Abstract
Introduction:
Improving the timeliness and completion of vaccination is key to reducing under-five childhood mortality. This study examines the prevalence of delayed vaccination for doses administered at birth, 6, 10, 14 weeks and 9 months of age and its association with under-vaccination among infants in sub-Saharan Africa (SSA).
Methods:
Pooling data across 33 SSA countries, we assessed vaccination timing and series completion for children 12–35 months who were included in the immunization module of Demographic and Health Surveys (DHS) conducted between 2010 and 2019. Survey design adjusted logistic regression was used to model likelihood of not fully completing the basic immunization schedule associated with dose-specific delays in vaccination. Data were obtained and analyzed in 05/2020.
Results:
Among children with complete date records (n=70,006), the proportion of children vaccinated with delays by a month or more was high: 25.9% for BGC (birth); 49.1% for the third dose of Pentavalent (Penta3 [14 weeks]) and 63.9% for the first dose of Measles (9 months). Late vaccination was more common for children born to mothers with lower levels of educational attainment (p<0.001) and wealth (p<0.001). Controlling for place, time, and socio-demographics, vaccination delays at any dose was significantly associated with not completing the immunization schedule by 12 months (BCG: adjusted odds ratio [aOR] 1.93, 95%CI 1.83–2.02; Penta3: 1.50, 95%CI 1.35–1.64; Measles: 3.76, 95%CI 3.37–4.15).
Conclusion:
Timely initiation of vaccination could contribute to higher rates of complete immunization schedules, improving the reach and impact of vaccination programs on child health outcomes in SSA.
Keywords: Vaccination timeliness, immunization programs, Demographic and Health Surveys, sub-Saharan Africa, fully immunized child
INTRODUCTION
Considerable progress has been made in reducing under-five mortality globally which has declined by 53% from 1990 to 2015.1 Despite this success, progress in sub-Saharan Africa has been slower: only 8 of 43 countries in the region met or exceeded the Millennium Development Goals (MDGs) related to childhood survival by 2015.2 Consequently, it is estimated that nearly two-thirds of sub-Saharan African (SSA) countries will need to accelerate improvement in order to achieve the updated goal of reducing under-five mortality to 25 or fewer deaths per 1,000 livebirths in every country by 2030 in line with the Sustainable Development Goals (SDGs).1
Inequities in vaccination are a major contributor to disparities in childhood health and survival.3,4 This is evidenced in SSA where some of the highest rates of childhood mortality globally (above 100 per 1,000 live births) coincide with fewer than one-third of countries reporting immunization schedule completion in infants above 60%.5 The low rates of age-appropriate vaccination directly threaten progress made in the control and elimination of vaccine-preventable diseases (VPDs) that contribute importantly to improving childhood survival.6,7
The World Health Organization (WHO) Expanded Programme on Immunization (EPI) recommends that young children in most countries globally receive one dose of Bacillus-Calmette-Guerin (BCG) at birth, three doses of Oral polio vaccine (Polio) and 3 doses of the Pentavalent (Penta) combination vaccine (i.e. diptheria-tetanus-pertusis [DPT] – Hepatitis B [HepB] – Haemophilus influenzae type b [Hib]) at 6 weeks, 10 weeks and 14 weeks and one dose of Measles-containing vaccine (measles) at 9 months of age.8 These recommendations are adapted to address the specific epidemiological profile at the country-level, but all countries in SSA at a minimum use this basic series and additionally some may also offer newer childhood vaccines. To achieve effective control of VPDs, high rates of both timely receipt and completion of the basic schedule is needed. In acknowledgement of this, the WHO’s Immunization Agenda 2030, which has put forth aspirational goals for national immunization programs in line with the SDG agenda, underscores the importance of both receiving vaccination altogether but also ensuring that access to on-time vaccination is available to target the age-specific vulnerabilities children have for each VPD covered in the schedule.9
Previous studies on timeliness and completion of childhood vaccination in SSA have focused on underlying determinants, including spatial and socio-demographic factors associated with low uptake or poor adherence to recommendations for age-specific vaccination.10–15 However, there has been no systematic assessment of the association between delayed vaccination and schedule completion outside of the context of high-income countries.16,17 Delayed or late vaccination poses public health risks both in terms of individual-level disease acquisition as well as community-level transmission as children remain susceptible to and reservoirs for VPDs for unnecessarily prolonged periods of time.18,19 In real-time, the level and duration of risk associated with delayed vaccination is unknown because the visibility of vaccination timing is limited when relying on administrative data.7 Across countries, vaccination coverage is estimated by aggregating reported administrative data on the total doses administered for each vaccine in the target population of surviving infants, estimated from census data, over a defined period of time.20 These aggregate measures of coverage mask age-specific vulnerabilities, and potentially obscure patterns of clustered risk that program managers and policymakers could address with a more granular view of adherence to age-specific vaccination recommendations.7 Importantly, although a less commonly explored implication, vaccination delays may also increase the likelihood of missing subsequent doses, and even dropping out of the schedule before concluding the full series of vaccines in the first year of life, as is recommended. Understanding the extent to which vaccine delays occur across the schedule and defining the role that delayed vaccination plays in completing all recommended vaccines could help inform strategies that reduce bottlenecks to achieving complete coverage of the childhood vaccination schedule, ultimately improving effective vaccination coverage and its impact on childhood survival. In this study, using data from the Demographic Health Surveys (DHS) conducted in 33 SSA countries, we sought to (1) estimate the prevalence of delayed vaccination at specific vaccination encounters in the schedule and to (2) explore the association between delays in dose-specific vaccination and the completion of the basic immunization schedule.
METHODS
Data sources and study population
Established in 1984, the DHS program collects nationally representative data on health and population demographics using standardized survey design approaches across participating countries.21 This widely used cross-sectional data source has been described in-depth elsewhere.22 For our study, we identified all publicly accessible DHS surveys conducted in SSA between 2010 and 2019, totaling 47 surveys from 33 countries (available as of 06/2020 at https://www.dhsprogram.com/data/available-datasets.cfm). We restricted our sample to one survey per country, including the most recent survey from each country in the analysis (Appendix Table 1).
DHS uses a multi-stage, unequal probability sampling scheme to identify a nationally representative sample of households.22 At the first stage, household clusters are selected based on probability proportional to the population area size from each rural or urban strata, defined by the host country. Then, after creating a complete listing of households within the cluster, approximately 30 households are randomly sampled from each. All women aged 15–49 who reside in the selected households are invited to participate in the survey.23 Data compiled from each survey is used to facilitate tracking of national, regional and global health indicators.22
Vaccination data are collected for living children who were born in the three to five years prior to the interview year.24 We used data collected from children aged 12–35 months at the time of interview, as this age group consistently participated in the vaccination module across the countries selected for inclusion. Due to the potential of correlated vaccination patterns among siblings, we restricted our sample to the youngest child in instances where multiple children from the same family were age-eligible (excluding 3.2% of the age-eligible sample).
Mothers are asked to report on their children’s status of vaccination receipt for each recommended vaccine in the national immunization schedule. To verify, interviewers review family health cards or children’s immunization records, when available, to confirm receipt and date of administration for all vaccines.25 Dates recorded on the vaccination card were used to assess timeliness and series completion. Children who did not have a card available at the time of interview or who had a card without record of complete or plausible vaccination dates were excluded from analysis.
Derived variables
The primary outcome of interest was completion of the recommended immunization schedule in the first year of life. All analyses used complete vaccination series status as the reference level. Many countries offer newer vaccines (e.g. rotavirus) and reinforcement doses for some antigens but given the variation across countries, we limited our evaluation to the vaccines recommended in the basic schedule and their respective timing intervals as defined by WHO and adopted by national immunization programs (Table 1). Incomplete vaccination schedules were defined as lacking any dose in the eight dose series, which includes BCG at birth, three doses each of Penta and Polio at 6, 10 and 14 weeks, respectively, and one dose of measles at 9 months.
Table 1.
