Abstract
The purpose of this study was to determine if a relationship exists among total serum and lipoprotein cholesterol concentration, the severity of amphotericin B (AmpB)-induced renal toxicity, and the serum pharmacokinetics of AmpB in hypercholesterolemic rabbits administered AmpB and AmpB lipid complex (ABLC). After 10 days of cholesterol-enriched diet (0.50% [wt/vol]) or regular rabbit diet (control), each rabbit was administered a single intravenous bolus of AmpB or ABLC (1.0 mg/kg of body weight). Blood samples were obtained before administration and serially thereafter for the assessment of serum pharmacokinetics, kidney toxicity, and serum lipoprotein distribution. Rabbits were humanely sacrificed after all blood samples were obtained, and tissues were harvested for drug analysis. Before drug treatment, cholesterol-fed rabbits demonstrated marked increases in total serum cholesterol and low-density lipoprotein (LDL) cholesterol levels compared with levels in rabbits on a regular diet. No significant differences in triglyceride levels were observed. A significant increase in serum creatinine levels was observed in cholesterol-fed and regular diet-fed rabbits administered AmpB. However, the magnitude of this increase was 2.5-fold greater in cholesterol-fed rabbits than in regular diet-fed rabbits. No significant differences in triglyceride levels were observed. A significant increase in serum creatinine levels was observed in cholesterol-fed and regular diet-fed rabbits administered ABLC. Whereas AmpB pharmacokinetics were significantly altered in cholesterol-fed rabbits administered free AmpB, similar AmpB pharmacokinetics were observed in both rabbit groups administered ABLC. Renal AmpB levels were significantly increased in cholesterol-fed rabbits administered AmpB compared with those in all other groups. Hepatic and lung AmpB levels were elevated in cholesterol-fed rabbits administered free AmpB compared to controls. In addition, hepatic, lung, and spleen AmpB levels were significantly decreased in cholesterol-fed rabbits administered ABLC compared to controls. An increased percentage of AmpB was recovered in LDL–very-low-density lipoprotein fraction when free AmpB was administered to cholesterol-fed rabbits compared with those in all other groups. These findings suggest that increases in cholesterol, specifically, LDL cholesterol levels, modify the disposition and renal toxicity of free AmpB. However, the pharmacokinetics and renal toxicity of ABLC were independent of elevations in total and LDL cholesterol levels.
Disseminated fungal infections such as candidiasis, histoplasmosis, and aspergillosis are on the rise (22). This increase is due in part to improved recognition and diagnosis of fungal infections but also due to the prolonged survival of patients with defects in their host defense mechanisms, including patients with cancer, organ transplant recipients, diabetics, and patients with AIDS (3, 22). In these patients, invasive fungal infections may account for as many as 30% of deaths (3). Despite the development of a number of new antifungal agents (9), amphotericin B (AmpB) formulated as a suspension remains one of the most effective agents in the treatment of systemic fungal infections (16). However, AmpB use is often limited by the development of kidney toxicity manifested by renal vasoconstriction with a significant decrease in glomerular filtration rate and renal plasma flow and by renal potassium and magnesium wasting (8).
Incorporation of many drugs, including chemotherapeutic and antifungal agents, into liposomes minimizes toxicity without loss in pharmacological effect (1, 14, 20, 25). In addition, when AmpB was complexed with lipid to form AmpB lipid complex (ABLC), it was selectively taken up by mononuclear phagocytes and delivered principally to the liver and the lung (15, 24). Survival of mice infected with Histoplasma capsulatum was greater with ABLC than with AmpB treatment, in part due to higher concentrations of AmpB in liver and lung tissue (24). Moreover, these animals were less toxic than infected mice administered equivalent amounts of AmpB. Recent studies by Bhamra et al. have suggested that the very low levels of circulating protein-bound AmpB that they observed after administration of ABLC to rats were a result of rapid tissue uptake leading to reduced toxicity (2).
There is growing evidence suggesting that elevations in serum low-density lipoprotein (LDL) cholesterol levels are associated with increases in AmpB-induced kidney toxicity (28–30) while increases in serum triglycerides are associated with a reduction in AmpB-induced kidney toxicity (5). Our preliminary studies have further suggested that this phenomenon might be due to the presence of high-affinity LDL receptors on kidney cells, which initiate the uptake of AmpB-LDL complex (28). Furthermore, we suggest that changes in lipoprotein lipid profile (cholesterol and triglycerides) (7, 10, 12, 26) that occur in patients with abnormal serum lipid levels (i.e., cancer, diabetic, and AIDS patients) alter the distribution of AmpB. To date, most studies which have investigated the importance of AmpB binding with serum lipids and lipoproteins, particularly serum LDL, in modifying AmpB-induced kidney toxicity have been conducted mainly with rats (5, 31, 32). However, the behavior of lipoproteins in rats is very different from that in other species (i.e., rabbits and humans). High-density lipoproteins (HDLs) are the major carrier of cholesterol in rats, while LDL is the major carrier of cholesterol in rabbits and humans (6). Furthermore, the activity of lipid transfer protein I (LTP I), a protein responsible for the transfer of serum lipid among different lipoprotein subfractions (17) and of AmpB from HDL to LDL (33), which is measurable in humans, is minimal in rats (11).
