Abstract
Objective
Despite 6%–20% of the adult population suffering from tinnitus, there is no standard treatment for it. Placenta extract has been used for various therapeutic purposes, including hearing loss. Here, we evaluate the effect of a novel neuroprotective protein composition (NPPC) extract on electrophysiological and molecular changes in the medial geniculate body (MGB) of tinnitus‐induced rats.
Methods
To evaluate the protein analysis by western blot, the rats were divided into three groups: (1) saline group (intraperitoneal injection of 200 mg/kg saline twice a day for 28 consecutive days, (2) chronic Na‐Sal group received sodium salicylate as in the first group, and (3) chronic treatment group (received salicylate 200 mg/kg twice daily for 2 weeks, followed by 0.4 mg NPPC daily from day 14 to day 28). Single‐unit recordings were performed on a separate group that was treated as in group 4. Gap‐prepulse inhibition of the acoustic startle (GPIAS) and pre‐pulse inhibition (PPI) was performed to confirm tinnitus in all groups at the baseline, 14th and 28th days.
Results
Western blot analysis showed that the expression of γ‐Aminobutyric acid Aα1 subunit (GABA Aα1), N‐methyl‐d‐aspartate receptor subtype 2B (NR2B or NMDAR2B), α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionic acid receptors subunit GluR1 (GluR1), and α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole propionic acid receptors subunit GluR2 (GluR2) decreased after Na‐Sal injection, while NPPC upregulated their expression. MGB units in rats with tinnitus showed decreased spontaneous firing rate, burst per minute, and a spike in a burst. After NPPC administration, neural activity patterns showed a significant positive effect of NPPC on tinnitus.
Conclusion
NPPC can play an effective role in the treatment of tinnitus in salicylate‐induced rats, and MGB is one of the brain areas involved in these processes.
Level of Evidence
NA.
Keywords: placenta extract, single unit recording, sodium salicylate, startle reflex, tinnitus
This study evaluated the effect of a novel neuroprotective protein compound (NPPC) on electrophysiological and molecular changes in the medial geniculate body (MGB) of tinnitus‐induced rats. The results showed NPPC can play an effective role in the treatment of tinnitus.
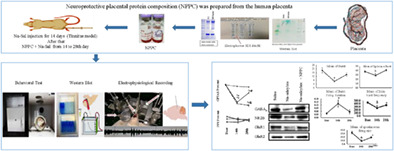
1. INTRODUCTION
Tinnitus is a phantom sound perception of an unorganized acoustical sensation without an external sound origin. It has been shown that 6%–20% of the adult population suffers from chronic tinnitus, which can affect a person's quality of life. 1 Since tinnitus is a heterogeneous problem with various factors affecting it, it is still without standard treatment, and its pathophysiology is in a haze of ambiguity. Sodium salicylate (Na‐Sal) is known as the main metabolite of aspirin. It is well‐established to cause tinnitus and sensorineural hearing loss, 2 when administered in high doses in humans 3 , 4 and animals. 5 , 6 The gap pre‐pulse inhibition of the acoustic startle (GPIAS) paradigm has been commonly validated by researchers to confirm behavioral evidence of tinnitus in laboratory rodents and has been used in various studies. 7 GPIAS consists of two phases, with and without a silent gap presented before the start of a pulse, under the background noise environment. In normal conditions, a silent gap inhibits the startle reflex, whereas in animals with tinnitus, the lack of inhibition of silent gaps indicates the presence of tinnitus due to the filling of the gaps with the tinnitus signal. 7 In vitro, studies have shown that Na‐Sal reduced the inhibitory postsynaptic current in neurons of the rat auditory cortex (AC) and specifically decreased the current‐evoked firing of gamma‐aminobutyric acid (GABAergic) interneurons without affecting glutamatergic pyramidal neurons. Na‐Sal also could increase the excitability of hippocampus neurons in rats by reducing GABAergic synaptic transmission inhibition but does not affect intrinsic membrane excitability in cortical area 1 (CA1) pyramidal neurons. 2 Some other previous studies have shown that Na‐Sal significantly reduces GABAergic inhibition mainly through suppressing GABAA receptor‐mediated responses and enhancing the expression and function of N‐methyl‐d‐aspartate (NMDA) receptors, which leads to increased neuronal excitation. 2 , 8 These studies show that excitatory‐inhibitory balance was disrupted by sodium salicylate consumption. The medial geniculate body (MGB), part of the auditory thalamus, is considered to have a processing role in central auditory perception. 9 A limited number of studies have been available to identify the effects of Na‐Sal on the MGB, and most of them have been limited to the cellular level. Therefore, the present study was designed to electrophysiologically investigate the MGB neural activity followed by Na‐Sal administration in the rat model. Yan‐Yan Su and colleagues recorded MGB neuronal activity by whole‐cell patch clamp, which showed that Na‐Sal (1.4 mM) changed the action potential, reduced membrane input resistance, suppressed current‐evoked firing, hyperpolarized the resting membrane potential, and altered rebound depolarization in MGB neurons. They suggest a possible role of the MGB in Na‐Sal‐induced tinnitus. 9 In another study, Basta and colleagues (2008) showed that the spontaneous firing rate after the local application of Na‐Sal increased. The change in firing rate was correlated with Na‐Sal concentration, which at higher doses could produce a greater percent increase in neuronal firing rate. 10 Animal and human placenta extract (HPE) have been used in traditional medicine for various therapeutic purposes including wound healing, cell proliferation, tissue regeneration, anti‐inflammatory, and antimicrobial properties, pain relief, hair growth, regeneration of dental pulp cells, health improvement, etc. 11 , 12 , 13 , 14 Therefore, several benefits of placenta extracts are due to their rich composition of bioactive compounds like a variety of amino acids, peptides, proteins, vitamins, growth factors, nutrients, and biological active ingredients such as enzymes, glycoproteins, lipids, minerals, steroid hormones, etc. 11 , 12 , 13 , 14 A porcine placental extract‐derived composition of unknown proteins, initially named X‐proteins (XP), was introduced by Alexander Anikin. He successfully managed the purification and preparation of principally new biologically active factors with prominent neuroprotective properties. 15 Poletaev et al. reported evident positive effects of X‐proteins (XP) in patients with brain ischemia associated with severe and steady‐state neurological deficits. Daily XP administration through 12–14 days leads to a decrease in motor, mental, and speech impairments and normalization of emotional state. These authors concluded that XP may be an inductor of regenerative/reparative processes in reversible damaged nervous tissue. 15
In the current study, the same extraction protocol of X‐proteins was used on the human placenta, named human neuroprotective protein composition (NPPC).
