Abstract
Introduction
Hematopoietic stem cell transplantation (HSCT) is an increasingly utilized therapy for malignant and non-malignant pediatric diseases. HSCT complications, including infection, organ dysfunction, and graft-versus-host-disease (GVHD) often require intensive care unit (ICU) therapies and are associated with mortality. Our aims were to identify the HSCT characteristics, complications and ICU therapies associated with (1) survival, and (2) survival changes over a ten-year period in a national dataset.
Methods
A national sample from the Health Facts (Cerner Corporation, Kansas City, MO) database from 2009 to 2018 was utilized. Inclusion criteria were age 30 days to <22 years and HSCT procedure code. For patients with >1 HSCT, the first was analyzed. Data included demographics, hospital length of stay (LOS), hospital outcome, transplant type and indication. HSCT complications included GVHD and infections. ICU therapies were positive pressure ventilation (PPV), vasoactive infusion, and dialysis. Primary outcome was survival to discharge. Statistical methods included bivariate analyses and multivariate logistic regression.
Results
473 patients underwent HSCT with 93% survival. 62% were allogeneic (89% survival) and 38% were autologous (98% survival). GVHD occurred in 33% of allogeneic HSCT. Infections occurred in 26% of all HSCT. ICU therapies included PPV (11% of patients), vasoactive (25%), and dialysis (3%). Decreased survival was associated with allogeneic HSCT (p < 0.01), GVHD (p = 0.02), infection (p < 0.01), and ICU therapies (p < 0.01). Survival improved from 89% (2009–2013) to 96% (2014–2018) (p < 0.01). Allogeneic survival improved (82%–94%, p < 0.01) while autologous survival was unchanged. Survival improvement over time was associated with decreasing infections (33%–21%, p < 0.01) and increasing vasoactive infusions (20%–28%, p = 0.05). On multivariate analysis, later time period was associated with improved survival (p < 0.01, adjusted OR 4.28).
Discussion
Hospital survival for HSCT improved from 89% to 96% from 2009 to 2018. Factors associated with mortality included allogeneic HSCT, GVHD, infections and ICU therapies. Improving survival coincided with decreasing infections and increasing vasoactive use.
Keywords: bone marrow transplant, hematopoietic stem cell transplantation, survival, outcomes, intensive care, infection, GVHD
Introduction
Hematopoietic stem cell transplantation (HSCT) is an established therapy for children with malignant and non-malignant diseases, including hematologic and solid tumors, bone marrow failure syndromes, immunodeficiencies, and genetic and metabolic disorders (1). As indications for HSCT broadened, transplant volumes have increased by 5%–10% per year; approximately 2,500 children currently undergo HSCT each year in the United States (1–3). However, HSCT carries substantial risk of treatment-related morbidity and mortality, including infectious complications, graft-versus-host disease (GVHD), and organ toxicity induced by preparatory regimens (1, 4, 5).
Approximately one third of patients require intensive care unit (ICU) management for HSCT complications (6–12). Mortality in the first 100 days is as high as 11% for allogeneic transplant and 4% for autologous transplant (1, 3, 5), an improvement from 15% and 7% respectively before 1991 (3). Other studies have revealed similar trends of improving survival over time (5, 13–17). Tracking change is particularly relevant given improvements in human leukocyte antigen-matching, reduced-intensity pre-transplant regimens, GVHD management, infection prophylaxis and treatment (5, 14, 15, 18) and ICU care. Importantly, the contribution of ICU care to these temporal trends has not been evaluated.
Our aims were to associate HSCT characteristics, HSCT complications and ICU therapies with (1) survival, and (2) survival changes over a ten-year time period in a national sample from 2009 to 2018 to assess if survival improved and if there are any changes in HSCT complications or practice associated with improvement.
Methods
Database and study design
This is a retrospective multicenter study using the Health Facts™ database (Cerner Corporation, Kansas City, MO). This database has de-identified clinical data from academic and nonacademic hospitals of varied sizes and locations in the United States with a Cerner data use agreement. The database includes demographic and admission information, diagnostic and procedure codes, laboratory results, medication and respiratory data, and hospital outcome. Health Facts™ has been successfully used in other longitudinal studies examining pediatric trends and practice (19, 20). This study was approved by the Children's National Hospital Institutional Review Board (Pro00009282) and granted a waiver of consent for de-identified data.
Inclusion criteria included encounters for patients age 30 days to less than 22 years admitted between January 1, 2009 and June 1, 2018 with at least one HSCT procedure code, indicating receipt of HSCT during the admission. The procedure codes used to define HSCT, associated diagnoses and some therapies are detailed in the Supplementary Appendix A. For patients with more than one HSCT encounter during the study period, only the first was included. Encounters were excluded if they had incomplete data (below).
Variables and outcome measures
The primary outcome was survival to hospital discharge. Demographic variables included age, sex, race, ethnicity, and hospital length of stay (LOS). HSCT variables included transplant type (autologous and allogeneic), year of transplant, underlying diagnoses necessitating the transplant and complications including GVHD and infection. ICU therapies included positive pressure ventilation (PPV), dialysis, and vasoactive agent infusion. Transplant type, GVHD and underlying diagnosis/indication for HSCT were identified from diagnostic and/or procedure codes. Diagnoses and transplant indications were grouped into categories including malignant hematologic diseases, solid tumors, non-malignant hematologic diseases, immunodeficiencies, and non-malignant other diseases. If more than one diagnosis was present, one was chosen based on clinical expertise and likelihood to necessitate HSCT by T.O and B.D. Infectious complications were identified from microbiology results and were categorized by the culture site as blood, respiratory, urine, skin and soft tissue, or other. Organism types included bacteria (gram positive and gram negative), viruses, and other (fungus, yeast, and mycobacteria); patients could have more than one organism identified. PPV (non-invasive and invasive) was determined from procedure codes and respiratory care data. Respiratory care data indicating PPV included >8 h of recorded ventilator settings. Dialysis (hemodialysis, peritoneal dialysis, urinary filtration, and vascular access for dialysis) was determined from procedure codes. Vasoactive agent infusion (epinephrine, norepinephrine, dopamine, dobutamine, milrinone, and/or vasopressin) was determined from medication administration data.