Furthermore, it has been suggested that AmpB’s pharmacokinetics may be a result of the slow release of AmpB from a tissue or organ site because of AmpB’s affinity to bind to cholesterol (5, 31) in serum lipoproteins or cell membranes. In addition, differences in the pharmacokinetics and tissue distributions of AmpB but not those of ABLC have been demonstrated between healthy rats and rats with diabetes-induced hyperlipidemia, suggesting independence of the liposomal delivery mechanism from the diabetic disease state and endogenous triglyceride and cholesterol levels (31).
The purpose of this study was to determine if a relationship exists among total serum and lipoprotein cholesterol concentration, the severity of AmpB-induced kidney toxicity, and the serum pharmacokinetics of AmpB in hypercholesterolemic rabbits administered AmpB and ABLC. It was our working hypothesis that an elevation in serum LDL cholesterol concentration increases the binding of AmpB with serum LDL, resulting in increased kidney toxicity. However, increases in serum LDL cholesterol concentration would not modify the kidney toxicity profile of ABLC.
MATERIALS AND METHODS
AmpB and ABLC formulations.
AmpB, which contains sodium deoxycholate (Fungizone) and is reconstituted in sterile water, was purchased from Bristol-Myers Squibb (Newark, N.J.). The method of preparing multilamellar liposomes containing AmpB (ABLC; Abelcet; The Liposome Company, Princeton, N.J.) has been described previously (2, 28). These liposomes use nontoxic phospholipids, dimyristoyl phosphatidylcholine and dimyristoyl phosphatidylglycerol, and are reconstituted in normal saline.
Cholesterol-fed rabbit model.
All rabbits used for this study were cared for in accordance with the principles promulgated by the Canadian Council on Animal Care and the University of British Columbia. They were housed within individual metabolism cages in a 12-h-dark-light-cycle animal facility with controlled temperature and humidity. Water and food (Purina Rabbit Chow 5001) were unrestricted throughout the study. All the rabbits were allowed 3 days to acclimate to their environment prior to experimentation. New Zealand White female rabbits (3.0 to 4.0 kg; Jeo-Bet Rabbits Ltd., Aldon, British Columbia, Canada) that exhibit hypercholesterolemia (induced by a cholesterol-enriched diet) were used (Table 1). The cholesterol-fed rabbits received Purina rabbit chow supplemented with 2.5% (wt/vol) coconut oil and 0.50% (wt/vol) cholesterol for 10 days prior to the experiment. This was an “ideal model” because no kidney or liver function and hematological profile abnormalities were observed in the cholesterol-fed and age-matched New Zealand White rabbits, and 3-ml blood samples were obtained without significant changes in blood flow (18, 19). Furthermore, the rabbit was the appropriate experimental animal to use in these studies because the behavior and structure of its lipoproteins are similar to those of humans (6). The operative technique for chronic catheter insertion was modified from that of Walsh and coworkers to include a heparin lock device (Harvard Apparatus Canada, Saint-Laurent, Quebec, Canada) (27). Briefly, a 2-cm incision was made in the right anterolateral cervical region about 3 cm posterior to the angle of the jaw to expose the external jugular vein. A segment of the vein was freed from subcutaneous fat just below the bifurcation of the internal and external maxillary veins. A catheter was then flushed with sterile saline and inserted carefully through an incision in the external jugular venous wall until the catheter cuff was continuous with the vein wall. Two silk suture ties were used to ligate the silastic catheter to the external jugular vein. After two-way flow was confirmed, the catheter was flushed with 1 ml of heparin (1,000 U/ml). Rabbits were then brought to the recovery room for postoperative observation.
TABLE 1.
Biochemical characteristics of serum and pharmacokinetic parameters of drug after a single intravenous dose of AmpB and ABLC (1 mg/kg) in control and cholesterol-fed (0.05% [wt/vol] cholesterol) rabbitsa
| Exptl group and drug | Biochemical characteristic
|
Pharmacokinetic parameter
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum creatinine (μmol/liter)
|
Total serum cholesterol with regard to drug administration (mg/dl)
|
Serum LDL cholesterol with regard to drug administration (mg/dl)
|
AUC (μg · h/ml) | t1/2α (h) | t1/2β (h) | MRT (h) | VSS (ml/kg) | CL (ml/h/kg) | |||||
| With regard to drug administration (μmol/liter)
|
% Change from baseline | ||||||||||||
| Before | 10 h after | Before | 10 h after | Before | 10 h after | ||||||||
| Control | |||||||||||||
| AmpB | 59 ± 12 | 92 ± 4c | +55.9 | 71 ± 35 | 66 ± 17 | 42 ± 7 | 37 ± 9 | 11.3 ± 2.5 | 0.2 ± 0.1 | 10.8 ± 3.5 | 14.1 ± 4.8 | 1,252 ± 187 | 88.8 ± 19.4 |
| ABLC | 56 ± 10 | 58 ± 6d | +3.6 | 52 ± 9 | 51 ± 8 | 40 ± 9 | 34 ± 7 | 2.1 ± 0.3d | 0.08 ± 0.04 | 0.7 ± 1.5 | 11.8 ± 2.3 | 5,542 ± 706d | 471.6 ± 58.6d |
| Cholesterol fed | |||||||||||||
| AmpB | 45 ± 17 | 112 ± 13bc | +148.9 | 528 ± 124b | 475 ± 88b | 330 ± 100b | 240 ± 67b | 24.7 ± 2.8b | 0.7 ± 0.1b | 12.0 ± 2.3 | 15.9 ± 3.0 | 643 ± 58b | 40.4 ± 4.5b |
| ABLC | 66 ± 19 | 84 ± 17b | +27.2 | 503 ± 58b | 389 ± 101b | 300 ± 68b | 210 ± 50b | 1.5 ± 0.3d | 0.3 ± 0.1bd | 6.7 ± 2.1d | 8.6 ± 2.7d | 5,900 ± 951d | 685 ± 118.1d |
Data are expressed as means ± standard deviations (n = 5). t1/2α and t1/2β, α and β half-lives, respectively. Note that serum creatinine is a measure of kidney toxicity; increases in serum creatinine concentration suggest elevation in kidney toxicity.
