Abstract
The working group, “Clinical Tissue Regeneration” of the German Society of Orthopedics and Traumatology (DGOU) issues this paper to update their guidelines.
Methods
Peer-reviewed literature was analyzed regarding different topics relevant to osteochondral lesions of the talus (OLTs) treatment. This process concluded with a statement for each topic reflecting the best scientific evidence available for a particular diagnostic or therapeutic concept, including the grade of recommendation. Besides the scientific evidence, all group members rated the statements to identify possible gaps between literature and current clinical practice.
Conclusion
In patients with minimal symptoms, OLT progression to ankle osteoarthritis is unlikely. Risk factors for progression are the depth of the lesion on MRI, subchondral cyst formation, and the extent of bone marrow edema. Conservative management is the adaptation of activities to the performance of the ankle joint. A follow-up imaging after 12 months helps not to miss any progression. It is impossible to estimate the probability of success of conservative management from initial symptoms and imaging. Cast immobilization is an option in OLTs in children, with a success rate of approximately 50%, although complete healing, estimated from imaging, is rare. In adults, improvement by conservative management ranges between 45% and 59%. Rest and restrictions for sports activities seem to be more successful than immobilization. Intra-articular injections of hyaluronic acid and platelet-rich plasma can improve pain and functional scores for more than 6 months. If 3 months of conservative management does not improve symptoms, surgery can be recommended.
Keywords: regulatory issues, general, articular cartilage, tissue, ankle, joint involved, other, non-surgical therapy, guidelines
Introduction
Osteochondral lesions of the talus (OLTs) affect the talar dome with varying involvement of the articular cartilage and subchondral bone. In 2017, the working group “Clinical Tissue Regeneration” of the German Society of Orthopedics and Traumatology (DGOU) published the first recommendation for treating OLTs. 1 Much further research has been done within the last 5 years. The rationale behind this update was to include recent results and the latest knowledge on the treatment algorithms and update the guidelines with the new literature. Due to a lack of evidence, in 2017, many of the recommendations were based on expert opinion. Meanwhile, more concepts are supported by an increasing number of scientific studies. Besides the continuous discussion within the working group, the development was also driven by several consensus meetings, including the “International Consensus Meeting on Cartilage Repair of the Ankle” which took place in Pittsburgh in 20172-12 and Dublin in 2019. 13
The working group on “Clinical Tissue Regeneration” of the DGOU issues the present paper. It represents the best evidence available for managing OLTs and updates the guidelines published in 2017. 1 This paper focuses on the etiology, classification, diagnostics, and conservative management of OLTs. Abbreviations are defined in Table 1 .
Table 1.
Abbreviations and Definitions.
| DGOU | German Society of Orthopedics and Traumatology |
| ESWT | Extracorporeal shockwave therapy |
| HA | Hyaluronic acid |
| ICRS | International Cartilage Regeneration and Joint Preservation Society |
| OA | Osteoarthritis |
| OLT | Osteochondral lesion of the talus |
| PRP | Platelet-rich plasma |
| SPECT | Single photon emission computed tomography |
| SPECT-CT | SPECT, in combination with a computed tomography scan |
| WBCT | Weightbearing computed tomography (syn. Weightbearing DVT—digital volume tomography) |
Method
The working group “Clinical Tissue Regeneration” of the DGOU brought together 60 orthopedic and trauma surgeons with a particular interest in treating articular cartilage lesions. According to their subspeciality, a subgroup of 29 focused on foot and ankle surgery. Under the leadership of the first author, literature was analyzed regarding different topics relevant to OLT treatment (PUBMED, Cochrane, Web of Science, Scopus, MEDLINE, University Library Munich). The following keywords were used in combination: ankle, talus, cartilage, damage, chondral, osteochondral, articular, injury, chondropathy, focal, defect, pain, classification, platelet-rich plasma (PRP), hyaluronic acid (HA), arthritis, degenerative, clinical examination, imaging, MRI, computed tomography (CT), weightbearing computed tomography (WBCT), single photon emission computed tomography in combination with a computed tomography scan (SPECT-CT), x-ray, progression, injection, immobilization, shock wave, extracorporeal shockwave therapy (ESWT), and magnetic field. Papers were collected for each topic, and the main conclusions were brought together. This process concluded with a statement for each topic reflecting the best scientific evidence available for a particular diagnostic or therapeutic concept. The level of evidence for the studies was analyzed, and a grade recommendation was given for each statement ( Tables 2 and 3 ).14 -16
Table 2.
Grades of Evidence.
| Therapeutic Study | Prognostic Study | Diagnostic Study | |
|---|---|---|---|
| Level I | • High-quality RCT with statistically significant difference or no statistically significant difference but narrow confidence intervals • Systematic review of Level I RCT (and study results were homogeneous) |
• High-quality prospective trial (all patients were enrolled at the same point in their disease with > 80% follow-up of enrolled patients) • Systematic review of Level I studies |
• Testing of previously developed diagnostic criteria in series of consecutive patients (with universally applied reference “gold” standard) • Systematic review of Level I studies |
| Level II | • Lesser quality RCT (<80% follow-up, no blinding, or improper randomization) • Prospective comparative study • Systematic review of Level II studies or Level I studies with inconsistent results |
• Retrospective study • Untreated controls from an RCT • Lesser quality prospective study (patients enrolled at different points in their disease or <80% follow-up) • Systematic review of Level II studies |
• Development of diagnostic criteria on basis of consecutive patients (with universally applied reference “gold” standard) • Systematic review of Level II studies |
| Level III | • Case-control study • Retrospective comparative study • Systematic review of Level III studies |
• Case-control study | • Study of nonconsecutive patients (without consistently applied reference “gold” standard) • Systematic review of Level III studies |
| Level IV | • Case series | • Case series | • Case-control study • Poor reference standard |
| Level V | • Expert opinion | • Expert opinion | • Expert opinion |
RCT = Randomized Controlled Trial.
Table 3.
