Abstract
Background
Actinium-225 (Ac-225) labelled PSMA RLT has been tested recently in metastatic castration-resistant prostate cancer (mCRPC), with encouraging results. Ac-225, being an alpha emitter, is expected to have higher efficacy and fewer side effects compared to the beta-emitters such as Lutetium-177. We have performed a meta-analysis to assess the therapeutic responses, survival effects, and significant side effects of Ac-225 PSMA RLT in patients with mCRPC.
Methodology
Systematic literature search was carried out from five electronic databases PubMed/MEDLINE, SCOPUS, EMBASE, Web of Science, and Cochrane Library until March 2021. Eight studies were found to be eligible for this metanalysis.
Results
Eight studies with 226 patients were analyzed in this metanalysis. 81% (95% CI 73–89) patients had a decline in PSA levels. 60% of the patients showed more than 50% PSA decline. Two studies assessed survival effects of radioligand naïve patients compared to patients who had received Lu-PSMA therapy previously and the pooled HR for radioligand naïve patients is 0.22. The most common toxicity reported was xerostomia in 167 patients out of 226 patients (73.9%, 95% CI 67.6–79.5%); however, most of them were confined to grade I and II levels. Other reported side effects include hematologic toxicity and nephrotoxicity.
Conclusion
Ac-PSMA RLT is a safe and potentially effective treatment option for patients with mCRPC.
Keywords: Actinium-225 prostate-specific membrane antigen radioligand therapy, Metanalysis, Metastatic castration-resistant prostate cancer
Highlights
-
•
This meta-analysis focuses on therapeutic responses, survival effects, and toxicity of Actinium-225 prostate-specific membrane antigen (Ac-225 PSMA) radioligand therapy in metastatic castration-resistant prostate cancer patients.
-
•
Patients enrolled in the studies underwent various local and systemic therapies before receiving 225Ac-PSMA.
Introduction
Carcinoma prostate is one of the most common causes of cancer-related death in the world. It is the 2nd most common cancer in the world and is showing rising trends [1, 2]. Patients with carcinoma prostate patients have a remarkable prognosis in the early stage, but with the advancement of stage, they tend to show resistance to almost all the standard lines of treatment [3]. Metastatic castration-resistant prostate cancer (mCRPC) is a known sequela in the disease spectrum of prostate cancer. Most patients with mCRPC present with progressively rising levels of prostate-specific antigen (PSA), extensive disease, and symptoms related to metastases. Therefore, more efficacious treatment options are needed. Docetaxel was among the first to be used in mCRPC and has been used for the last 2 decades. With time, new molecules such as cabazitaxel, abiraterone, enzalutamide have been tested for this purpose. Prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) has been a significant addition to this group. Many studies on RLT including metanalyses have shown encouraging results with Lu-177 PSMA [4–6]. Recently, Actinium-225 (Ac-225) labelled PSMA RLT has also been tried in mCRPC, which has shown very encouraging results. Ac-225, being an alpha emitter, is expected to have higher efficacy and fewer side effects compared to the beta-emitters such as Lutetium-177. Alpha-emitters seem to be a promising therapeutic option due to their characteristics of being short-range, high-energy particles with significantly higher linear energy transfer compared to beta-particles. The short-range causes less tissue damage to adjacent healthy tissues as compared to beta-particles. We present here a meta-analysis on therapeutic responses, survival effects and significant side effects of Ac-225 PSMA RLT in the patients with mCRPC.
Materials and Methods
Selection and Eligibility Criteria
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) standards were followed to evaluate and describe an evidence-based minimum set of items for reporting in systematic reviews and meta-analysis [7]. The predefined PICO (P-Populations/People/Patient/Problem, I-Intervention(s), C-Comparison, O-Outcome) worksheet and search strategy were used to perform this meta-analysis (Table 1) [8]. Both prospective and retrospective studies were extracted and evaluated.
Table 1.
PICO worksheet (radioligand therapy with 225Ac-PSMA for metastatic castration-resistant prostate cancer)
| P Patient | Patients with metastatic castration-resistant prostate cancer (mCRPC), those have taken 225Ac-PSMA alpha radioligand therapy |
| I Intervention | 225Ac-PSMA radioligand therapy |
| C Comparison | Studies in which patients were given 225Ac-PSMA therapy along with comparison was done with 177Lu-PSMA radioligand therapy or 223Ra-dichloride |
| O Outcome | Safety and efficacy of 225Ac-PSMA therapy, biochemical response, clinical response, survival, and toxicity |
PSMA, prostate-specific membrane antigen.
Search Strategy
A systematic literature search was done in 5 electronic databases PubMed/MEDLINE, SCOPUS, EMBASE, Web of Science, and Cochrane Library until March 2021. We used a search algorithm based on combination of key terms and Boolean operators. Keywords were as follows: ((PSMA[Ti]) OR (“prostate specific membrane antigen“[Ti]) OR (PSMA-617[Ti])) AND ((Actinium[Ti]) OR (“Actinium 225”[Ti]) OR (Ac225[Ti]) OR (Ac-225[Ti]) OR (Ac[Ti]) OR (Alpha Therapy[Ti]) OR (α-therapy[Ti])). No language restriction was followed for the broader search; however, only studies in English were included in this study.
Selection of Studies
Titles and abstracts of searched articles were screened according to our inclusion and exclusion criteria. Potential eligible studies were included for data extraction after full-text review. These three stages of screening were performed independently by two reviewers who selected those articles in meta-analysis which met the following inclusion criteria: (1) original (clinical study or trial) studies of 225Ac-PSMA RLT, (2) studies with patient number >9, (3) studies with biochemical response, imaging data, survival data, and toxicity data. Reviewers excluded studies having following characteristics: (1) studies on radiotracers other than Ac-225 PSMA, (2) review articles, comments, abstracts presented at seminars, editorials, or letters, (3) case studies, (4) papers on radiochemistry, dosimetry, and bio-distribution, (5) case series with sample size <9 patients, (6) studies on Tandem therapy.
