Abstract
In the past 5 years, important advances have been made in the scientific understanding and clinical management of cholangiocarcinoma (CCA). The cellular immune landscape of CCA has been characterized and tumour subsets with distinct immune microenvironments have been defined using molecular approaches. Among these subsets, the identification of ‘immune-desert’ tumours that are relatively devoid of immune cells emphasizes the need to consider the tumour immune microenvironment in the development of immunotherapy approaches. Progress has also made in identifying the complex heterogeneity and diverse functions of cancer-associated fibroblasts in this desmoplastic cancer. Assays measuring circulating cell-free DNA and cell-free tumour DNA are emerging as clinical tools for detection and monitoring of the disease. Molecularly targeted therapy for CCA has now become a reality, with three drugs targeting oncogenic fibroblast growth factor receptor 2 (FGFR2) fusions and one targeting neomorphic, gain-of-function variants of isocitrate dehydrogenase 1 (IDH1) obtaining regulatory approval. By contrast, immunotherapy using immune-checkpoint inhibitors has produced disappointing results in patients with CCA, underscoring the requirement for novel immune-based treatment strategies. Finally, liver transplantation for early stage intrahepatic CCA under research protocols is emerging as a viable therapeutic option in selected patients. This Review highlights and provides in-depth information on these advances.
Introduction
Cholangiocarcinoma (CCA) is an epithelial cell malignancy of the liver and biliary tract. Histopathologically, this cancer is characterized by features of cholangiocyte differentiation (cholangiocytes are the epithelial cells that line the intrahepatic and extrahepatic bile ducts). The incidence of CCA is increasing in several countries globally, and given its high lethality with 5-year overall survival (OS) ranging from 7% to 20%, this disease has attracted considerable scientific and clinical interest1,2. Indeed, several outstanding reviews focusing on CCA have been published in the past few years2,3. These reviews richly describe the three anatomical subsets of the disease — namely, perihilar cholangiocarcinoma (pCCA), intrahepatic cholangiocarcinoma (iCCA) and distal cholangiocarcinoma (dCCA) — and their epidemiology, histopathology, genetics and molecular drivers. These recent reviews also summarize the clinical diagnosis, staging and treatment of CCA. Yet given a virtual explosion of published manuscripts (>6,000 publications) since the last Review of CCA in this journal in 2018 (ref. 4), an update is warranted.
This update is focused on what we believe are the most pertinent scientific and clinical advances made in the past 5 years. CCA is a highly desmoplastic cancer characterized by a rich tumour immune microenvironment (TIME)2. We highlight the deepening understanding of the TIME based on single-cell RNA sequencing (scRNA-seq) and immunophenotyping. The desmoplasia of CCA is the result of an abundance of cancer-associated fibroblasts (CAFs)5, which have not been fully described in prior reviews, and the nature and role of CAFs in CCA pathogenesis will be emphasized herein. Assessments of circulating tumour cells and cell-free DNA (cfDNA) are becoming important in the diagnosis and monitoring of this malignancy6,7, and are therefore also covered in this Review. Potent oncogenic drivers of iCCA include fibroblast growth factor receptor 2 (FGFR2) gene fusions and neomorphic, gain-of-function variants of the genes encoding isocitrate dehydrogenase (IDH), specifically IDH1 (refs. 8,9). Importantly, both FGFR2 fusions and IDH1 mutations are targetable, and approved therapies are now available for the targeted treatment of CCAs harbouring these genetic aberrations10–17. We discuss the clinical efficacy of these molecularly targeted therapies in detail, considering that information continues to evolve regarding their efficacy and sequential use within the treatment armamentarium for advanced-stage CCA. Finally, emerging information regarding the efficacy of liver transplantation for early stage iCCAs, especially those occurring in the context of chronic liver disease, are reviewed18. We hope that our focused update on these particularly germane topics will be of high value to scientists and clinicians studying CCA and caring for patients afflicted with this complex and nefarious cancer.
The TIME of CCA
Immunosuppressive barriers
CCAs are dense, desmoplastic tumours with a prominent stroma comprising CAFs and innate and adaptive immune cells, among other cell types, that can have tumour-promoting or tumour-suppressive roles depending upon the context19. The TIME of CCA is complex with intricate and extensive crosstalk between the various stromal and immune components19. Emerging literature suggests that the CCA microenvironment is ‘immune cold’, with a paucity of cytotoxic immune cells and an abundance of immunosuppressive elements, particularly myeloid cells20–24. Indeed, CCAs have a decreased abundance of cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells relative to adjacent tumour-free liver tissues, whereas the density of regulatory T (Treg) cells is increased24. Moreover, the expression of immune-checkpoint molecules such as PD-1 and its ligand PD-L1 as well as CTLA4 is increased on the tumour-infiltrating immune cells, indicating an immunosuppressive milieu20,23–25. CCAs also have a high level of intratumoural transcriptomic heterogeneity, probably owing to the presence of multiple different cell types that promote immunosuppression26. These immunosuppressive elements within the TIME (Fig. 1) facilitate tumour growth and progression, immune evasion and resistance to chemotherapy, and probably contribute to the limited efficacy of immune-checkpoint inhibitor (ICI) monotherapy in clinical trials20,27,28.
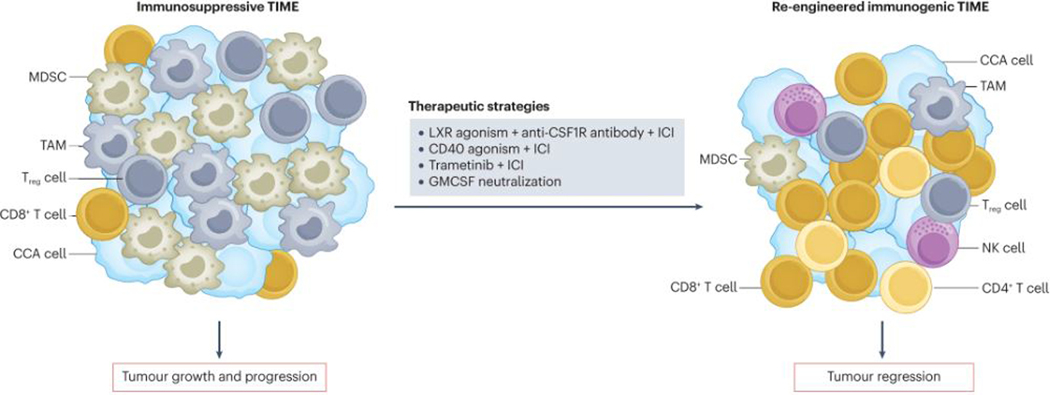
Fig. 1 |. Therapeutically re-engineering the TIME of CCA to reduce tumour burden.
The cholangiocarcinoma (CCA) tumour immune microenvironment (TIME) is poorly immunogenic with an abundance of immunosuppressive cell types such as myeloid-derived suppressor cells (MDSCs), tumour-associated macrophages (TAMs) and regulatory T (Treg) cells that foster tumour growth and progression. Immunotherapeutic strategies that have been investigated in preclinical studies using mouse models include neutralization of granulocyte–macrophage colony-stimulating factor (GMCSF), liver X-receptor (LXR) agonism to inhibit MDSCs combined with macrophage inhibition using anti-colony-stimulating factor 1 receptor (CSF1R) antibodies and immune-checkpoint inhibitors (ICIs), as well as the combination of ICIs with CD40 agonism or MEK inhibition with trametinib. These immunotherapeutic strategies have the potential to re-engineer the CCA TIME to an immunogenic one that is less favourable to the tumour. NK, natural killer.
Tumour-associated macrophages (TAMs) are the predominant immunosuppressive myeloid cell type in the TIME of CCA20,21. Moreover, an abundance of TAMs is associated with poor patient outcomes21. A high ratio of monocytes to lymphocytes in the blood is an independent predictor of unfavourable outcomes in patients with CCA21,29–31. Furthermore, patients with iCCAs harbouring a high percentage of macrophages expressing CD206, a scavenger receptor expressed on TAMs, and a low percentage expressing CD86, a co-stimulatory signal for T cells, have a higher risk of disease recurrence and worse OS following surgical resection than those with CD206-low, CD86-high tumours32.
TAMs mediate tumour initiation and progression via diverse pathways, and multiple mechanisms are involved in TAM accumulation within the TIME. Kupffer cells, which are resident macrophages in the liver, can facilitate cholangiocyte proliferation and oncogenic transformation by secreting TNF that triggers JNK signalling in the cholangiocellular compartment33. Accordingly, JNK inhibition attenuates iCCA development in preclinical models33. In addition, inflammatory macrophages in the TIME promote a WNT-high state that favours tumour growth and progression across several preclinical models of CCA34. Consequently, either WNT inhibition or macrophage depletion markedly reduces the tumour burden in these models34. TNF-like weak inducer of apoptosis (TWEAK) predominantly produced by immune cells, including macrophages, can induce secretion of monocyte chemoattractant protein 1 (MCP1, also known as CCL2) by CCA cells and thereby promotes macrophage recruitment to the CCA TIME and induces their polarization to a pro-tumour CD206+ TAM phenotype, in association with an increase in tumour growth35. Indeed, although resident macrophages have an essential role in CCA development, the majority of TAMs in established murine tumours are recruited from peripheral monocytes20. Expression of PD-L1 is an important mechanism of tumour immune evasion, and PD-L1 expression within the tumour front is associated with unfavourable OS following surgical resection of iCCA23. Monocyte-derived TAMs in the CCA TIME abundantly express PD-L1 and are in fact the main source of PD-L1 in human CCAs, and these recruited PD-L1+ TAMs are essential for CCA progression in mouse models20. However, genetic disruption of TAM recruitment or pharmacological inhibition of TAM function did not reduce CCA tumour burden owing to a compensatory emergence of another immunosuppressive myeloid cell population, myeloid-derived suppressor cells (MDSCs).
MDSCs are a heterogeneous population of immature myeloid cells categorized as either monocytic (M-MDSCs) or granulocytic (G-MDSCs), with the latter also referred to as polymorphonuclear MDSCs (PMN-MDSCs)36. MDSCs foster tumour growth and have potent immunosuppressive properties that contribute to CTL and NK cell dysfunction in cancer20,36. Notably, patients with CCA have higher circulating levels of CD14+CD11b+HLA-DR− MDSCs than individuals without known cancer37. Moreover, high levels of MDSCs in the peripheral blood are associated with resistance to ICIs in patients with melanoma38. In preclinical models of colitis or primary sclerosing cholangitis, which is an important risk factor for CCA, intestinal barrier dysfunction and gut dysbiosis enable bacterial cells and endotoxins to accumulate in the liver22. Consequently, hepatocytes secrete the chemokine CXCL1, which recruits CXCR2+ PMN-MDSCs that accelerate the development of CCA22. This effect can be abrogated through antibiotic treatment or neutralization of CXCL1 (ref. 22). These findings highlight the interplay between the gut microbiota, intestinal barrier function and the CCA TIME.
Insights from multiomics analyses
Emerging single-cell and bulk multiomics studies have begun to illuminate the complex heterogeneity of the CCA TIME, and its relationship with distinct molecular subtypes of the disease and correlation with patient prognosis, as well as its modulation through immunotherapy. scRNA-seq of 144,878 cells from 14 iCCA specimens revealed two tumour subtypes based on differential expression of the markers S100P and SPP1 (also known as osteopontin)39. Each subtype had a distinct TIME and correlated with a different patient prognosis. For example, S100P+SPP1− iCCAs (termed perihilar large-duct-type tumours) had decreased infiltration of CD4+ T cells and CD56+ NK cells while having an increased abundance of CCL18+ macrophages and PD-1+CD8+ T cells relative to the other tumour subtype39. By contrast, S100P−SPP1+ iCCAs (peripheral small-duct-type) had enhanced infiltration of SPP1+ macrophages39. Moreover, S100P−SPP1+ iCCAs were associated with a less aggressive tumour phenotype (including lower levels of serum CA19–9 and CEA and tumour Ki67 expression, as well as a lower prevalence of lymph node metastasis and advanced-stage disease) and better OS (based on immunohistochemistry microarray data from 186 patients and RNA-seq data from 66 patients)39. In another multiomics analysis of 151 iCCAs using proteomics analysis, whole-exome sequencing and scRNA-seq, the tumours were classified into three molecular subtypes: chromatin remodelling, metabolism and chronic inflammation40. The chronic inflammation subtype was characterized by the presence of APOE+C1QB+ TAMs and associated with poor patient outcomes40. By comparison, the metabolism subtype had frequent mutations in KMT2D (encoding histone-lysine N-methyltransferase 2D) and was associated with lower inflammatory activity40.