P < 0.05 versus control rabbits.
P < 0.05 versus value before drug administration.
P < 0.05 versus AmpB treatment.
Serum lipoprotein separation.
The strategy for separating serum into lipoprotein (HDL, LDL, very-low-density lipoprotein [VLDL] and lipoprotein-deficient [LPD]) fractions was step gradient ultracentrifugation (33). Rabbit serum samples (3.0 ml) from the 0.25-h blood collection were placed into centrifuge tubes, and their solvent densities were adjusted to 1.25 g/ml by the addition of solid sodium bromide (0.34 g/ml of serum). Once the sodium bromide had dissolved into the serum, 2.8 ml of the highest-density sodium bromide solution (density of 1.21 g/ml, which represents the HDL fraction) was layered on top of the serum solution. Then, 2.8 ml of the second sodium bromide solution (density of 1.063 g/ml, which represents the LDL fraction) was layered on top of the sample, followed by 2.8 ml of the third sodium bromide solution (density of 1.006 g/ml, which represents the VLDL and chylomicron fraction). Upon completion of layering with the sodium bromide density solutions, four distinct regions of progressively greater densities (from the top to the bottom of the tube) were observed. All sodium bromide solutions were kept at 4°C prior to the layering of the density gradient. The centrifuge tubes were placed in an SW-41 Ti swinging bucket rotor (Beckman Canada) and centrifuged at 40,000 rpm (288,000 × g; k factor = 128), at a temperature of 15°C for 18 h (L8-80 M; Beckman Canada). Following ultracentrifugation, each density layer was removed by using a Pasteur pipette and the volume of each lipoprotein fraction was measured.
To ensure that the lipoprotein distribution of AmpB was a result of its association with each lipoprotein and not a result of the density of the formulation, the distribution of AmpB formulation reconstituted in sterile water (Fungizone) and ABLC reconstituted in normal saline within LPD serum was determined. The majority of AmpB (>90%) was found in the density range of >1.21 g/ml, suggesting that the AmpB distribution within the ultracentrifuge tubes following incubation in rabbit serum is not a function of formulation density (data not shown).
Characterization of lipoproteins.
Lipoprotein preparations were characterized with respect to lipid and protein composition. Cholesterol (esterified and unesterified), triglyceride, and protein were quantitated by established colorimetric and fluorometric techniques as previously described (31, 33).
Measurement of AmpB.
AmpB levels in serum, tissue, and lipoprotein fractions were analyzed by high-pressure liquid chromatography as previously described (31, 33).
Assessment of renal function.
To assess renal function, serum creatinine concentrations prior to and 10 h following the administration of AmpB or ABLC were measured by standard enzymatic reactions (Sigma Chemical, St. Louis, Mo.). For the purposes of this study and based on our preliminary studies with rats (31) and humans (29), the criterion for measurable kidney toxicity was set as a 50% increase in serum creatinine concentration from baseline. Ten hours was chosen because initial studies demonstrated that, following the administration of a single intravenous bolus of AmpB (1 mg/kg of body weight) to rabbits, serum creatinine reached its maximum elevation from baseline 10 h following the dose (data not shown).
Experimental design.
Cholesterol-fed (n = 10) or normolipidemic (n = 10) female New Zealand White rabbits (3 to 4 kg) were administered a single intravenous dose through the jugular vein of either AmpB or ABLC (1 mg/kg). Preliminary studies have shown that an AmpB dose of 1 mg/kg is sufficient to treat experimental candidiasis and yet exhibit measurable kidney toxicity (31–33). In addition, four cholesterol-fed and normolipidemic rabbits were administered the vehicle controls sterile water and normal saline. Following AmpB or ABLC administration, serial blood samples were obtained and stored in centrifuge tubes prior to and 0.25, 0.5, 1, 2, 4, 8, 12, 24, and 48 h after the injection. Serum was harvested and stored at 4°C prior to analysis to prevent any redistribution of drug. Preliminary studies have shown that AmpB is not redistributed between lipoprotein fractions at 4°C (33). After the 48-h sample, each rabbit was humanely sacrificed and the liver, right kidney, lung, spleen, and heart were removed, dried, and weighed. Each organ was stored at −20°C until analysis.