Data Extraction
Two reviewers evaluated the following details from finalized papers into a predefined Microsoft Excel sheet; first author, publication year, study design (prospective or retrospective), number of patients, median or mean age of patients, number of cycles of therapy, administered activity, extent of disease, Gleason score, baseline level of tumor marker (PSA), median follow-up duration, biochemical response (any PSA decline and >50% PSA decline), objective imaging response, overall survival (OS), progression-free survival (PFS), toxicity, and number of deaths.
Quality Assessment
The quality assessment of the included studies was done by two authors independently as per the Newcastle-Ottawa score for non-randomized cohort studies [9]. The quality scale is comprised of three domains: (1) selection, (2) comparability, and (3) outcome. Grading of quality of the three domains was done by using star system. All the disagreements were discussed and resolved to achieve a consensus.
Statistical Analysis
Continuous variables were represented in the form of median with interquartile range (IQR). Demographic data, biochemical response data PSA decline (both any and >50%), imaging response data (using both RECIST and PERCIST), survival data like OS, PFS, and toxicity were expressed in tabular form. Publication bias among studies was assessed by using funnel plots [10]. To minimize the heterogeneity among the studies, random-effects model was used while doing statistical analysis [11]. Therapeutic response parameters like any PSA decline, more than 50% PSA decline after the end of 225Ac-PSMA RLT, OS, and PFS, were represented in forest plots. All the proportions were expressed with 95% confidence intervals (CIs). The I2 statistic was used for evaluation of heterogeneity among the studies [12]. A p value of <0.05 was considered significant. Statistical analyses of funnel plots and forest plots of response, efficacy, survival, and toxicity were conducted using the STATA software (version 16, StataCorp). Survival data were extracted from the graphical representation of the Kaplan-Meier curves by an Engauge Digitizer (http://digitizer.sourceforge.net) to evaluate the hazard ratio (HR). The pooled survival effects were assessed as the log HRs of PFS using the software RevMan (Version 5.4.1).
Results
Selection and Eligibility Criteria
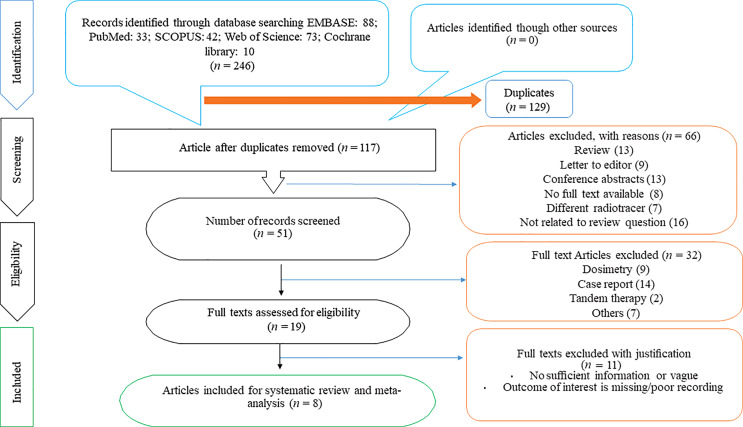
The initial computerized search of PubMed/MEDLINE, EMBASE, SCOPUS, Web of Science, and Cochrane Library resulted in 246 relevant records. Out of these, 129 were excluded as they were duplicated. The titles of rest of 117 articles were reviewed, and 51 were selected for abstract review. Of these, 19 were selected for full-text review and 11 of these were found to be irrelevant as per the inclusion criteria and so were excluded. Finally, eight studies [13–20] were found eligible for this present review. The process of selecting the relevant studies in this review is depicted in the PRISMA flowchart (Fig. 1).
Fig. 1.
PRISMA flowchart depicting the process of selecting relevant studies for this review.
Study Characteristics
Table 2 describes the study characteristics of each of the included articles in terms of publication year, study design (whether prospective or retrospective), scan done prior to therapy initiation, ligand used, sample size, mean or median age of sample, administered activity, total number of therapies given, extent of metastasis and median Gleason score. Only one study [16] was prospective and seven of the eight studies [13–15, 17–20] were retrospective. Patients enrolled in the studies underwent various local and systemic therapies before receiving 225Ac-PSMA. These therapies were variable among the studies and not of a single type. Radical prostatectomy was done in patients in four out of eight studies (n = 4) [14–17]. Most of the studies involved patients who received prior chemotherapy with either docetaxel or cabazitaxel or a combination of both (n = 7) [13, 15–20], antiandrogen therapy with either abiraterone or enzalutamide or combination of both (n = 7) [13, 15–20] and 177Lu-PSMA (n = 7) [14–20]. Other prior therapies received by the patients were androgen deprivation therapy (n = 4), which included medical castration (LHRH analog or LHRH antagonist) or surgical castration [14, 16–18], prior radiotherapy (n = 5) [14–18]. Additionally, prior therapy with 223RaCl2 (n = 4) [13, 17, 19, 20] and bone modifying agents (bisphosphonates/denosumab) (n = 2) [16, 18] were administered to the patients, and two studies mentioned other unspecified treatment given to the patients prior to 225Ac-PSMA [13, 19]. Sathekge et al. [14] included patients with widespread metastasis who did not receive any therapy prior to recruitment for 225Ac-PSMA. Most of the studies used 68Ga-PSMA-11 positron-emission tomography (PET)/CT (n = 5) for lesion characterization prior to therapy initiation; one study [13] had done 68Ga-PSMA-11 PET/CT or 99mTc-MIP-1427 scanning (n = 1), Zacherl et al. [17] mentioned using 18F-PSMA-1007 PET (n = 1). However, no specific radionuclide was mentioned in the study by Feuerecker et al. [19] and the term PSMA-ligand PET was used (n = 1). Most of the studies (n = 7) used PSMA-617 agent in 225Ac-PSMA RLT, only one study [17] used PSMA-I&T. The median of sample size of selected studies was 22 (IQR, 16–31). The pooled median age of the selected studies was 69.85 years (IQR, 68.75–71.37 years). Kratochwil et al. [13] adopted a uniform-dose approach 100 kBq/kg of body weight. Studies using variable dose of PSMA RLT, had doses ranging from 4 to 62.9 MBq/cycle. Sathekge et al. [15] and Doelen et al. [20] initiated therapy by administrating 8 MBq, later deescalated. Median number of therapies given was 3 cycles (range 1–8 cycles) and depended on patient’s condition. Extent of metastatic disease was mentioned in percentage.
