Abstract
Introduction
Fibroadenomas are solid, mobile, and non-tender benign breast lumps, with highest prevalence amongst young women aged between 15 and 35. Symptoms can include discomfort, and they can become problematic, particularly when they enlarge, resulting in many referrals for biopsies, with fibroadenomas accounting for 30–75% of the cases. Diagnosis is based on triple assessment that involves a clinical examination, ultrasound imaging, and mammography, as well as core needle biopsies. Current management includes observation for 6–12 months, with the indication of definitive surgery, in cases that are older than 35 years or with fibroadenoma persistence. Serious adverse effects of surgery might include nipple areolar distortion, scarring, and damage to the breast tissue, as well as the risks associated with surgery and anesthesia, making it a non-feasible option.
Methods
A literature search was performed on the databases Embase, MEDLINE/PubMed, Google Scholar, and Ovid for English language papers published between January 1, 2000, and March 17, 2021. A structured protocol was employed to devise a comprehensive search strategy with keywords and Boolean operators defined by the research question. The keywords used for the search were “HIFU”, “High Intensity Focused Ultrasound,” “Fibroadenoma,” “Breast,” “Lesion.” This review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Recently, a thermal ablative technique, high intensity focused ultrasound (HIFU), was found to be a safe, noninvasive, and technically successful alternative, having displayed promising outcomes in reducing the volume of fibroadenomas, pain experienced by patients, and the length of hospitalization. Quality of life improvement was also evidenced, exhibited by the disappearance of symptoms, and enhanced physical activity post-intervention, in addition to patients’ satisfaction with the cosmetic results and future recommendation of the procedure to other patients.
Conclusion
Overall, HIFU is a well-tolerated treatment associated, with low risk of complications, that can potentially include erythema, skin discoloration, and bruising with the majority of these self-resolving shortly after the procedure.
Keywords: Ultrasound, High intensity focused ultrasound, Breast, Efficacy, Side effects, Fibroadenoma
Introduction
Fibroadenomas (FA) are the most common benign breast lesions in women, accounting for between 30% and 75% of all breast biopsies [1, 2]. The most common age of occurrence of FA is between 20 and 30 [1, 3]. FA will affect approximately 1 in 10 women during their lifetime [1, 4–6]. The diagnosis of FA is based upon clinical examination, ultrasound imaging, and core needle biopsy, which are collectively referred to as the triple assessment test [2, 5–7]. Previously, treatment of FA consisted of conservative management with a follow-up of 6–12 months, followed by surgical excision if the patient is over the age of 35 and the FA has not regressed [2]. Surgical excision of FA may potentially cause nipple areolar distortion, scarring, or breast volume loss [8]. The Association of Breast Surgery (ABS) in the UK recommended fibroadenoma excision only for cellular fibroepithelial lesions, lesions with persistent pain, and/or rapidly growing lesions with a core biopsy of fibroadenoma due to their association of these lesions with phyllodes tumor [9]. Factors distinguishing phyllodes tumors from fibroadenomas consisted of the presenting symptoms (persistent palpable breast mass or breast pain), rapidly growing lesions, hyperdense mass on mammogram, and the presence of round cysts or clefts within the mass on ultrasound scans [10]. Implementation of the excision policy recommended by ABS could potentially increase the risk of missing another potentially invasive diagnosis, and hence appropriate safety netting advice should be provided to identify suspicious rapidly growing lesions [9]. Another recent retrospective review by Barakzai et al. [11] evaluated conservative management of fibroadenomas over 5 years and reported the lack of practicality of watchful waiting in the majority of patients due to high patient attrition rate (lack of follow-up) and potential disease progression. Additionally, only 10% of the patients who completed the 5-years observation period had complete resolution of their fibroadenoma [11].
Vacuum-assisted biopsy and cryoablation are non-surgical, minimally invasive techniques that were shown to be safe and effective in the treatment of FA [12–14] and thus are potential alternatives to surgery. A more novel and noninvasive FA treatment uses high intensity focused ultrasound (HIFU), which is a thermal ablative technique that clinical studies show is feasible, safe, and effective in the treatment of benign and malignant tumors of the brain, breast, bone, liver, pancreas, kidney, thyroid, parathyroid, uterus, and prostate [15–21]. With HIFU, an ultrasound beam that propagates through soft tissue is focused on the target tissue, increasing the temperature to 60–95°C, leading to protein denaturation and coagulative necrosis without damaging adjacent tissues or disturbing skin integrity [20, 22, 23]. HIFU treatment can provide a noninvasive alternative to surgical excision that avoids anesthesia and surgery-related complications [24].
HIFU treatment for breast FA is especially useful for noninvasive ablation due to breast fibroadenomas’ proximity to the skin [4]. A general drawback to the HIFU technique is that it has a prolonged treatment time of up to 3 h as suggested by data from systematic reviews [25, 26]; however, it can be done in a single session and minimize recovery time, thus time spent in hospital is lower overall. The aim of this systematic review was to outline the procedure, outcomes, and complications associated with the use of HIFU as a treatment for breast fibroadenoma and reach a conclusion regarding the efficacy and safety of the technique.
Methods
A literature search was performed on the databases Embase, MEDLINE/PubMed, Google Scholar, and Ovid for English language papers published between January 1, 2000, and March 17, 2021. A structured protocol was employed to devise a comprehensive search strategy with keywords and Boolean operators defined by the research question. The keywords used for the search were “HIFU,” “High Intensity Focused Ultrasound,” “Fibroadenoma,” “Breast,” “Lesion.” This review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and the PRISMA 2020 checklist has been completed (available at https://doi.org/10.1159/000524738).