Several studies have suggested potential mechanisms of tinnitus and related pathological changes associated with Na‐Sal administration. According to reported studies, chronic tinnitus induced by Na‐Sal could be related to extensive changes in the auditory pathways at electrophysiological, histological, and molecular levels. 16 , 17 To our knowledge of the potential efficacy of HPE mentioned earlier, this is the first study postulating that NPPC may ameliorate pathological tinnitus changes induced by Na‐Sal. This study aimed to investigate whether Na‐Sal can alter the neural discharge properties of MGB neurons, the levels of GABAARα1, NR2B subunits, mGluR1, and mGluR2 subunits, and also whether NPPC can ameliorate those pathological changes in the MGB level.
2. MATERIALS AND METHODS
2.1. Animals
In this study, 29 male Wistar rats weighing 250–350 g were used. We housed two to three rats per cage under standard conditions: 12 h of light/dark cycles (lights were on from 7:00 AM) access to standard food and water ad libitum. Room temperature and humidity were monitored and kept in the standard range of 21–22°C and 50%–55%, respectively. All interventions are done according to animal rights and the National Institutes of Health guide regarding the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). In addition, the study was approved by the Ethical Committee of Research Vice‐Chancellor, Iran University of Medical Science (Ethical approval number: IR.IUMS.REC.1398.233).
2.2. Groups and design
Rats were divided into three groups. A chronic Na‐Sal induced group, a chronic treatment group, and a saline group as a sham‐control group. The chronic Na‐Sal induced group (7 rats) received twice‐daily intraperitoneal injections of sodium salicylate (Sigma–Aldrich, Shanghai, China) (200 mg/kg) at 08:00 AM and 4 PM for 28 consecutive days. The chronic treatment group (7 rats) received sodium salicylate. Just like the previous group except that for 14 consecutive days and then in addition to sodium salicylate, they also received a single dosage of NPPC (0.4 mg/kg) intraperitoneally in the second 14 consecutive days. Whereas the saline group (7 rats) received IP injections of saline solution (200 mg/kg) at the same time points. All three groups underwent the GPIAS and PPI test (for evaluation of hearing function and confirmation of tinnitus), on the first day (baseline), on the 14th day (after modeling), and finally on the 28th day (after treatment). In the end, samples were extracted for protein expression analysis. A separate group of 8 rats was considered for the single‐unit recording. On the baseline, on the 14th (after 2 weeks of sodium salicylate injection and tinnitus model confirmation) and 28th day (after 2 weeks of NPPC injection), the single‐unit recordings were done (Figure 1).
FIGURE 1.
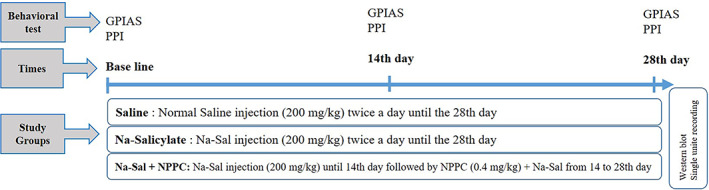
Schematic diagram of the study's method.
2.3. Preparation of neuroprotective placental protein composition (NPPC)
The human placenta for NPPC preparation was procured from SinaCell Company, officially certified by the Food and Drug Agency of Iran for the ethical distribution of donated human placenta and placental derivatives for medical and research use. The non‐hydrolyzed protein extraction and NPPC preparation from the human placenta was performed according to the methodology described by Poletaev et al. 15 with minor modifications, including the replacement of DE‐52 cellulose resin with DEAE‐Sepharose Fast Flow resin (Cytiva, USA) without further size‐based fractionation of the obtained protein composition. The final total protein concentration (measured by a QuBit 3.0 fluorometer instrument, Life Technologies, USA) and pH were set at ~1 mg/mL and ~7.0, respectively.
2.4. Startle responses system
GPIAS and PPI tests were administered to investigate tinnitus behavior by using the Acoustic Startle Reflex Starter Package system for mice or rats (SR‐LAB System—San Diego Instruments, San Diego, CA). The system consists of an amplifier, a sound‐attenuating chamber, two speakers, which were placed on the ceiling of the acoustic chamber, a stimulus generation panel with a platform table, and a force and pressure sensors with the piezoelectric load cell. The rats were placed in an acrylic holder that was installed inside the sound‐attenuating chamber. The panel stimuli consisted of white broadband noises (BBN) presented at the sound pressure level (SPL) of 60 dB and an acoustic startle, by a 20‐ms burst of white noise at an SPL of 115 dB, produced by loudspeakers. The startle response amplitude (peak‐to‐peak values) was amplified and then digitized by a computer to follow the corresponding analysis using SR‐LAB software (SR‐LAB System—San Diego Instruments, San Diego, CA) connected to a PC. Each GPIAS session consisted of 12 trials with a gap and 12 trials without a gap, which were performed in 15 separate measurements. The duration of the gap lasted 50 ms (1 ms rise/fall time) which was generated at the level of 100 ms prior to the startle stimulus. The interval between each startling stimulus was 15–20 s, and each test lasted almost 15 min. 18 In the PPI session, 15 pulses, 10 prepulses and 8 no‐stimulus trials were performed. The startle stimulus was randomly presented in two pulse‐alone conditions and when combined with prepulse stimuli (60 dB SPL broadband noise, 50 ms duration, 1 ms rise/fall time, and 100 ms preceded before startle stimulus). The PPI test was done after the GPIAS test and lasted 15 min. 18 A schematic picture of the GPIAS and PPI paradigms is shown in Figure 2. The GPIAS results were calculated by accounting for the average ratio of the gap trials versus no‐gap trials according to the below formula: [(AvgTnogap − AvgTgap)/AvgTnogap × 100%], (AvgTgap = the average amplitude during the gap trials and AvgTnogap = the average amplitude of the no‐gap trials). For the calculation of PPI percent, the average ratio of the startle responses versus pre‐pulse trials was obtained for each SPL using the below formula:
(AvgTpre‐pulse = the average amplitude during the pre‐pulse trials, and AvgTstartle = the average amplitude of the startle trials). 18 , 19
FIGURE 2.
