation vessel that must be periodically cleaned out. Modern distillation units also have activated carbon filters that can trap volatile contaminants [200].
6.2.2. Reverse Osmosis
Small, under-the-sink, home water treatment systems that use the reverse osmosis process are widely available for do-it-yourself and professional installation. These small systems are fully automated and make use of the existing household water pressure (40–60 psi) to force water through a semipermeable membrane, storing it in a reservoir for later use. As with industrial systems, membrane fouling and scaling require periodic (and more frequent) washing. Up to nine volumes of water may be lost during this cycle for every volume of water produced depending on the influent water quality. High water TDS and hardness decrease RO system performance, significantly increasing household water consumption. In areas of the US with very hard water, a softening pretreatment may be needed. When used to lower arsenic or other primary contaminants, homeowners should test their water before and after RO treatment and regularly thereafter to make sure that arsenic or any other primary drinking water standards are met.
6.2.3. Iron Filters
Small POU in-line GFH filters are slowly becoming available to homeowners to filter arsenic out of water. However, their arsenic-filtering capacity is very much dependent on the concentrations of several other water ions commonly present in water, such as silica, sulfate, and fluoride, and other anions, as previously mentioned. Therefore, the homeowner should test arsenic levels in the treated water periodically to check the arsenic-removal efficiency of these filters over time. These filters do not generate any waste while in use, but they cannot be regenerated. Thus, when exhausted, they should be handled and disposed of as potentially hazardous materials [200].
6.3. Summary—Challenges to Removal of Arsenic from Drinking Water
The EPA lowered the arsenic drinking water standard of arsenic in 2001 from 50 μg/L to 10 μg/L. Ideally, drinking water should not have any arsenic, but reducing the levels of this element below 10 μg/L is difficult and expensive. This is because, as discussed above, arsenic exists in several forms in water and complex multistep treatments are often required to reduce arsenic levels in water. Because of its geological origins and arsenic’s affinity for iron hydroxides and aluminum oxides (alumina), large-scale treatment has traditionally focused on the use of coagulation, coprecipitation, and sorption of arsenic using iron-based chemicals and alumina. Although new iron, other metal-based, and hybrid nanomaterials (silica and activated charcoal with metal coatings and Fe, Ti, and Zr nanoparticles for example) are being developed for arsenic capture, their high cost and varying efficacies remain a challenge. Anion exchange resins remain an expensive but efficient method to lower arsenic concentrations in water. Membrane filtration systems such as nanofiltration are increasing in performance, with lower energy costs and lower levels of other water contaminants, such as salts, nitrate, metals, and many organic contaminants in addition to arsenic. For most of these approaches, an added concern is that the environmentally safe and cost-effective disposal of arsenic-contaminated solid and liquid residues, sorbents, and brines remains a challenge. Small-scale treatment systems such as POUs are increasing in popularity, in particular home RO systems. These systems also have associated costs due to maintenance requirements, increased water use, and water testing costs.
Funding:
This work was supported by the National Institute of Environmental Health Sciences Superfund Research Program through grant P42ES004940.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Data Availability Statement:
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
References
- 1.World Economic Forum. “The Global Risks Report 2021”. Available online: https://www.weforum.org/agenda/2021/01/global-risks-report-2021/ (accessed on 25 April 2023).
- 2.World Health Organization; United Nations Children’s Fund. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2017: Special Focus on Inequalities. World Health Organization. 2019. Available online: https://apps.who.int/iris/handle/10665/329370 (accessed on 25 April 2023). [Google Scholar]
- 3.Li P; Karunanidhi D; Subramani T; Srinivasamoorthy K Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol 2021, 80, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO; FAO; IAEA. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996. Available online: https://apps.who.int/iris/handle/10665/37931 (accessed on 25 April 2023). [Google Scholar]
- 5.Tchounwou PB; Yedjou CG; Patlolla AK; Sutton DJ Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch A, Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waseem A; Arshad J; Iqbal F; Sajjad A; Mehmood Z; Murtaza G Pollution status of Pakistan: A retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed Res. Int 2014, 2014, 813206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacquart T; Frisbie S; Mitchell E; Grigg L; Cole C; Small C; Sarkar B Multiple inorganic toxic substances contaminating the groundwater of Myingyan Township, Myanmar: Arsenic, manganese, fluoride, iron, and uranium. Sci. Total Environ 2015, 517, 232–245. [DOI] [PubMed] [Google Scholar]
- 8.Rehman K; Fatima F; Waheed I; Akash MSH Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem 2018, 119, 157–184. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson MS; Bommarito P; George A; Yelton S; Cable P; Coyte R; Karr J; Vengosh A; Gray KM; Fry RC Assessment of inorganic contamination of private wells and demonstration of effective filter-based reduction: A pilot-study in Stokes County, North Carolina. Environ. Res 2019, 177, 108618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotfi S; Chakit M; Belghyti D Groundwater Quality and Pollution Index for Heavy Metals in Saïs Plain, Morocco. J. Health Poll 2020, 10, 200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATSDR (Agency for Toxic Substances Disease Registry). Substance Priority List (1997). Available online: https://www.atsdr.cdc.gov/spl/resources/1997_atsdr_substance_priority_list.html (accessed on 25 April 2023).
- 12.Bullard RD Dumping in Dixie: Race, Class and Environmental Quality; Westview Press: Boulder, CO, USA, 1990. [Google Scholar]
- 13.Bullard RD Environmental Justice in the 21st Century: Race Still Matters. Phylon 2001, 49, 151–171. [Google Scholar]
- 14.U.S. Department of Health & Human Services, Biden-Harris Administration Establishes HHS Office of Environmental Justice. 2022. Available online: https://www.hhs.gov/about/news/2022/05/31/biden-harris-administration-establishes-hhs-office-of-environmental-justice.html (accessed on 25 April 2023).
- 15.World Health Organization. Social Determinants of Health. Available online: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 25 April 2023).
- 16.Office of Disease Prevention and Health Promotion, Healthy People 2030. Available online: https://health.gov/healthypeople (accessed on 25 April 2023).
- 17.CDC/ATSDR. Social Vulnerability Index. 2022. Available online: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed on 25 April 2023).