Grades of Recommendation.
| A | Good evidence (Level I studies with consistent findings) for or against recommending intervention. |
| B | Fair evidence (Level II or III studies with consistent findings) for or against recommending intervention. |
| C | Conflicting or poor-quality evidence (Level IV or V studies) not allowing a recommendation for or against intervention. |
| I | There is insufficient evidence to make a recommendation. |
In the second step, the group members were asked to rate the different statements according to their clinical practice. The goal was to identify possible gaps between clinical experience and evidence in the literature. Blinded electronic surveys were distributed to all group members. The participants could agree or disagree with the statements, comment on the statements, and provide additional references. Based on the participants’ input, statements were revised if additional literature was provided and sent for a second vote. The process ended with a statement on the different topics, based on the best evidence available, together with a grade of recommendation based on the quality of the studies supporting each statement. In addition, agreement among the 29 experts was given, reflecting the current clinical practice and experience. 17
Etiology and Location
OLT summarizes the large variety of pathologies at the talus. As the abbreviation implies, the constant factors are bone and cartilage involvement. However, there is no single etiology of OLTs. In the active pediatric and young adult population, osteochondritis dissecans is the predominant presentation. It is considered an atraumatic, idiopathic phenomenon. Etiopathogenetic theories include local ischemia, aberrant endochondral ossification of the secondary subarticular physis, repetitive microtrauma, and genetic predisposition.18,19
In the adult population, OLTs can be caused by avascular necrosis, 20 systemic vascular diseases, trauma,21,22 chronic microtrauma, 23 endocrine or metabolic factors, 24 Vitamin D deficiency, 25 degenerative joint disease, 26 and malalignment. 27 There seems to be also a genetic predisposition, as some patients present nearly identical pathologies in both ankle joints.28 -30 Flick and Gould 23 reported a history of trauma in 98% of the lateral and 70% of the medial lesions.
van Dijk et al. 31 hypothesized the progression of a cartilage lesion to a subchondral cyst. Elias et al. 32 investigated data from 424 patients characterizing the location of the OLT and reported that 96.2% of all lesions affected either the medial (62.8%) or the lateral (33.4%) talar dome, with 53% of the lesions located at the central third of the medial talar dome.
There is only a minor focus on different etiologies in papers dealing with OLT treatment. If an underlying cause can be improved, it should be included in the treatment concept.
Statement: The causes of OLTs are diverse. The predominant location is the central third of the medial talar dome. Many of the causative factors cannot be changed. However, remediating any causative factors should be part of the treatment concept, if possible.
Grade of Recommendation: B
Diagnostics
Clinical Examination
Typically, the patients report deep pain in the ankle joint, usually increasing with activity. They may present with swelling and tenderness to palpation. The location of the pain is not necessarily related to the location of the OLT. Pain is typically felt in the ankle joint during or after weightbearing.12,33,34 Especially in unstable lesions, locking of the joint can also be a symptom. 33
The clinical examination in patients suspecting an osteochondral lesion of the talus should include hindfoot alignment, lower extremity alignment, the ankle’s range of motion, and ankle stability. These factors are essential for deciding the final treatment concept. 27 ,35 -37
Statement: The evaluation of hindfoot alignment, ankle stability, and range of motion should be part of the clinical assessment.
Grade of Recommendation: B
Imaging
Various diagnostic imaging modalities have been described in the literature. These include radiography (weightbearing, non-weightbearing, anteroposterior (AP), mortise, lateral, heel rise), CT, WBCT, MRI, CT arthrography, MRI arthrography, scintigraphy, ultrasound, SPECT, and SPECT-CT.
Weightbearing x-rays of the ankle (AP, mortise) help evaluate the leg’s and the hindfoot’s mechanical axis. Weightbearing x-rays are more critical for planning the treatment strategy rather than for detecting a cartilage lesion. X-ray has a low sensitivity to cartilage defects. Cartilage defects can only be suspected if they have already caused secondary bony pathologies like cysts, loose bodies, or osteochondral fragments. 38 Therefore, about 50% of OLTs cannot be identified on plain x-rays. 39
MRI is the best imaging modality to visualize cartilage defects and bone edema. MRI imaging has significantly improved since the first papers were published in the early 1990s. 40 The development of the last years was driven by the introduction of 3 Tesla scanners where even routine (2D) sequences with an in-plane resolution of less than 0.5 mm can be acquired. 41 In a study on cadaver specimens, the sensitivity of cartilage lesion images varied from 50% at 1.5-Tesla and 75% at 3-Tesla. 42
For the ankle examination, the patient is placed supine with the ankle in a neutral position. High-resolution imaging is ideally achieved using dedicated multichannel coils. A small image field of 12 to 16 cm and slice thicknesses of maximum 3 mm in 3 spatial directions is essential. In particular, proton density (PD) or intermediate-weighted, fat-suppressed sequences are used in the sagittal, axial, and coronal slice planes. 43 These sequences are supplemented by a coronal T1-weighted sequence and a sagittal planned 3-dimensional (3-D) sequence. A routine intravenous contrast agent is not needed to assess post-traumatic cartilage damage. 41
Current 3-D sequences promise a further gain in spatial resolution and secondary reconstruction possibilities.44 -47 T2- or PD-weighted, fat-suppressed SPACE sequence (Sampling Perfection with Application Optimized Contrast with Different Flip Angle Evolution) or a T2*-weighted MEDIC (Multi-Echo Data Image Combination) sequence IS excellent for cartilage imaging. 48 Many of those sequences have not been introduced in daily routine imaging. However, these sequences can be recommended as a supplement, especially in complex or unclear cases.
The orthopedic surgeon should be aware that for cartilage lesions, the sensitivity of MRI was 91% and specificity was 55% for the Outerbridge grading scale in a study performed by Staats et al. 49 For the Berndt and Harty classification system, sensitivity was 91% and specificity was 28%. An intact cartilage surface reported by the radiologist in MRI may not necessarily represent the truth. This is important as some treatments (e.g., retrograde drilling) are only indicated in patients with intact cartilage surfaces.
CT gives the best image of any bony pathology. With new detector technology, the radiation for a high-quality CT scan has been significantly reduced during the last few years. Regarding bone pathologies, MRI tends to overestimate the defect size due to the bone edema pattern, whereas CT gives a precise picture of the width and depth of a bony defect and any other bony pathology. 12 Moreover, a CT scan in maximum plantar flexion can be used to assess the accessibility of the lesion with arthroscopy, ventral arthrotomy, or osteotomy. 50 Ultra-high resolution axial slices with an increment of 0.3 mm and a thickness of 0.6 mm produce a high-quality primary data set with an axial and secondary reconstruction of 1.0 mm. CT arthrography with the injection of a contrast agent into the joint space improves detection and direct visualization of cartilage defects at the ankle and can be a relevant tool for treatment decisions in unclear cases. 51
A very recent development is the introduction of WBCT.52,53 The information on WBCT is not limited to the bony pathology as in traditional CT scans but also includes information about hindfoot mechanics and axis. It, therefore, can replace standard weightbearing x-rays and traditional CT scans. An additional advantage is the low radiation dose compared with a routine CT scan. 54
Statement: The standard assessment for evaluating cartilage lesions includes MRI to visualize bone edema and the cartilage in combination with weightbearing x-rays or WBCT. Traditional CT visualizes the bony pathology more precisely than MRI; however, it does not provide information on the mechanical axis and foot position under weightbearing conditions. Although many new MRI protocols have been published within the last few years, the sensitivity and specificity of MRI regarding cartilage lesions have limitations.
