Abstract
Background:
Across multiple levels of investigation, there appears to be convergent neuronal processes underlying substance use and other motivated behaviors (i.e., the pursuit and consumption of rewarding substances). The consumption of alcohol and sweet, high-fat food engages many of the same brain regions; especially, the ventral striatum. In the current study, we hypothesized that ventral striatal local field potentials (LFPs) recorded during self-administration sessions could be used to detect when the consumption of 10% ethanol (EtOH) or sweet-fat food (SF) was occurring compared to all other behaviors, including naturalistic controls (i.e., water or house-chow).
Methods:
We used an intermittent limited access approach to condition Sprague-Dawley rats to consume either EtOH or SF while we recorded LFPs. We used machine learning and simple logistic regressions to determine if LFP features could classify when consumption of each substance was occurring, as well as whether a general model could predict consumption of both substances. We report performance as the average area under the receiver operator characteristic curve (AUROC).
Results:
Consumption of a single substance was differentiable from all other behaviors (EtOH = 0.84 and SF = 0.83, p < 0.01). Models built from the combined dataset (general [gen]) did modestly overall (gen→gen = 0.68, p < 0.05), and did not detect the consumption of both substances with similar performance (gen→SF = 0.5 and gen→EtOH = 0.63, p > 0.05).
Conclusions:
The success of models classifying EtOH or SF consumption versus all other behavior/naturalistic controls, in juxtaposition to the poor performance of generalized models, suggest that the neural activity reflected in ventral striatal oscillations is distinct between EtOH and SF consumption.
Keywords: ventral striatum, local field potentials, machine learning, substance use, biomarker
Introduction
Prior work has established the critical role of ventral tegmental area mediated dopamine modulation of nucleus accumbens (NAc) activity in the regulation of motivated behaviors, particularly in the pursuit of highly rewarding substances (e.g., palatable food or drugs of abuse). Molecular changes in this “reward circuit” appear to correspond with systems level changes in brain activity, as measured by fMRI bold signals or neural oscillations. Since the NAc plays a well-established role in encoding the pursuit and receipt of rewarding substances (Koob and Volkow, 2010; “The neurobiology of drug addiction,” 1997), it is no surprise that the NAc is a strong moderator of both hedonic, or sweet/fatty food (SF) and ethanol (EtOH) intake (Imperato and Di Chiara, 1986; Saper et al., 2002). For example, both SF and EtOH reliably alter the firing rate of NAc medium spiny neurons (Janak et al., 1999; Robinson and Carelli, 2008; Taha and Fields, 2005; Tellez et al., 2012) and increase extracellular dopamine levels (Doyon et al., 2003; Gambarana et al., 2003; Hernandez and Hoebel, 1988; Imperato and Di Chiara, 1986; Weiss et al., 1993; Wilson et al., 1995), like most addictive drugs (Di Chiara et al., 2004; Di Chiara and Imperato, 1988). Similarly, the repeated consumption of addictive substances or palatable food has been shown to result in similar adaptations within the NAc (e.g., decreased expression of dopamine D2 receptors). There are notable differences between EtOH and sucrose which is commonly used as a control to measure the effects of EtOH (Bachtell et al., 1999) or used to ensure that an experimental manipulation is altering the rewarding value of EtOH specifically and not altering reward processing in general (Rezvani et al., 2014). However, the goal in comparing EtOH to SF is to determine whether there are any electrophysiological biomarkers that generalize across substances, regardless of the known differences between how those substances affect brain activity.
Despite the above evidence supporting convergent neuronal processes underpinning the consumption of different rewarding substances, it remains unclear if the temporal organization of NAc neuronal activity, as captured by neural oscillations, is similarly convergent across substances. The ensembles of NAc neurons whose activity contributes to the regulation of reward-driven consummatory behavior partially overlap, with some neurons responding to multiple substances. For example, the activity of NAc medium spiny neurons display similar patterns of firing around periods of consumption with only minor variation across substances (Carelli et al., 2000). Similarly, while the phasic release of dopamine within the NAc across substances has shown variation in magnitude, the overlapping release dynamics are the same.
High temporal resolution, systems-level neural data from the ventral striatum during EtOH and SF consumption is limited. However, there is evidence that EtOH consumption leads to detectable changes in NAc LFPs which are more pronounced in alcohol preferring rats (McCane et al., 2018). There are also shifts in NAc LFPs when rats receive a food reward (Hernandez and Cheer, 2012; McCane et al., 2018), and we’ve previously demonstrated that LFPs recorded from the NAc can be used to differentiate epochs of binge eating (consumption) from all other behavior, as well as predict if feeding is about to occur (Dwiel et al., 2019). Similar prediction models have been used to trigger DBS of the nucleus accumbens (NAc) to reduce the amount of palatable food consumed in a rodent model of binge eating (Wu et al., 2018), and this approach is being implemented in a clinical trial for loss-of-control eating in patients with treatment-resistant obesity.
Having already found NAc derived LFP biomarkers of SF consumption, we hypothesized that the same would be possible for rats conditioned to drink 10% EtOH (Henricks et al., 2019a, 2019b). We also hypothesized that a single predictive model may be able to use LFPs to differentiate both EtOH and SF consumption from all other behaviors, including water and house chow consumption. To test these hypotheses, we constructed predictive models built to detect consumption from LFP data recorded during limited access SF or EtOH sessions. The performance of the resulting generalized vs. substance-specific models sheds light on the similarities and differences in the temporal dynamics of ventral striatal processing between EtOH and SF consumption. It is important to note that many of the studies evaluating NAc responses to different rewarding substances have utilized operant based self-administration models that contextualize the substances as rewards for some behavior (e.g., lever-pressing) thus eliciting signals representing features associated with those behaviors that may not be related to EtOH of SF consumption (Saddoris et al., 2015). In the current study, we aimed to model more naturalistic, internally initiated epochs of consumption that may be more clinically relevant to substance use. Further, since poly-substance use is common in clinical populations, it is important to know if there is a predictable, shared signature for the use of more than one substance that can serve as future biomarkers to trigger interventions.
Materials and Methods
Three cohorts of 60-day old Sprague-Dawley rats (Charles River, Shrewsbury, MA) were individually housed using a reverse 12-hour light/dark schedule with house chow (HC) and water (H2O) available ad libitum. We carried out all experiments in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications no. 80–23, revised in 1996) and approved by the Institutional Animal Care and Use Committee at Dartmouth College.
SF Experiment:
The presented feeding data represent a novel analysis from previously published experiments (Doucette et al., 2018; Dwiel et al., 2019). Briefly, we conditioned a cohort (n = 9 males) to binge SF using an intermittent limited access paradigm without food restriction. When HC was made available with the SF diet, there was minimal to no HC consumption during the limited access sessions. In order to get enough epochs of HC consumption for model building we restricted access to HC in the home cage for 24 hours and provided access to either the HC or SF in the limited access sessions. Due to the near complete preference for the SF diet over the HC, we could not collect consumption data for both diets within the same session. Data for both SF and HC were collected following 24 hours of HC restriction to keep hunger levels comparable with rats prior to HC and SF access.
EtOH Experiment:
As with the feeding data, the drinking data are a novel analysis of data collected from previously published studies (Henricks et al., 2022, 2019b). We conditioned a cohort (n = 4 males and 5 females) to drink 10% EtOH using intermittent limited access. For these experiments both EtOH and H2O were available within the same limited access sessions and animals consumed enough of each to be able to build our models.
General methods
Electrode implantation
Surgery and electrode construction was similar to our previous experiments (Doucette et al., 2015); briefly, we built custom electrode arrays with a custom milled plastic base and polyimide tubing that guides a 50 μm nichrome wire to each brain location. We implanted the electrodes such that the polyimide tubing and wires were below the skull, with the plastic base attached to the skull surface with acrylic cement. We targeted the first cohort’s (feeding) electrodes to the bilateral NAc shell (AP 1.2 mm; ML ±1; and DV −7.6 mm relative to bregma) and the NAc core (AP 1.2 mm; ML ±2.4 mm; and DV −7.6 mm relative to bregma). We targeted the second and third cohort’s (drinking) electrodes to the bilateral rat medial prefrontal cortex (infralimbic cortex - AP 3.4 mm; ML ±0.75 mm; and DV −5 mm relative to bregma) and NAc shell. Therefore, all cohorts shared bilateral NAc shell LFPs and we first limited our model building to these shared features.
