Abstract
Introduction
This pilot study aimed to investigate the effects of using an app-based certified medical product named PINK! on breast cancer patients and survivors. The objectives were to measure psychological distress, physical activity, and therapy-related fatigue of patients using PINK! to identify trends and develop a study design for a subsequent multicentric proof of efficacy RCT.
Materials and Methods
PINK! offers individualized, evidence-based therapy and side-effect management, mindfulness-based stress reduction, nutritional and psychological education, physical activity tracking, and motivational exercises to implement lifestyle changes sustainably in daily routine. A prospective, intraindividual RCT was performed with n = 60 patients in 2021 at Comprehensive Cancer Center Munich. Patients with BC were included independent of the stage of diseases. The intervention group got access to PINK! over 12 weeks. Control group served as a waiting-list comparison to “standard of care.”
Results
Primary efficacy variable analysis revealed a relative average decrease of 32.9% in psychological distress, which corresponds to a statistically significant reduction (p < 0.001) within 12 weeks compared to the control group. Linear regressions within usage groups showed a correlation of high app usage and a reduction of psychological distress. Fatigue data presented a statistically significant antifatigue efficacy (p < 0.001) and physical activity increased by 63.9%.
Conclusion
App-based supportive care offers a promising, low-threshold, and cost-efficient opportunity to improve psychological well-being, quality of life, fatigue, and physical activity. More research is needed to implement eHealth solutions in clinical cancer care.
Keywords: Breast cancer, Psychological distress, Fatigue, App-based intervention, Physical activity, eHealth, Digitale Gesundheitsanwendung, PINK!
Introduction
Although the long-term survival has increased within the last decades and the individualization of treatment options has improved substantially, breast cancer is still the leading malignant disease of women worldwide and the number of cases still increases. Research of the last years focused on the development of personalized therapeutic concepts. Therapeutic decisions are mainly based on molecular and histological characteristics of the tumor. The main goal is to find optimal treatment pathways or to tailor treatments especially for early breast cancer (EBC) patients, with respect to long-term toxicity-related side effects and improving quality of life. An integration of multimodal therapeutic concepts for EBC patients leads to full recovery in more than 70–80%. Treatment of metastatic breast cancer (MBC) aims to improve progression-free survival, overall survival, and therapy-related side effects as well as to improve symptom control and quality of life. Both EBC and MBC patients suffer from psychological and social distress after diagnosis, during therapy, and in aftercare even 1 year after diagnosis [1, 2].
However, actual treatments can result in poor health-related quality of life, adherence, psychological distress, fatigue, and lower physical activity levels. Generating further evidence in research of psycho-oncological interventions and digital behavioral coaching is one of the main goals of this project. The current status of research clearly shows that increased psychological well-being and health-related quality of life are associated with better treatment responses and significant improvement of disease-free survival rates [3, 4]. Psychological well-being and patient empowerment are complex and very individual factors in patients with a serious disease such as breast cancer. It can be improved by lifestyle behaviors such as health-promoting nutrition [5], physical activity [6, 7], and stress reduction [8–10] as well as side-effect management, self-management, empowerment, patient engagement, and education [11]. Patient education improves patient’s health literacy that is by definition the knowledge, motivation, and ability to find, understand, assess, and apply health information in order to make decisions about health-related topics in daily routine [12].
The PINK! app offers individualized, evidence-based therapy and side-effect management, mindfulness-based stress reduction, nutritional and psychological education, physical activity tracking, and motivational exercises to implement lifestyle changes sustainably in daily routine of breast cancer patients. Side-effect management supports patient empowerment, which in turn reduces anxiety and depression symptoms [13, 14]. Mindfulness-based stress reduction interventions have been found efficacious in treating psychological distress among cancer patients and survivors [8, 10, 13, 14]. Patient education has been shown in many studies as very effective in terms of reducing therapy-related side effects such as fatigue and psychological distress [9, 15]. Patient education also leads to increased physical activity in patients receiving systematic therapy and in survivors. Moreover, recent research shows that physical activity decreases the risk of mortality from breast cancer by up to 40% [6]. The biological mechanisms explaining these associations are still not well investigated even though many studies that prove those findings exist. Especially psychological distress, physical activity, and fatigue seem to be related [16–19].
A substantial proportion of breast cancer patients are dealing with fatigue symptoms, which are partly triggered by the diseases itself and the treatment and what results in psychologically and physically impairments. Current research shows that improving physical activity during and after therapy leads to improved psychological well-being and a significant reduction of fatigue symptoms [16, 17].