Table 2.
Study characteristics
| No. | Study | Year | Study type | Scan prior to therapy | Therapy agent | Patients, n | Age, years | Administered activity | Therapy, n | Extent of metastasis, % | Gleason Score |
Primary outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Kratochwil et al. [13] | 2018 | Retrospective | 68Ga-PSMA-11 PET/CT or 99mTc-MIP-1427 (planar whole-body, torso SPECT/CT) | PSMA–617 | 40 | Median, 70 |
100 kBq/kg of body weight | 3/up to 5 cycles | Bone metastasis (97.5), visceral metastasis (40) [lung (22.5), liver (22.5), brain (5), other (7.5)] | NR | Efficacy of 225Ac-PSMA-617 therapy | NR |
| 2 | Sathekge et al. [14] | 2019 | Retrospective | 68Ga-PSMA-11 PET/CT | PSMA-617 | 17 | Mean±SD, 64.5±9.7 | Mean, 7.4±1.5 MBq | Median, 3 (range, 2–6 cycles) | Bone metastases (82), lymph nodes (50), visceral metastases (12) [lungs (6), brain, and liver (6)] | Median, 9 (range 6–10) | Efficacy of 225Ac-PSMA-617 therapy | 13 (11–15) months |
| 3 | Sathekge et al. [15] | 2020 | Retrospective | 68Ga-PSMA-11 PET/CT | PSMA-617 | 73 | Median, 69 (range, 45–85) | Initial-8 MBq, later deescalated to 7,6,4 MBq | Median, 3 (range, 1–8 cycles) | Bone metastases (90), visceral metastases [lung (3) liver (5) brain (1)] | Median, 8.00 (IQR 7.00–9.00) | Overall and disease-free survival | 9.0 (2.0–22) months |
| 4 | Yadav et al. [16] | 2020 | Prospective | 68Ga-PSMA-11 PET/CT | PSMA–617 | 28 | Mean, 69.7±SD 9.4 (range, 46–87) | Mean, 26.5±12 MBq (range, 9.25–62.9 MBq) | Median, 3 (range, 1–7 cycles) | Bone metastases (96), lymph nodes, [Iliac and abdominal (50) Thoracic to iliac (35.7)], lung metastases (10.7) brain (7), liver (10.7), adrenals (4) |
Median, 9 (range 7–10) | Efficacy and safety of 225Ac-PSMA-617 therapy | 10 (5.0–22) months |
| 5 | Zacherl et al. [17] | 2020 | Retrospective | 18F-PSMA-1007 PET | PSMA-I&T | 18 | Median (IQR), 75 (64–80) | Median, 7.8 MBq (6.0– 8.5) | Median, 2 (range: 1–5 cycles) | Bone (93), lymph node (71), visceral metastasis (21) [liver (7) lung (21) other organs (7)] |
NR | Efficacy and toxicity | 23.6 (8.0–77) weeks |
| 6 | Satapathy et al. [18] | 2020 | Retrospective | 68Ga-PSMA-11 PET/CT | PSMA-617 | 11 | Median (IQR), 68 (62–76) | Median, 8.3 MBq (IQR 5.6–20.4 MBq) | Range, 1–4 cycles | Local nodes (82) Distant nodes (27) Skeletal (100) |
Median, (IQR) 8 (7–9) | Health-related quality-of-life outcomes after 225Ac-PSMA-617 therapy | NR |
| 7 | Feuerecker et al. [19] | 2020 | Retrospective | NR | PSMA-617 | 26 | Median (IQR) 72.5 (63–75.75) | Median, 9 MBq (IQR) 8–10 | Median, 2 (IQR 1.3–3.0) cycles | Lymph node (88) Bone (100) Bone only (8) Visceral (42) [liver (19) lung (23) other (19)] |
Median, (IQR) 8 (7–9) | Safety, activity and adverse events | 7.0 (2.4–16) months |
| 8 | Doelen et al. [20] | 2020 | Retrospective | 68Ga-PSMA-11 PET/CT | PSMA-617 | 13 | Median (IQR) 71 (64−77) | Initial-8 MBq, later deescalated to 6 MBq | Median, 3 (range, 1–5) cycles | Bone metastases (100) Bone metastases only (23.1) Locoregional lymph node metastases (76.9) Visceral metastases (61.5) |
NR | Efficacy of 225Ac-PSMA TAT and impact on quality of life | NR |
PSMA, prostate-specific membrane antigen; kBq, Kilobecquerel; MBq, Megabecquerel; NR, not reported; IQR, interquartile range.
Data Extraction
All 8 articles reported a decline in PSA levels after completion of therapy and only 4 [13, 14, 16, 17] of the 8 articles reported a decline after the 1st cycle (Table 4). Only four studies [14–16, 20] reported imaging response data (Table 5). Similarly, only five studies [13, 15, 16, 19, 20] assessed data on OS and PFS. Toxicity data of all the papers are shown in Table 6.
Table 4.