Behavioral measures
Following recovery from surgery (~1 week), we conditioned rats using intermittent limited access to either: 1) SF (Figure 1A), containing 4.6 kcal/g and 19% protein, 36.2% carbohydrates, and 44.8% fat by calories (Teklad Diets 06415, South Easton, MA); or 2) 10% EtOH (Figure 1B), as described previously (Dwiel et al., 2019). After 16–20 sessions the rats consumed a stable and significant amount of SF (54±12% of their daily caloric intake; mean ± 1 standard deviation). On average, rats drank 0.49±0.38 g/kg of EtOH (mean ± 1 standard deviation) corresponding to a BEC of about 10 mg/dl (Li et al., 2011). We weighed the SF and HC or EtOH and H2O before and after all sessions to calculate the amount consumed during the session (Figure 1C and D insets, respectively). However, as previously demonstrated by our group, a subset of animals escalated their EtOH consumption significantly while others did not, which reflects what others have shown regarding significant individual variability in alcohol preference among rats (Henricks, Dwiel, 2019). All animals were included in the analysis. Figure 1C and D illustrates the percent of rats actively consuming SF or EtOH over the course of a limited access session at the end of conditioning.
Figure 1.
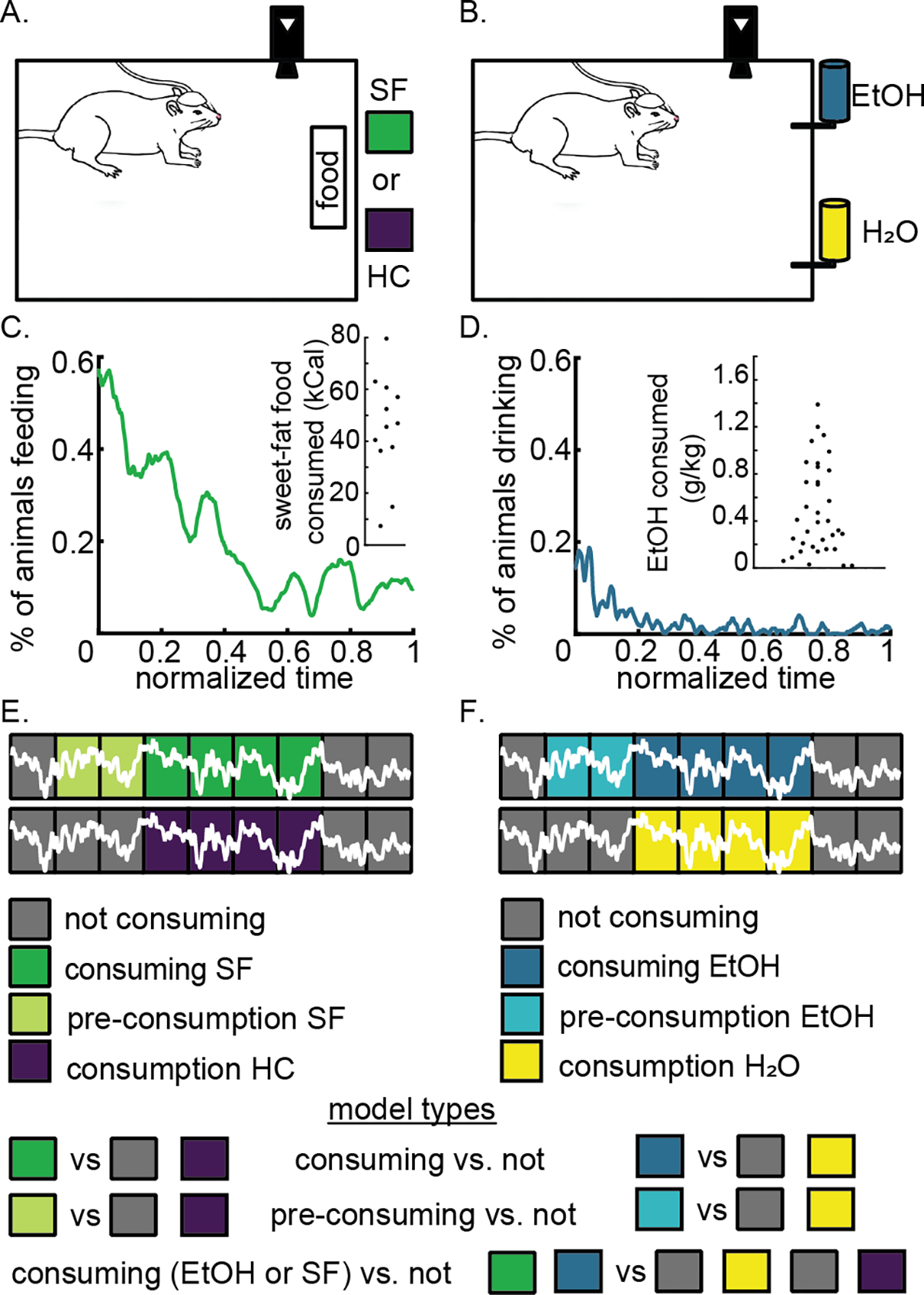
Naturalistic feeding and drinking behavior synchronized to local field potential recording to build models predicting consuming sweet-fat food (SF) or 10% ethanol (EtOH). Panel A depicts the experimental setup in which we recorded video and local field potentials while rats had free access to either SF or house chow (HC) within the recording chamber. Panel B depicts a similar setup for drinking behavior except that rats could have access to water (H2O) and EtOH simultaneously. Panels C and D illustrate the percent of rats engaged in SF or EtOH consumption through normalized session time and the amount of SF or EtOH consumed inset. Panels E and F depict how local field potentials are divided into 5 second bins that are aligned within hand-scored behavioral intervals (green and blue) and calculated behavioral intervals (light green and light blue). We used this segmentation of the data to construct models as outlined in model types (below E and F).
Local field potential recording and processing
We tethered rats in an 18”x12”x24” chamber through a commutator to a Plexon data acquisition system (Plexon, Plano, TX) with time-synchronized video for offline analysis. We manually scored videos that were time-locked to the neural data to identify intervals of food or liquid consumption using Plexon Cineplex software (Figure 1A and B). We used custom code written for Matlab R2019a for all LFP signal processing as previously reported (Dwiel et al., 2019), and used the following frequency bands: delta = 1–4 Hz, theta = 5–10 Hz, alpha = 11–14 Hz, beta = 15–30 Hz, low gamma = 45–65 Hz, and high gamma = 70–90 Hz. To compare recordings across rats and across days, we normalized power per frequency band as the percent of total power of the signal from 1 to 90 Hz, and normalized coherence per frequency band and channel pair as the z-score from the average coherence within each channel pair. From the NAc shell electrodes (2 signals) shared across cohorts we calculated 18 features: 12 power features (6 from each brain hemisphere representing the 6 frequency bands) and 6 coherence features (one from each frequency band between left and right NAc shell).
Verification of electrode placement
We euthanized rats at the end of the experiment using CO2. For histologic verification of electrode placement we removed the brains, flash froze in 2-methylbutane, sectioned with a cryostat, mounted on slides, and stained with thionine (Doucette et al., 2015). No rats required exclusion based on electrode location (Doucette et al., 2018; Henricks et al., 2019b).
Model building overview
We aligned non-overlapping 5-second bins of LFP data to fit within one of four possible behavioral categories: 1. “consuming” for SF or EtOH; 2. “control” for house chow (HC) or H2O; 3. “pre-consumption” for SF or EtOH or; 4. “not” for all other behavior (i.e., not “consuming”, not “control”, and not “pre-consumption”). We defined pre-consumption bins as those occurring up to 1 minute prior to consumption initiation and we discarded them if they overlapped with a previous consuming bin (Figure 1E and F). We included H2O and HC data in our model training to control for potential noise generated due to the physical act of consuming (e.g., chewing and swallowing), as well as signals that are not specific to the consumption of SF or EtOH, but are features of eating and drinking in general. We used the same number of bins from each rat (weighting bins as needed) and balanced the number of bins for each group (e.g., consuming vs. not+control) to prevent the creation of a majority classifier; this resulted in the equivalent of 40 bins of either SF or EtOH, 20 bins of HC or H2O, and 20 bins of all other behaviors from each rat.
We implemented the classifier lasso using cvglmnet() with 10-fold cross validation and validated the model using left-out data (excluded from model building) to calculate the classification probabilities with predict(), constructing a receiver operator characteristic (ROC) curve and calculating the AUROC with the function perfcurve(). We built these models with the shared 18 LFP features and used multiple iterations of random data selection for model building and testing with the reported performances being the average of the 100 iterations with 95% confidence intervals. We used statistical inference by permutation to assess the by-chance accuracy of the models. Specifically, we used Monte Carlo sampling to shuffle the assignment of predictors (i.e., LFP features) and outcome variables (e.g., consuming vs. not+control) from the same data sets described above. We compared each real model’s mean performance (AUROC) to a distribution made from the performance of the permuted models. We calculated P-values with p = b+1/m+1, where b = number of permuted instances greater than the group mean and m = the total number of permutations. We used the lasso algorithm for all models except when we built single feature logistic models, glmfit(), using each LFP feature individually to quantify the amount of predictive information of each feature alone. This allowed us to determine if the same features were similarly predictive across datasets.