- 18.Adger WN Vulnerability. Global Environ. Change 2006, 16, 268–281. [Google Scholar]
- 19.Cutter SL Vulnerability to Environmental Hazards. Prog. Hum. Geogr 1996, 20, 29–539. [Google Scholar]
- 20.Ramírez-Andreotta MD; Walls R; Youens-Clark K; Blumberg K; Isaacs KE; Kaufmann D; Maier RM Alleviating Environmental Health Disparities through Community Science and Data Integration. Front. Sust. Food Sys 2021, 5, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeedi P; Petersohn I; Salpea P; Malanda B; Karuranga S; Unwin N; Colagiuri S; Guariguata L; Motala AA; Ogurtsova K; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract 2019, 157, 107843. [DOI] [PubMed] [Google Scholar]
- 22.Nordstrom DK Public Health. Worldwide Occurrences of Arsenic in Ground Water. Science 2002, 296, 2143–2145. [DOI] [PubMed] [Google Scholar]
- 23.Cullen WR; Reimer KJ Arsenic Speciation in the Environment. Chem. Rev 1989, 89, 713–764. [Google Scholar]
- 24.Shankar S; Shanker U; Shikha. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J 2014, 2014, 304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naujokas MF; Anderson B; Ahsan H; Aposhian HV; Graziano JH; Thompson C; Suk WA The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect 2013, 121, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Focazio MJ; Welch AH; Watkins SA; Helsel DR; Horn MA A Retrospective Analysis on the Occurrence of Arsenic in Ground-Water Resources of the United States and Limitations in Drinking-Water-Supply-Characterizations; Water-Resources Investigations Report 99–4279; US Geological Survey: Reston, VA, USA, 2000. [Google Scholar]
- 27.Huang L; Wu H; van der Kuijp TJ The Health Effects of Exposure to Arsenic-Contaminated Drinking Water: A Review by Global Geographical Distribution. Int. J. Environ. Health Res 2015, 25, 432–452. [DOI] [PubMed] [Google Scholar]
- 28.Navas-Acien A; Silbergeld EK; Streeter RA; Clark JM; Burke TA; Guallar E Arsenic Exposure and Type 2 Diabetes: A Systematic Review of the Experimental and Epidemiological Evidence. Environ. Health Perspect 2006, 114, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill-Briggs F; Adler NE; Berkowitz SA; Chin MH; Gary-Webb TL; Navas-Acien A; Thornton PL; Haire-Joshu D Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramírez-Andreotta MD; Brusseau ML; Beamer P; Maier RM Home Gardening Near a Mining Site in an Arsenic-Endemic Region of Arizona: Assessing Arsenic Exposure Dose and Risk Via Ingestion of Home Garden Vegetables, Soils, and Water. Sci. Total Environ 2013, 454–455, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manjón I; Ramírez-Andreotta MD; Saez AE; Root RA; Hild J; Janes K; Alexander-Ozinskas A Ingestion and Inhalation of Metal(loid)s Through Preschool Gardening: An Exposure and Risk Assessment in Legacy Mining Communities. Sci. Total Environ 2020, 718, 134639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Chavez T; Rine K; Almusawi R; O’Brien-Metzger R; Ramírez-Andreotta MD; Betterton E; Saez AE Outdoor/Indoor Contaminant Transport by Atmospheric Dust and Aerosol at an Active Smelter Site. Water Air Soil Pollut. 2021, 232, 226. [Google Scholar]
- 33.Heusinkveld D; Ramírez-Andreotta MD; Rodriguez-Chavez TB; Saez EA; Betterton E; Rine K Assessing a Children’s Integrated Exposure Model in an Active Mining Town. Expo. Health 2021, 13, 517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 25 April 2023).
- 35.National Diabetes Association. The Cost of Diabetes. Available online: https://diabetes.org/about-us/statistics/cost-diabetes (accessed on 25 April 2023).
- 36.Smith NM; Lee R; Heitkemper DT; Cafferky KD; Haque A; Henderson AK Inorganic Arsenic in Cooked Rice and Vegetables from Bangladeshi Households. Sci. Total Environ 2006, 370, 294–301. [DOI] [PubMed] [Google Scholar]
- 37.Prohaska T; Stingeder G Speciation of Arsenic. In Handbook of Elemental Speciation II—Species in the Environment, Food, Medicine and Occupational Health; Cornelis R, Ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2005; pp. 69–85. [Google Scholar]
- 38.Muñoz O; Bastias JM; Araya M; Morales A; Orellana C; Rebolledo R Estimation of the Dietary Intake of Cadmium, Lead, Mercury, and Arsenic by the Population of Santiago (Chile) Using a Total Diet Study. Food Chem. Toxicol 2005, 43, 1647–1655. [DOI] [PubMed] [Google Scholar]
- 39.Yost LJ; Schoof RA; Aucoin R Intake of Inorganic Arsenic in the North American Diet. Hum. Ecol. Risk Assess 1998, 4, 137–152. [Google Scholar]
- 40.Diaz OP; Leyton I; Muñoz O; Nuñez N; Devesa V; Súñer MA; Veles D; Mpntor R Contribution of Water, Bread and Vegetables (Raw and Cooked) to Dietary Intake of Inorganic Arsenic in a Rural Village of Northern Chile. J. Agric. Food Chem 2004, 52, 1773–1779. [DOI] [PubMed] [Google Scholar]
- 41.Diaz OP; Arcos R; Tapia Y; Pastene R; Velez D; Devesa V; Montoro R; Aguilera V; Becerra M Estimation of Arsenic Intake from Drinking Water and Food (Raw and Cooked) in a Rural Village of Northern Chile. Urine as a Biomarker of Recent Exposure. Intl. J. Environ. Res. Public Health 2015, 12, 5614–5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laparra JM; Vélez D; Barberá R; Farré R; Montoro R Bioavailability of Inorganic Arsenic in Cooked Rice: Practical Aspects for Human Risk Assessments. J. Agric. Food Chem 2005, 53, 8829–8833. [DOI] [PubMed] [Google Scholar]
- 43.Bundschuh J; Nath B; Battacharya P; Liu CW; Armienta MA; López MV; Lopez DL; Jean JS; Cornejo L; Macedo LF; et al. Arsenic in the Human Food Chain: The Latin America Perspective. Sci. Total Environ 2012, 429, 92–106. [DOI] [PubMed] [Google Scholar]
- 44.Chung JY; Yu SD; Hong YS Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.mindat.org. The Mineralogy of Arsenic. Available online: https://www.mindat.org/element/Arsenic (accessed on 25 April 2023).