Inclusion and Exclusion Criteria
Using a predefined PICO framework (population, intervention, comparison, outcomes) a list of criteria was devised to guide the inclusion and exclusion of studies. The initial search was not strictly limited, and adherence to medical subject heading terms formed the main criterion for inclusion. Electronic publications ahead of print or in-process were accepted as eligible. Abstracts, editorials, or case reports were excluded. Also, included studies were limited to those reported in English language or translated. Sought outcomes included reduction in fibroadenoma size, palpability, pain scores, and complications. After removal of duplicates, additional records were manually identified and added to the final list of eligible studies. Exclusion criteria included (1) article not English language; (2) case reports, editorials, or reviews; (3) patients under 18 years old; (4) non-human subjects; and (5) not relevant to fibroadenoma.
Data Extraction
A standardized form for data extraction was designed for collection of study variables and outcomes. Two investigators (O.M. and A.N.) independently performed database searches to identify eligible papers. Papers were screened by title and abstract, and following shortlisting, abstract and full text screening were performed to identify the papers meeting the inclusion and exclusion criteria. Extraction was performed by A.M. and A.G., and this was iteratively reviewed and modified for discrepancies and clarity by O.M. and A.N. Extraction of parameters was organized to reflect the PICO framework, collecting data on populations such as sample size and patient clinical details such as age, ethnicity, number, and palpability of fibroadenomas. Procedure details included power, frequency, aperture size, transducer diameter, sonification time, and treatment time. Data accuracy was checked and appropriately amended by O.M. and A.N.
Data Analysis
All data were analyzed using SPSS (SPSS Statistics, Version 15.0, Armonk, NY: IBM Corp.). Univariate inferential statistics were used to describe data, with weighted averages, means, and ranges used as the main summary statistics.
Quality Assessment
Two investigators (M.A. and A.M.) scored all studies using the Newcastle-Ottawa scale for assessing the quality of nonrandomized studies. Following full text screening, studies were scores out of 9 based on selection, comparability, and exposure/outcome. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was used to assess the quality of the single RCT in our review.
Description of Studies
Kovatcheva et al. [27] performed a multi-center prospective cohort study that included 42 women with 51 FAs in one or both breasts confirmed histologically via core needle biopsy. Most patients underwent a single HIFU treatment session. Some of the patients with multiple fibroadenoma in both breasts had to undergo an additional HIFU session for the other breast.
Peek et al. [28] performed a single-center prospective case-control study that included 40 patients each with a symptomatic fibroadenoma. Patients were divided into 2 equal groups, the first undergoing US-guided HIFU under local anesthesia, with the other group not receiving any treatment. Results were assessed via ultrasound 6 months later for both groups.
Kovatcheva [29] performed a single-center prospective cohort study that included 20 patients with 26 fibroadenomas. Patients underwent 1 or 2 HIFU sessions, depending on their follow-up results within the first 6 months after the procedure. Follow-up duration was relatively long, at up to 24 months after the initial procedure.
Peek et al. [30] performed a single-center prospective cohort study that included 51 patients with 53 symptomatic fibroadenomas. Circumferential HIFU treatment was performed as a means of shortening treatment times compared to the classical technique.
Hahn et al. [31] performed a single-center prospective cohort study that included 27 patients with histologically confirmed fibroadenomas. Each patient received one session of HIFU under local anesthesia. Twelve months after the procedure, core needle biopsy was offered if patients showed indistinct residuals on ultrasound.
Imankulov et al. [32] performed a single-center prospective randomized trial that included 80 women with breast fibroadenomas. Patients were evenly and randomly allocated into 2 groups. The first group underwent HIFU therapy, while the second group performed partial resection. SF-36 questionnaire was used to assess the patient’s quality of life after treatment.
Kwong et al. [33] performed a single-center prospective cohort that included 60 patients with biopsy-confirmed fibroadenoma. Follow-up was scheduled for up to 12 months. In patients with an increased fibroadenoma volume surgical excision was performed.
Results
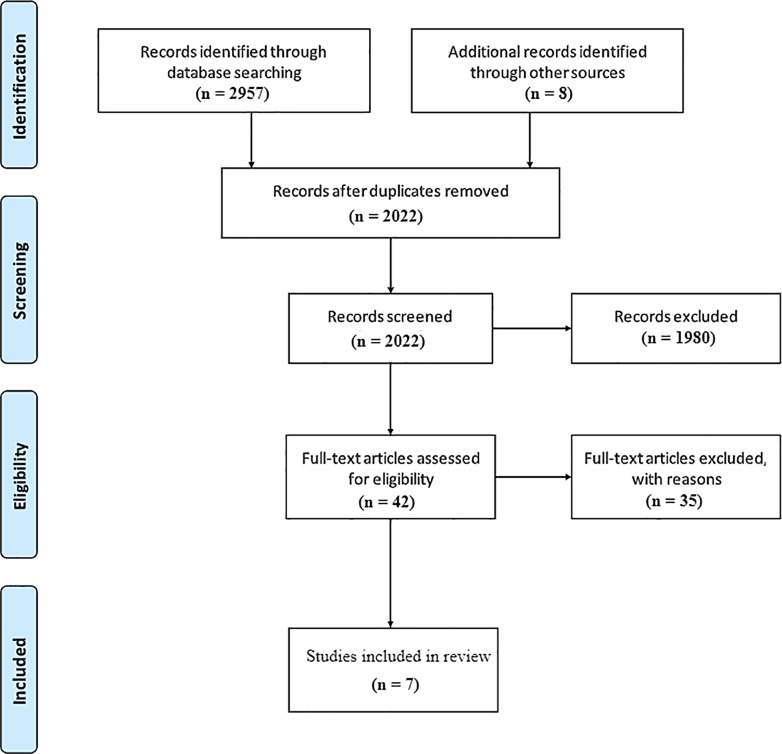
Figure 1 shows the study selection flow diagram according to PRISMA statement.