Summary of prostate-specific antigen (PSA) response of 8 studies
| Reference No. | Baseline PSA, ng/mL | Patients evaluated for PSA decline, n | Cycles, N | Follow-up interval, weeks |
PSA decline after 1st cycle | PSA decline after completion of RLT | ||
|---|---|---|---|---|---|---|---|---|
| any PSA | >50% PSA decline | any PSA | >50% PSA decline | |||||
| [13] | Median 169 | 38 | NR | 4 | 33/38 (87%) | 24/38 (63%) | 22/38 (57.8%) | 18/38 (47.3%) |
| [14] | Median, 49.08 (range 1.20–1,300.69) | 17 | 59 | 4 | 14/17 (82.3%) | 13/17 (76.4%) | 16/17 (94.1%) | 15/17 (88.2%) |
| [15] | Median, 57.2 | 73 | 210 | 4 | NR | NR | 60/73 (82%) | 51/73 (70%) |
| [16] | Median (25–75% IQR) 222.2 (47–443.2) | 28 | 85 | 3–4 | 25/28 (89.2%) | 7/28 (25%) | 22/28 (78.6%) | 11/28 (39%) |
| [17] | Median, 176 (range 13.4–1,146) | 14 | 38 | 4–8 | 8/14 (57%) | 3/14 (21%) | NR | NR |
| [18] | Median (IQR), 158 (35–840) | 11 | 25 | 3 | NR | NR | 8/11 (72.7%) | 5/11 (46%) |
| [19] | Median (IQR), 331 (142–682) | 26 | 61 | 4–8 | NR | NR | 23/26 (95% CI 70–97%) | 17/26 (95% CI 46–81%) |
| [20] | Median (IQR), 878 (203−1,611) | 13 | NR | NR | NR | NR | 11/13 (84.6%) | 9/13 (69%) |
PSA, prostate-specific antigen; ng/mL, nanogram/milliliter; RLT, radioligand therapy; NR, not reported; IQR, interquartile range; CI, confidence interval.
Table 5.
Overview of imaging results, clinical response, and survival data across 8 studies
| Reference No. | Patients, N | RECIST | PERCIST | Deaths, n | OS, months | PFS, months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients evaluated, n | CR or PR | SD | PD | Patients evaluated, n | CR or PR | SD | PD | |||||
| [13] | 40 | NR | NR | NR | NR | NR | NR | NR | NR | 2 | >12 | 6 |
| [14] | 17 | NR | NR | NR | NR | 17 | 15 | 0 | 2 | 3 | NR | NR |
| [15] | 73 | NR | NR | NR | NR | 73 | NR | NR | 23 | 13 | 18 (95% CI, 16.2–19.9) | 15.2 (95% CI, 13.1–17.4 |
| [16] | 28 | NR | NR | NR | NR | 22 | 12 | 2 | 8 | 6 | 17 (95% CI: 16- upper limit not reached) | 12 (95% CI: 9–13) |
| [17] | 18 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [18] | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [19] | 26 | NR | NR | NR | NR | NR | NR | NR | NR | 18 (95% CI 49–84%) | 7.7 (95% CI 4.5–12.1) | 4.1 (95% CI 3.0–14.8) |
| [20] | 13 | 6 | 3 | 1 | 2 | 7 | 6 | 0 | 1 | 11(85%) | 8.5 | 5.5 |
RECIST, Response Evaluation Criteria in Solid Tumors; PERCIST, PET Response Criteria in Solid Tumors; OS, overall survival, PFS, progression-free survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NR, not reported; CI, confidence interval.
Table 6.
Overview of toxicity profile across 8 studies
| Reference No. | Patients, N | Salivary gland toxicity (xerostomia) | Hematologic toxicity | Nephrotoxicity/renal failure | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| haemoglobin/anaemia | WBC count/leukopenia | platelets/thrombocytopenia | ||||||||||||||
| grade I and II | grade III | grade IV | grade I and II | grade III | grade IV | grade I and II | grade III | grade IV | grade I and II | grade III | grade IV | grade I and II | grade III | grade IV | ||
| [13] | 40 | Severe xerostomia-15 | Intolerable xerostomia-4 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| [14] | 17 | 17 | 0 | 0 | NR | 1 | 0 | NR | NR | NR | NR | NR | NR | NR | 0 | 1 |
| [15] | 73 | 62 | 0 | 0 | 22 | 5 | 0 | 7 | 2 | 0 | 6 | 1 | 0 | 18 | 3 | 2 |
| [16] | 28 | 8 | 0 | 0 | 27 | 1 | 0 | 11 | 0 | 0 | 4 | 0 | 0 | 4 | 0 | 0 |
| [17] | 18 | 13 | 0 | ND | 11 | 3 | 0 | 4 | 1 | 0 | 6 | 0 | 0 | 2 | 0 | 0 |
| [18] | 11 | 8 | 1 | 0 | 8 | 1 | 0 | 5 | 0 | 0 | 5 | 2 | 0 | 1 | 1 | 0 |
| [19] | 26 | 26 | 0 | ND | 6 | 8 | 1 | 6 | 7 | 0 | 9 | 3 | 2 | 5 | 0 | 0 |
| [20] | 13 | 13 | 0 | 0 | NR | 0 | 0 | NR | 0 | 0 | NR | 0 | 0 | NR | 0 | 0 |
NR, not reported; ND, not defined.
Quality Assessment
Quality assessment of the included studies was performed according to the Newcastle-Ottawa score. The quality assessment scoring of all the included studies is depicted with supportive explanation in Table 3. All patients included in the study had mCRPC and had undergone multiple prior treatments. Follow-up was done for at least 6 months. Two studies [15, 16] compared the survivals between patients in pre 177Lu-PSMA therapy group and RLT naïve therapy group. All the included studies were of good quality as per the Newcastle-Ottawa Scale, with 6 of the 8 [13, 14, 17–20] having a total score of 7 stars each and 2 of the 8 articles [15, 16] scored 8 stars each.
Table 3.