- 46.Savage KS; Tingle TN; O’Day PA; Waychunas GA; Bird DK Arsenic Speciation in Pyrite and Secondary Weathering Phases, Mother Lode Gold District, Tuolumne County, California. Appl. Geochem 2000, 15, 1219–1244. [Google Scholar]
- 47.Deditius AP; Utsunomiya S; Renock D; Ewing RC; Ramana CV; Becker U; Kesler SE A Proposed New Type of Arsenian Pyrite: Composition, Nanostructure and Geological Significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar]
- 48.Chen CJ; Chuang YC; Lin TM; Wu HY Malignant Neoplasms Among Residents of a Blackfoot Disease-Endemic Area in Taiwan: High-Arsenic Atesian Well Water and Cancers. Cancer Res. 1985, 45 Pt 2, 5895–5899. [PubMed] [Google Scholar]
- 49.Saha KC Chronic Arsenical Dermatoses From Tube- Well Water In West Bengal During 1983–87. Indian J. Dermatol 1995, 40, 1–12. [Google Scholar]
- 50.Smith AH; Lingas EO; Rahman M Contamination of Drinking-Water by Arsenic in Bangladesh: A Public Health Emergency. Bull. World Health Org 2000, 78, 1093–1103. [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell KM; Nordstrom DK Arsenic Speciation and Sorption in Natural Environments. Rev. Mineral. Geochem 2014, 79, 185–216. [Google Scholar]
- 52.Foster AL; Kim CS Arsenic Speciation in Solids Using X-ray Absorption Spectroscopy. Rev. Mineral. Geochem 2014, 79, 257–369. [Google Scholar]
- 53.Polizzotto ML; Kocar BD; Benner SG; Sampson M; Fendorf S Near-Surface Wetland Sediments as a Source of Arsenic Release to Ground Water in Asia. Nature 2008, 454, 505–508. [DOI] [PubMed] [Google Scholar]
- 54.Masuda H Arsenic Cycling in the Earth’s Crust and Hydrosphere: Interaction Between Naturally Occurring Arsenic and Human Activities. Prog. Earth Planet. Sci 2018, 5, 68. [Google Scholar]
- 55.Chowdhury UK; Biswas BK; Chowdhury TR; Samanta G; Mandal BK; Basu GC; Chanda CR; Lodh D; Saha KC; Mukherjee SK Groundwater Arsenic Contamination in Bangladesh and West Bengal, India. Environ. Health Perspec 2000, 108, 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charlet L; Polya DA Arsenic in Shallow, Reducing Groundwaters in Southern Asia: An Environmental Health Disaster. Elements 2006, 2, 91–96. [Google Scholar]
- 57.Nriagu JO (Ed.) Arsenic in the Environment (Part II, Human Health and Ecosystem Effects); Wiley Interscience: Hoboken, NJ, USA, 1994; ISBN 978-0-471-30436-4. [Google Scholar]
- 58.Berg M; Tran HC; Nguyen TC; Pham HV; Schertenleib R; Giger W Arsenic Contamination of Groundwater and Drinking Water in Vietnam: A Human Hhealth Threat. Environ. Sci. Technol 2001, 35, 2621–2626. [DOI] [PubMed] [Google Scholar]
- 59.Nickson RT; McArthur JM; Shrestha B; Kyaw-Myint TO; Lowry D Arsenic and Other Drinking Water Quality Issues, Muzaffargarh District, Pakistan. Appl. Geochem 2005, 20, 55–68. [Google Scholar]
- 60.Chen SL; Dzeng SR; Yang MH; Chiu KH; Shieh GM; Wai CM Arsenic Species in Groundwaters of the Blackfoot Disease Area, Taiwan. Environ. Sci. Technol 1994, 28, 877–881. [DOI] [PubMed] [Google Scholar]
- 61.Ayotte JD; Medalie L; Qi SL; Backer LC; Nolan BT Estimating the High-Arsenic Domestic-Well Population in the Conterminous United States. Environ. Sci. Technol 2017, 51, 12443–12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grantham DA; Jones JF Arsenic Contamination of Water wells in Nova Scotia. J.-Am. Water Work. Assoc 1977, 69, 653–657. [Google Scholar]
- 63.Bundschuh J; Farias B; Martin R; Storniolo A; Bhattacharya P; Cortes J; Bonorino G; Albouy R Groundwater Arsenic in the Chaco-Pampean Plain, Argentina: Case Study from Robles County, Santiago del Estero Province. Appl. Geochem 2004, 19, 231–243. [Google Scholar]
- 64.Bundschuh J; Litter MI; Parvez F; Román-Ross G; Nicolli HB; Jean JS; Liu CW; López D; Armienta MA; Guilherme LRG; et al. One Century of Arsenic Exposure in Latin America: A Review of History and Occurrence from 14 Countries. Sci. Total Environ 2012, 429, 2–35. [DOI] [PubMed] [Google Scholar]
- 65.Smedley P; Kinniburgh D; Macdonald D; Nicolli H; Barros A; Tullio J; Pearce J; Alonso M Arsenic Associations in Sediments from the Loess Aquifer of La Pampa, Argentina. Appl. Geochem 2005, 20, 989–1016. [Google Scholar]
- 66.Smedley PL; Kinniburgh DG A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem 2002, 17, 517–568. [Google Scholar]
- 67.Morin G; Calas G Arsenic in Soils, Mine Tailings, and Former Industrial Sites. Elements 2006, 2, 97–101. [Google Scholar]
- 68.Root RA; Vlassopoulos D; Rivera NA; Rafferty MT; Andrews C; O’Day PA Speciation and Natural Attenuation of Arsenic and Iron in a Tidally Influenced Shallow Aquifer. Geochim. Cosmochim. Acta 2009, 73, 5528–5553. [Google Scholar]
- 69.O’Day PA; Vlassopoulos D; Root R; Rivera N The Influence of Sulfur and Iron on Dissolved Arsenic Concentrations in the Shallow Subsurface Under Changing Redox Conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 13703–13708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Day PA Chemistry and Mineralogy of Arsenic. Elements 2006, 2, 77–83. [Google Scholar]
- 71.Welch AH; Lico MS Factors Controlling as and U in Shallow Ground Water, Southern Carson Desert, Nevada. Appl. Geochem 1998, 13, 521–539. [Google Scholar]
- 72.Demergasso CS; Ching GD; Escudero LG; Mur JJP; Alio CP Microbial Precipitation of Arsenic Sulfides in Andean Salt Flats. Geomicrobiol. J 2007, 24, 111–123. [Google Scholar]
- 73.Pierce ML; Moore CB Adsorption of Arsenite and Arsenate on Amorphous Iron Hydroxide. Water Res. 1982, 16, 1247–1253. [Google Scholar]
- 74.Fuller CC; Davis JA; Waychunas GA Surface Chemistry of Ferrihydrite: Part 2. Kinetics of Arsenate Adsorption and Coprecipitation. Geochim. Cosmochim. Acta 1993, 57, 2271–2282. [Google Scholar]
- 75.Dixit S; Hering JG Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environ. Sci. Technol 2003, 37, 4182–4189. [DOI] [PubMed] [Google Scholar]