Fig. 1.
Study selection flow diagram according to PRISMA statement.
Patient Characteristics and Quality Assessment
A total of 260 patients were included in our systematic review encompassing 7 studies researching the role of HIFU in treating fibroadenoma (Table 1). Ages ranged from 28.9 ± 9.1–37.6 (19, 60) years, with patients being mostly of European descent. The total number of fibroadenomas treated by HIFU was 277, with most being palpable by the patient’s pre-intervention. The quality of the papers was assessed using Newcastle-Ottawa and GRADE tools (Table 2), with three papers scoring a 7, two papers scoring an 8, one paper scoring a 5, and our only RCT scoring moderate on GRADE.
Table 1.
Patient demographic data
| Study | Journal | Number of patients | Number of fibroadenomas | Age | Nationality | BMI, kg/m2 | Palpable fibroadenomas, % |
|---|---|---|---|---|---|---|---|
| Kovatcheva et al. (2015) [27] | Journal of Therapeutic Ultrasound | 42 | 51 | 32 (16, 52) | European | NA | 97.6 |
| Peek et al. (2016) [28] | International Journal of Hyperthermia | 20 | 20 | 30.3±7.5 | British | NA | 100 |
| Kovatcheva et al. (2017) [29] | Journal of Therapeutic Ultrasound | 20 | 26 | 29.0±10.2 | European | 20.5±3.4 | NA |
| Peek et al. (2017) [30] | International Journal of Hyperthermia | 51 | 53 | 29.8±7.2 | British | NA | NA |
| Hahn et al. (2018) [31] | International Journal of Hyperthermia | 27 | NA | 28.9±9.1 | German | 22.2±2.3 | 85.1 |
| Imankulov et al. (2018) [32] | Journal of Pakistan Medical Association | 40 | NA | 30.00±1.75 | Kazakh | NA | NA |
| Kwong et al. (2021) [33] | The Breast Journal | 60 | 60 | 37.6 (19, 60) | European | NA | NA |
Table 2.
Quality assessment of studies
| Study | Selection | Comparability | Exposure/Outcome | Overall |
|---|---|---|---|---|
| Kovatcheva et al. (2015) [27] | *** | ** | *** | 8 |
| Peek et al. (2016) [28] | *** | ** | ** | 7 |
| Kovatcheva et al. (2017) [29] | ** | ** | *** | 7 |
| Peek et al. (2017) [30] | *** | ** | *** | 8 |
| Hahn et al. (2018) [31] | ** | ** | *** | 7 |
| Imankulov et al. (2018) [32] | NA | NA | NA | Moderate |
| Kwong et al. (2021) [33] | ** | ** | * | 5 |
HIFU Treatment Protocols, Technical, and Exposure Parameters
Generally, the treatment of fibroadenoma with HIFU consists of several steps including pre-treatment radial and antiradial ultrasound scans, to locate the targeted fibroadenoma compartment, relative to its center and depth below the skin. Fibroadenoma volume was measured, and target units were also planned on the ultrasound display screen [27–31]. The breast was initially supported in supine or lateral decubitus positions, by the Breast support and immobilization system SenoPad (Theraclion, Paris, France), depending on the fibroadenoma location [27, 29]. Subsequently, the number of pulses and time for treatment are automatically calculated by the ultrasound-guided Echopulse device (Theraclion, Malakoff, France) [27–31], which was the most used device in the trials included [27–31]; it is dedicated to treat breast fibroadenomas, as well as thyroid nodules occasionally [28]. There was variation in the type of anesthesia administered. Two studies used intravenous fentanyl or midazolam, with propofol to achieve conscious sedation (n = 62) [27, 29]. Additional analgesia was sometimes used, including an infusion of ketoprofen [27] or posttreatment ibuprofen [28, 31] if patients were still in pain. Other studies [28, 30, 31, 33] used subcutaneous local anesthesia (1.0% lidocaine with adrenaline and 0.25–0.5% bupivacaine, ratio 1:1) (n = 145). Ropivacaine was also used (n = 13) [31] as it was more potent and had a longer-lasting analgesic effect. The procedure was then initiated with the pre-selected energy level, which was determined by the energy required to achieve hyperechoic marks in the center of the fibroadenoma on ultrasound as sign of tissue damage [27–31]. High-energy (200 W) was recommended for optimal ablation in one study [32]. However, other trials used lower dosages of mean power ranging from 29.1 ± 7.3–60 W [28–31, 33]. Lesions were ablated under ultrasound guidance using a 7.5–12 MHz diagnostic ultrasound transducer [28, 30, 31]. A 3.0 MHz therapeutic transducer with a diameter of 56 mm and an aperture size of 11 mm for coaxial imaging transducer was used to produce energy pulses, with each being used to ablate an ellipsoid lesion of approximately 9 mm length and 2 mm width [28–31]. Both transducers were equipped with a balloon filled with cooling fluid, ensuring both ultrasound coupling and skin cooling, to prevent skin burns [29, 31].