Identifying predictive LFP features
To quantify how much predictive information was contained within each LFP feature, we also built the consuming vs. not+control and pre-consumption vs. not+control models using each LFP feature by itself with 100 iterations of sub-sampling and weighting and compared the average AUROC to distributions of AUROCs obtained from permuted single feature models. To compare the performance of each feature between datasets we represented the direction of the correlation between the LFP features and consumption by giving the AUROCs a sign (e.g., if increased beta power from the right NAc shell correlated with drinking EtOH, then the AUROC would be positive). In this way, we can visualize features with an AUROC above 0.5 on a 2-dimensional Cartesian plot such that features that correlate with the behavior in the same direction across feeding and drinking models will lie in quadrants I and III.
Due to the multicollinear nature of LFP features, building models with single features does not account for features jointly contributing information. To account for this, we applied a correlated feature perturbation method (Bârzan et al., 2022) in which sets of collinear features are permuted together. Briefly, after training the models on the full feature data (all 18 features) we calculated the pairwise correlations between all features in the test data. For each of the 18 features we defined a feature set as the features with whom it had a correlation coefficient of at least 0.5. Next, we permuted this feature set in the test data and calculated the performance of the original model on the permuted test data. To quantify the effect of permuting these features we then subtracted this performance from the performance on non-permuted data, providing a change in AUROC. As there were a different number of features in the different feature sets (i.e., 1 to 10 features) we checked if the decrease in AUROC correlated with the number of features permuted using the Spearman coefficient for the feeding and drinking, both separately and combined.
Building models for specific substances – consuming vs. not+control
First, we analyzed the feeding dataset and drinking dataset separately to determine if LFPs during consuming SF or EtOH were differentiable from LFPs during all other behaviors (not) + consuming the respective naturalistic control (i.e., HC or H2O). From each rat, we used 40 total bins: 20 consuming bins (i.e., consumption of SF or EtOH), 10 naturalistic control bins (i.e., HC or H2O), and 10 not bins (i.e., all other behaviors). To minimize overfitting we: 1) held out a random 20% of the data for a test set (80:20); or 2) held out each rat as a test set (leave-one out; LOO). For the 80/20 split, we repeated the sub-sampling and weighting process 100 times. For LOO, we repeated the sub-sampling/weighting 100 times for each of the held-out rats. We also split the test set into consuming vs. naturalistic control and consuming vs. not to assess if the models were actually differentiating the consumption of EtOH or sweet-fat food from their naturalistic controls. We first built models using the LFP shared across cohorts (18 features from bilateral NAc shell) to ensure that performances were directly comparable. Models using more features specific to each cohort are presented in supplemental figures.
Sex differences in predictors of EtOH drinking
To explore possible sex differences in the relationship between LFPs and epochs of EtOH consumption. From each rat, we used 20 bins of LFP data: 10 drinking EtOH, 5 drinking water, and 5 not drinking anything. For each sex, we built 500 models by 100 iterations of sub-sampling and weighting for each rat left out for testing. We then tested these models on the left-out rats of the same sex or rats from the opposite sex to determine if models performed better within sex.
Building models that generalize across substances
To determine if we could build a model to detect times of consumption of either SF or EtOH, we used the 18 LFP features shared between the SF and EtOH cohorts; 12 power features and 6 coherence features from bilateral NAc shell. We then created training and test sets using both 80/20 and LOO. For LOO, we had 81 training/test sets representing all possible iterations that included a rat from each cohort (i.e., feeding and drinking) in the left-out test data. Even though the datasets for SF and EtOH were recorded from different rats at different times, the models were trained such that each rat is contributing data on each side of the binomial classification (consumption [SF or EtOH] vs. not+naturalistic control). This ensures that LFP feature value differences across rats, and recordings occurring at different times under different contexts, are not able to contribute information that would influence model performance.
Pre-consumption vs. not
For greater temporal resolution, we calculated LFP features using a 5-second window with 80% overlap (i.e., ‘advancing’ the window by 1 second), up to a minute before feeding or drinking began. We built the models using 20 bins from each rat: 10 pre-consumption bins that immediately preceded consumption (i.e., the first 5 seconds before consumption) and 10 not bins at least 1 minute away from consumption (Figure 1E and F). We used 100 iterations of holding out 20% of the data for the test set. Again, we first built pre-consumption vs. not models using the shared LFP features and then built models using all available LFP features from each cohort.
Visualizing LFP feature changes around consuming epochs
To visualize the dynamics of features of interest, we used the normalized feature values from all recordings around both feeding and drinking, up to 1 minute before and after consumption occurred. Due to the differences in the average bout length of consumption between feeding and drinking, with feeding bouts being longer than drinking bouts, we used 9 seconds of data at the beginning and end of feeding while we used 5 seconds for drinking. For the sake of visualization, the variance is only displayed for the feature of interest around the behavior and we plot the mean of that feature across all other behaviors (‘not’).
Results
Common LFP features predicting consumption vs. not+control
Feeding
Figure 2A illustrates that LFPs recorded from the bilateral NAc shell are capable of classifying consuming SF from all other behavior including consuming HC. The 80/20 models classified consuming SF with an average AUROC of 0.77±0.01 and permuted models performed with an average AUROC of 0.51±0.02 (p = 0.01; Figure 2A). Including the LFPs from the NAc core led to a performance of 0.8±0.01 vs. permuted 0.51±0.02 (p = 0.01; Supplemental Figure 1A). When we used LOO testing on the common features, the performance for consuming SF vs. not + control classification was 0.76±0.01 and the corresponding permuted performance was 0.50±0.01 (p = 0.07; Supplemental Figure 2A). LOO testing with all features had a performance of 0.68±0.01 vs. 0.51±0.01 (p > 0.05; Supplemental Figure 3A). Splitting the test sets revealed that the model was equally able to differentiate SF from other behaviors and SF vs. HC (Figure 2B, p = 0.01; Supplemental Figure 1B). Single feature analysis for 80:20 models revealed the top features used by the feeding model to be SLd, SRd, SLa, SLb, SRb, and SLhg power (Extended Data Table 1; Supplemental Figure 5B).
Figure 2.