Evolving data also suggest that the CCA TIME can influence response to immunotherapy. Multiplexed single-cell transcriptomic and epitope sequencing of 200,000 peripheral blood mononuclear cells from nine patients with CCA and eight matched donors without known cancer demonstrated high levels of a unique population of CD14+ monocytes, termed CD14CTX cells, in patients with tumours resistant to anti-PD-1 antibodies41. The CD14CTX cells had increased expression of immunosuppressive cytokines and chemotactic molecules, and induced expression of SOCS3 in CD4+ T cells, thereby inhibiting their functional activity. The presence of CD14CTX cell is also of prognostic relevance, given that tumours expressing a CD14CTX gene signature were associated with unfavourable OS in patients with CCA as well as in those with other ICI-refractory cancers41.
Overcoming the immunosuppressive TIME
Preclinical studies have provided insights into potential strategies to circumvent immunosuppressive barriers in the TIME of CCAs. TAMs and MDSCs seem to have an integral role in the CCA TIME; therefore, combined targeting of both of these cell populations is a rational therapeutic approach (Fig. 1). Activation of the transcription factor liver-X receptor (LXR) restricts the survival and abundance of MDSCs42. In a syngeneic orthotopic mouse model of CCA, pharmacological targeting of MDSCs using either an anti-Ly6G antibody or the LXR agonist GW3965 combined with targeting of TAMs using an anti-CSF1R antibody augmented the efficacy of anti-PD-1 antibodies, with consequent prolongation of survival20. Neutralization of granulocyte–macrophage colony-stimulating factor (GMCSF) is another approach that has demonstrated promise in preclinical models of iCCA21. GMCSF has an integral role in myeloid cell programming, and higher expression of this cytokine is associated with unfavourable OS in patients with iCCA following surgical resection21. Accordingly, antibody-mediated GMCSF blockade curtailed tumour growth in an orthotopic model of CCA by decreasing TAM abundance and repolarizing immunosuppressive TAMs and MDSCs in a manner that facilitated the T cell response21.
CD40 is a receptor that is expressed on antigen-presenting cells, including dendritic cells (DCs) as well as macrophages, and promotes DC activation and macrophage re-education to an antitumour, inflammatory phenotype43. In several different preclinical models of iCCA, combination of an agonistic anti-CD40 antibody with an anti-PD-1 antibody markedly reduced tumour burden, in association with increases in the abundance and activation of macrophages and DCs as well as cytotoxic cell types including CD8+ T cells, NK cells and CD4+ T cells; most of these cell types were functionally implicated in the antitumour response through depletion experiments44. Furthermore, CD40 agonism combined with PD-1 antagonism enhanced the efficacy of gemcitabine–cisplatin chemotherapy, with consequent increased mouse survival compared to gemcitabine–cisplatin alone44.
Other systemic therapies can also prime the TIME and potentiate the response to ICIs. For example, MEK inhibition with trametinib upregulates tumour cell expression of MHC class I (MHC I) molecules and PD-L1. Hence, the combination of trametinib and an anti-PD-1 antibody had enhanced antitumour efficacy compared with that of either agent alone in a mouse model of iCCA45. In aggregate, the results of these preclinical studies support the concept that therapeutic strategies that modulate the CCA TIME by shifting the balance from immuno-suppressive factors in favour of cytotoxic elements can augment the antitumour response and the efficacy of ICIs (Fig. 1).
Desmoplasia and CAFs in CCA
One of the main features of CCA is its highly desmoplastic nature with an accumulation of CAFs and excessive deposition of extracellular matrix (ECM) in the TIME, which contributes to the poor prognosis of patients with this disease27,46–49. CAFs intercommunicate with a wide variety of cell types including cancer cells, immune cells and endothelial cells, all interacting in a multidirectional network influencing cholangiocarcinogenesis27,50. Indeed, CAFs are one of the most abundant cell types in the TIME of CCA and contribute to tumour neoangiogenesis, immunomodulation and stiffness by secreting growth factors, cytokines, exosomes and ECM proteins; therefore, CAFs have long been considered a putative target for therapy in CCA5,48,49,51. However, therapeutic targeting of CAFs in other tumour types has thus far failed to improve patient outcomes, highlighting the need for a more meticulous characterization of these cells52. Indeed, despite their overall tumour-promoting role in CCA5,51,53, specific CAF mediators have now been shown to exert tumour-promoting or tumour-restricting effects5, adding more complexity to an already intricate picture.
CAF heterogeneity
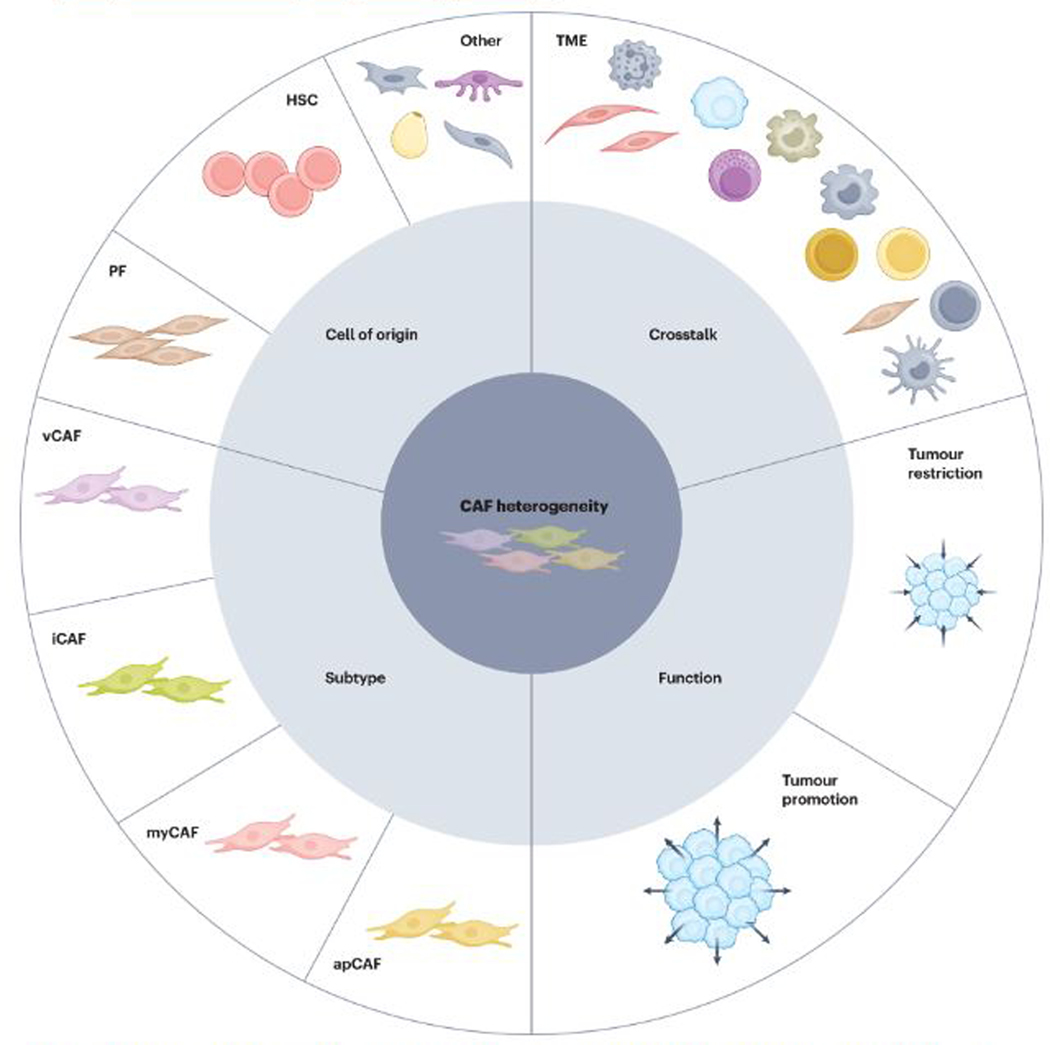
CAFs are heterogeneous in their origins, transcriptomes and functions (Fig. 2), as observed in several tumour types46,48,54,55. Mainly originating from resident fibroblasts in the liver, CAFs in the CCA TIME demonstrate considerable plasticity; for example, becoming activated in response to the dynamic microenvironment during cholangiocarcinogenesis5,51. Classically identified based on expression of α-smooth muscle actin (α-SMA), CAFs are also characterized by expression of collagen type I α1 chain (COL1A1), fibroblast activation protein, platelet-derived growth factor receptor-α (PDGFRα) and PDGFRβ, among other proteins5,26,52,54,56–59. Heterogeneity in CAF biomarkers has been confirmed by data from both bulk RNA-seq and scRNA-seq transcriptomic studies, which also outline the coexistence of transcriptomically diverse CAF subtypes in the TIME of CCA5,57,58. Despite the lack of a common nomenclature, the presence of specific CAF subtypes enriched in genes and pathways related to either a myofibroblastic or an inflammatory phenotype (myCAFs and iCAFs, respectively) has been confirmed in all such studies5,57,58. Moreover, the identification of intermediate CAF populations arising from the same progenitor cell points towards the existence of transient rather than static CAF subtypes, thus linking CAF heterogeneity to CAF plasticity5. Despite the abundant evidence of the overall tumour-promoting role of CAFs in CCA5,51,53, specific CAF mediators such as COL1A1 have been shown to restrain rather than support tumorigenesis5,56,60, thus challenging the historical paradigm and highlighting the need to further investigate the functionality of specific CAF subtypes.
Fig. 2 |. Different shades of CAF heterogeneity in CCA.
Cholangiocarcinoma (CCA) is a highly desmoplastic tumour type characterized by a rich stroma with an abundance of fibroblasts and their molecular products. These cancer-associated fibroblasts (CAFs) are heterogeneous in several aspects including their cell of origin, mainly arising from hepatic stellate cells (HSCs) and portal fibroblasts (PFs). Moreover, transcriptomically diverse CAF subtypes have been described to coexist in the same tumour and include vascular CAFs (vCAF), inflammatory CAFs (iCAF), myofibroblastic CAFs (myCAF) and antigen-presenting CAFs (apCAF). CAF heterogeneity is also reflected in their functionality, with evidence for subtypes with tumour-promoting and/or tumour-restricting activities. Furthermore, diverse CAF subtypes are likely to interact differentially with tumour cells and other cells in the tumour microenvironment (TME) during tumorigenesis.
Therapeutic opportunities
New therapeutic strategies are urgently needed to improve outcomes in patients with CCA, and CAFs are considered an attractive target for therapy because they actively participate in cholangiocarcinogenesis3,27,46,49,59. With the emerging evidence of CAF heterogeneity, however, targeting these cells becomes more challenging and makes strategies aimed at their global targeting infeasible51. As evidenced by data from studies reported in the past 2 years, interfering with the cancer cell–CAF crosstalk and the development of dual therapies aimed at targeting both CAFs and cancer cells seem to be the most promising therapeutic strategy5,56,57,60. Mediators of this direct cross-talk that promote tumour growth include hyaluronan produced by myCAFs and hepatocyte growth factor (HGF) secreted by iCAFs, which can stimulate cancer cells via various hyaluronan receptors and MET, respectively5,56. Nevertheless, myCAFs also secrete type I collagen that can counteract these tumour-promoting effects5, as shown through conditional deletion of Col1a1 in this cell type in mouse models of other desmoplastic cancers such as pancreatic ductal adenocarcinoma60, probably by forming a physical barrier that mechanically restrains tumour invasion56. These opposing effects highlight the complex role of CAFs in the TIME; a comprehensive understanding of these dual effects would guide the development of more effective therapeutic strategies. For example, therapeutic strategies that target tumour-promoting CAF mediators (such as HGF) while maintaining the effect of tumour-suppressive mediators (such as type I collagen) have the potential to transform CAFs from a pro-tumour to an antitumour cell population. Furthermore, stratification of CCAs based on patient-specific mutational profiles and TIME characteristics57 might provide new insights to advance towards precision medicine. Therefore, a better understanding of CAF heterogeneity and plasticity is needed to develop new therapeutic approaches aimed at targeting CCA taking into consideration the complexity of its TIME.