Pharmacokinetic analysis.
The pharmacokinetic parameters mean residence time (MRT), total body clearance (CL), and volume of distribution at steady state (VSS) were estimated by compartmental analysis using the WINNONLIN nonlinear estimation program (23). It was concluded that the AmpB serum concentration data fit a two-compartment model based on goodness of fit and residual sum of square estimations using the WINNONLIN program. Concentrations of AmpB in serum were plotted against time on log-linear graph paper and α and terminal half-lives were estimated by the method of residuals (23). Area under the AmpB concentration-time curve (AUC) was estimated by trapedzoidal rule (23).
Statistical analysis.
AmpB pharmacokinetics, tissue concentration, lipoprotein distribution, serum creatinine concentration, and lipid levels were compared between drug treatment and animal groups by analysis of variance (PCANOVA; Human Systems Dynamics). Critical differences were assessed by Tukey post hoc tests. A difference was considered significant if the probability of chance explaining the results was reduced to less than 5% (P < 0.05). All data was expressed as means ± standard deviations.
RESULTS
Mean weight of cholesterol-fed rabbits was not significantly different from that of regular diet-fed rabbits prior to drug administration (3.77 ± 0.35 versus 3.21 ± 0.24 kg). Similarly, kidney, liver, lung, spleen, and heart weights were not different between cholesterol-fed and regular diet-fed rabbits (data not shown).
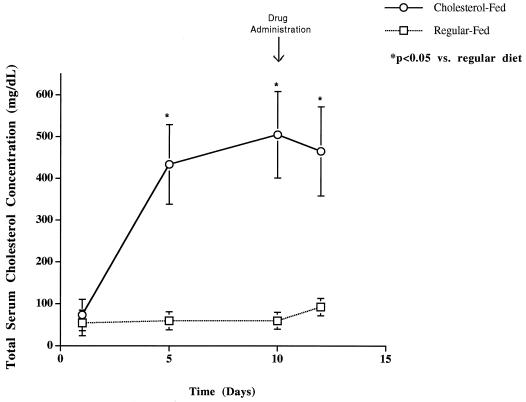
Total and LDL serum cholesterol concentrations were significantly higher in cholesterol-fed than in regular diet-fed rabbits prior to and 10 h following drug administration (Table 1). However, serum creatinine levels were not significantly different between cholesterol-fed and regular diet-fed rabbits prior to drug administration (Table 1). Significant increases in percentages of baseline serum creatinine levels were observed in cholesterol-fed and regular diet-fed rabbits administered AmpB (Table 1); no significant differences from baseline were found in cholesterol-fed or regular diet-fed rabbits administered ABLC (Table 1). Increases in total serum and LDL cholesterol levels were observed in rabbits receiving a cholesterol-enriched diet (0.5% [wt/vol]) for 10 days compared to rabbits receiving a regular diet (Fig. 1 and Table 1). However, no differences in total serum or lipoprotein triglyceride levels were observed (data not shown).
FIG. 1.
Total serum cholesterol concentration in rabbits fed a cholesterol-enriched diet (0.5% [wt/vol]) and a regular diet for 12 days. Data are shown as means ± standard deviations (n = 10). ∗, P < 0.05 versus regular diet-fed rabbits.
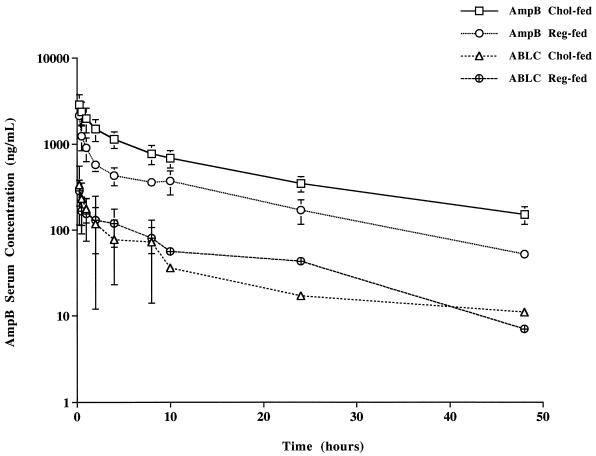
AUC after a single intravenous dose of AmpB in cholesterol-fed rabbits was significantly higher than the AUC in regular diet-fed rabbits, whereas no significant differences were observed in AUC in rabbits administered ABLC (Table 1 and Fig. 2). The α half-life was prolonged in cholesterol-fed rabbits administered AmpB and ABLC compared to that for regular diet-fed rabbits administered AmpB and ABLC, respectively (Table 1). VSS was lower in cholesterol-fed rabbits than in regular diet-fed rabbits administered AmpB (Table 1). However, VSS was greater in rabbits administered ABLC than in rabbits administered AmpB (Table 1). Systemic CL was decreased in cholesterol-fed rabbits compared to regular diet-fed rabbits administered AmpB (Table 1). However, CL was elevated in rabbits administered ABLC compared to rabbits administered AmpB (Table 1). The α and β half-lives and MRT were shorter in cholesterol-fed rabbits administered ABLC than in cholesterol-fed rabbits administered AmpB (Table 1). In addition, AmpB AUC was significantly lower while VSS and CL were significantly greater in cholesterol-fed rabbits administered ABLC than in cholesterol-fed rabbits administered AmpB (Table 1).