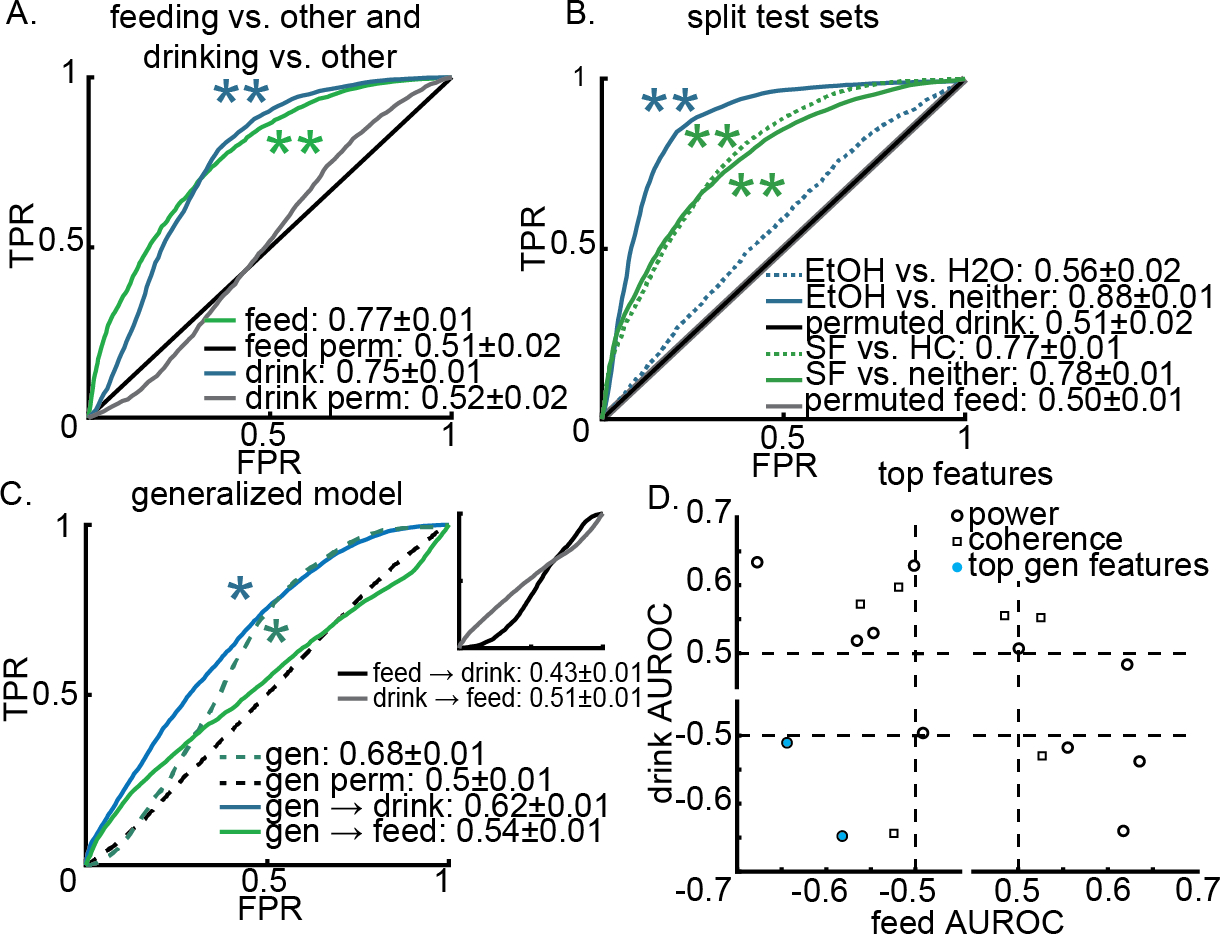
Local field potentials (LFPs) recorded in the nucleus accumbens (NAc) shell can predict sweet-fat food (SF) or 10% ethanol (EtOH) consumption versus all other behavior more accurately as separate models than as a single general model. Panel A illustrates the performance of models predicting SF (green) or EtOH (blue) consumption versus all other behavior using only LFP features from the bilateral NAc shell. These receiver operator characteristic (ROC) curves illustrate how the true positive rate (TPR) varies in relation to the false positive rate (FPR). The average area under the ROC curve (AUROC) is shown ± 95% confidence intervals. Panel B shows the performance of the feed and drink models from A on split test sets; e.g., the performance of the drink model on differentiating EtOH from H2O (dashed blue) or differentiating EtOH from other, non-drinking, behaviors (solid blue). Panel C shows the performance of generalized (gen) models built and tested on a combination of the feeding and drinking datasets (dashed teal curve). The blue (drinking) and green (feeding) curves illustrate how the general model performs on those specific types of data. The inset shows the performance of feeding models on the drinking dataset and vice versa. Panel D depicts how each of the 18 LFP features perform (AUROC) when we build and test models on each dataset separately. The sign of the AUROC value indicates the direction of feature change during consumption – if features change in the same way for both types of consumption they would be in quadrants I and III. The blue features (right delta power and left high gamma power) are the most important features in the generalized models. See Extended Data Table 1 for more details, Supplemental Figure 1 for models using all available features, and Supplemental Figure 2 for models using LOO testing.
Table 1.
Single feature performance from 80:20 models classifying feeding and drinking. Model performance presented as average area under the receiver operator characteristic curve (AUROC) of the model built with a given feature. Sign of the performance delineates the direction of the correlation. S = shell; R = right; L = left; d = delta; t = theta; a = alpha; b = beta; lg = low gamma; and hg = high gamma. Power features have one site while coherence have two; e.g., ‘SLd −0.68’ means that a model could use the decrease in delta power at shell left to classify feeding behavior and achieve an AUROC of 0.68 on the left out 20% of the data. Bold features outperformed corresponding permuted data sets with p <0.05; Supplemental Figure 5.
| feed | drink | ||
|---|---|---|---|
| SLd | −0.68 | SLSRhg | −0.65 |
| SRd | −0.64 | SLhg | −0.65 |
| SLb | 0.64 | SRb | −0.65 |
| SLa | 0.62 | SLd | 0.63 |
| SRb | 0.62 | SLt | 0.62 |
| SLhg | −0.58 | SLSRb | 0.59 |
| SRt | −0.57 | SLSRa | 0.57 |
| SLSRa | −0.56 | SLSRt | 0.56 |
| SRa | 0.56 | SLb | −0.56 |
| SRhg | −0.55 | SLSRd | 0.56 |
| SLSRlg | 0.53 | SRa | −0.54 |
| SLSRt | 0.53 | SRd | −0.53 |
| SLSRhg | −0.52 | SRhg | 0.53 |
| SLSRb | −0.52 | SLSRlg | −0.52 |
| SLt | −0.50 | SRt | 0.51 |
| SRlg | 0.50 | SRlg | 0.50 |
| SLlg | −0.49 | SLlg | −0.50 |
| SLSRd | 0.49 | SLa | 0.47 |
Drinking
Figure 2A illustrates that LFPs recorded from the bilateral NAc shell are capable of classifying brain activity recorded during consuming EtOH from all other behavior including consuming H2O; however, splitting the test sets revealed that the performance was largely coming from differentiating EtOH from other behaviors and not from H2O. The 80/20 models classified consuming EtOH with an average AUROC of 0.75±0.01 and permuted models performed with an average AUROC of 0.52±0.02 (p = 0.01; Figure 2A). Including the cortical LFPs led to a performance of 0.84±0.01 vs. permuted 0.51±0.02 (p = 0.01; Supplemental Figure 1A). When we used LOO testing on the common features, the performance for consuming EtOH vs. not + control classification was 0.72±0.01 and the corresponding permuted performance was 0.49±0.02 (p > 0.05; Supplemental Figure 2A). LOO testing with all features had a performance of 0.81±0.01 vs. 0.51±0.02 (p > 0.05; Supplemental Figure 3A). Splitting the test sets revealed that the model was primarily differentiating EtOH from other behaviors (Figure 2B; p < 0.01), and performed poorly at differentiating between EtOH and H2O (Figure 2B; p > 0.05). However, including cortical LFPs improved this differentiation (0.69±0.01, p < 0.05; Supplemental Figure 1B). Single feature analysis for 80:20 models revealed the top features used by the drinking model to be SLd, SLt, SLb, and SLhg power and SLSRb and SLSRhg coherence (Extended Data Table 1; Supplemental Figure 5A).
Testing models across datasets
When either 80/20 or LOO approaches were evaluated on data of the other substance type the performances dropped to chance level (i.e., AUROC = 0.5) or below (Figure 2C inset and Supplemental Figure 2C inset). The “drink→feed” (built on drinking data and tested on feeding data) had an average AUROC of 0.51±0.01 (80/20) and 0.49±0.01 (LOO). The “feed→drink” performances had an average AUROC of 0.43±0.01 (80/20) and 0.43±0.01 (LOO). While either feeding or drinking behavior could be classified, these models did not generalize across substance types. To determine if a single model could be capable of predicting both SF and EtOH consumption from all other behavior a model was trained on the combination of both datasets.
Testing models built to generalize across substances
When both datasets (feeding and drinking) were used in training the model, consumption of either SF or EtOH could be differentiated from all other behavior, including HC and H2O consumption (Figure 2C). The performance of models predicting SF+EtOH vs. not+control had an average AUROC of 0.68±0.01 for 80:20 with a corresponding permuted performance of 0.5±0.01 (p = 0.01; Figure 2C). When these generalized models were tested on the individual datasets (e.g., gen → feed) the AUROC on the feeding data was 0.54±0.01 and 0.62±0.01 on the drinking data (p > 0.05 and p = 0.02; Figure 2C).Using the LOO approach, the performance of models predicting SF+EtOH vs. not+control had an average AUROC of 0.66±0.03 with a corresponding permuted performance of 0.52±0.03 (p > 0.5; Supplemental Figure 2C). When the LOO generalized models were tested on the individual datasets the AUROC on the feeding data was 0.5±0.04 and 0.63±0.04 on the drinking data (p > 0.05; Supplemental Figure 2C).
To explore why the generalized models performed poorly compared to the substance specific models, and performed better on one dataset than the other (i.e., drink > feed) the performance of single feature logistic models built and tested on drinking or feeding data were compared (Figure 2D; Extended Data Table 1 and 2). Many single LFP feature models performed better than chance for both substance types, but the direction of change around consumption was frequently in the opposite direction (quadrants II and IV in Figure 2D). Very few features changed in the same direction around consumption of EtOH and SF as indicated by the small number of features in quadrants I and III. The same pattern emerged from the LOO models as well (Supplemental Figure 2D). The single feature analysis highlighted the importance of decreased delta power predicting feeding and decreased high gamma coherence and power predicting drinking (Extended Data Table 1 and 2). Permuting the correlated feature sets (Supplemental Figure 4A–B) revealed a similar pattern with permuting low frequency power (delta through beta) feature sets causing the largest decreases in AUROCs for predicting feeding while permuting feature sets with high gamma coherence led to larger decreases in AUROC for predicting drinking (Extended Data Table 3). However, the high gamma coherence feature sets also contained lower frequency coherence (i.e., theta and beta; Extended Data Table 3). Although there was a significant correlation between the number of features permuted and the decrease in AUROC for the feeding data, this correlation did not hold for the drinking data or the combined, feeding and drinking, data (Supplemental Figure 4C).