- 76.Gao X; Root RA; Farrell J; Ela W; Chorover J Effect of Silicic Acid on Arsenate and Arsenite Retention Mechanisms on 6-L Ferrihydrite: A Spectroscopic and Batch Adsorption Approach. Appl. Geochem 2013, 38, 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waychunas GA; Rea BA; Fuller CC; Davis JA Surface Chemistry of Ferrihydrite: Part 1. EXAFS Studies of the Geometry of Coprecipitated and Adsorbed Arsenate. Geochim. Cosmochim. Acta 1993, 57, 2251–2269. [Google Scholar]
- 78.Root RA; Dixit S; Campbell KM; Jew AD; Hering JG; O’Day PA Arsenic Sequestration by Sorption Processes in High-Iron Sediments. Geochim. Cosmochim. Acta 2007, 71, 5782–5803. [Google Scholar]
- 79.Root RA; Fathordoobadi S; Alday F; Ela W; Chorover J Microscale Speciation of Arsenic and Iron in Ferric-Based Sorbents Subjected to Simulated Landfill Conditions. Environ. Sci. Technol 2013, 47, 12992–13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez-Freire L; Moore SE; Sierra-Alvarez R; Root RA; Chorover J; Field JA Arsenic Remediation by Formation of Arsenic Sulfide Minerals in a Continuous Anaerobic Bioreactor. Biotechnol. Bioeng 2016, 113, 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmann D; Krumholz LR; Hemond HF; Lovley DR; Morel FMM Microbial Mobilization of Arsenic from Sediments of the Aberjona Watershed. Environ. Sci. Technol 1997, 31, 2923–2930. [Google Scholar]
- 82.Zobrist J; Dowdle PR; Davis JA; Oremland RS Mobilization of Arsenite by Dissimilatory Reduction of Adsorbed Arsenate. Environ. Sci. Technol 2000, 34, 4747–4753. [Google Scholar]
- 83.Podgorski J; Berg M Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [DOI] [PubMed] [Google Scholar]
- 84.Manning BA; Fendorf SA; Goldberg S Surface Structures and Stability of Arsenic (III) on Goethite: Spectroscopic Evidence for Inner-Sphere Complexes. Environ. Sci. Technol 1998, 32, 2383–2388. [Google Scholar]
- 85.Bose P; Sharma A Role of Iron in Controlling Speciation and Mobilization of Arsenic in Subsurface Environment. Water Res. 2002, 36, 4916–4926. [DOI] [PubMed] [Google Scholar]
- 86.Bradham K; Scheckel K; Nelson C; Seales P; Lee G; Hughes M; Miller B; Yeow A; Gilmore T; Serda S; et al. Relative Bioavailability and Bioaccessibility and Speciation of Arsenic in Contaminated Soils. Environ. Health Perspect 2011, 119, 1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azcue JM; Nriagu JO; Schiff S Role of Sediment Porewater in the Cycling of Arsenic in a Mine-Polluted Lake. Environ. Int 1994, 20, 517–527. [Google Scholar]
- 88.Inskeep WP; Macur RE; Harrison G; Bostick BC; Fendorf S Biomineralization of As(V)-Hydrous Ferric Oxyhydroxide in Microbial Mats of an Acid-Sulfate-Chloride Geothermal Spring, Yellowstone National Park. Geochim. Cosmochim. Acta 2004, 68, 3141–3155. [Google Scholar]
- 89.Cherry JA; Shaikh AU; Tallman DE; Nicholson RV Arsenic Species as an Indicator of Redox Conditions in Groundwater. Devel. Water Sci 1979, 12, 373–392. [Google Scholar]
- 90.Manning BA; Goldberg S Modeling Competitive Adsorption of Arsenate with Phosphate and Molybdate on Oxide Minerals. Soil Sci. Soc. Amer. J 1996, 60, 121–131. [Google Scholar]
- 91.Kneebone PE; Hering JG Behavior of Arsenic and Other Redox-Sensitive Elements in Crowley Lake, CA: A Reservoir in the Los Angeles Aqueduct System. Environ. Sci. Technol 2000, 34, 4307–4312. [Google Scholar]
- 92.Beak DG; Wilkin RT Performance of a Zero valent Iron Reactive Barrier for the Treatment of Arsenic in Groundwater: Part 2. Geochemical Modeling and Solid Phase Studies. J. Contam. Hydrol 2009, 106, 15–28. [DOI] [PubMed] [Google Scholar]
- 93.Dzombak DA; Morel FMM Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: New York, NY, USA, 1990; p. 416. [Google Scholar]
- 94.Newman DK; Kennedy EK; Coates JD; Ahmann D; Ellis DJ; Lovley DR; Morel FMM Dissimilatory Arsenate and Sulfate Reduction in Desulfotomaculum auripigmentum sp. nov. Arch. Microbiol 1997, 168, 380–388. [DOI] [PubMed] [Google Scholar]
- 95.Kocar BD; Fendorf S Thermodynamic Constraints on Reductive Reactions Influencing the Biogeochemistry of Arsenic in Soils and Sediments. Environ. Sci. Technol 2009, 43, 4871–4877. [DOI] [PubMed] [Google Scholar]
- 96.Oremland RS; Stolz JF Arsenic, Microbes and Contaminated Aquifers. Trends Microbiol. 2005, 13, 45–49. [DOI] [PubMed] [Google Scholar]
- 97.Stollenwerk KG; Breit GN; Welch AH; Foster AL Remediation of Arsenic-Contaminated Groundwater by Oxidized Sediments in Bangladesh. Geochim. Cosmochim. Acta 2006, 70 (Suppl. 18), A617. [Google Scholar]
- 98.Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2004, 27 (Suppl. 1), s5–s10. [DOI] [PubMed] [Google Scholar]
- 99.DeFronzo R; Ferrannini E; Groop L; Henry RR; Herman WH; Holst JJ; Hu FB; Kahn CR; Raz I; Shulman GI; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Prim 2015, 1, 15019. [DOI] [PubMed] [Google Scholar]
- 100.Katsarou A; Gudbjörnsdottir S; Rawshani A; Dabelea D; Bonifacio E; Anderson BJ; Jacobsen LM; Schatz DA; Lernmark A Type 1 Diabetes Mellitus. Nat. Rev. Dis. Prim 2017, 3, 17016. [DOI] [PubMed] [Google Scholar]
- 101.Chen YW; Yang CY; Huang CF; Hung DZ; Leung YM; Liu SH Heavy Metals, Islet Function and Diabetes Development. Islets 2009, 1, 169–176. [DOI] [PubMed] [Google Scholar]
- 102.Maull EA; Ahsan H; Edwards J; Longnecker MP; Navas-Acien A; Pi J; Silbergeld EK; Styblo M; Tseng CH; Thayer KA; et al. Evaluation of the Association Between Arsenic and Diabetes: A National Toxicology Program Workshop Review. Environ. Health Perspect 2012, 120, 1658–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Del Razo LM; García-Vargas GG; Valenzuela OL; Castellanos EH; Sánchez-Peña LC; Currier JM; Drobná Z; Loomis D; Stýblo M Exposure to Arsenic in Drinking Water is Associated with Increased Prevalence of Diabetes: A Cross-Sectional Study in the Zimapán and Lagunera Regions in Mexico. Environ. Health 2011, 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lai MS; Hsueh YM; Chen CJ; Shyu MP; Chen SY; Kuo TL; Wu MM; Tai TY Ingested Inorganic Arsenic and Prevalence of Diabetes Mellitus. Am. J. Epidemiol 1994, 139, 484–492. [DOI] [PubMed] [Google Scholar]
