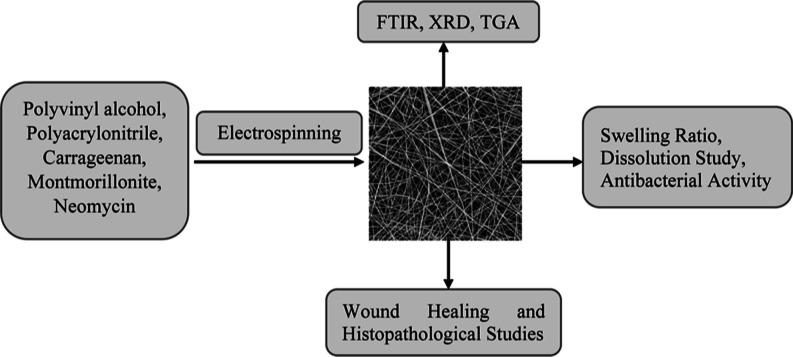
Abstract
Background: Skin wounds affect millions of individuals around the world, and their treatment is expensive. Objective: The purpose of this study was to make neomycin-loaded CG/PVA/PAN (NCPP) nanofibers to improve wound healing. Methods: The NCPP nanofibers were characterized by using thermogravimetric analysis (TGA), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy, and X-ray diffraction. Drug solubility, dissolution, swelling ratio, erosion, and antibacterial studies were performed. The in vivo wound healing study of nanofibers was performed in a rabbit model and was supported by % age wound closure and histopathology. Results: The results of SEM showed some sort of agglomeration on the surface of fibers, while TGA showed 10% more stability for drug-loaded nanofibers. The drug permeation study indicated that the formulation with 15% PVA showed a controlled release profile of the drug. The NCPP nanofibers had an appreciable water retention capability. The NCPP nanofibers showed appreciable antibacterial activity against Enterococcus faecalis (Gram-positive bacteria) and Klebsiella pneumonia (Gram-negative bacteria). The wound healing study showed the better healing properties of NCPP nanofibers within 15 days. Conclusion: The findings helped us to conclude that the NCPP nanofibers were successfully fabricated and found to have a promising role in infected wound healing.
1. Introduction
A wound refers to an injury to tissues or the skin due to some microbial, thermal, physical, or chemical disorder.1 The skin functions as a route for topical drug delivery because it is the biggest and most easily accessible organs in the human body.2 Many known drug delivery systems are directed through enteral channels, i.e., tablets, granules, and capsules, whereas some are directed through parenteral routes, including intraarterial, intravenous, and intramuscular. Some channels and administration approaches have disadvantages, such as first-pass metabolism and pain.3 To overcome these problems, medications can be administered directly to the skin.
Recently, electrospun nanofibers of numerous polymers have been extensively studied for wound healing. The nanofibers have an extraordinary surface area, adjustable porosity, and manageable size and shape. Electrospun nanofibers have a porous structure that resembles the extracellular matrix (ECM). The pores of the nanofibers also help in the exchange of nutrients. The ECM-like structure of nanofibers provides the best cellular environment for wound healing and also assists in the coordination of numerous biological changes during epidermal restoration. To enhance healing, nanofibers can be functionalized with various bioactive agents.4
Drug-loaded natural or synthetic polymeric nanofibers may be used to enhance wound healing and prevent wound infection.5,6 During the wound healing process, the wound must be protected from bacterial infection. Thus, antibiotic-releasing nanofibrous mats have seemed like a worthy nanomaterial. Due to their lightweight and flexibility, nanofibers play a considerable role in protecting the wound from any type of tensity.7 These nanofibers are very significant for the sustained delivery of drugs. For better loading capacity and better delivery of drugs, some functional modifications of nanofibers are made.8
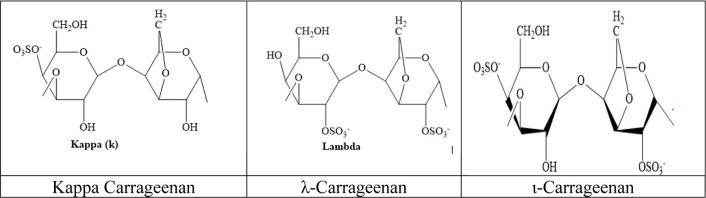
Carrageenan (CG; Figure 1) is a sulfated polysaccharide of d-galactose and 3,6-anhydro-d-galactose. It is extracted from red seaweeds. Carrageenan is available mainly in three forms: Iota carrageenan (ι), Kappa carrageenan (κ), and Lambda carrageenan (λ), based on the category of seaweed utilized as a source. Carrageenan, kappa and iota-CG, is used to form thermally reversible gels due to the presence of anhydro bridges.9 λ-carrageenan is used as a thickening agent.10,11
Figure 1.
Chemical structures of Kappa carrageenan and λ-carrageenan and ι-varrageenan.