Table 2.
Single feature performance from leave-one out (LOO) models classifying feeding and drinking. Model performance presented as average area under the receiver operator characteristic curve (AUROC) of the model built with a given feature. Sign of the performance delineates the direction of the correlation. S = shell; R = right; L = left; d = delta; t = theta; a = alpha; b = beta; lg = low gamma; and hg = high gamma. Power features have one site while coherence have two; e.g., ‘SLd -0.70’ means that a model could use the decrease in delta power at shell left to classify feeding behavior and achieve an AUROC of 0.70 in the left out rat.
| feed | drink | ||
|---|---|---|---|
| SLd | −0.70 | SRb | −0.67 |
| SRd | −0.67 | SLSRhg | −0.66 |
| SLb | 0.65 | SLSRt | 0.62 |
| SRb | 0.63 | SLhg | −0.61 |
| SLa | 0.62 | SLSRb | 0.60 |
| SLSRa | −0.61 | SLSRa | 0.59 |
| SLhg | −0.60 | SLSRd | 0.58 |
| SRhg | −0.57 | SLb | −0.58 |
| SRa | 0.56 | SLd | 0.58 |
| SLSRlg | 0.55 | SLt | 0.57 |
| SRt | −0.55 | SRa | −0.55 |
| SLSRb | −0.52 | SRhg | 0.52 |
| SLSRt | 0.50 | SLSRlg | −0.52 |
| SLt | −0.49 | SLlg | 0.49 |
| SLSRhg | −0.47 | SRd | −0.47 |
| SLlg | −0.47 | SRt | 0.47 |
| SRlg | 0.44 | SRlg | 0.46 |
| SLSRd | 0.38 | SLa | 0.44 |
Table 3.
Decrease in model performance with permuted correlated feature sets. Bold feature indicates the feature compared to all other features. For example, feature group 1 in the feeding data was obtained by computing the correlation coefficient between SLd and all other features and those with R>0.5 (SLt, SRd, SRt) were included in the feature group. When these features were permuted, the model performance (area under the receiver operator characteristic curve; AUROC) decreased by 0.25. See Supplemental Figure 4 for correlation matrices. S = shell; R = right; L = left; d = delta; t = theta; a = alpha; b = beta; lg = low gamma; and hg = high gamma.
| feed | drink | |||
|---|---|---|---|---|
|
|
||||
| feature group | features | drop in AUROC | features | drop in AUROC |
| 1 | SLd, SLt, SRd, SRt | 0.25 | SLd, SLt, SLa, SLb, SRd, SRt | 0.18 |
| 2 | SLd, SLt, SLa, SLb, SRd, SRt, SRa, SRb | 0.25 | SLd, SLt, SLa, SLb, SRd, SRt | 0.18 |
| 3 | SLt, SLa, SLb, SRt, SRa, SRb | 0.24 | SLd, SLt, SLa, SLb, SRd, SRt, SRa, SRb | 0.19 |
| 4 | SLt, SLa, SLb, SRt, SRa, SRb | 0.24 | SLd, SLt, SLa, SLb, SLlg, SLhg, SRd, SRt, SRa, SRb | 0.19 |
| 5 | SLlg, SLhg, SRlg | 0.22 | SLb, SLlg, SLhg, SRlg, SLhg | 0.18 |
| 6 | SLlg, SLhg, SRhg | 0.23 | SLb, SLlg, SLhg, SRlg, SRhg | 0.18 |
| 7 | SLd, SLt, SRd, SRt | 0.25 | SLd, SLt, SLa, SLb, SRd, SRt, SRa, SRb | 0.18 |
| 8 | SLd, SLt, SLa, SLb, SRd, SRt, SRa, SRb | 0.24 | SLd, SLt, SLa, SLb, SRd, SRt, SRa, SRb | 0.18 |
| 9 | SLt, SLa, SLb, SRt, SRa, SRb | 0.25 | SLt, SLa, SLb, SRd, SRt, SRa, SRb | 0.19 |
| 10 | SLt, SLa, SLb, Srt, SRa, SRb | 0.24 | SLt, SLa, SLb, SRd, SRt, SRa, SRb, SRlg, SRhg | 0.18 |
| 11 | SLlg, SRlg | 0.22 | SLlg, SLhg, SRt, SRb, SRlg, SRhg | 0.18 |
| 12 | SLhg, SRhg | 0.23 | SLlg, SLhg, SRb, SRlg, SRhg | 0.18 |
| 13 | SLSRd | 0.21 | SLSRd | 0.20 |
| 14 | SLSRt, SLSRhg | 0.22 | SLSRt, SLSRhg | 0.24 |
| 15 | SLSRa | 0.24 | SLSRa | 0.22 |
| 16 | SLSRb, SLSRhg | 0.22 | SLSRb, SLSRhg | 0.19 |
| 17 | SLSRlg | 0.22 | SLSRlg | 0.17 |
| 18 | SLSRt, SLSRb, SLSRhg | 0.22 | SLSRt, SLSRb, SLSRhg | 0.23 |
Sex differences in classifying EtOH drinking
Models trained and tested within sex out performed chance (M→M = 0.8±0.02, p = 0.036; F→F = 0.75±0.01, p = 0.028) and when these sex-specific models were tested across sexes, performance dropped similarly for both sexes (M→F = 0.74±0.02, p = 0.08; F→M = 0.73±0.02, p = 0.08). When the model built on both sexes (Figure 3A blue curve) was tested on each sex independently (Figure 3A), the model still outperformed chance (drink→F = 0.84±0.01, p = 0.01; drink→M = 0.83±0.01, p = 0.01). Models using LOO testing showed a similar pattern of performance (Supplemental Figure 1C and D).
Figure 3.
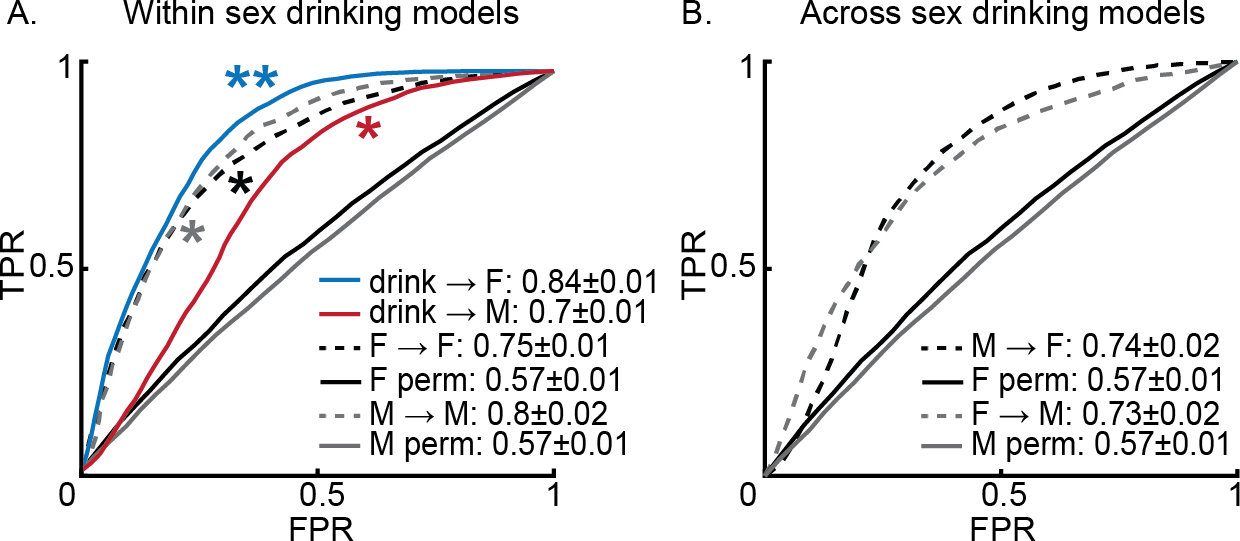
Local field potentials (LFPs) recorded in the nucleus accumbens (NAc) shell can predict 10% ethanol (EtOH) drinking vs. all other behaviors including drinking water equally across sexes. Panel A shows the performance of the drinking model from Figure 2A on test sets split by sex (blue and red lines) as well as the performance of models restricted to female (black dashed and solid) or male (gray dashed and solid) data (p > 0.05). Panel B shows the performance of the within-sex models, shown in A, tested across sexes (male to female [M→F]; female to male [F→M]). ** p ≤ 0.01, * p ≤ 0.05
Common LFP features predicting pre-consumption vs. not
LFPs recorded immediately preceding consumption identified intervals of imminent SF or EtOH consumption.
