Abstract
Background
Data from the National Health and Nutrition Examination Survey (NHANES) has not been previously utilized to study the prevalence of tinnitus and depression among adults over 20 years old, nor the impact of tinnitus on depression.
Objective
The aim of this study was to evaluate the relationship between tinnitus and depression among adults in the United States.
Materials and Methods
This cross‐sectional study drew upon data from the 2005–2018 NHANES, incorporating adults aged 20 and older who had completed the tinnitus and depression questionnaire. Depression was assessed using the PHQ‐9 questionnaire. Multivariate logistic regression models, subgroup analyses, and sensitivity analyses were performed to examine the association between tinnitus and depression.
Results
This nationally representative study included 10,409 participants, of whom 17.69% reported experiencing tinnitus. The prevalence of depression was 6.2% among those without tinnitus and 15.1% among those with tinnitus (p < .0001). Accounting for potential confounders such as demographic and socioeconomic variables, participants who experienced tinnitus were more likely to exhibit depression symptoms (adjusted odds ratio = 2.0, 95% confidence interval = 1.61–2.48). Subgroup analyses further suggested that tinnitus was associated with an increased prevalence of depression across all subgroups. Sensitivity analysis affirmed these findings.
Conclusions
This study suggests that there is a significant association between tinnitus and the risk of depression in the adult population of the United States, emphasizing the importance of psychological factors in the clinical management of tinnitus.
Level of Evidence
2b.
Keywords: depression, NHANES, tinnitus
This study used data from the NHANES to examine the link between tinnitus and depression among US adults. It found that tinnitus was associated with a higher risk of depression, even after adjusting for other factors. The study suggested that psychological factors should be considered in the treatment of tinnitus.
1. INTRODUCTION
Tinnitus is a condition where individuals perceive sounds without any external source being present. This condition can affect individuals at any age. 1 Tinnitus is not considered a disease but rather a symptom that can be mediated by multiple etiologies, including otitis media, hearing loss, and Meniere's disease. Tinnitus might also be triggered by nervous disorders, over‐exposure to noise, certain medications, head trauma, or circulatory system disorders. 2 , 3 It is estimated that tinnitus affects 10–30% of the US and global population. 4 , 5 , 6 , 7 Furthermore, the prevalence of tinnitus among younger individuals has been on the rise over the past decade, possibly as a result of increased exposure to potentially damaging recreational noise. 3
Depression is a major psychiatric disorder worldwide, accounting for 45 million disability‐adjusted life years in 2019. 8 In the past decade, severe depression has escalated among adults in the United States. 9 Tinnitus can have a profound impact on health‐related quality of life, with most patients reporting a mild impact. On the other hand, other tinnitus patients have reported anxiety, depression, and even suicidal ideation. It is estimated that at least 10% of tinnitus patients are severely impacted and seek medical evaluation. 2 , 3 , 10 Existing tinnitus therapies offer limited benefit, focusing mainly on reducing the burden of tinnitus on the patient.
The relationship between tinnitus and depression is complex. Tinnitus has been linked to psychological and psychiatric disorders, particularly depression, in previously published studies. 11 , 12 , 13 In the United States, the National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey of the general public. There has been only one study that has used the NHANES database to investigate the relationship between tinnitus and depression, focusing on older populations. 14 The objective of our study was to leverage a nationally representative sample of US adults aged 20 and above to examine the association between depression and tinnitus. We aimed to comprehensively assess the evidence of depression in tinnitus patients, quantify the degree of depression in this population, and inform clinical practice and public health interventions.
2. MATERIALS AND METHODS
2.1. Participants
This cross‐sectional study utilized data from the National Health and Nutrition Examination Survey (NHANES) from 2005 to 2018 to explore the association between tinnitus and depression in adults aged 20 and above. NHANES applied a complex, stratified, multiple‐stage probability cluster sampling design to survey a nationally representative sample. All participants provided written informed consent and the NHANES protocol was approved by the Institutional Review Board for Disease Control and Prevention Research Ethics, Interviews and examinations were performed according to standardized protocols.
In the 2005–2006 NHANES, 2009–2010 NHANES, and 2017–2018 NHANES cycles, tinnitus status was evaluated in participants 12–19 years of age and 70 years of age or older. In the 2011–2012 NHANES, and 2015–2016 NHANES cycles, tinnitus status was assessed in participants aged 20–69. These NHANES cycles only publicly released depression data for adults aged 20 or older, thus our analyses were conducted based on individuals within this age group. The study omitted participants with incomplete depression or tinnitus data. The flowchart presented in Figure 1 outlines the participant selection process.
FIGURE 1.
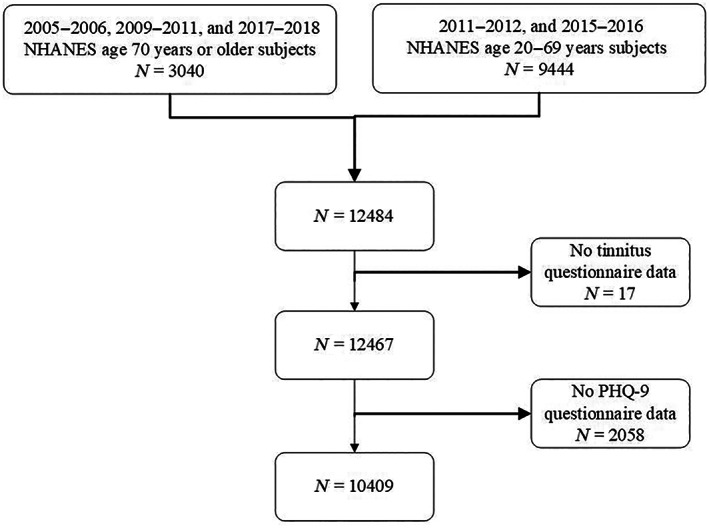
Flowchart study population.