The polymer matrix of carrageenan has been studied in the pharmaceutical industry as an oral extended-release tablet, as a novel extrusion that assists in the preparation of pellets, and as a stabilizer in nanoparticle systems. It has also been employed for tissue regeneration and cell transport with medicinal bio macromolecules. As a gelling agent, it has been used in sustained drug release and muco-adhesion.12 CG, due to its biocompatible nature and consolidation behavior, is generally used to prolong drug release from formulation. The required characteristics for drug release, pH-independent release, and zero-order release cannot be achieved if the CG matrix is employed alone.13 So, to overcome these limitations for achieving an optimal drug release profile, other polymers are used that interact with CG. Moreover, combinations of different forms of CG are also an effective system for prolonged medication release. The medication solubility and oral bioavailability of less soluble drugs are also enhanced due to the ability of CG to absorb water.14 The neomycin-loaded nanofibers of CG in combination with polyacrylonitrile and pullulan have been reported showing considerable wound healing and antibacterial activity.14
Poly(vinyl alcohol) (PVA) is a synthetic, nontoxic, and water-soluble polymer with good biocompatibility and biodegradability. Moreover, the inability of natural polymers to be electrospun can be overcome by using PVA, resulting in smooth fibers.15 PVA possesses high thermal stability, chemical resistance, and mechanical properties. PVA is extensively used for biomedical applications such as wound dressings, drug delivery, and tissue adhesion barriers.16 The swelling ability and elasticity of PVA enhance its biocompatibility.17,18 Electrospun PVA nanofibers have gained much attention in antimicrobial and wound healing applications, and their properties can be improved by blending them with a variety of materials, such as natural plant extract and other polymers.19 Kharaghani et al. synthesized a 2-fold drug delivery system, comprised of PVA–PAN core–shell nanofibers loaded with anti-inflammation and antibiotic drugs, for wound healing.20 PVA nanofibers showing some healing ability, due to their extracellular matrix-like structure, have also been reported.21
The nanocomposites of polyacrylonitrile (PAN) are biocompatible, promoting bone marrow stem cell (BMSC) growth, maintaining cellular activity, and providing positive encouragement to regulate cellular proliferation.22 PAN is a very important synthetic polymer due to the ease of fabrication of its nanofibers, which have better thermal and mechanical properties. Fayemi et al. reported the wound healing activity of polyacrylonitrile nanofibers with varying concentrations of Moringa oleifera leaf extract.23
Montmorillonite (MMT) is a natural inorganic material or hydrated aluminosilicate clay mineral. The addition of MMT as a nanofiller to the polymeric solution increases its mechanical and thermal properties.24 Due to the weak Van der Waal interaction between layered structures of montmorillonite polymeric molecules, they can be easily incorporated between layers.25 MMT has a large surface area with good swelling ability, good absorption capacity, cation exchange capability, and drug-carrying ability.26
Neomycin has average effectiveness against bacterial infections. It has been used to create an ion-exchange nanofibrous mat with wound healing potential.27 The fabrication of neomycin-loaded nanofibers was reported and found to enhance wound healing by releasing neomycin.28
The objective of the study was to fabricate and characterize the nanocomposite polymeric fibers, i.e., neomycin-loaded CG/PVA/PAN (NCPP) nanofibers, and to study their potential role in infected wound healing.
2. Material and Methods
Poly(vinyl alcohol) (Mw = 72,000), polyacrylonitrile (Mw = 150,000), carrageenan (Mw = 12,000), and montmorillonite (surface area = 250 m2/g), all were obtained from Sigma-Aldrich. DMF was purchased from Lab scan, Lahore, Pakistan. Neomycin was gifted by Saffron Pharmaceuticals, Faisalabad, Pakistan.
2.1. Preparation of Polymeric Solutions
The polymeric solutions of PVA, PAN, and CG were prepared separately in dimethylformamide (DMF). PVA solution (8% w/v) was formulated by dissolving an adequate amount of PVA into 5 mL of DMF. The solution was sonicated for about an hour and heated with stirring at 45 °C for about 130 min. PAN solution (8% w/v) is obtained by dissolving the required amount into 3 mL of DMF. The solution was heated at 45–60 °C for about an hour with constant stirring. CG solution (8% w/v) was formulated by adding the proper amount of CG to 5 mL of DMF. The solution was sonicated for about an hour and placed on a hot plate at 45 °C with constant stirring for about an hour. All of these solutions were mixed and heated with constant heating for about 1 h at 45 °C. The 1% MMT mixture was mixed separately with the desired amount of DMF. This mixture was then added to a polymeric mixture with vigorous stirring and minimum heating. 0.1% drug was added to the mixture of three polymeric solutions. The solution was agitated constantly for about 10 h to get a completely homogeneous solution. The different formulations of solution are shown in Table 1.
Table 1. Composition of Various Formulations.
| sr. no. | formulations | polymeric solution weight (%) | neomycin (mg) | MMT (%) | total amount of solute (g) | PAN/CG/PVA |
|---|---|---|---|---|---|---|
| 1 | PAN-60% | 8 | 1.2 | 1 | 1.2 | 60:20:20 |
| 2 | PAN-70% (L) | 8 | 1.2 | 1 | 1.2 | 70:15:15 (with drug) |
| 3 | PAN-70% (U) | 8 | 0 | 1 | 1.2 | 70:15:15 (without drug) |
| 4 | PAN-80% | 8 | 1.2 | 1 | 1.2 | 80:10:10 |
| 5 | PAN-90% | 8 | 1.2 | 1 | 1.2 | 90:5:5 |
2.2. Electrospinning of the Polymeric Solution
In the electrospinning machine (FLUIDNA TEK LE-10 model), the prepared polymeric solution was spun by applying an electric field to fabricate nanofibers. The homogeneous solution was injected into a 5 mL syringe with a diameter of 11.99 mm. The optimal process parameters were applied for fabricating nanofibers. These parameters were kept constant for all formulations. When a high voltage was applied, a charged solution jet in the shape of a Tylor cone was ejected from the spinneret. The jet of charged solution traveled toward the collector under the action of the applied field. The solid nanofibers were deposited on the metallic collecting plate, which was covered by aluminum foil. Fibers were collected from aluminum foil as a membrane after complete drying.
2.3. Characterization Techniques
The neomycin-loaded CG/PVA/PAN (NCPP) nanofibers were subjected to the following characterization techniques.
2.3.1. Fourier Transformed Infrared Spectroscopy
Fourier transformed infrared (FT-IR) spectroscopy was performed to study the functional groups in the sample, as each vibration corresponds to a specific chemical bond. The spectra were scanned from 4000 to 1000 cm–1 using “FT/IR-6600 type A”. The FTIR method scans materials using infrared light from electromagnetic radiation, and the resulting IR spectra disclose their chemical properties. The bonds between different elements in a molecule absorb light at different frequencies.
2.3.2. Scanning Electron Microscopy
The topology and morphology of nanofibers were investigated using Jeol, JSM 6400F. When the sample’s surface is targeted with a focused electron beam, signals are produced that reveal the morphological features of the sample. High ranges of pressure and temperature and less than 1 nm resolution are required for its working. Scanning electron microscopy (SEM) is a nondestructive technique in which there is no volume loss of sample, so it is possible to analyze the same sample repeatedly.
2.3.3. X-ray Diffraction
X-ray diffraction (XRD) is used to investigate the nature of material, i.e., whether it is amorphous or crystalline. XRD can be used for many applications, such as the analysis of thin films to find out the crystalline structure and sample texture. The physical character of nanofibers was determined using a Philips XPERT PRO 3040/60. The scanning range for 2 was 5 to 80°.
2.3.4. Thermogravimetric Analysis
The thermal stability of the fabricated nanofibers was investigated using a Q600 SDT thermogravimetric analyzer. The measurement of thermal stability, material decomposition, filler’s content in polymers, and the percent composition of components in materials can be investigated by thermogravimetric analysis (TGA) analysis. The samples were thermally decomposed at temperatures ranging from 20 to 700 °C at a rate of 10 °C per minute. The weight loss versus temperature was recorded and analyzed for nanofibers.