Newcastle-Ottawa quality assessment scoring of included studies
| Reference No. | Selection | Comparability | Outcome | Quality score | |||||
|---|---|---|---|---|---|---|---|---|---|
| representativeness quality score of exposed cohort | selection of the non-exposed cohort from same source as exposed cohort | ascertainment of exposure | outcome of interest was not present at start of the study | comparability of cohorts | assessment of outcome | follow-up long enough for outcome to occur (median duration of follow-up ≥6 months) | adequacy of follow-up | ||
| [13] | ★ | – | ★ | ★ | ★ | ★ | ★ | ★ | 7★ Good |
| [14] | ★ | – | ★ | ★ | ★ | ★ | ★ | ★ | 7★ Good |
| [15] | ★ | – | ★ | ★ | ★★ | ★ | ★ | ★ | 8★ Good |
| [16] | ★ | – | ★ | ★ | ★★ | ★ | ★ | ★ | 8★ Good |
| [17] | ★ | – | ★ | ★ | ★ | ★ | ★ | ★ | 7★ Good |
| [18] | ★ | – | ★ | ★ | ★ | ★ | ★ | ★ | 7★ Good |
| [19] | ★ | – | ★ | ★ | ★ | ★ | ★ | ★ | 7★ Good |
| [20] | ★ | – | ★ | ★ | ★ | ★ | ★ | ★ | 7★ Good |
Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
Therapeutic Efficacy in Terms of PSA Response at the End of Treatment: Any PSA Decline
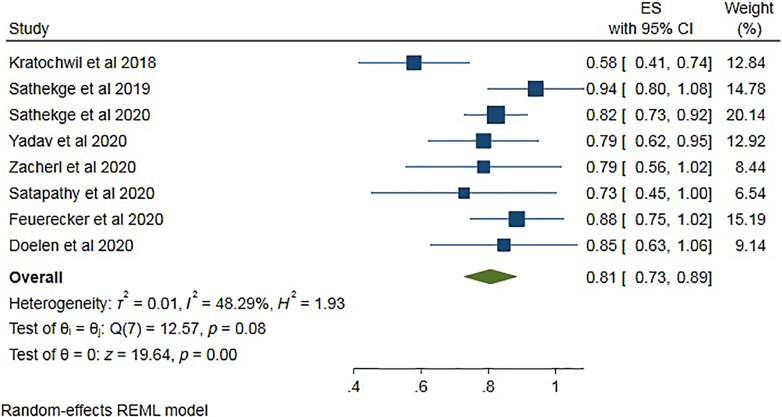
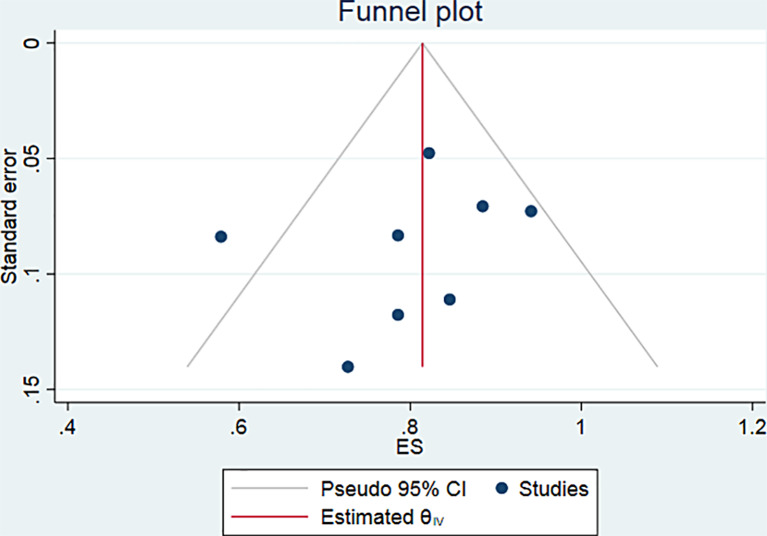
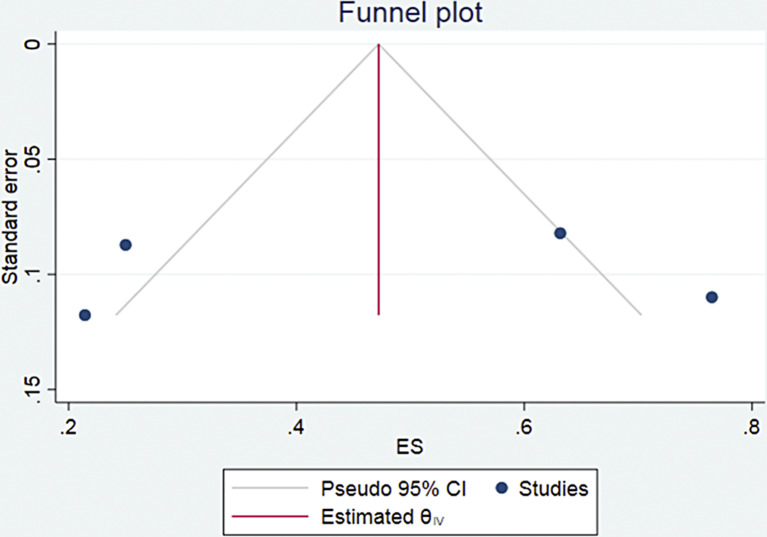
All the eight studies comprising 226 patients were included in the evaluation of any PSA decline at the end of treatment (Table 4). A total of 183 of the 226 patients showed any PSA decline. Pooled analysis was done using forest plot (Fig. 2), which showed overall 81% (95% CI 73–89) patients showed any PSA decline at the treatment course. The funnel plot (Fig. 3) for the proportion of any PSA decline showed no significant publication bias with a p value of 0.5 in egger’s test. The included studies showed significant heterogeneity with I2 = 48.29% (p = 0.08).
Fig. 2.
Forest plot for pooled analysis of the patients showing any PSA decline during treatment.
Fig. 3.
Funnel plot for the patients showing any PSA decline during treatment.
More than 50% PSA Decline
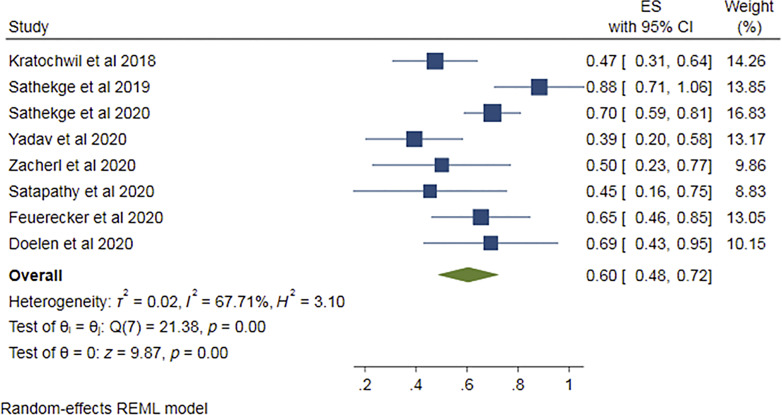
All the eight studies [13–20] provided data regarding >50% PSA decline. The pooled proportion was found out to be 0.60 (95% CI 0.48–0.72) (Fig. 4), i.e., 60% of the patients showed more than 50% PSA decline. Funnel plot (Fig. 5) for this proportion showed no significant publication bias and “p” value in Egger’s test being 0.4. I2 value was 67.71% (p = 0.00), suggesting notable heterogeneity among studies.