PINK! aims to support patients with breast cancer in every stage of disease and therapy in order to empower patients to become an active role during their therapy and in aftercare independent of factors such as stage of disease, type of therapy, age, place of residence, or the clinic the patient is being treated or was treated. Using PINK! offers patients a personalized, time- and location-independent coaching. Investigating the personalized app-based therapeutic concept of PINK!, not only with patients in initial therapy (surgery, chemotherapy neoadjuvant or adjuvant, post-neoadjuvant; aapecitabine, abemaciclib, bisphosphonate, endocrine therapy +/− GbRH, radiation) but also in aftercare and adjuvant treatment, was the main goal of this pilot study. The main focus was set on the connection of psychological distress, fatigue, and physical activity. Besides that, the feasibility of implementing an App-based support in clinical cancer routine of a large German breast cancer center was investigated. We hypothesized that PINK! empowers breast cancer patient in therapy and aftercare to increase their level of physical activity and reduce at the same time psychological distress and fatigue symptoms compared to patients in the control group.
Materials and Methods
General
In total, 60 women with breast cancer were recruited to this pilot study at the LMU Breast Cancer Center, Munich in August 2021. Participants in this study had to meet the following inclusion criteria: (1) they were at least 18 years old, (2) they were German speaking as the app is in German, (3) they had a breast cancer diagnosis and are in therapy or aftercare at least 12 more weeks (study duration time for each patient), (4) they had an e-mail address and were willing to answer 4 digital questionnaires, and (5) they had a smartphone to use PINK!. Furthermore, the following exclusion criteria were defined: (1) strong psychological distress at baseline (PHQ-9 > 20) and (2) participation in other clinical study with digital support and/or measurements of quality of life. The primary endpoint PHQ-9 is a multipurpose instrument for screening, diagnosing, monitoring, and measuring the severity of depression.
Population
Randomization was 1:2 to control and intervention groups. Control group served as a waiting-list group starting PINK! use after 12 weeks and as comparison to “standard of care” (primary endpoint). “Standard of care” means best practice care. Nine of 60 patients were MBC patients: 39 patients were undergoing chemotherapy and 21 were in aftercare. Undergoing chemotherapy means that they were in therapy for the entire time of the study (12 weeks). There was no other specific definition of therapy. All types of therapy ways (neoadjuvant, adjuvant, post-neoadjuvant, and metastatic) were included. Patients that were defined as “in aftercare” had their surgery performed at least 6 months ago. They completed surgery, chemotherapy, and, if necessary, radiotherapy, and optional received adjuvant endocrine, adjuvant oral Her2-targeted therapy, or no therapy during aftercare. All patients in the subgroup “aftercare” were EBC patients.
The PINK app pilot study has been approved by the Medical Ethical Committee of the LMU University of Munich, Germany, on August 19, 2021 (Reference number: 21-0757). The trial has been registered on October 6, 2021 on the DRKS Trial Registry (DRKS00025811).
Procedure
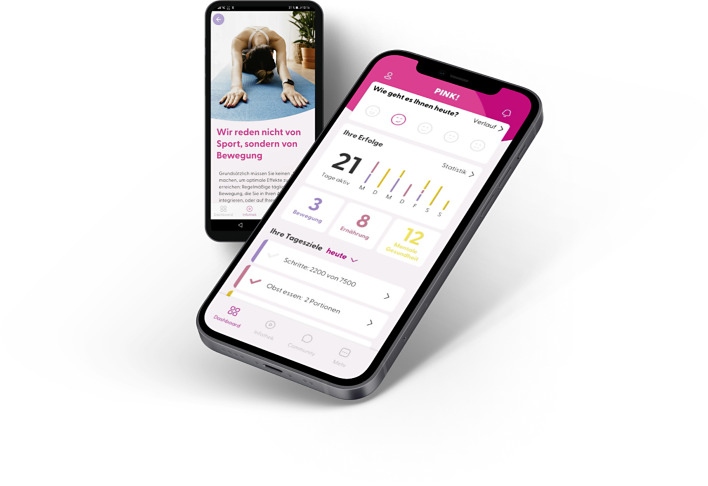
Patients were identified, informed, and included in the study at LMU Breast Cancer Center. All patients in this study signed a written informed consent to participate in this study. After baseline documentation and the patients’ first questionnaire, they were randomized in an intervention or a control group. Patients in the intervention group got access and introduction in PINK! app and started immediately using it. PINK! offers personal coaching, evidence-based therapy and side-effect management, mindfulness-based stress reduction, nutritional and psychological education, physical activity tracking, and motivational exercises to implement lifestyle changes sustainably in daily routine. Patients were instructed to use the app as often as they need it during daily routine. There was no predefined usage time they were supposed to achieve. Figure 1 shows the main PINK! Coach App functionalities.