2.4. Pharmaceutical Studies
2.4.1. Calibration Curve
Double-distilled water, having a pH of 6.6, was used to set up the calibration curve of neomycin with different dilutions. 100 mg of drug was added to 100 mL of water to prepare a stock solution. From this stock solution, ten dilutions ranging from 1 to 10 μg/mL were prepared. A UV Spectrophotometer was used to measure the absorbance of dilutions at 304 nm. The double-distilled water was taken as a blank.
2.4.2. Swelling Studies
The swelling capacities of the formulations were determined by soaking a calculated (15 mg) patch of nanofibers in double-distilled water. Patches were weighed, submerged in Petri dishes, and allowed to swell for about an hour. To check their swelling ratio, fibers were picked up from water every 15 min. The fibers were inflated over filter paper to dry and weigh again. The following formula will be used to study the swelling ratio
where Ws = wet weight and Wd = final dry weight
2.4.3. Erosion Studies
The loss of the weight of swollen polymers is regarded as an erosion study. The wet sample was kept in air for about 24 h and weighed at different time intervals. The process was repeated in triplicate. The erosion study was done by using the formula
where Wo = wet weight and Wf = final dry weight.
2.4.4. Drug Release Study
About 15 mg of NCPP was immersed in 30 mL of distilled water having pH 6.6. The solution of NCPP nanofibers was placed in an incubator at 37 °C. After incubation, they were stirred at about 250 rpm. The samples were taken at the time interval of 0.5, 1, 1.5, and 2 h to analyze drug release. These samples were tested at 304 nm using a UV Spectrophotometer.
2.4.5. Antibacterial Study
Using the agar disc method, the antibacterial action of NCPP nanofibers was investigated. The prepared media was placed in an autoclave along with Petri plates for about 2.5 h to sterilize. About 20 mL of agar media was placed in each Petri plate using laminar flow and kept for solidification at ambient temperature. After solidification, plates were incubated for about 24 h. The next day, plates were infected with bacteria from both Gram-positive and Gram-negative strains and again incubated for about 24 h. Bacterial colonies grew on Petri dishes, which were inoculated into an inoculum of nutrient broth. The Petri dishes were swapped with the incubated inoculum, and drug-loaded sample disc were placed in Petri plates. The zones of inhibition (ZOI) were measured (in mm) after 24 h of incubation.
2.4.6. Wound Healing
Each study protocol followed was authorized by the Research Ethics Committee of COMSATS University Islamabad (CUI), Lahore Campus, with an approval no.880/CUI/PHM-2021, and conducted according to international guidelines for animal use in lab studies.
The in vivo study was performed on rabbits to study the wound healing properties of the NCPP nanofibers and to compare the effectiveness of these nanofibers with the marketed formulation for wound healing (Quench). Four male rabbits of approximately 3–4 kg weight were selected for each of the following groups: control, standard, and two samples (NCPP nanofibers and drug-free nanofibers). Ketamine hydrochloride and xylene were used to anesthetize the rabbits. Two specific dorsal areas of each rabbit were shaved to get hair-free, clear skin. These areas were then washed with the 70% ethanol–water solution to minimize rashes which appeared during the shaving process. With the help of a sterilized biopsy punch, two wounds (burn wounds) were created with a 0.5 cm full thickness in an area of about 3–4 cm2. The standard wound was infected with bacterial strains, the control wound was kept noninfected, and the animals’ ears were colored for their identification. One of the four rabbit groups was kept as a control, so no medication was applied to it, while the second was treated with NCPP nanofibers, the third with neomycin-free nanofibers, and so forth with the marketed formulation, Quench. The change in the wound size, as a percentage of the original wound area, was measured using the formula given below
where Ao = original wound area and A = wound area after a fixed time
After 3 days of complete healing, histology was performed to check the progress of healing.27,29 Rabbits were sacrificed, and the tissue samples were isolated and then histopathologically tested the next day.
3. Results and Discussions
3.1. Preparation of Neomycin Smart Release Nanofibers
We aimed to develop neomycin-release nanofibers to treat burn wounds, which are susceptible to bacterial infection. We selected the neomycin antibiotic, which has excellent antibacterial properties, and then we selected the biocompatible polymers PVA and PAN for increasing the water resistance properties of PVA for drug-resistant bacteria. Carrageenan was selected as a capping agent for increasing the stabilization of nanofibers. Due to the ion exchange phenomenon of neomycin, the drug was readily released from the nanofibers, followed by sustained release due to the interaction of neomycin with carrageenan, which has gelling property.
3.2. Physical Characterizations
3.2.1. FTIR Spectroscopy
Fourier transform infrared (FTIR) spectroscopy of nanofibers was done to study the chemical interactions between the PVA/CG/PAN nanocomposites and Neomycin (Figure 2). The FTIR analysis of the prepared nanofibers, as shown in Figure 2, confirms the link between the polymer and the drug. The transmittance peak at 3320 cm–1 is due to the OH of PVA, at 2916 cm–1 is due to CH-asymmetric, at 2231 cm–1 is due to the CN of PAN, at 1608 cm–1 is due to C=C (conjugated carbon bonds), at the range of 1449 cm–1 is due to the S=O group, and at 1072 cm–1 is due to NH2. Similar information has already been reported.27
Figure 2.
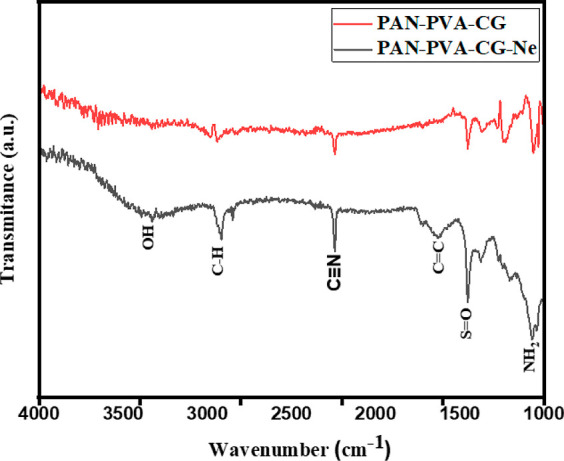
FTIR spectra of nanofibers.