Fig. 4.
Forest plot for pooled analysis of patients showing >50% PSA decline.
Fig. 5.
Funnel plot for the patients showing >50% PSA decline.
PSA Response after 1st Cycle of Therapy
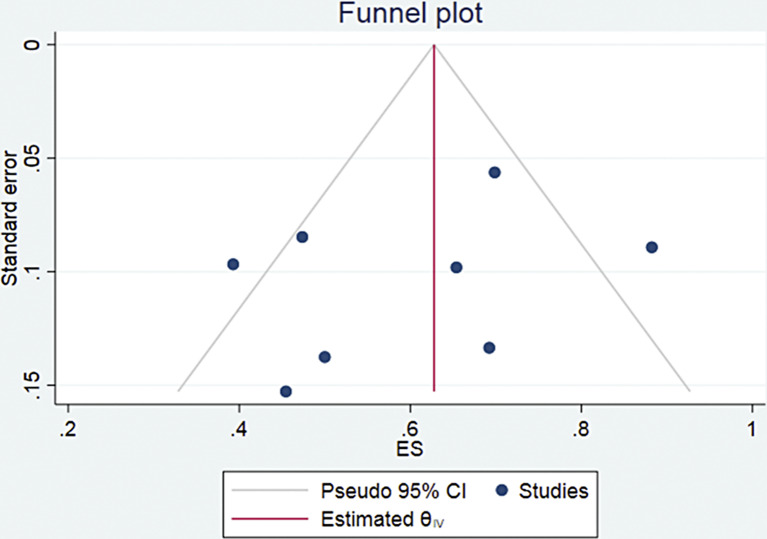
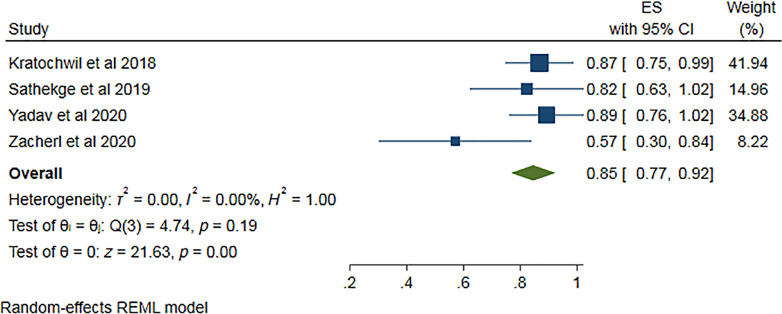
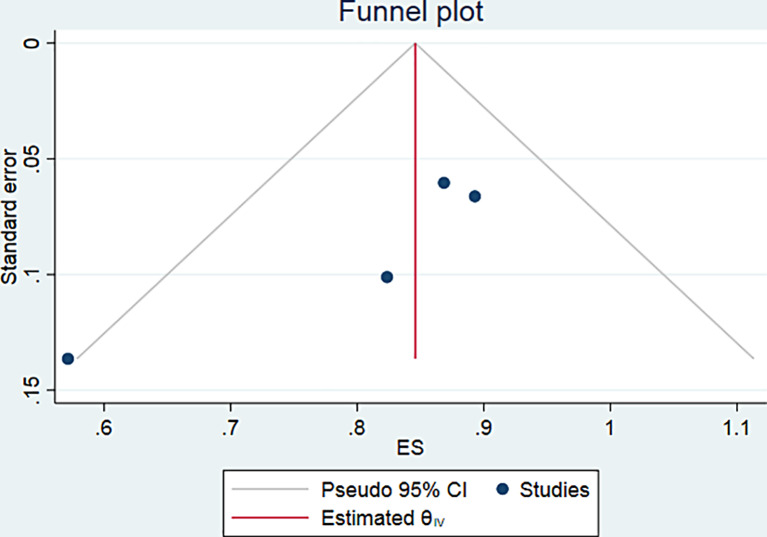
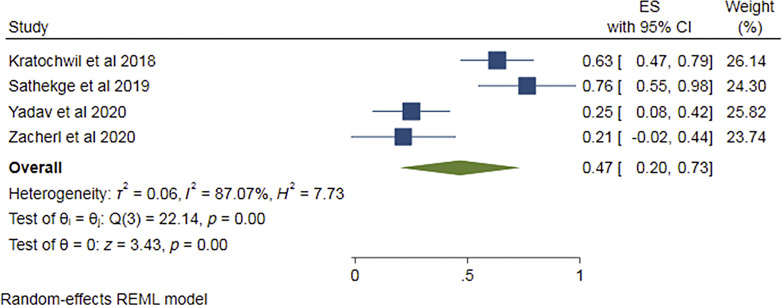
Four of the eight studies [13–17] provided PSA response data after 1st cycle of Ac-PSMA therapy comprising 97 patients. Pooled analysis was done using forest plot (Fig. 6), which showed overall 85% (95% CI 77–92%) patients showed any PSA decline after 1st cycle. The funnel plot (Fig. 7) for the proportion of any PSA decline showed minimal publication bias with a p value of 0.051 in Egger’s test. The included studies showed no significant heterogeneity with I2 = 0.00% (p = 0.00). For >50% PSA decline after 1st cycle of Ac-PSMA therapy, pooled analysis using forest plot (Fig. 8) showed overall 47% (95% CI 20–73%) patients showed >50% PSA decline after 1st cycle. The funnel plot (Fig. 9) for the proportion of >50% PSA decline showed no significant publication bias with a p value of 0.87 in egger’s test. The included studies showed significant heterogeneity among the studies with I2 = 87.07% (p = 0.00).
Fig. 6.
Forest plot for pooled analysis of patients showing any PSA decline after the 1st cycle of therapy.
Fig. 7.
Funnel plot for patients showing any PSA decline after the 1st cycle of therapy.
Fig. 8.