Age-specific recommendations for the basic immunization schedule endorsed by WHO*
| Age at administration | Vaccines | Minimum acceptable age (days) | Delays initiated (age in days) |
|---|---|---|---|
| Birth | BCG, OPV0 | 0 days | Greater or equal to 28 days |
| 6 [8] weeks | Penta1, OPV1 | 42 [56] days | Greater than 70 [84] days |
| 10 [12/16] weeks | Penta2, OPV2 | Age in days at previous dose + 28 | Greater than 98 [112/140] days |
| 14 [16/24] weeks | Penta3, OPV3 | Age in days at previous dose + 28 | Greater than 126 [140/196] days |
| 9 months | Measles | 252 days | Greater than 280 days |
Four countries in the sample use the schedules denoted in brackets
We evaluated dose-specific vaccination timeliness by creating a three-way categorization that reflected adherence or non-adherence to the age-specific recommendations for each dose.9 Doses administered were defined as ‘on-time’, ‘delayed, as a first instance’ of delayed vaccination in the schedule, or ‘delayed, with prior instances’ of delay at prior vaccination encounters. Any dose that was recorded as having been administered 4 or more weeks after the recommended age was considered delayed. Age (in days) at vaccination was used as the cut-off for on-time versus delayed vaccination, and history of delayed vaccination at any prior dose was used to assign children to ‘delayed, with prior instances’.
We derived age in days at vaccination by subtracting the child’s birthdate from the vaccination date recorded on a child’s immunization card. Where month and/or year of birth were missing, we cross-referenced other available dates collected in the surveys to define plausibility bounds. For cases in which the day of birth was missing but the date of BCG vaccination complete (n = 14,243), age at vaccination was imputed drawing from the distribution of known values for age at BCG vaccination and then birthdate was back-calculated from the imputed age in days and date at BCG vaccination. Appendix Table 2 summarizes overall missingness for values of variables included in analysis and provides further information on the day of birth imputation procedure.
Known predictors of vaccination timeliness and completion were also explored and used as covariates in analysis. We defined birth setting as: institutional delivery in public sector setting; institutional delivery in private sector setting; non-institutional delivery with presence of skilled healthcare attendant; non-institutional delivery with traditional birth attendant; non-institutional delivery with no assistance. We assessed each child’s rank in the birth order, adjusting for multiples. We also explored missed opportunities for vaccination when recommended co-administration of Polio and Pentavalent did not occur. Maternal educational attainment, parental marital status, household wealth and residence location were assessed using the categorical definitions defined by DHS.26
Statistical analysis
We compared delayed vaccination across levels of child characteristics, adjusting the proportion of delayed administration of BCG, Penta1–3 and measles for survey design, and tested the significance of differences with chi-square tests of independence. Using multinomial logistic regression, we further explored predictors for categories of delayed vaccination: (1) delayed, first instance versus on-time and (2) delayed, prior instance versus on-time. Then, we assessed the association between dose-specific delays and schedule completion, separately evaluating late or delayed receipt of BCG, Penta1, Penta2, Penta3 and measles in a set of logistic regression models that included children conditional on having received the vaccine. Odds Ratios (ORs), average marginal effects (AMEs) and predicted probabilities of the outcome were estimated for first instance of delayed vaccination and repeated delays in vaccination. AMEs and predicted probabilities of the outcome allow for making more appropriate comparisons across models due to our inability to assume that unobserved heterogeneity is the same across model samples conditional on having received a vaccine, e.g. children who receive BCG differ from children who receive doses later in the schedule. Covariates that were identified as significantly associated with vaccination delays were retained for controls in the adjusted models exploring associations between dose-specific delays and schedule completion. Necessitating a control for time and place in the multi-country pooled models, we included indicator dummy variables for each country and continuous variables for year of interview and child’s age at interview. Excluding the observations that underwent imputation for date of birth, we repeated our outcome models as a sensitivity analysis. We also explored country stratified models to evaluate the heterogeneity in association measures across countries in our pooled sample. All analyses used country-specific sampling weights and survey design strata variables to account for the complex sample design. Unweighted case frequencies and weighted proportions are reported. All analyses were conducted in Stata 16.1 (StataCorp LLC, College Station, TX).
RESULTS
A total of 136,745 children aged 12–35 months were surveyed in the most recent DHS waves during the period between 2010 and 2019 across the 33 countries included. After selecting the youngest child from households with multiple-age eligible children, the availability of vaccination records in 132,405 children was assessed. Across country surveys, the median proportion of age-eligible children who had a vaccination card available at the time of interview was 58% (IQR 46–63%). In total, 61,399 age-eligible children were excluded from analysis, owing either to having no vaccination card available (n=53,659) or implausible and/or missing vaccination dates recorded throughout their records (n=8,740). While characteristics of children stratified on the restriction criteria did not differ substantially between groups, our analytic sample (n=70,006) represented children who had considerably higher rates of vaccination schedule completion overall at the time of interview than children excluded from analysis. All characteristics of children included and excluded from our sample are described in Table 2.
Table 2.
Characteristics of children 12–35 months according to data availability for assessing vaccination status. Only children with complete dates included in analytic sample.
| Card verification (n=78,746) | Maternal recall (n=53,659) | Overall (n=132,405) | ||
|---|---|---|---|---|
| Complete dates | Incomplete dates | |||
| Child’s age | 70,006 | 8,740 | 53,659 | 132,405 |
| 12–23 months | 50% | 50% | 50% | 50% |
| 24–35 months | 50% | 50% | 50% | 50% |
| Child’s sex | 70,006 | 8,740 | 53,659 | 132,405 |
| Male | 57% | 55% | 45% | 52% |
| Female | 43% | 45% | 55% | 48% |
| Birth order | 70,006 | 8,740 | 53,659 | 132,405 |
| 1st | 23% | 22% | 22% | 23% |
| 75% 2nd to 3rd | 37% | 36% | 34% | 36% |
| 4th to 5th | 29% | 29% | 30% | 29% |
| 6th + | 11% | 13% | 14% | 12% |
| Birth setting | 69,180 | 8,616 | 53,049 | 130,845 |
| Institutional, public | 66% | 61% | 50% | 59% |
| Institutional, private | 9% | 8% | 8% | 9% |
| Home, skilled attendant | 2% | 3% | 3% | 2% |
| Home, traditional attendant | 21% | 25% | 34% | 26% |
| Home, no attendant | 3% | 3% | 5% | 4% |
| Vaccination status, according to card or recall | 70,006 | 8,740 | 53,659 | 132,405 |
| Fully vaccinated | 21% | 26% | 78% | 43% |
| Not fully vaccinated | 79% | 74% | 22% | 57% |
| Maternal age (at child’s birth) | 70,006 | 8,740 | 53,659 | 132,405 |
| Under 19 | 15% | 17% | 18% | 16% |
| 20–29 | 52% | 52% | 52% | 52% |
| 30–39 | 29% | 28% | 26% | 28% |
| 40–49 | 4% | 4% | 4% | 4% |
| Maternal education | 69,995 | 8,739 | 53,655 | 132,389 |
| None | 36% | 37% | 44% | 39% |
| Primary | 34% | 35% | 29% | 32% |
| Secondary | 27% | 25% | 24% | 25% |
| Higher | 3% | 3% | 3% | 3% |
| Household wealth quint. | 70,357 | 8,389 | 53,659 | 132,405 |
| Poorest | 20% | 22% | 25% | 22% |
| Poorer | 21% | 22% | 22% | 22% |
| Middle | 21% | 21% | 19% | 20% |
| Richer | 20% | 19% | 18% | 19% |
| Richest | 18% | 16% | 15% | 17% |
| Place of residence | 70,357 | 8,389 | 53,659 | 132,405 |
| Urban | 34% | 34% | 33% | 34% |
| Rural | 66% | 66% | 67% | 66% |
In terms of under-vaccination in the sample, the proportion of children missing recommended doses or receiving delayed doses increased with each subsequent visit across the vaccination milestone visits, using BCG, Penta1–3 and measles vaccination status as representative of the five vaccine administration encounters across the schedule since Penta1–3 are administered concomitantly with Polio1–3 (Figure 1). While <1% of children received no vaccines in their first year of life, the other 20% of children who did not complete their schedule by 12 months age had missed an important number of doses when considering the full 8 dose recommended series: 5% missing 4–7 doses; 6% missing 2–3 doses and 9% missing at least one dose (Not shown).
Figure 1.