Fig. 1.
PINK! Coach App Screens.
Intervention
PINK! offers multimodal content in 3 categories: nutrition, physical activity, and mental health. Content is provided as articles, videos, podcasts, and daily goals to achieve. Those daily goals are steps counts, nutritional habits, physical exercises, MBSR exercises, and more. The patients decide themselves if and how many goals they try to achieve each day. The goal is to motivate patients to start changing daily lifestyle habits. All information is evidence-based, validated, and permanently updated based on recent research results, German Breast Cancer Guidelines (AGO S3 guidelines), and the PINK! Research Expert Board [12].
Questionnaires were all standardized. They were sent out with Redcap as database after 4, 8, and 12 weeks. Control group got access to PINK! after completing T3 questionnaire (12 weeks). Primary endpoint of this pilot study was psychological distress measured by validated PHQ-9. Secondary outcomes were subscores of EORTC-QLQ-C30 such as fatigue and physical activity level (International Physical Activity Questionnaire [IPAQ]) [20–22]. Additionally, the app usage time was measured with usage time in minutes per week to define usage groups. Demographic and medical information (marital status, age, gender, cancer type, therapy type, treatment, date of surgery, chemotherapy start and radiation therapy, TNM) was collected at baseline.
Objectives
The primary objective of this study was that using PINK! over 12 weeks during therapy or in aftercare leads to a significant reduction of psychological distress in patients with breast cancer compared to control group that provided comparison to “standard of care.” Moreover, we expected as secondary objectives an increase of physical activity level and a decrease of fatigue symptoms in the intervention group. Finally, this pilot study aimed to investigate the feasibility of integrating a digital lifestyle solution in clinical routine cancer care at a large breast cancer center. Based on those findings, a multicentric randomized-controlled trial was designed to investigate effects of PINK! in a larger patient cohort and specified subgroups.
Statistics and Endpoints
To investigate changes on the primary outcome PHQ-9 score and secondary outcomes (fatigue and physical activity level), a paired samples t-test was used both between baseline and posttreatment. The PHQ-9 is a multipurpose instrument for screening, diagnosing, monitoring, and measuring the severity of depression. The total score is calculated by assigning scores of 0, 1, 2, and 3, to the response categories of “not at all,” “several days,” “more than half the days,” and “nearly every day,” respectively. PHQ-9 total score for the nine items ranges from 0 to 27. PHQ-9 total score was determined by adding up item scores. Total scores of 5, 10, 15, and 20 represent cutpoints for mild, moderate, moderately severe, and severe depression, respectively [20].
Fatigue was measured with a subscore of EORTC-QLQ-C30. Fatigue is one of the symptom scales of this questionnaire. To calculate the symptom score, the raw score needs to be calculated to do a linear transformation and obtain a value between 0 and 100. Symptomatic scales as the fatigue scale should be interpreted as follows: 100 corresponds to a high level of symptoms, while 0 corresponds to no symptoms at all [21].
IPAQ is a survey to describe physical activity levels. There are two forms of output from scoring the IPAQ. Results can be reported in categories (low activity levels, moderate activity levels, or high activity levels) or as a continuous variable (MET minutes a week). In this study, we choose to use MET minutes. MET minutes represent the amount of energy expended carrying out physical activity. The activity level is considered as high if patients achieve at least 3,000 MET minutes per week. As in this study the intervals of questionnaires are 4 weeks, the data presented below refer to the MET minutes within 4 weeks. At least 600 MET minutes per week is considered as the moderate activity level. To calculate MET minutes a week, multiply the MET value of the questionnaire given (remember walking = 3.3, moderate activity = 4, vigorous activity = 8) by the minutes the activity was carried out and again by the number of days that the activity was undertaken [22].