3.2.2. Scanning Electron Microscopy
The morphological features of the nanofibers with drug and without drug were studied by scanning electron micrographs and are shown in Figure 3, which illustrates the surface properties of MMT-reinforced PVA/CG/PAN nanofibers with and without drugs. A detailed examination of the SEM image indicates that the fiber’s surface is not smooth but rather comprises micrometer-sized agglomerations, which are most likely caused by the presence of carrageenan particles.30 The SEM micrograph shows the randomly oriented, cylindrical, twisted, and beads-like morphology of nanofibers with an even distribution of MMT. There are no clump formations observed, which means that the drug has been finely distributed within the fibers. However, some irregular patches were also observed in NCPP nanofibers.31 A slight change in diameter was observed in NCPP nanofibers comprising of drug.32 SEM morphology reveals the nonwoven structure of nanofibers.
Figure 3.
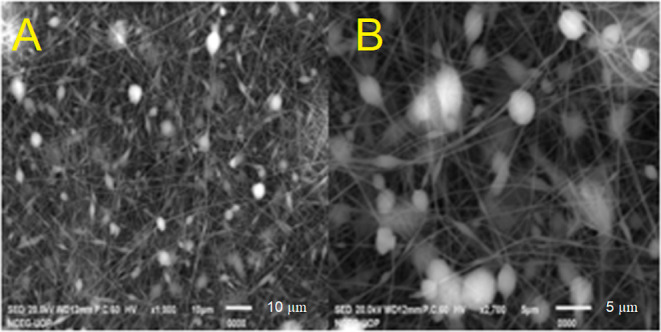
SEM micrographs of NCPP nanofibers (A) and drug-free nanofibers (B).
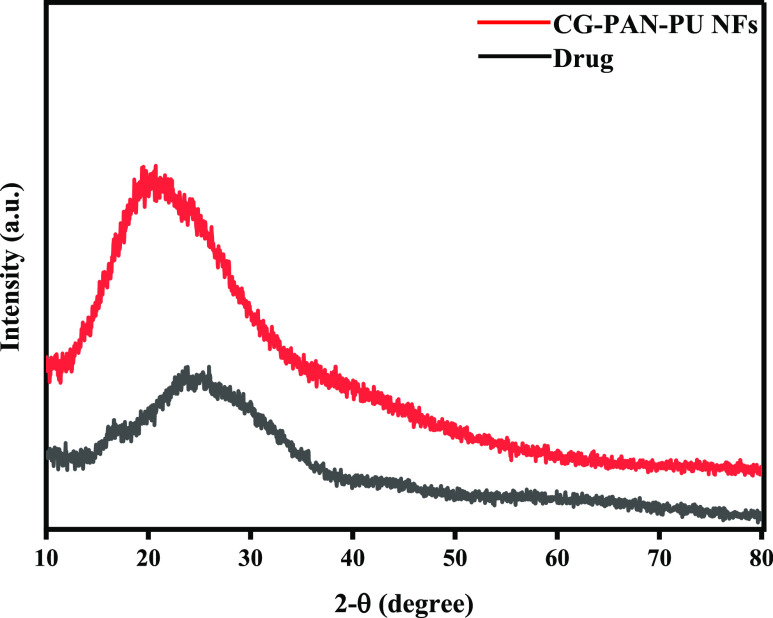
3.2.3. XRD Spectroscopy
An XRD study was performed to check the crystalline or amorphous nature of drug-loaded SCP-02(a) nanofibers and Neomycin. Figure 4 shows the nature of the nanofiber composites. According to the literature, pure semicrystalline PVA shows a peak at 20–22° and pure PAN shows a peak at 17°. In Figure 4, two small peaks were observed, which are due to the hybrid composites of PAN and PVA.32 The broadening of peaks is due to the quick solidification of nanofibers during electrospining, which hampers the formation of crystalline structures. At a value of roughly 20°, there is a distinctive broad amorphous peak. This is typical of an amorphous nature, and it has been observed in a variety of polymers with an amorphous area. Furthermore, the inorganic salts like KCl in the kappa carrageenan may explain the formation of a sharp peak at value 2 of 28.4.33 The overall diffractogram shows the amorphous nature of neomycin and drug-free nanofibers.
Figure 4.
XRD pattern of nanofibers.
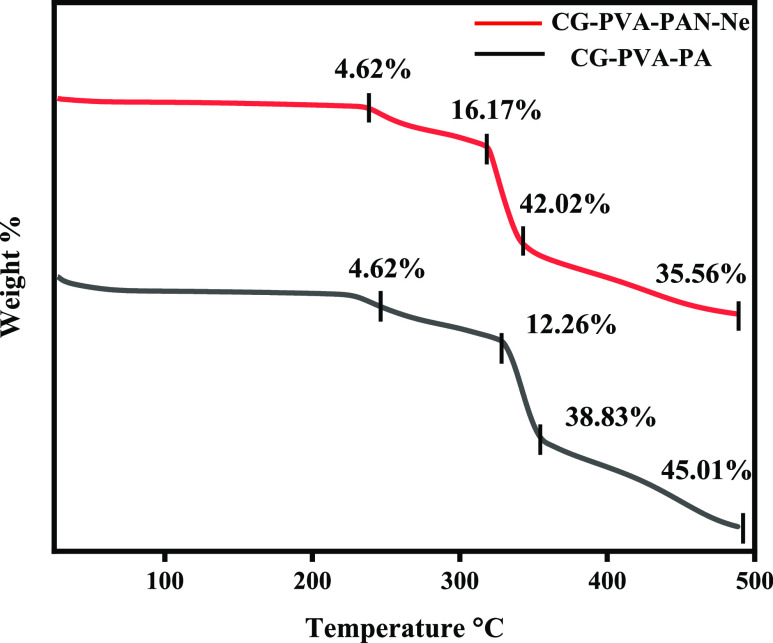
3.2.4. Thermogravimetric Analysis
The TGA of NCPP and drug-free nanofibers was studied to check the thermal stability of the nanofibers. Figure 5 shows the weight loss parameters of the NCPP and drug-free nanofibers. A three-step decomposition was observed in each case. Both NCPP and drug-free nanofibers showed higher stability as weight loss occurred at higher temperatures above 200 °C. In the case of NCPP nanofibers, the first weight loss (4.62%) occurred at about 240 °C due to the biodegradable polymer CG. The second weight loss (16.17%) is at 310 °C due to the semisynthetic polymer PVA. Third-step decomposition (weight loss of 42.02%) at 350 °C is due to PAN. Neomycin was found to be heat-stable. This graph demonstrated that the higher stability of nanofibers is due to PAN. The higher melting point of PAN is due to the cyclization reaction of the nitrile group. In the case of drug-free nanofibers, weight loss is different, but the reason for decomposition is the same for all three polymers.34
Figure 5.