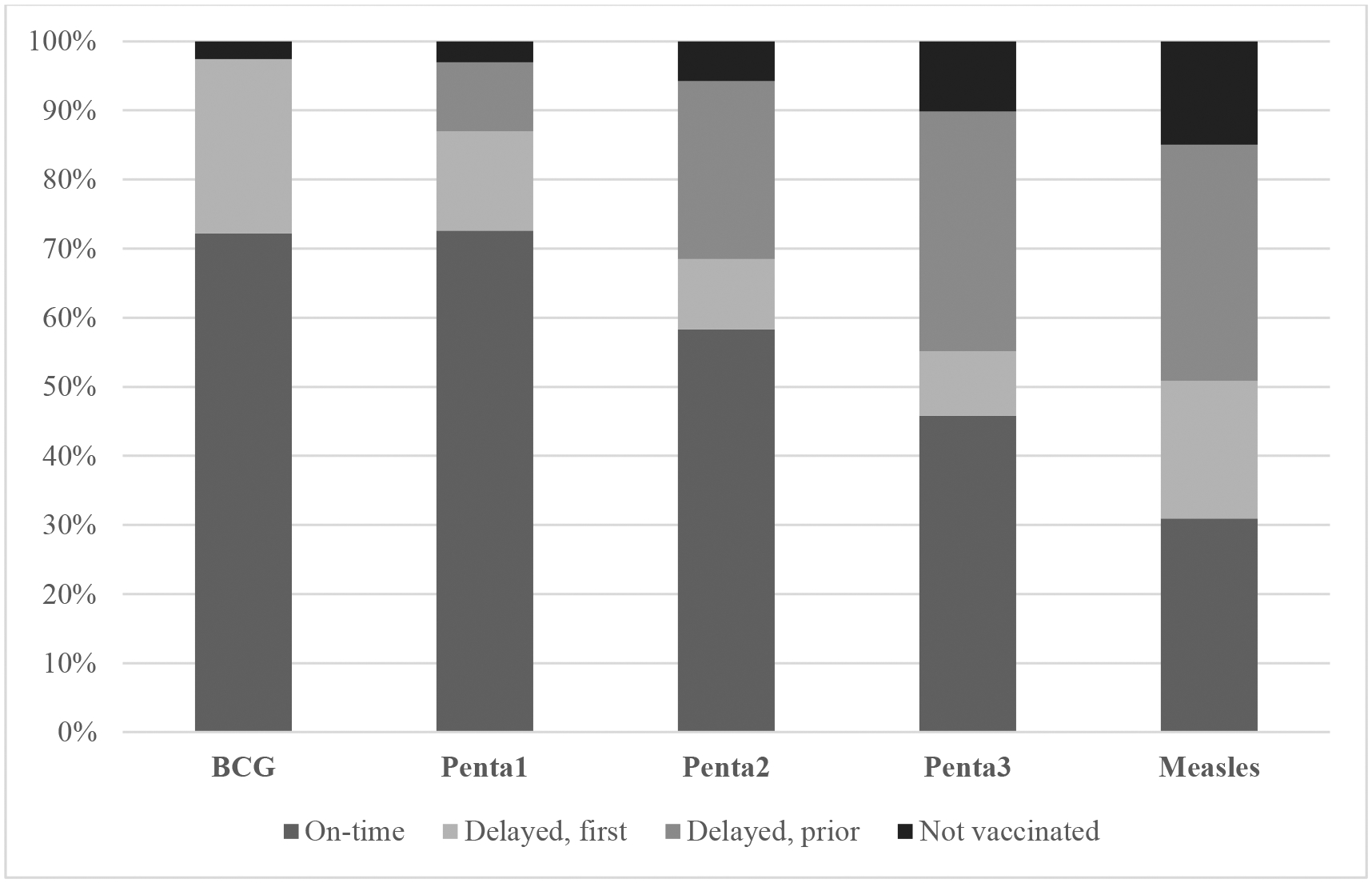
Percentage of children by vaccination status across the recommended series in the pooled analytic sample, weighted using country weights provided by DHS.
Among vaccinated children, late administration by 4 or more weeks was: 25.9% for BCG; 23.5%, 38.2%, 49.1% for the first, second and third doses of Penta, and 63.6% for measles (Table 3). The proportion of children receiving delayed vaccination repeatedly across the schedule was consistently highest for children who were of higher birth order (7th +) or born in non-institutional settings with no skilled assistance. In contrast, the proportion of delayed vaccination trended substantially lower for children born to mothers with higher levels of educational attainment and household wealth. For example, in the wealthiest households, only 35.3% of children were delayed for Penta3 vaccination compared to 58.7% in the poorest households. Similarly, there was more than a 30-point difference in the prevalence of delayed Penta3 vaccination between children of mothers who had high educational attainment (24.4%) versus no education (60.8%). For children who were vaccinated against measles, though late, the proportion affected by delays did not vary as substantially across childhood and maternal predictors as was observed for other vaccination visits. Nonetheless, except for parental marital status and child sex, all sociodemographic characteristics demonstrated some level of significant association with delayed vaccination, either as a first instance or following prior delays. (p < 0.05 [see Appendix Table 3]).
Table 3.
Proportiona of children 12–35 months with delayed vaccination across the immunization series stratified by descriptive characteristics (N=70,006b)
| BCG | Penta, 1st dose | Pent, 2nd dose | Pent, 3rd dose | Measles, 1st dose | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Birth) | (6 or 8 wk) | (10, 12 or 16 wk) | (14, 16 or 24 wk) | (9 mo.) | ||||||
| % | p e | % | p e | % | p e | % | p e | % | p e | |
| Overall c | 25.9% | 23.5% | 38.2% | 49.1% | 63.6% | |||||
| Child’s sex | 0.33 | 0.24 | 0.46 | 0.51 | 0.23 | |||||
| Male | 25.7% | 24.9% | 38.4% | 49.3% | 63.9% | |||||
| Female | 26.1% | 25.4% | 38.0% | 49.0% | 63.3% | |||||
| Child’s age (at interview) | 0.20 | 0.61 | 0.05 | 0.02 | 0.01 | |||||
| 12–23 months | 25.7% | 25.1% | 37.8% | 48.6% | 62.8% | |||||
| 24–35 months | 26.2% | 25.3% | 38.8% | 49.9% | 64.6% | |||||
| Birth order | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| 1st | 21.8% | 20.9% | 32.8% | 42.9% | 59.3% | |||||
| 2nd to 3rd | 23.2% | 22.7% | 35.3% | 46.4% | 63.5% | |||||
| 4th to 5th | 29.0% | 28.4% | 42.2% | 53.4% | 66.2% | |||||
| 6th + | 35.9% | 34.1% | 49.5% | 61.4% | 66.9% | |||||
| Birth setting | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Institutional delivery, public | 19.8% | 0.2109 | 33.9% | 45.3% | 62.2% | |||||
| Institutional delivery, private | 18.9% | 0.1782 | 29.3% | 39.0% | 64.2% | |||||
| Home delivery, skilled attend. | 35.7% | 0.3164 | 45.7% | 54.9% | 62.2% | |||||
| Home delivery, trad.l attend. | 45.7% | 0.3942 | 54.1% | 65.2% | 67.7% | |||||
| Home delivery, no attend. | 48.9% | 0.3948 | 54.4% | 64.7% | 70.2% | |||||
| Fully immunized | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Incomplete | 36.0% | 40.6% | 55.6% | 60.9% | 71.2% | |||||
| Complete | 23.5% | 21.6% | 38.4% | 47.5% | 63.0% | |||||
| Not coadministered with Polio1 | - | 41.1% | <0.001 | 52.2% | <0.001 | 58.1% | 66.9% | |||
| Not coadministered with Polio2 | - | - | 48.6% | <0.001 | 53.3% | 64.8% | ||||
| Not coadministered with Polio3 | - | - | - | 53.3% | 66.0% | |||||
| Mother’s age (at childbirth) | <0.001 | <0.001 | 1 | <0.001 | <0.001 | <0.001 | ||||
| Under 20 | 28.0% | 26.3% | 40.3% | 51.1% | 61.7% | |||||
| 20–29 | 25.2% | 24.6% | 36.9% | 47.9% | 63.3% | |||||
| 30–39 | 25.6% | 25.1% | 38.8% | 49.6% | 64.7% | |||||
| 40–44 | 29.1% | 28.4% | 43.1% | 54.0% | 66.8% | |||||
| Mother’s educational attainment | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| None | 33.3% | 33.3% | 48.8% | 60.8% | 64.1% | |||||
| Primary | 26.6% | 23.9% | 37.1% | 48.4% | 65.7% | |||||
| Secondary | 17.4% | 17.8% | 28.7% | 38.8% | 61.3% | |||||
| Higher | 10.1% | 11.4% | 17.9% | 24.4% | 57.6% | |||||
| Mother’s marital status | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Never married | 18.2% | 19.6% | 30.8% | 38.3% | 58.0% | |||||
| Formerly married | 25.1% | 25.3% | 39.8% | 50.5% | 65.6% | |||||
| Currently married | 26.6% | 25.6% | 38.7% | 50.0% | 64.0% | |||||
| Household wealth quintile | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Poorest | 34.3% | 31.9% | 46.8% | 58.7% | 66.9% | |||||
| Poorer | 31.5% | 29.2% | 43.3% | 54.4% | 64.9% | |||||
| Middle | 27.1% | 25.5% | 39.2% | 50.7% | 63.5% | |||||
| Richer | 21.3% | 21.8% | 34.9% | 45.8% | 62.4% | |||||
| Richest | 14.1% | 16.6% | 25.8% | 35.3% | 60.2% | |||||
| Place of residence | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Rural | 31.0% | 28.0% | 42.1% | 53.6% | 64.7% | |||||
| Urban | 16.3% | 19.7% | 30.9% | 40.7% | 61.5% | |||||
| Observations d | 67,335 | 66,849 | 65,036 | 62,271 | 57,501 | |||||
All proportions in the pooled sample account for each country’s survey design using sampling weights provided by DHS.