2.2. Tinnitus
In this study, participants were asked whether they had experienced tinnitus in the past 12 months, including a ringing, roaring, or buzzing in the ears or head lasting for at least 5 min. Responding “yes” to this tinnitus question initiated subsequent questions. In response to the ensuing question of how long they had been bothered by tinnitus, there were five possible responses: less than 3 months, 3 months to a year, 1–4 years, 5–9 years, and 10 years or more. Another question delved into whether listening to loud sounds or loud music prompted an episode of tinnitus. Another question probed whether they were disturbed by tinnitus when sleeping. When asked about the extent of the tinnitus symptoms, respondents could select from no problem, a small problem, a moderate problem, a big problem, or a very big problem. In the 2011–2012 NHANES, 2015–2016 NHANES, and 2017–2018 NHANES cycles, participants were asked about the frequency of their tinnitus episodes, with options including less frequently than once a month, at least once a month, at least once a week, at least once a day and almost always.
2.3. Depression
In this study, depression symptoms were evaluated using the Patient Health Questionnaire (PHQ‐9). The PHQ‐9 is a nine‐item depression screening instrument that measures the frequency of depression symptoms over the past 2 weeks. Each symptom question is associated with a point ranging from 0 to 3, based on the response categories “not at all,” “several days,” “more than half the days,” and “nearly every day.” Depression severity was categorized by using established thresholds from the total PHQ‐9 score, with no depression (0–4), mild depression (5–9), moderate depression (10–14), moderately severe depression (15–19), and severe depression (20–27) observed. For the analyses presented, using the PHQ‐9, depression status was bifurcated as <10 (no or mild depression symptoms) or ≥10 (moderate or severe depression symptoms, respectively). Major depression was identified with this cutoff value due to its high sensitivity and specificity. 15
2.4. Covariates
Information about age, sex, and race was collected using standardized questionnaires during in‐person interviews. Data were collected on health‐related behaviors including smoking and alcohol use. Participants were asked to answer questions about whether they had smoked at least 100 cigarettes in their lifetime and whether they were currently smoking. Based on their responses to those questions, participants were classified as nonsmokers (<100 cigarettes in life), former smokers (>100 cigarettes but not currently smoking), or current smokers (smoking “some days” or “every day”). “Never drinkers” were coded as those who answered “no” to drinking alcohol in the past 12 months and in their entire lives. “Former drinkers” were those who answered “yes” to drinking in their lifetime but had not consumed alcohol in the past 12 months. “Current drinkers” were those who answered “yes” to drinking at least 12 alcoholic drinks in the past year or in their lifetime, and who consumed alcohol at least once in the past year. “Current drinkers” were classified into the following groups: mild, moderate, and heavy. At the time of the study, moderate drinking was defined as two drinks per day for women and three drinks per day for men or binge drinking (≥4 drinks on same occasion for females, ≥5 drinks on same occasion for males) more than 2 days but less than 5 days a month. Females who consumed three drinks per day or more, and males who consumed four drinks per day or more, or binge drank five or more days a month, were considered heavy users of alcohol. Participants were classified according to their education level: those with less than a high school diploma, those with a high school or equivalent diploma, and those with a degree higher than a high school diploma. There were six categories of marital status: widowed, living with a partner, divorced, separated, never married, and married. Ratio of family income to poverty level (Poverty index quartiles) was categorized as ≤1, 1–3.5, or >3. Hearing quality was assessed by answering “which statement best describes your hearing?” with answers “excellent,” “good,” “a little trouble,” “moderate trouble,” “a lot of trouble,” and “deaf.” The latter three answers were categorized as having subjective hearing loss (HL). The study also included variables related to medical comorbidities such as body mass index (BMI; categorized as 25 kg/m2, 25–30 kg/m2, or >30 kg/m2), diabetes, and hypertension. Those with elevated blood pressure (systolic over 140 mmHg or diastolic over 90 mmHg) or on blood pressure‐lowering medication were considered to have elevated blood pressure. The diagnosis of diabetes was based on either of the following: (1) Told by their doctor that they were diabetic. (2) Fasting blood glucose level (mmol/l) was > = 7.0. (3) Two‐hour OGTT blood glucose level (mmol/l) was > = 11.1. (4) Glycosylated hemoglobin HbA1c (%) was over 6.5. (5) Random blood glucose (mmol/l) was > = 11.1. (6) Taking diabetes medication or insulin.