Feeding
The performance of models built to identify the 5 seconds immediately preceding SF consumption versus not outperformed permuted models and demonstrated declining performance with temporal distance from the start of SF consumption (Figure 4A). To visualize how the model performance increases approaching feeding initiation related to predictive LFP features, we visualized an individual LFP feature around SF consumption (Figure 4B). The horizontal black line indicates the ‘not’ average feature value and the dashed green horizontal line the SF consumption average feature value. In this example, high gamma power in the left NAc shell drops leading up to the consumption of SF food. To confirm that LFP features used to predict imminent SF consumption were likely capturing the same LFP changes that manifest during consumption, we determined the performance of single feature logistic models built and tested on SF consumption or pre-consumption data sets. We found that many single LFP feature models perform better than chance for both model types and that the direction of change frequently occurred in the same direction (quadrants I and III in Figure 4C).
Figure 4.
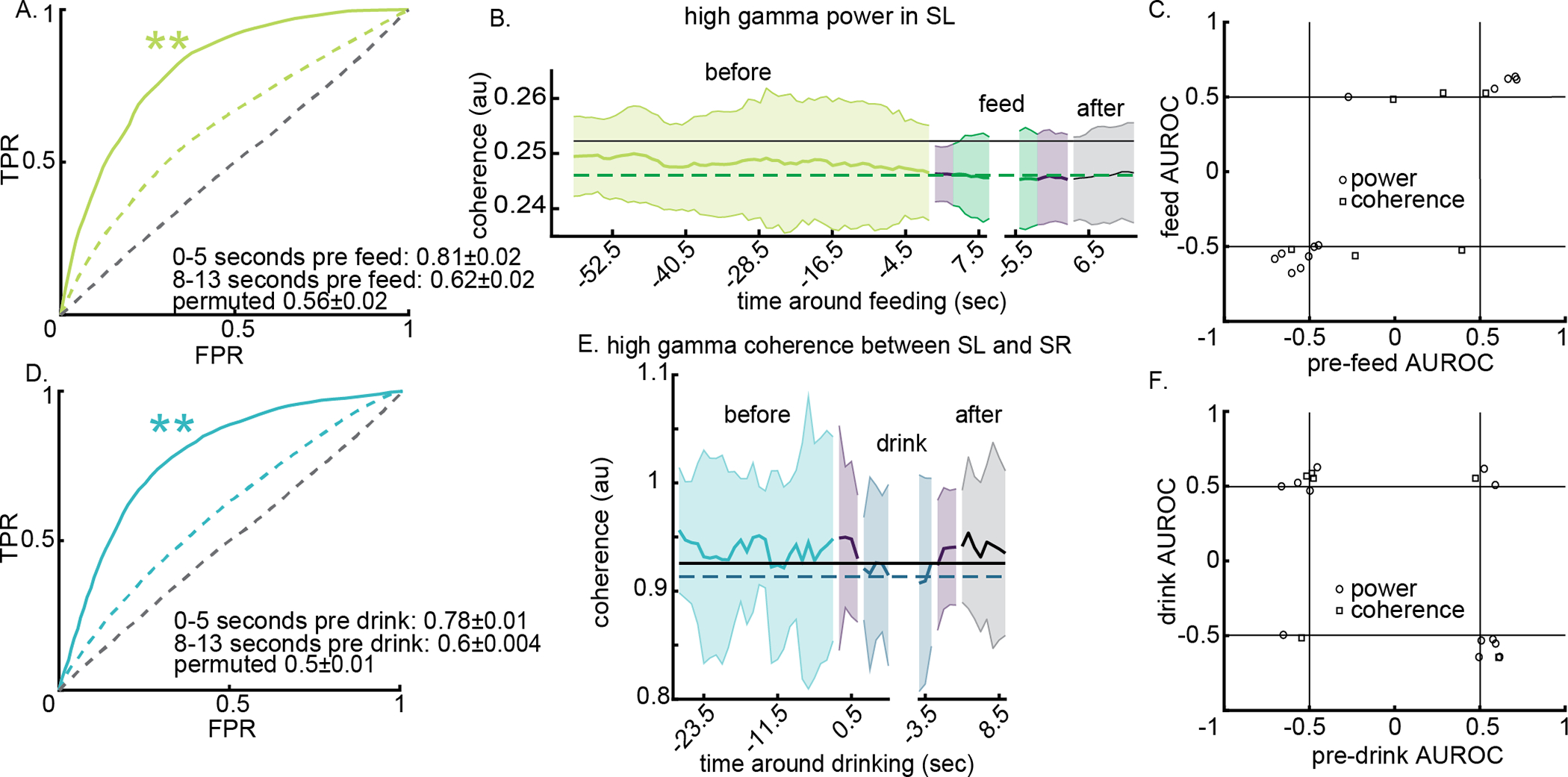
Local field potentials (LFPs) recorded from rat ventral striatal regions are able to predict that a rat is about to consume sweet-fat food (SF) or 10% ethanol (EtOH). Panels A and D illustrate model performance when trained on data from 0 to 5 seconds before feeding (A) or drinking (D) and tested on left out data from 0 to 5 seconds before the behavior and 8–13 seconds before the behavior. In both cases, it is possible to differentiate pre-feeding or pre-drinking from all other behaviors (A, p = 0.01; D, p = 0.01). However, these models fail when tested on data from 8 to 13 seconds before the behavior (A, p = 0.11; D, p = 0.08). Panels B and E are examples of how single LFP features vary around intervals of eating SF (B) and drinking EtOH (E). Light green and light blue indicate feature values from 5 second bins prior to consumption, purple segments indicate data bins that straddle two behavior categories – the 5 second analytical window includes time in both behaviors – and black/gray indicates time after consumption interval. Solid black/gray lines indicate the average feature value during all other behaviors and dashed horizontal lines indicate average feature value during consumption; variance not shown. Panels C and F show the performance of individual LFP features (AUROC) and the direction of feature change in the corresponding consumption (y-axis) and pre-consumption (x-axis) models for feeding (C) and drinking (F). ** p < 0.01
Drinking
The performance of models built to identify the 5 seconds immediately preceding consuming EtOH versus not outperformed permuted models (Figure 4A, p = 0.01) and performance decreased with temporal distance from the start of EtOH consumption (Figure 4D). As shown for the feeding data, we visualized a top feature from EtOH pre-consumption models around intervals of consuming EtOH (Figure 4E). The black horizontal line indicates the not average feature value and the dashed blue horizontal line the EtOH consumption average feature value. In this example, high gamma coherence between left and right shell shows no ramping changes leading up to EtOH consumption and instead undergoes a rapid change upon initiation of EtOH consumption. The performance of single feature logistic models built and tested on EtOH consumption or pre-consumption data sets showed that few features correlated with behavior in the same direction (Figure 4F).
Discussion
These results suggest that neural oscillations recorded from the NAc shell are predictive of when the consumption of EtOH or SF is occurring compared to all other behaviors, although EtOH was more difficult to differentiate from its naturalistic control of H2O than SF was from HC when only using striatal LFPs. Models built to predict consumption of both substances reveal that the features of NAc shell oscillations containing information about EtOH consumption are mostly distinct from those that are predictive of SF consumption. Further, LFP features recorded from the NAc shell change in advance of SF or EtOH consumption allowing for the prediction of imminent (up to 5 seconds before) consumption of both substances, but these models could not predict consumption using data from ~10 seconds before consumption. Overall, this work demonstrates that systems-level brain activity (i.e., neural oscillations) can identify periods of consumption (monitoring biomarker) of at least two distinct rewarding substances (SF and EtOH) from all other behaviors in real-time, when models are trained on data for that specific substance. Models attempting to classify consumption of either substance (EtOH and SF) from a general model showed poorer performance, suggesting that there are unique oscillatory patterns associated with SF and EtOH consumption. These results suggest that the temporal organization of activity underpinning the recorded oscillations is largely distinct between EtOH and SF consumption.
Predictive features
Although there was little overlap in LFP features that could differentiate EtOH and SF consumption from naturalistic controls, decreased NAcS delta and high gamma power correlated with consumption for both substances (Figure 2D and Extended Data Table 1). Beta power was also predictive of consumption for both substances, but in opposite directions with decreased beta power during drinking and increased during feeding (Extended Data Table 1). The correlated feature perturbation revealed that decreased low frequency power correlated with feeding while decreased high frequency coherence correlated with drinking (Extended Data Table 3). The single feature analysis shows that although none of the features are able to perform as well as the full model, they still perform better than chance (e.g., for feeding, SLd AUROC = 0.68 vs. full feature model AUROC= 0.77; and for drinking, SLSR hg AUROC = 0.65 vs. full feature model AUROC = 0.75). Similarly, the correlations between features (Supplemental Figure 4 A–B) suggest that the LFP features used here have non-trivial redundancy. Together these data suggest that relatively simple models (i.e., models using few features) can predict feeding/drinking behaviors. Although these findings suggest that there may not be a specific change in a single given feature, the behaviors seem to be represented by more specific changes than full-spectrum shifts (i.e., globally decreased power or coherence).