Statistical analysis
Variables were assessed individually for their association to hospital survival using bivariate analysis. Bivariate tests included Pearson's χ2 or Fisher's Exact for categorical variables and Wilcoxon rank sums tests for continuous variables. Post hoc multiple comparisons were performed if the primary comparison was significant.
The study period was divided into two 5-year intervals to assess change over time, 2009–2013 and 2014–2018. Bivariate analyses were performed for demographic, HSCT, ICU care variables, and survival to assess changes between the two time periods.
Multivariable logistic regression was used to investigate the effect of time period, selected demographics, HSCT, and ICU therapy variables on hospital survival. Variables significant at the 0.2 level in the bivariate analyses of survival were included in the multivariable logistic regression model.
Odds ratios and adjusted odds ratios are reported. Statistical significance was declared at the 0.05 alpha level. Results were expressed as medians with 25th–75th percentiles or counts with percentages. All statistical analyses were conducted using JMP® (version 16.1, SAS, Cary, North Carolina, USA).
Results
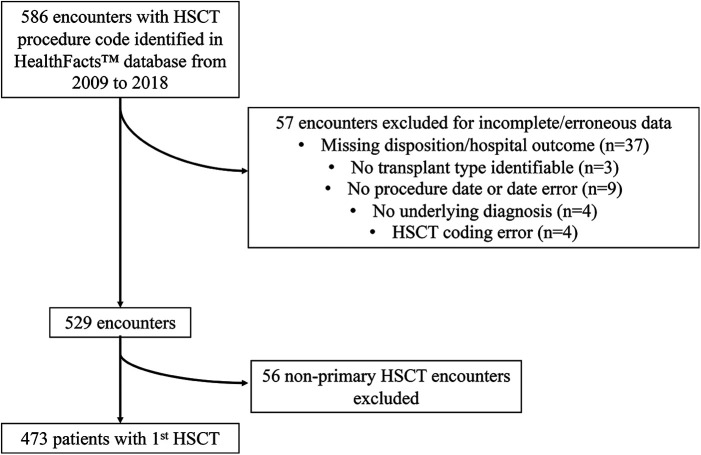
A total of 586 encounters met the study inclusion criteria. Fifty-seven encounters were excluded for incomplete data (Figure 1). Only the first HSCT procedure for each patient was included, with 56 subsequent HSCT encounters excluded. The final sample had 473 patients with hospital survival of 93%. Demographic and HSCT variable data are shown in Table 1. Median age was 8 years [3–15]. There were 284 (60%) males, and 322 (68%) patients were Caucasian. Transplant type was allogeneic for 293 patients (62%). Underlying diagnoses and HSCT indications included malignant hematologic diseases (47%), solid tumors (33%), non-malignant hematologic diseases (14%), immunodeficiency syndromes (4%) and other non-malignant diseases (2%). Hospital LOS was 32 days [23–47] and differed between survivors [31 days (23–43)] and deaths [85 days (63–116)] (p < 0.01).
Figure 1.
Patient inclusion diagram. HSCT, hematopoietic stem cell transplantation.
Table 1.
Demographic and hematopoietic stem cell transplant variables and their association with survival.
| All patients (n = 473) |
Survivors (n = 438) |
Deaths (n = 35) |
p (1) | OR of Survival [95% CI] |
|
|---|---|---|---|---|---|
| Demographic | |||||
| Age, median [25%ile–75%ile], years | 8 [3–15] | 8 [3–15] | 6 [1–15] | 0.41 (2) | |
| Male, n (%) | 284 (60) | 265 (93) | 19 (7) | 0.47 (3) | 1.29 [0.65–2.58] |
| Female, n (%) | 189 (40) | 173 (92) | 16 (8) | ||
| Race and ethnicity, n (%) | |||||
| Caucasian | 322 (68) | 299 (93) | 23 (7) | 0.96 (4) | |
| African American | 69 (15) | 63 (91) | 6 (9) | ||
| Asian/Pacific Islander | 21 (4) | 20 (95) | 1 (5) | ||
| Hispanic | 26 (6) | 24 (92) | 2 (8) | ||
| Other/Unknown | 35 (7) | 32 (91) | 3 (9) | ||
| Hospital LOS, median [25%ile–75%ile], days | 32 [23–47] | 31 [23–43] | 85 [63–116] | <0.01 (5) | |
| Transplant Type | |||||
| Allogeneic, n (%) | 293 (62) | 262 (89) | 31 (11) | <0.01 (6) | 0.19 [0.07–0.55] |
| Autologous, n (%) | 180 (38) | 176 (98) | 4 (2) | ||
| Transplant Indication | 0.02 (7) | ||||
| Malignant Hematologic, n (%) | 221 (47) | 199 (90) | 22 (10) | REF (7) | |
| Solid tumor, n (%) | 157 (33) | 153 (97) | 4 (3) | <0.01 (7) | 4.23 [1.43–12.53] |
| Non-malignant Hematologic, n (%) | 64 (14) | 59 (92) | 5 (8) | 0.81 (7) | 1.30 [0.47–3.59] |
| Immunodeficiency, n (%) | 21 (4) | 18 (86) | 3 (14) | 0.46 (7) | 0.66 [0.18–2.43] |
| Non-malignant other, n (%) | 10 (2) | 9 (90) | 1 (10) | 1.00 (7) | 0.99 [0.12–8.23] |
OR, odds ratio; CI, confidence interval; NS, not significant, LOS, length of stay; REF, reference group.
(1) Continuous variables compared with Wilcoxon rank sums tests. Categorical variables were compared with Pearson's χ2 or Fisher's Exact, and post hoc multiple comparisons were performed when the primary comparison was significant (see Methods).
(2) Comparison of age medians, survivors vs. deaths.
(3) Comparison of sex distributions, survivors vs. deaths.
(4) Comparison of race and ethnicity distributions, survivors vs. deaths.
(5) Comparison of hospital LOS medians, survivors vs. deaths.
(6) Comparison of transplant type distributions, survivors vs. deaths.
(7) Comparison of transplant indication distributions, survivors vs. deaths. Malignant hematologic subgroup served as reference group for post hoc multiple comparisons. See Supplementary Appendix for individual diagnoses included in each transplant indication subgroup.