- 105.Tsai SM; Wang TN; Ko Y-C Mortality for Certain Diseases in Areas with High Levels of Arsenic in Drinking Water. Arch. Environ. Health 1999, 54, 186–193. [DOI] [PubMed] [Google Scholar]
- 106.Jovanovic D; Rasic-Milutinovic Z; Paunovic K; Jakovljevic B; Plavsic S; Milosevic J Low Levels of Arsenic in Drinking Water and Type 2 Diabetes in Middle Banat Region, Serbia. Int. J. Hyg. Environ. Health 2013, 216, 50–55. [DOI] [PubMed] [Google Scholar]
- 107.Islam R; Khan I; Hassan SN; McEvoy M; D’Este C; Attia J; Peel R; Sultana M; Akter S; Milton AH Association Between Type 2 Diabetes and Chronic Arsenic Exposure in Drinking Water: A Cross Sectional Study in Bangladesh. Environ. Health 2012, 11, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meliker JR; Wahl RL; Cameron LL; Nriagu JO Arsenic in Drinking Water and Cerebrovascular Disease, Diabetes Mellitus, and Kidney Disease in Michigan: A Standardized Mortality Ratio Analysis. Environ. Health 2007, 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balakrishnan P; Navas-Acien A; Haack K; Vaidya D; Umans JG; Best LG; Goessler W; Francesconi KA; Franceschini N; North KE; et al. Arsenic-Gene Interactions and Beta-Cell Function in the Strong Heart Family Study. Toxicol. Appl. Pharmacol 2018, 348, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Domingo-Relloso A; Makhani K; Riffo-Campos AL; Tellez-Plaza M; Klein KO; Subedi P; Zhao J; Moon KA; Bozack AK; Haack K; et al. Arsenic Exposure, Blood DNA Methylation, and Cardiovascular Disease. Circ. Res 2022, 131, e51–e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spratlen MJ; Grau-Perez M; Umans JG; Yracheta J; Best LG; Francesconi K; Goessler W; Balakrishnan P; Cole SA; Gamble MV; et al. Arsenic, one carbon metabolism and diabetes-related outcomes in the Strong Heart Family Study. Environ. Int 2018, 121 Pt 1, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang K; Xun P; Carnethon M; Carson AP; Lu L; Zhu J; He K Low to Moderate Toenail Arsenic Levels in Young Adulthood and Incidence of Diabetes Later in Life: Findings from the CARDIA Trace Element Study. Environ. Res 2019, 171, 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nizam S; Kato M; Yatsuya H; Khalequzzaman M; Ohnuma S; Naito H; Nakajima T Differences in Urinary Arsenic Metabolites Between Diabetic and Non-Diabetic Subjects in Bangladesh. Int. J. Environ. Res. Public Health 2013, 10, 1006–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nabi AH; Rahman MM; Islam LN Evaluation of Biochemical Changes in Chronic Arsenic Poisoning Among Bangladeshi Patients. Int. J. Environ. Res. Public Health 2005, 2, 385–393. [DOI] [PubMed] [Google Scholar]
- 115.Rahman M; Tondel M; Ahmad SA; Axelson O Diabetes Mellitus Associated with Arsenic Exposure in Bangladesh. Amer. J. Epidemiol 1998, 148, 198–203. [DOI] [PubMed] [Google Scholar]
- 116.Rahman M; Tondel M; Ahmad SA; Chowdhury IA; Faruquee MH; Axelson O Hypertension and Arsenic Exposure in Bangladesh. Hypertension 1999, 33, 74–78. [DOI] [PubMed] [Google Scholar]
- 117.Jensen GE; Hansen ML Occupational Arsenic Exposure and Glycosylated Haemoglobin. Analyst 1998, 123, 77–80. [DOI] [PubMed] [Google Scholar]
- 118.Eick SM; Ferreccio C; Acevedo J; Castriota F; Cordero JF; Roh T; Smith AH; Smith MT; Steinmaus C Socioeconomic Status and the Association Between Arsenic Exposure and Type 2 Diabetes. Environ. Res 2019, 172, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X; Gao D; Zhang G; Zhang X; Li Q; Gao Q; Chen R; Xu S; Huang L; Zhang Y; et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ. Int 2020, 135, 105370. [DOI] [PubMed] [Google Scholar]
- 120.Jia X; Zhang L; Zhao J; Ren M; Li Z; Wang J; Wang S; Liu Y; An H; Li Y; et al. Associations between endocrine-disrupting heavy metals in maternal hair and gestational diabetes mellitus: A nested case-control study in China. Environ. Int 2021, 157, 106770. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Y; Chen T; Zhang Y; Hu Q; Wang X; Chang H; Mao JH; Snijders AM; Xia Y Contribution of trace element exposure to gestational diabetes mellitus through disturbing the gut microbiome. Environ. Int 2021, 153, 106520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xia X; Liang C; Sheng J; Yan S; Huang K; Li Z; Pan W; Tao R; Hao J; Zhu B; et al. Association between serum arsenic levels and gestational diabetes mellitus: A population-based birth cohort study. Environ. Poll 2018, 235, 850–856. [DOI] [PubMed] [Google Scholar]
- 123.Lucio M; Barbir R; Vučić Lovrenčić M; Canecki Varžić S; Ljubić S; Smirčić Duvnjak L; Šerić V; Milić M; Tariba Lovaković B; Krivohlavek A; et al. Association Between Arsenic Exposure and Biomarkers of Type 2 Diabetes Mellitus in a Croatian Population: A Comparative Observational Pilot Study. Sci. Total Environ 2020, 720, 137575. [DOI] [PubMed] [Google Scholar]
- 124.Velmurugan G; Swaminathan K; Mohanraj S; Dhivakar M; Veerasekar G; Alexander T; Cherian M; Palaniswami NG; Pradeep T Association of Co-Accumulation of Arsenic and Organophosphate Insecticides with Diabetes and Atherosclerosis in a Rural Agricultural Community: KMCH-NNCD-I study. Acta Diabetol. 2020, 57, 1159–1168. [DOI] [PubMed] [Google Scholar]
- 125.Bartoli D; Battista G; De Santis M; Iaia TE; Orsi D; Tarchi M; Pirastu R; Valiani M Cohort Study of Art Glass Workers in Tuscany, Italy: Mortality from Non-Malignant Diseases. Occup. Med 1998, 48, 441–445. [DOI] [PubMed] [Google Scholar]
- 126.Coronado-González JA; Del Razo LM; García-Vargas G; Sanmiguel-Salazar F; Escobedo-de la Peña J Inorganic Arsenic Exposure and Type 2 Diabetes Mellitus in Mexico. Environ. Res 2007, 104, 383–389. [DOI] [PubMed] [Google Scholar]