Forest plot for pooled analysis of patients showing >50% PSA decline after the 1st cycle of therapy.
Fig. 9.
Funnel plot for patients showing >50% PSA decline after the 1st cycle of therapy.
Survival
Not all studies provided data regarding OS and PFS (Table 5). Only five of the eight studies [13, 15, 16, 19, 20] presented information on OS and PFS. In these five studies, median PFS and pooled median PFS were found out to be 6 months (IQR 5.5–7 months). In the eight studies, out of total 222 patients, among which PSA progression was assessed, overall, 47 patients (i.e., 21.2%, 95% CI 16–27%) showed a progression of disease. However, radiological and/or molecular (PERCIST) response was assessed only in four studies [14–16, 20]. Overall, 34 patients of 119 patients (28.6%, 95% CI 21–37%) showed a progression of disease in molecular imaging findings. Three studies [14, 16, 20] assessed CR/PR and SD proportion; 33 of 44 patients showed either CR/PR; only 2 patients showed SD after the treatment. Although five [13, 15, 16, 19, 20] studies have mentioned the OS, one [13] of them could not achieve the median OS. The pooled median OS of the four [15, 16, 19, 20] studies was 12.75 months (IQR 8.1–17.5 months).
Hazard Ratio
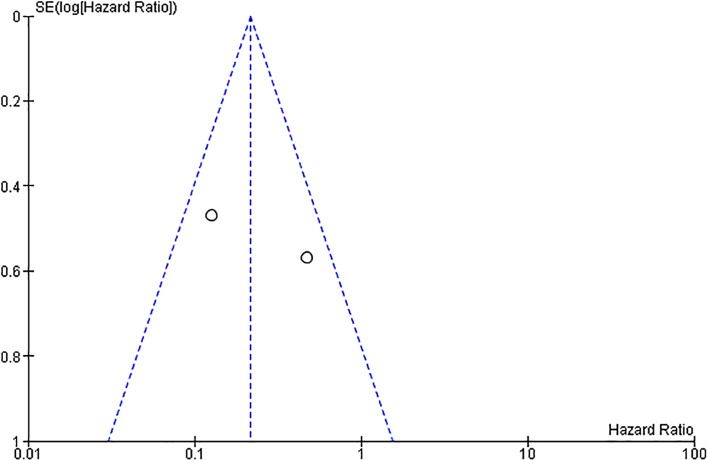
Two studies [15, 16] assessed survival effects of radioligand naïve patients versus patients who received Lu-PSMA therapy before. The pooled HR for radioligand naïve patients is 0.22 with significance, using fixed-effect model (95% CI, 0.11–0.44; p < 0.0001) (Fig. 10). The I2 statistics were 68% (Fig. 11), suggesting significant heterogeneity between the studies.
Fig. 10.
Forest plot for pooled hazard ratio analysis of radioligand naïve patients.
Fig. 11.
Funnel plot for hazard ratio of survival effect in radioligand-naïve patients versus patients who received prior radioligand therapy.
Safety Assessment
Most of the studies showed low-grade toxicity due to Ac-PSMA RLT (Table 6). The most common toxicity reported being xerostomia occurring in 167 patients out of 226 patients (73.9%, 95% CI 67.6–79.5%); however, most of them are confined to grade I and II levels. Other reported side effects include hematologic toxicity and nephrotoxicity. All the studies reported xerostomia, and only seven [14–20] reported hematologic toxicity and nephrotoxicity. Anemia grade III or higher was seen in 20 of 186 patients (10.75%, 95% CI 6.7–16.1%) in seven studies [14–20], leukopenia in 10 of 169 patients (5.9%, 95% CI 2.9–10.6%) in six studies [15–20], thrombocytopenia in 8 of 169 patients (4.73%, 95% CI 2.1–9.1%) in six studies [15–20] and nephrotoxicity in 7 out of 186 patients (3.76%, 95% CI 1.5–7.6%). In one study [13], xerostomia was reported as “severe” and “intolerable” rather than any specific grade. In the rest of the studies, only one patient showed grade 3 or more xerostomia [18].
Discussion
This systematic review with meta-analysis is focused on the therapeutic responses, survival effects, and significant side effects of 225Ac-PSMA RLT in mCRPC. The results showed a significant efficacy of Ac-PSMA RLT with reported PSA decline after completion of therapy in all the eight studies. Response rates with 225Ac-PSMA RLT are found to be higher than that reported of 177Lu-PSMA RLT in a previously published meta-analysis of mCRPC patients (60% vs. 46%, respectively). Overall, 81% patients demonstrated any PSA decline at the treatment course with 60% of the patients experiencing more than 50% PSA decline. After the first cycle, 85% of the patients exhibited any PSA decline and 47% patients showed >50% PSA decline reported in four of the studies [13, 14, 16, 17]. In four studies [14–16, 20] reporting imaging response data, 28.6% of patients showed disease progression based on molecular imaging findings, while 21.2% of patients assessed for PSA progression demonstrated disease progression. Median PFS assessed in five studies [13, 15, 16, 19, 20] was found to be 6 months. Pooled median OS from four studies [15, 16, 19, 20] was 12.75 months. However, one study [13] failed to achieve the median OS. Toxicity was assessed in all the studies; with the most frequently reported toxicity being xerostomia (73.9%), mostly confined to grade I and II. This can be explained by physiological expression of PSMA in salivary glands [21]. Only one patient showed grade 3 or higher xerostomia. Anemia of grade III or more was seen in 10.75% patients [14–20], leukopenia in 5.9% [15–20], thrombocytopenia in 4.73% [15–20], and nephrotoxicity in 3.76% patients [14–20]. Clinically significant treatment-related toxicity was seen in a limited number of the patients, which in combination with a favorable treatment response, signifies a relatively better quality-of-life for such patients. Two studies [15, 16] assessed survival effects between radioligand naïve patients versus those who received Lu-PSMA therapy previously. Pooled HR for radioligand naïve patients was found to be 0.22. The results are significant given that these studies on 225Ac-PSMA also included patients who had already progressed on 177Lu-PSMA and showed clinical benefit after alpha therapy. In 177Lu-PSMA RLT, prior chemotherapy has been reported to be a worse predictor of survival outcomes and response [22, 23]. So, it may be possible that 225Ac-PSMA RLT at an earlier stage of the disease could produce favorable outcomes.