Multiple imputation algorithms (predictive mean matching) were used to deal with missing values at post-assessment [23, 24]. At first, we performed an intention-to-treat (ITT) analysis including both adherent and nonadherent patients. Adherent patients were all patients that at least finished baseline and T3 (12 weeks primary endpoint). Second, we analyzed data sets and changes in scores for only adherent patients. Due to the small number of patients, the ITT analysis was chosen for this paper. To measure the effect size for the dependent samples t-test analyses, Cohen's d was calculated as follows: Cohen’s d = mean difference/standard deviation of the difference [25, 26]. A significance level was set at p ≤ 0.05. Usage groups were defined as high, medium, and low according to average usage time in minutes over 12 weeks. Linear regression was performed to investigate the correlation of usage time and changes in primary endpoint. The demographic, medical history, and outcome variables were described using frequency and descriptive statistics. Analyses were performed using SPSS Version 27.0.0.
Results
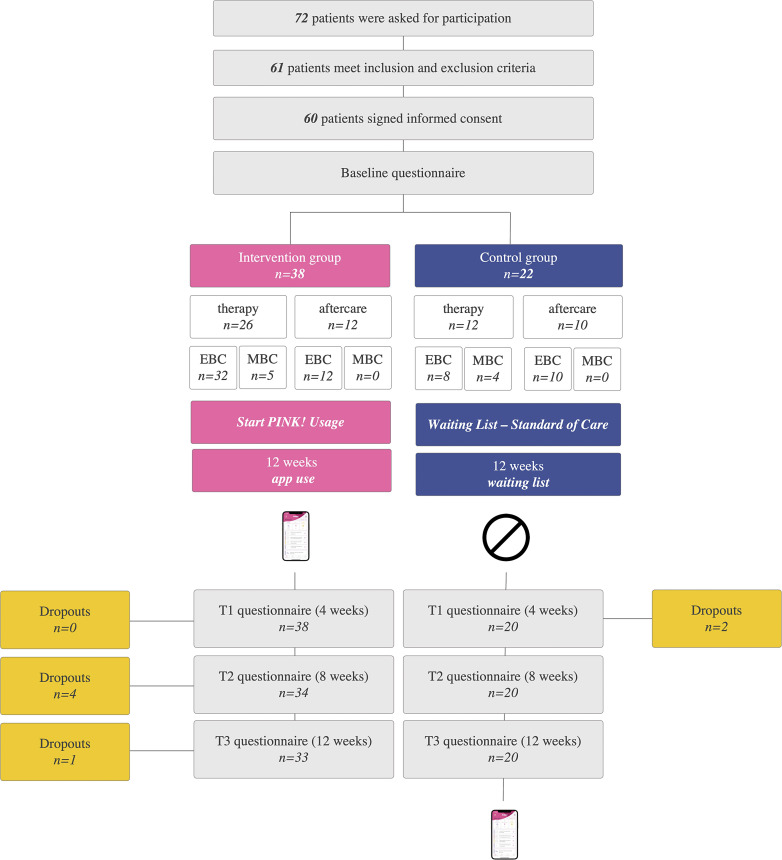
In August 2021, in total 72 patients were asked to participate in this pilot study. 61 patients met the inclusion and did not meet exclusion criteria. 60 patients signed the informed consent, answered the baseline questionnaire, and were randomized to one of the study groups. 38 patients were randomized to the intervention group and 22 to the control group whereby 39 were in therapy and 21 in aftercare. Over 12 weeks, there were 5 dropouts in the intervention group and 2 in the control group, which corresponds to 11.7% dropout rate. Figure 2 displays the recruitment flowchart.
Fig. 2.
Flowchart of recruitment, 1:2 randomization to intervention and control groups (waiting list) and 1:2 randomization to “therapy” and “aftercare” subgroups. Control group started PINK! usage after 12 weeks; intervention group at baseline.
Table 1 displays baseline characteristics and therapy status of all mITT EBC patients. All patients received chemotherapy and had surgery. Nine of 53 mITT patients were MBC patients who underwent first-line chemotherapy. Table 2 displays baseline characteristics of patients with MBC in this pilot study.
Table 1.