Number of children age-eligible for inclusion who had a vaccination card available at the time of interview with complete dates for administered doses of BCG, Penta3, Polio3 and Measles if received.
‘Overall’ reflects the proportion of children vaccinated late, according to cutoffs defined in Table 1, regardless of being the first instance of delay or having a prior history of delayed vaccination, among children who were vaccinated.
Differences in the number of observations between vaccination doses reflect dropout due to not receiving a dose or listwise deletion due to missing values for covariates/predictors. For BCG, 1,863 children did not receive the dose and another 808 children were excluded due to missing values for birth setting and/or maternal education; For Pentavalent, 1,982 (dose 1), 3,900 (dose 2) and 6,977 (dose 3) children did not receive doses in the vaccination series, and another 811, 796 and 758 children were excluded from the respective analytic samples due to incomplete vaccination dates, or missing values for birth setting or maternal education. For measles, 10,593 children did not receive the vaccine and another 729 children were excluded due to missing values for birth setting and/or maternal education.
p-values are calculated from chi-square test for independence between levels of categorical characteristics
Adjusted logistic regression models showed that children with delayed vaccination were at increased odds of not finishing their schedules by 12 months of age compared to children who received on-time vaccination (Table 4). The magnitude of this association was highest for children who received delayed vaccination against measles as the first occurrence of delay in the schedule (adjusted odds ratio [aOR]: 3.76; 95% CI 3.37 – 4.15) or following a pattern of delayed vaccination across the schedule (aOR: 8.21; 95% CI 7.50 – 8.91) compared to on-time vaccination in children. However, children who were both delayed in receiving measles and did not complete their schedules by 12 months of age often did finish their schedules at an older age. The median age of measles vaccination for these children was 13.25 months, 4.25 months after the recommended age.
Table 4.
Association between dose-specific delayed vaccination and not completing the basic immunization schedule by 12 months of age in children 12–35 months across 33 countries in sub-Saharan Africa. Logistic regressiona results presented as Odds Ratios (ORb) and Average Marginal Effects (AMEc).
| BCGd | Penta1 | Penta2 | Penta3 | Measles | BCGd | Penta1 | Penta2 | Penta3 | Measles | |
|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio (OR) | Average Marginal Effect (AME) | |||||||||
| (95%CI) | (95%CI) | |||||||||
| Delayed, first instance | ||||||||||
| (ref = on-time) | 1.93*** | 1.99*** | 1.88*** | 1.50*** | 3.76*** | 0.129*** | 0.131*** | 0.106*** | 0.056*** | 0.106*** |
| (1.83–2.02) | (1.83–2.14) | (1.74–2.02) | (1.36–1.63) | (3.37–4.15) | (0.11–0.15) | (0.11–0.15) | (0.08–0.12) | (0.04–0.07 | (0.09–0.12) | |
| Delayed, prior instance | ||||||||||
| (ref = on-time) | - | 2.91*** | 2.79*** | 2.46*** | 8.21*** | 0.212*** | 0.186*** | 0.143*** | 0.215*** | |
| (2.71–3.12) | (2.63–2.94) | (2.32–2.60) | (7.50–8.91) | (0.19–0.23) | (0.17–0.21) | (0.12–0.16) | (0.20–0.23) | |||
| Observations | 67,335 | 66,849 | 65,036 | 62,271 | 58,684 | 67,408 | 66,849 | 65,036 | 62,271 | 57,501 |
p<0.05
p<0.01
p<0.001
For consistency across models for BCG, Penta1, Penta2, Penta3 and Measles, all models adjust for: continuous child age, birth order and setting; mother’s age at childbirth and educational attainment by time of interview; and household wealth quintile and location (rural/urban); survey year and country. Models for Penta and Measles include controls for missed opportunities of vaccination associated with recommended concomitant vaccination of Penta and Polio at 6, 10 and 14 weeks. Not shown.
Odds of not completing the basic immunization schedule by 12 months of age (i.e. receiving BCG, Penta1–3, Polio1–3 and Measles1) are compared between children who receive delayed vaccination and children who are vaccinated on-time. The three-level delay category captures two types of delay: first instance of delayed receipt in the schedule and delayed at a given instance after having experienced delays at prior vaccination instances.
AME shows the average change in probability of the outcome when making a discrete level change in the categorical predictor defining delayed vaccination versus on-time vaccination, i.e. how much higher (or lower) the expected mean probability of not completing the vaccination series is in the study population when a child is delayed (either first instance or with prior delays) in receiving a specific vaccine dose versus receiving the dose on time holding all other variables at their observed values.
The only type of delay recognized for BCG is first instance because it is the first dose (administered at birth) in the series.
Delays as a first instance at the initial dose of Penta (recommended at 6 weeks) were associated with a significantly higher probability of not following through on the complete schedule (13.1%), even slightly more so than delayed initiation of the BCG at birth (Table 4). Additionally, reported patterns of repeated delays across the childhood schedule predicted even higher probabilities of drop-off from the recommended series compared to children who were receiving on-time vaccination (Table 4). Figure 2 graphically shows that both ‘first instance’ delays and ‘with prior’ delays at the first dose of Pentavalent significantly predicted incompletion rates which was sustained for delays at Penta2, Penta3 and measles, though with predictions of the probability of incompletion declining with each subsequent dose. Excluding children who had had their dates of birth imputed, the findings from the outcome models were consistent with the full analytic sample models [not shown]. In the country stratified models, we found that there was variation across countries in the magnitude of association between dose-specific delays and not finishing the basic childhood vaccination schedule. However, delays compared to on-time doses consistently increased the likelihood of not completing the schedule.
Figure 2.