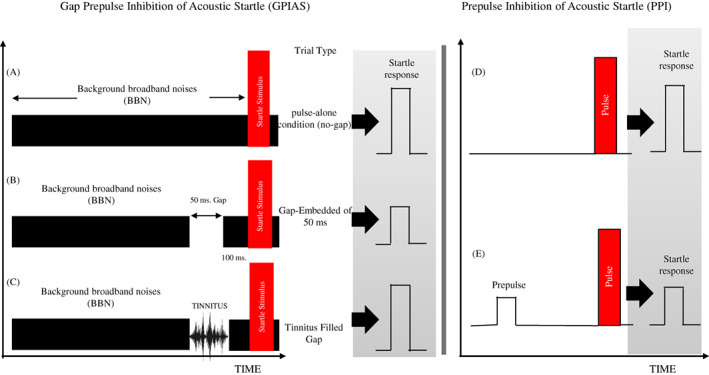
Schematic picture of the GPIAS and PPI paradigms is shown to illustrate the stimulus conditions and presumption results for the three conditions no‐gap, gap, and tinnitus.
2.5. Western blotting
In this project, the results of the expression of inhibitory and excitatory proteins of the MGB in the three groups were evaluated. The implementation of this laboratory method consisted of three main steps that were performed: first, transfer to gel electrophoresis, second to transfer to polyvinylidene difluoride (PVDF) membrane, and third, identification of specific proteins. For the immunoblot test, fresh MGB tissue was first removed from the rats' brains in the shortest possible time and homogenized. Homogenization was performed in a cold buffer and on ice. The homogenized tissue was centrifuged (14,000 rpm at 4°C for 15 min). The Brad Ford method was used to measure the total protein concentration in the relevant tissue samples. 25 μg of each sample was used for Western blotting. Tissue samples were loaded and electrophoresed in 12% SDS‐PAGE gel. After electrophoresis, the proteins were separated in the gel and then transferred to PVDF paper. The next step was the blocking process. Then, the primary antibody–antigen complex was kept overnight at 4°C. Finally, after washing, the secondary antibody–antigen complex appeared on the radiographic film using the Enhanced Chemiluminescence (ECL) system with the emergence and fixation solutions, and after digitizing the images, the density of the bands was measured by the Lab works software. 20 Western blot experiments were performed using MGB tissues to evaluate the effect of Na‐Sal and NPPC on the level of GABA Aα1, NR2B, GluR1 and GluR2 subunits in this auditory structure. Bands of immunoblots for all subunits were normalized against β‐actin.
2.6. Acoustic stimuli and single unit recording
Rats were anesthetized with chloral hydrate. The animal was then placed in a stereotaxic machine and the tungsten‐coated metal electrode was placed, in the area of the auditory thalamus based on the Paxinos atlas. 21 The acoustic stimuli were delivered by a calibrated loudspeaker (DT48, Beyer Dynamic, Heilbronn, Germany) via a plastic cone located in the outer ear canal by using an Audiology Lab system (Otoconsult, Frankfurt a. M., Germany). Initially sound signals to identify the Characteristic Frequency (CF) of neuronal units of the auditory thalamic nucleus with the following characteristics: tone‐burst stimulation, duration of 50 ms, 4 ms rise/fall time, frequency steps (1–42 kHz), and intensities (0–90 dB) with 10 dB SPL steps were used. Immediately after recording the response map (Figure 3), it was analyzed using a custom‐written MATLAB program (The MathWorks Inc. United States) 22 to extract the CF. After determining the CF, sound stimuli (based on the CF) were presented for 1 h through a microphone placed in the left ear canal, and the single unit signal was recorded through the recording electrode placed in the right (Figure 4). It should be mentioned that the opposite ear was plugged. The results of the processes performed based on the codes written in the results section are reported separately.
FIGURE 3.
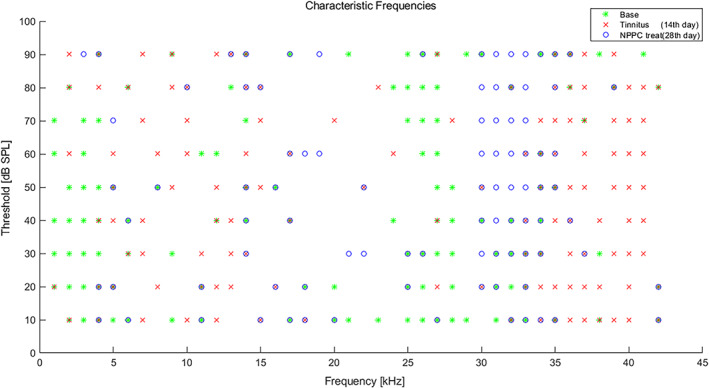
Characteristic frequencies (CFs) and MGB unit thresholds at baseline, 14th and 28th day: scatter plot of individual MGB unit thresholds at their CFs from the base (green) and 14th (red) and 28th days (blue) rats. The average threshold at CFs of MGB unit recording in three situations (Base, 14th and 28th days) did not exhibit any significant difference (p > .05).
FIGURE 4.
An example of a rat in the stereotaxic system with electrodes and a microphone. The arrows indicate the electrode and insertion phone place.
2.7. Preprocessing and extracting the spikes and bursts
Extracting the spikes and bursts from the single unit signal requires a preprocessing procedure. The single unit data first was imported into MATLAB (The MathWorks Inc., USA) 22 and filtered using a zero‐phase bandpass Finite Impulse Response (FIR) filter between 300 and 6000 Hz frequencies. Then, a thresholding method developed specifically for the spikes differentiated them from the background noise. 23 To extract bursts from the detected spikes, we utilized a definition of burst and the autonomous approach presented by Chen et al. 24 This method groups Inter‐Spike Intervals (ISIs) into bursts by employing an iterative algorithm. In particular, we defined and calculated spikes in a burst as the number of spikes within each burst, spontaneous firing rate as the rate of spikes per second prior to the onset of the first stimulation, burst firing duration as the time length of each burst, burst firing/minute as the number of bursts per minute, and intraburst frequency as the number of spikes per second within each burst. 25
2.8. Statistical analysis
The sample size was calculated using the “equation of resources” approach. 26 GPIAS, PPI, and Single unit recording data were calculated using a Repeated Measures ANOVA analysis of variance to determine main effect interactions among experimental groups. Protein expression data, between experimental groups and control, were determined by one‐way ANOVA, followed by Bonferroni. p < .05 was considered statistically significant. All results were presented as mean ± SEM (Standard Error of the Mean) and were performed using SPSS version 16 software (IBM‐SPSS, Inc., Chicago, IL, USA).