Molecular diagnosis using blood and bile
Clinical gaps exists in the early diagnosis of CCA as well as the monitoring of disease progression and treatment responses. Utilization of blood-based liquid biopsy assays has shown promise in detecting multiple types of early stage cancers61,62. Among various classes of liquid biopsy, circulating tumour DNA (ctDNA), a fraction of cfDNA originating from tumour cells, has been studied most extensively for early cancer detection. Indeed, ctDNA in the blood might serve as a diagnostic marker for CCA6,63. A proof-of-concept retrospective study published in 2021 investigated a panel of ctDNA methylation markers for the detection of CCA specifically6. The methylated DNA markers were identified as differentially methylated regions in frozen CCA specimens versus matched adjacent benign biliary epithelia and liver parenchyma tissues, and a total of nine markers were selected from this tissue comparison6. Subsequently, this panel of methylated DNA markers was blindly tested in plasma cfDNA samples from 54 patients with pCCA and five with dCCA as well as from control groups comprising patients with primary sclerosing cholangitis or individuals without known liver disease (n = 22 and 95, respectively)6. The cfDNA methylation panel had a sensitivity of 76% for the detection of CCA at a specificity of 94%6. The panel correctly identified 64% of patients with CCAs amenable to transplantation or surgical resection and 63% of CCAs among patients with low serum CA19-9 levels (≤100 U/ml)6, highlighting the potential utility of ctDNA methylation assays for the detection of early stage CCA. In addition, methylation of OPCML and HOXD9 in cfDNA was assessed in a cohort of 40 patients and compared with a disease control group comprising patients with non-malignant biliary diseases such as cholelithiasis63. The combination of OPCML and HOXD9 markers had a sensitivity and specificity of 62.5% and 100%, respectively, for the diagnosis of CCA63. These findings require prospective validation in larger cohorts before methylated ctDNA markers can be used for routine clinical care.
Genetic and epigenetic alterations detected in cfDNA are strongly correlated with those present in tumour tissue7,64; therefore, tumour-specific somatic mutations and epigenetic changes in plasma ctDNA could potentially be utilized for minimally invasive longitudinal monitoring of dynamic changes in tumour biology and surveillance for disease progression. In a multicentre retrospective analysis of 138 blood-derived cfDNA samples from 124 patients with CCA (70% of whom had iCCA) using a 73-gene panel, 105 samples (76%) had one or more detectable genetic alterations after excluding variants of unknown significance, and 76 samples (55%) had therapeutically relevant alterations, including BRAF mutations, ERBB2 (also known as HER2) amplifications, FGFR2 fusions or mutations and IDH1 mutations65. Thus, ctDNA could be a valuable resource for tumour molecular profiling, especially when obtaining tumour tissue samples is technically challenging or unsafe, which is a major challenge in managing CCA. In addition, ctDNA can be used to monitor response to chemotherapy and targeted therapy. A proof-of-concept study of serial plasma cfDNA testing in eight patients with pancreaticobiliary cancers demonstrated that changes in cfDNA mutant allele fraction correlate well with changes in CA19–9 levels (Pearson’s r = 0.69 for interval slopes, r = 0.93 for interval differences), suggesting that ctDNA levels reflect changes in disease burden over time during treatment7. Furthermore, longitudinal profiling of ctDNA can be performed to track the emergence of resistance to chemotherapy and, therefore, potentially facilitate the timely use of second-line or third-line treatments for CCA7,64. Thus, determination of cancer-derived cfDNAs present in blood is a promising and emerging technology for the management of patients with CCAs.
Confirmation of malignant biliary stricture is another major clinical challenge in patients with indeterminate biliary strictures detected using non-invasive imaging. The diagnostic work-up in these patients involves endoscopic retrograde cholangiopancreatography (ERCP), but the sensitivity of traditional methods such as ERCP-guided brush cytology or biopsy sampling for the detection of malignant biliary stricture is <40%66,67. To address this diagnostic dilemma, molecular characterization of biliary brush specimens and/or bile has been evaluated as a tool for the diagnosis of malignancy. Fluorescence in situ hybridization (FISH) analysis of biliary brush specimens for chromosome 1q21, 7p12, 8q24 and 9p21 aberrations was found to improve the accuracy in diagnosing malignant biliary stricture, achieving 65% sensitivity and a specificity of 93%67. Moreover, somatic KRAS mutations and/or loss-of-heterozygosity alterations across ten tumour-suppressor genes detected in DNA isolated from biliary brush specimens have been shown to be excellent diagnostic biomarkers for malignant biliary strictures68. In this prospective study involving 100 patients with biliary strictures (41% with malignancy), routine FISH identified nine of 28 malignancies not detected on cytology analysis; mutation profiling of cell-free supernatant fluid of the brush specimen identified an additional eight malignancies not detected by FISH, increasing the overall sensitivity to 51% versus 32% with cytology alone, whilst maintaining 100% specificity68. These data highlight the complementary role of these diagnostic assays. Considering the rapid evolution of next-generation sequencing (NGS) capacity, a single-centre prospective study in 252 patients with biliary stricture utilized BiliSeq, a targeted NGS panel encompassing 28 genes, to screen for mutations in biliary brush cytology samples with or without biliary biopsy specimens; the assay had good performance for the detection of CCA with 73% sensitivity and 100% specificity69. Notably, BiliSeq had a sensitivity of 83% in patients with primary sclerosing cholangitis69. For comparison, the sensitivity and specificity of elevated serum CA19–9 alone were 76% and 69%, respectively, and for pathological evaluation alone were 48% and 99%69. In another prospective study, NGS-based mutational analysis of cfDNA from ERCP-derived bile samples using a 52-gene panel had a sensitivity of 96.4% and a specificity of 69.2% for the detection of CCA in 68 patients with suspicious biliary strictures70.
Altered DNA methylation markers in biliary brush specimens and/or bile samples from patients with indeterminate biliary strictures might also improve the diagnosis of CCA. The first proof-of-concept case–control study in patients with CCA (n = 15 and 34 in the training and validation cohorts, respectively) and control patients with primary sclerosing cholangitis (n = 20 and 34) reported that a panel of four methylation markers (in CDO1, CNRIP1, SEPT9 and VIM) measured in DNA from biliary brush specimens had a sensitivity of 85% and a specificity of 98% for the detection of CCA (area under the receiver operating characteristic curve (AUC) 0.944)71. Several smaller case–control studies have shown the outstanding performance of DNA methylation biomarkers (for example, at loci associated with EMX1 or HOXD8) in biliary brush and/or bile samples, with AUC values of 0.98–1 for the detection of malignant biliary strictures6,72. Similarly, profiling of microRNAs in bile samples has been investigated, with promising results for the detection of CCA (AUC 0.652, 0.730, 0.765, 0.975 and 0.975 for miR-92a-3p, miR-30d-5p, miR-944, miR-9 and miR-145*, respectively)73,74.
The data from these initial biomarker studies have revealed the great promise of ctDNA as a novel biomarker for CCA detection. We anticipate that such liquid biopsy assays will further improve the diagnosis of CCA in routine clinical practice once larger-scale external validation studies are completed in the near future. Given that comprehensive molecular profiling is recommended in the routine clinical care of patients with advanced-stage CCA, ctDNA analysis might also serve as a minimally invasive test for the selection of molecularly targeted treatments. Plasma ctDNA will be particularly helpful in identifying patients with CCAs harbouring IDH1 mutations or FGFR2 fusions, given the availability of approved therapies predicated on these alterations65. In an NGS-based analysis involving 1,671 patients with advanced-stage CCA, genetic alterations in cfDNA were detected in samples from 84%, with targetable alterations in cfDNA detected in samples from 44%75. The concordance between cfDNA and tumour tissue analysis for the detection of IDH1 and BRAFV600E mutations was 87% and 100%, respectively, but was only 18% for FGFR2 fusions75. Although molecular analyses of blood, biliary brush and/or bile specimens can facilitate the implementation of precision medicine in patients with CCA, important challenges continue to limit widespread clinical use of such assays. First, numerous methods can be used to detect methylation markers or mutations in cfDNA, with substantial variation in sample type (for example, biliary brush cytology versus cell-free supernatant fluid versus bile), handling and processing, and analysis. Standardized ctDNA assay protocols that can capture the full spectrum of mutation and methylation with high sensitivity and specificity need to be developed and validated in order to ensure robust and generalizable results. Second, most current supporting evidence comes from smallcohort, single-centre, case–control studies. Given the low incidence of CCA, these issues necessitate large-scale, collaborative, multicentre prospective studies using standardized protocols.
Emerging molecular classifications of CCA
The prevalence of molecular profiling has enabled the definition of transcriptome-based classes of iCCA and pCCA76–78. Importantly, the molecular subclasses might predict patient outcomes or response to certain therapeutic approaches. For example, a genomic and transcriptomic characterization of 104 surgically resected iCCA specimens revealed two distinct tumour classes associated with different clinical outcomes and with the absence or presence of mutations in KRAS or BRAF76. Specifically, the 5-year OS in 51 patients with ‘cluster 1’ class tumours according to a transcriptomic classifier based on 238 significantly differentially expressed genes was 72% compared with 30% in 53 patients with cluster 2 tumours (P < 0.0007)76. Patients with cluster 2 tumours also had a shorter median time to recurrence (13.7 ± 6.3 months versus 22.7 ± 18.1 months; P = 0.001)76. Integration of KRAS/BRAF mutational status with the 238-gene classifier grouped all 18 evaluable patients with such mutations into the poor prognosis cluster 2 (ref. 76). Integrative molecular analyses have also been applied for the detection of biological classes of CCA with differential activation of certain signalling pathways. One such analysis of 149 iCCAs defined two principal tumour classes: an inflammation class (38%) and a proliferation class (62%)78. Features of the inflammation class included activation of inflammatory signalling pathways, overexpression of cytokines and activation of STAT3, whereas the proliferation class was characterized by activation of oncogenic signalling pathways, KRAS/BRAF mutations, and gene expression signatures previously associated with poor outcomes in patients with hepatocellular carcinoma78. Patients with proliferation class iCCAs had significantly worse OS than patients with inflammation class tumours (median 24.3 months versus 47.2 months; P = 0.048)78. Similarly, an integrated genomic analysis of pCCA and dCCA tumour specimens (n = 189) enabled the definition of four distinct classes: metabolic, proliferation, mesenchymal and immune77. Tumours of the metabolic class (comprising 19% of cases) had a hepatocyte-like phenotype with enrichment of genes related to bile acid metabolism77. dCCAs were more likely to be categorized into the proliferation class (accounting for 23% of tumours overall), which was defined by mTOR signalling, HER2 alterations and enrichment for MYC targets77. Patients classified in the mesenchymal class (47%) had unfavourable OS and enrichment of gene signatures associated with epithelial-to-mesenchymal transition77. The immune class (11%) was characterized by high levels of lymphocyte infiltration and PD-1/PD-L1 expression, as well as other transcriptomic features associated with increased responsiveness to ICI in other tumour types77.