TGA curves of nanofibers.
3.3. Pharmaceutical Studies
3.3.1. Calibration Curve
The calibration curve of neomycin is shown in Figure 6. R2 value was 0.9971.
Figure 6.
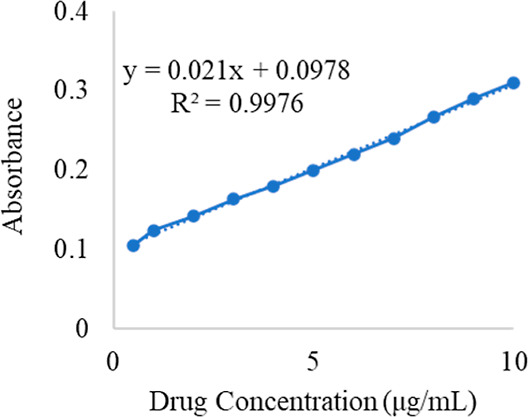
Calibration curve of Ne.
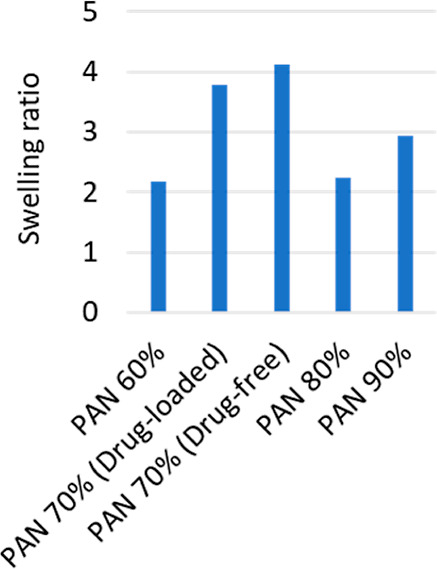
3.3.2. Swelling Ratio
The swelling ratio of various formulations was measured to determine which formulation swells more. Antibacterial wound dressings are known for their capacity to absorb water and retain moisture, which helps to keep the wound optimally moist and speed wound healing. Figure 7 shows that drug-free nanofibers swelled at a substantially faster pace than NCPP nanofibers, with the swelling peaking in the first 5 min. Furthermore, the fabricated NCPP nanofibers had an appreciable water retention capability at all-time intervals. All the studies showed that the nanofibrous material had a considerable moisture-absorbing capacity.35 Drug-free nanofibers (PAN70%) were thin and hence had a higher surface-to-volume ratio. This causes more interaction of the nanofibers with water and swells more as compared to NCPP nanofibers.
Figure 7.
Swelling studies of nanofibers.
3.3.3. Drug Dissolution Study
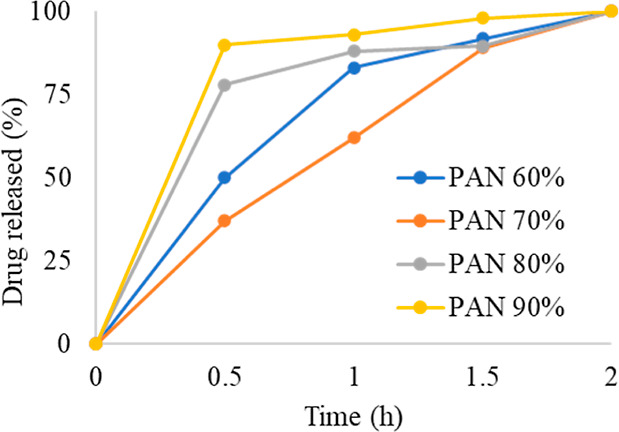
Dissolution studies of different formulations were performed to find out the drug release behavior. Drug solubility, an equilibrium measure, is an important predictor of dissolution rate, which is an indication of absorption for many drugs. Thus, determining the dissolution profiles of different nanofiber formulations is a central goal of the current research.36Figure 8 shows the drug release of different formulations at different time points. Although there is a burst effect in the drug release profiles, formulation PAN70% shows a relatively controlled drug release behavior for 2 h of dissolution. Slow drug release is effective and has excellent wound healing potential. This controlled release is due to the jelling property of the carrageenan, as it forms a compact mass with the drug; thus, controlled release is the property of the carrageenan.34,37 The controlled release behavior of nanofibers also depends on the nature of the incorporated drug. Apart from the physical and chemical properties of the drug, its release rate can also be affected by the rates of diffusion and matrix’ degradation after being embedded in the polymer matrix.37 In the present study, the drug release pattern was affected by the amount of PAN used for the fabrication of nanofibers. The drug release rate was controlled in the formulation containing 70% PAN, which showed 40% neomycin release during the initial 30 min. In contrast, other formulations showed 60–90% drug release at the same time.38
Figure 8.
Drug dissolution study of all of the formulations.
3.3.4. Antibacterial Activity
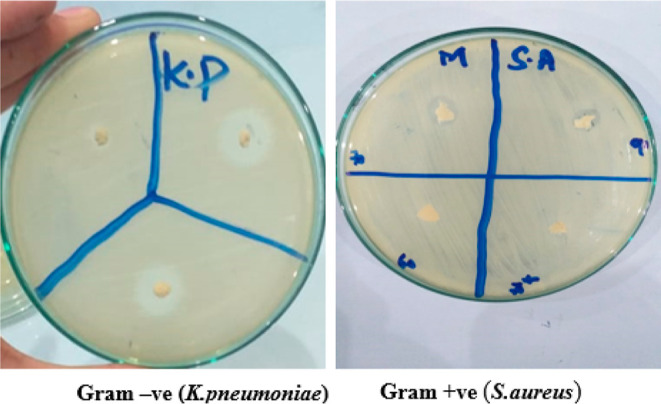
The antibacterial activity of different formulations was performed against Staphylococcus aureus (S. aureus) and Klebsiella pneumonia (K. pneumonia) (Figure 9, Table 2). The antibacterial efficacy of NCPP nanofibers was evaluated using the zone of inhibition. This suggests that NCPP fibers have a promising antibacterial effect and can be employed for wound healing.30 The fabricated nanofibers showed significantly (p < 0.05) greater antibacterial efficacy against K. pneumonia as compared to S. aureus. These findings suggest that these nanofibers could be useful in wound healing.
Figure 9.
Antibacterial study of nanofibers.