During instrumental behaviors (e.g., pressing levers for reward) or during food reward receipt there is evidence for increased striatal low frequency power (Donnelly et al., 2014; Gruber et al., 2009); however, in our experiment the rats are not performing a task or receiving a reward per se, suggesting that there may be an intrinsic difference between these instrumental behaviors and outcomes and naturalistic feeding behavior as represented by low frequency striatal oscillations. There is also some evidence that increased dopamine signaling correlates with decreased delta power in the nucleus accumbens (Shi et al., 2022; Zhang et al., 2021), suggesting that there may actually be decreased dopamine signaling SF consumption which is characterized in part by decreased delta power. High gamma power has also been shown to correlate with behavior during a spatial task with transient increases in power upon reward receipt followed by a marked, and longer lasting, decline in power (van der Meer and Redish, 2009). Less is known about the acute effects of EtOH on striatal oscillations, however, there is evidence that EtOH disrupts corticostriatal synchrony, particular at theta frequency (McCane et al., 2018) which we see correlated with the reduced high gamma coherence (Extended Data Table 3), and this disruption may be connected to the increased EtOH consumption seen in alcohol preferring (P) rats. However, it must be noted that oscillations alone do not provide enough information to fully explicate the neurobiological mechanisms at play, but these experiments were designed to determine if oscillations could be used to predict behavior.
Implications for substance use disorders
A shared feature of addictive substances, like alcohol, is that they activate common brain regions – particularly the NAc – in ways that bias these networks to select behavior supporting continued substance use (Koob and Volkow, 2016). Though not traditionally considered an “addictive substance,” highly palatable food activates these networks in similar ways (Balleine, 2005), and can lead to maladaptive feeding behaviors like that seen in binge-eating disorder (Leigh and Morris, 2018). The NAc is known to play a role in the intake of hedonically desirable food (Zhang and Kelley, 2002, 2000) and this likely contributes to our ability to detect SF consumption using striatal LFPs. Further, while rats will readily consume palatable foods with little to no training (Corwin et al., 2011), EtOH requires relatively extensive limited access conditioning or sucrose fading to stimulate escalated consumption, which may explain the differences in consumption dynamics shown in Figure 1C and D. Although it is promising that NAc oscillations can identify periods of SF and EtOH consumption, an interesting next step would be to extend these findings to other addictive substances (e.g., cocaine, opioids, or nicotine). It will also be important to determine whether chronic intermittent exposure to EtOH, which leads to behavioral and neurobiological changes indicative of dependence, change the oscillatory dynamics of the NAc around drinking. Further, the consumption of SF is more easily differentiated both from HC consumption and all other behaviors while EtOH consumption is difficult to differentiate from H2O consumption; suggesting that SF consumption has a more unique neural representation than EtOH consumption. However, by including cortical LFPs, the difference between EtOH and H2O greatly improved, suggesting that this difference is represented outside of the NAcS alone. Overall, these data are an important demonstration that brain oscillations can identify when SF or EtOH is occurring (i.e., serve as a monitoring biomarker), which is a critical first step in the development of next generation closed-loop and adaptive treatment systems that could intervene to reduce the probability of problematic substance use.
Why is imminent EtOH consumption different from current consumption?
When we directly compared the features used for detecting current drinking and imminent drinking, we found little overlap, with many features correlating in opposite directions. Further, when we plotted features used by the model through time around the behaviors of interest, we found that the temporal dynamics of these features were radically different around SF consumption versus around EtOH drinking. Many of the features had slow changes leading up to the beginning of feeding and then would slowly return back to the average during all other behavior after SF feeding ended; however, in the case of EtOH drinking, the top features exhibited rapid shifts that were outside of the temporal resolution of the models we used to predict imminent behavior. Given that rats have a lower EtOH/H2O preference compared to SF/HC and must be conditioned in some way to escalate drinking (Priddy et al., 2017; Wayner et al., 1972; Wise, 1973), the striatal networks may be less or differently engaged in generating the appetitive drive. Although using a brief limited access paradigm did induce some rats to escalate EtOH consumption, the volumes ranged across individuals from clinically relevant (0.08 mg/dl) to almost zero (Figure 1D, inset). As mentioned above, our future work to predict imminent EtOH drinking in rats will be done in EtOH dependent animals, which may engage reward-related circuits (such as the ventral striatum) more so than in non-dependent animals. Alternatively, rodent strains bred for greater natural EtOH preference (e.g., P-rats) could also be investigated.
Sex differences in EtOH drinking
While there are known sex differences in EtOH drinking behaviors (Henricks et al., 2019b; Lancaster and Spiegel, 1992; Nieto and Kosten, 2017), our combined sex models suggest that NAc oscillations can be used to classify epochs of EtOH drinking in a sex independent manner and that, in general, the models are not relying on sex specific LFP features. However, our group has observed sex differences in the ability of corticostriatal oscillations to predict the amount of EtOH consumed in a session (Henricks et al., 2019a, 2019b). This suggests that while some aspects of drinking are processed in the brain in a sex-specific manner, information that predicts when EtOH drinking occurs or is about to occur is not dependent on sex.
Limitations and future directions
The SF data presented here was a re-analysis of a previously published dataset (Dwiel et al., 2019) that did not include female rats, thus preventing an assessment of sex specific LFP features of SF consumption. Future work would need to address the generalizability of these models to female rats as we evaluated here for the EtOH models. The presented EtOH data suggest that there is a significant amount of overlapping information contained within NAc LFPs. The overall number of rats used was relatively low (feeding: n = 9; drinking n = 9) which may have limited conclusions of model generalization across individuals. This likely explains the differences in model performance between the 80:20 and LOO testing strategies. However, our sample sizes ultimately came from the 5-second windows of clean data, with 100s of samples per self-administration session, and provided sufficient data for training models. Further, since the relationship between neural oscillations and behavior was correlational, a vital next step would be to manipulate the LFP features used to predict consuming SF or EtOH and determine if predicted behavior changes occur, as has been demonstrated in Parkinson’s disease with beta frequency oscillations (Chen et al., 2020; Kehnemouyi et al., 2021; Yin et al., 2021). Similarly, it could be the case that the amount of the substances consumed (EtOH or SF) could have an impact on the behavior of the rats which would then be reflected in the LFPs. Addressing this question will require a more thorough behavioral analysis paired with electrophysiology, and is an important future direction. Despite these limitations, however, these data support the potential of neural oscillations as monitoring biomarkers of SF and EtOH consumption and suggest differences between SF and EtOH in the temporal structure of neural activity around periods of consumption.
Although Sprague-Dawley rats, used here, will drink EtOH, there are other strains, like Wistar and P rats, that are known to drink more EtOH (Martinetti et al., 2007) and prefer higher concentrations of EtOH (Martinetti et al., 2006). We chose to use Sprague-Dawley rats in these experiments so that the data from the feeding and drinking cohorts could be directly compared, but future work would benefit from including other rat strains (e.g., Wistar, P, high-alcohol-drinking, or Marchigian Sardinian alcohol-preferring rats) to ensure our findings are not due to Sprague-Dawley rats’s relative distaste for alcohol (McBride et al., 2014). However, some strains that prefer alcohol exhibit increased anxiety- and depression-like behaviors which would also confound interpretation of the data (Borruto et al., 2021).
Conclusions
The consumption of EtOH and SF is reflected in neural oscillations recorded in the NAc, and allows for the classification of when either behavior is occurring compared to all other behavior, including naturalistic controls (ie., drinking water or eating less palatable house chow). Although both models outperformed chance, performance in the drinking model was largely driven by features that were related to drinking of EtOH and H2O while the performance in the feeding model used features specific to consuming SF and not HC. This means that SF consumption has a more unique representation in ventral striatal oscillations than EtOH consumption, which overlaps heavily with drinking H2O. Further, although a generalized model could be made to detect the consumption of multiple substances, this generalized model did not perform equally well on each substance. These data highlight the differences in how the ventral striatum represents the consumption of EtOH and SF and show that although there is potential for finding biomarkers related to substance use, it may be difficult to build a model that performs well detecting multiple substances.
Supplementary Material
Acknowledgments
This work was supported through an NIAAA training grant (F31AA027441; LD), the Hitchcock Foundation (AH), a NIDA T32 training grant (DA037202; AH), an LRP grant from NIH NCATS (KL2TR001088; WD), and a K08 grant from NIMH (1K08MH117347–01A1; WD). The authors have no competing financial interests to report.