The increasing emphasis in oncology on immunotherapeutic approaches has highlighted the integral role of the TIME and the potential clinical significance of immune-based classifications of CCA. A TIME-based classification developed using transcriptomics from a training set comprising 198 iCCA specimens identified four immune subclasses associated with distinct immune escape mechanisms and patient outcomes: immune-desert, immunogenomic, myeloid and mesenchymal79. This classification was validated using an independent set of 368 iCCAs and through immunohistochemical analysis of a further 64 iCCA samples79. In alignment with the concept that the CCA TIME is ‘immune cold’, the dominant subclass (present in approximately 45% of patients overall) was the immune-desert subset characterized by low expression of TIME gene signatures79 (Table 1). By contrast, the immunogenomic subclass was enriched for infiltration of adaptive and innate immune cells and activation of inflammatory and immune-checkpoint pathways, and was associated with the most favourable patient outcomes79. The myeloid subset featured enrichment of monocyte-derived and myeloid gene signatures, whereas the mesenchymal subclass was enrichment for fibroblast gene signatures79. Similarly, a Stroma, Tumour and Immune Microenvironment (STIM) classification of iCCA, which as its name implies integrates stromal, tumour and immune microenvironmental elements, categorized the preponderance of patients into non-inflamed tumour classes (65%)57. Of the five STIM classes of iCCA identified (Table 1), two inflamed classes were defined as ‘immune classical’ (accounting for 10% of patients) and ‘inflammatory stroma’ (~25%), with the latter characterized by a rich stroma (extensive desmoplasia), T cell exhaustion and KRAS mutations57. The three non-inflamed classes are: (1) the ‘desert-like’ class (20%) with a paucity of immune cells but enrichment of Treg cells; (2) the ‘hepatic stem-like’ class (35%) with abundant M2-like macrophages and enrichment for IDH and BAP1 mutations and FGFR2 fusions; and (3) the ‘tumour classical’ class (10%) characterized by cell cycle pathway activation and associated with unfavourable patient outcomes57. Although these classifications assist in the broad categorization of CCA subtypes and support the notion that CCAs typically have an immunosuppressive TIME, their clinical utility is currently unclear. Prospective validation of these classifications is needed before they can be used in routine patient care. For example, clinical trials stratifying patients based on these classifications with correlation to particular treatments would help to clarify their potential utility in the management of CCA.
Table 1 |.
Immune classifications of iCCA based on RNA-sequencing data
| Subclass | Proportion of iCCAs (%) | Key features | Prognostic association (median OS, months) |
|---|---|---|---|
| Classification based on immune gene expression signatures 79 | |||
| Immune-desert | 46–48 | TME signatures with weak expression of immune and myofibroblast signatures | 42 |
| Immunogenomic | 9–13 | Signatures of recruited innate immune cells, adaptive immune cells and activated fibroblasts Activation of inflammatory pathways |
73 |
| Myeloid | 13–19 | Strong expression of monocyte-derived and/or other myeloid signatures Low expression of lymphoid signatures | 25 |
| Mesenchymal | 22–28 | Strong expression of fibroblast signatures | 19 |
| Stroma, Tumour and Immune Microenvironment (STIM) classification 57 | |||
| Immune classical | ~10 | High immune infiltration (63%) Moderate stromal infiltration (27%) | –a |
| Inflammatory stroma | ~25 | Moderate immune infiltration (43%) High stromal infiltration (50%) Abundant desmoplastic reaction and ECM deposition High stiffness Activated inflammatory stroma T cell exhaustion |
– |
| Hepatic stem-like | ~35 | Low immune infiltration (28%) Low stromal infiltration (17%) Abundant tumour-promoting macrophages |
– |
| Tumour classical | ~10 | Low immune infiltration (22%) Low stromal infiltration (12%) Activation of cell cycle pathways | – |
| Desert-like | ~20 | Low immune infiltration (22%) Low stromal infiltration (11%) Regulatory T cell enrichment | – |
ECM, extracellular matrix; iCCA, intrahepatic cholangiocarcinoma; OS, overall survival; TME, tumour microenvironment.
In a multivariate analysis, the STIM classes were not independent predictors of outcome.
Advances in the treatment of CCA
Given that the objective of this Review is to provide an overview of pertinent clinical updates since our last Review in the journal4, here we focus on progress made over the past 5 years in systemic and non-systemic therapeutic strategies for CCA. Locoregional therapies including radiotherapy, transarterial chemoembolization and radioembolization were highlighted in our prior Review4. Since then, no major advances in locoregional treatments for CCA have been made, and thus such therapies are not included in this update.
Molecularly targeted therapies
Several high-throughput genomic sequencing analyses have elucidated the distinct mutational landscapes of iCCA, pCCA and dCCA, thus revealing differences in biology between these anatomical subtypes of the disease80–84. Actionable alterations such as FGFR2 fusions and IDH1 mutations occur almost exclusively in iCCAs, whereas pCCAs and dCCAs more commonly harbour HER2 amplifications and KRAS mutations80–84. Mutation frequencies among CCAs vary geographically and by aetiology, with Asian populations having lower frequencies of IDH1 mutations than non-Asian populations, and liver fluke-associated CCAs having a lower prevalence of IDH1 mutations and a higher prevalence of TP53 mutations than those with other aetiologies85–87.
The identification of actionable genetic alterations in approximately 40–50% of iCCAs and 15–20% of pCCAs and dCCAs88 has transformed the therapeutic landscape forpatients with advanced-stage CCA. Mutational profiling is usually performed on tumour tissue specimens, but given failure rates of up to 27% in CCA biopsies, liquid biopsy analysis of ctDNA might serve as an additional tool for identifying therapeutic targets, as described above64,65,89. Genetic alterations that define the eligibility of patients with CCA for molecularly targeted therapies based on FDA approvals or recommendations in the National Comprehensive Cancer Network (NCCN) guidelines are discussed below90.
FGFR2 fusions and other rearrangements.
FGFR signalling has key roles in various physiological processes and drives cell proliferation, growth and survival, thus making this pathway susceptible to hijack by cancer cells91,92. FGFR2 fusions and other rearrangements, which were first reported in CCAs in 2013 and subsequently found to be present in 13–14% of iCCAs93–95, constitute one of the most highly actionable alterations in this disease. The first two molecularly targeted therapies to gain regulatory approval for the treatment of CCA were the ATP-competitive, reversible FGFR inhibitors pemigatinib and infigratinib13,14. These oral small-molecule inhibitors demonstrated overall response rates (ORRs) of 35.5% and 23.1%, respectively, in single-arm phase II trials involving patients with previously treated advanced-stage CCAs harbouring FGFR2 fusions or rearrangements10,11 (Table 2), resulting in FDA Accelerated Approval for this indication in April 2020 and May 2021, respectively13,14. However, the development of infigratinib has been halted according to a manufacturer’s press release96. A third selective FGFR inhibitor, futibatinib, binds covalently and irreversibly to the P-loop cysteine residue in the FGFR kinase domain and achieved an ORR of 41.7%, with responses lasting a median of 9.7 months, in a similar phase II study involving patients with previously treated advanced-stage FGFR2-rearranged iCCA12. These data led to FDA Accelerated Approval of futibatinib for this indication in September 2022 (ref. 15). The safety and efficacy of derazantinib, an FGFR1–3 inhibitor, has also been assessed in a phase II trial involving 103 patients with advanced-stage CCA harbouring an FGFR2 fusion who had received at least one line of prior treatment97. In this study, derazantinib was associated with an ORR of 21.4%, a disease control rate of 74.8% and a median OS of 15.5 months97.
Table 2 |.
Efficacy of molecularly targeted therapies in patients with CCA
| Agent | Study phase | Number of patientsa | ORR (%) | DCR (%) | Median PFS (months) | Median OS (months) | Ref. |
|---|---|---|---|---|---|---|---|
| FGFR inhibitors | |||||||
| Pemigatinibb | II | 107 | 35.5 | 82.2 | 6.9 | NA | 10 |
| Infigratinibb | II | 108 | 23.1 | 84.3 | 7.3 | 12.2 | 11 |
| Futibatinibb | II | 103 | 41.7 | 82.5 | 9.0 | 21.7 | 12 |
| Derazantinib | I/II | 103 | 21.4 | 74.8 | 8.0 | 15.5 | 97 |
| IDH inhibitors | |||||||
| Ivosidenibb | III | 124 | 2.4 | 53.2 | 2.7 | 10.8 | 16 |
| BRAF inhibitors | |||||||
| Dabrafenib plus trametinib (MEK inhibitor)b,c | II | 43 | 46.5 | 85.4 | 9.0 | 14.0 | 111 |
| Vemurafenibc | II | 9 | 33.3 | NA | NA | NA | 113 |
| HER2-targeted agents | |||||||
| Trastuzumab plus pertuzumab (monoclonal antibodies)c,d | II | 39 | 23.1 | 51.3 | 4.0 | 10.9 | 115 |
| Trastuzumab deruxtecan (antibody–drug conjugate)c | II | 24 | 36.4 | 81.8 | 4.4 | 7.1 | 116 |
| Neratinib (pan-ErbB tyrosine kinase inhibitor)c | II | 25 (11 with CCA) | 16 | 28 | 2.8 (1.4 in CCA subgroup) | 5.4 (5.4 in CCA subgroup) | 118 |
| Zanidatamab (bispecific antibody)c | I | 17 | 47 | 65 | NA | NA | 119 |
CCA, cholangiocarcinoma; DCR, disease control rate; NA, not available; ORR, objective response rate; PFS, progression-free survival.
Number of patients with target molecular aberrations: FGFR2 fusions and/or rearrangements for FGFR inhibitors, IDH1 R132 mutations for IDH inhibitors; BRAFV600E mutations for BRAF inhibitors; and ERBB2 (also known as HER2) amplification and/or overexpression for HER2-targeted therapies.
FDA approved for use in patients with previously treated advanced-stage CCAs harbouring the target oncogenic alteration.
Data for these agents come from patients with CCAs as well as other biliary tract cancers.
Listed in National Comprehensive Cancer Network guidelines as subsequent-line therapy for patients with advanced-stage CCA following disease progression on recommended initial treatements90.
Acquired resistance to FGFR inhibitors limits the effectiveness of these agents, which are consistently associated with median progression-free survival (PFS) durations of 7–9 months10–12,97 (Table 2). Polyclonal secondary mutations in the FGFR2 kinase domain are a common resistance mechanism98–101. Futibatinib has shown potent preclinical activity against many of these mutations and has shown clinical activity after progression of CCA on treatment with reversible FGFR inhibitors101–103. Additional next-generation inhibitors, such as the FGFR2-selective inhibitor RLY-4008 (NCT04526106) and the multikinase inhibitor tinengotinib (also known as TT-00420; NCT04919642), are currently under investigation to assess their efficacy in overcoming resistance to prior FGFR inhibitors104–106. RLY-4008 is a highly selective FGFR2 inhibitor that has activity against primary FGFR2 alterations and common resistance mutations106. In a first-in-human study involving 35 patients with FGFR2-altered CCA, this agent had encouraging clinical activity, with >10% tumour shrinkage observed in nine (56%) of 16 patients with CCA previous treated with an FGFR inhibitor106.
Other FGFR inhibitors under investigation in patients with CCA include the pan-FGFR inhibitors erdafitinib (NCT02699606) and KIN-3248 (which has activity against FGFR2 gatekeeper, molecular brake and activation loop mutations105; NCT05242822), the multikinase inhibitor derazantinib(NCT03230318), the bivalent FGFR1–3 inhibitor tasurgratinib (E7090, which blocks FGF–FGFR interactions; NCT04238715), and the FGFR1–3 inhibitor HMPL-453 (NCT04353375). Moreover, phase III trials comparing the approved FGFR inhibitors against frontline gemcitabine and cisplatin chemotherapy are currently ongoing (pemigatinib (NCT03656536), infigratinib (NCT03773302) and futibatinib (NCT04093362)), as are early phase trials ofcombination therapies including FGFR inhibitors (gemcitabine and cisplatin plus pemigatinib or ivosidenib (NCT04088188) and derazantinib plus atezolizumab (NCT05174650)).
IDH1 mutations.