Survival to hospital discharge for allogeneic HSCT was 89% compared to 98% for autologous HSCT [p < 0.01, OR 0.19 (0.07–0.55)] (Table 1). Compared to malignant hematologic diseases with 90% survival (reference group), solid tumors had improved survival of 97% [p < 0.01, OR 4.23 (1.43–12.53)]. There were no differences in survival between the other HSCT indication groups and the malignant hematologic reference group.
The major complications of GVHD and infections were associated with decreased survival to discharge. GVHD occurred in 96 patients (33% of allogeneic HSCT) and infections in 125 (26% of all HSCT) (Table 2). Survival for allogeneic HSCT patients with GVHD was 83% compared to 92% survival for those without GVHD [p = 0.02, OR 0.41 (0.19–0.87)]. Infectious complications were associated with decreased survival [p < 0.01, OR 0.27 (0.13–0.54)], with 85% survival if one or more infectious complication occurred and 95% survival if no infectious complications occurred. Among the infectious complication types, positive blood [p < 0.01, OR 0.23 (0.11–0.47)], respiratory [p < 0.01, OR 0.10 (0.04–0.23)], and urine cultures [p = 0.05, OR 0.39 (0.15–1.02)] were associated with decreased survival. The lowest survival (63%) was in patients with a positive respiratory culture.
Table 2.
Hematopoietic stem cell transplant complications and ICU therapies and their association with survival.
| N (%) (Total n = 473) | Survival (%) | p (1) | OR of Survival [95% CI] | |
|---|---|---|---|---|
| Complication | ||||
| GVHD (2) | 96 (33) | 80 (83) | 0.02 | 0.41 [0.19–0.87] |
| No GVHD | 197 (67) | 182 (92) | ||
| Any infectious complication | 125 (26) | 106 (85) | <0.01 | 0.27 [0.13–0.54] |
| No infectious complication | 348 (74) | 332 (95) | ||
| Blood culture positive | 87 (18) | 71 (82) | <0.01 | 0.23 [0.11–0.47] |
| No blood culture positive | 386 (82) | 367 (95) | ||
| Respiratory culture positive | 30 (6) | 19 (63) | <0.01 | 0.10 [0.04–0.23] |
| No respiratory culture positive | 443 (94) | 419 (95) | ||
| Urine culture positive | 39 (8) | 33 (85) | 0.05 | 0.39 [0.15–1.02] |
| No urine culture positive | 434 (92) | 405 (93) | ||
| Skin/soft tissue culture positive | 10 (2) | 8 (80) | 0.16 | 0.31 [0.06–1.50] |
| No skin/soft tissue culture positive | 463 (98) | 430 (93) | ||
| Other culture positive | 11 (2) | 9 (82) | 0.19 | 0.35 [0.07–1.67] |
| No other culture positive | 462 (98) | 429 (93) | ||
| GVHD plus infectious complication (3) | 39 (27) | 29 (74) | 0.08 | 0.45 [0.18–1.11] |
| GVHD or infection alone | 105 (73) | 91 (87) | ||
| ICU Therapies | ||||
| Any ICU Therapy | 143 (30) | 111 (78) | <0.01 | 0.03 [0.01–0.11] |
| No ICU Therapy | 330 (70) | 327 (99) | ||
| PPV (4) | 53 (11) | 28 (53) | <0.01 | 0.03 [0.01–0.06] |
| No PPV | 420 (89) | 410 (98) | ||
| Vasoactive infusion (5) | 116 (25) | 91 (78) | <0.01 | 0.10 [0.05–0.23] |
| No vasoactive infusion | 357 (75) | 347 (97) | ||
| Dialysis (6) | 16 (3) | 6 (38) | <0.01 | 0.03 [0.01–0.10] |
| No Dialysis | 457 (97) | 432 (95) | ||
| Number of ICU Therapies | <0.01 | |||
| 1 ICU Therapy (7) | 109 (76) | 99 (91) | REF | 1 |
| 2 ICU Therapies | 27 (19) | 11 (41) | <0.01 | 0.07 [0.03–0.19] |
| 3 ICU Therapies | 7 (5) | 1 (14) | <0.01 | 0.02 [0.002–0.15] |
ICU, intensive care unit; OR: odds ratio; CI, confidence interval; GVHD, graft-versus-host-disease; PPV, positive pressure ventilation.
(1) Categorical variables were compared with Pearson's χ2 or Fisher's Exact.
(2) Reported as percent of allogeneic transplants (total n = 293).
(3) For allogeneic transplant recipients, those with both GVHD and infectious complication were compared to those with GVHD or infectious complication alone (total n = 144).
(4) PPV includes invasive and non-invasive modalities.
(5) Vasoactive infusions include epinephrine, norepinephrine, dopamine, dobutamine, milrinone, and/or vasopressin.
(6) Dialysis includes hemodialysis, peritoneal dialysis, urinary filtration and related procedures (see Supplementary Methods and Appendix).
(7) For patients receiving ICU therapies (n = 143), receipt of 1, 2, or 3 therapies were compared.
ICU therapies included PPV in 53 patients (11%), vasoactive agent infusion in 116 patients (25%), and dialysis in 16 patients (3%) (Table 2). Survival was 53% for those receiving PPV, 78% for those receiving vasoactive agent infusions, and 38% for those receiving dialysis. Receiving one or more ICU therapies was associated with decreased survival [p < 0.01, OR 0.03 (0.01–0.11)]. An increasing number of ICU therapies was associated with worse survival, with 91% survival for one ICU therapy, 41% survival for two ICU therapies, and 14% survival for three ICU therapies (p < 0.01).
There was a significant improvement in survival over the 10-year period, from 89% in the early time period (2009–2013) to 96% in the late time period (2014–2018) [p < 0.01, OR 2.72 (1.32–5.61)] (Table 3). In particular, allogeneic HSCT survival increased from 82% to 94% [p < 0.01, OR 3.51 (1.59–7.77)], while autologous HSCT survival remained unchanged at 98%. The demographic and transplant variables were similar between the time periods with no significant differences in age, sex, transplant type, transplant indication, or LOS. GVHD was not different between the time periods but infectious complications were significantly reduced from 33% to 21% [p < 0.01, OR 0.54 (0.36–0.81)]. Of the ICU therapies, there was a trend towards an increase in vasoactive agent infusions from 20% to 28% [p = 0.05, OR 1.54 (1.00–2.37)] and a decrease in the use of PPV from 14% to 9% [p = 0.07, OR 0.59 (0.33–1.05)].