3. RESULTS
3.1. The effect of Na‐Sal and NPPC on tinnitus‐like behaviors in rats
Salicylate‐induced tinnitus was measured using the GPIAS test (n = 7 rats). A PPI experiment was performed in parallel with the GPIAS experiment to control that the GPIAS deficits were not due to auditory deficits and that rats could hear background and prepulse noise. Animals that fail the GPIAS test but pass the PPI test are thought to have tinnitus. 27 There were no significant differences in the PPI values among the three groups: Saline, Na‐Sal, and, NPPC, as well as within each group among the three conditions: baseline, 14th day, and 28th day. It indicated that these animals had normal hearing in all of the groups and conditions (Figure 5A). Rats in the Na‐Sal group which received sodium salicylate for 28 days, showed a significant decrease in GPIAS values from the first day to the 14th day gradually, compared to the saline group and baseline condition (p ≤ .001, p ≤ .001), this reduction continued from the 14th day to the 28th day, compared to the saline group and baseline condition (p ≤ .001, p ≤ .002, Figure 5B). Rats in the NPPC group that received sodium salicylate for 14 days showed a significant decrease in GPIAS values between the first day to the 14th day compared to the saline group and baseline condition (p ≤ .000, p ≤ .000, Figure 5B). However, treatment with sodium salicylate and NPPC between the 14th day to the 28th day significantly increased GPIAS values compared to the Na‐Sal group (p ≤ .000, Figure 5B).
FIGURE 5.
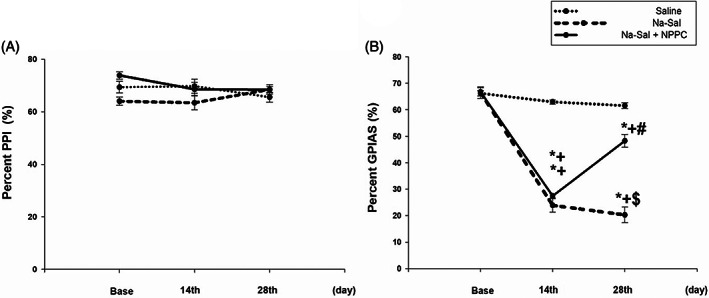
PPI and GPIAS test at baseline and after Na‐Sal and NPPC administration. (A) Chronic effects of Na‐Sal and NPPC on pre‐pulse inhibition (PPI) values as a criterion for the hearing evaluation. The animal in the Na‐Sal group received only Na‐Sal (200 mg/kg) in all over 28 days. While the rats in the treatment group received Na‐Sal in the first 14 days, and Na‐Sal (200 mg/kg) and also NPPC (0.4 mg/kg) in the second 14 days. There were no significant differences in the PPI values neither among the three groups (Saline, Na‐Sal and treatment) nor intra groups (baseline, 14 days and 28 days) (n = 7, p < .05). (B) Chronic effects of Na‐Sal and NPPC on Gap pre pulse inhibition of acoustic startle (GPIAS) values as a criterion for tinnitus confirmation. Saline injection had no effect on GPIAS performance (n = 7, p < .05). In contrast, injection of Na‐Sal at a dose of 200 mg/kg caused a significant reduction in GPIAS performance on both the 14th day and the 28th day in the Na‐Sal group, compared with the baseline and the two comparison groups (n = 7, p < .05). The same matter happened in the treatment group on the 14th day. While the injection of Na‐Sal (200 mg/kg) with NPPC at a dose of 0.4 mg/kg caused a significant increase in GPIAS performance on the 28th day (n = 7, p < .05). Data are the mean ± SEM (two‐way repeated measures analysis of variance‐post hoc Tukey's test).
It is indicated that animals in both Na‐Sal and NPPC groups, were experiencing Na‐Sal‐induced tinnitus on the 14th day, and animals in the NPPC group improved in their perception of Na‐Sal‐induced tinnitus gradually between the 14th day to the 28th day. It is noteworthy there were no significant differences in GPIAS values between baseline conditions in the three groups and between various conditions in the saline group (Figure 5B).
3.2. The effect of Na‐Sal and NPPC on overall levels of GABA Aα1, NR2B, GluR,1 and GluR2 expression in MGB
The effect of Na‐Sal and NPPC treatment on the inhibitory and excitatory system was investigated by Western blot of GABA Aα1, NR2B, GluR1, and GluR2 which are respectively, a major inhibitory and most important excitatory neurotransmitter in the central auditory pathway. GABA Aα1 protein expression significantly decreased in the Na‐Sal group in comparison to the saline (p ≤ .001) and increased in the Na‐Sal+PPC group vs. Na‐Sal group (p = .001, respectively) (Figure 6A). The statistical analysis showed neuroactivity that the NR2B, GluR1, and GluR2 proteins expression levels in the Na‐Sal group were decreased vs. saline groups (p ≤ .001, p ≤ .01, p ≤ .01, respectively), while the overexpression of proteins was observed in Na‐Sal+NPPC group compared to Na‐Sal group (p ≤ .001, p ≤ .05, p ≤ .05, Figure 6B–D). Taken together, the results indicated that Na‐Sal reduces both excitatory and inhibitory responses mostly through its actions on GABAA and NMDA receptors in the MGB, and NPPC increases excitability and inhibitory responses.
FIGURE 6.