Initially pulse was directed to the center of the fibroadenoma, to detect the hyperechoic region [28]. Repeated HIFU pulses were then directed to the lesion, with no hyperechoic marks required, to cover the full volume, resulting in the treatment duration being dependent on the fibroadenoma size [27–31]. Some studies only ablated the circumference of the fibroadenoma (two circumferential rings), and the center was deselected [28, 30, 31]. This resulted in reduced treatment time by an average of 37.5% (20.1%) [28]. The Echopulse device treats only one central target volume each session treatment, to avoid thermal damage to skin and pectoral muscle, and to cover the most central part of the fibroadenoma [28, 30]. Most fibroadenomas require only one target volume, as this is sufficient to cover the whole volume of the lesion [28]. One study performed two HIFU treatments in one of its groups to assess further reduction in volume and hence efficacy of treatment [29]. Average sonification time ranged from 32.9 ± 9.8–38 ± 12 min [28, 30, 31], while mean total procedural duration accounting for sonification, cooling, and repositioning [29] ranged from 60.6 ± 22.8–118 min [27–31] (Table 3). After the final pulse, ultrasound examination with color doppler can be performed to assess changes in volume [27]. Patients then usually wait for 2 h after the treatment, receiving appropriate analgesia if required and ice pads, to be monitored for pain or side effects [27–31]. Patients were assessed for pain using different scales including visual analog scale (VAS) [27–31], SF-36 [32], and Likert scales [33]. Efficacy and safety were assessed according to changes in the fibroadenoma volume, improvement in symptoms, lesion palpability, cosmetic concerns; as well as complications [27–31, 33]. Outcomes affecting quality of life were evaluated in one study [32]. Patients were then followed-up for a duration which varied (6–24 months) [27–33].
Table 3.
Treatment protocols, technical, and exposure parameters
| Study | Anesthesia | Power, W | Frequency, MHz | Aperture size, mm | Transducer diameter, mm | Sonification time, min | Treatment time, min |
|---|---|---|---|---|---|---|---|
| Kovatcheva et al. (2015) [27] | Sedation | NA | NA | NA | NA | NA | 118 (60, 255) |
| Peek et al. (2016) [28] | Local | 33.3±4.8 | 3 | 11 | 56 | 34.6±10.5 | 68.7±10.5 |
| Kovatcheva et al. (2017) [29] | Sedation | 60 | 3 | NA | 56 | NA | 60.6±22.8 |
| Peek et al. (2017) [30] | Local | 29.1±7.3 | 3 | 11 | NA | 32.9±9.8 | 66±16.5 |
| Hahn et al. (2018) [31] | Local | 40 | 3 | 11 | 56 | 38±12 | 80±19 |
| Imankulov et al. (2018) [32] | NA | 200 | NA | NA | NA | NA | NA |
| Kwong et al. (2021) [33] | Local | 30±5 | NA | NA | NA | NA | NA |
Outcomes
The overall technical success of the HIFU procedure in our studies was calculated as a mean of 93.10% [27–33] (Table 4). The main outcome measured to assess the efficacy of HIFU was the fibroadenoma volume reduction, with most studies included reporting a significant reduction (p < 0.05) in final volume, ranging from 43.20% to 90.47%, as displayed in Table 5 [27–31]. Kwong et al. [33] also reported a significant reduction in fibroadenoma volume (p = 0.046). The mean reduction in fibroadenoma volume at 12 months was 71.56% [27, 29–31]. One study reported a further reduction in the volume of the lesion when providing a second HIFU therapy to its subjects, which was not particularly evidenced at 6 months; however, more prominent when followed up at 12 and 24 months [29]. Volume reduction was lower in one study by Peek et al. [30], compared to other trials, with the significance not being reported.
Table 4.
Technical success rate of HIFU ablation of fibroadenomas
| Study | Total number of patients treated | Technical success, % |
|---|---|---|
| Kovatcheva et al. (2015) [27] | 42 | 100 |
| Peek et al. (2016) [28] | 20 | 80 |
| Kovatcheva et al. (2017) [29] | 20 | 100 |
| Peek et al. (2017) [30] | 51 | 83 |
| Hahn et al. (2018) [31] | 27 | 96 |
| Imankulov et al. (2018) [32] | 40 | 100 |
| Kwong et al. (2021) [33] | 60 | 93 |
| Weighted mean | 93.10 |
Table 5.