Table 3.
Trends over time in hematopoietic stem cell transplant indications, complications, intensive care unit therapies and outcomes.
| Early Time Period (2009–2013) (n = 204) |
Late Time Period (2014–2018) (n = 269) |
p (1) | Odds Ratio [95% CI] |
|
|---|---|---|---|---|
| Demographics | ||||
| Age, median [25%ile–75%ile], years | 7 [3–15] | 8 [3–14] | 0.73 | |
| Male (n, column %) | 116 (57) | 168 (62) | 0.22 | 0.79 [0.55–1.15] |
| Female (n, column %) | 88 (43) | 101 (38) | ||
| Transplant type | ||||
| Allogeneic (n, column %) | 119 (58) | 174 (65) | 0.16 (2) | 0.76 [0.53–1.11] |
| Autologous (n, column %) | 85 (42) | 95 (35) | ||
| Hospital outcome | ||||
| Overall | ||||
| Survivors (n, column %) | 181 (89) | 257 (96) | <0.01 (3) | 2.72 [1.32–5.61] |
| Deaths (n, column %) | 23 (11) | 12 (4) | ||
| Allogeneic | ||||
| Survivors (n, %allogeneic/column) | 98 (82) | 164 (94) | <0.01 (4) | 3.51 [1.59–7.77] |
| Deaths (n, %allogeneic/column) | 21 (18) | 10 (6) | ||
| Autologous | ||||
| Survivors (n, % autologous/column) | 83 (98) | 93 (98) | 1.00 (5) | 1.12 [0.15–8.13] |
| Deaths (n, % autologous/column) | 2 (2) | 2 (2) | ||
| Hospital LOS, median [25%ile–75%ile], days | 31 [23–47] | 33 [24–48] | 0.47 | |
| Transplant Indication (6) | 0.10 | |||
| Malignant Hematologic (n, column %) | 83 (41) | 138 (51) | ||
| Solid tumor (n, column %) | 76 (37) | 81 (30) | ||
| Non-malignant Hematologic (n, column %) | 29 (14) | 35 (13) | ||
| Immunodeficiency (n, column %) | 9 (4) | 12 (4) | ||
| Non-malignant other (n, column %) | 7 (3) | 3 (1) | ||
| Transplant Complication | ||||
| GVHD (n, %allogeneic transplant/column) (7) | 39 (33) | 57 (33) | 1.00 | 1.00 [0.61–1.64] |
| No GVHD (n, %allogeneic transplant/column) (7) | 80 (67) | 117 (67) | ||
| Any infectious complication (n, column %) | 68 (33) | 57 (21) | <0.01 | 0.54 [0.36–0.81] |
| No Infectious complication (n, column %) | 136 (67) | 212 (79) | ||
| ICU Therapies | ||||
| Any ICU Therapy (n, column %) | 58 (28) | 85 (32) | 0.46 | 0.16 [0.78–1.73] |
| No ICU Therapy (n, column %) | 146 (72) | 184 (68) | ||
| PPV (n, column %) | 29 (14) | 24 (9) | 0.07 | 0.59 [0.33–1.05] |
| No PPV (n, column %) | 175 (86) | 245 (91) | ||
| Dialysis (n, column %) | 8 (4) | 8 (3) | 0.57 | 0.75 [0.28–2.04] |
| No Dialysis (n, column %) | 196 (96) | 261 (97) | ||
| Vasoactive infusion (n, column %) | 41 (20) | 75 (28) | 0.05 | 1.54 [1.00–2.37] |
| No Vasoactive infusion (n, column %) | 163 (80) | 194 (72) | ||
CI, confidence interval; NS, not significant; LOS, length of stay; GVHD, graft-versus-host-disease; ICU, intensive care unit; PPV, positive pressure ventilation.
(1) Variable distributions in the early versus late time periods were compared. Continuous variables were compared with Wilcoxon rank sums tests, and categorical variables were compared with Pearson's χ2 or Fisher's Exact.
(2) Comparison of transplant type distribution (allogeneic/autologous), early vs. late time periods.
(3) Comparison of survival distribution for all transplant types, early vs. late time periods.
(4) Comparison of survival distribution for allogeneic transplants, early vs. late time periods.
(5) Comparison of survival distribution for autologous transplants, early vs. late time periods.
(6) See Supplementary Appendix for individual diagnoses included in each transplant indication subgroup.
(7) GVHD reported as percent of allogeneic transplants (total n = 293).
The results of the multivariable logistic regression investigating the effect of time period on hospital survival, controlled for HSCT type, indication, GVHD, infectious complications and ICU therapies, are shown in Table 4 (Online). The adjusted OR for survival in the late time period relative to the early time period was 4.44 [1.43–13.77] (p < 0.01). ICU therapies were associated with decreased survival on multivariate analysis including PPV [p < 0.01, adjusted OR 0.07 (0.02–0.19)], vasoactive infusion [p < 0.01, adjusted OR 0.08 (0.03–0.24)], and dialysis [p = 0.01, adjusted OR 0.12 (0.02–0.68)].
Table 4.
Hematopoietic stem cell transplant variables including transplant time period and their association with survival: A multivariable logistic regression.
| Variable | p | aOR Survival (95% CI) |
|---|---|---|
| PPV | <0.01 | 0.07 [0.02–0.19] |
| Vasoactive infusion | <0.01 | 0.08 [0.03–0.24] |
| Time period (2014–2018) | <0.01 | 4.44 [1.43–13.77] |
| Dialysis | 0.01 | 0.12 [0.02–0.68] |
| Transplant type/GVHD | ||
| Autologous transplant | REF | 1 |
| Allogeneic transplant without GVHD | 0.61 | 0.41 [0.01–12.71] |
| Allogeneic transplant with GVHD | 0.36 | 0.19 [0.01–6.51] |
| Infectious Complication | 0.56 | 0.72 [0.24–2.15] |
| Transplant Indication | ||
| Malignant Hematologic | REF | 1 |
| Solid tumor | 0.84 | 0.70 [0.02–22.21] |
| Non-malignant Hematologic | 0.50 | 0.60 [0.14–2.61] |
| Immunodeficiency | 0.61 | 1.60 [0.26–10.00] |
| Non-malignant other | 0.43 | 2.99 [0.20–45.37] |
aOR, adjusted odds ratio; CI, confidence interval; PPV, positive pressure ventilation; GVHD, graft-versus-host-disease; REF, reference group.