Grade of Recommendation: B
Classification
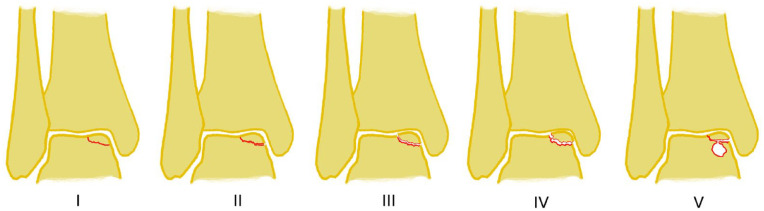
The most popular classification system for OLTs, based on plain x-rays of the ankle, was published by Berndt and Harty in 1959. 21 The classification system was based on plain x-rays of the ankle. In 1993, Loomer et al. 39 added the type with the subchondral cyst based on CT imaging ( Fig. 1 ).
Figure 1.
Classification by Berndt and Harty (stages I-IV), modified by Loomer (stage V).
Two other grading systems frequently found in the literature are based on the arthroscopic appearance of the cartilage surface. The arthroscopic grading system was primarily published by Pritsch et al. 55 and later modified by Takao et al. 56 ( Table 4 ).
Table 4.
Pritsch Classification, and Pritsch Classification Modified by Takao.
| Pritsch Classification | Pritsch Modified by Takao | Arthroscopic Evaluation |
|---|---|---|
| I | 0 | Intact overlaying cartilage |
| II | I | Soft cartilage |
| III | II | Frayed overlaying cartilage |
| III | Detached fragment is in place | |
| IV | Dislocated fragment |
With MRI becoming more accessible in the 1990s, MRI-based classification systems have become increasingly popular. The Hepple et al. 57 classification is based on the initial classification system of Berndt and Harty but introduces additional traumatic, cystic, or idiopathic subtypes of OLTs ( Table 5 ). The Mintz et al. 58 classification correlates the MRI with arthroscopic findings.
Table 5.
Hepple Classification (MRI).
| Stage | MRI Characteristics |
|---|---|
| I | Articular cartilage damage only |
| IIA | Cartilage damage with underlying fracture and surrounding bony edema |
| IIB | IIA without bony edema |
| III | Detached but undisplaced fragment |
| IV | Detached and displaced fragment |
| V | Subchondral cyst formation |
Today, the ICRS (International Cartilage Regeneration and Joint Preservation Society) 59 Classification and the Outerbridge 60 classification are used to grade cartilage lesions independent from a particular joint ( Fig. 2 ).
Figure 2.
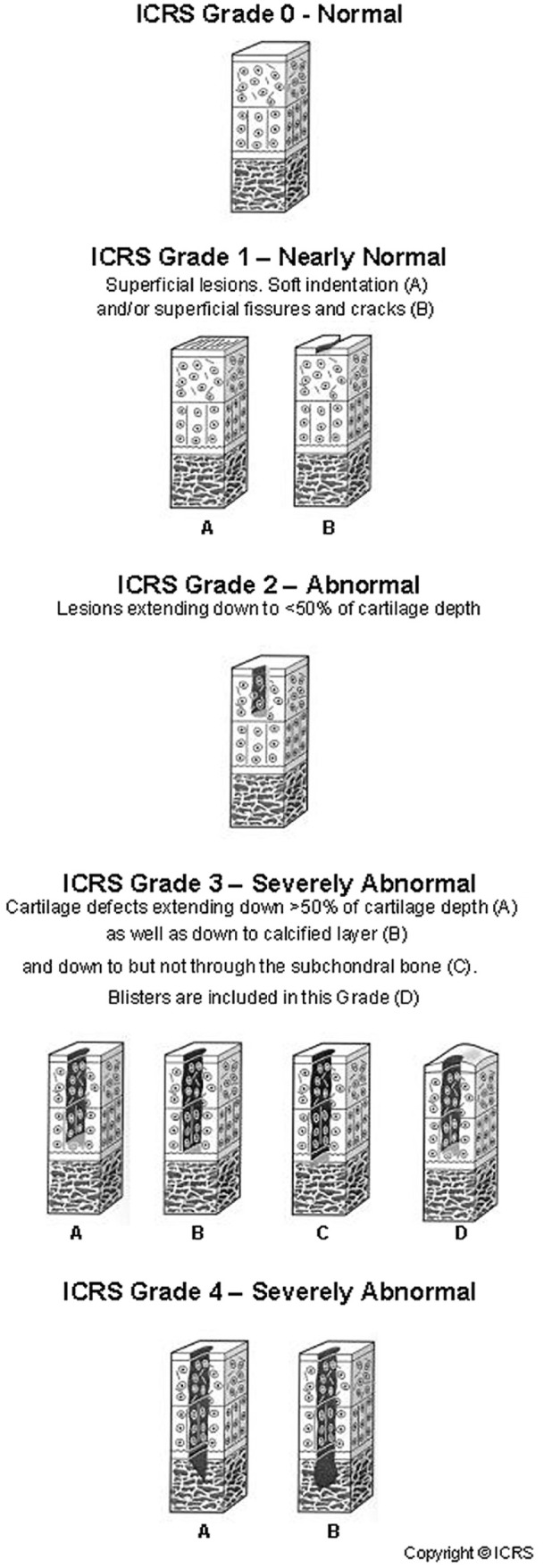
ICRS classification. ICRS = International Cartilage Regeneration and Joint Preservation Society.
The Giannini et al. 61 classification was developed specifically for the ankle joint. This comprehensive classification distinguishes between acute and chronic lesions, intact, or damaged cartilage surface, and includes the size of the lesion and the depth of a bony defect. Subchondral cysts with intact cartilage surfaces can also be classified. Massive OLTs are characterized by a lesion larger than 3 cm² and a depth of more than 1 cm ( Table 6 ).
Table 6.
Giannini classification.
| Type of Lesion | Surface | Extension | Treatment |
|---|---|---|---|
| Acute | |||
| I | Damaged | <1 cm² | Debridement |
| II | Damaged | ≥1 cm² | Fixation |
| Chronic | |||
| 0 | Intact | Any | Drilling |
| I | Damaged | <1.5 cm² | Microfractures |
| II | Damaged | ≥1.5 cm² | Cartilage replacement |
| IIA | Damaged | ≥1.5, >5 mm deep cm² | Cartilage replacement and bone graft |
| III | Damaged | ≥1 cm² | Osteochondral massive graft |
Besides the size of the lesion, the localization may influence the treatment options. Therefore, any documentation of the localization is recommended. Typically, location and size are documented by the imaging of a patient. For scientific studies, the location can be categorized using a 9-zone anatomic grid scheme (medial/central/lateral and anterior/central/posterior), as described by Elias et al. 32 The lesion size can be estimated in 3 planes, including surface area and depth of the lesion. 12 It is mandatory to document the source of the measurement as findings in arthroscopy, open surgery, MRI, and CT may differ.38,51,58,62,63
Statement: The descriptive classification system of Berndt and Harty, modified by Loomer, can still be recommended, as well as the Giannini Classification, ICRS Classification, and Outerbridge Classification. Especially for scientific studies, location and size should be documented, including the source of the measurements.