R132-mutant IDH1 acquires a neomorphic activity that converts α-ketoglutarate to 2-hydroxyglutarate, an oncometabolite that inhibits histone and DNA demethylases and results in widespread epigenetic alterations and oncogenesis107. The frequency of IDH1 mutations in CCAs varies widely across studies and was found to be 13.1% in iCCAs and 0.8% in pCCAs and dCCAs in a systematic review of 45 studies85. Ivosidenib, an orally administered small-molecule inhibitor of mutant IDH1, gained FDA approval for the treatment of chemorefractory IDH1-mutated CCA in August 2021 based on the results of a phase III trial showing an improvement in PFS over placebo (median 2.7 versus 1.4 months; HR 0.37, 95% CI 0.25–0.54; P < 0.0001)16,17 (Table 2). OS was not significantly improved (HR 0.79, 95% CI 0.56–1.12; P = 0.093); however, the fact that 70% of patients in the placebo group crossed over to receive ivosidenib following disease progression is likely to have confounded this result16. Additional IDH1 inhibitors under investigation in patients with CCA include olutasidenib (FT-2102; NCT03684811), LY3410738 (NCT04521686) and HMPL-306 (NCT04762602). Given that IDH1 mutations can lead to deficiencies in the repair of double-stranded DNA damage108,109, several ongoing trials of poly(ADP-ribose) polymerase (PARP) inhibitors are also including patients with IDH1-mutant CCA (olaparib (NCT03212274). olaparib plus the ATR inhibitor ceralasertib (NCT03878095) and olaparib plus durvalumab (NCT03991832)).
BRAFV600E mutations.
BRAFV600E mutations occur in up to 5% of biliary tract cancers (BTCs), primarily in iCCAs110. Treatment with the BRAF inhibitor dabrafenib in combination with the MEK inhibitor trametinib led to an ORR of 47% and a median PFS of 9.0 months in the BTC cohort of the phase II ROAR basket trial111 (Table 2). In June 2022, this combination gained FDA approval for use in patients with previously treated non-colorectal solid tumours, thus including CCAs, harbouring a BRAFV600E mutation and no satisfactory alternative treatment options, based on data from ROAR and several other trials112. Data on BRAF inhibitors as monotherapy for BTCs are limited, although vemurafenib induced partial responses in three of nine patients with BRAFV600E-mutant BTCs in another basket trial113.
HER2 alterations.
HER2 belongs to the ErbB family of receptor tyrosine kinases and is overexpressed more frequently in pCCAs and dCCAs (17.4%) and gallbladder cancers (19.1%) than in iCCAs (4.8%)114. The combination of two anti-HER2 antibodies, trastuzumab and pertuzumab, achieved an ORR of 23% and a median PFS of 4.0 months in 39 patients with HER2-amplified and/or overexpressing BTCs in the phase IIa MyPathway basket study115 (Table 2), gaining a listing in the NCCN guidelines as a recommended therapy for previously treated HER2-positive BTCs90. The antibody–drug conjugate trastuzumab deruxtecan demonstrated an ORR of 36.4% (including two complete responses (CRs)), a median PFS of 4.4 months and a median OS of 7.1 months in a similar phase II trial population (n = 22)116, and a partial response was also seen in one of eight patients with HER2-low disease. Trastuzumab deruxtecan is being further explored in patients with BTCs in an ongoing phase II trial (NCT04482309). Another phase II trial evaluated the efficacy of combination folinic acid, 5-fluorouracil and oxaliplatin (FOLFOX) chemotherapy plus trastuzumab in the second-line setting in 34 patients with HER2-positive BTCs; the ORR was 29.4%, median PFS was 5.1 months and median OS was 10.7 months117.
Additionally, the oral, irreversible, pan-ErbB tyrosine kinase inhibitor (TKI) neratinib has been evaluated in patients with previously treated HER2-mutant CCA (as opposed to merely HER2-positive disease) as part of the SUMMIT basket trial118. Results from the phase II BTC cohort of this trial, which included 11 patients with CCAs, demonstrated an ORR of 16% in all 25 patients evaluated, with a median PFS of 2.8 months and median OS of 5.4 months (1.4 months and 5.4 months, respectively, in the CCA subgroup)118 (Table 2).
The novel bispecific anti-HER2 antibody zanidatamab, which targets the same epitopes as trastuzumab and pertuzumab, achieved an interim ORR of 47% and a median response duration of 6.6 months in 17 patients with advanced-stage HER2-positive BTCs119 (Table 2), and this agent is being further evaluated in studies including patients with CCAs and other BTCs (NCT04466891 and NCT03929666). Other therapeutic strategies currently being evaluated in trials involving patients with HER2-overexpressing CCAs include the combination of trastuzumab and the HER2 TKI tucatinib (NCT04579380) and novel antibody–drug conjugates (A166 (NCT03602079) and zanidatamab zovodotin (NCT03821233)).
NTRK fusions.
NTRK fusions occur in approximately 0.2% of CCAs and are highly actionable alterations in solid tumours120. These NTRK fusions are therapeutically actionable using the TRK inhibitors larotrectinib and entrectinib, which received FDA approval agnostic of tumour histology based on efficacy across cancer types (summary ORR of 75% with larotrectinib and 57% with entrectinib); partial responses were observed in two of three patients with CCA included in the pivotal trials of these agents121–124.
RET fusions.
RET fusions are also very rare in CCAs, with a prevalence of 0.15% in iCCA and 0.11% in pCCA/dCCA125. The phase I/II ARROW trial evaluated the potent, selective of RET inhibitor pralsetinib in 29 patients with advanced-stage RET-altered solid tumours126. Of the three patients with CCA included in this basket study cohort, two had a partial response and one had stable disease126, gaining pralsetinib a listing on the NCCN guidelines90. The safety and efficacy of selpercatinib, another highly selective RET inhibitor, are being evaluated in patients with RET fusion-positive cancers, including two patients with CCA, in the ongoing phase I/II LIBRETTO-001 basket trial127.
Immunotherapies
The role of ICI-based immunotherapy for the treatment of CCA has changed drastically over the past few years128. It is important to differentiate the role of immunotherapy for CCA according to two separate scenarios129.
The first scenario is related to exploration of immunotherapies in a tumour-agnostic manner, whereby selection of patients relies on specific molecular alterations, regardless of the primary tumour site or histology. In this regard, the ICI pembrolizumab is indicated for use in patients harbouring CCAs with deficient mismatch repair (dMMR) and/or high microsatellite instability (MSI-H)130–132. Pembrolizumab, an anti-PD-1 antibody, had an ORR of 53% (21% CR rate) in a cohort of 86 patients with advanced-stage dMMR solid tumours; four patients with CCA were included in this cohort and one had a CR, with the other three having stable disease130. An updated analysis of the multicohort phase II KEYNOTE-158 study of pembrolizumab in patients with advanced-stage MSI-H/dMMR non-colorectal cancers included data from 22 patients with CCA131. In the CCA cohort, pembrolizumab was associated with an ORR of 40.8% (CR rate 13.5%), a median response duration of 30.6 months, a median PFS of 4.2 months and median OS of 19.4 months, with a 3-year OS of 30.3%131. In addition, ICIs have notable activity against cancers with a high tumour mutational burden (TMB). Indeed, pembrolizumab has also been granted histology-agnostic approval by the FDA for patients with a high TMB (≥10 mutations per megabase)133, based on a reported ORR of 29% (CR rate 4%) in a cohort of 102 patients with previously treated advanced-stage solid tumours (not specified how many had CCA); responses were durable, lasting ≥12 months in 57% of patients and ≥24 months in 50%134. Unfortunately, these tumour-agnostic approaches are applicable to only a very limited proportion of patients with CCA, given that <5% of this patient population have dMMR/MSI-H or TMB-high tumours129. Therefore, developing and applying immunotherapies in a way that would enable a wider spectrum of patients to benefit is of substantial importance.
Indeed, the second scenario is the development of immunotherapy in unselected populations of patients with advanced-stage CCA. This approach is mainly based on translational research work suggesting a potential role for immune-directed therapies in the treatment of CCA and other BTCs, considering that markers of a pre-existing antitumour T cell response are generally associated with a favourable prognosis in patients with these cancers135–138. Hence, first efforts in this regard were mainly focused on the use of monotherapy approaches with ICIs. Unfortunately, this strategy had limited efficacy in patients with BTCs, with an ORR of 5.8%, a median PFS of 2.0 months and median OS of 7.4 months with single-agent pembrolizumab in the phase II KEYNOTE-158 study28. Moreover, the efficacy was not clearly better in patients with PD-L1-positive tumours (ORR 6.6% and 13.0% in KEYNOTE-158 and KEYNOTE-028, respectively)28. Given these findings, alternative approaches have been pursued, mainly in the form of combining ICIs with cytotoxic chemotherapy.
Preliminary results from a retrospective study by Sun and colleagues139 suggested better outcomes with the addition of anti-PD-1 antibodies to chemotherapy (38 patients) than with anti-PD-1 antibodies alone (20 patients) or chemotherapy alone (19 patients); the median PFS durations were 5.1 months, 2.2 months and 2.4 months, respectively, and the median OS was 14.9 months versus 4.1 months versus 6.0 months. Additional phase II studies in patients with previously untreated advanced-stage BTCs supported this rationale140–142. In the largest of these phase II studies, Oh and colleagues142 recruited 124 patients to three different treatment arms: (1) chemotherapy (gemcitabine plus cisplatin) followed by concurrent chemotherapy and dual ICI therapy with the anti-PD-L1 antibody durvalumab and the anti-CTLA4 antibody tremelimumab from cycle 2 (30 patients); (2) concurrent chemotherapy plus durvalumab from cycle 1 (47 patients); or (3) concurrent chemotherapy plus durvalumab and tremelimumab from cycle 1 (47 patients). Whenever tremelimumab was administered, a maximum of four cycles were given. The primary end point of the study was ORR, which was highest in the arms in which ICIs were administered from cycle 1 onwards (ORRs of 50%, 72% and 70% in arms 1, 2 and 3, respectively); however, the median PFS was similar between arms (12.8 months, 11.8 months and 12.3 months, respectively)142. In view of a more favourable toxicity profile without compromised efficacy in the arm without tremelimumab, the combination of gemcitabine, cisplatin and durvalumab was selected for further assessment in the phase III TOPAZ-1 trial.
In TOPAZ-1, 685 patients with treatment-naive advanced-stage CCAs or gallbladder cancer were randomly assigned to receive gemcitabine and cisplatin plus either durvalumab or placebo143. The primary end point was OS, which was significantly improved with durvalumab (median 12.8 months versus 11.5 months in the placebo group; 24-month OS 24.9% versus 10.4%; HR 0.80, 95% CI 0.66–0.97; P = 0.021)143. The durvalumab group also had improvements in PFS (median 7.2 months versus 5.7 months; HR 0.75, 95% CI 0.63–0.89; P = 0.001) and ORR (26.7% versus 18.7%; OR 1.60, 95% CI 1.11–2.31)143. This improved activity was reported without additional toxicity (for example, grade 3−4 adverse events in 75.7% versus 77.8% of patients)143, and with a trend towards a longer time to deterioration in patient-reported global health status according to EORTC QLQ C30 scores favouring the durvalumab arm (HR 0.87, 95% CI 0.69–1.12; P = 0.279)144. Interestingly, subgroup analyses did not reveal significant differences in outcomes depending on PD-L1 expression143, primary tumour site145, disease status (that is, initially unresectable versus recurrent)146 or geographical area (Asian versus rest of the world)147. On the basis of these results, the FDA approved the combination of gemcitabine, cisplatin and durvalumab in 2022 (ref. 148), and the latest NCCN guidelines for hepatobiliary cancer (version 1.2022)90 recommend this regimen as the preferred first-line treatment option for advanced-stage CCA. Similarly, the KEYNOTE-966 trial, the largest phase III trial in patients with CCA to date, evaluated the combination of chemotherapy and ICI therapy in the first-line treatment of patients with unresectable, locally advanced or metastatic CCA or gallbladder cancer149. In this randomized, double-blind trial, 533 patients were allocated to receive pembrolizumab plus gemcitabine and cisplatin and 536 patients to receive placebo plus gemcitabine and cisplatin149. The primary end point was median OS, which was 12.7 months in the pembrolizumab plus gemcitabine and cisplatin group compared with 10.9 months in the placebo group (HR 0.83, 95% CI 0.72–0.95; one-sided P = 0.0034)149. In contrast to the TOPAZ-1 trial, maintenance gemcitabine was allowed in both arms without a maximum duration while cisplatin was administered for a maximum of eight cycles149. This may have accounted for the ORR being the same in both arms (29%)149. On the basis of these two trials, the role of ICIs in the first-line treatment of advanced-stage CCA has been firmly established.