Discussion
We observed a 93% survival after hospital admission for pediatric HSCT in a large multicenter sample in the United States from 2009 to 2018. Survival was 89% for allogeneic HSCT and 98% for autologous HSCT. Clinical variables associated with decreased survival included allogeneic HSCT, complications of GVHD and infection, and indicators of severity of illness post-HSCT including ICU therapies of PPV, vasoactive agent infusion and dialysis. Survival significantly improved from 89% (2009–2013) to 96% (2014–2018); in particular allogeneic HSCT survival improved (82%–94%) while autologous HSCT survival remained unchanged. Survival improvement was accompanied by decreasing infectious complications and increasing vasoactive agent use over time. After adjusting for HSCT variables, HSCT complications, and ICU therapies in a multivariable regression, time period was a significant predictor of survival (p < 0.01) with an adjusted OR of 4.44 [1.43–13.77].
Early treatment-related mortality, often standardized to 100 days following HSCT, is generally attributable to organ toxicity from the transplant conditioning regimen, infection during the period of immunosuppression, and GVHD, as opposed to relapse-related mortality which generally occurs later post-transplant. Since there is no risk of GVHD for autologous HSCT, there is no need for prophylactic immunosuppression with decreased risk of infection as a result (21). Mortality at 100 days is as high as 11% for allogenic HSCT and 4% for autologous HSCT (1, 3, 5) which has improved over time (3, 5, 13–16). We observed 11% and 2% hospital mortality for allogeneic and autologous HSCT respectively at median hospital day 85 [63–116]. Because these data were acquired from a multi-institutional database, we used HSCT admission hospital survival as a proxy for early (100-day) treatment-related mortality.
These findings support the trends of decreasing HSCT complications and improving survival noted over the last several decades (3–5, 13, 14, 16, 17, 22–25). Outcome improvement over time is presumably related, in part, to advancements in HSCT care including reduced intensity conditioning (3, 5, 15, 24, 26) higher resolution human leukocyte antigen-matching (18, 27, 28), expanded agents for bacterial, viral and fungal prophylaxis and treatment, enhanced detection of infection (29–36), and novel GVHD prophylaxis and treatment strategies (14, 24, 37–42). In particular, we identified that infectious complications were significantly reduced over time which was temporally associated with improving survival over time. However, infectious complications were still frequent and were associated with decreased survival, with the worst survival seen for respiratory infections (63%). Respiratory infections have a high mortality in HSCT patients (43, 44) and both animal and human data suggest defects in the pulmonary immune response following HSCT may be contributing (45).
A total of 17%–35% of children require ICU care following HSCT (9–12) and outcomes for post-HSCT ICU patients have improved over time in parallel with HSCT survival (46, 47). For instance, survival of mechanically ventilated HSCT patients has steadily increased from 9%–14% in the 1970–1980s (48–50), to 12%–47% in the 1980–1990s (51–54), and to 18%–58% in the 1990s to early 2000s (6, 10, 17, 47, 55, 56) with current estimates of 39%–58% survival (8, 9, 11, 57–60). In our 2009–2018 cohort, 30% of patients received at least one ICU therapy in the immediate post-HSCT period. Survival was 53% for patients receiving PPV, 78% for patients receiving at least one vasoactive agent infusion, and 38% for patients receiving dialysis, comparable to other recent studies (8, 9, 57, 58). We also found that survival decreased with an increasing number of ICU therapies received: 91% of patients receiving only one therapy survived, while 41% of those receiving two therapies survived, and 14% of those receiving all three therapies survived.
Our observation that increased vasoactive agent use was temporally associated with improvement in survival is novel and may represent a practice shift towards more liberal vasoactive use. Emphasis on early recognition of sepsis with prompt initiation of vasoactive treatments, including peripheral delivery (a modification to guidelines in 2007) (61), may have contributed to increasing use and be partly responsible for this observation. This finding may also relate to the potential harmful effects of fluid overload post-HSCT and recommendations for conservative fluid management (62–66) which could have influenced increasing use of vasoactive agent infusions. Additionally, we observed a trend towards decreasing use of PPV over time during the study period. Decreasing use of mechanical ventilation in this population has been previously reported (17, 67) in conjunction with improving survival. Decreasing infections (that may manifest as deterioration requiring PPV) may be contributing. The impact of non-invasive PPV use on this trend is unclear. In our cohort, only 3 patients received only non-invasive PPV, limiting our inferences on the overall PPV trend.
There are limitations to this study. First, national databases, while providing large samples from multiple sites, usually lack the granularity present in single site data. Therefore, some important HSCT variables could not be analyzed, including donor source, matching, and conditioning regimen. Second, our use of positive culture results as evidence of infectious complications did not include clinical corroboration and presumably missed culture-negative infections or mis-assigned instances of contamination. Third, respiratory cultures may be more likely sent for mechanically ventilated patients, contributing to the low survival seen in this group. Fourth, while we were able to assess ICU therapies (PPV, vasoactive agent infusion, dialysis), we were not able to assess other details of ICU care such as admission and discharge dates, indications for admission or therapies, or other measures of severity of illness. Comorbid diagnoses and some therapies were deduced from diagnosis and procedure codes, potentially missing those that were not coded/billed. For example, the specific diagnostic code for hepatic veno-occlusive disease was introduced in 2015 and therefore was not assessed in this study. Finally, we could not ascertain cause of death or outcome after discharge (including 100-day mortality for survivors discharged before 100 days).