Grade of Recommendation: B
Conservative Management Strategies
Natural Progression
OLTs are not necessarily related to clinical symptoms. Bezuglov et al. 64 reviewed a cohort of 37 asymptomatic professional soccer players and found osteochondral lesions in 42% of the athletes. So far, there is no evidence that OLT progresses to ankle osteoarthritis (OA). Klammer et al. 65 followed 48 patients with OLT for a minimum of 2 years (mean = 52 months; range, 27-124 months) and noted that the minimally symptomatic OLT did not progress over time when treated non-operatively. Weigelt et al. 66 followed 22 patients for 11 to 20 years, in whom the progression of OA was analyzed based on plain ankle radiographs at the initial presentation and the final follow-up (van Dijk et al. 67 classification). At the final follow-up, 11 cases (73%) showed no progression of OA while 4 (27%) showed progression by 1 grade, and 38% of the patients reduced their sports activities because of sports-related symptoms. However, it is unlikely that OLT will disappear, and OLT may progress with increasing symptoms. Risk factors for progression are the depth of the lesion on MRI, subchondral cyst formation, and the extent of bone marrow edema. 65 A follow-up imaging is recommended after 12 months, even in asymptomatic patients, to avoid missing any progression.
Statement: OLTs with minimal symptoms are unlikely to progress to OA, especially if the modification of activities leads to a symptom-free situation. Based on MRI findings alone, there is no need to urge patients with minimal or no symptoms for prophylactic surgery. After 12 months, a follow-up imaging with MRI prevents missing any asymptomatic progression of the lesion.
Grade of Recommendation: B
Immobilization
There are 2 systematic reviews on the conservative management of OLTs. Zengerink et al. 68 calculated a success rate of 45% among patients who had conservative management with rest. Patients who underwent cast immobilization ranging from 3 weeks to 4 months had a success rate of 53%. Tol et al. 69 reported good or excellent results in 59% of patients with rest and restrictions for sports activities with or without nonsteroidal anti-inflammatory drugs (NSAIDs). The cohort with cast immobilization for 3 weeks to 4 months presented with good or excellent results in 41%. Shearer et al. 70 analyzed conservative management in chronic Stage V OLTs (Loomer classification). 39 They found a minimal relation between the morphological findings and clinical symptoms. They concluded that the conservative strategy should be limited to 3 months in patients with persistent symptoms.
In an investigation of OLTs in children, Perumal et al. 71 reported on 32 patients with an average age of 12 years. After 6 months of conservative management with cast immobilization and unloading, 77% continued to have persistent lesions on radiographs, 16% demonstrated complete clinical and radiographic healing, and 6% had severe pain after cast removal that required surgery. In those patients with persistent radiographic lesions and after an extra 6 months of nonoperative treatment, 42% had to undergo surgery for unhealed lesions and pain, whereas 46% had no symptoms despite persistent lesions on radiographs.
Statement: Reduction of activity is a strategy to reduce symptoms in OLTs. There is no evidence that cast immobilization leads to better results than reducing activities to a level with minimal or no symptoms. The overall success rate seems to be about 50%. There is only a limited correlation between the morphological appearance in imaging and the clinical symptoms. Surgery can be recommended if patients do not improve within 3 months of conservative management.
Grade of Recommendation: B
Injections
There is only limited literature on the effect of injections in OLTs. 72 Mei-Dan et al. 73 performed a prospective study on the effect of HA injection. They followed 15 patients, 60% of whom presented with stage III lesions (Cheng-Ferkel classification), 74 for 26 weeks after receiving 3 weekly intra-articular HA injections. They reported a significant improvement in pain and functional scores, with the effect of the injection lasting for more than 6 months with minimal adverse events. A second study compared injections of HA and PRP. Although both injections improved pain and functional scores, PRP led to a significantly better outcome than HA. However, further scrutiny of the data in a meta-analysis revealed that the study was underpowered. Although there is strong evidence of an improvement, it is impossible to conclude the superiority of PRP over HA. 72
In a retrospective cohort study of 49 patients with OLT grade I to III (Berndt and Harty 21 classification), Akpancar and Gul 75 compared PRP injections and prolotherapy, noting that both the injection of PRP and prolotherapy had a similar effect on pain and functional scores, extending 1 year. They reported excellent or good outcomes in 88.8% of the patients in the prolotherapy group, while 90.9% of the patients in the PRP group reported excellent or good outcomes. As we could not find any research on the potentially harmful effect of the 25% dextrose solution for intra-articular injection on the cartilage, further research is needed until prolotherapy can be recommended as a treatment for OLTs.
Statement: Injection therapy can improve pain and function in OLTs with PRP and HA demonstrating a similar positive effect on pain and functional scores. The effect extends approximately 6 months after injecting HA and 12 months after injecting PRP.
Grade of Recommendation: B
Extracorporeal Shockwave Therapy
After some promising results in animal studies, 76 there is increasing discussion of how ESWT might be used to treat OLTs. Zhang et al. 77 evaluated the efficiency of focused ESWT in patients with persistent pain 3 months after arthroscopic microfracture for OLTs and reported that the visual analog scale and American Orthopaedic Foot and Ankle Society (AOFAS) score significantly improved 12 weeks after ESWT and at the last follow-up (mean follow-up: 27.8 months). In addition, areas of lesions (sagittal plane MRI) were distinctly reduced at the last follow-up. Gao et al. 78 reported combined therapy with focused ESWT and retrograde bone marrow–derived cell transplantation for OLT Hepple grade I to III with a follow-up for more than 2 years. They saw a better reduction in pain and a significantly higher AOFAS score in the group that received additional focused ESWT. Thus, the authors concluded that the combined technique is a highly effective therapeutic option in OLTs with intact cartilage. In both studies, the ESWT was applied transmalleolar to medial talar lesions.
Statement: So far, there are no studies on shockwave as a standalone treatment option for OLTs. Focused ESWT can be considered a complementary treatment in patients with persistent pain after surgical treatment or adjunctive to stimulate tissue regeneration.
Grade of Recommendation: C
Magnetic Field
Some recent studies examined the effect of electromagnetic fields on enhancing osteochondral repair in rabbits. 79 Based on basic science research, there might be an effect on the healing in OLTs, 80 but there is no data available on the application in humans.
Statement: Currently, no data are available to support the use of an electromagnetic field in treating OLTs in humans.