2.5. Statistical analyses
For the analysis of NHANES data, we followed NHCS guidelines. To procure unbiased national estimates, the interview weight variable (WTINT2YR) was incorporated in all analyses. To adjust the standard errors (SEs) for the complex survey design, we employed the Primary Sampling Unit variable (SDMVPSU) and the stratification variable (SDMVSTRA). A logistic regression was deployed to estimate unadjusted and adjusted odds ratios (ORs) for assessing the potential association between tinnitus and depression. Multivariable logistic regression models with depression as dependent variables and tinnitus as the independent variable were adjusted for gender, age, race, ratio of family income to the poverty level, educational level, marital status, smoking status, alcohol use, diabetes, hypertension, body mass index, and subjective hearing condition. In addition, we executed stratified and interaction analyses to appraise effects within different subgroups. Moreover, we carried out sensitivity analyses. The current literature defines depression according to PHQ‐9 ≥ 10 and PHQ‐9 ≥ 5 as critical values. We used PHQ‐9 ≥ 10 as a criterion for determining depression and PHQ‐9 ≥ 5 for sensitivity analysis. This study included both acute and chronic tinnitus participants. There is no universally accepted definition of chronic tinnitus, which can range from a minimum of 3 months to a maximum of 12 months. In this study, a sensitivity analysis was undertaken to contrast the difference in the prevalence of depression between participants enduring chronic tinnitus (duration of tinnitus exceeding 3 months or 1 year) and those devoid of tinnitus, based on a tinnitus questionnaire. Statistical analyses were executed using R language (version 4.1.3), and analysis of complex sample survey data was performed using the survey package. The level of significance was established at 0.05 for all analyses utilizing a two‐sided test.
3. RESULTS
Following the survey methodology, the study sample included 10,409 adults aged 20 years and above, representing 197,057,173 non‐institutionalized civilian US adults aged 20 years and older (weighted calculation). Figure 1 presents the flowchart for participant inclusion. Among the participants, 17.69% reported experiencing tinnitus. Extrapolating this percentage to the non‐institutionalized civilian US adult population yields an estimated 35 million individuals. Collectively, tinnitus patients were significantly older than the general populace, with an average age of 53.64 (0.54) years compared to 46.38 (0.40) years for the general populace (p < .001). Additionally, tinnitus showed a higher prevalence in males than in females within this study population (p < .001), and those afflicted with tinnitus presented poorer subjective self‐reported hearing (p < .001). Furthermore, tinnitus and non‐tinnitus groups exhibited differences in relation to race, education level, marital status, smoking habits, alcohol consumption, diabetes, hypertension, and weight (all p values <.01). In contrast, no difference was observed in family economic status between the two groups. The aforementioned data are delineated in Table 1.
TABLE 1.
Demographic information.
| Variables, n (%) a | Total | No tinnitus | Tinnitus | p value b |
|---|---|---|---|---|
| 8609 (82.31) | 1800 (17.69) | |||
| Gender | .01 | |||
| Female | 5214 (51.30) | 4364 (52.26) | 850 (46.82) | |
| Male | 5195 (48.70) | 4245 (47.74) | 950 (53.18) | |
| Age | <.0001 | |||
| 20 ~ 29 | 1658 (18.71) | 1522 (20.89) | 136 (8.56) | |
| 30 ~ 39 | 1569 (16.81) | 1386 (17.84) | 183 (12.02) | |
| 40 ~ 49 | 1541 (18.39) | 1319 (18.92) | 222 (15.95) | |
| 50 ~ 59 | 1547 (19.04) | 1242 (17.67) | 305 (25.42) | |
| 60 ~ 69 | 1675 (15.46) | 1293 (14.03) | 382 (22.11) | |
| 70 ~ 79 | 1522 (7.92) | 1161 (7.25) | 361 (11.06) | |
| 80~ | 897 (3.66) | 686 (3.40) | 211 (4.87) | |
| Race | <.0001 | |||
| Non‐Hispanic White | 4212 (67.05) | 3307 (65.45) | 905 (74.49) | |
| Non‐Hispanic Black | 2347 (11.16) | 2026 (11.83) | 321 (8.06) | |
| Mexican American | 1374 (8.08) | 1117 (8.30) | 257 (7.03) | |
| Other race | 2476 (13.71) | 2159 (14.42) | 317 (10.42) | |
| Poverty index quartiles | .96 | |||