Table 2. Zone of Inhibition Shown by Different Nanofibers Showing Their Antibacterial Efficacy.
| formulations codes | mean zone
of inhibition (mm) ± SD |
|
|---|---|---|
| Klebsiella pneumonia | Staphylococcus aureus | |
| S-CP 01 | 14.5 ± 0×.5 | 7.8 ± 0.3 |
| S-CP 02 | 16.2 ± 0.6 | 9.5 ± 0.5 |
| S-CP 03 | 14.6 ± 0.4 | 8.1 ± 0.3 |
| S-CP 04 | 14.2 ± 0.5 | 8.9 ± 0.4 |
| standard (Ne) | 22.4 ± 0.7 | 17.8 ± 0.6 |
3.3.5. Wound Healing Study
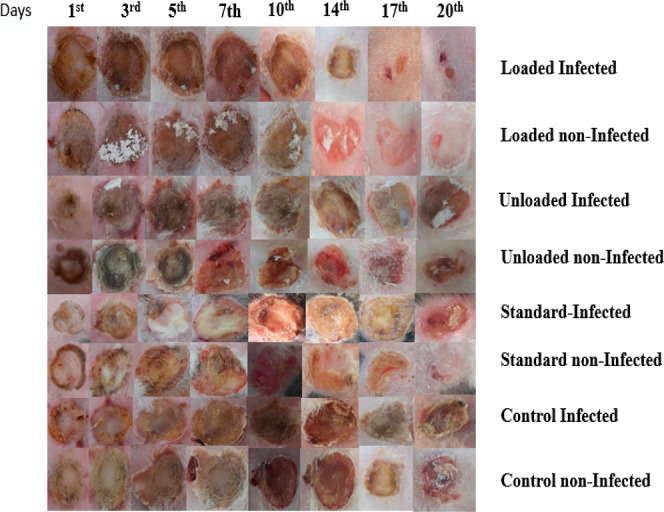
In this study, NCPP nanofibers were tested to check their healing properties. The wound healing area was measured every other day with the help of a scale. Wounds treated with NCPP membranes, drug-free nanofiber film, and positive control (Quench) and untreated wounds (negative control) were studied to evaluate the wound healing capability of different formulations. Photographs were taken on the day of implantation and every day afterward until the wound healed fully, as shown in Figure 10. The drug-free film showed a dry wound after 3 days of treatment, whereas the wounds of the negative and positive controls were still red and wet. On day 5, wound healing rates for the NCPP nanofibers, drug-free nanofibers, positive control, and negative control samples were 65, 60, 20, and 15%, respectively. On day 7, the conditions of the NCPP nanofibers and drug-free nanofibers were observed to be better as compared to the positive and negative controls. On day 14, the wound size of the NCPP nanofibers was observed to be very small as compared to others, which shows their excellent healing properties. The wound conditions of the positive and negative controls were not good enough even on the 18th day of the study. Their wound sizes were not small, and the wounds were still red and wet, as shown in Figure 11. On the other hand, the wound size was very negligible, and it was completely healed on the last day. Similar findings have already been reported for nanofibers loaded with therapeutic moieties.35 Initially, bacteria that were present at the wound site were killed by the burst release of drugs from the nanofibrous structure. Later, drug release was controlled for several days to prevent wound infection. The incorporation of drugs into a polymeric matrix enhances antibacterial activity by increasing affinity for bacteria due to the high surface area of nanofibers.39
Figure 10.
Wound area measurements of various formulations.
Figure 11.
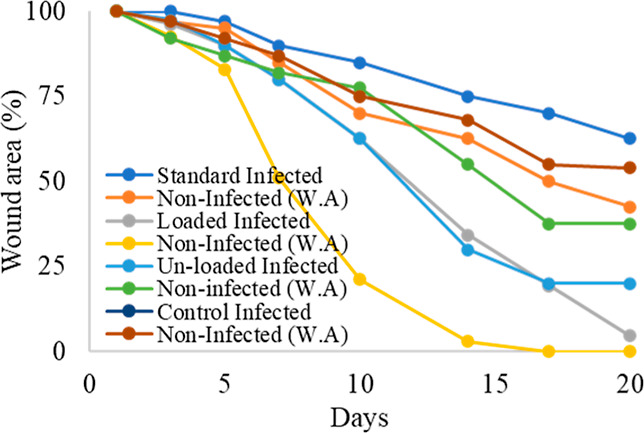
Wound healing curves.
3.3.6. Histopathological Studies
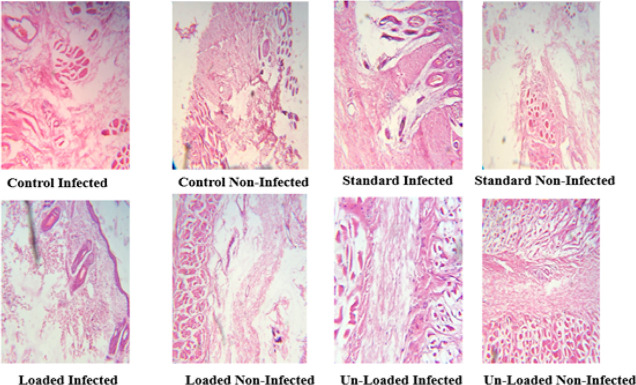
For histopathological examinations, the excised skin tissue was fixed in a 10% formalin solution on the 18th day of treatment. Hematoxylin–Eosin (H&E) was used to stain the samples and Masson’s trichrome, which were then examined using an optical microscope with a digital camera, OLAMPUS DP 72. The effectiveness of the therapies on the wound healing process was assessed using histological investigations. According to histological observations stained with H&E, epithelial growth was dramatically enhanced in wounds treated with NCPP nanofibers. Complete wound healing was detected in NCPP nanofibers after 18 days of treatment. Histological investigations revealed a more efficient healing with greater granular tissue production, more compact collagen, and new skin covering by the stratum corneum.35 In addition, there were negligible signs of inflammation and necrosis. This study showed an appreciable healing rate by using NCPP nanofibers within 15 to 17 days. Histopathological images in Figure 12 at higher magnification are displayed for better clarity.
Figure 12.
Histological images of the wounded tissues.
The combination of biodegradable polymers with neomycin is a new platform for developing advanced strategies to develop sustained-release nanofibers. The use of wound dressings to deliver antibiotics to the wound site is useful due to their ability to provide tissue compatibility, least bacterial resistance, and wound healing acceleration. Due to the cationic exchange property of neomycin, it is readily released from the nanofiber mat and retains its antibacterial activity, thereby accelerating wound healing.