Grade of Recommendation: I
Discussion
The etiology of OLTs covers a wide range of possible causative factors. Many potential risk factors are intrinsic, with limited possibilities for therapeutic improvement. While one causative factor might be chronic overloading of the talar dome, in most papers published on treatment outcomes, etiology is of minor relevance. Hopefully, this may change over the coming years with a better understanding of the underlying problems. For example, it can be estimated that a blood supply problem at the talar dome with bone necrosis may trigger other treatment algorithms than an acute traumatic fracture of the talar dome. Increasing histologic probes taken during OLT treatment may further enlighten possible categories of causes. Other causative factors might be chronic overloading of the talar dome.
Imaging has improved with new MRI sequences and WBCT, and while many new MRI imaging sequences are of scientific interest, they have not been widely established in the daily diagnostic routine. The standard MRI of the ankle requires 3 planes, including sagittal, coronal, and transversal planes with T1 and T2 sequences. 3T systems with high-resolution coil systems provide the best image quality, while open, low-field MRIs are insufficient to provide the information needed to plan the treatment.
There has been no relevant change in the classification systems over the last few years, so the descriptive classification system of Berndt and Harty, modified by Loomer ( Fig. 1 ), can still be recommended. Also, the Giannini Classification ( Table 6 ), ICRS Classification ( Fig. 2 ), and Outerbridge Classification are helpful tools. Especially for scientific studies, location and size should be documented, including the source of the measurements, as findings in arthroscopy, open surgery, MRI, and CT may differ significantly.38,51,58,62,63
The published literature on the conservative management of OLTs shows increasing evidence for the different treatment options. However, the maximum grade of recommendation never exceeds level B—fair evidence. Studies supporting a grade of recommendation A are extremely difficult to perform, requiring level I RCTs with similar findings or a meta-analysis.
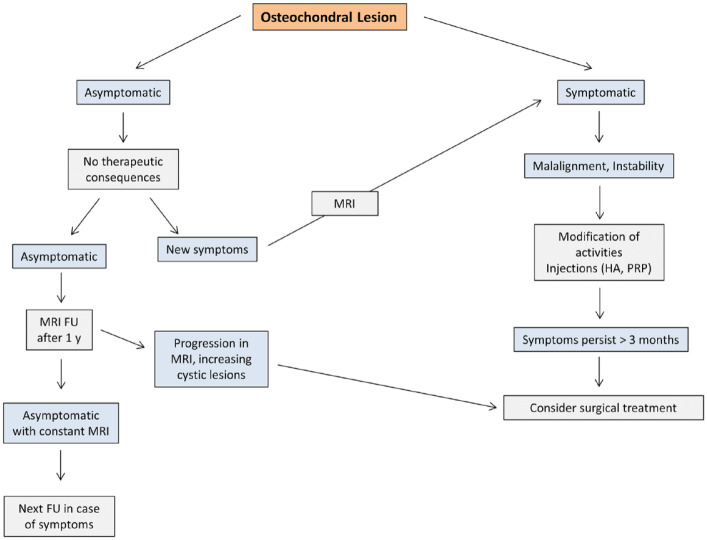
The treatment algorithm based on the findings of the study is shown in Figure 3 . There are several key messages regarding conservative treatment, which are in general also supported by the voting of the expert group ( Table 7 ):
Figure 3.
Suggested conservative treatment algorithm for osteochondral lesions of the talus. HA = hyaluronic acid; PRP = platelet-rich plasma; FU = Follow-up.
Table 7.
Statements, Grade of Recommendation, LOE of the Best Study on the Topic, and Agreement on the Statements Among the Experts.
| Statement | Grade of Recommendation | Highest LOE | Agreement Among the Experts (%) | ||||
|---|---|---|---|---|---|---|---|
| Totally Agree | Somehow Agree | Neither Agree nor Disagree | Somehow Disagree | Totally Disagree | |||
| The causes of OLTs are diverse. The predominant location is the central third of the medial talar dome. Many of the causative factors cannot be changed. However, remediating any causative factors should be part of the treatment concept, if possible. | B | III | 96 | 4 | 0 | 0 | 0 |
| The evaluation of hindfoot alignment, ankle stability, and range of motion should be part of the clinical assessment. | B | III | 92 | 8 | 0 | 0 | 0 |
| The standard assessment for evaluating cartilage lesions includes MRI to visualize bone edema and the cartilage in combination with weightbearing x-rays or WBCT. Traditional CT visualizes the bony pathology more precisely than MRI; however, it does not provide information on the mechanical axis and foot position under weightbearing conditions. Although many new MRI protocols have been published within the last few years, the sensitivity and specificity of MRI regarding cartilage lesions have limitations. | B | III | 85 | 11 | 4 | 0 | 0 |
| The descriptive classification system of Berndt and Harty, modified by Loomer, can still be recommended, as well as the Giannini Classification, ICRS Classification, and Outerbridge Classification. Especially for scientific studies, location and size should be documented, including the source of the measurements. | B | III | 85 | 15 | 0 | 0 | 0 |
| OLTs with minimal symptoms are unlikely to progress to OA, especially if the modification of activities leads to a symptom-free situation. Based on MRI findings alone, there is no need to urge patients with minimal or no symptoms for prophylactic surgery. After 12 months, a follow-up imaging with MRI prevents missing any asymptomatic progression of the lesion. | B | III | 85 | 15 | 0 | 0 | 0 |
| Reduction of activity is a strategy to reduce symptoms in OLTs. There is no evidence that cast immobilization leads to better results than reducing activities to a level with minimal or no symptoms. The overall success rate seems to be about 50%. There is only a limited correlation between the morphological appearance in imaging and the clinical symptoms. Surgery can be recommended if patients do not improve within 3 months of conservative management. | B | II | 73 | 27 | 0 | 0 | 0 |
| Injection therapy can improve pain and function in OLTs with PRP and HA demonstrating a similar positive effect on pain and functional scores. The effect extends approximately 6 months after injecting HA and 12 months after injecting PRP. | B | II | 42 | 42 | 8 | 8 | 0 |
| So far, there are no studies on shockwave as a standalone treatment option for OLTs. Focused ESWT can be considered a complementary treatment in patients with persistent pain after surgical treatment or adjunctive to stimulate tissue regeneration. | C | IV | 58 | 31 | 11 | 0 | 0 |
| Currently, no data are available to support the use of an electromagnetic field in treating OLTs in humans. | I | V | 81 | 4 | 15 | 0 | 0 |
LOE = level of evidence; OLT = osteochondral lesion of the talus; WBCT = weightbearing computed tomography; CT = computed tomography; ICRS = International Cartilage Regeneration and Joint Preservation Society; OA = osteoarthritis; PRP = platelet-rich plasma; HA = hyaluronic acid; ESWT = extracorporeal shockwave therapy.