- 127.Mendez MA; González-Horta C; Sánchez-Ramírez B; Ballinas-Casarrubias L; Cerón RH; Morales DV; Terrazas FA; Ishida MC; Gutiérrez-Torres DS; Saunders R; et al. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross-Sectional Study in Chihuahua, Mexico. Environ. Health Perspec 2016, 124, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ruiz-Navarro ML; Navarro-Alarcón M; Lopez González-de la Serrana H; Pérez-Valero V; López-Martinez MC Urine Arsenic Concentrations in Healthy Adults as Indicators of Environmental Contamination: Relation with Some Pathologies. Sci. Total Environ 1998, 216, 55–61. [DOI] [PubMed] [Google Scholar]
- 129.Grau-Perez M; Navas-Acien A; Galan-Chilet I; Briongos-Figuero LS; Morchon-Simon D; Bermudez JD; Crainiceanu CM; de Marco G; Rentero-Garrido P; Garcia-Barrera T; et al. Arsenic Exposure, Diabetes-Related Genes and Diabetes Prevalence in a General Population from Spain. Environ. Poll 2018, 235, 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lagerkvist BJ; Zetterlund B Assessment of Exposure to Arsenic Among Smelter Workers: A Five-Year Follow-Up. Am. J. Indus. Med 1994, 25, 477–488. [DOI] [PubMed] [Google Scholar]
- 131.Rahman M; Axelson O Diabetes Mellitus and Arsenic Exposure: A Second Look at Case-Control Data from a Swedish Copper Smelter. Occup. Environ. Med 1995, 52, 773–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rahman M; Wingren G; Axelson O Diabetes Mellitus Among Swedish Art Glass Workers- An Effect of Arsenic Exposure? Scand. J. Work Environ. Health 1996, 22, 146–149. [DOI] [PubMed] [Google Scholar]
- 133.Tseng CH; Tai TY; Chong CK; Tseng CP; Lai MS; Lin BJ; Chiou HY; Hsueh YM; Hsu KH; Chen CJ Long-Term Arsenic Exposure and Incidence of Non-Insulin-Dependent Diabetes Mellitus: A Cohort Study in Arseniasis-Hyperendemic Villages in Taiwan. Environ. Health Perspec 2000, 108, 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang SL; Chiou JM; Chen CJ; Tseng CH; Chou WL; Wang CC; Wu TN; Chang LW Prevalence of Non-Insulin-Dependent Diabetes Mellitus and Related Vascular Diseases in Southwestern Arseniasis-Endemic and Nonendemic Areas in Taiwan. Environ. Health Perspec 2003, 111, 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chiu HF; Chang CC; Tsai SS; Yang CY Does Arsenic Exposure Increase the Risk for Diabetes Mellitus? J. Occup. Environ. Med 2006, 48, 63–67. [DOI] [PubMed] [Google Scholar]
- 136.Chen JW; Wang SL; Wang YH; Sun CW; Huang YL; Chen CJ; Li WF Arsenic Methylation, GSTO1 Polymorphisms, and Metabolic Syndrome in an Arseniasis Endemic Area of Southwestern Taiwan. Chemosphere 2012, 88, 432–438. [DOI] [PubMed] [Google Scholar]
- 137.Ward MI; Pim B Trace Element Concentrations in Blood Plasma from Diabetic Patients and Normal Individuals. Biol. Trace Elem. Res 1984, 6, 469–487. [DOI] [PubMed] [Google Scholar]
- 138.Kuo CC; Howard BV; Umans JG; Gribble MO; Best LG; Francesconi KA; Goessler W; Lee E; Guallar E; Navas-Acien A Arsenic Exposure, Arsenic Metabolism, and Incident Diabetes in the Strong Heart Study. Diabetes Care. 2015, 38, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mabuchi K; Lilienfeld AM; Snell LM Cancer and Occupational Exposure to Arsenic: A Study of Psticide Workers. Prev. Med 1980, 9, 51–77. [DOI] [PubMed] [Google Scholar]
- 140.Lubin JH; Pottern LM; Stone BJ; Fraumeni JF Respiratory Cancer in a Cohort of Copper Smelter Workers: Results from more than 50 Years of Follow-Up. Am. J. Epidem 2000, 151, 554–565. [DOI] [PubMed] [Google Scholar]
- 141.Tollestrup K; Frost FJ; Harter LC; McMillan GP Mortality Among Children Residing near the American Smelting and Refining Company (ASARCO) Copper Smelter in Ruston, Washington. Arch. Environ. Health 2003, 58, 683–691. [DOI] [PubMed] [Google Scholar]
- 142.Lewis DR; Southwick JW; Ouellet-Hellstrom R; Rench J; Calderon RL Drinking water arsenic in Utah: A cohort Mortality Study. Environ. Health Perspec 1999, 107, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zierold KM; Knobeloch L; Anderson H Prevalence of Chronic Diseases in Adults Exposed to Arsenic-Contaminated Drinking Water. Am. J. Public Health 2004, 94, 1936–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Navas-Acien A; Silbergeld EK; Pastor-Barriuso R; Guallar E Arsenic Exposure and Prevalence of Type 2 Diabetes in US Adults. JAMA 2008, 300, 814–822. [DOI] [PubMed] [Google Scholar]
- 145.Gribble MO; Howard BV; Umans JG; Shara NM; Francesconi KA; Goessler W; Crainiceanu CM; Silbergeld EK; Guallar E; Navas-Acien A Arsenic Exposure, Diabetes Prevalence, and Diabetes Control in the Strong Heart Study. Am. J. Epidem 2012, 176, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bulka CM; Persky VW; Daviglus ML; Durazo-Arvizu RA; Argos M Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res 2019, 168, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen WJ; Davis EM; Stoner JA; Robledo C; Goodman JR; Garwe T; Janitz AE; Xu C; Hwang J; Peck JD Urinary total arsenic and arsenic methylation capacity in pregnancy and gestational diabetes mellitus: A case-control study. Chemosphere 2021, 271, 129828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grau-Pérez M; Kuo CC; Spratlen M; Thayer KA; Mendez MA; Hamman RF; Dabelea D; Adgate JL; Knowler WC; Bell RA; et al. The Association of Arsenic Exposure and Metabolism with Type 1 and Type 2 Diabetes in Youth: The SEARCH Case-Control Study. Diabetes Care 2017, 40, 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Macfarlane WM; Smith SB; James RF; Clifton AD; Doza YN; Cohen P; Docherty K The p38/Reactivating Kinase Mitogen-Activated Protein Kinase Cascade Mediates the Activation of the Transcription Factor Insulin Upstream Factor 1 and Insulin Gene Transcription by High Glucose in Pancreatic Beta-Cells. J. Biol. Chem 1997, 272, 20936–20944. [DOI] [PubMed] [Google Scholar]
- 150.Elrick LJ; Docherty K Phosphorylation-Dependent Nucleocytoplasmic Shuttling of Pancreatic Duodenal Homeobox-1. Diabetes 2001, 50, 2244–2252. [DOI] [PubMed] [Google Scholar]
- 151.Salazard B; Bellon L; Jean S; Maraninchi M; El-Yazidi C; Orsière T; Margotat A; Botta A; Bergé-Lefranc JL Low-Level Arsenite Activates the Transcription of Genes Involved in Adipose Differentiation. Cell Biol. Toxicol 2004, 20, 375–385. [DOI] [PubMed] [Google Scholar]