| <1.3 | 2993 (20.13) | 2421 (21.37) | 572 (21.72) | |
| 1.3–3.5 | 3781 (34.36) | 3119 (36.59) | 662 (36.45) | |
| >3.5 | 2784 (39.46) | 2354 (42.04) | 430 (41.83) | |
| Educational level | .01 | |||
| <12th grade | 2418 (14.59) | 1930 (14.15) | 488 (16.65) | |
| 12th grade | 2329 (20.83) | 1887 (20.08) | 442 (24.33) | |
| >12th grade | 5656 (64.56) | 4787 (65.77) | 869 (59.02) | |
| Marital Status | <.0001 | |||
| Married | 5219 (54.19) | 4338 (54.29) | 881 (53.78) | |
| Living with partner | 842 (9.06) | 723 (9.46) | 119 (7.17) | |
| Separated | 349 (2.41) | 277 (2.33) | 72 (2.76) | |
| Divorced | 1078 (10.14) | 836 (9.04) | 242 (15.25) | |
| Widowed | 997 (5.41) | 773 (5.10) | 224 (6.83) | |
| Never married | 1920 (18.79) | 1660 (19.77) | 260 (14.22) | |
| Smoke status | <.001 | |||
| Never | 5785 (55.52) | 4942 (56.99) | 843 (48.78) | |
| Former | 2675 (25.15) | 2104 (24.64) | 571 (27.57) | |
| Now | 1941 (19.30) | 1557 (18.37) | 384 (23.65) | |
| Alcohol use | <.001 | |||
| Never | 1580 (10.83) | 1361 (11.34) | 219 (9.44) | |
| Former | 1683 (13.18) | 1317 (12.57) | 366 (17.16) | |
| Mild | 3490 (35.94) | 2874 (35.87) | 616 (39.47) | |
| Moderate | 1447 (17.10) | 1216 (17.92) | 231 (14.76) | |
| Heavy | 1879 (21.41) | 1581 (22.30) | 298 (19.18) | |
| Diabetes | 2164 (14.87) | 1683 (13.91) | 481 (20.20) | <.0001 |
| Hypertension | 4628 (36.61) | 3586 (33.64) | 1042 (50.45) | <.0001 |
| Body mass index c | <.0001 | |||
| Normal weight | 2980 (28.83) | 2590 (30.18) | 390 (23.69) | |
| Overweight | 3378 (32.44) | 2822 (33.15) | 556 (30.43) | |
| Obese | 3934 (38.03) | 3110 (36.67) | 824 (45.88) | |
| Subjective hearing condition | <.0001 | |||
| Excellent | 3490 (34.84) | 3209 (38.99) | 281 (15.58) | |
| Good | 4258 (41.51) | 3690 (43.54) | 568 (32.08) | |
| A little trouble | 1613 (15.50) | 1107 (12.46) | 506 (29.65) | |
| Bad | 1046 (8.13) | 602 (5.01) | 444 (22.70) | |
| Depression (PHQ ≥10) | 872 (7.81) | 581 (6.22) | 291 (15.19) | <.0001 |
| Depression severity d | <.0001 | |||
| None | 7929 (77.17) | 6803 (79.97) | 1126 (64.14) | |
| Mild | 1608 (15.02) | 1225 (13.80) | 383 (20.67) | |
| Moderate | 537 (4.76) | 380 (4.01) | 157 (8.27) | |
| Moderate–severe | 224 (2.04) | 143 (1.59) | 81 (4.15) | |
| Severe | 111 (1.00) | 58 (0.62) | 53 (2.77) |
Unweighted N.o. (Weighted %).
Chi‐squared test with Rao & Scott's second‐order correction.
Body mass index: Normal weight (BMI ≤ 24), overweight (25 ≤ BMI < 30), obese (BMI ≥ 30).
Depression severity: none (PHQ score 0–4), mild (PHQ score 5–9), moderate (PHQ score 10–14), moderate–severe (PHQ score 15–19), severe (PHQ score 20–27).
As shown in Table S1, 37.09% of those afflicted by tinnitus had endured symptoms for over a decade, 32.28% experienced it virtually daily, and 39.70% reported that tinnitus impeded their sleep. Among individuals with tinnitus, 12.51% developed the condition subsequent to noise exposure. Among participants with tinnitus, 8.81% perceived the condition as a big problem or even a very big problem.
In the nationally representative sample shown in Table S2, depression affected 7.81% of adults aged 20 and above. Depression was more prevalent among tinnitus patients than in the general populace, with 6.22% of those devoid of tinnitus experiencing depression as compared to 15.19% of those diagnosed with tinnitus (p < .001).
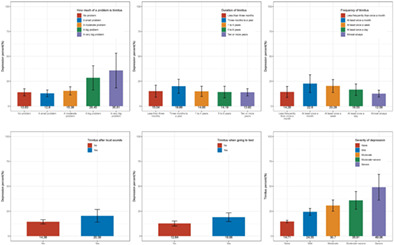
Figure 2 illustrates the observed variations in the prevalence of depression among tinnitus patients with different characteristics. This was specifically found in people with tinnitus reportedly affecting sleep and in people with tinnitus caused by noise exposure. Individuals who perceived tinnitus as a serious problem showed a higher prevalence of depression, suggesting a potential association between tinnitus severity and depression prevalence. Similarly, an association was found between severe depression and a higher prevalence of tinnitus. Tinnitus duration and course did not show a statistical difference from the prevalence of depression.
FIGURE 2.
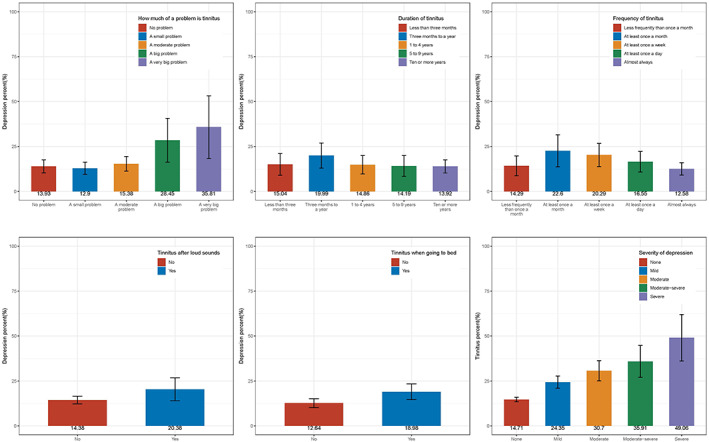
Prevalence of depression in different states of tinnitus, and prevalence of tinnitus across different degrees of depression.