Conclusion
Hospital survival following HSCT was 93% in a recent multicenter national sample from 2009 to 2018. Factors associated with decreased survival included allogeneic HSCT, GVHD, infectious complications and ICU therapies. Survival significantly improved over time, from 89% to 96%, particularly for allogeneic HSCT. In addition, improving survival was associated with decreasing infectious complications and increasing vasoactive agent use.
Funding Statement
Supported in part by the National Institute for Child Health and Human Development [K23 HD105978–01 (AP)]. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute for Child Health and Human Development or the National Institutes of Health. The funder did not participate in the work.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
TO, AP, BD, and MP contributed to the study design, methodology, data curation, and formal analysis. TO and MP contributed to drafting of the initial manuscript. TO, AP, BD, and MP contributed to reviewing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1247792/full#supplementary-material
References
- 1.Auletta JJ, Kou J, Chen M, Shaw BE. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. (2021). [Cited 2022 Nov 17]. Available at: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx.
- 2.Number of transplants by year per age group. Last updated: April 13, 2022. [Internet]. [Cited 2022 Nov 17]. Center for international blood and marrow transplant, a contractor for the C.W. bill young cell transplantation program operated through the U. S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau. Available at: https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report.
- 3.Miano M, Labopin M, Hartmann O, Angelucci E, Cornish J, Gluckman E, et al. Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European group for blood and marrow transplantation. Bone Marrow Transplant. (2007) 39(2):89–99. 10.1038/sj.bmt.1705550 [DOI] [PubMed] [Google Scholar]
- 4.Zaucha-Prazmo A, Gozdzik J, Debski R, Drabko K, Sadurska E, Kowalczyk JR. Transplant-related mortality and survival in children with malignancies treated with allogeneic hematopoietic stem cell transplantation. A multicenter analysis. Pediatr Transplant. (2018) 22(3):e13158. 10.1111/petr.13158 [DOI] [PubMed] [Google Scholar]
- 5.Styczyński J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. (2020) 55(1):126–36. 10.1038/s41409-019-0624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kache S, Weiss IK, Moore TB. Changing outcomes for children requiring intensive care following hematopoietic stem cell transplantation. Pediatr Transplant. (2006) 10(3):299–303. 10.1111/j.1399-3046.2005.00453.x [DOI] [PubMed] [Google Scholar]
- 7.Jacobe SJ, Hassan A, Veys P, Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. (2003) 31(5):1299–305. 10.1097/01.CCM.0000060011.88230.C8 [DOI] [PubMed] [Google Scholar]
- 8.Zinter MS, Logan BR, Fretham C, Sapru A, Abraham A, Aljurf MD, et al. Comprehensive prognostication in critically ill pediatric hematopoietic cell transplant patients: results from merging the center for international blood and marrow transplant research (CIBMTR) and virtual pediatric systems (VPS) registries. Biol Blood Marrow Transplant. (2020) 26(2):333–42. 10.1016/j.bbmt.2019.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chima RS, Daniels RC, Kim MO, Li D, Wheeler DS, Davies SM, et al. Improved outcomes for stem cell transplant recipients requiring pediatric intensive care. Pediatr Crit Care Med. (2012) 13:e336–42. 10.1097/PCC.0b013e318253c945 [DOI] [PubMed] [Google Scholar]
- 10.Lamas A, Otheo E, Ros P, Vázquez JL, Maldonado MS, Muñoz A, et al. Prognosis of child recipients of hematopoietic stem cell transplantation requiring intensive care. Intensive Care Med. (2003) 29(1):91–6. 10.1007/s00134-002-1549-2 [DOI] [PubMed] [Google Scholar]
- 11.Duncan CN, Lehmann LE, Cheifetz IM, Greathouse K, Haight AE, Hall MW, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med. (2013) 14(3):261–7. 10.1097/PCC.0b013e3182720601 [DOI] [PubMed] [Google Scholar]
- 12.Diaz MA, González-Vicent M, Sevilla J. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation hemoglobinopathies view project blocking oncogene expression through CRISPR/Cas9 system view project. (2014). Available at: http://www.haematologica.ws/2002_03/292.htm.
- 13.Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. (2011) 29(7):805–13. 10.1200/JCO.2010.32.5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. (2010) 363(22):2091–101. 10.1056/NEJMoa1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratwohl A, Baldomero H, Passweg J, Urbano-Ispizua A, Baur A, Elvy P, et al. Increasing used of reduced intensity conditioning transplants: report of the 2001 EBMT activity survey. Bone Marrow Transplant. (2002) 30(12):813–31. 10.1038/sj.bmt.1703819 [DOI] [PubMed] [Google Scholar]
- 16.Bacigalupo A, Sormani MP, Lamparelli T, Gualandi F, Occhini D, Bregante S, et al. Reducing transplant-related mortality after allogeneic hematopoietic stem cell transplantation. Haematologica. (2004) 89(10):1238–47. [PubMed] [Google Scholar]
- 17.Bratton SL, Van DH, Statler KD, Pulsipher MA, McArthur J, Keenan HT. Lower hospital mortality and complications after pediatric hematopoietic stem cell transplantation. Crit Care Med. (2008) 36(3):923–7. 10.1097/01.CCM.0B013E318161FAC1 [DOI] [PubMed] [Google Scholar]