4. Conclusions
Neomycin has been effectively incorporated into MMT-reinforced PVA/CG/PAN nanofibers by an electrospinning process. One of the primary requirements of biomaterials is their biocompatibility. An ideal wound dressing exhibits cell proliferation and cell adhesion. Biopolymers are preferred materials to produce nanofibers because of their adhesion properties and biocompatibility. Synthetic polymers such as PVA and PAN are highly useful for biomedical applications. The incorporation of carrageenan in PVA and PAN enhances the biocompatibility of fabricated nanofibers.40 Carrageenan is used as a capping and reducing agent for nanofiber stabilization and targeted drug delivery.41 In vivo and in vitro cytotoxicity studies of carrageenan proved that it is safe for biological applications with negligible inflammatory responses.42
The NCPP nanofibers are thermally stable, have considerable activity against bacterial strains, and are an interesting tool for wound healing. Drug-loaded hybrid polymeric (PVA/CG/PAN) nanofibers can absorb the exudate, keep wounds dry, and accelerate the healing process. Even the neomycin-free nanofibers have excellent healing ability.
Acknowledgments
The authors are thankful to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R73), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
All the data has been incorporated into this article.
Author Contributions
¶ Equal contribution.
The authors are thankful to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R73), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
References
- Chen S.; Liu B.; Carlson M. A.; Gombart A. F.; Reilly D. A.; Xie J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. 10.2217/nnm-2017-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. Superpowered skin. Nature 2018, 563, S84–S85. 10.1038/d41586-018-07429-3. [DOI] [PubMed] [Google Scholar]
- Torres-Martinez E. J.; Cornejo Bravo J. M.; Serrano Medina A.; Pérez González G. L.; Villarreal Gómez L. J. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Delivery 2018, 15, 1360–1374. 10.2174/1567201815666180723114326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J.; Peng C.; Li C.; Qi Z.; Zhan J.; Pan S. Dual bio-active factors with adhesion function modified electrospun fibrous scaffold for skin wound and infections therapeutics. Sci. Rep. 2021, 11, 457. 10.1038/s41598-020-80269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.; Duan X.-P.; Li Y.-M.; Yang D.-P.; Long Y.-Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng., C 2017, 76, 1413–1423. 10.1016/j.msec.2017.03.034. [DOI] [PubMed] [Google Scholar]
- Cui H.; Chai Y.; Yu Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res., Part A 2019, 107, 1849–1859. 10.1002/jbm.a.36688. [DOI] [PubMed] [Google Scholar]
- Tottoli E. M.; Dorati R.; Genta I.; Chiesa E.; Pisani S.; Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. 10.3390/pharmaceutics12080735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topuz F.; Uyar T. Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 2018, 11, 6. 10.3390/pharmaceutics11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Zaveri T.; Ziegler G. R.; Hayes J. E. User preferences in a carrageenan-based vaginal drug delivery system. PLoS One 2013, 8, e54975 10.1371/journal.pone.0054975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michna A.; Maciejewska-Prończuk J.; Pomorska A.; Wasilewska M.; Kilicer T.; Witt J.; Ozcan O. Effect of the Anchoring Layer and Transport Type on the Adsorption Kinetics of Lambda Carrageenan. J. Phys. Chem. B 2021, 125, 7797–7808. 10.1021/acs.jpcb.1c03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallamilli T.; Ketomaeki M.; Prozeller D.; Mars J.; Morsbach S.; Mezger M.; Vilgis T. Complex coacervation of food grade antimicrobial lauric arginate with lambda carrageenan. Curr. Res. Food Sci. 2021, 4, 53–62. 10.1016/j.crfs.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Ni R.; Shao Y.; Mao S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. 10.1016/j.carbpol.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Alshahrani A.; Alharbi A.; Alnasser S.; Almihdar M.; Alsuhybani M.; AlOtaibi B. Enhanced heavy metals removal by a novel carbon nanotubes buckypaper membrane containing a mixture of two biopolymers: Chitosan and i-carrageenan. Sep. Purif. Technol. 2021, 276, 119300. 10.1016/j.seppur.2021.119300. [DOI] [Google Scholar]
- Goonoo N.; Khanbabaee B.; Steuber M.; Bhaw-Luximon A.; Jonas U.; Pietsch U.; Jhurry D.; Schönherr H. κ-Carrageenan enhances the biomineralization and osteogenic differentiation of electrospun polyhydroxybutyrate and polyhydroxybutyrate valerate fibers. Biomacromolecules 2017, 18, 1563–1573. 10.1021/acs.biomac.7b00150. [DOI] [PubMed] [Google Scholar]
- Adeli H.; Khorasani M. T.; Parvazinia M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. 10.1016/j.ijbiomac.2018.10.115. [DOI] [PubMed] [Google Scholar]
- Arefian M.; Hojjati M.; Tajzad I.; Mokhtarzade A.; Mazhar M.; Jamavari A. A review of Polyvinyl alcohol/Carboxymethyl cellulose (PVA/CMC) composites for various applications. J. Compos. Sci. 2020, 2, 69–76. 10.29252/jcc.2.2.2. [DOI] [Google Scholar]
- Kashyap N.; Kumar N.; Kumar M. R. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carrier Syst. 2005, 22, 107. 10.1615/critrevtherdrugcarriersyst.v22.i2.10. [DOI] [PubMed] [Google Scholar]
- Nagarkar R.; Patel J. Polyvinyl alcohol: A comprehensive study. Acta Sci. Pharm. Sci. 2019, 3, 34–44. [Google Scholar]
- Nokhasteh S.; Molavi A. M.; Khorsand-Ghayeni M.; Sadeghi-Avalshahr A. Preparation of PVA/Chitosan samples by electrospinning and film casting methods and evaluating the effect of surface morphology on their antibacterial behavior. Mater. Res. Express 2020, 7, 015401. 10.1088/2053-1591/ab572c. [DOI] [Google Scholar]