FIG. 2.
AmpB serum concentration-versus-time curve on a log-linear graph following a single intravenous dose of AmpB or ABLC (1 mg/kg) to cholesterol-fed and regular diet-fed rabbits. Data are shown as means ± standard deviations (n = 5).
Kidney tissue concentrations of AmpB were greater in cholesterol-fed rabbits administered AmpB than in other groups (Table 2). Likewise, liver and lung concentrations of AmpB were greater in cholesterol-fed than in regular diet-fed rabbits administered AmpB (Table 2). In agreement with our previous studies with diabetic rats (22), lung AmpB concentrations 48 h after a single intravenous administration of AmpB were markedly lower than those after ABLC administration in animals fed a regular diet (Table 2). Both the liver and lung had significantly lower concentrations of AmpB in cholesterol-fed rabbits than in regular diet-fed rabbits following the administration of ABLC (Table 2). Spleen AmpB concentrations were significantly lower in cholesterol-fed rabbits than in regular diet-fed rabbits administered ABLC (Table 2). Heart AmpB concentrations were significantly greater in cholesterol-fed rabbits than in regular diet-fed rabbits administered AmpB (Table 2). In regular diet-fed rabbits, AmpB liver, lung, and spleen concentrations were significantly greater in rabbits administered ABLC than in those administered AmpB (Table 2). However, in cholesterol-fed rabbits AmpB kidney, liver, and heart concentrations were significantly lower in rabbits administered ABLC than in those administered AmpB (Table 2).
TABLE 2.
AmpB tissue distribution following a single intravenous dose of free AmpB and ABLC (1 mg/kg) in control and cholesterol-fed (0.5% [wt/vol] cholesterol) rabbitsa
| Tissue | μg of AmpB/g of tissue for rabbit group administered drug
|
|||
|---|---|---|---|---|
| Control
|
Cholesterol
|
|||
| AmpB | ABLC | AmpB | ABLC | |
| Kidney | 1.07 ± 0.17* | 0.87 ± 0.14 | 1.49 ± 0.20*b | 0.90 ± 0.17c |
| Liver | 2.54 ± 0.54 | 4.81 ± 0.86c | 4.41 ± 0.81b | 2.74 ± 0.51bc |
| Lung | 0.88 ± 0.10** | 2.72 ± 1.19c | 1.59 ± 0.49*b | 1.06 ± 0.26**b |
| Spleen | 3.50 ± 1.65 | 6.85 ± 1.73*c | 2.26 ± 2.01 | 3.57 ± 0.58b |
| Heart | 0.07 ± 0.06 | 0.05 ± 0.06 | 0.44 ± 0.09b | 0.10 ± 0.06c |
Data are means ± standard deviations (n = 5 for all except those designated by ∗ [n = 4] and ∗∗ [n = 3]). Rabbits in the control group were fed a regular diet.
P < 0.05 versus control rabbits.
P < 0.05 versus free AmpB.
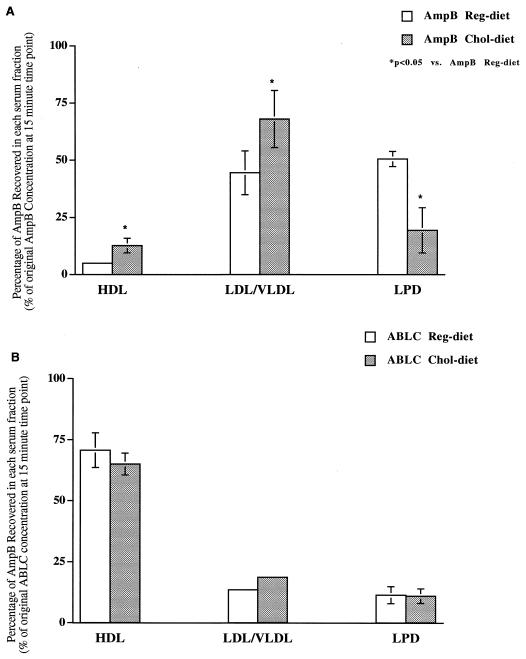
The in vivo serum distribution of AmpB was determined at 15 min following the administration of AmpB and ABLC. A greater percentage of AmpB was recovered in the HDL and LDL-VLDL fractions following the administration of AmpB to cholesterol-fed rabbits than that for regular diet-fed rabbits (Fig. 3A). However, a lower percentage of AmpB was recovered in the LPD serum fraction (which contains albumin and α-1-glycoprotein) following administration to cholesterol-fed rabbits than after that to regular diet-fed rabbits (Fig. 3A). No differences in serum distribution were observed following the administration of ABLC to cholesterol-fed and regular diet-fed rabbits (Fig. 3B).
FIG. 3.