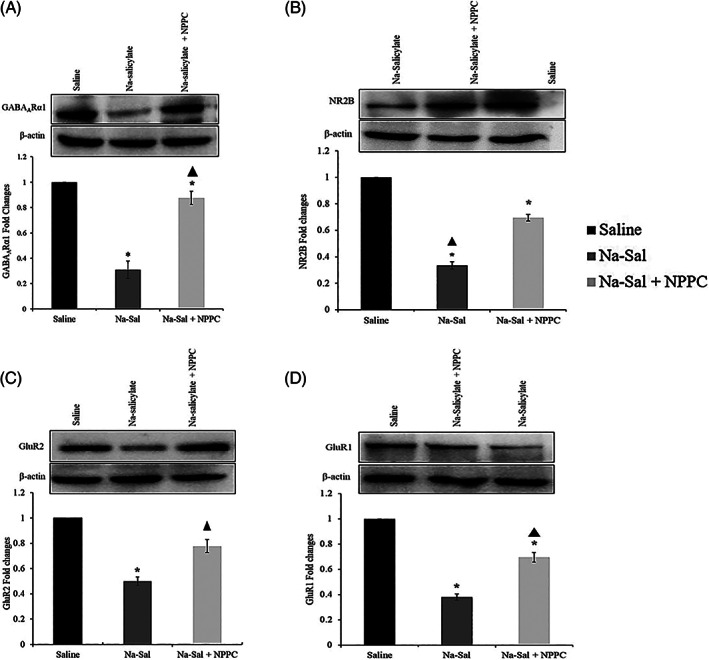
Western blots' analysis of inhibitory and excitatory subunits in Medial Geniculate Body (MGB) tissue. (A) Western blots' analysis of GABAARα1 subunit in MGB tissue immunoreactivity against the GABAARα1 subunit in Medial Geniculate Body (MGB) tissue from the saline group (Control), Na‐Sal group (Tinnitus) and the treatment group; the plot showing immunoreactivity against the GABAARα1 subunit in the MGB. It can be observed that the level of the GABAARa1 in MGB tissue in the Na‐Sal group significantly decreased, while it increased in the treatment group and the control group. (B) Immunoreactivity against the NR2B subunit in MGB tissue from the saline group (Control), Na‐Sal group (Tinnitus) and the treatment group; the plot showing immunoreactivity against the NR2B subunit in the MGB. It can be observed that the level of the NR2B subunit in MGB tissue decreased in the Na‐Sal group, while it increased in the treatment group, compared with the control group. (C) Immunoreactivity against the GluR2 subunit in MGB tissue from the saline group (Control), Na‐Sal group (Tinnitus) and the treatment group; the plot showing immunoreactivity against the GluR1 subunit in the MGB. It can be observed that the level of the GluR2 subunit in MGB tissue decreased in the NA‐Sal group, while it increased in the treatment group, compared with the control group. (D) Immunoreactivity against the GluR1 subunit in MGB tissue from the saline group (Control), Na‐Sal group (Tinnitus) and the treatment group; the plot showing immunoreactivity against the GluR1 subunit in the MGB. It can be observed that the level of the GluR1 subunit in MGB tissue decreased in the Na‐Sal group, while it increased in the treatment group, compared with the control group. Data are the mean ± SEM (one‐way repeated measures analysis of variance‐post hoc Tukey's test).
3.3. The effect of Na‐Sal and NPPC on the neuroactivity in the MGB structure
Figure 3 shows the response map of the individual MGBs at their CFs of animals exposed to noise in the three situations before the intervention (baseline), after the intervention (day 14) and after the administration of NPPC (day 28). There was no significant difference between the mean threshold of MGB unit recorded from the base (29.14 ± 2.1 dB), 14th (33.00 ± 2.3 dB), and 28th days (30.85 ± 2.8 dB), (p > .05).
Comparison of MGB unit burst recording between the base (baseline condition) and 14th day (salicylate‐induced animals) revealed a significant tinnitus‐related decrease in (1) mean bursts per minute (18.37 ± 2.70, base vs. 6.86 ± 1.44, 14th day; p ≤ .002, Figure 7B); (2) mean spikes in a burst (3.70 ± 0.26, base vs. 2.84 ± 0.20, 14th day; p ≤ .037; Figure 7C); (3) and intraburst frequency (438 ± 22.70 s, control base, vs. 263.60 ± 19.51, 14th day; p = .001, Figure 7E). However, the mean duration of the burst (0.04 ± 0.01 s, control, vs. 0.134 ± 0.035 s, tinnitus; p ≤ .052; Figure 7D) was increased. In addition, on the 28th day, after treatment with NPPC showed an increase in the mean bursts (14.039 ± 2.03, p ≤ .126), spikes in a burst (4.08 ± 0.25, p ≤ .005), and intraburst frequency (356.11 ± 24.4, p = .055) compared to day 14. Also, the mean duration of the burst (0.056 ± 0.020, p = .342) after adding NPPC was reduced. The results suggest that NPPC may be effective in modulating MGN neuronal activity after the administration of sodium salicylate. The mean spontaneous firing rate (SFR) recorded from the base condition of animals (32.2 ± 3) was significantly decreased on the 14th day (8.68 ± 0.92, p ≤ .001). While after NPPC administration (on the 28th day), the mean firing rate (18.98 ± 2.03, p ≤ .001) showed a significant increase (Figure 7F).
FIGURE 7.
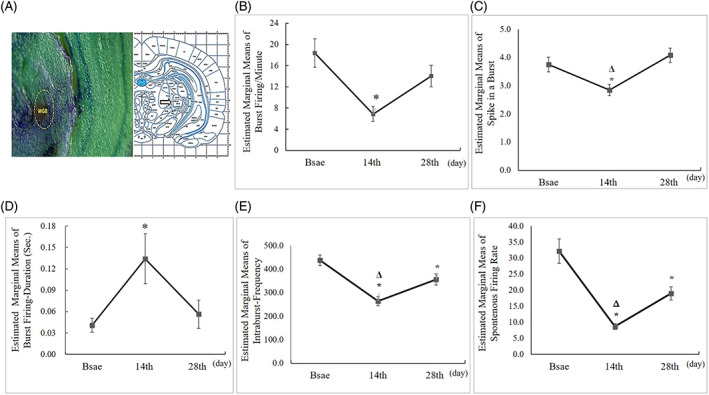
Chronic effects of Na‐Sal and NPPC on MGB unit recordings. (A) photomicrograph shows histological verification of recording site (MGB) and the schematic equivalent is taken from the atlas of Paxinos, 6th Edn. Amsterdam, published online, 2007. (B, E, F) The analysis of MGB Units recordings revealed that there was a significant decrease in the mean number of Burst firing‐per‐minute, Intra‐Burst‐Frequency and Firing Rate on the 14th day condition compared to the baseline and 28th day, whereas there was no significant difference between the baseline and 28th day (one‐way analysis of variance). (C) But there was no significant difference in the mean number of the spike in a burst between every three conditions (one‐way analysis of variance). (D) In addition, there was a significant increase in the mean of burst duration in seconds on the 14th‐day condition compared to baseline and the 28th day, whereas there was no significant difference between baseline and the 28th day (one‐way analysis of variance). The star indicates the significant difference value between the baseline and the 14th day, and the triangle indicates the significant difference value between the 14th day and the 28th day (p < .05).