- 18.Spellman S, Setterholm M, Maiers M, Noreen H, Oudshoorn M, Fernandez-Viña M, et al. Advances in the selection of HLA-compatible donors: refinements in HLA typing and matching over the first 20 years of the national marrow donor program registry. Biol Blood Marrow Transplant. (2008) 14:37–44. 10.1016/j.bbmt.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 19.Heneghan JA, Trujillo Rivera EA, Zeng-Treitler Q, Faruqe F, Morizono H, Bost JE, et al. Medications for children receiving intensive care: a national sample. Pediatr Crit Care Med. (2020) 21(9):e679–85. 10.1097/PCC.0000000000002391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel AK, Gai J, Trujillo-Rivera E, Faruqe F, Kim D, Bost JE, et al. Association of intravenous Acetaminophen administration with the duration of intravenous opioid use among hospitalized pediatric patients. JAMA Netw Open. (2021) 4(12):e2138420. 10.1001/jamanetworkopen.2021.38420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Champlin R. Selection of autologous or allogeneic transplantation. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler Jr TS, Holland JF, et al., editors. Cancer medicine. 6th Edn. Hamilton (oN): BC Decker; (2003). [Google Scholar]
- 22.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant. (2005) 36:757–69. [DOI] [PubMed] [Google Scholar]
- 23.Brissot E, Rialland F, Cahu X, Strullu M, Corradini N, Thomas C, et al. Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: a three-decade experience of a single institution. Bone Marrow Transplant. (2016) 51(2):267–72. 10.1038/bmt.2015.250 [DOI] [PubMed] [Google Scholar]
- 24.Hahn T, McCarthy PL, Hassebroek A, Bredeson C, Gajewski JL, Hale GA, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. (2013) 31(19):2437–49. 10.1200/JCO.2012.46.6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Pereda MA, Bala N, Nagarajan S. In-hospital mortality of hematopoietic stem cell transplantation among children with nonmalignancies: a nationwide study in the United States from 2000 to 2012. Pediatr Blood Cancer. (2019) 66(6):e27626. 10.1002/pbc.27626 [DOI] [PubMed] [Google Scholar]
- 26.Pulsipher MA, Boucher KM, Wall D, Frangoul H, Duval M, Goyal RK, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the pediatric blood and marrow transplant consortium study ONC0313. Blood. (2009) 114(7):1429–36. 10.1182/blood-2009-01-196303 [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. (2007) 110(13):4576–83. 10.1182/blood-2007-06-097386 [DOI] [PubMed] [Google Scholar]
- 28.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. (2004) 104(7):1923–30. 10.1182/blood-2004-03-0803 [DOI] [PubMed] [Google Scholar]
- 29.Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C, et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European conference on infections in leukemia. Haematologica. (2013) 98(12):1826–35. 10.3324/haematol.2013.091025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Averbuch D, Cordonnier C, Livermore DM, Mikulska M, Orasch C, Viscoli C, et al. Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European conference on infections in leukemia (ECIL-4, 2011). Haematologica. (2013) 98(12):1836–47. 10.3324/haematol.2013.091330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, et al. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. (2017) 102(3):433–44. 10.3324/haematol.2016.152900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winston DJ, Young JAH, Pullarkat V, Papanicolaou GA, Vij R, Vance E, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. (2008) 111(11):5403–10. 10.1182/blood-2007-11-121558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marty FM, Winston DJ, Rowley SD, Vance E, Papanicolaou GA, Mullane KM, et al. CMX001 to prevent cytomegalovirus disease in hematopoietic-cell transplantation. N Engl J Med. (2013) 369(13):1227–36. 10.1056/NEJMoa1303688 [DOI] [PubMed] [Google Scholar]
- 34.Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. (2017) 377(25):2433–44. 10.1056/NEJMoa1706640 [DOI] [PubMed] [Google Scholar]
- 35.Einsele H, Ehninger G, Hebart H, Wittkowski K, Schuler U, Jahn G, et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood. (1995) 86(7):2815–20. 10.1182/blood.V86.7.2815.2815 [DOI] [PubMed] [Google Scholar]
- 36.Hebart H, Löffler J, Meisner C, Serey F, Schmidt D, Böhme A, et al. Early detection of aspergillus infection after allogeneic stem cell transplantation by polymerase chain reaction screening. J Infect Dis. (2000) 181(5):1713–9. 10.1086/315435 [DOI] [PubMed] [Google Scholar]
- 37.Yanik G, Levine JE, Ratanatharathorn V, Dunn R, Ferrara J, Hutchinson RJ. Tacrolimus (FK506) and methotrexate as prophylaxis for acute graft-versus-host disease in pediatric allogeneic stem cell transplantation. Bone Marrow Transplant. (2000) 26(2):161–7. 10.1038/sj.bmt.1702472 [DOI] [PubMed] [Google Scholar]
- 38.Mielcarek M, Storer BE, Boeckh M, Carpenter PA, McDonald GB, Deeg HJ, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. (2009) 113(13):2888–94. 10.1182/blood-2008-07-168401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hockenbery DM, Cruickshank S, Rodell TC, Gooley T, Schuening F, Rowley S, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. (2007) 109(10):4557–63. 10.1182/blood-2006-05-021139 [DOI] [PubMed] [Google Scholar]
- 40.Kasper C, Sayer HG, Mügge LO, Schilling K, Scholl S, Issa MC, et al. Combined standard graft-versus-host disease (GvHD) prophylaxis with mycophenolate mofetil (MMF) in allogeneic peripheral blood stem cell transplantation from unrelated donors. Bone Marrow Transplant. (2004) 33(1):65–9. 10.1038/sj.bmt.1704299 [DOI] [PubMed] [Google Scholar]
- 41.Nash RA, Pin¨eiro LA, Storb R, Deeg HJ, Fitzsimmons WE, Furlong T, et al. FK506 in combination with methotrexate for the prevention of graft- versus-host disease after marrow transplantation from matched unrelated donors. Blood. (1996) 88(9):3634–41. [PubMed] [Google Scholar]
- 42.Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis. (1995) 171(6):1545–52. 10.1093/infdis/171.6.1545 [DOI] [PubMed] [Google Scholar]
- 43.Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. (2010) 24:257–72. 10.1016/j.idc.2010.01.010 [DOI] [PubMed] [Google Scholar]
- 44.Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. (2006) 27:297–309. 10.1055/s-2006-945530 [DOI] [PubMed] [Google Scholar]