In vivo serum distribution at 15 min following the administration of AmpB (A) or ABLC (B) to cholesterol-fed or regular diet-fed rabbits. Data shown are means ± standard deviations (n = 5). ∗, P < 0.05 versus AmpB or ABLC regular diet. HDL, high-density lipoproteins; LDL/VLDL, low- and very low-density lipoproteins; LPD, lipoprotein-deficient serum, which includes albumin and α-1-glycoprotein.
DISCUSSION
The administration of AmpB has been limited by its dose-dependent kidney toxicity, which has not been predictable by monitoring serum drug concentration (22). To date, it has been assumed that the serum drug concentration is directly related to concentration at the site of action. Error in this assumption may be due to underlying or changing disease states or altered drug protein binding parameters. Since AmpB is an example of a drug that binds to lipoproteins both in vivo and in vitro (33), we studied the influence of experimentally induced hypercholesterolemia on drug disposition and toxicity in rabbits.
There were considerable differences in the disposition, lipoprotein distribution, and tissue distribution of AmpB following the administration of free AmpB to hypercholesterolemic rabbits compared to their normolipidemic counterparts. AUC was elevated in hypercholesterolemic rabbits. This result could be explained by the fact that the systemic clearance of free AmpB was significantly lower in hypercholesterolemic rabbits. Furthermore, the volume of distribution of free AmpB was significantly lower in hypercholesterolemic than in normolipidemic rabbits, suggesting binding differences accounting for changes in disposition.
Since AmpB is significantly bound to lipoproteins, we expected a greater AUC with a reduction in clearance in the presence of hypercholesterolemia. We hypothesize that this may be due to the drug’s preferential association with LDLs, which are increased in hypercholesterolemia. Consistent with this hypothesis, we observed a greater percentage of AmpB recovered in the VLDL-LDL fraction when the drug was administered to hypercholesterolemic rabbits than when it was administered to normolipidemic rabbits (Fig. 3A). These findings suggest that VLDL-LDL may be an important mediator of drug disposition.
In addition, we hypothesize that AmpB’s associations with lipoproteins have a major impact on the safety of this drug since AmpB is often administered to patients with abnormal serum cholesterol and triglyceride metabolism (7, 10, 12, 26). There is growing evidence that supports our hypothesis that increases in cholesterol concentrations increase the renal toxicity of AmpB, while an elevation in serum triglyceride levels decreases AmpB-induced renal toxicity. Specifically, when AmpB was administered to patients with leukemia (13) and immunocompromised patients who exhibited lower plasma cholesterol concentrations (<100 mg/dl) (21), AmpB-induced renal toxicity was decreased. Chabot and coworkers observed no measurable renal toxicity when AmpB was administered to cancer patients who exhibited hypocholesterolemia (4). Our preliminary findings with humans suggest that patients with higher serum LDL cholesterol levels and in turn a greater binding of AmpB with serum LDL are more susceptible to AmpB-induced kidney toxicity (29). In this study, increased AUC of AmpB in hypercholesterolemic rabbits administered free AmpB was associated with increased renal toxicity. Similarly, AmpB levels in renal tissue of these rabbits were greater than those found in normolipidemic rabbits. In contrast, renal toxicity was not observed in either rabbit group administered ABLC, which is supported by similar levels of AmpB being found in renal tissue (Table 2). Taken together with the lipoprotein distribution data, it appears that the increased association of AmpB with lipoproteins in hypercholesterolemia (Fig. 3A) magnifies AmpB-induced renal toxicity.
The pharmacokinetics and tissue distributions of ABLC were not markedly altered in the presence of hypercholesterolemia. Whereas the transport of free AmpB was influenced by LDL cholesterol concentrations, preferential uptake of ABLC into the reticuloendothelial system is most likely independent of LDL cholesterol levels. Furthermore, we have observed that a greater percentage of AmpB associated with serum HDL when ABLC was administered to these animals. An increase in LDL cholesterol levels did not alter this distribution (Fig. 3B). In contrast to observations with free AmpB administration, no change in renal toxicity was found with ABLC dosing. This data is consistent with our previous work with rats (31) and with others demonstrating a nephroprotective effect of AmpB delivered in a lipid complex (13, 24).
Bhamra and coworkers have observed similar concentration-time curves of AmpB and ABLC following administration to rats (2) as we did following administration to rabbits (Fig. 2). They further reported that when rat plasma was spiked with free AmpB and incubated for 3 h at 37°C most of the drug was associated with the VLDL and LPD plasma fractions. In addition, the distribution of released AmpB from ABLC resulted in a greater association with the HDL fraction and less association with the VLDL fraction immediately after spiking. More than 50% of AmpB from samples spiked with ABLC or AmpB was associated with the LPD plasma fraction. These findings are in disagreement with our results (Fig. 3). The differences could be attributed to two factors: (i) their lipoprotein distribution was determined in vitro while our lipoprotein distribution was determined in vivo and (ii) their studies were completed in rat plasma while our studies were completed in rabbit serum. Rabbits are the appropriate experimental animals to use when determining lipoprotein distribution because the behavior and structure of rabbit lipoproteins (6) and LTP I function (11) are similar to those for humans. However, the behavior of lipoproteins in rats is very different from that in humans. HDLs are the major carrier of cholesterol in rats while LDL is the major carrier of cholesterol in rabbits and humans (6). Furthermore, the activity of a lipid transfer protein (LTP I), a protein responsible for the transfer of serum lipid among different lipoprotein subfractions (17) and of AmpB from HDL to LDL (33), while measurable in rabbits and humans, is minimal in rats (11).