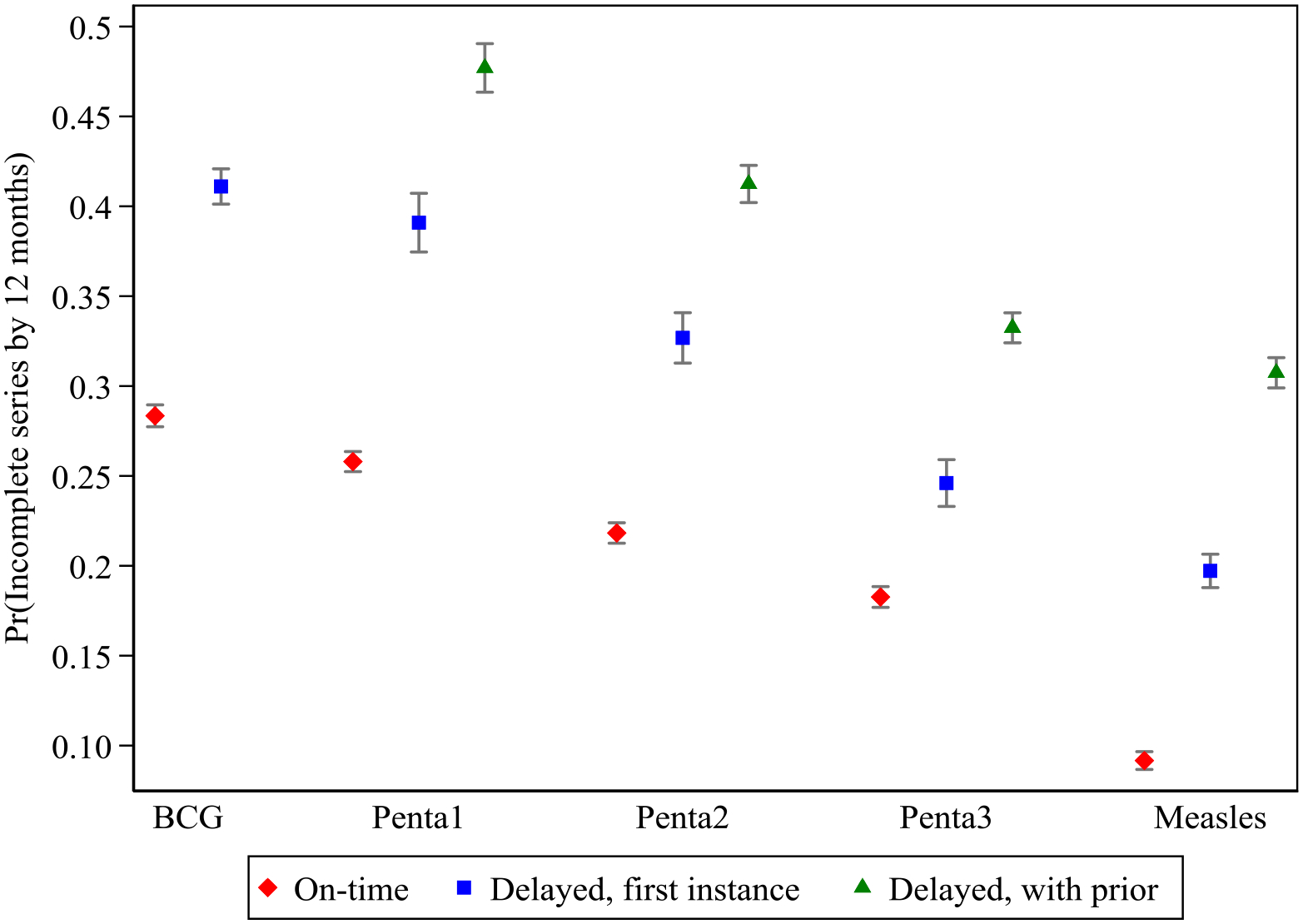
Predicted probability of not being fully vaccinated by 12 months of age for categories of vaccination timeliness at each dose: on-time, delayed (first instance) or delayed (with prior instances).
DISCUSSION
Assessment of vaccination timeliness is essential to identifying age-specific risks to vaccine-preventable diseases, which continue to contribute to under-five mortality in sub-Saharan Africa.27,28 Furthermore, defining the role that late or delayed vaccination plays in hindering the completion of the recommended schedule in the first year of life is useful for evidencing the need for programmatic interventions that target timely vaccination as a means to improving protective coverage overall. While uptake of specific vaccine doses has improved, i.e. Penta3 increased over the past decade from 77% to 81% in Eastern and Southern Africa and 65% to 70% in West and Central Africa, aggregate measures of coverage are an imprecise predictor of the population risk profile for vaccine-preventable diseases as they do not account for the timing of vaccination and the resulting age-specific protection, or lack thereof when delays lead to additional delays or dropout.29 In this study, we explored the association between children having dose-specific delays and completing their immunization schedules before 12 months of age. Using nationally representative data from surveys conducted between 2010 and 2019 across 33 sub-Saharan African nations, our findings suggest that dose-specific delays are common and that those delays lead to a significantly higher probability of dropping off the schedule, resulting in prolonged susceptibility to specific VPDs beyond the first year of a child’s life.
To the authors’ knowledge, previous studies on the determinants of under-vaccination in SSA have not considered the role of adherence to age-specific vaccination recommendations, besides on-time vaccination at birth. Studies in both low-income and higher-income settings have found that the risk of programmatic dropout associated with delayed initiation of vaccination at birth is significant.16,17,30 In our study, delays in vaccination with any dose was significantly associated with increased likelihood of non-completion of the immunization schedule. Across immunization programs in SSA, education and outreach designed to improve community demand for on-time vaccination services could lessen the programmatic burden of follow-up when children fall behind in their schedules and reduce the risk of under-vaccination. However, vaccine stock-outs and other service disruptions are often unavoidable barriers to access. In these scenarios, outreach and catch-up campaigns remain important for bringing children up-to-date on their vaccination.
It is worth clarifying that some delays may result from intentional adjustments to the schedule for individual children following delayed initiation of a multi-dose series. This is because a 4-week interval is recommended between doses to avoid blunting the immune response.8 Nonetheless, in our assessment, delays were predictive of subsequent delays that extended beyond the necessary interval between doses and even predictive of drop-out, both of which can contribute to under-vaccination after the first year of life. For example, only 12% of all Penta2 doses and 14% of all Penta3 doses considered delayed were administered late due to following the appropriate time interval recommended between delayed receipt of the previous dose and the subsequent dose in the series. All other delays for these doses fell outside the recommended adjusted interval.
Consistent with immunization research in SSA14,15,31–33 we also found that delayed vaccination was most prevalent among families with socioeconomic and educational disadvantages. Although, notably, the proportion of children who received late measles vaccination as a first instance of delay did not differ as substantially across wealth and maternal education as compared to the variation in delayed vaccination observed across socioeconomic strata for earlier doses in the schedule. Instead, there was consistently high levels of delay for receipt of measles, particularly among children who did not complete the schedule by 12 months, i.e. missed a previous dose or were delayed in their measles vaccination well past the infant period. This finding is notable because immunization programs have long measured their success by the population coverage achieved with the third dose of Penta instead of measles, which is the last dose in the basic schedule. As a result, immunization program performance may appear to be improving, yet when delayed Penta doses result in delaying the single measles dose recommended before 12 months of age, the threat of a measles resurgence becomes an important concern and one that has come to recent fruition in a number of SSA countries.34.
Considering existing challenges to reducing under-vaccination in the context of the destabilizing threat that pandemic spread of SARS-CoV-2 poses for weak public health systems, immunization programs, with the support of their national governments, must consider how to prioritize timely vaccination throughout the course of the schedule to ensure age-specific protection and to increase the likelihood of completing all recommended vaccines. While standard outreach activities may not be feasible, continued emphasis on education for mothers and providers about the contingency plans for completing their infants’ immunization schedules, either through campaigns or health facility visits, will be needed. Where substantial concern for interrupted immunization activity may exist35, immunization programs could also consider vaccinating against measles at younger infant ages in settings that warrant such an approach.8
Despite contributing a new perspective on vaccination timeliness and completion in SSA, our approach and data sources used to study this association has some limitations. Like most vaccination research using survey data, we excluded children who lacked complete vaccination histories, either living children who did not have complete vaccination records available or children who had died prior to the interview. Both sub-populations likely differ substantially in their overall health, risk factors and access to immunization from children with complete records, which limits the generalizability of our findings. Assuming that delayed vaccination is correlated with access to services and availability of a vaccination card is an indicator of access, we might also assume that delayed vaccination and drop-out may even more frequently occur in children who do not have records. This would lead to under-estimating the prevalence of delays and their contribution to overall completion rates. On the other hand, in the absence of electronic immunization registries, our study may have incorrectly classified vaccination outcomes if dates were not correct or doses administered doses were not documented. Though, data quality measures are embedded in the DHS program to change implausible dates to missing and survey data is generally considered the gold-standard for assessing immunization uptake36,37. Although the surveys are cross-sectional, the availability of vaccination dates for our sample allowed us to establish the sequential timing of vaccine administration across the schedule and temporally associate delays, classified as a first-time delay or prior delays, with vaccination schedule completion as the ultimate outcome in the timing sequence. Finally, programming constraints and barriers to access predictive of under-vaccination undoubtedly vary across countries in SSA. While we explored the heterogeneity in the magnitude and direction of our main effects across countries, identifying and adjusting for country-specific observed and unobserved confounding was outside the scope of our aim to generally establish delays as predictive of overall vaccination status in SSA. Future studies on the country-specific nuances of each program could contribute more precise recommendations on how to intervene in cases where clear patterns of bottlenecks in schedule completion arise due to dose-specific delays.