While both 225Ac-PSMA and 177Lu-PSMA have shown promising results in clinical studies, 225Ac-PSMA is a relatively newer agent that is still being evaluated in ongoing clinical trials. 225Ac-based targeted alpha-radiation therapy may be more effective for disseminated metastatic disease due to its short range, high LET, and reduced damage to healthy tissues compared to 177Lu-PSMA including red bone marrow. However, a higher rate of xerostomia is observed with 225Ac-PSMA [13–16, 18–20]. Kratochwil et al. [13] divided patients into two groups (Lu-PSMA vs. Ac-PSMA therapy groups) based on the pattern of expression on prior imaging. As such, patient selection criteria for Ac-PSMA may vary depending on the specific trial and its stage of development and may evolve over time as more clinical data becomes available. Additional research is needed to determine the specific patient population that may benefit the most from a particular radiolabeled therapy. In our meta-analysis, seven out of eight studies [14–20] included patients who received prior Lu-PSMA therapy and concluded that Ac-PSMA can be useful in patients refractory to Lu-PSMA. Understanding the mechanisms of radioresistance in prostate cancer cells could pave the way for treatment strategies aimed at inhibiting radioresistance, potentially enhancing the effectiveness of therapies such as 177Lu-PSMA-617, 177Lu-PSMA-I&T, and 225Ac-PSMA-617.
This meta-analysis is accompanied by certain limitations. The included studies were single-arm observational studies on small patient populations thereby limiting the strength of these observations and had an inherently high risk of bias. Only 4 out of 8 selected studies reported PSA decline after 1st cycle, which is too small to draw a conclusion by metanalysis including the funnel plot analysis. Also, assessing pooled HR using only 2 papers about the survival effects has a limited significance. There was heterogeneity of the study design, patients’ clinical stage, degree of PSMA expression, and follow‐up; for example, in one study [13] 70% of the patients were treated by docetaxel, whereas another study [14] included chemotherapy-naive patients. However, we tried to account for the statistical heterogeneities by using the random-effects model in our analysis.
Conclusions
RLT is the new addition to the armamentarium of treatment options available for metastatic prostate cancer. Among RLTs, Ac-225 PSMA therapy is one of the latest developments. Being an alpha emitter, Ac-225 labelled RLT shows promising efficacy and reasonable safety profile in the management of metastatic prostate cancer.
Statement of Ethics
Ethical approval was obtained from the Institutional Ethics Committee.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Funding Sources
None.
Author Contributions
Dr. Girish Kumar Parida: study design and statistical analysis; Mr. Raj Abhisek Panda: data collection and writing the manuscript; Dr. Komal Bishnoi: writing the manuscript; and Dr. Kanhaiyalal Agrawal: guiding and mentoring.
Funding Statement
None.
Data Availability Statement
As this is a metanalysis, all the data are available as published papers.
References
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends--an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2. Jain S, Saxena S, Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–605. 10.1016/j.mgene.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300, 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4. Kim YJ, Kim YI. Therapeutic responses and survival effects of 177Lu-PSMA-617 radioligand therapy in metastatic castrate-resistant prostate cancer: a meta-analysis. Clin Nucl Med. 2018 Oct;43(10):728–34. 10.1097/RLU.0000000000002210. [DOI] [PubMed] [Google Scholar]
- 5. Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019 Aug;213(2):275–85. 10.2214/AJR.18.20845. [DOI] [PubMed] [Google Scholar]
- 6. Von Eyben FE, Bauman G, Von Eyben R, Rahbar K, Soydal C, Haug AR, et al. Optimizing PSMA radioligand therapy for patients with metastatic castration-resistant prostate cancer. a systematic review and meta-analysis. Int J Mol Sci. 2020 Nov 28;21(23):9054. 10.3390/ijms21239054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sackett DL. Evidence-based medicine: how to practice and teach EBM. Philadelphia, PA, USA: WB Saunders Company; 1997. [Google Scholar]
- 9. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and metaanalysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 10. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–34. . [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N. Meta-analysis in clinical trials. Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in metaanalyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59(5):795–802. 10.2967/jnumed.117.203539. [DOI] [PubMed] [Google Scholar]
- 14. Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46(1):129–38. 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving 225Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61(1):62–9. 10.2967/jnumed.119.229229. [DOI] [PubMed] [Google Scholar]
- 16. Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant prostate cancer patients. Theranostics. 2020;10(20):9364–77. 10.7150/thno.48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zacherl MJ, Gildehaus FJ, Mittlmeier L, Böning G, Gosewisch A, Wenter V, et al. First clinical results for PSMA-targeted α-therapy using 225Ac-PSMA-I&T in advanced-mCRPC patients. J Nucl Med. 2021;62(5):669–74. 10.2967/jnumed.120.251017. [DOI] [PubMed] [Google Scholar]
- 18. Satapathy S, Mittal BR, Sood A, Das CK, Singh SK, Mavuduru RS, et al. Health-related quality-of-life outcomes with actinium-225-prostate specific membrane antigen-617 therapy in patients with heavily pretreated metastatic castration-resistant prostate cancer. Indian J Nucl Med. 2020;35(4):299–304. 10.4103/ijnm.IJNM_130_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol. 2021;79(3):343–50. 10.1016/j.eururo.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 20. van der Doelen MJ, Mehra N, van Oort IM, Looijen-Salamon MG, Janssen MJR, Custers JAE, et al. Clinical outcomes and molecular profiling of advanced metastatic castration-resistant prostate cancer patients treated with 225Ac-PSMA-617 targeted alpha-radiation therapy. Urol Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 21. Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 22. Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60(7):955–62. 10.2967/jnumed.118.216820. [DOI] [PubMed] [Google Scholar]
- 23. Kessel K, Seifert R, Schäfers M, Weckesser M, Schlack K, Boegemann M, et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics. 2019;9(17):4841–8. 10.7150/thno.35759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As this is a metanalysis, all the data are available as published papers.