In conclusion, we have demonstrated significant differences in the pharmacokinetics, serum lipoprotein and tissue distributions, and drug-induced renal toxicities of free AmpB in hypercholesterolemic rabbits. However, the pharmacokinetics, lipoprotein distributions, and extents of AmpB-induced renal toxicity following ABLC administration were unchanged in the hypercholesterolemic model, suggesting an independence of this delivery mechanism from serum lipoprotein cholesterol levels.
ACKNOWLEDGMENTS
This study was supported with funding from the Medical Research Council of Canada (grants MA-14484 and MT-14484 to K.M.W. and P.H.P.).
We thank Michael Boyd from the Acute Care Animal Unit at the University of British Columbia for his surgical assistance.
REFERENCES
- 1.Balazsovits J A, Mayer L D, Bally M B, Cullis P R, McDonnell M, Ginsberg R S, Falk R E. Analysis of the effect of liposomal encapsulation on the vesicant properties, acute and cardiac toxicities, and antitumor efficacy of doxorubicin. Cancer Chemother Pharmacol. 1989;23:81–86. doi: 10.1007/BF00273522. [DOI] [PubMed] [Google Scholar]
- 2.Bhamra R, Sa’ad A, Bolcsak L E, Janoff A S, Swenson C E. Behavior of amphotericin B lipid complex in plasma in vitro and in the circulation in rats. Antimicrob Agents Chemother. 1997;41:886–892. doi: 10.1128/aac.41.5.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodey G P. Infection in cancer patients: a continuing association. Am J Med. 1986;81:11–26. doi: 10.1016/0002-9343(86)90510-3. [DOI] [PubMed] [Google Scholar]
- 4.Chabot G G, Pazdur R, Valeriote F A, Baker L H H. Pharmacokinetics and toxicity of continuous infusion of amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 5.Chavanet P, Joly V, Rigaud D, Bolard J, Carbon C, Yenni P. Influence of diet on experimental toxicity of amphotericin B deoxycholate. Antimicrob Agents Chemother. 1994;38:963–968. doi: 10.1128/aac.38.5.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis R A, Vance J E. Structure, assembly and secretion of lipoproteins. In: Vance D E, Vance J E, editors. Biochemistry of lipids, lipoproteins and membranes. New York, N.Y: Elsevier; 1996. pp. 473–493. [Google Scholar]
- 7.Feingold K R, Krauss R M, Pang M, Doerrler W, Jensen P, Grunfeld C. The hypertriglyceridemia of acquired immunodeficiency syndrome is associated with an increased prevalence of low-density lipoprotein subclass pattern. J Clin Endocrinol Metab. 1993;76:1423–1431. doi: 10.1210/jcem.76.6.8501146. [DOI] [PubMed] [Google Scholar]
- 8.Gardier A M, Mathe D, Guedeney X, Barre J, Benvenutti C, Navarro N, Vernillet L, Loisance D, Cachera J P, Jacotot B, Tillement J P. Effects of plasma lipid levels on blood distribution and pharmacokinetics of cyclosporin A. Ther Drug Monit. 1993;15:274–280. doi: 10.1097/00007691-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gates C, Pinney R J. Amphotericin B and its delivery by liposomal and lipid formulations. J Clin Pharm Ther. 1993;18:147–153. doi: 10.1111/j.1365-2710.1993.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 10.Grunfeld C, Pang M, Doerrler W, Shigenaga J K, Jensen P, Feingold K R. Lipids, lipoproteins, triglyceride clearance and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:2045–2051. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 11.Ha Y C, Barter P J. Differences in plasma cholesteryl ester transfer activity in sixteen vertebrate species. Comp Biochem Physiol. 1982;71:265–269. doi: 10.1016/0305-0491(82)90252-8. [DOI] [PubMed] [Google Scholar]
- 12.Kritchevsky S B, Wilcosky T C, Morris D L, Truong K N, Tyroler H A. Changes in plasma lipid and lipoprotein cholesterol and weight prior to the diagnosis of cancer. Cancer Res. 1991;51:3198–3203. [PubMed] [Google Scholar]
- 13.Lopez-Berestein G. Liposomes as carriers of antifungal drugs. Ann N Y Acad Sci. 1988;544:590–597. doi: 10.1111/j.1749-6632.1988.tb40459.x. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Berestein G, Mehta R, Hopfer R L, Mills K, Kasi L, Mehta K, Fainstein V, Luna M, Hersh E M, Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposomal-encapsulated amphotericin B. J Infect Dis. 1983;147:939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- 15.Lopez-Berestein G, Rosenblum M G, Mehta R. Altered tissue distribution of amphotericin B by liposomal encapsulation: comparison of normal mice to mice infected with Candida albicans. Cancer Drug Delivery. 1984;1:199–205. doi: 10.1089/cdd.1984.1.199. [DOI] [PubMed] [Google Scholar]