Several other clinical trials of ICIs are ongoing in patients with BTCs128. Beyond their addition to chemotherapy, combinations of ICIs with TKIs have also been tested, albeit early results have been mediocre. For example, a small-cohort phase II study found an ORR of only 10% with the combination of lenvatinib plus pembrolizumab in patients with previously treated advanced-stage BTCs150. Nevertheless, other ongoing clinical trials are exploring this potential alternative, chemotherapy-free ICI-based therapeutic strategy (NCT04211168).
Thus, the future of immunotherapy for CCA looks bright for the first time. Efforts to identify predictive biomarkers for patient selection and to understand the mechanisms of resistance will be paramount in order to realize the full potential of ICIs and other immunotherapies.
Liver transplantation for iCCA
Transplant oncology is a developing field that merges the disciplines of organ transplantation and oncology151. iCCA is one of the paradigms of transplant oncology, but remains a formal contraindication for liver transplantation outside clinical trials in most jurisdictions. Two distinct populations of patients with iCCA might benefit from liver transplantation: (1) patients with decompensated cirrhosis who have a liver mass with imaging characteristics that are not diagnostic of hepatocellular carcinoma and are found to have iCCA following biopsy sampling; and (2) those with multifocal and large iCCAs usually occurring in otherwise healthy livers.
In the past 10 years, enthusiasm to incorporate iCCA as an indication for liver transplantation has increased after data from several retrospective studies demonstrated excellent outcomes in patients with cirrhosis and with ‘very early’ and ‘early’ iCCAs (defined as single tumours ≤2 cm and ≤5 cm, respectively) who received a liver transplant (Table 3). Most of these studies involved patients either initially misdiagnosed with hepatocellular carcinoma or with an incidental iCCA mass found on explant pathology. A prospective phase II trial of liver transplantation in patients with cirrhosis and very early iCCA (NCT02878473) is now underway (Table 3); however, accruing patients has been challenging owing to the difficulty of listing such patients for transplantation and finding an allograft. Before liver transplantation can be recommended for such patients outside clinical trials, several questions remain to be answered. First, do patients with a known diagnosis of iCCA have similar outcomes to those in whom iCCA was diagnosed incidentally? Second, should these patients undergo neoadjuvant treatments? Third, should this transplant indication be limited to single iCCAs of ≤2 cm or could this be expanded to those with single larger tumours?
Table 3 |.
Summary of key contemporary studies of liver transplantation in patients with Icca
| Study (year of publication) | Tumour size (n) | iCCAs discovered incidentally/initially misdiagnosed as HCC | Patients with cirrhosis (n) | OS | RFS | Recurrence rate | Chemotherapy treatment |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Retrospective studies in patients with incidental or misdiagnosed small iCCAs, mainly associated with cirrhosis | |||||||
|
| |||||||
| Sapisochin et al. (2014)156 | Single ≤2 cm (8) Multiple or single >2 cm (21) |
23 (79%)/6 (21%) | 29 (100%) | Overall: 79% at 1 year; 61% at 3 years; 45% at 5 years. ≤2 cm tumour group: 100% at 1 year; 73% at 3 years; 73% at 5 years. >2 cm tumour group: 71% at 1 year; 43% at 3 years; 34% at 5 years |
NR | Overall: 11% at 1 year; 29% at 3 years; 29% at 5 years. ≤2 cm tumour group: 0% at 1 year; 0% at 3 years; 0% at 5 years. >2 cm tumour group: 26% at 1 year; 42% at 3 years; 42% at 5 years |
None |
|
| |||||||
| Sapisochin et al. (2016)157 | Single >2 cm or multiple tumours of any size (33) | 8 (24%)/25 (76%) | 33 (100%) | 1 year: 79% 3 years: 50% 5 years: 45% |
NR | 1 year: 30% 3 years: 47% 5 years: 61% |
None |
|
| |||||||
| Single ≤2 cm (15) |
7 (47%)/8 (53%) | 15 (100%) | 1 year: 93% 3 years: 84% 5 years: 65% |
NR | 1 year: 7% 3 years: 18% 5 years: 18% |
None | |
|
| |||||||
| Jung et al. (2017)158 | Single ≤2 cm (4) Multiple ≤2 cm (2) Single >2 cm (10) |
1 (6%)/14 (88%) | 12 (13%) | 1 year: 81% (63%a) 3 years: 52% (63%a) 5 years: 52% |
NR | 1 year:56% (50%a) 3 years:56% (50%a) 5 years:78% |
None |
|
| |||||||
| De Martin et al. (2020)159 | Largest nodule ≤5 cm (49; 24 patients with iCCA only combined with 25 patients with mixed HCC–iCCA for analyses) | 5 (10%)/44 (90%) | 49 (100%) | 1 year: 90% (88%b, 92%c) 3 years: 76% (65%b, 87%c) 5 years: 67% (65%b, 69%c) |
1 year: 87% (81%b, 78%c) 3 years: 79% (74%b, 69%c) 5 years: 75% (74%b, 55%c) |
NR | None |
|
| |||||||
| Retrospective studies in patients with large multifocal iCCAs diagnosed prior to liver transplantation | |||||||
|
| |||||||
| Lunsford et al. (2018)160 | Among 6 patients: median number of tumours 4 (IQR 3.0–5.8); median cumulative diameter 10.5 cm (IQR 7.0–13.5 cm) | NA | 1 (17%) | 1 year: 100% 3 years: 83.3% 5 years: 83.3% |
1 year: 50% 3 years: 50% 5 years: 50% |
1 year: 50% 3 years: 50% 5 years: 50% |
Yes, neoadjuvant and adjuvant |
|
| |||||||
| Ito et al. (2022)161 | 22 of 31 patients (71%) had tumours larger ≥5 cm |
NA | 14 (45%) | 1 year: 80% 3 years: 63% 5 years: 49% 5-year OS was 100% in patients who received both neoadjuvant chemotherapy and locoregional therapy, compared with 77%, 57% and 41% at 1, 3 and 5 years, respectively in those who did not or received neoadjuvant locoregional therapy alone |
5 years 42% | NR | Yes, neoadjuvant, sometimes combined with locoregional therapy (10 patients received neoadjuvant locoregional therapy with or without neoadjuvant chemotherapy); 11 patients received adjuvant chemotherapy |
|
| |||||||
| Retrospective studies in patients with large multifocal iCCAs diagnosed prior to liver transplantation | |||||||
|
| |||||||
| McMillan et al. (2022)152 | Among 18 patients: median number of tumours 2 (1–11); median tumour size 10.4 cm (2.5–19.9 cm) | NA | 6 (33%) | 1 year: 100% 3 years: 71% 5 years: 57% |
3 years: 52% | 7 (38.9%); median time to recurrence 11 months (range 4–42 months) |
Yes, neoadjuvant and adjuvant |
|
| |||||||
| Ongoing prospective clinical trials | |||||||
|
| |||||||
| University Health Network Toronto phase II multicentre trial NCT02878473 (NA) |
Single iCCA ≤2 cm with no extrahepatic disease or vascular invasion (target of 30 patients who are not candidates for tumour resection and have serum CA19.9 levels ≤ 100 ng/ml) | NA | 100% | NR (5-year OS is primary end point) | NR (secondary end point) | NR (secondary end point) | NR |
| Oslo University phase II TESLA trial NCT04556214 (NA) | NA; histologically proven iCCA with no extrahepatic disease, vascular invasion or lymph node involvement (target of 15 patients who are not candidates for tumour resection) | NA | NR | NR (3-year OS is primary end point) | NR (secondary end point) | NR | Patients must have stable disease after ≥6 months of systemic chemotherapy or locoregional treatment |
|
| |||||||
| University Health Network Toronto phase II single-centre Trial NCT04195503 (NA) | NA; histologically proven iCCA with no extrahepatic disease, vascular invasion or lymph node involvement (target of 10 patients who are not candidates for tumour resection and have a potential live liver donor) | NA | NR | NR (5-year OS is primary end point; 1-year OS is secondary end point) | NR (5-year RFS is secondary end point) | NR | Patients must have stable disease or tumour regression lasting ≥6 months after systemic chemotherapy or locoregional treatment |
HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; IQR, interquartile range; NA, not applicable; NR, not reported; OS, overall survival; RFS, recurrence-free survival.
Data for patients with iCCA alone (n = 8), as opposed to concurrent iCCA and HCC (n = 7) or mixed HCC–iCCA (n = 1).
Data for patients with iCCA and mixed HCC–iCCA tumours >2 cm but ≤5 cm (n = 24).
Data for patients with iCCA ≤2 cm and mixed HCC–iCCA tumours (n = 25).
With advances in the understanding of iCCA biology and improvements in patient management, the concept of considering liver transplantation has also been revisited for patients with unresectable iCCA, no extrahepatic disease and a sustained response to systemic and/or local therapies18. The largest prospective study to date involved a series of 65 patients referred for liver transplantation evaluation; 37 patients met the criteria and were listed, although five patients were subsequently excluded owing to conversion to resectable disease with the stipulated neoadjuvant chemotherapy, and 18 eventually received a liver transplant152. The results of that study demonstrated a 5-year OS of 57% and a 3-year disease-free survival of 52% after transplantation152. Several questions remain, and these results need to be externally validated, although they are encouraging and will serve as a baseline in future studies to identify the optimal candidates for liver transplantation153. To our knowledge, several centres are now considering liver transplantation as an option for patients with unresectable locally advanced iCCA who have a sustained response to systemic and/or local therapies and no evidence of extrahepatic disease at any time point. With the development of molecularly targeted therapies for iCCA and a better understanding of mutations associated with a favourable prognosis, such as FGFR genetic aberrations154, the proportion of patients who might be eligible for liver transplantation in the future might increase. When considering alternative treatments for these patients, the options are extremely limited owing to very poor outcomes with resection or hepatic artery infusion pump chemotherapy155.
In the next few years, more data on liver transplantation for iCCA will be published and this treatment modality is expected to become available to patients at many centres. Until then, liver transplantation should be performed under strict research protocols with stringent inclusion criteria.
Conclusions
As is evident from this update, many crucial scientific and clinical questions remain despite the recent progress in CCA research and management. Although the TIME presents potential targets for the treatment of CCA, the limited antitumour activity of ICIs in this disease highlights the need for a more in-depth and fundamental understanding of the immunosuppressive cell populations. Further information will also be needed to stratify TIME-based subgroups for a precision therapy approach, and perhaps some subtypes such as the immune-desert tumours will prove difficult if not impossible to target effectively. Given the heterogeneity of CAFs in CCAs, targeting this cell population is also likely to prove more difficult than originally conceptualized. Better diagnostic modalities are necessary for pCCA and perhaps the emerging technology of measuring cfDNA and ctDNA will address this problem; the use of such assays would also greatly facilitate monitoring of disease activity following surgical resection or during systemic therapy. Indeed, we are optimistic that these liquid biopsy assays will achieve clinical utility in the management of CCA. Although an important advance, targeted therapies for patients with FGFR2 fusions or IDH1 mutations are often met with intrinsic resistance or a short durability of response owing to acquired resistance; combinatorial approaches will need to be examined in model systems and patients in order to improve outcomes. The use of neoadjuvant and adjuvant therapies in combination with surgery and/or locoregional therapies to improve outcomes is also an unmet need that warrants further studies. Finally, selection of patients who will benefit from liver transplantation will require national organ allocation systems to acknowledge patients with iCCA as candidates for this procedure. We look forward to future updates on CCA, which will hopefully report solutions to these unmet needs in patients with this disease.
Key points.
Cholangiocarcinomas (CCAs) are highly desmoplastic cancers characterized by a tumour microenvironment that is poorly immunogenic, with an abundance of immunosuppressive cell types such as myeloid-derived suppressor cells and tumour-associated macrophages.
The desmoplasia of CCAs is associated with an abundance of heterogeneous cancer-associated fibroblasts (CAFs), with several transcriptomically diverse CAF subtypes identified.
Assessments of circulating tumour cells and cell-free tumour DNA are becoming important in the diagnosis and monitoring of CCA.