Overall percentage decrease of fibroadenoma volume
| Study | Overall percentage decrease of fibroadenoma | |||||
|---|---|---|---|---|---|---|
| 6 months, % | p value | 12 months, % | p value | 24 months, % | p value | |
| Kovatcheva et al. (2015) [27] | 59.20 | <0.001 | 72.50 | <0.001 | N/A | N/A |
| Peek et al. (2016) [28] | 43.50 | N/A | N/A | N/A | N/A | N/A |
| Kovatcheva et al. (2017) [29] | 58.04 | N/A | 71.02 | 0.02 | 77.32 | 0.025 |
| Kovatcheva et al. (2017) [2 HIFU] [29] | 50.44 | N/A | 86.28 | 0.02 | 90.47 | 0.025 |
| Peek et al. (2017) [30] | 35 | <0.005 | 43.20 | <0.005 | N/A | N/A |
| Hahn et al. (2018) [31] | 61.60 | N/A | 84.80 | N/A | N/A | N/A |
| Imankulov et al. (2018) [32] | N/A | N/A | N/A | N/A | N/A | N/A |
| Kwong et al. (2021) [33] | N/A | N/A | N/A | 0.046 | N/A | N/A |
Imankulov et al. [32] did not evaluate the fibroadenoma volume reduction following ablation; however, they conducted morphological studies that revealed significant coagulation necrosis of the lesions at the periphery, both in vitro and in vivo. Quality of life improvement post-HIFU was exhibited by a reduction in pain, being 66.7% higher in the control group, and enhanced physical activity (44.7% higher in HIFU group), as well as 52% reduction in hospitalization length in patients receiving treatment, compared to surgical resection [32]. Pain score was reported by all trials [27–33], however, using different scoring scales with variable ranges. Overall, most studies displayed a reduction in pain post-HIFU, even though the mean procedural pain reported by patients was relatively high in four studies [28, 30, 31, 33]. Kwong et al. [33] reported a significant reduction in pain, relative to baseline (p = 0.044) with a gradual reduction in pain after HIFU from a mean procedural pain of 5.88 to 1.9 one day after the procedure and 0.58 1 week after intervention using Likert scale (0–10). Similarly, Peek et al. [28] have shown a reduction from 6.4 (SD 3.2) during treatment to 1.6 (SD 1.9) post-HIFU, using VAS (0–10). Additionally, patients included in another study by Peek et al. [30] reported a reduction in pain posttreatment (1.6 (SD 1.8) compared to 7.1 (SD 2.6) using VAS (0–10). However, Hahn et al. [31] have shown an increase in pain score 30 min posttreatment, relative to baseline (29.6 [SD 20] compared to 22.3 [SD 23.5]). However, pain was not documented on follow-up, hence it was inconclusive. Moreover, patients who received a second HIFU therapy experienced a further reduction in pain sensation (34.9 ± 17.9 vs. 40.7 ± 24.6), using VAS (0–100) [29].
Finally, all patients were satisfied with the cosmetic results [29, 31]. 95% [29] and 89% [31] of the patients were completely satisfied with the symptoms disappearing and would consider HIFU again. 96% of the patients would recommend the procedure [31].
Complications
Overall, HIFU was deemed a safe and well-tolerated interventional treatment with a low risk of causing serious adverse effects. Generally, most of the complications self-resolve with only a minority persisting [27–33]. Kovatcheva et al. [27] reported a low procedural mean VAS pain score 29.7 ± 27.5 mm (0–80), and patients did not require posttreatment analgesia [27, 29]. In terms of side effects, skin hyperpigmentation was deemed one of the most significant complications post-HIFU, with 4 patients having persistent skin hyperpigmentation at 6 months [28], and 6 cases of hyperpigmentation that developed later in one study [30] and endured at 12 months. 20 patients (7.7%) suffered erythema after the intervention, however, all were transient and resolved within the follow-up period [28–30]. Additionally, 8 patients (3.1%) suffered superficial skin burns [28–30, 33], with one of the cases having received two HIFU sessions [29] and developing skin hyperpigmentation concurrently. Other complications reported included skin bruising (n = 15), ecchymosis (n = 9), treatment site dimpling and numbness (n = 6), skin hypopigmentation (n = 2); and a case of both skin blistering and pain in the ipsilateral axilla [28–30]. Moreover, 4 patients (n = 20) who were treated twice by HIFU [29] suffered mild subcutaneous edema. However, these other complications all resolved within follow-up duration. Furthermore, Hahn et al. [31] reported one case with severe pain despite analgesia, secondary to an indurated fat nodule that was excised 18 months post-HIFU, with no evidence of fibroadenoma cells. Similarly, Kovatcheva et al. [27] reported another case of indurated fat nodule that persisted until the end of the trial. Finally, no HIFU-related complications were reported by Imankulov et al. [32].
Discussion
High-intensity focused ultrasound (HIFU) is a promising, noninvasive, well-tolerated treatment for breast fibroadenoma (FA). This review has found that HIFU treatment for breast FA has a high technical success rate of 93.10% (weighted mean). FA volume reduction size, one of the main assessments for the treatment efficacy, was significant (p < 0.050) in all the articles where it was measured [27–31], and the overall FA volume percentage decrease ranged from 35.00% to 61.60% after a 6-month follow-up period. Furthermore, circumferential ablation using HIFU, which is utilized by some of the studies, in which the FA is isolated from its blood supply, results in reduced treatment time, greater FA volume reduction, and less damage to neighboring tissues. In those articles, FA volume further regressed over time as measured in later follow-ups, and the largest FA volume reduction over time was reported by Kovatcheva et al. [29], which was a reduction of 50.44% after 6 months, 86.28% (p = 0.020) after 12 months, and 90.47% (p = 0.025) after 24 months. This suggests that circumferential HIFU ablation specifically and effectively targets the FA and provides adequate coagulative necrosis, successfully ablating the tumor. The successful ablation of the FAs was also evidenced by the reduction of the number of palpable masses post-intervention, which was reported by three of our papers [27, 28, 31] as most of the palpable fibroadenomas were not palpable after the treatment, and the majority of those that still were had a reduction in size as reported by the patients. The patient’s satisfaction with the treatment also points to its efficacy as 95% [29] and 89% [31] of the patients were completely satisfied with the symptoms disappearing and would consider HIFU treatment again, and 96% of the patients would recommend the procedure [31]. The successful ablation was also shown cosmetically as 100% of the patients were satisfied with the cosmetic results [29, 31].