- 16.Meyer R D. Current role of therapy with amphotericin B. Clin Infect Dis. 1992;14:S154–S160. doi: 10.1093/clinids/14.supplement_1.s154. [DOI] [PubMed] [Google Scholar]
- 17.Morton R E, Zilversmit D A. Purification and characterization of lipid transfer protein(s) from human lipoprotein-deficient plasma. J Lipid Res. 1982;23:1058–1067. [PubMed] [Google Scholar]
- 18.Norido F, Zatta A, Fiorito C, Prosdocimi M, Weber G. Hematologic and biochemical analysis profiles of selectively bred WHHL rabbits. Lab Anim Sci. 1993;43:319–323. [PubMed] [Google Scholar]
- 19.O’Meara N M, Devery R A, Owens D, Collins P B, Johnson A H, Tomkin G H. Serum lipoproteins and cholesterol metabolism in two hypercholesterolemic rabbit models. Diabetologia. 1991;34:139–143. doi: 10.1007/BF00418266. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Soler R, Khokhar A R, Hacker M P, Lopez-Berestein G. Toxicity and antitumor activity of cis-bis-cyclopentenecarboxylato-1,2-diaminocyclohexane platinum (II) encapsulated in multilamellar vesicles. Cancer Res. 1986;46:6269–6273. [PubMed] [Google Scholar]
- 21.Pontaini D R, Sun D, Brown J W, Shahied S I, Plescia O J, Schaffner C P, Lopez-Berestein G, Sarin P S. Inhibition of HIV replication by liposomal encapsulated amphotericin B. Antivir Res. 1989;11:119–125. doi: 10.1016/0166-3542(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 22.Rothon D A, Mathias R G, Schechter M T. Prevalence of HIV infection in provincial prisons in British Columbia. Can Med Assoc J. 1994;151:S154–S160. [PMC free article] [PubMed] [Google Scholar]
- 23.Shargel L, Yu A B C. Multicompartment models. In: Shargel L, Yu A B C, editors. Applied biopharmaceutics and pharmacokinetics. Norwalk, Conn: Appleton & Lange; 1985. pp. 51–67. [Google Scholar]
- 24.Taylor R L, Williams D M, Craven P C, Graybill J R, Drutz D J, Magee W E. Amphotericin B in liposomes: a novel therapy for histoplasmosis. Am Rev Respir Dis. 1982;125:610–611. doi: 10.1164/arrd.1982.125.5.610. [DOI] [PubMed] [Google Scholar]
- 25.Vadiei K, Lopez-Berestein G, Perez-Soler R, Luke D R. Tissue distribution and in vivo immunosuppressive activity of liposomal cyclosporine. Drug Metab Dispos. 1991;19:1147–1151. [PubMed] [Google Scholar]
- 26.Vitols S, Gahrton G, Bjorkholm M, Peterson C. Hypercholesterolemia in malignancy due to elevated low-density lipoprotein receptor activity in tumor cells: evidence from studies in patients with leukemia. Lancet. 1985;iv:1150–1154. doi: 10.1016/s0140-6736(85)92679-0. [DOI] [PubMed] [Google Scholar]
- 27.Walsh T J, Bacher J, Pizzo P A. Chronic silastic central venous catheterization for reduction, maintenance and support of persistent granulocytopenia in rabbits. Lab Anim Sci. 1988;38:467–471. [PubMed] [Google Scholar]
- 28.Wasan K M, Rosenblum M G, Cheung L, Lopez-Berestein G. Influence of lipoproteins on renal cytotoxicity and antifungal activity of amphotericin B. Antimicrob Agents Chemother. 1994;38:223–227. doi: 10.1128/aac.38.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wasan K M, Conklin J S. Enhanced amphotericin B nephrotoxicity in intensive care patients with elevated levels of low-density lipoprotein cholesterol. Clin Infect Dis. 1997;24:78–80. doi: 10.1093/clinids/24.1.78. [DOI] [PubMed] [Google Scholar]
- 30.Wasan K M, Cassidy S M. The role of plasma lipoproteins in modifying the biological activity of hydrophobic drugs. J Pharm Sci. 1998;87:411–424. doi: 10.1021/js970407a. [DOI] [PubMed] [Google Scholar]
- 31.Wasan K M, Vadiei K, Lopez-Berestein G, Luke D R. Pharmacokinetics, tissue distribution, and toxicity of free and liposomal amphotericin B in diabetic rats. J Infect Dis. 1990;161:562–566. doi: 10.1093/infdis/161.3.562. [DOI] [PubMed] [Google Scholar]
- 32.Wasan K M, Grossie V B, Jr, Lopez-Berestein G. Concentrations in serum and distribution in tissue of free and liposomal amphotericin B in rats during continuous Intralipid infusion. Antimicrob Agents Chemother. 1994;38:2224–2226. doi: 10.1128/aac.38.9.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasan K M, Morton R E, Rosenblum M G, Lopez-Berestein G. Decreased toxicity of liposomal amphotericin B is due to the association of amphotericin B with high density lipoproteins: role of lipid transfer protein. J Pharm Sci. 1994;83:1006–1010. doi: 10.1002/jps.2600830716. [DOI] [PubMed] [Google Scholar]