CONCLUSION
Our study identified delayed vaccination at birth and delays in subsequent doses as important impediments to completing the routine schedule in SSA. While children in sub-Saharan Africa who have contact with the immunization program likely have higher probability of survival associated with general health services access, the benefit of on-time and full immunization of individuals extends beyond the individuals themselves. Targeting on-time delivery of vaccines across the immunization schedule among individuals and communities may contribute to achieving greater levels of protection at the population-level.
Appendix Table 1.
Countries and sample sizes covered in Demographic Health Surveys (DHS) in the region of sub-Saharan Africa from 2010–2019.
| Country | Year | DHS Wave | Children 12–35m in sample | Observations used in analysis | % sample used in analysis |
|---|---|---|---|---|---|
| Angola | 2015–16 | 7 | 5524 | 1798 | 33% |
| Burkina Faso | 2010 | 6 | 5467 | 4076 | 75% |
| Benin | 2017–18 | 7 | 4865 | 3066 | 63% |
| Burundi | 2016–17 | 7 | 4980 | 3128 | 63% |
| Congo Dem. Republic | 2013–14 | 6 | 6858 | 729 | 11% |
| Congo | 2011–12 | 6 | 3569 | 1300 | 36% |
| Cote D’Ivoire | 2011–12 | 6 | 2841 | 1547 | 54% |
| Cameroon | 2011 | 6 | 4361 | 1995 | 46% |
| Ethiopia | 2016 | 7 | 3855 | 1647 | 43% |
| Gabon | 2012 | 6 | 2344 | 1250 | 53% |
| Ghana | 2014 | 6 | 2262 | 1770 | 78% |
| Gambia | 2013 | 6 | 3133 | 2474 | 79% |
| Guinea | 2018 | 7 | 2677 | 1307 | 49% |
| Kenya | 2014 | 6 | 8068 | 5068 | 63% |
| Comoros | 2012 | 6 | 1210 | 640 | 53% |
| Liberia | 2013 | 6 | 2709 | 1107 | 41% |
| Lesotho | 2014 | 6 | 1228 | 857 | 70% |
| Mali | 2018 | 7 | 3675 | 1396 | 38% |
| Malawi | 2015–16 | 7 | 6500 | 4053 | 62% |
| Mozambique | 2011 | 6 | 4233 | 2894 | 68% |
| Nigeria | 2018 | 7 | 11893 | 3515 | 30% |
| Niger | 2012 | 6 | 4525 | 2178 | 48% |
| Namibia | 2013 | 6 | 1973 | 1077 | 55% |
| Rwanda | 2014–15 | 6 | 3070 | 2437 | 79% |
| Sierra Leon | 2013 | 6 | 4096 | 2573 | 63% |
| Senegal | 2017 | 7 | 4616 | 2833 | 61% |
| Chad | 2014–15 | 6 | 6200 | 897 | 14% |
| Togo | 2013–14 | 6 | 2678 | 1579 | 59% |
| Tanzania | 2015–16 | 7 | 4034 | 2731 | 68% |
| Uganda | 2016 | 7 | 5838 | 3392 | 58% |
| South Africa | 2016 | 7 | 1346 | 632 | 47% |
| Zambia | 2018–19 | 7 | 3811 | 2397 | 63% |
| Zimbabwe | 2015 | 7 | 2307 | 1663 | 72% |
| Median | 3855 | 1798 | 58% | ||
| First Quartile | 1210 | 1300 | 46% | ||
| Third Quartile | 4980 | 2833 | 63% | ||
| Total | 136746 | 70006 | 51% |
Appendix Table 2.
Summary of missing values*** for variables used as controls in analysis or used to derive outcome and predictor variables in the age-eligible and vaccination card-holding sample (n=72,263).
| Analytic variables | Missing, freq. | Missing, % | Influential country survey samples* |
|---|---|---|---|
| Birthdate | 965 | 1.23% | - |
| Day | 965 | 1.23% | - |
| Month | - | 0.00% | - |
| Year | - | 0.00% | - |
| BCG date** | 3,397 | 4.31% | DRC, Malawi, Mozambique, Nigeria |
| Penta1 date** | 2,867 | 3.64% | Malawi, Nigeria |
| Penta2 date** | 2,945 | 3.74% | Malawi, Nigeria |
| Penta3 date** | 3,158 | 4.01% | Malawi, Nigeria |
| Polio1 date** | 3,285 | 4.17% | Malawi, Nigeria |
| Polio2 date** | 3,421 | 4.34% | Malawi, Nigeria |
| Polio3 date** | 3,787 | 4.81% | DRC, Malawi, Nigeria |
| Measles1 date** | 5,084 | 6.46% | DRC, Kenya, Malawi, Nigeria, Rwanda |
| Age (in days) at BCG receipt | 3,404 | 4.32% | DRC, Malawi, Mozambique, Nigeria |
| Age (in days) at Penta1 receipt | 2,894 | 3.68% | Malawi, Nigeria |
| Age (in days) at Penta2 receipt | 2,953 | 3.75% | Malawi, Nigeria |
| Age (in days) at Penta3 receipt | 3,163 | 4.02% | Malawi, Nigeria |
| Age (in days) at Polio1 receipt | 3,322 | 4.22% | Malawi, Nigeria |
| Age (in days) at Polio2 receipt | 3,432 | 4.36% | Malawi, Nigeria |
| Age (in days) at Polio3 receipt | 3,797 | 4.82% | DRC, Malawi, Nigeria |
| Age (in days) at Measles1 receipt | 5,164 | 6.56% | DRC, Kenya, Malawi, Nigeria, Rwanda |
| Fully immunized | - | 0.00% | - |
| Fully immunized by 12 months of age | 5,988 | 7.60% | Burundi, DRC, Kenya, Malawi, Mozambique, Nigeria, Rwanda, Tanzania, Zambia |
| Child’s age | - | 0.00% | - |
| Child’s sex | - | 0.00% | - |
| Child’s birth setting | 950 | 1.21% | - |
| Child’s birth order | - | 0.00% | - |
| Mother’s age | - | 0.00% | - |
| Mother’s education | 12 | 0.02% | - |
| Mother’s marital status | - | 0.00% | - |
| Household wealth | - | 0.00% | - |
| Year of survey | - | 0.00% | - |
| Country of survey | - | 0.00% | - |
| Survey weight | - | 0.00% | - |
Countries that contribute >250 missing values to variables with missingness >1%
Missing values for vaccination date among children who have a card available for review at interview will vary across the schedule if there are both missing and non-missing dates within the same child
This table summarizes the frequency of missing values after imputing day of birth for children sampled during DHS wave 6 data collection, which did not collect day of birth for 100% of children sampled. Day of month at birth was imputed for 14,243 children who lacked reporting for this variable. Observations with non-missing date of BCG administration that had occurred within the 6-month interval following month and year of birth were subject to the hotdeck imputation, where age (in days) at BCG administration was imputed using the distribution of known values for age at BCG administration among observations with non-missing values, matching on the month and year of BCG vaccination.
Appendix Table 3.
Multinomial logistic regression results of factors associated with first instance/prior delays compared to on-time vaccination by vaccine. Results reported as adjusted Odds Ratios (aOR) and robust standard errors in parentheses.