The procedural parameters of the HIFU treatment, that include power (W), frequency (MHz), aperture size (mm), transducer diameter (mm), and sonification time (min), were similar in most of the articles studied that reported them, which suggests a standardization of methods used in HIFU treatment that provides a greater safety and efficacy in the performance of this procedure; however, as not all the studies reported their HIFU parameters, it is difficult to draw conclusions. The frequency was the same (3 MHz) in all the articles where it was reported, as well as the aperture size (11 mm) and transducer diameter (56 mm). The power used ranged from 29.1 W ± 7.3–60 W in most articles but one [32], where the power used was 200 W. This was due to using an in vitro experiment to determine how much power was necessary to provide adequate ablation. 100 W was determined to not be adequate in vitro, 200 W was determined to be adequate, and 300 W was determined to be too damaging. A drawback of this determination is that in vitro conditions for HIFU ablation do not necessarily reflect the conditions in vivo and could result in surrounding tissue damage. Imankulov et al. [32], however, report that zero incidences of complications have occurred. Mean sonification times and total treatment times ranged from 32.9 to 38.0 min and 60.6–118.0 min, respectively. Sonication time is defined as the total time tissue is subjected to HIFU, while the total treatment time is defined as the time from the first sonication to the last. A drawback of HIFU treatment is the prolonged treatment time, which, according to Peek et al. [25, 26], can be up to 3 h. However, the articles studied show a lower treatment time overall in comparison to Peek et al. [25, 26]. This may in part be due to the use of circumferential ablation, which isolates FA from blood circulation and therefore targets it more effectively, reducing time needed for sufficient ablation. Technical success rates, which we have defined as a reduction in volume of fibroadenomas that was sustained by the end of the follow-up period, were extremely high across the studies that reported them, at 93.1%. This demonstrates the efficacy of HIFU as almost all patients had a reduction in volume that was maintained for at least 6 months and up to 2 years.
HIFU’s advantages over surgical excision were more clearly outlined in the study performed by Imankulov et al. [32]. The study’s main assessment was concerned with the effect of HIFU treatment on patients’ quality of life. This was assessed by comparing pain, hospitalization length, and physical activity post-procedurally between a group receiving HIFU treatment and a control group receiving surgical excision, where the HIFU group had 66.7% greater pain reduction than the control group, 44.7% higher physical activity levels, and 52% less hospitalization length. This is consistent with the patient approval and satisfaction ratings of HIFU treatment. All articles studied pain scores of varying scales, with many displaying relatively high procedural pain. However, in almost all studies, this pain was transient and decreased rapidly in the post-operative period, often reducing below the baseline, pre-operative pain [28, 30, 31, 33]. One study by Hahn et al. [31] displayed an increase of pain score from 22.3 (SD 23.5) to 29.6 (SD 20); however, this is inconclusive due to the lack of documentation of pain scores on follow-up, as the pain was only documented 30 min posttreatment, so it is difficult to comment on the significance of this result. One reason that might explain the relatively high procedural pain is the lack of adequate analgesia during the intervention. Two papers by Kovatcheva et al. [27, 29] stated that the patients did not require postoperative analgesia, suggesting that their pain was more tolerable. These 2 patient populations were sedated, unlike the other studies where the patient received local anesthetic and required further pain management. As suggested by Peek et al. [28, 30], this may be due to the inadequate effect of local anesthetics, as the pulses of HIFU are directly targeted at the local sensory pain receptors, thus causing pain even though local anesthetic (usually lidocaine) was administered beforehand. More research is required to determine the optimal method of pain control during HIFU procedures, whether it is sedation or another method which has not been trialed yet, such as pre-operative pain killers.
HIFU was shown to be a well-tolerated, noninvasive, low-risk treatment with a low incidence of complications. Most complications were self-resolving. In a few cases where subcutaneous fat nodules were a complication, surgical excision was required [27, 31]. Hyperpigmentation was the most common persistent complication; however, many of its cases self-resolved. 3.1% of patients suffered from first degree skin burns, with no patients suffering deeper injuries reported. This shows that the cooling fluid filled balloons utilized by the transducers, as well as the ice used in the immediate follow-up period were both successful in limiting the short-term consequences of HIFU treatment. However, several patients still suffered from a delayed reaction to HIFU in the form of hyperpigmentation, the most common persistent complication. This shows that while the cooling measures taken were successful in the short term, they did not prevent long term damage to the tissues. This displays the need for further research on optimum cooling methods, with long term follow-up to ensure complications do not arise later. A possible way of achieving this effect is suggested by Gupta & Srivastava [34], where optimizing the scanning pathways during HIFU treatment aids in uniformly distributing the energy throughout the lesions, leading to simultaneous destruction of the affected lesions. Furthermore, as the heating is more optimized, it may lead to shorter treatment times if the cooling period can be shortened or eliminated if it is not needed, this resolves one of the HIFU drawbacks mentioned by Peek et al. [28] This solution has not been tried on humans; however, it is a promising and novel approach and warrants a human trial in the future. Post-treatment pain was reduced and a majority of the complications that arose self-resolved, supporting the use of HIFU being safe, well-tolerated and noninvasive. However, the low number and adversity of complications from HIFU might be discouraging the thorough reporting of side-effects as studies are more likely to report a result of none-to-few complications if they deem that the complications are not serious or frequent enough. This may be the reason that Imankulov et al. [32] reported no HIFU-related complications, despite using a much higher power of 200 W in their HIFU procedure.