In asymptomatic patients and patients with minimal symptoms, OLT progression to ankle OA is unlikely.64 -66 Risk factors for progression are the depth of the lesion on MRI, subchondral cyst formation, and the extent of bone marrow edema. 65 To some extent, conservative management is the adaptation of activities to the performance of the ankle joint. 69 A follow-up imaging after 12 months helps to monitor any progression.
It is impossible to estimate the probability of success of conservative management from initial symptoms and imaging. 70
Cast immobilization is an option in OLTs in children, 71 with a success rate of approximately 50%, although, based on imaging, complete healing is only seen in 6% of the patients.
In adults, the success rate of conservative management ranges from 45% to 59%.68,69 Rest and restrictions for sports activities seem to be more successful than immobilization in adults, contrary to the findings in children where cast immobilization is an option.
Intra-articular injections of HA and PRP can improve pain and functional scores for more than 6 months.72,73,81
If 3 months of conservative management does not improve symptoms, it is unlikely that further conservative management will be successful, and surgery can be recommended. 70
Focused ESWT can be considered a complementary treatment in patients with persistent pain after surgical treatment or adjunctive to stimulate tissue regeneration. So far, no studies support ESWT as a standalone treatment for OLTs.76,78
The literature does not support the use of an electromagnetic field in the treatment of OLTs in humans.
Footnotes
Acknowledgments and Funding: The publication funds of the Martin-Luther-University Halle-Wittenberg covered the open access fees.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Peter Angele: Consultant, Arthrex, Aesculap; Christoph Becher: Consultant, Plasmaconcept; Oliver Gottschalk: Paid speaker, Geistlich; Daniel Günther: Paid speaker, Geistlich, Arthrex, Codon; Peter Müller: BBraun Aesculap; Markus Walther: Paid speaker, Geistlich. The remaining authors have nothing to disclose.
Ethical Approval: Not applicable.
ORCID iDs: Markus Walther  https://orcid.org/0000-0003-1122-1303
https://orcid.org/0000-0003-1122-1303
Oliver Gottschalk  https://orcid.org/0000-0002-6512-6275
https://orcid.org/0000-0002-6512-6275
Matthias Steinwachs  https://orcid.org/0000-0002-2287-1654
https://orcid.org/0000-0002-2287-1654
Christian Plaass  https://orcid.org/0000-0003-0568-2074
https://orcid.org/0000-0003-0568-2074
Peter Angele  https://orcid.org/0000-0002-2335-1087
https://orcid.org/0000-0002-2335-1087
References
- 1. Aurich M, Albrecht D, Angele P, Becher C, Fickert S, Fritz J, et al. Treatment of osteochondral lesions in the ankle: a guideline from the group “clinical tissue regeneration” of the German Society of Orthopaedics and Traumatology (DGOU). Z Orthop Unfall. 2017;155(1):92-9. [DOI] [PubMed] [Google Scholar]
- 2. Rothrauff BB, Murawski CD, Angthong C, Becher C, Nehrer S, Niemeyer P, et al. Scaffold-based therapies: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):41S-47S. [DOI] [PubMed] [Google Scholar]
- 3. Hurley ET, Murawski CD, Paul J, Marangon A, Prado MP, Xu X, et al. Osteochondral autograft: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):28S-34S. [DOI] [PubMed] [Google Scholar]
- 4. D’Hooghe P, Murawski CD, Boakye LAT, Osei-Hwedieh DO, Drakos MC, Hertel J, et al. Rehabilitation and return to sports: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):61S-73S. [DOI] [PubMed] [Google Scholar]
- 5. van Dijk PAD, Murawski CD, Hunt KJ, Andrews CL, Longo UG, McCollum G, et al. Post-treatment follow-up, imaging, and outcome scores: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):68S-73S. [DOI] [PubMed] [Google Scholar]
- 6. Mittwede PN, Murawski CD, Ackermann J, Görtz S, Hintermann B, Kim HJ, et al. Revision and salvage management: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):54S-60S. [DOI] [PubMed] [Google Scholar]
- 7. Dombrowski ME, Yasui Y, Murawski CD, Fortier LA, Giza E, Haleem AM, et al. Conservative management and biological treatment strategies: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):9S-15S. [DOI] [PubMed] [Google Scholar]
- 8. Shimozono Y, Brown AJ, Batista JP, Murawski CD, Gomaa M, Kong SW, et al. Subchondral pathology: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):48S-53S. [DOI] [PubMed] [Google Scholar]
- 9. Reilingh ML, Murawski CD, DiGiovanni CW, Dahmen J, Ferrao PNF, Lambers KTA, et al. Fixation techniques: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):23S-27S. [DOI] [PubMed] [Google Scholar]
- 10. Smyth NA, Murawski CD, Adams SB, Jr, Berlet GC, Buda R, Labib SA, et al. Osteochondral allograft: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):35S-40S. [DOI] [PubMed] [Google Scholar]
- 11. Hannon CP, Bayer S, Murawski CD, Canata GL, Clanton TO, Haverkamp D, et al. Debridement, curettage, and bone marrow stimulation: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):16S-22S. [DOI] [PubMed] [Google Scholar]
- 12. van Bergen CJA, Baur OL, Murawski CD, Spennacchio P, Carreira DS, Kearns SR, et al. Diagnosis: history, physical examination, imaging, and arthroscopy: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(Suppl 1):3S-8S. [DOI] [PubMed] [Google Scholar]
- 13. Dahmen J, Bayer S, Toale J, Mulvin C, Hurley ET, Batista J, et al. Osteochondral lesions of the tibial plafond and ankle instability with ankle cartilage lesions: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2022;43(3):448-52. [DOI] [PubMed] [Google Scholar]
- 14. Wright JG, Swiontkowski M, Heckman JD. Levels of evidence. J Bone Joint Surg Br. 2006;88(9):1264. [DOI] [PubMed] [Google Scholar]
- 15. Wright JG, Einhorn TA, Heckman JD. Grades of recommendation. J Bone Joint Surg Am. 2005;87(9):1909-10. [DOI] [PubMed] [Google Scholar]
- 16. Slobogean GP, Dielwart C, Johal HS, Shantz JA, Mulpuri K. Levels of evidence at the Orthopaedic Trauma Association annual meetings. J Orthop Trauma. 2013;27(9):e208-12. [DOI] [PubMed] [Google Scholar]
- 17. Turoff M, Linstone HA. The Delphi method: techniques and applications. Reading (MA): Addison-Wesley; 1975. [Google Scholar]