References
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE (1999) Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 847, 157–165. [DOI] [PubMed] [Google Scholar]
- Balleine BW (2005) Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol. Behav. 86, 717–730. [DOI] [PubMed] [Google Scholar]
- Bârzan H, Ichim A-M, Moca VV, Mureşan RC (2022) Time-Frequency Representations of Brain Oscillations: Which One Is Better? Front. Neuroinform. 16, 871904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borruto AM, Stopponi S, Li H, Weiss F, Roberto M, Ciccocioppo R (2021) Genetically selected alcohol-preferring msP rats to study alcohol use disorder: Anything lost in translation? Neuropharmacology 186, 108446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ (2000) Evidence that separate neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J. Neurosci. 20, 4255–4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Gong C, Tian Y, Orlov N, Zhang J, Guo Y, Xu S, Jiang C, Hao H, Neumann W-J, Kühn AA, Liu H, Li L (2020) Neuromodulation effects of deep brain stimulation on beta rhythm: A longitudinal local field potential study. Brain Stimul. 13, 1784–1792. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM (2011) Feeding and reward: perspectives from three rat models of binge eating. Physiol. Behav. 104, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47 Suppl 1, 227–241. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 85, 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly NA, Holtzman T, Rich PD, Nevado-Holgado AJ, Fernando ABP, Van Dijck G, Holzhammer T, Paul O, Ruther P, Paulsen O, Robbins TW, Dalley JW (2014) Oscillatory activity in the medial prefrontal cortex and nucleus accumbens correlates with impulsivity and reward outcome. PLoS One 9, e111300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette WT, Dwiel L, Boyce JE, Simon AA, Khokhar JY, Green AI (2018) Machine Learning Based Classification of Deep Brain Stimulation Outcomes in a Rat Model of Binge Eating Using Ventral Striatal Oscillations. Front. Psychiatry 9, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette WT, Khokhar JY, Green AI (2015) Nucleus accumbens deep brain stimulation in a rat model of binge eating. Transl. Psychiatry 5, e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA (2003) Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol. Clin. Exp. Res. 27, 1573–1582. [DOI] [PubMed] [Google Scholar]
- Dwiel LL, Khokhar JY, Connerney MA, Green AI, Doucette WT (2019) Finding the balance between model complexity and performance: Using ventral striatal oscillations to classify feeding behavior in rats. PLoS Comput. Biol. 15, e1006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambarana C, Masi F, Leggio B, Grappi S, Nanni G, Scheggi S, De Montis MG, Tagliamonte A (2003) Acquisition of a palatable-food-sustained appetitive behavior in satiated rats is dependent on the dopaminergic response to this food in limbic areas. Neuroscience 121, 179–187. [DOI] [PubMed] [Google Scholar]
- Gruber AJ, Hussain RJ, O’Donnell P (2009) The nucleus accumbens: a switchboard for goal-directed behaviors. PLoS One 4, e5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks AM, Dwiel LL, Deveau NH, Simon AA, Ruiz-Jaquez MJ, Green AI, Doucette WT (2019a) Corticostriatal Oscillations Predict High vs. Low Drinkers in a Rat Model of Limited Access Alcohol Consumption. Front. Syst. Neurosci. 13, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks AM, Sullivan EDK, Dwiel LL, Keus KM, Adner ED, Green AI, Doucette WT (2019b) Sex differences in the ability of corticostriatal oscillations to predict rodent alcohol consumption. Biol. Sex Differ. 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricks AM, Sullivan EDK, Dwiel LL, Li JY, Wallin DJ, Khokhar JY, Doucette WT (2022) Maternal immune activation and adolescent alcohol exposure increase alcohol drinking and disrupt cortical-striatal-hippocampal oscillations in adult offspring. Transl. Psychiatry 12, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Cheer JF (2012) Effect of CB1 receptor blockade on food-reinforced responding and associated nucleus accumbens neuronal activity in rats. J. Neurosci. 32, 11467–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG (1988) Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 42, 1705–1712. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G (1986) Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J. Pharmacol. Exp. Ther. 239, 219–228. [PubMed] [Google Scholar]
- Janak PH, Chang JY, Woodward DJ (1999) Neuronal spike activity in the nucleus accumbens of behaving rats during ethanol self-administration. Brain Res. 817, 172–184. [DOI] [PubMed] [Google Scholar]
- Kehnemouyi YM, Wilkins KB, Anidi CM, Anderson RW, Afzal MF, Bronte-Stewart HM (2021) Modulation of beta bursts in subthalamic sensorimotor circuits predicts improvement in bradykinesia. Brain 144, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of Addiction. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS (1992) Sex differences in pattern of drinking. Alcohol 9, 415–420. [DOI] [PubMed] [Google Scholar]
- Leigh SJ and Morris MJ (2018) ‘The role of reward circuitry and food addition in the obesity epidemic: An update’, Biological Psychology, 131, pp. 31–42. [DOI] [PubMed] [Google Scholar]
- Li J, Zou Y, Ye J-H (2011) Low frequency electroacupuncture selectively decreases voluntarily ethanol intake in rats. Brain Res. Bull. 86, 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinetti MP, Khan Z, Lewis MJ (2007) Matching law choice analyses of ethanol and sucrose consumption in alcohol-preferring (P), nonpreferring (NP), and Sprague-Dawley (SD) rats. Alcohol. Clin. Exp. Res. 31, 1338–1348. [DOI] [PubMed] [Google Scholar]
- Martinetti MP, Lowery EG, Vona SR, Wichnick AM, Adler RA, Finch DG (2006) Limited-access consumption of ascending ethanol concentrations in alcohol-preferring, nonpreferring, and Sprague-Dawley rats. Alcohol. Clin. Exp. Res. 30, 836–843. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li T-K (2014) The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol 48, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCane AM, Ahn S, Rubchinsky LL, Janetsian-Fritz SS, Linsenbardt DN, Czachowski CL, Lapish CC (2018) COMT Inhibition Alters Cue-Evoked Oscillatory Dynamics during Alcohol Drinking in the Rat. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto SJ, Kosten TA (2017) Female Sprague-Dawley rats display greater appetitive and consummatory responses to alcohol. Behav. Brain Res. 327, 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priddy BM, Carmack SA, Thomas LC, Vendruscolo JCM, Koob GF, Vendruscolo LF (2017) Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol. Biochem. Behav. 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Cauley MC, Levin ED (2014) Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol. Biochem. Behav. 125, 8–14. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Carelli RM (2008) Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur. J. Neurosci. 28, 1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM (2015) Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation. J. Neurosci. 35, 11572–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang M, Xiao L, Gui L, Zheng W, Bai L, Su B, Li B, Xu Y, Pan W, Zhang J, Wang W (2022) Potential therapeutic mechanism of deep brain stimulation of the nucleus accumbens in obsessive-compulsive disorder. Front. Cell. Neurosci. 16, 1057887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, Fields HL (2005) Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J. Neurosci. 25, 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez LA, Perez IO, Simon SA, Gutierrez R (2012) Transitions between sleep and feeding states in rat ventral striatum neurons. J. Neurophysiol. 108, 1739–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The neurobiology of drug addiction (1997) The Journal of Neuropsychiatry and Clinical Neurosciences. [DOI] [PubMed] [Google Scholar]
- van der Meer MAA, Redish AD (2009) Low and High Gamma Oscillations in Rat Ventral Striatum have Distinct Relationships to Behavior, Reward, and Spiking Activity on a Learned Spatial Decision Task. Front. Integr. Neurosci. 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cott A (1972) A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol. Behav. 8, 345–362. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF (1993) Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J. Pharmacol. Exp. Ther. 267, 250–258. [PubMed] [Google Scholar]
- Wilson C, Nomikos GG, Collu M, Fibiger HC (1995) Dopaminergic correlates of motivated behavior: importance of drive. J. Neurosci. 15, 5169–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29, 203–210. [DOI] [PubMed] [Google Scholar]
- Wu H, Miller KJ, Blumenfeld Z, Williams NR, Ravikumar VK, Lee KE, Kakusa B, Sacchet MD, Wintermark M, Christoffel DJ, Rutt BK, Bronte-Stewart H, Knutson B, Malenka RC, Halpern CH (2018) Closing the loop on impulsivity via nucleus accumbens delta-band activity in mice and man. Proc. Natl. Acad. Sci. U. S. A. 115, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Zhu G, Zhao B, Bai Y, Jiang Y, Neumann W-J, Kühn AA, Zhang J (2021) Local field potentials in Parkinson’s disease: A frequency-based review. Neurobiol. Dis. 155, 105372. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley A (2002) Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE (2000) Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and Fos expression. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gui H, Duan Z, Yu T, Zhang J, Liang X, Liu C (2021) Dopamine D1 Receptor in the Nucleus Accumbens Modulates the Emergence from Propofol Anesthesia in Rat. Neurochem. Res. 46, 1435–1446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