4. DISCUSSION
For the first time, this study investigated the effect of a placenta extract (NPPC) on sodium salicylate‐induced changes in GABAA and NMDA receptor expression and its evoked electrical activities in MGB neurons in rats. Our results showed that Na‐Sal administration could induce tinnitus behaviors and decrease the level of GABAA and NMDA receptor subunits such as GABAAα1, NR2B, GluR1, and GluR2 as well as some parameters of neuronal activity, including bursts per minute, spikes per burst, burst frequency, and firing rate in rat MGB neurons. Studies have shown that Na‐Sal can pass the blood–brain barrier and reach the cerebrospinal fluid in animals treated with high doses of Na‐Sal. 28 As a result, when Na‐Sal is applied systemically, it not only acts on the cochlea but can also directly affect the several regions of the brain within and outside the central auditory system, including the MGB, to produce tinnitus. 5 , 9 Our findings showed NPPC might treat Na‐Sal‐induced ototoxic effects and reverse its effects. In the present study, a high dose of Na‐Sal (400 mg/kg/day) reliably induced tinnitus‐like behavior in Wistar rats, as observed in the behavioral data analysis. The data demonstrated that Na‐Sal inhibited startle responses in the GPIAS parameter, while the PPI parameter remained unaffected (Figure 5). These findings align with previous studies indicating that long‐term administration of salicylate at a dosage of 400 mg/kg can induce tinnitus in rats. 29 In experimental tinnitus, inflammation is believed to play a role in the underlying pathophysiology. 30 Two inflammatory cytokines, TNF‐α and IL‐1, are elevated throughout the auditory pathway in animal models of tinnitus. 31 , 32 , 33 , 34 These cytokines have been shown to impact both inhibitory and excitatory neurotransmission. TNF‐α, for instance, affects inhibitory GABA receptors by reducing their strength through the endocytosis of GABA receptors, resulting in a decrease in surface GABA receptors. 35 IL‐1β has been observed to suppress GABA‐induced currents. 36 TNF‐α and IL‐1β also influence excitatory neurotransmission. The precise effect of TNF‐α on the excitatory NMDA receptor is still a topic of debate, as both decreased and increased NMDA receptor currents have been reported. 36 , 37 , 38 , 39 Our findings demonstrate that Na‐Sal decreases the expression of NMDA and GABA receptor subunits. Conversely, NPPC (human placental extract) increases the expression of excitatory and inhibitory receptor subunits and modifies the effects of salicylate on them. Our findings align with a study conducted by Yan‐Yan Su et al., where they demonstrated that Na‐Sal decreased both excitatory and inhibitory postsynaptic responses in a salicylate‐induced tinnitus model using whole‐cell patch‐clamp recordings in MGB slices. 9 Human placenta extract (HPE) possesses various beneficial properties, including anti‐inflammatory, analgesic, antioxidant, cellular and radioactive protection, anti‐allergic, repair, and proliferation stimulation effects. 12 , 40 , 41 , 42 Notably, HPE has been demonstrated to have anti‐inflammatory effects. A study conducted by Lee et al. (2010) revealed that HPE significantly inhibited inflammatory factors such as TNF‐α, cyclooxygenase‐2, and nitric oxide in cellular and animal models of inflammation. 43 Therefore, in our study, it is plausible that Na‐Sal‐induced inflammatory factors contributed to the decreased expression of NMDA and GABA receptor subunits. Additionally, NPPC (human placental extract) could reverse the molecular changes induced by Na‐Sal due to its anti‐inflammatory properties. Further investigations are warranted to explore the relationship between the anti‐inflammatory effects of HPE and the suppression of tinnitus, providing more insights into the role of HPE in tinnitus alleviation. The current study demonstrated that NPPC could reverse and enhance the alterations induced by Na‐Sal in various electrophysiological parameters, including bursts per minute, spikes per burst, burst duration per second, burst frequency, and firing rate in MGB units.
In this regard, Yan‐Yan Su and colleagues (2012) employed whole‐cell patch‐clamp recordings in MGB slices to investigate the effects of Na‐Sal (1.4 mM) on action potentials and current‐evoked firing in MGB neurons. 9 They observed that Na‐Sal modulated action potentials and suppressed current‐evoked firing in MGB neurons. Following salicylate administration, both excitatory and inhibitory currents decreased in the MGB. Although Na‐Sal reduces MGB excitability, it induces tinnitus‐like behavior in rats. The reduction in MGB excitability results in decreased thalamic inputs to the auditory cortex. As these inputs preferentially activate inhibitory neurons in the cerebral cortex rather than excitatory neurons, 44 , 45 , 46 , 47 the reduction in these inputs leads to increased excitatory activity in the auditory cortex. In other words, the fluctuation in the input caused by Na‐Sal may alter the balance between inhibition and excitation, shifting it toward the excitatory side and ultimately increasing the excitability in auditory cortical neural networks. Besides, using the single‐unit recording method, Kalappa et al. (2014) investigated the activity of MGB neurons in an animal model of sound‐induced tinnitus in awake rats. They reported an increase in the spontaneous firing rate of neurons and an increase in the slope of the activity level graph of neurons in the MGB region. 48 Their results contradicted the findings of our study. The difference between the results of these two studies can be linked to several points. (1) The recording method, in the present study, the neural activities caused by acoustical stimulation were recorded, while in the study of Kalappa et al (2014), those responses caused by the baseline neuronal stimulation and or the spontaneous activities that are not driven by an external stimulus were reported. (2) Different methods of inducing tinnitus in animal models, in the current study, the salicylate‐induced tinnitus model was used to investigate the neural activities, while in the report of the Kalappa study, the results of the experimental model of sound‐induced tinnitus were analyzed. (3) In the present study, single units were recorded from anesthetized rats but not from awake rats as in Kalappa's study. (4) Another reason for the difference between the current study's results and Kapala's maybe because of the presence of a variety of neuronal types in the MGB and the complexity of determining the exact role of specific neurons in causing tinnitus. Also, we found that salicylate‐induced changes in various electrophysiological characteristics were modified by NPPC administration. The study of Girotto and Malinverni is in agreement with the results of the present study and shows that placenta extract can increase electrophysiological activities. 49 However, despite the above results, how the changes caused by salicylate induced can be recovered by NPPC is not clear precisely and needs further studies. In addition, given the essential role of the MGB in tinnitus, 50 the effect of NPPC on the MGB was investigated in this study. The effect of NPPC on other parts of the auditory pathway requires further investigation.