- 18. Chau MM, Klimstra MA, Wise KL, Ellermann JM, Tóth F, Carlson CS, et al. Osteochondritis dissecans: current understanding of epidemiology, etiology, management, and outcomes. J Bone Joint Surg Am. 2021;103(12):1132-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38(2):392-404. [DOI] [PubMed] [Google Scholar]
- 20. Szaro P, Geijer M, Solidakis N. Traumatic and non-traumatic bone marrow edema in ankle MRI: a pictorial essay. Insights Imaging. 2020;11(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41-A:988-1020. [PubMed] [Google Scholar]
- 22. Galli MM, Protzman NM, Mandelker EM, Malhotra AD, Schwartz E, Brigido SA. Examining the relation of osteochondral lesions of the talus to ligamentous and lateral ankle tendinous pathologic features: a comprehensive MRI review in an asymptomatic lateral ankle population. J Foot Ankle Surg. 2014;53(4):429-33. [DOI] [PubMed] [Google Scholar]
- 23. Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5(4):165-85. [DOI] [PubMed] [Google Scholar]
- 24. Pick MP. Familial osteochondritis dissecans. J Bone Joint Surg Br. 1955;37-B(1):142-5. [DOI] [PubMed] [Google Scholar]
- 25. Fraissler L, Boelch SP, Schäfer T, Walcher M, Arnholdt J, Maier G, et al. Vitamin D deficiency in patients with idiopathic and traumatic osteochondritis dissecans of the talus. Foot Ankle Int. 2019;40(11):1309-18. [DOI] [PubMed] [Google Scholar]
- 26. Thordarson DB. Talar body fractures. Orthop Clin North Am. 2001;32(1):65-77, viii. [DOI] [PubMed] [Google Scholar]
- 27. Knupp M, Stufkens SA, van Bergen CJ, Blankevoort L, Bolliger L, van Dijk CN, et al. Effect of supramalleolar varus and valgus deformities on the tibiotalar joint: a cadaveric study. Foot Ankle Int. 2011;32(6):609-15. [DOI] [PubMed] [Google Scholar]
- 28. Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1979;140:131-6. [PubMed] [Google Scholar]
- 29. Kessler JI, Weiss JM, Nikizad H, Gyurdzhyan S, Jacobs JC, Jr, Bebchuk JD, et al. Osteochondritis dissecans of the ankle in children and adolescents: demographics and epidemiology. Am J Sports Med. 2014;42(9):2165-71. [DOI] [PubMed] [Google Scholar]
- 30. Hermanson E, Ferkel RD. Bilateral osteochondral lesions of the talus. Foot Ankle Int. 2009;30(8):723-7. [DOI] [PubMed] [Google Scholar]
- 31. van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. The natural history of osteochondral lesions in the ankle. Instr Course Lect. 2010;59:375-86. [PubMed] [Google Scholar]
- 32. Elias I, Zoga AC, Morrison WB, Besser MP, Schweitzer ME, Raikin SM. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28(2):154-61. [DOI] [PubMed] [Google Scholar]
- 33. Looze CA, Capo J, Ryan MK, Begly JP, Chapman C, Swanson D, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. 2017;8(1):19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gianakos AL, Yasui Y, Hannon CP, Kennedy JG. Current management of talar osteochondral lesions. World J Orthop. 2017;8(1):12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ackermann J, Casari FA, Germann C, Weigelt L, Wirth SH, Viehöfer AF. Autologous matrix-induced chondrogenesis with lateral ligament stabilization for osteochondral lesions of the talus in patients with ankle instability. Orthop J Sports Med. 2021;9(5):23259671211007439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Körner D, Ateschrang A, Schröter S, Aurich M, Becher C, Walther M, et al. Concomitant ankle instability has a negative impact on the quality of life in patients with osteochondral lesions of the talus: data from the German Cartilage Registry (KnorpelRegister DGOU). Knee Surg Sports Traumatol Arthrosc. 2020;28(10):3339-46. [DOI] [PubMed] [Google Scholar]
- 37. Gotze C, Nieder C, Felder H, Peterlein CD, Migliorini F. AMIC for traumatic focal osteochondral defect of the talar shoulder: a 5 years follow-up prospective cohort study. BMC Musculoskelet Disord. 2021;22(1):638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Bergen CJ, Gerards RM, Opdam KT, Terra MP, Kerkhoffs GM. Diagnosing, planning and evaluating osteochondral ankle defects with imaging modalities. World J Orthop. 2015;6(11):944-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med. 1993;21(1):13-9. [DOI] [PubMed] [Google Scholar]
- 40. Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7(1):101-4. [DOI] [PubMed] [Google Scholar]
- 41. Weber MA, Wünnemann F, Jungmann PM, Kuni B, Rehnitz C. Modern cartilage imaging of the ankle. Rofo. 2017;189(10):945-56. [DOI] [PubMed] [Google Scholar]
- 42. Barr C, Bauer JS, Malfair D, Ma B, Henning TD, Steinbach L, et al. MR imaging of the ankle at 3 Tesla and 1.5 Tesla: protocol optimization and application to cartilage, ligament and tendon pathology in cadaver specimens. Eur Radiol. 2007;17(6):1518-28. [DOI] [PubMed] [Google Scholar]
- 43. Joshy S, Abdulkadir U, Chaganti S, Sullivan B, Hariharan K. Accuracy of MRI scan in the diagnosis of ligamentous and chondral pathology in the ankle. Foot Ankle Surg. 2010;16(2):78-80. [DOI] [PubMed] [Google Scholar]
- 44. Tao H, Zhang Y, Hu Y, Li Q, Hua Y, Lu R, et al. Cartilage matrix changes in hindfoot joints in chronic ankle instability patients after anatomic repair using T2-mapping: initial experience with 3-year follow-up. J Magn Reson Imaging. 2022;55:234-43. [DOI] [PubMed] [Google Scholar]
- 45. Abrar DB, Schleich C, Radke KL, Frenken M, Stabinska J, Ljimani A, et al. Detection of early cartilage degeneration in the tibiotalar joint using 3 T gagCEST imaging: a feasibility study. MAGMA. 2021;34(2):249-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Behzadi C, Maas KJ, Welsch G, Kaul M, Schoen G, Laqmani A, et al. Quantitative T2 * relaxation time analysis of articular cartilage of the tibiotalar joint in professional football players and healthy volunteers at 3T MRI. J Magn Reson Imaging. 2018;47:372-79. [DOI] [PubMed] [Google Scholar]
- 47. Tao H, Hu Y, Qiao Y, Ma K, Yan X, Hua Y, et al. T2-mapping evaluation of early cartilage alteration of talus for chronic lateral ankle instability with isolated anterior talofibular ligament tear or combined with calcaneofibular ligament tear. J Magn Reson Imaging. 2018;47:69-77. [DOI] [PubMed] [Google Scholar]
- 48. Xing W, Sheng J, Chen WH, Tian JM, Zhang LR, Wang DQ. Reproducibility and accuracy of quantitative assessment of articular cartilage volume measurements with 3.0 Tesla magnetic resonance imaging. Chin Med J (Engl). 2011;124(8):1251-6. [PubMed] [Google Scholar]