In Table 2, multivariate logistic regression models indicated an increased association of depression with tinnitus (adjusted odds ratio (OR) = 2.0, 95% confidence interval (CI) = 1.61–2.48). Furthermore, women were more likely than men to suffer from depression. Unmarried, living with a partner, divorced, widowed, and separated people were more likely to experience depression than married people. Poor hearing was associated with an increased likelihood of depression, as was low household income. Non‐Hispanic Black and Mexican Americans were less likely to experience depression than Non‐Hispanic White participants. In terms of depression risk, there was no significant difference observed between individuals aged 20–29 and those aged 30–69. However, a considerable reduction in depression likelihood was evident among participants aged 70 and above. Depression was more common in people with diabetes and in people who smoked. People who had consumed alcohol in the past and were not currently drinking were more likely to experience depression. Depression was not associated with high blood pressure, obesity, or education level. The stratified analysis presented in Table 3 illustrates an elevated association with depression related to tinnitus across various demographic and health‐related subgroups. Regardless of variations in age, gender, race, poverty index quartiles, education level, marital status, diabetes and hypertension status, smoking and alcohol usage, body mass index, and subjective hearing condition, individuals with tinnitus consistently showed a higher association with depression. This consistent trend emphasizes the potentially far‐reaching influence of tinnitus on depression across a diverse demographic range. The sensitivity analyses of depression and tinnitus are presented in Table 4. The cut‐off value for depression in this study was PHQ‐9 ≥ 10. In the current literature, depression was defined according to PHQ‐9 ≥ 10 or PHQ‐9 ≥ 5 as critical values. For sensitivity analysis, a PHQ >5 value was used as the diagnostic cutoff value for depression. According to multivariate logistic regression models, individuals with tinnitus were more likely to experience depression (adjusted OR = 1.80; 95% CI = 1.50–2.15; p < .001). Chronic tinnitus is not universally defined, but most literature classifies this as a period lasting from a minimum of 3 months to a maximum of 12 months. Furthermore, we compared depression rates between individuals with chronic tinnitus (lasting more than 3 months or 12 months) and those without it. The adjusted ORs were 2.01 (95% CI, 1.59–2.54) and 1.89 (95% CI, 1.44–2.49).
TABLE 2.
Univariate and multivariate analysis of risk factors for depression.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Tinnitus | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.70 (2.26, 3.22) | <.0001 | 2.00 (1.61, 2.48) | <.001 |
| Gender | ||||
| Male | Ref. | Ref. | Ref. | Ref. |
| Female | 1.71 (1.42, 2.05) | <.0001 | 2.13 (1.75, 2.59) | <.001 |
| Age | ||||
| 20 ~ 29 | Ref. | Ref. | Ref. | Ref. |
| 30 ~ 39 | 1.21 (0.85, 1.73) | .27 | 1.37 (0.92, 2.02) | .11 |
| 40 ~ 49 | 1.14 (0.76, 1.73) | .53 | 1.24 (0.82, 1.88) | .3 |
| 50 ~ 59 | 1.45 (1.09, 1.93) | .01 | 1.21 (0.78, 1.86) | .4 |
| 60 ~ 69 | 1.11 (0.71, 1.74) | .64 | 0.90 (0.52, 1.57) | .7 |
| 70 ~ 79 | 0.76 (0.51, 1.13) | .17 | 0.42 (0.23, 0.75) | .004 |
| 80~ | 0.81 (0.55, 1.20) | .30 | 0.31 (0.15, 0.65) | .003 |
| Race | ||||
| Non‐Hispanic White | Ref. | Ref. | Ref. | Ref. |
| Non‐Hispanic Black | 1.19 (0.94, 1.50) | .15 | 0.71 (0.54, 0.94) | .016 |
| Mexican American | 0.89 (0.67, 1.19) | .43 | 0.58 (0.39, 0.85) | .006 |
| Other Race | 1.23 (0.88, 1.72) | .23 | 1.10 (0.75, 1.60) | .6 |
| Poverty index quartiles | ||||
| <1.3 | Ref. | Ref. | Ref. | Ref. |
| 1.3–3.5 | 0.46 (0.36, 0.58) | <.0001 | 0.59 (0.46, 0.76) | <.001 |
| >3.5 | 0.19 (0.14, 0.26) | <.0001 | 0.31 (0.21, 0.44) | <.001 |
| Educational level | ||||
| <12th grade | Ref. | Ref. | – | |
| 12th grade | 0.70 (0.57, 0.86) | .001 | 0.88 (0.67, 1.15) | .3 |
| >12th grade | 0.46 (0.36, 0.59) | <.0001 | 0.87 (0.63, 1.20) | .4 |
| Marital status | ||||
| Married | Ref. | Ref. | Ref. | Ref. |
| Living with partner | 2.16 (1.60, 2.93) | <.0001 | 1.52 (1.04, 2.23) | .031 |
| Separated | 4.35 (3.22, 5.88) | <.0001 | 2.31 (1.70, 3.14) | <.001 |
| Divorced | 2.70 (2.08, 3.49) | <.0001 | 1.38 (1.01, 1.89) | .045 |
| Widowed | 2.63 (1.85, 3.72) | <.0001 | 2.27 (1.32, 3.91) | .004 |
| Never married | 2.38 (1.88, 3.03) | <.0001 | 2.28 (1.67, 3.11) | <.001 |
| Diabetes | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.83 (1.52, 2.21) | <.0001 | 1.42 (1.10, 1.82) | .008 |
| Hypertension | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.57 (1.29, 1.90) | <.0001 | 1.21 (0.96, 1.52) | .10 |
| Smoke Status | ||||
| Never | Ref. | Ref. | Ref. | Ref. |
| Former | 1.31 (0.99, 1.74) | .05 | 1.29 (0.92, 1.82) | .13 |
| Now | 3.46 (2.67, 4.48) | <.0001 | 2.52 (1.88, 3.37) | <.001 |
| Alcohol use | ||||
| Never | Ref. | Ref. | Ref. | Ref. |
| Former | 1.78 (1.25, 2.54) | .002 | 1.55 (1.02, 2.36) | .040 |
| Mild | 0.72 (0.55, 0.94) | .02 | 0.92 (0.64, 1.32) | .6 |
| Moderate | 0.99 (0.68, 1.44) | .96 | 0.98 (0.63, 1.54) | >.9 |
| Heavy | 1.49 (1.13, 1.97) | .01 | 1.18 (0.80, 1.74) | .4 |
| Body mass index | ||||
| Normal weight | Ref. | Ref. | Ref. | Ref. |
| Overweight | 0.86 (0.64, 1.14) | .28 | 0.99 (0.71, 1.38) | >.9 |
| Obese | 1.42 (1.09, 1.84) | .01 | 1.24 (0.93, 1.65) | .14 |
| Subjective hearing condition | ||||
| Excellent | Ref. | Ref. | Ref. | Ref. |
| Good | 1.63 (1.25, 2.12) | <.001 | 1.55 (1.14, 2.12) | .006 |
| A little trouble | 2.46 (1.87, 3.24) | <.0001 | 2.13 (1.46, 3.12) | <.001 |
| Bad | 3.59 (2.56, 5.04) | <.0001 | 3.75 (2.44, 5.74) | <.001 |
TABLE 3.