5. CONCLUSIONS
The present study shows that neuroprotective protein composition (NPPC) extract may modulate molecular and electrophysiological changes associated with salicylate‐induced tinnitus. Salicylate reduces the expression of excitatory and inhibitory receptor subunits. It alters neuroelectrical activity such as spontaneous firing rate, burst per minute, and the spike in a burst in the medial geniculate nucleus of tinnitus rats. However, the application of NPPC increases the expression of NMDA and GABA receptor subunits, leading to positive therapeutic effects on neural activity. Further research is required to uncover the mechanism underlying this promising therapeutic approach.
FUNDING INFORMATION
This study was financially supported by the Iran National Science Foundation (INSF, Synergy Grant Code no. insf‐98020383‐1400/03/23 and 95830795), and ENT and Head and Neck Research Center, Hazrate Rasoul Akram Hospital, the Five Senses Institute, School of Medicine, Iran University of Medical Sciences, Tehran, Iran (Ethical approval no. IR.IUMS.REC.1398.233).
CONFLICT OF INTEREST STATEMENT
The authors have no financial relationships or conflicts of interest to disclose.
ACKNOWLEDGMENTS
The generous support of the Iran National Science Foundation (INSF), as well as ENT and Head & Neck Research Center, Iran University of Medical Sciences to assign credit to the research line auditory neuroscience, is gratefully acknowledged. Also, all authors would like to appreciate the late Professor Alexander Borisovich Poletaev (*06 November 1951—† March 6th, 2021). He was the first to report the evident positive effects of the protein fraction of porcine placental extract introduced by Alexander Anikin, in terms of having prominent neuroprotective properties, initially named X‐proteins (XP). He also generously provided the placenta extract extraction method to this research group. The authors further thank all colleagues as well as administrative personnel at Shefa Neuroscience Institute, Tehran, Iran, who contributed to data collection.
Farhadi M, Gorji A, Mirsalehi M, et al. Electrophysiological and molecular changes following neuroprotective placental protein administration on tinnitus‐induced rats. Laryngoscope Investigative Otolaryngology. 2023;8(5):1410‐1420. doi: 10.1002/lio2.1156
Zeinab Akbarnejad and Saeid Mahmoudian contributed equally.
Contributor Information
Zeinab Akbarnejad, Email: akbarnejad.z@iums.ac.ir, Email: zeinab.akbarnejad8@gmail.com.
Saeid Mahmoudian, Email: mahmoudian.s@iums.ac.ir, Email: saeid.mahmoudian@gmail.com.
REFERENCES
- 1. Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36(2):239‐248. [DOI] [PubMed] [Google Scholar]
- 2. Gong N, Zhang M, Zhang XB, Chen L, Sun GC, Xu TL. The aspirin metabolite salicylate enhances neuronal excitation in rat hippocampal CA1 area through reducing GABAergic inhibition. Neuropharmacology. 2008;54(2):454‐463. doi: 10.1016/j.neuropharm.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 3. Halla JT, Hardin JG. Salicylate ototoxicity in patients with rheumatoid arthritis: a controlled study. Ann Rheum Dis. 1988;47(2):134‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Myers EN, Bernstein JM. Salicylate ototoxicity: a clinical and experimental study. Arch Otolaryngol. 1965;82(5):483‐493. [DOI] [PubMed] [Google Scholar]
- 5. Stolzberg D, Salvi RJ, Allman BL. Salicylate toxicity model of tinnitus. Front Syst Neurosci. 2012;6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang T, Kunze C, Dunlop MJ. Salicylate increases fitness cost associated with MarA‐mediated antibiotic resistance. Biophys J. 2019;117(3):563‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu H, Vikhe Patil K, Han C, et al. GLAST deficiency in mice exacerbates gap detection deficits in a model of salicylate‐induced tinnitus. Front Behav Neurosci. 2016;10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu J‐Y, Sastry BR. Benzodiazepine involvement in LTP of the GABA‐ergic IPSC in rat hippocampal CA1 neurons. Brain Res. 2005;1062(1–2):134‐143. [DOI] [PubMed] [Google Scholar]
- 9. Su Y‐Y, Luo B, Jin Y, et al. Altered neuronal intrinsic properties and reduced synaptic transmission of the rat's medial geniculate body in salicylate‐induced tinnitus. PLoS One. 2012;7:e46969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basta D, Goetze R, Ernst A. Effects of salicylate application on the spontaneous activity in brain slices of the mouse cochlear nucleus, medial geniculate body and primary auditory cortex. Hear Res. 2008;240(1–2):42‐51. doi: 10.1016/j.heares.2008.02.005 [DOI] [PubMed] [Google Scholar]
- 11. Gurgel LA, Santos FA, Rao VSN. Effects of human placental extract on chemical and thermal nociception in mice. Eur J Pain. 2000;4(4):403‐408. [DOI] [PubMed] [Google Scholar]
- 12. Jazayeri MH, Barzaman K, Nedaeinia R, Aghaie T, Motallebnezhad M. Human placental extract attenuates neurological symptoms in the experimental autoimmune encephalomyelitis model of multiple sclerosis—a putative approach in MS disease? Auto Immun Highlights. 2020;11(1):14. doi: 10.1186/s13317-020-00137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chakraborty D, Basu JM, Sen P, Sundar S, Roy S. Human placental extract offers protection against experimental visceral leishmaniasis: a pilot study for a phase‐I clinical trial. Ann Trop Med Parasitol. 2008;102(1):21‐38. [DOI] [PubMed] [Google Scholar]
- 14. Qu N, Zhang T‐T, Wen S‐S, et al. The application of selective neck dissection while preserving the cutaneous branches of cervical plexus in the surgical treatment of differentiated thyroid cancer—experiences from thousands of cases. Ann Transl Med. 2019;7(7):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poletaev AB, Arapov NA. Rehabilitation of patients after stroke: new remedy? Pharmacol Online. 2006;3:73‐79. [Google Scholar]
- 16. Oh K‐W, Qian T, Brenner DA, Lemasters JJ. Salicylate enhances necrosis and apoptosis mediated by the mitochondrial permeability transition. Toxicol Sci. 2003;73(1):44‐52. [DOI] [PubMed] [Google Scholar]
- 17. Abd‐Elhakim YM, Abdel‐Motal SM, Malhat SM, et al. Curcumin mitigates neurotoxic and neurobehavioral changes of gentamicin and sodium salicylate in rats by adjusting oxidative stress and apoptosis. Life Sci. 2021;265:118824. [DOI] [PubMed] [Google Scholar]