- Kharaghani D.; Gitigard P.; Ohtani H.; Kim K. O.; Ullah S.; Saito Y.; Khan M. Q.; Kim I. S. Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci. Rep. 2019, 9, 12640–12711. 10.1038/s41598-019-49132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiri A.; Saidin S.; Sani M. H.; Al-Ashwal R. H. Epidermal and fibroblast growth factors incorporated polyvinyl alcohol electrospun nanofibers as biological dressing scaffold. Sci. Rep. 2021, 11, 5634. 10.1038/s41598-021-85149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider M. K.; Sun L.; Ullah A.; Ullah S.; Suzuki Y.; Park S.; Kato Y.; Tamada Y.; Kim I. S. Polyacrylonitrile/Carbon Black nanoparticle/Nano-Hydroxyapatite (PAN/nCB/HA) composite nanofibrous matrix as a potential biomaterial scaffold for bone regenerative applications. Mater. Today Commun. 2021, 27, 102259. 10.1016/j.mtcomm.2021.102259. [DOI] [Google Scholar]
- Fayemi O. E.; Ekennia A. C.; Katata-Seru L.; Ebokaiwe A. P.; Ijomone O. M.; Onwudiwe D. C.; Ebenso E. E. Antimicrobial and wound healing properties of polyacrylonitrile-moringa extract nanofibers. ACS Omega 2018, 3, 4791–4797. 10.1021/acsomega.7b01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhajekar R. M.; Jogi B. F.; Nirantar S. R. Preparation and characterization of PAEK based polymer nanocomposites in the presence of MMT clay as nanofiller to study tensile and impact properties. Mater. Today: Proc. 2018, 5, 6848–6854. 10.1016/j.matpr.2017.11.345. [DOI] [Google Scholar]
- Kavimani V.; Stalin B.; Gopal P. M.; Ravichandran M.; Karthick A.; Bharani M. Application of r-GO-MMT Hybrid Nanofillers for Improving Strength and Flame Retardancy of Epoxy/Glass Fibre Composites. Adv. Polym. Technol. 2021, 2021, 2021–2029. 10.1155/2021/6627743. [DOI] [Google Scholar]
- Barikloo H.; Ahmadi E.; Ahmadi S. Evaluation of PE/POE/PA6 blends containing silica and clay toward nano composite packaging film. J. Food Meas. Char. 2021, 15, 2297–2308. 10.1007/s11694-020-00781-x. [DOI] [Google Scholar]
- Kataria K.; Gupta A.; Rath G.; Mathur R.; Dhakate S. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int. J. Pharm. 2014, 469, 102–110. 10.1016/j.ijpharm.2014.04.047. [DOI] [PubMed] [Google Scholar]
- Nitanan T.; Akkaramongkolporn P.; Rojanarata T.; Ngawhirunpat T.; Opanasopit P. Neomycin-loaded poly (styrene sulfonic acid-co-maleic acid)(PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int. J. Pharm. 2013, 448, 71–78. 10.1016/j.ijpharm.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Kaler A.; Mittal A. K.; Katariya M.; Harde H.; Agrawal A. K.; Jain S.; Banerjee U. C. An investigation of in vivo wound healing activity of biologically synthesized silver nanoparticles. J. Nanopart. Res. 2014, 16, 2605–2610. 10.1007/s11051-014-2605-x. [DOI] [Google Scholar]
- Bajpai S.; Daheriya P. Kappa-carrageenan/PVA filmswith antibacterial properties: Part 1. Optimization of preparation conditions and preliminary drug release studies. J. Macromol. Sci., Part A: Pure Appl.Chem. 2014, 51, 286–295. 10.1080/10601325.2014.882687. [DOI] [Google Scholar]
- Singh M.; Jonnalagadda S. Design and characterization of 3D printed, neomycin-eluting poly-L-lactide mats for wound-healing applications. J. Mater. Sci.: Mater. Med. 2021, 32, 44. 10.1007/s10856-021-06509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M. N.; Ullah A.; Haider M.; Hussain N.; Ullah S.; Hashmi M.; Khan M. Q.; Kim I. S. Evaluating antibacterial efficacy and biocompatibility of PAN nanofibers loaded with diclofenac sodium salt. Polymers 2021, 13, 510. 10.3390/polym13040510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K.; Kaneko Y.; Kadokawa J. . i. Novel gelling systems of κ-ι-and λ-carrageenans and their composite gels with cellulose using ionic liquid. Macromol. Biosci. 2009, 9, 376–382. 10.1002/mabi.200800179. [DOI] [PubMed] [Google Scholar]
- Roy S.; Rhim J.-W. Fabrication of copper sulfide nanoparticles and limonene incorporated pullulan/carrageenan-based film with improved mechanical and antibacterial properties. Polymers 2020, 12, 2665. 10.3390/polym12112665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norouzi M. A.; Montazer M.; Harifi T.; Karimi P. Flower buds like PVA/ZnO composite nanofibers assembly: Antibacterial, in vivo wound healing, cytotoxicity and histological studies. Polym. Test. 2021, 93, 106914. 10.1016/j.polymertesting.2020.106914. [DOI] [Google Scholar]
- Dubey P.; Barker S. A.; Craig D. Q. Design and characterization of cyclosporine A-loaded nanofibers for enhanced drug dissolution. ACS Omega 2020, 5, 1003–1013. 10.1021/acsomega.9b02616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T.; Wan X.; Yu D.-G.; Wang K.; Yang Y.; Liu Z.-P. Electrospun lipid-coated medicated nanocomposites for an improved drug sustained-release profile. Mater. Des. 2019, 162, 70–79. 10.1016/j.matdes.2018.11.036. [DOI] [Google Scholar]
- Samal K.; Mahapatra S.; Hibzur Ali M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. 10.1016/j.nexus.2022.100076. [DOI] [Google Scholar]
- Amiri N.; Ajami S.; Shahroodi A.; Jannatabadi N.; Amiri Darban S.; Fazly Bazzaz B. S.; Pishavar E.; Kalalinia F.; Movaffagh J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656. 10.1016/j.ijbiomac.2020.06.195. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Mu L.; Zhao X.; Han Y.; Guo B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16 (8), 13022–13036. 10.1021/acsnano.2c05557. [DOI] [PubMed] [Google Scholar]
- Das N.; Kumar A.; Rayavarapu R. G. The role of deep eutectic solvents and carrageenan in synthesizing biocompatible anisotropic metal nanoparticles. Beilstein J. Nanotechnol. 2021, 12, 924–938. 10.3762/bjnano.12.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Bai L.; Yang Y.; Yin Z.; Guo B. Biodegradable gelatin/silver nanoparticle composite cryogel with excellent antibacterial and antibiofilm activity and hemostasis for Pseudomonas aeruginosa-infected burn wound healing. J. Colloid Interface Sci. 2022, 608 (Pt 3), 2278–2289. 10.1016/j.jcis.2021.10.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data has been incorporated into this article.