- 49. Staats K, Sabeti-Aschraf M, Apprich S, Platzgummer H, Puchner SE, Holinka J, et al. Preoperative MRI is helpful but not sufficient to detect associated lesions in patients with chronic ankle instability. Knee Surg Sports Traumatol Arthrosc. 2018;26:2103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Bergen CJ, Tuijthof GJ, Blankevoort L, Maas M, Kerkhoffs GM, van Dijk CN. Computed tomography of the ankle in full plantar flexion: a reliable method for preoperative planning of arthroscopic access to osteochondral defects of the talus. Arthroscopy. 2012;28(7):985-92. [DOI] [PubMed] [Google Scholar]
- 51. Kirschke JS, Braun S, Baum T, Holwein C, Schaeffeler C, Imhoff AB, et al. Diagnostic value of CT arthrography for evaluation of osteochondral lesions at the ankle. Biomed Res Int. 2016;2016:3594253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lintz F, Beaudet P, Richardi G, Brilhault J. Weight-bearing CT in foot and ankle pathology. Orthop Traumatol Surg Res. 2021;107(Suppl 1):102772. [DOI] [PubMed] [Google Scholar]
- 53. Conti MS, Ellis SJ. Weight-bearing CT scans in foot and ankle surgery. J Am Acad Orthop Surg. 2020;28(14):e595-603. [DOI] [PubMed] [Google Scholar]
- 54. Siewerdsen JH, Uneri A, Hernandez AM, Burkett GW, Boone JM. Cone-beam CT dose and imaging performance evaluation with a modular, multipurpose phantom. Med Phys. 2020;47(2):467-79. [DOI] [PubMed] [Google Scholar]
- 55. Pritsch M, Horoshovski H, Farine I. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1986;68(6):862-5. [PubMed] [Google Scholar]
- 56. Takao M, Uchio Y, Kakimaru H, Kumahashi N, Ochi M. Arthroscopic drilling with debridement of remaining cartilage for osteochondral lesions of the talar dome in unstable ankles. Am J Sports Med. 2004;32(2):332-6. [DOI] [PubMed] [Google Scholar]
- 57. Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. 1999;20(12):789-93. [DOI] [PubMed] [Google Scholar]
- 58. Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003;19(4):353-9. [DOI] [PubMed] [Google Scholar]
- 59. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58-69. [DOI] [PubMed] [Google Scholar]
- 60. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752-7. [DOI] [PubMed] [Google Scholar]
- 61. Giannini S, Buda R, Faldini C, Vannini F, Bevoni R, Grandi G, et al. Surgical treatment of osteochondral lesions of the talus in young active patients. J Bone Joint Surg Am. 2005;87(Suppl 2):28-41. [DOI] [PubMed] [Google Scholar]
- 62. Lee KB, Bai LB, Park JG, Yoon TR. A comparison of arthroscopic and MRI findings in staging of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2008;16(11):1047-51. [DOI] [PubMed] [Google Scholar]
- 63. Roßbach BP, Paulus AC, Niethammer TR, Wegener V, Gülecyüz MF, Jansson V, et al. Discrepancy between morphological findings in juvenile osteochondritis dissecans (OCD): a comparison of magnetic resonance imaging (MRI) and arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1259-64. [DOI] [PubMed] [Google Scholar]
- 64. Bezuglov E, Khaitin V, Lazarev A, Brodskaia A, Lyubushkina A, Kubacheva K, et al. Asymptomatic foot and ankle abnormalities in elite professional soccer players. Orthop J Sports Med. 2021;9(1):2325967120979994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Klammer G, Maquieira GJ, Spahn S, Vigfusson V, Zanetti M, Espinosa N. Natural history of nonoperatively treated osteochondral lesions of the talus. Foot Ankle Int. 2015;36(1):24-31. [DOI] [PubMed] [Google Scholar]
- 66. Weigelt L, Laux CJ, Urbanschitz L, Espinosa N, Klammer G, Götschi T, et al. Long-term prognosis after successful nonoperative treatment of osteochondral lesions of the talus: an observational 14-year follow-up study. Orthop J Sports Med. 2020;8(6):2325967120924183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Dijk CN, Verhagen RA, Tol JL. Arthroscopy for problems after ankle fracture. J Bone Joint Surg Br. 1997;79(2):280-4. [DOI] [PubMed] [Google Scholar]
- 68. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21(2):119-26. [DOI] [PubMed] [Google Scholar]
- 70. Shearer C, Loomer R, Clement D. Nonoperatively managed stage 5 osteochondral talar lesions. Foot Ankle Int. 2002;23(7):651-4. [DOI] [PubMed] [Google Scholar]
- 71. Perumal V, Wall E, Babekir N. Juvenile osteochondritis dissecans of the talus. J Pediatr Orthop. 2007;27(7):821-5. [DOI] [PubMed] [Google Scholar]
- 72. Boffa A, Previtali D, Di Laura Frattura G, Vannini F, Candrian C, Filardo G. Evidence on ankle injections for osteochondral lesions and osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2021;45(2):509-23. [DOI] [PubMed] [Google Scholar]
- 73. Mei-Dan O, Maoz G, Swartzon M, Onel E, Kish B, Nyska M, et al. Treatment of osteochondritis dissecans of the ankle with hyaluronic acid injections: a prospective study. Foot Ankle Int. 2008;29(12):1171-8. [DOI] [PubMed] [Google Scholar]
- 74. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750-62. [DOI] [PubMed] [Google Scholar]
- 75. Akpancar S, Gul D. Comparison of platelet rich plasma and prolotherapy in the management of osteochondral lesions of the talus: a retrospective cohort study. Med Sci Monit. 2019;25:5640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qi H, Jin S, Yin C, Chen L, Sun L, Liu Y. Radial extracorporeal shock wave therapy promotes osteochondral regeneration of knee joints in rabbits. Exp Ther Med. 2018;16(4):3478-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang C, Huang H, Yang L, Duan X. Extracorporeal shock wave therapy for pain relief after arthroscopic treatment of osteochondral lesions of talus. J Foot Ankle Surg. 2020;59(1):190-4. [DOI] [PubMed] [Google Scholar]
- 78. Gao F, Chen N, Sun W, Wang B, Shi Z, Cheng L, et al. Combined therapy with shock wave and retrograde bone marrow-derived cell transplantation for osteochondral lesions of the talus. Sci Rep. 2017;7(1):2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yan J, Liu C, Tu C, Zhang R, Tang X, Li H, et al. Hydrogel-hydroxyapatite-monomeric collagen type-I scaffold with low-frequency electromagnetic field treatment enhances osteochondral repair in rabbits. Stem Cell Res Ther. 2021;12(1):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15(1):96-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534-41. [DOI] [PubMed] [Google Scholar]