The most significant limitations in all the articles studied were the short follow-up times and the small sample sizes. Both these factors contribute to a deficiency in the assessment of outcomes and complications. This is especially important for HIFU treatment as complications such as hyperpigmentation were shown to develop at later follow-ups [28–30]. Longer and more frequent follow-ups, as well as greater sample sizes, would provide a more conclusive result that displays HIFU treatment’s safety and efficacy. Additionally, more outcomes related to the patients’ quality of life, recovery duration, and duration of hospitalization are required in future studies to better assess the efficacy and safety of HIFU for the treatment of fibroadenomas. Another possible limitation is the inhomogeneous reporting of the outcomes, making the quantitative analysis and comparison challenging. To enhance and standardize future analysis in treating fibroadenomas, it is recommended that future trials include at least the following: patient’s pre-treatment characteristics (demographics, number, volume and palpability of fibroadenomas, pre-treatment pain score, and quality of life scores using validated measurement scales), HIFU procedure characteristics, outcomes (volume and palpability of fibroadenomas, posttreatment pain, and quality of life scores), complications, and follow-up duration. It is also recommended to report the volume change of each patient individually, such as in Peek et al. [28] papers [30], so that more detailed analyses can be conducted. The reporting of palpability, pain, and quality of life assessment using quantified methods will help to assess the real-life effect of HIFU treatment on the treatment of fibroadenoma, instead of using volume as a surrogate, which may not be accurate.
Conclusion
HIFU is an emerging technique in the ablation of fibroadenomas that has tangible benefits in reducing the volume of fibroadenomas and reducing the symptom burden on patients. It can do this in a safe manner that exposes patients to a low risk of complications. Circumferential ablation of fibroadenomas is an evolution of this idea that displays promise in improving results and reducing treatment time of traditional HIFU ablation. However, more human trials are required, preferably randomized controlled trials, to compare the effects of HIFU therapy to surgical or no treatment and to accurately assess the benefit of this new procedure. These trials need to follow up the patients for longer, have homogenous reporting of methodology and outcomes, and most importantly, use measures such as quantitative assessment of pain and Quality of Life scores to determine the clinical benefit of HIFU treatment rather than using volume reduction as a surrogate. HIFU is rapidly growing in popularity, and further research is required to understand the optimal methods of utilizing it.
Statement of Ethics
An ethics statement was not required for this study type, no human or animal subjects or materials were used.
Conflict of Interest Statement
The author(s) declared that they have no competing interests.
Funding Sources
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for authorship. Ahmed R. Gonnah and Omar Masoud are the first authors in this paper. Study concept and design: Ahmed R. Gonnah and Omar Masoud. Analysis and interpretation of data: Ahmed R. Gonnah, Omar Masoud, Mohammed AbdelWahab, and Ahmed ElMosalamy. Data acquisition: Ahmed R. Gonnah, Omar Masoud, Ahmed ElMosalamy, Mohammed AbdelWahab, and Abdulrahman Al-Naseem. Supervisor(s): not applicable. All authors read and approved the final manuscript.
Funding Statement
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
References
- 1. Dent DM, Cant PJ. Fibroadenoma. World J Surg. 1989;13(6):706–10. 10.1007/BF01658418. [DOI] [PubMed] [Google Scholar]
- 2. Greenberg R, Skornick Y, Kaplan O. Management of breast fibroadenomas. J Gen Intern Med. 1998;13(9):640–5. 10.1046/j.1525-1497.1998.cr188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larsen TK, Faurschou JP, Bak M, Ryttov NF. Fibroadenoma of the breast-modern strategy of treatment. Ugeskr Laeger. 2003;165(19):1979–83. [PubMed] [Google Scholar]
- 4. Fine RE, Staren ED. Percutaneous radiofrequency-tassisted excision of fibroadenomas. Am J Surg. 2006;192(4):545–7. 10.1016/j.amjsurg.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 5. Cerrato F, Labow BI. Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg. 2013;27(1):23–5. 10.1055/s-0033-1343992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sperber, F, Blank, A, Metser, U, Flusser, G, Klausner, JM, Lev-Chelouche, D. Diagnosis and treatment of breast fibroadenomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg. 2003;138(7), 796–800. [DOI] [PubMed] [Google Scholar]
- 7. Brenin DR. Management of the palpable breast mass. In: Harris J, Lippman M, Morrow M, Osborne CK, editors. Diseases of the breast. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 37. [Google Scholar]
- 8. Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol. 2005;6(3):145–57. 10.1016/S1470-2045(05)01765-1. [DOI] [PubMed] [Google Scholar]