Potent oncogenic drivers of intrahepatic CCAs (iCCAs) include fibroblast growth factor receptor 2 (FGFR2) gene fusions and neomorphic, gain-of-function variants of isocitrate dehydrogenase 1 (IDH1). FGFR2 fusions and IDH1 mutations are targetable, and approved molecularly targeted therapies are now available for the treatment of CCAs harbouring these genetic aberrations.
The results of immune-checkpoint inhibitor monotherapy in patients with CCA have been disappointing, although the combination of gemcitabine, cisplatin and durvalumab has been approved in the first-line setting for the treatment of advanced-stage CCA.
Liver transplantation is an emerging option for the subset of patients with early stage iCCA occurring in the setting of cirrhosis.
Acknowledgements
The work of the authors is supported by the US National Institutes of Health (NIH)/National Cancer Institute (NCI) grants SPORE P50 CA210964 (to S.I.I. and G.J.G.) and 1K08CA236874 (to S.I.I.). The work of S.A. is supported by a fellowship from “la Caixa” Foundation (ID 100010434) and by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 847648. The work of L.G. is supported by American Cancer Society Clinical Scientist Development Grant 134013-CSDG-19-163-01-TBG and NIH/NCI Gastrointestinal Cancer SPORE P50 CA127003. A.L. has received funding from The Christie Charity and the European Union’s Horizon 2020 research and innovation programme (grant no. 82551, ESCALON). J.D.Y. has received an American College of Gastroenterology Junior Faculty Development Award and a U.S. Department of Defense Peer Reviewed Cancer Research Program Career Development Award (CA 191051).
Competing interests
S.I.I. serves as an adviser and consultant to AstraZeneca. L.G. has received research funding (via her institution) from Adaptimmune, Bayer, Bristol Myers Squibb, Eisai, Eli Lilly, Genentech, Incyte, Leap Therapeutics, Loxo Oncology, Macrogenics, Merck, Novartis, Nucana, QED Therapeutics, Relay Therapeutics, Servier and Taiho Oncology, and serves as an adviser or consultant to Alentis Therapeutics, AstraZeneca, Black Diamond, Exelixis, Genentech, H3Biomedicine, Incyte, QED Therapeutics, Servier, Sirtex Medical, Taiho Oncology and Transthera Bio. A.L. declares travel and educational support from Advanced Accelerator Applications, Bayer, Delcath, Ipsen, Mylan, Novartis, Pfizer and SirtEx; speaker’s honoraria from Advanced Accelerator Applications, AstraZeneca, Eisai, Incyte, Ipsen, Merck, Pfizer, QED Therapeutics and Servier; and advisory or consultancy roles with Albireo Pharma, AstraZeneca, Boehringer Ingelheim, Boston Scientific, Eisai, GENFIT, Ipsen, Nutricia, QED Therapeutics, Roche, Servier and TransThera Biosciences; and is a member of the Knowledge Network and NETConnect Initiatives funded by Ipsen. G.S. has received grant support from Roche, and declares consultancy roles with AstraZeneca, Chiesi, Evidera, Integra, Novartis and Roche. S.A., J.D.Y. and G.J.G. declare no competing interests.
Footnotes
Additional information
Peer review information Nature Reviews Clinical Oncology thanks C. Berasain, H. Francis, T. Greten, and J. Harding for their contribution to the peer review of this work.
Related links
References
- 1.Banales JM et al. Cholangiocarcinoma: state-of-the-art knowledge and challenges. Liver Int. 39, 5–6 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Brindley PJ et al. Cholangiocarcinoma. Nat. Rev. Dis. Prim 7, 65 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banales JM et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol 17, 557–588 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi S, Khan SA, Hallemeier CL, Kelley RK & Gores GJ Cholangiocarcinoma– evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol 15, 95–111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Affo S et al. Promotion of cholangiocarcinoma growth by diverse cancer-associated fibroblast subpopulations. Cancer Cell 39, 883 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang JD et al. DNA methylation markers for detection of cholangiocarcinoma: discovery, validation, and clinical testing in biliary brushings and plasma. Hepatol. Commun 5, 1448–1459 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zill OA et al. Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discov. 5, 1040–1048 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu MJ, Shi L, Merritt J, Zhu AX & Bardeesy N Biology of IDH mutant cholangiocarcinoma. Hepatology 75, 1322–1337 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Kelley RK, Bridgewater J, Gores GJ & Zhu AX Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol 72, 353–363 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Abou-Alfa GK et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 21, 671–684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Javle M et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol 6, 803–815 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Goyal L et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N. Engl. J. Med 388, 228–239 (2023). [DOI] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. FDA; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approvalpemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (2020). [Google Scholar]
- 14.US Food and Drug Administration. FDA grants accelerated approval to infigratinib for metastatic cholangiocarcinoma. FDA; https://www.fda.gov/drugs/resourcesinformation-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastaticcholangiocarcinoma (2021). [Google Scholar]
- 15.US Food and Drug Administration. FDA grants accelerated approval to futibatinib for cholangiocarcinoma. FDA; https://www.fda.gov/drugs/resources-information-approveddrugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma (2022). [Google Scholar]
- 16.Abou-Alfa GK et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 21, 796–807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. FDA approves ivosidenib for advanced or metastatic cholangiocarcinoma. FDA; https://www.fda.gov/drugs/resources-information-approveddrugs/fda-approves-ivosidenib-advanced-or-metastatic-cholangiocarcinoma (2022). [Google Scholar]
- 18.Gravely AK, Vibert E & Sapisochin G Surgical treatment of intrahepatic cholangiocarcinoma. J. Hepatol 10.1016/j.jhep.2022.01.004 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Loeuillard E, Gores GJ & Ilyas SI Cholangiocarcinoma: what are the most valuable therapeutic targets – cancer-associated fibroblasts, immune cells, or beyond T cells? Expert. Opin. Ther. Targets 25, 835–845 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeuillard E et al. Targeting tumor-associated macrophages and granulocytic myeloidderived suppressor cells augments PD-1 blockade in cholangiocarcinoma. J. Clin. Invest 130, 5380–5396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruffolo LI et al. GM-CSF drives myelopoiesis, recruitment and polarisation of tumour-associated macrophages in cholangiocarcinoma and systemic blockade facilitates antitumour immunity. Gut 71, 1386–1398 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 11, 1248–1267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gani F et al. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol 23, 2610–2617 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Zhou G et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J. Hepatol 71, 753–762 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Ghidini M et al. Characterisation of the immune-related transcriptome in resected biliary tract cancers. Eur. J. Cancer 86, 158–165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L et al. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36, 418–430.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabris L, Sato K, Alpini G & Strazzabosco M The tumor microenvironment in cholangiocarcinoma progression. Hepatology 73, 75–85 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piha-Paul SA et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 147, 2190–2198 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Buettner S et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery 164, 411–418 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Peng D et al. Lymphocyte to monocyte ratio predicts resectability and early recurrence of Bismuth-Corlette type IV hilar cholangiocarcinoma. J. Gastrointest. Surg 24, 330–340 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsilimigras DI et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB 22, 1667–1674 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Sun D et al. CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ 8, e8458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan D et al. Kupffer cell-derived TNF triggers cholangiocellular tumorigenesis through JNK due to chronic mitochondrial dysfunction and ROS. Cancer Cell 31, 771–789.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulter L et al. WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. J. Clin. Invest 125, 1269–1285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dwyer BJ et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J. Hepatol 74, 860–872 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Veglia F, Perego M & Gabrilovich D Myeloid-derived suppressor cells coming of age. Nat. Immunol 19, 108–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu XD et al. Circulating myeloid-derived suppressor cells in patients with pancreatic cancer. Hepatobiliary Pancreat. Dis. Int 15, 99–105 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Meyer C et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother 63, 247–257 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song G et al. Single-cell transcriptomic analysis suggests two molecularly subtypes of intrahepatic cholangiocarcinoma. Nat. Commun 13, 1642 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bao X et al. Molecular subgroups of intrahepatic cholangiocarcinoma discovered by single-cell RNA sequencing-assisted multiomics analysis. Cancer Immunol. Res 10, 811–828 (2022). [DOI] [PubMed] [Google Scholar]
- 41.Keenan BP et al. Circulating monocytes associated with anti-PD-1 resistance in human biliary cancer induce T cell paralysis. Cell Rep. 40, 111384 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tavazoie MF et al. LXR/ApoE activation restricts innate immune suppression in cancer. Cell 172, 825–840.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vonderheide RH CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med 71, 47–58 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Diggs LP et al. CD40-mediated immune cell activation enhances response to anti-PD-1 in murine intrahepatic cholangiocarcinoma. J. Hepatol 74, 1145–1154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wabitsch S et al. Anti-PD-1 in combination with trametinib suppresses tumor growth and improves survival of intrahepatic cholangiocarcinoma in mice. Cell Mol. Gastroenterol. Hepatol 12, 1166–1178 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Affo S, Yu LX & Schwabe RF The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol 12, 153–186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brivio S, Cadamuro M, Strazzabosco M & Fabris L Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World J. Hepatol 9, 455–468 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirica AE, Campbell DJ & Dumur CI Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol 27, 276–284 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Sirica AE & Gores GJ Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology 59, 2397–2402 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabris L et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int. 39, 63–78 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mertens JC et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 73, 897–907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y, McAndrews KM & Kalluri R Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol 18, 792–804 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell DJ, Dumur CI, Lamour NF, Dewitt JL & Sirica AE Novel organotypic culture model of cholangiocarcinoma progression. Hepatol. Res 42, 1119–1130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Biffi G & Tuveson DA Diversity and biology of cancer-associated fibroblasts. Physiol. Rev 101, 147–176 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharjee S et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Invest 10.1172/JCI146987 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin-Serrano MA et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 10.1136/gutjnl-2021-326514 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang M et al. Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. J. Hepatol 73, 1118–1130 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Ravichandra A, Bhattacharjee S & Affo S Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma progression and therapeutic resistance. Adv. Cancer Res. 156, 201–226 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Chen Y et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39, 548–565.e6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu MC et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol 31, 745–759 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cohen JD et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasenang W, Chaiyarit P, Proungvitaya S & Limpaiboon T Serum cell-free DNA methylation of OPCML and HOXD9 as a biomarker that may aid in differential diagnosis between cholangiocarcinoma and other biliary diseases. Clin. Epigenetics 11, 39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ettrich TJ et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci. Rep 9, 13261 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mody K et al. Circulating tumor DNA profiling of advanced biliary tract cancers. JCO Precis. Oncol 3, 1–9 (2019). [DOI] [PubMed] [Google Scholar]
- 66.Moreno Luna LE et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 131, 1064–1072 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barr Fritcher EG et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology 149, 1813–1824.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Gonda TA et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin. Gastroenterol. Hepatol 15, 913–919.e1 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Singhi AD et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 69, 52–61 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arechederra M et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 71, 1141–1151 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andresen K et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology 61, 1651–1659 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loi E et al. HOXD8 hypermethylation as a fully sensitive and specific biomarker for biliary tract cancer detectable in tissue and bile samples. Br. J. Cancer 126, 1783–1794 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shigehara K et al. Real-time PCR-based analysis of the human bile microRNAome identifies miR-9 as a potential diagnostic biomarker for biliary tract cancer. PLoS ONE 6, e23584 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han HS et al. Bile-derived circulating extracellular miR-30d-5p and miR-92a-3p as potential biomarkers for cholangiocarcinoma. Hepatobiliary Pancreat. Dis. Int 19, 41–50 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Berchuck JE et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann. Oncol 33, 1269–1283 (2022). [DOI] [PubMed] [Google Scholar]