A stratified analysis of tinnitus's effect on depression.
| Character | No Tinnitus | Tinnitus | p for interaction |
|---|---|---|---|
| Gender | 0.992 | ||
| Female | Ref. | 1.942 (1.489, 2.533) | |
| Male | Ref. | 2.278 (1.623, 3.198) | |
| Age | 0.33 | ||
| 20 ~ 29 | Ref. | 1.655 (0.803, 3.414) | |
| 30 ~ 39 | Ref. | 1.738 (0.816, 3.704) | |
| 40 ~ 49 | Ref. | 2.251 (0.865, 5.863) | |
| 50 ~ 59 | Ref. | 2.848 (1.319, 6.147) | |
| 60 ~ 69 | Ref. | 1.634 (0.765, 3.489) | |
| 70 ~ 79 | Ref. | 1.385 (0.702, 2.733) | |
| 80~ | Ref. | 2.478 (0.996, 6.166) | |
| Race | 0.781 | ||
| Non‐Hispanic White | Ref. | 1.910 (1.390, 2.625) | |
| Non‐Hispanic Black | Ref. | 2.127 (1.273, 3.552) | |
| Mexican American | Ref. | 2.141 (0.965, 4.751) | |
| Other race | Ref. | 2.533 (1.344, 4.771) | |
| Poverty index quartiles | 0.803 | ||
| <1.3 | Ref. | 1.790 (1.359, 2.358) | |
| 1.3–3.5 | Ref. | 2.150 (1.383, 3.343) | |
| >3.5 | Ref. | 2.320 (1.198, 4.490) | |
| Educational level | 0.68 | ||
| <12th grade | Ref. | 2.388 (1.519, 3.754) | |
| 12th grade | Ref. | 2.176 (1.223, 3.870) | |
| >12th grade | Ref. | 1.809 (1.197, 2.732) | |
| Marital status | 0.074 | ||
| Married | Ref. | 2.730 (1.693, 4.402) | |
| Living with partner | Ref. | 1.887 (0.745, 4.776) | |
| Separated | Ref. | 2.768 (0.885, 8.661) | |
| Divorced | Ref. | 3.132 (1.806, 5.429) | |
| Widowed | Ref. | 1.042 (0.533, 2.040) | |
| Never married | Ref. | 1.296 (0.736, 2.284) | |
| Diabetes | 0.619 | ||
| No | Ref. | 2.112 (1.604, 2.783) | |
| Yes | Ref. | 1.842 (1.122, 3.025) | |
| Hypertension | 0.657 | ||
| No | Ref. | 1.808 (1.220, 2.679) | |
| Yes | Ref. | 2.167 (1.592, 2.948) | |
| Smoke status | 0.289 | ||
| Never | Ref. | 1.900 (1.130, 3.193) | |
| Former | Ref. | 1.633 (0.994, 2.683) | |
| Now | Ref. | 2.369 (1.630, 3.443) | |
| Alcohol use | 0.786 | ||
| Never | Ref. | 2.694 (1.383, 5.247) | |
| Former | Ref. | 1.895 (1.149, 3.125) | |
| Mild | Ref. | 2.502 (1.522, 4.116) | |
| Moderate | Ref. | 1.945 (0.781, 4.843) | |
| Heavy | Ref. | 1.847 (0.878, 3.885) | |
| Body mass index | 0.095 | ||
| Normal weight | Ref. | 1.411 (0.738, 2.697) | |
| Overweight | Ref. | 3.323 (1.803, 6.124) | |
| Obese | Ref. | 1.753 (1.218, 2.523) | |
| Subjective hearing condition | 0.686 | ||
| Excellent | Ref. | 2.656 (1.481, 4.761) | |
| Good | Ref. | 2.020 (1.292, 3.159) | |
| A little trouble | Ref. | 2.018 (1.100, 3.700) | |
| Bad | Ref. | 1.745 (0.900, 3.385) |
TABLE 4.