- 9. Lee A, James J, Whisker L, Rakha EA, Ellis IO. Which lesions with a radiological or core biopsy diagnosis of fibroadenoma should be excised? Ann R Coll Surg Engl. 2021 Dec 23;104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wiratkapun C, Piyapan P, Lertsithichai P, Larbcharoensub N. Fibroadenoma versus phyllodes tumor: distinguishing factors in patients diagnosed with fibroepithelial lesions after a core needle biopsy. Diagn Interv Radiol. 2014;20(1):27–33. 10.5152/dir.2013.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barakzai N, Mansoor E, Buccimazza I. Is conservative management of fibroadenomas feasible? 5-year results from the Durban Breast Unit. S Afr J Surg. 2021 Jun;59(2):41–6. [PubMed] [Google Scholar]
- 12. Thurley P, Evans A, Hamilton L, James J, Wilson R. Patient satisfaction and efficacy of vacuum-assisted excision biopsy of fibroadenomas. Clin Radiol. 2009;64(4):381–5. 10.1016/j.crad.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 13. Hahn M, Pavlista D, Danes J, Klein R, Golatta M, Harcos A, et al. Ultrasound guided cryoablation of fibroadenomas. Ultraschall Med. 2013;34(1):64–8. 10.1055/s-0032-1325460. [DOI] [PubMed] [Google Scholar]
- 14. Grady I, Gorsuch H, Wilburn-Bailey S. Long-term outcome of benign fibroadenomas treated by ultrasound-guided percutaneous excision. Breast J. 2008;14(3):275–8. 10.1111/j.1524-4741.2008.00574.x. [DOI] [PubMed] [Google Scholar]
- 15. Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol. 2011;2(1):8–27. 10.5306/wjco.v2.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–85. 10.1148/radiology.219.1.r01ap02176. [DOI] [PubMed] [Google Scholar]
- 17. Esnault O, Franc B, Ménégaux F, Rouxel A, De Kerviler E, Bourrier P, et al. High-intensity focused ultrasound ablation of thyroid nodules: first human feasibility study. Thyroid. 2011;21(9):965–73. 10.1089/thy.2011.0141. [DOI] [PubMed] [Google Scholar]
- 18. Kovatcheva R, Vlahov J, Stoinov J, Lacoste F, Ortuno C, Zaletel K. US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol. 2014;24(9):2052–8. 10.1007/s00330-014-3252-4. [DOI] [PubMed] [Google Scholar]
- 19. Wu F, Wang ZB, Cao YD, Chen WZ, Bai J, Zou JZ, et al. A randomised clinical trial of high-intensity focused ultrasound ablation for the treatment of patients with localised breast cancer. Br J Cancer. 2003;89(12):2227–33. 10.1038/sj.bjc.6601411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmitz AC, Gianfelice D, Daniel BL, Mali WP, van den Bosch MA. Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol. 2008;18(7):1431–41. 10.1007/s00330-008-0906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maloney E, Hwang JH. Emerging HIFU applications in cancer therapy. Int J Hyperthermia. 2015;31(3):302–9. 10.3109/02656736.2014.969789. [DOI] [PubMed] [Google Scholar]
- 22. Haar GT, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. 10.1080/02656730601186138. [DOI] [PubMed] [Google Scholar]
- 23. Kim SH, Jung SE, Kim HL, Hahn ST, Park GS, Park WC. The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int J Hyperthermia. 2010;26(6):594–603. 10.3109/02656736.2010.481275. [DOI] [PubMed] [Google Scholar]
- 24. Payne A, Todd N, Minalga E, Wang Y, Diakite M, Hadley R, et al. In vivo evaluation of a breast-specific magnetic resonance guided focused ultrasound system in a goat udder model. Med Phys. 2013;40(7):073302. 10.1118/1.4811103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peek MCL, Ahmed M, Napoli A, ten Haken B, McWilliams S, Usiskin SI, et al. Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer. Br J Surg. 2015;102(8):873–82; discussion 882. 10.1002/bjs.9793. [DOI] [PubMed] [Google Scholar]
- 26. Peek MCL, Ahmed M, Napoli A, Usiskin S, Baker R, Douek M. Minimally invasive ablative techniques in the treatment of breast cancer: a systematic review and meta-analysis. Int J Hyperthermia. 2017;33(2):191–202. 10.1080/02656736.2016.1230232. [DOI] [PubMed] [Google Scholar]
- 27. Kovatcheva R, Guglielmina JN, Abehsera M, Boulanger L, Laurent N, Poncelet E. Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound. 2015;3(1):1. 10.1186/s40349-014-0022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peek MCL, Ahmed M, Scudder J, Baker R, Pinder SE, Douek M; HIFU-F Trialists’ Group . High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. Int J Hyperthermia. 2016;32(8):881–8. 10.1080/02656736.2016.1212278. [DOI] [PubMed] [Google Scholar]
- 29. Kovatcheva R, Zaletel K, Vlahov J, Stoinov J. Long-term efficacy of ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma. J Ther Ultrasound. 2017;5:1. 10.1186/s40349-017-0083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peek MCL, Ahmed M, Scudder J, Baker R, Charalampoudis P, Pinder SE, et al. High-intensity focused ultrasound in the treatment of breast fibroadenomata (HIFU-F trial). Int J Hyperthermia. 2018;34(7):1002–9. 10.1080/02656736.2017.1373865. [DOI] [PubMed] [Google Scholar]
- 31. Hahn M, Fugunt R, Schoenfisch B, Oberlechner E, Gruber IV, Hoopmann U, et al. High intensity focused ultrasound (HIFU) for the treatment of symptomatic breast fibroadenoma. Int J Hyperthermia. 2018;35(1):463–70. 10.1080/02656736.2018.1508757. [DOI] [PubMed] [Google Scholar]
- 32. Imankulov S, Tuganbekov T, Razbadauskas A, Seidagaliyeva Z. HIFU treatment for fibroadenoma: a clinical study at national scientific research centre, astana, Kazakhstan. J Pak Med Assoc. 2018;68(9):1378–80. [PubMed] [Google Scholar]
- 33. Kwong A, Co M, Chen C, Wu A. Prospective clinical trial on high-intensity focused ultrasound for the treatment of breast fibroadenoma. Breast J. 2021;27(3):294–6. 10.1111/tbj.14166. [DOI] [PubMed] [Google Scholar]
- 34. Gupta P, Srivastava A. Numerical study on the possible scanning pathways to optimize thermal impacts during multiple sonication of HIFU. IEEE Trans Biomed Eng. 2020;68(7). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.