- 45.Zinter MS, Hume JR. Effects of hematopoietic cell transplantation on the pulmonary immune response to infection. Front Pediatr. (2021) 9:634566. 10.3389/fped.2021.634566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hume J, Gupta S, Steiner M. Historical outcomes of pediatric hematopoietic stem cell transplantation patients requiring critical care. J Pediatr Intensive Care. (2015) 03(03):83–90. 10.3233/PIC-14099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamburro RF, Barfield RC, Shaffer ML, Rajasekaran S, Woodard P, Morrison RR, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. (2008) 9(3):270–7. 10.1097/PCC.0b013e31816c7260 [DOI] [PubMed] [Google Scholar]
- 48.Warwick AB, Mertens AC, Ou Shu X, Ramsay NKC, Neglia JP. Outcomes following mechanical ventilation in children undergoing bone marrow transplantation. Bone Marrow Transplant. (1998) 22(8):787–94. 10.1038/sj.bmt.1701417 [DOI] [PubMed] [Google Scholar]
- 49.Todd K, Wiley F, Landaw E, Gajewski J, Bellamy PE, Harrison RE, et al. Survival outcome among 54 intubated pediatric bone marrow transplant patients. Crit Care Med. (1994) 22(1):171–6. 10.1097/00003246-199401000-00030 [DOI] [PubMed] [Google Scholar]
- 50.Nichols DG, Walker LK, Wingard JR, Bender KS, Bezman M, Zahurak ML, et al. Predictors of acute respiratory failure after bone marrow transplantation in children. Crit Care Med. (1994) 22(9):1485–91. 10.1097/00003246-199409000-00021 [DOI] [PubMed] [Google Scholar]
- 51.Rossi R, Shemie SD, Calderwood S. Prognosis of pediatric bone marrow transplant recipients requiring mechanical ventilation. Crit Care Med. (1999) 27(6):1181–6. 10.1097/00003246-199906000-00048 [DOI] [PubMed] [Google Scholar]
- 52.Keenan HT, Bratton SL, Martin LD, Crawford SW, Weiss NS. Outcome of children who require mechanical ventilatory support after bone marrow transplantation. Crit Care Med. (2000) 28(3):830–5. 10.1097/00003246-200003000-00036 [DOI] [PubMed] [Google Scholar]
- 53.Hagen SA, Craig DM, Martin PL, Plumer DD, Gentile MA, Schulman SR, et al. Mechanically ventilated pediatric stem cell transplant recipients: effect of cord blood transplant and organ dysfunction on outcome. Pediatr Crit Care Med. (2003) 4(2):206–13. 10.1097/01.PCC.0000043293.83440.79 [DOI] [PubMed] [Google Scholar]
- 54.Bojko T, Notterman DA, Greenwald BM, de Bruin WJ, Magid MS, Godwin T. Acute hypoxemic respiratory failure in children following bone marrow transplantation: an outcome and pathologic study. Crit Care Med. (1995) 23(4):755–9. 10.1097/00003246-199504000-00026 [DOI] [PubMed] [Google Scholar]
- 55.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. (2009) 15(7):817–26. 10.1016/j.bbmt.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 56.van Gestel JPJ, Bollen CW, Bierings MB, Boelens JJ, Wulffraat NM, van Vught AJ. Survival in a recent cohort of mechanically ventilated pediatric allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. (2008) 14(12):1385–93. 10.1016/j.bbmt.2008.09.020 [DOI] [PubMed] [Google Scholar]
- 57.Balit CR, Horan R, Dorofaeff T, Frndova H, Doyle J, Cox PN. Pediatric hematopoietic stem cell transplant and intensive care: have things changed? Pediatr Crit Care Med. (2016) 17(3):e109–16. 10.1097/PCC.0000000000000607 [DOI] [PubMed] [Google Scholar]
- 58.van Gestel JPJ, Bierings MB, Dauger S, Dalle JH, Pavlíček P, Sedláček P, et al. Outcome of invasive mechanical ventilation after pediatric allogeneic hematopoietic SCT: results from a prospective, multicenter registry. Bone Marrow Transplant. (2014) 49(10):1287–92. 10.1038/bmt.2014.147 [DOI] [PubMed] [Google Scholar]
- 59.Rowan CM, Gertz SJ, McArthur J, Fitzgerald JC, Nitu ME, Loomis A, et al. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: a multicenter study. Pediatr Crit Care Med. (2016) 17(4):294–302. 10.1097/PCC.0000000000000673 [DOI] [PubMed] [Google Scholar]
- 60.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. (2015) 43(9):1986–94. 10.1097/CCM.0000000000001085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American college of critical care medicine. Crit Care Med. (2009) 37(2):666–88. 10.1097/CCM.0b013e31819323c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mahadeo KM, McArthur J, Adams RH, Radhi M, Angelo J, Jeyapalan A, et al. Consensus report by the pediatric acute lung injury and sepsis investigators and pediatric blood and marrow transplant consortium joint working committees on supportive care guidelines for management of veno-occlusive disease in children and adolescents: part 2—focus on ascites, fluid and electrolytes, renal, and transfusion issues. Biol Blood Marrow Transplant. (2017) 23(12):2023–33. 10.1016/j.bbmt.2017.08.014 [DOI] [PubMed] [Google Scholar]
- 63.Shimomura Y, Morita M, Hashimoto H, Ishikawa T. Fluid overload early after allogeneic hematopoietic stem cell transplantation was associated with poor survival. Blood. (2017) 130:3233. 10.1182/blood.V130.Suppl_1.3233.3233 [DOI] [Google Scholar]
- 64.Tomaske M, Bosk A, Eyrich M, Bader P, Niethammer D. Risks of mortality in children admitted to the paediatric intensive care unit after haematopoietic stem cell transplantation. Br J Haematol. (2003) 121(6):886–91. 10.1046/j.1365-2141.2003.04390.x [DOI] [PubMed] [Google Scholar]
- 65.Rowan CM, Nitu ME, Moser EAS, Swigonski NL, Renbarger JL. Weight gain and supplemental O2: risk factors during the hematopoietic cell transplant admission in pediatric patients. Pediatr Blood Cancer. (2017) 64(11). 10.1002/pbc.26561 [DOI] [PubMed] [Google Scholar]
- 66.Benoit G, Phan V, Duval M, Champagne M, Litalien C, Merouani A. Fluid balance of pediatric hematopoietic stem cell transplant recipients and intensive care unit admission. Pediatr Nephrol. (2007) 22(3):441–7. 10.1007/s00467-006-0331-z [DOI] [PubMed] [Google Scholar]
- 67.van Gestel JP, Bollen CW, van der Tweel I, Boelens JJ, van Vught AJ. Intensive care unit mortality trends in children after hematopoietic stem cell transplantation: a meta-regression analysis. Crit Care Med. (2008) 36(10):2898–904. 10.1097/CCM.0b013e318186a34a [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.