- 18. Turner JG, Brozoski TJ, Bauer CA, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120(1):188‐195. [DOI] [PubMed] [Google Scholar]
- 19. Yang G, Lobarinas E, Zhang L, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226(1–2):244‐253. [DOI] [PubMed] [Google Scholar]
- 20. Afrazi S, Esmaeili‐Mahani S. Allopregnanolone suppresses diabetes‐induced neuropathic pain and motor deficit through inhibition of GABAA receptor down‐regulation in the spinal cord of diabetic rats. Iran J Basic Med Sci. 2014;17(5):312‐317. [PMC free article] [PubMed] [Google Scholar]
- 21. Paxinos G, Watson C. The Rat Brain Atlas in Stereotaxic Coordinates. 6th ed. Academic Press; 2007. [Google Scholar]
- 22. The MathWorks Inc . MATLAB version: 9.0 (R2016a). The MathWorks Inc; 2016. [Google Scholar]
- 23. Quiroga RQ, Nadasdy Z, Ben‐Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Comput. 2004;16(8):1661‐1687. [DOI] [PubMed] [Google Scholar]
- 24. Chen L, Deng Y, Luo W, Wang Z, Zeng S. Detection of bursts in neuronal spike trains by the mean inter‐spike interval method. Prog Nat Sci. 2009;19(2):229‐235. [Google Scholar]
- 25. Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86(11):2564‐2578. doi: 10.1002/jnr.21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24(5):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulders WHAM, Barry KM, Robertson D. Effects of furosemide on cochlear neural activity, central hyperactivity and behavioural tinnitus after cochlear trauma in Guinea pig. PLoS One. 2014;9(5):e97948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silverstein H, Bernstein JM, Davies DG. IX salicylate ototoxicity: a biochemical and electrophysiological study. Ann Otol Rhinol Laryngol. 1967;76(1):118‐128. [DOI] [PubMed] [Google Scholar]
- 29. Yi B, Hu S, Zuo C, et al. Effects of long‐term salicylate administration on synaptic ultrastructure and metabolic activity in the rat CNS. Sci Rep. 2016;6(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mennink LM, Aalbers MW, van Dijk P, van Dijk JMC. The role of inflammation in tinnitus: a systematic review and meta‐analysis. J Clin Med. 2022;11(4):30‐34. doi: 10.3390/jcm11041000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu S‐S, Mei L, Chen J‐Y, Huang Z‐W, Wu H. Effects of salicylate on the inflammatory genes expression and synaptic ultrastructure in the cochlear nucleus of rats. Inflammation. 2014;37:365‐373. [DOI] [PubMed] [Google Scholar]
- 32. Chen X, Zheng L. Expression of pro‐inflammatory cytokines in the auditory cortex of rats with salicylate‐induced tinnitus. Mol Med Rep. 2017;16(4):5643‐5648. [DOI] [PubMed] [Google Scholar]
- 33. Hwang J‐H, Chen J‐C, Chan Y‐C. Effects of C‐phycocyanin and spirulina on salicylate‐induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS One. 2013;8(3):e58215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jang CH, Lee S, Park IY, Song A, Moon C, Cho G‐W. Memantine attenuates salicylate‐induced tinnitus possibly by reducing NR2B expression in auditory cortex of rat. Exp Neurobiol. 2019;28(4):495‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor‐α. J Neurosci. 2005;25(12):3219‐3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawasaki Y, Zhang L, Cheng J‐K, Ji R‐R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin‐1β, interleukin‐6, and tumor necrosis factor‐α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28(20):5189‐5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Furukawa K, Mattson MP. The transcription factor NF‐κB mediates increases in calcium currents and decreases in NMDA‐and AMPA/kainate‐induced currents induced by tumor necrosis factor‐α in hippocampal neurons. J Neurochem. 1998;70(5):1876‐1886. [DOI] [PubMed] [Google Scholar]
- 38. Jara JH, Singh BB, Floden AM, Combs CK. Tumor necrosis factor alpha stimulates NMDA receptor activity in mouse cortical neurons resulting in ERK‐dependent death. J Neurochem. 2007;100(5):1407‐1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wheeler D, Knapp E, Bandaru VVR, et al. Tumor necrosis factor‐α‐induced neutral sphingomyelinase‐2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109(5):1237‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rozanova S, Cherkashina Y, Repina S, Rozanova K, Nardid O. Protective effect of placenta extracts against nitrite‐induced oxidative stress in human erythrocytes. Cell Mol Biol Lett. 2012;17:240‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim TH, Choi J‐Y, Kim KH, et al. Hominis placenta suppresses acute lung inflammation by activating Nrf2. Am J Chin Med. 2018;46(4):801‐817. [DOI] [PubMed] [Google Scholar]
- 42. Muluye RA, Bian Y, Wang L, et al. Placenta peptide can protect mitochondrial dysfunction through inhibiting ROS and TNF‐α generation, by maintaining mitochondrial dynamic network and by increasing IL‐6 level during chronic fatigue. Front Pharmacol. 2016;7:328. doi: 10.3389/fphar.2016.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee K‐H, Kim T‐H, Lee W‐C, Kim SH, Lee SY, Lee S‐M. Anti‐inflammatory and analgesic effects of human placenta extract. Nat Prod Res. 2011;25(11):1090‐1100. [DOI] [PubMed] [Google Scholar]
- 44. Sun W, Lu J, Stolzberg D, et al. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159(1):325‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Su Y‐Y, Luo B, Wang H‐T, Chen L. Differential effects of sodium salicylate on current‐evoked firing of pyramidal neurons and fast‐spiking interneurons in slices of rat auditory cortex. Hear Res. 2009;253(1–2):60‐66. [DOI] [PubMed] [Google Scholar]
- 46. Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10(4):462‐468. [DOI] [PubMed] [Google Scholar]
- 47. Steriade M. The GABAergic reticular nucleus: a preferential target of corticothalamic projections. Proc Natl Acad Sci U S A. 2001;98(7):3625‐3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalappa BI, Brozoski TJ, Turner JG, Caspary DM. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J Physiol. 2014;592(22):5065‐5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Girotto G, Malinverni W. Use of placental extract for the treatment of myopic and senile chorio‐retinal dystrophies. Int J Tissue React. 1982;4(2):169‐172. [PubMed] [Google Scholar]
- 50. Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96(26):15222‐15227. [DOI] [PMC free article] [PubMed] [Google Scholar]