| BCG | Penta1 | Penta 2 | Penta3 | Measles | |||||
|---|---|---|---|---|---|---|---|---|---|
| Compared to on-time receipt | |||||||||
| First delay | First delay | Prior delays | First delay | Prior delays | First delay | Prior delays | First delay | Prior delays | |
| Child’s sex (ref=male) | |||||||||
| Female | 1.01 | 1.01 | 1.02 | 0.90* | 1 | 1.01 | 0.96 | 1 | 0.96 |
| (0.02) | (0.04) | (0.03) | (0.03) | (0.03) | (0.04) | (0.02) | (0.03) | (0.02) | |
| Child’s birth order (ref=1st) | |||||||||
| Second | 1.05 | 1.05 | 1.12* | 1.22*** | 1.06 | 1.08 | 1.14** | 1.17*** | 1.29*** |
| (0.04) | (0.06) | (0.06) | (0.07) | (0.04) | (0.06) | (0.04) | (0.05) | (0.05) | |
| Third | 1.20*** | 1.26*** | 1.34*** | 1.22** | 1.29*** | 1.17* | 1.29*** | 1.25*** | 1.51*** |
| (0.06) | (0.09) | (0.08) | (0.08) | (0.06) | (0.08) | (0.06) | (0.07) | (0.07) | |
| Fourth or higher order | 1.39*** | 1.48*** | 1.62*** | 1.28** | 1.62*** | 1.27** | 1.63*** | 1.12 | 1.64*** |
| (0.09) | (0.14) | (0.12) | (0.12) | (0.10) | (0.12) | (0.10) | (0.09) | (0.11) | |
| Birth setting (ref=Institutional, public) | |||||||||
| Institutional, private | 1.12* | 0.98 | 1.05 | 1.02 | 1.02 | 0.94 | 1 | 1.03 | 1 |
| (0.06) | (0.08) | (0.08) | (0.07) | (0.06) | (0.07) | (0.05) | (0.05) | (0.05) | |
| Non-institutional, skilled attendant | 1.73*** | 0.99 | 1.42*** | 0.94 | 1.36** | 0.9 | 1.18 | 0.88 | 1.18 |
| (0.15) | (0.13) | (0.14) | (0.12) | (0.14) | (0.14) | (0.13) | (0.12) | (0.12) | |
| Non-institutional, traditional attendant | 2.12*** | 1.07 | 1.98*** | 1.05 | 1.67*** | 1.02 | 1.60*** | 0.72*** | 1.33*** |
| (0.07) | (0.05) | (0.07) | (0.05) | (0.06) | (0.05) | (0.06) | (0.03) | (0.05) | |
| Non-institutional, no attendant | 2.14*** | 1.01 | 2.04*** | 1.08 | 1.78*** | 0.96 | 1.66*** | 0.83 | 1.49*** |
| (0.15) | (0.11) | (0.15) | (0.12) | (0.10) | (0.13) | (0.12) | (0.08) | (0.11) | |
| Mother’s age at childbirth (ref=15–19) | |||||||||
| 20–29 | 0.86*** | 0.97 | 0.83*** | 0.79*** | 0.88*** | 0.93 | 0.85*** | 1.01 | 0.88** |
| (0.04) | (0.06) | (0.04) | (0.05) | (0.04) | (0.06) | (0.04) | (0.05) | (0.04) | |
| 30–29 | 0.76*** | 0.81** | 0.72*** | 0.87 | 0.75*** | 0.88 | 0.76*** | 1.04 | 0.80*** |
| (0.04) | (0.07) | (0.05) | (0.06) | (0.04) | (0.07) | (0.04) | (0.07) | (0.05) | |
| 44–44 | 0.76*** | 0.83 | 0.66*** | 0.86 | 0.71*** | 0.86 | 0.70*** | 1.1 | 0.82* |
| (0.06) | (0.10) | (0.06) | (0.10) | (0.06) | (0.10) | (0.06) | (0.11) | (0.07) | |
| Mother’s educational attainment (ref=none) | |||||||||
| Primary | 0.92** | 0.85*** | 0.84*** | 0.86** | 0.82** | 0.90* | 0.81*** | 1.13** | 0.97 |
| (0.03) | (0.04) | (0.03) | (0.04) | (0.03) | (0.05) | (0.03) | (0.05) | (0.03) | |
| Secondary | 0.79*** | 0.68*** | 0.67*** | 0.65*** | 0.64*** | 0.78*** | 0.61*** | 1.27*** | 0.87** |
| (0.03) | (0.05) | (0.04) | (0.04) | (0.03) | (0.05) | (0.03) | (0.06) | (0.04) | |
| Higher | 0.56*** | 0.58*** | 0.48*** | 0.47*** | 0.47*** | 0.61** | 0.40*** | 1.24* | 0.74*** |
| (0.07) | (0.09) | (0.09) | (0.08) | (0.05) | (0.09) | (0.04) | (0.11) | (0.07) | |
| Mother’s marital status (ref=never married) | |||||||||
| Married, currently | 1.00 | 0.95 | 0.99 | 1 | 0.98 | 1.28** | 1.07 | 0.96 | 1.01 |
| (0.06) | (0.08) | (0.08) | (0.08) | (0.06) | (0.11) | (0.06) | (0.06) | (0.06) | |
| Married, formerly | 1.01 | 1.1 | 1 | 1.13 | 1.09 | 1.23 | 1.19* | 0.91 | 1.09 |
| (0.08) | (0.12) | (0.10) | (0.12) | (0.09) | (0.13) | (0.09) | (0.08) | (0.09) | |
| Household wealth quintile (ref=poorest) | |||||||||
| Poorer wealth quintile | 0.91** | 0.87** | 0.88** | 0.94 | 0.85*** | 0.9 | 0.83*** | 0.98 | 0.91* |
| (0.03) | (0.05) | (0.04) | (0.05) | (0.03) | (0.05) | (0.03) | (0.04) | (0.03) | |
| Middle wealth quintile | 0.80*** | 0.80*** | 0.78*** | 0.91 | 0.76*** | 0.91 | 0.76*** | 1.02 | 0.87*** |
| (0.03) | (0.05) | (0.04) | (0.05) | (0.03) | (0.05) | (0.03) | (0.05) | (0.03) | |
| Richer wealth quintile | 0.69*** | 0.78*** | 0.69*** | 0.92 | 0.69*** | 0.88* | 0.70*** | 1.04 | 0.84*** |
| (0.03) | (0.05) | (0.04) | (0.06) | (0.03) | (0.05) | (0.03) | (0.05) | (0.04) | |
| Richest wealth quintile | 0.51*** | 0.80** | 0.54*** | 0.74*** | 0.56*** | 0.74*** | 0.55*** | 1.16* | 0.72*** |
| (0.03) | (0.07) | (0.04) | (0.06) | (0.03) | (0.06) | (0.03) | (0.07) | (0.04) | |
| Residence location (ref=urban) | |||||||||
| Rural | 1.45*** | 1.15** | 1.33*** | 1.06 | 1.35*** | 1.05 | 1.32*** | 0.86** | 1.18*** |
| (0.06) | (0.06) | (0.07) | (0.06) | (0.06) | (0.06) | (0.05) | (0.04) | (0.05) | |
| Year of interview | 0.90* | 0.84* | 0.88 | 1.08 | 0.89* | 0.94 | 0.96 | 0.98 | 0.92 |
| (0.05) | (0.06) | (0.06) | (0.07) | (0.05) | (0.06) | (0.05) | (0.05) | (0.05) | |
| Child’s age in months | 1.01*** | 1 | 1.01*** | 1.01** | 1.01*** | 1 | 1.01*** | 1 | 1.01*** |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | |
| Missed opportunity for vaccination (ref=Polio and Penta co-administered) | |||||||||
| At 6 weeks | - | 0.36*** | 0.55*** | 1.27* | 0.60*** | 1.38** | 0.74*** | 1.28*** | 0.97 |
| (0.02) | (0.03) | (0.12) | (0.04) | (0.13) | (0.05) | (0.10) | (0.06) | ||
| At 10 weeks | - | 0.54*** | 0.73*** | 1 | 0.84** | 1.30*** | 0.91 | ||
| (0.05) | (0.05) | (0.10) | (0.05) | (0.10) | (0.06) | ||||
| At 14 weeks | - | 0.73*** | 0.87** | 1.25*** | 0.74*** | ||||
| (0.06) | (0.05) | (0.09) | (0.04) | ||||||
| Observations | 67,335 | 66,849 | 65,036 | 62,271 | 58,684 | ||||
p<0.05
p<0.01
p<0.001
Footnotes
Disclosure of conflicts: Authors disclose no conflicts of interest. Co-authors Boulton and Wagner are guest editors to the special issue and therefore the authors request separate referee process for this submission.
Contributor Information
Cara Bess Janusz, School of Public Health, University of Michigan, Ann Arbor, Michigan.
Margaret Frye, University of Michigan, Ann Arbor, Michigan.
Martin K. Mutua, Statistician, Epidemiologist, Immunization, Data Measurements and Evaluation, African Population and Health Research Center, Nairobi, Kenya.
Abram L. Wagner, Global Public Health Faculty Associate, Lieberthal Rogel Center for Chinese Studies Center Associate, University of Michigan, Ann Arbor, MI.
Mousumi Banerjee, School of Public Health, Director of Biostatistics, Center for Healthcare Outcomes & Policy, University of Michigan, Ann Arbor, MI.
Matthew L. Boulton, School of Public Health, Professor of Internal Medicine, Infectious Disease Division, Michigan Medicine, Ann Arbor, Michigan.