Sensitivity analysis.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Character | OR (95% CI) | p value | OR (95% CI) | p value |
| Definition of depression as PHQ‐9 ≥ 5 | ||||
| Tinnitus | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.23 (1.95, 2.56) | <.0001 | 1.80 (1.50, 2.15) | <.001 |
| Depression risk for people with tinnitus over 3 months compared with those without | ||||
| Tinnitus | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.66 (2.26, 3.14) | <.0001 | 2.01 (1.59, 2.54) | <.001 |
| Depression risk for people with tinnitus over 12 months compared with those without | ||||
| Tinnitus | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Yes | 2.51 (2.04, 3.08) | <.0001 | 1.89 (1.44, 2.49) | <.001 |
4. DISCUSSION
Previously, only one study using NHANES data had found a significant association between tinnitus and depression in the older adult population (over the age of 70). 14 In this study, we investigated the association between tinnitus and depression in the US adult population aged 20 years and older based on the NHANES database. Tinnitus and depression were significantly correlated in our study, with 17.69% of US adults reporting tinnitus and 7.81% indicating depression. The association between tinnitus and depression was reported by previous studies. 10 , 11 , 14 , 16 , 17 , 18 This study is the first nationally representative report of the relationship between depression and tinnitus in the general adult population.
Additionally, this study corroborates earlier research, reflecting that females tend to show a higher susceptibility to depressive symptoms compared to males. 11 , 18 , 19 It is possible that the higher female burden is due to socioeconomic factors or unique gender‐related biopsychological phenotypes. Depression can also be influenced by age, race, diabetes, hearing loss, smoking, and alcohol consumption. Therefore, we examined depression prevalence by gender, age, and other subgroup analyses in populations both with and without tinnitus. A higher prevalence of depression was demonstrated in populations with tinnitus than in those without, this held true across different subgroups. Contrary to a contemporaneous Korean study, our study did not identify a higher risk of depression among the female tinnitus population compared to the male tinnitus population. 11 Cultural and social differences in the population might have contributed to this.
Furthermore, our study identified an association between a subgroup of tinnitus (where sleep was negatively impacted) and an increased likelihood of depression. This association has been previously reported 14 but has also been a recurring theme across similar studies. 11 , 20 It is not difficult to rationalize that people with tinnitus who have trouble sleeping might be more likely to experience fatigue and unrest, thus increasing their psychological burdens. 20 , 21 Depression also appears to be more likely in patients with a sub‐group of tinnitus triggered by exposure to loud sounds or loud music. Tinnitus resulting from loud sounds might be more psychologically taxing compared to other tinnitus etiologies. Interestingly, we did not find an association between the course and frequency of tinnitus and depression. This might suggest that some individuals adapt to the constant presence of tinnitus, rather than experiencing an increased likelihood of depression as a result of prolonged tinnitus.
A positive correlation was identified between self‐perceived tinnitus severity and depression severity. It was also observed that depression scores were positively correlated with tinnitus prevalence. The relationship between tinnitus and depression can be complex. Experiencing tinnitus might contribute to the onset of depression, which in turn may intensify tinnitus. According to prevailing theories, tinnitus may potentially trigger depression in individuals predisposed to depression, and psychological processes can simultaneously worsen tinnitus. 17 , 22 , 23 , 24 Tinnitus is not solely produced by the peripheral auditory system but is also transmitted and interpreted by the central nervous system. 25 The auditory, central, and peripheral nervous systems all participate in the production of tinnitus. Tinnitus‐related stress has been reported to modify limbic–cortical pathways in the brain, possibly leading to a more depressed state. 26 , 27 Thus, tinnitus and depression might be physiologically interconnected, as depression and other psychosomatic disorders are associated with abnormal limbic system activity.
The study does present some limitations. First, this study is a secondary review of a cross‐sectional study, thus, causality could not be determined. The second limitation is that the NHANES did not employ a standard questionnaire/scale for measuring tinnitus severity. Despite these limitations, this study investigated the association between tinnitus and depression using the most recent and nationally representative database of US adults. The results obtained are, therefore, highly likely to be representative of the US adult population, as substantiated by the results of a sensitivity analysis.
5. CONCLUSION
This study revealed a significant correlation between tinnitus and depression symptoms in US adults over 20 years of age. An association was identified where tinnitus patients showed a significantly higher potential for depression than the general population, which heightens the importance of psychological factors in the clinical management of tinnitus.
FUNDING INFORMATION
The authors have indicated that they have no financial relationships relevant to this article to disclose.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no conflict of interest.
Supporting information
Table S1. Characteristics of the tinnitus population included in the study.
Table S2. Depression in tinnitus and non‐tinnitus populations.
Lin X, Liu Y, Chen Z, et al. Association between depression and tinnitus in US adults: A nationally representative sample. Laryngoscope Investigative Otolaryngology. 2023;8(5):1365‐1375. doi: 10.1002/lio2.1134
Xing Lin and Yang Liu have contributed equally to this work and share first authorship.