- 76.Andersen JB et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology 142, 1021–1031.e15 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Montal R et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J. Hepatol 73, 315–327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sia D et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 144, 829–840 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Job S et al. Identification of four immune subtypes characterized by distinct composition and functions of tumor microenvironment in intrahepatic cholangiocarcinoma. Hepatology 72, 965–981 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lowery MA et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin. Cancer Res. 24, 4154–4161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weinberg BA et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J. Gastrointest. Oncol 10, 652–662 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wardell CP et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J. Hepatol 68, 959–969 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Jusakul A et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 7, 1116–1135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nakamura H et al. Genomic spectra of biliary tract cancer. Nat. Genet 47, 1003–1010 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Boscoe AN, Rolland C & Kelley RK Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J. Gastrointest. Oncol 10, 751–765 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaisaingmongkol J et al. Common molecular subtypes among Asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell 32, 57–70.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan-On W et al. Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nat. Genet 45, 1474–1478 (2013). [DOI] [PubMed] [Google Scholar]
- 88.Bekaii-Saab TS, Bridgewater J & Normanno N Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann. Oncol 32, 1111–1126 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Lamarca A et al. Molecular profiling in daily clinical practice: practicalities in advanced cholangiocarcinoma and other biliary tract cancers. J. Clin. Med 10.3390/jcm9092854 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.National Comprehensive Cancer Network. NCCN guidelines: hepatobiliary cancer. NCCN; https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1438 (2022). [Google Scholar]
- 91.Haugsten EM, Wiedlocha A, Olsnes S & Wesche J Roles of fibroblast growth factor receptors in carcinogenesis. Mol. Cancer Res. 8, 1439–1452 (2010). [DOI] [PubMed] [Google Scholar]
- 92.Babina IS & Turner NC Advances and challenges in targeting FGFR signalling in cancer. Nat. Rev. Cancer 17, 318–332 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Wu YM et al. Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov. 3, 636–647 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graham RP et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum. Pathol 45, 1630–1638 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Arai Y et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 59, 1427–1434 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Becker Z BridgeBio says it’s undeterred after Truseltiq partner withdraws application, discontinues drug. FIERCE Pharma https://www.fiercepharma.com/pharma/bridgebios-cancer-drug-truseltiqs-future-unclear-partner-helsinn-withdrawing-nda (2022). [Google Scholar]
- 97.Droz Dit Busset M et al. Derazantinib for patients with intrahepatic cholangiocarcinoma harboring FGFR2 fusions/rearrangements: primary results from the phase II study FIDES-01 [abstract 47P]. Ann. Oncol 32 (Suppl. 5), S376 (2021). [Google Scholar]
- 98.Krook MA et al. Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma. Mol. Cancer Ther. 19, 847–857 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krook MA et al. Tumor heterogeneity and acquired drug resistance in FGFR2-fusion-positive cholangiocarcinoma through rapid research autopsy. Cold Spring Harb. Mol. Case Stud. 10.1101/mcs.a004002 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goyal L et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 7, 252–263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goyal L et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 9, 1064–1079 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sootome H et al. Futibatinib is a novel irreversible FGFR 1–4 inhibitor that shows selective antitumor activity against FGFR-deregulated tumors. Cancer Res. 80, 4986–4997 (2020). [DOI] [PubMed] [Google Scholar]
- 103.Meric-Bernstam F et al. Futibatinib, an irreversible FGFR1–4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose-expansion study. Cancer Discov. 12, 402–415 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casaletto J et al. RLY-4008, a novel precision therapy for FGFR2-driven cancers designed to potently and selectively inhibit FGFR2 and FGFR2 resistance mutations [abstract]. Cancer Res. 81 (Suppl. 13), 1455 (2021). [Google Scholar]
- 105.Franovic A et al. Activity of KIN-3248, a next-generation pan-FGFR inhibitor, against acquired FGFR-gatekeeper and molecular-brake drug resistance mutations [abstract]. J. Clin. Oncol 40 (Suppl. 4), 461 (2022). [Google Scholar]
- 106.Goyal L et al. First results of RLY-4008, a potent and highly selective FGFR2 inhibitor in a first-in-human study in patients with FGFR2-altered cholangiocarcinoma and multiple solid tumors [abstract]. Mol. Cancer Ther. 20 (Suppl. 12), P02–02 (2021). [Google Scholar]
- 107.Lu C & Thompson CB Metabolic regulation of epigenetics. Cell Metab. 16, 9–17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inoue S et al. Mutant IDH1 downregulates ATM and alters DNA repair and sensitivity to DNA damage independent of TET2. Cancer Cell 30, 337–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sulkowski PL et al. 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci. Transl. Med 10.1126/scitranslmed.aal2463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valle JW, Lamarca A, Goyal L, Barriuso J & Zhu AX New horizons for precision medicine in biliary tract cancers. Cancer Discov. 7, 943–962 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Subbiah V et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 21, 1234–1243 (2020). [DOI] [PubMed] [Google Scholar]
- 112.US Food and Drug Administration. FDA grants accelerated approval of dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. FDA; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (2022). [Google Scholar]
- 113.Subbiah V et al. Pan-cancer efficacy of vemurafenib in BRAFV600-mutant non-melanoma cancers. Cancer Discov. 10, 657–663 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galdy S et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 36, 141–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Javle M et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 22, 1290–1300 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Ohba A et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): an investigator-initiated multicenter phase 2 study (HERB trial) [abstract]. J. Clin. Oncol 40 (Suppl. 16), 4006 (2022). [Google Scholar]
- 117.Lee CK et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19–14). Lancet Gastroenterol. Hepatol 10.1016/S2468-1253(22)00335-1 (2022). [DOI] [PubMed] [Google Scholar]
- 118.Harding JJ et al. Targeting HER2 mutation-positive advanced biliary tract cancers with neratinib: final results from the phase 2 SUMMIT basket trial. J. Clin. Oncol 40, 4079–4079 (2022). [Google Scholar]
- 119.Meric-Bernstam F et al. Zanidatamab (ZW25) in HER2-positive biliary tract cancers (BTCs): results from a phase I study [abstract]. J. Clin. Oncol 39 (Suppl. 3), 299 (2021). [Google Scholar]
- 120.Westphalen CB et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis. Oncol 5, 69 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Drilon A et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N. Engl. J. Med 378, 731–739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.US Food and Drug Administration. FDA approves larotrectinib for solid tumors with NTRK gene fusions. FDA; https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions-0 (2018). [Google Scholar]
- 123.Doebele RC et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 21, 271–282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.US Food and Drug Administration. FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. FDA; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (2019). [Google Scholar]
- 125.Parimi V et al. Genomic landscape of 891 RET fusions detected across diverse solid tumor types. NPJ Precis. Oncol 7, 10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Subbiah V et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat. Med 28, 1640–1645 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Subbiah V et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol. 23, 1261–1273 (2022). [DOI] [PubMed] [Google Scholar]
- 128.Valle JW, Kelley RK, Nervi B, Oh DY & Zhu AX Biliary tract cancer. Lancet 397, 428–444 (2021). [DOI] [PubMed] [Google Scholar]
- 129.Lamarca A, Edeline J & Goyal L How I treat biliary tract cancer. ESMO Open 7, 100378 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Le DT et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maio M et al. Pembrolizumab in microsatellite instability high or mismatch repair deficient cancers: updated analysis from the phase 2 KEYNOTE-158 study. Ann. Oncol 10.1016/j.annonc.2022.05.519 (2022). [DOI] [PubMed] [Google Scholar]
- 132.US Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. FDA; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (2017). [Google Scholar]
- 133.US Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. FDA; https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (2020). [Google Scholar]
- 134.Marabelle A et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Ma K et al. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol. Lett 14, 250–256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu Y et al. Programmed death ligand 1 expression in human intrahepatic cholangiocarcinoma and its association with prognosis and CD8+ T-cell immune responses. Cancer Manag. Res 10, 4113–4123 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fluxa P et al. High CD8+ and absence of Foxp3+ T lymphocytes infiltration in gallbladder tumors correlate with prolonged patients survival. BMC Cancer 18, 243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goeppert B et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br. J. Cancer 113, 1343–1349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun D et al. Anti-PD-1 therapy combined with chemotherapy in patients with advanced biliary tract cancer. Cancer Immunol. Immunother 68, 1527–1535 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sahai V et al. A multicenter randomized phase II study of nivolumab in combination with gemcitabine/cisplatin or ipilimumab as first-line therapy for patients with advanced unresectable biliary tract cancer (BilT-01) [abstract]. J. Clin. Oncol 38 (Suppl. 15), 4582 (2020). [Google Scholar]
- 141.Liu T et al. Toripalimab with chemotherapy as first-line treatment for advanced biliary tract tumors: a preliminary analysis of safety and efficacy of an open-label phase II clinical study [abstract 53P]. Ann. Oncol 31 (Suppl. 4), S261 (2020). [Google Scholar]
- 142.Oh DY et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: an open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol 7, 522–532 (2022). [DOI] [PubMed] [Google Scholar]
- 143.Oh D-Y et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 10.1056/EVIDoa2200015 (2022). [DOI] [PubMed] [Google Scholar]
- 144.Burris HA et al. Patient-reported outcomes for the phase 3 TOPAZ-1 study of durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer [abstract]. J. Clin. Oncol 40 (Suppl. 16), 4070 (2022). [Google Scholar]
- 145.He A et al. Outcomes by primary tumour location in patients with advanced biliary tract cancer treated with durvalumab or placebo plus gemcitabine and cisplatin in the phase 3 TOPAZ-1 study [abstract O-1]. Ann. Oncol 33 (Suppl. 4), S378 (2022). [Google Scholar]
- 146.Okusaka T et al. Outcomes by disease status in patients with advanced biliary tract cancer treated wtih durvalumab or placebo plus gemcitabine and cisplatin in the phase 3 TOPAZ-1 study [abstract 93P]. Ann. Oncol 33 (Suppl. 9), S1471 (2022). [Google Scholar]
- 147.Vogel A et al. Regional subgroup analysis of the phase 3 TOPAZ-1 study of durvalumab (D) plus gemcitabine and cisplatin (GC) in advanced biliary tract cancer (BTC) [abstract]. J. Clin. Oncol 40 (Suppl. 16), 4075 (2022). [Google Scholar]
- 148.US Food and Drug Administration. FDA D.I.S.C.O. burst edition: FDA approval of Imfinzi (durvalumab) for adult patients with locally advanced or metastatic biliary tract cancer. FDA; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-imfinzi-durvalumab-adult-patients-locally-advanced-or (2022). [Google Scholar]
- 149.Kelley RK et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 10.1016/S0140-6736(23)00727-4 (2023). [DOI] [PubMed] [Google Scholar]
- 150.Villaneuva L et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study [abstract]. J. Clin. Oncol 39 (Suppl. 3), 321 (2021). [Google Scholar]
- 151.Sapisochin G et al. Transplant oncology in primary and metastatic liver tumors: principles, evidence, and opportunities. Ann. Surg 273, 483–493 (2021). [DOI] [PubMed] [Google Scholar]
- 152.McMillan RR et al. Survival following liver transplantation for locally advanced, unresectable intrahepatic cholangiocarcinoma. Am. J. Transpl 22, 823–832 (2022). [DOI] [PubMed] [Google Scholar]
- 153.Ivanics T, Toso C, Ilyas SI & Sapisochin G Transplant oncology in locally advanced intrahepatic cholangiocarcinoma: one more step on a long road. Am. J. Transpl 22, 685–686 (2022). [DOI] [PubMed] [Google Scholar]
- 154.Jain A et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis. Oncol 2, 1–12 (2018). [DOI] [PubMed] [Google Scholar]
- 155.Franssen S et al. Comparison of hepatic arterial infusion pump chemotherapy vs resection for patients with multifocal intrahepatic cholangiocarcinoma. JAMA Surg. 10.1001/jamasurg.2022.1298 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sapisochin G et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am. J. Transpl 14, 660–667 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Sapisochin G et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 64, 1178–1188 (2016). [DOI] [PubMed] [Google Scholar]
- 158.Jung DH et al. Clinicopathological features and prognosis of intrahepatic cholangiocarcinoma after liver transplantation and resection. Ann. Transpl 22, 42–52 (2017). [DOI] [PubMed] [Google Scholar]
- 159.De Martin E et al. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl. 26, 785–798 (2020). [DOI] [PubMed] [Google Scholar]
- 160.Lunsford KE et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol. Hepatol 3, 337–348 (2018). [DOI] [PubMed] [Google Scholar]
- 161.Ito T et al. A 3-decade, single-center experience of liver transplantation for cholangiocarcinoma: impact of era, tumor size, location, and neoadjuvant therapy. Liver Transpl. 28, 386–396 (2022). [DOI] [PubMed] [Google Scholar]