- 152.Wauson EM; Langan AS; Vorce RL Sodium Arsenite Inhibits and Reverses Expression of Adipogenic and Fat Cell-Specific Genes During in vitro Adipogenesis. Toxicol. Sci 2002, 65, 211–219. [DOI] [PubMed] [Google Scholar]
- 153.Walton FS; Harmon AW; Paul DS; Drobna Z; Patel YM; Styblo M Inhibition of Insulin-Dependent Glucose Uptake by Trivalent Arsenicals: Possible Mechanism of Arsenic-Induced Diabetes. Toxicol. Appl. Pharmacol 2004, 198, 424–433. [DOI] [PubMed] [Google Scholar]
- 154.Paul DS; Hernández-Zavala A; Walton FS; Adair BM; Dědina J; Matoušek T; Stýblo M Examination of the Effects of Arsenic on Glucose Homeostasis in Cell culture and Animal Studies: Development of a Mouse Model for Arsenic-Induced Diabetes. Toxicol. Appl. Pharm 2007, 222, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yen YP; Tsai KS; Chen YW; Huang CF; Yang RS; Liu SH Arsenic Inhibits Myogenic Differentiation and Muscle Regeneration. Environ. Health Perspect 2010, 118, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu S; Guo X; Wu B; Yu H; Zhang X; Li M Arsenic Induces Diabetic Effects Through Beta-Cell Dysfunction and Increased Gluconeogenesis in Mice. Sci. Rep 2014, 4, 6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang CF; Yang CY; Chan DC; Wang CC; Huang KH; Wu CC; Tsai KS; Yang RS; Liu SH Arsenic Exposure and Glucose Intolerance/Insulin Resistance in Estrogen-Deficient Female Mice. Environ. Health Perspect 2015, 123, 1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Díaz-Villaseñor A; Cruz L; Cebrián A; Hernández-Ramírez RU; Hiriart M; García-Vargas G; Bassol S; Sordo M; Gandolfi AJ; Klimecki WT; et al. Arsenic Exposure and Calpain-10 Polymorphisms Impair the Function of Pancreatic Beta-Cells in Humans: A Pilot Study of Risk Factors for T2DM. PLoS ONE 2013, 8, e51642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Divya SP; Pratheeshkumar P; Son YO; Roy RV; Hitron JA; Kim D; Dai J; Wang L; Asha P; Huang B; et al. Arsenic Induces Insulin Resistance in Mouse Adipocytes and Myotubes Via Oxidative Stress-Regulated Mitochondrial Sirt3-FOXO3a Signaling Pathway. Toxicological. Sci 2015, 146, 290–300. [DOI] [PubMed] [Google Scholar]
- 160.Sowell MO; Boggs KP; Robinson KA; Dutton SL; Buse MG Effects of Insulin and Phospholipase C in Control and Denervated Rat Skeletal Muscle. Am. J. Physiol 1991, 260 Pt 1, E247–E256. [DOI] [PubMed] [Google Scholar]
- 161.Ambrosio F; Brown E; Stolz D; Ferrari R; Goodpaster B; Deasy B; Distefano G; Roperti A; Cheikhi A; Garciafigueroa Y; et al. Arsenic Induces Sustained Impairment of Skeletal Muscle and Muscle Progenitor Cell Ultrastructure and Bioenergetics. Free Radic. Biol. Med 2014, 74, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McDowell HE; Walker T; Hajduch E; Christie G; Batty IH; Downes CP; Hundal HS Inositol Phospholipid 3-Kinase is Activated by Cellular Stress But is Not Required for rhe Stress-Induced Activation of Glucose Transport in L6 Rat Skeletal Muscle Cells. Eur. J. Biochem 1997, 247, 306–313. [DOI] [PubMed] [Google Scholar]
- 163.Farkhondeh T; Samarghandian S; Azimi-Nezhad M The Role of Arsenic in Obesity and Diabetes. J. Cell. Physiol 2019, 234, 12516–12529. [DOI] [PubMed] [Google Scholar]
- 164.Gong Y; Liu J; Xue Y; Zhuang Z; Qian S; Zhou W; Li X; Qian J; Ding G; Sun Z Non-Monotonic Dose-Response Effects of Arsenic on Glucose Metabolism. Toxicol. Appl. Pharmacol 2019, 377, 114605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Adebayo AO; Zandbergen F; Kozul-Horvath CD; Gruppuso PA; Hamilton JW Chronic exposure to low-dose arsenic modulates lipogenic gene expression in mice. J. Biochem. Mol. Toxicol 2015, 29, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Singh DP; Yadav SK; Patel K; Patel S; Patil GP; Bijalwan V; Singh G; Palkhade R; Kondepudi KK; Boparai RK; et al. Short-Term Trivalent Arsenic and Hexavalent Chromium Exposures Induce Gut Dysbiosis and Transcriptional Alteration in Adipose Tissue of Mice. Mol. Biol. Rep 2023, 50, 1033–1044. [DOI] [PubMed] [Google Scholar]
- 167.Liu P; Dodson M; Li H; Schmidlin CJ; Shakya A; Wei Y; Garcia JGN; Chapman E; Kiela PR; Zhang QY; et al. Non-Canonical NRF2 Activation Promotes a Pro-Diabetic Shift in Hepatic Glucose Metabolism. Mol. Metab 2021, 51, 101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hu Y; Jin X; Snow ET Effect of Arsenic on Transcription Factor Ap-1 and Nf-Kappab DNA Binding Activity and Related Gene Expression. Toxicol. Lett 2002, 133, 33–45. [DOI] [PubMed] [Google Scholar]
- 169.Yang C; Frenkel K Arsenic-Mediated Cellular Signal Transduction, Transcription Factor Activation, and Aberrant Gene Expression: Implications in Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol 2002, 21, 331–342. [PubMed] [Google Scholar]
- 170.Lee YS; Lee EK; Oh HH; Choi CS; Kim S; Jun HS Sodium Meta-Arsenite Ameliorates Hyperglycemia in Obese Diabetic Db/Db Mice by Inhibition of Hepatic Gluconeogenesis. J. Diabetes Res 2014, 2014, 961732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Aposhian HV Biochemical Toxicology of Arsenic. In Reviews in Biochemical Toxicology; Hodgson E, Bend JR, Philpot RM, Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 10, pp. 265–289. [Google Scholar]
- 172.NRC (National Research Council). Arsenic in Drinking Water; National Academy Press: Washington, DC, USA, 1999. [Google Scholar]
- 173.Kennedy EP; Lehninger AL Oxidation of Fatty Acids and Tricarboxylic Acid Cycle Intermediates by Isolated Rat Liver Mitochondria. J. Biol. Chem 1949, 179, 957–972. [PubMed] [Google Scholar]
- 174.Wu MM; Chiou HY; Wang TW; Hsueh YM; Wang IH; Chen CJ; Lee TC Association of Blood Arsenic Levels with Increased Reactive Oxidants and Decreased Antioxidant Capacity in a Human Population of Northeastern Taiwan. Environ. Health Perspec 2001, 109, 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thomas DJ; Styblo M; Lin S The Cellular Metabolism and Systemic Toxicity of Arsenic. Toxicol. Appl. Pharmacol 2001, 176, 127–144. [DOI] [PubMed] [Google Scholar]