REFERENCES
- 1. Lewis S, Chowdhury E, Stockdale D, Kennedy V. Assessment and management of tinnitus: summary of NICE guidance. BMJ. 2020;368:m976. [DOI] [PubMed] [Google Scholar]
- 2. Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(2 Suppl):S1‐S40. [DOI] [PubMed] [Google Scholar]
- 3. Bauer CA. Tinnitus. N Engl J Med. 2018;378(13):1224‐1231. [DOI] [PubMed] [Google Scholar]
- 4. Lewkowski K, Heyworth J, Ytterstad E, Williams W, Goulios H, Fritschi L. The prevalence of tinnitus in the Australian working population. Med J Aust. 2022;216(4):189‐193. [DOI] [PubMed] [Google Scholar]
- 5. Stohler NA, Reinau D, Jick SS, Bodmer D, Meier CR. A study on the epidemiology of tinnitus in the United Kingdom. Clin Epidemiol. 2019;11:855‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schubert NMA, Rosmalen JGM, Van Dijk P, et al. A retrospective cross‐sectional study on tinnitus prevalence and disease associations in the Dutch population‐based cohort lifelines. Hear Res. 2021;411:108355. [DOI] [PubMed] [Google Scholar]
- 7. Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu B, Zhang X, Wang C, Sun M, Jin L, Liu X. Trends in depression among adults in the United States, NHANES 2005–2016. J Affect Disord. 2020;263:609‐620. [DOI] [PubMed] [Google Scholar]
- 10. Han KM, Ko YH, Shin C, et al. Tinnitus, depression, and suicidal ideation in adults: A nationally representative general population sample. J Psychiatr Res. 2018;98:124‐132. [DOI] [PubMed] [Google Scholar]
- 11. Park M, Kang SH, Nari F, Park EC, Jang SI. Association between tinnitus and depressive symptoms in the south Korean population. PloS One. 2021;16(12):e0261257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams AP, Gourishetti SC, Flaherty MA, Eisenman DJ. Anxiety, depression, and symptom severity in patients with pulsatile and non‐pulsatile tinnitus. Laryngoscope. 2022;133:683‐688. [DOI] [PubMed] [Google Scholar]
- 13. Boecking B, Biehl R, Brueggemann P, Mazurek B. Health‐related quality of life, depressive symptoms, anxiety, and somatization symptoms in male and female patients with chronic tinnitus. J Clin Med. 2021;10(13):2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loprinzi PD, Maskalick S, Brown K, Gilham B. Association between depression and tinnitus in a nationally representative sample of US older adults. Aging Ment Health. 2013;17(6):714‐717. [DOI] [PubMed] [Google Scholar]
- 15. Diep C, Bhat V, Wijeysundera DN, Clarke HA, Ladha KS. The association between recent cannabis use and suicidal ideation in adults: A population‐based analysis of the NHANES from 2005 to 2018. Can J Psychiatry. 2022;67(4):259‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng YF, Xirasagar S, Kuo NW, Lin HC. Tinnitus and risk of attempted suicide: A one year follow‐up study. J Affect Disord. 2023;322:141‐145. [DOI] [PubMed] [Google Scholar]
- 17. Salazar JW, Meisel K, Smith ER, Quiggle A, McCoy DB, Amans MR. Depression in patients with tinnitus: A systematic review. Otolaryngol Head Neck Surg. 2019;161(1):28‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CE, Chen LF, Chou PH, Chung CH. Increased prevalence and risk of anxiety disorders in adults with tinnitus: A population‐based study in Taiwan. Gen Hosp Psychiatry. 2018;50:131‐136. [DOI] [PubMed] [Google Scholar]
- 19. Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017;4(2):146‐158. [DOI] [PubMed] [Google Scholar]
- 20. Li YL, Hsu YC, Lin CY, Wu JL. Sleep disturbance and psychological distress in adult patients with tinnitus. J Formos Med Assoc. 2022;121(5):995‐1002. [DOI] [PubMed] [Google Scholar]
- 21. Gu H, Kong W, Yin H, et al. Prevalence of sleep impairment in patients with tinnitus: a systematic review and single‐arm meta‐analysis. Eur Arch Otorhinolaryngol. 2022;279(5):2211‐2221. [DOI] [PubMed] [Google Scholar]
- 22. Ooms E, Meganck R, Vanheule S, Vinck B, Watelet JB, Dhooge I. Tinnitus severity and the relation to depressive symptoms: a critical study. Otolaryngol Head Neck Surg. 2011;145(2):276‐281. [DOI] [PubMed] [Google Scholar]
- 23. Trevis KJ, Mclachlan NM, Wilson SJ. Psychological mediators of chronic tinnitus: the critical role of depression. J Affect Disord. 2016;204:234‐240. [DOI] [PubMed] [Google Scholar]
- 24. Trevis KJ, Mclachlan NM, Wilson SJ. A systematic review and meta‐analysis of psychological functioning in chronic tinnitus. Clin Psychol Rev. 2018;60:62‐86. [DOI] [PubMed] [Google Scholar]
- 25. Han BI, Lee HW, Kim TY, Lim JS, Shin KS. Tinnitus: characteristics, causes, mechanisms, and treatments. J Clin Neurol. 2009;5(1):11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boecking B, Von Sass J, Sieveking A, et al. Tinnitus‐related distress and pain perceptions in patients with chronic tinnitus—do psychological factors constitute a link? PloS One. 2020;15(6):e0234807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vanneste S, Plazier M, Der Loo E, et al. The neural correlates of tinnitus‐related distress. Neuroimage. 2010;52(2):470‐480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the tinnitus population included in the study.
Table S2. Depression in tinnitus and non‐tinnitus populations.