REFERENCES
- 1.You D, Hug L, Ejdemyr S, et al. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: A systematic analysis by the un Inter-Agency Group for Child Mortality Estimation. Lancet. 2015;386(10010):2275–2286. doi: 10.1016/S0140-6736(15)00120-8 [DOI] [PubMed] [Google Scholar]
- 2.Victora CG, Harris Requejo J, Barros AJD, et al. Countdown to 2015: a decade of tracking progress for maternal, newborn, and child survival. Lancet. 2016;387:2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang AY, Riumallo-Herl C, Perales NA, et al. The equity impact vaccines may have on averting deaths and medical impoverishment in developing countries. Health Aff. 2018;37(2):316–324. doi: 10.1377/hlthaff.2017.0861 [DOI] [PubMed] [Google Scholar]
- 4.Chopra M, Bhutta Z, Blanc DC, et al. Addressing the persistent inequities in immunization coverage. Bull World Health Organ. 2020;98(2):146–148. doi: 10.2471/BLT.19.241620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ICF International. The DHS Program STATcompiler. MEASURE DHS. http://www.statcompiler.com. Published 2015. Accessed April 20, 2020. [Google Scholar]
- 6.Sartorius B, Cohen C, Chirwa T, Ntshoe G, Puren A, Hofman K. Identifying high-risk areas for sporadic measles outbreaks: lessons from South Africa. Bull World Health Organ. 2013;91(3):174–183. doi: 10.2471/BLT.12.110726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373(9674):1543–1549. doi: 10.1016/S0140-6736(09)60317-2 [DOI] [PubMed] [Google Scholar]
- 8.World Health Orgranization. Recommended Routine Immunizations for Children; 2019. http://www.who.int/immunization/documents/positionpapers/for.
- 9.World Health Organization. Immunisation Agenda 2030: A Global Strategy to Leave No One Behind; 2020. [DOI] [PubMed]
- 10.Akmatov MK, Kimani-Murage E, Pessler F, et al. Evaluation of invalid vaccine doses in 31 countries of the WHO African Region. Vaccine. 2015;33(7):892–901. doi: 10.1016/j.vaccine.2014.10.089 [DOI] [PubMed] [Google Scholar]
- 11.Miyahara R, Jasseh M, Gomez P, et al. Barriers to timely administration of birth dose vaccines in The Gambia, West Africa. Vaccine. 2016;34(29):3335–3341. doi: 10.1016/j.vaccine.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mbengue MAS, Mboup A, Ly ID, et al. Vaccination coverage and immunization timeliness among children aged 12–23 months in Senegal: a Kaplan-Meier and Cox regression analysis approach. Pan Afr Med J. 2017;27(Supp 3):8. doi: 10.11604/pamj.supp.2017.27.3.11534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashbaugh HR, Hoff NA, Doshi RH, et al. Predictors of measles vaccination coverage among children 6–59 months of age in the Democratic Republic of the Congo. Vaccine. 2018;36(4):587–593. doi: 10.1016/j.vaccine.2017.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters NB, Wagner AL, Carlson BF, Boulton ML. Vaccination timeliness and co-administration among Kenyan children. Vaccine. 2018;36(11):1353–1360. doi: 10.1016/j.vaccine.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 15.Boulton ML, Carlson BF, Wagner AL, Porth JM, Gebremeskel B, Abeje Y. Vaccination timeliness among newborns and infants in Ethiopia. PLoS One. 2019;14(2):1–13. doi: 10.1371/journal.pone.0212408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiely M, Boulianne N, Talbot D, et al. Impact of vaccine delays at the 2, 4, 6 and 12 month visits on incomplete vaccination status by 24 months of age in Quebec, Canada. BMC Public Health. 2018;18(1):1–15. doi: 10.1186/s12889-018-6235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yusuf H, Daniels D, Smith P, Coronado V, Rodewald L. Association Between Administration of of the Hepatitis B and 4 : 3 : 1 : 3 Vaccine Series. Jama. 2000;284(8):978–983. [DOI] [PubMed] [Google Scholar]
- 18.Guerra FA. Delays in Immunization Have Potentially Serious Health Consequences. Pediatr Drugs. 2007;9(3):143–148. [DOI] [PubMed] [Google Scholar]
- 19.Zaman SMA, Howie SRC, Ochoge M, et al. Impact of routine vaccination against Haemophilus influenzae type b in The Gambia: 20 years after its introduction. J Glob Health. 2020;10(1). doi: 10.7189/jogh.10.010416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cutts FT, Izurieta HS, Rhoda DA. Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys. PLoS Med. 2013;10(5). doi: 10.1371/journal.pmed.1001404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ICF International. The DHS Program: Who We Are. https://www.dhsprogram.com/Who-We-Are/About-Us.cfm. Published 2020. Accessed April 20, 2020.
- 22.Corsi DJ, Neuman M, Finlay JE, Subramanian SV. Demographic and health surveys: A profile. Int J Epidemiol. 2012;41(6):1602–1613. doi: 10.1093/ije/dys184 [DOI] [PubMed] [Google Scholar]
- 23.ICF International. Demographic and Health Survey Sampling and Household Listing Manual. Calverton, Maryland, USA; 2012. [Google Scholar]
- 24.Croft TN, Marshall AMJ, Allen CK. Guide to DHS Statistics, DHS-7. Rockville, Maryland, USA; 2018. http://www.measuredhs.com/pubs/pdf/DHSG1/Guide_to_DHS_Statistics_29Oct2012_DHSG1.pdf%5Cnhttp://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.431.8235&rep=rep1&type=pdf. [Google Scholar]
- 25.ICF International. Demographic and Health Surveys Methodology: Interviewer’s Manual. Calverton, Maryland, USA; 2012. https://www.dhsprogram.com/pubs/pdf/DHSM1/DHS6_Interviewer_Manual_19Oct2012_DHSM1.pdf. [Google Scholar]
- 26.International ICF. Standard Recode Manual for DHS 6. ICF International/MEASURE DHS; 2013. [Google Scholar]
- 27.McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Heal. 2019;7(1):e47–e57. doi: 10.1016/S2214-109X(18)30408-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troeger C, Blacker BF, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1211–1228. doi: 10.1016/S1473-3099(18)30362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. WHO Immunization, Vaccines and Biologicals Measles Surveillance Data. http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/#. Published 2019. Accessed August 6, 2019.
- 30.Emmanuel OW, Samuel AA, Helen KL. Determinants of childhood vaccination completion at a peri-urban hospital in Kenya, December 2013-January 2014: a case control study. Pan Afr Med J. 2015;20(277). doi: 10.11604/pamj.2015.20.277.5664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Odutola A, Afolabi MO, Ogundare EO, et al. Risk factors for delay in age-appropriate vaccinations among Gambian children. BMC Health Serv Res. 2015;15(1):346. doi: 10.1186/s12913-015-1015-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akmatov MK, Mikolajczyk RT. Timeliness of childhood vaccinations in 31 low and middle-income countries. J Epidemiol Community Health. 2012;66(7). doi: 10.1136/jech.2010.124651 [DOI] [PubMed] [Google Scholar]
- 33.Bangura JB, Xiao S, Qiu D, Ouyang F, Chen L. Barriers to childhood immunization in sub-Saharan Africa: A systematic review. BMC Public Health. 2020;20(1108). doi: 10.1186/s12889-020-09169-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scobie HM, Ilunga BK, Mulumba A, et al. Antecedent causes of a measles resurgence in the Democratic Republic of the Congo. Pan Afr Med J. 2015;21(30). doi: 10.11604/pamj.2015.21.30.6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson R COVID-19 disrupts vaccine delivery. Lancet Infect Dis. 2020;20(5):546. doi: 10.1016/S1473-3099(20)30304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danovaro-Holliday MC, Dansereau E, Rhoda DA, Brown DW, Cutts FT, Gacic-Dobo M. Collecting and using reliable vaccination coverage survey estimates: Summary and recommendations from the “Meeting to share lessons learnt from the roll-out of the updated WHO Vaccination Coverage Cluster Survey Reference Manual and to set an operational. Vaccine. 2018;36(34):5150–5159. doi: 10.1016/j.vaccine.2018.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutts FT, Claquin P, Danovaro-Holliday MC, Rhoda DA. Monitoring vaccination coverage: Defining the role of surveys. Vaccine. 2016;34(35):4103–4109. doi: 10.1016/j.vaccine.2016.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]