- 176.Pan WC; Kile ML; Seow WJ; Lin X; Quamruzzaman Q; Rahman M; Mahiuddin G; Mostofa G; Lu Q; Christiani DC Genetic susceptible locus in NOTCH2 interacts with arsenic in drinking water on risk of type 2 diabetes. PLoS ONE 2013, 8, e70792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Argos M Arsenic Exposure and Epigenetic Alterations: Recent Findings Based on the Illumina 450K DNA Methylation Array. Curr. Environ. Health Rep 2015, 2, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Michailidi C; Hayashi M; Datta S; Sen T; Zenner K; Oladeru O; Brait M; Izumchenko E; Baras A; VandenBussche C; et al. Involvement of Epigenetics and EMT-Related miRNA in Arsenic-Induced Neoplastic Transformation and Their Potential Clinical Use. Cancer Prev. Res 2015, 8, 208–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bailey KA; Wu MC; Ward WO; Smeester L; Rager JE; García-Vargas G; Del Razo LM; Drobná Z; Stýblo M; Fry RC Arsenic and the Epigenome: Interindividual Differences in Arsenic Metabolism Related to Distinct Patterns of DNA Methylation. J. Biochem. Mol. Tox 2013, 27, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rager JE; Bailey KA; Smeester L; Miller SK; Parker JS; Laine JE; Drobná Z; Currier J; Douillet C; Olshan AF; et al. Prenatal Arsenic Exposure and the Epigenome: Altered MicroRNAs Associated with Innate and Adaptive Immune Signaling in Newborn Cord Blood. Environ. Mol. Mutagen 2014, 55, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ren X; Gaile DP; Gong Z; Qiu W; Ge Y; Zhang C; Huang C; Yan H; Olson JR; Kavanagh TJ; et al. Arsenic Responsive Micrornas In Vivo and Their Potential Involvement in Arsenic-Induced Oxidative Stress. Toxicol. Appl. Pharmacol 2015, 283, 198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Abernathy CO; Liu YP; Longfellow D; Aposhian HV; Beck B; Fowler B; Goyer R; Menzer R; Rossman T; Thompson C; et al. Arsenic: Health Effects, Mechanisms of Actions, and Research Issues. Environ. Health Perspec 1999, 107, 593–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.All About Arsenic. 2022. Available online: https://www.allaboutarsenic.org/ (accessed on 25 April 2023).
- 184.Bailey C; Farrell A; Purty T; Taylor A; Disney J Development of Privacy Features on Anecdata.org, a Free Citizen Science Platform for Collecting Datasets for Climate Change and Related Projects. Front. Clim 2021, 3, 620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O’Fallon L; Finn S Citizen Science and Community-Engaged Research in Environmental Public Health. Lab Matters 2015, 4, 5. [Google Scholar]
- 186.Pandya R A Framework for Engaging Diverse Communities in Citizen Science in the US. Front. Ecol. Environ 2012, 10, 314–331. [Google Scholar]
- 187.Cooper CB; Hawn CL; Larson LR; Parrish JK; Bowser G; Cavalier D; Dunn RR; Haklay M; Gupta KK; Jelks NO; et al. Inclusion in Citizen Science: The Conundrum of Rebranding. Science 2021, 372, 1386–1388. [Google Scholar]
- 188.Davis LF; Ramírez-Andreotta MD Participatory Research for Environmental Justice: A Critical Interpretive Synthesis. Environ. Health Perspec 2021, 129, 026001-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wallerstein N; Duran B; Oetzel JG; Minkler M Community-Based Participatory Research for Health: Advancing Social and Health Equity; John Wiley & Sons: San Francisco, CA, USA, 2017. [Google Scholar]
- 190.Ramirez-Andreotta M; Brusseau M; Artiola J; Maier R; Gandolfi A Building a Co-Created Citizen Science Program with Gardeners Neighboring a Superfund Site: The Gardenroots Case Study. Int. Public Health J 2015, 7, 139. [PMC free article] [PubMed] [Google Scholar]
- 191.Ramirez-Andreotta MD; Lothrop N; Wilkinson ST; Root RA; Artiola JF; Klimecki W; Loh M Analyzing Patterns of Community Interest at a Legacy Mining Waste Site to Assess and Inform Environmental Health Literacy Efforts. J. Environ. Stud. Sci 2016, 6, 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sandhaus S; Kaufmann D; Ramirez-Andreotta MD Public Participation, Trust and Data Sharing: Gardens as Hubs for Citizen Science and Environmental Health Literacy Efforts. Int. J. Sci. Edu. Part B 2019, 9, 54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zeider K; Van Overmeiren N; Rine KP; Sandhaus S; Sáez EA; Sorooshian A; Muñoz HCS; Ramírez-Andreotta MD Foliar Surfaces as Dust and Aerosol Pollution Monitors: An Assessment by a Mining Site. Sci. Total Environ 2021, 790, 148164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sorg TJ; Chen ASC; Wang L Arsenic Species in Drinking Water Wells in the USA with High Arsenic Concentrations. Water Res. 2014, 48, 156–169. [DOI] [PubMed] [Google Scholar]
- 195.USEPA. Arsenic Mitigation Strategies. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/train5-mitigation.pdf (accessed on 25 April 2023).
- 196.Sorg T; Wang L; Chen A The Costs of Small Drinking Water Systems Removing Arsenic from Groundwater. J. Water Supply Res. Technol.-Aqua 2015, 64, 219–234. [Google Scholar]
- 197.Nicomel NR; Leus K; Folens K; Van Der Voort P; Laing GD Technologies for Arsenic Removal from Water: Curren Status and Future Perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fields KA; Chen A; Wang L Arsenic Removal from Drinking Water by Coagulation/Filtration and Lime Softening Plants. EPA/600/R-00/063. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NRMRL&dirEntryId=63171 (accessed on 25 April 2023).
- 199.Singh AK Approaches for Removal of Arsenic from Groundwater of Northeastern India. Curr. Sci 2007, 92, 1506–1515. [Google Scholar]
- 200.Artiola JF; Wilkinson ST How to Lower the Levels of Arsenic in Well Water: What Choices do Arizona Consumers Have? Available online: https://extension.arizona.edu/pubs/how-lower-levels-arsenic-well-water-what-choices-do-arizona-consumer-shave (accessed on 25 April 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.