Baseline characteristics of study EBC participants n = 53, mITT data set EBC n = 44
| Parameter | Value | Intervention | Control | Therapy | Aftercare |
|---|---|---|---|---|---|
| Number of patients EBC | n (%) | mITT n = 28 (%) | mITT n = 16 (%) | mITT n = 24 | mITT n = 20 |
| Age | years | 49,4 | 49,9 | 49,2 | 49,4 |
| T | pT1 | 18 (64.3) | 6 (37.5) | 13 | 10 |
| pT2-T4d | 10 (35.7) | 10 (62.5) | 11 | 10 | |
| N | pN0 | 21 (75.0) | 8 (50.0) | 17 | 12 |
| pN+ | 7 (25.0) | 8 (50.0) | 7 | 8 | |
| M | M0 | 28 (100.0) | 16 (100.0) | 24 | 20 |
| HR | Luminal A | 2 (7.1) | 2 (12.5) | 0 | 4 |
| Luminal B | 13 (46.4) | 11 (68.8) | 11 | 13 | |
| TNBC | TNBC | 7 (25.0) | 2 (12.5) | 8 | 1 |
| HER2 | Her2+ | 6 (21.4) | 1 (6.3) | 5 | 2 |
| Therapy status | neoadjuvant | 21 (75.0) | 12 (75.0) | 18 | 15 |
| Adjuvant | 5 (17.9) | 2 (12.5) | 6 | 1 | |
| Endocrine therapy only + BP | 2 (7.1) | 2 (12.5) | 0 | 4 |
mITT, modified intention to treat data set.
Table 2.
Baseline characteristics of MBC patients of study cohort
| Parameter | Value | Intervention | Control |
|---|---|---|---|
| Number of patients MBC | n (%) | mITT n = 5 | mITT n = 4 |
| Age | Years | 47,0 | 49,0 |
| HR | Positive | 4 | 4 |
| Negative | 1 | 0 | |
| HER2 | Positive | 3 | 3 |
| Negative | 2 | 1 | |
| TNBC | 1 | 0 | |
| Therapy status | In therapy | 9 | 0 |
| In aftercare | 0 | 9 |
Primary Endpoint PHQ-9
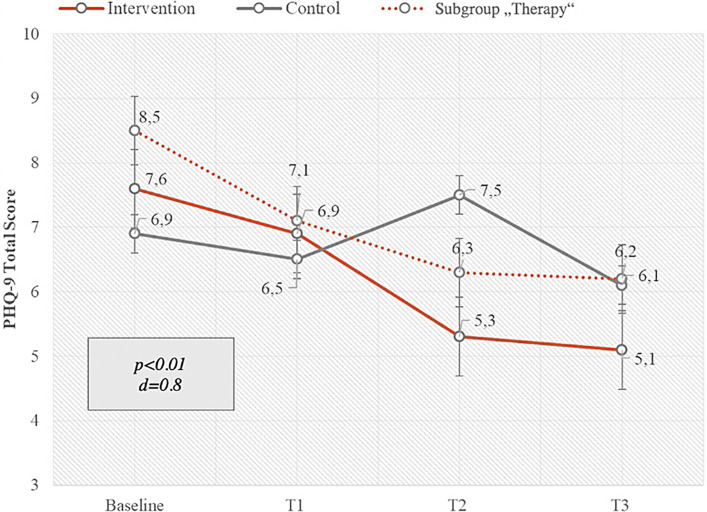
Primary endpoint of this pilot study was a group comparison of PHQ-9 total scores in intervention and control groups after 12 weeks of app usage in the intervention group. The PHQ-9 is a multipurpose instrument for screening, diagnosing, monitoring, and measuring the severity of depression. A paired samples t-test in the mITT analysis indicated that patients experienced significantly less psychological distress after the intervention than before the intervention, with a large effect size (Cohen’s d) of 0.8 and a p value <0.01. PHQ-9 total score decreased by 2.5 score points in the intervention group and by 0.8 after 12 weeks. Compared to baseline values, this reduction is equivalent to 32.9%. A subgroup analysis of patients in therapy showed also a significant reduction of psychological distress after 12 weeks as well as a higher value of PHQ-9 total score at baseline. Patients in therapy showed a decrease of 2.3 score points in PHQ-9. These effects were lower in the subgroup of aftercare patients as this group started at a lower level of psychological distress at baseline. Figure 3 shows the changes of the primary endpoint PHQ-9 average score points in the intervention and control groups over 12 weeks.
Fig. 3.
Changes of primary endpoint PHQ-9 total score over 12 weeks’ intervention versus control and versus intervention subgroup “therapy” (mITT) with SD, p value, and Cohen’s d effect size: intervention group n = 33, control group n = 20, and subgroup therapy n = 24.
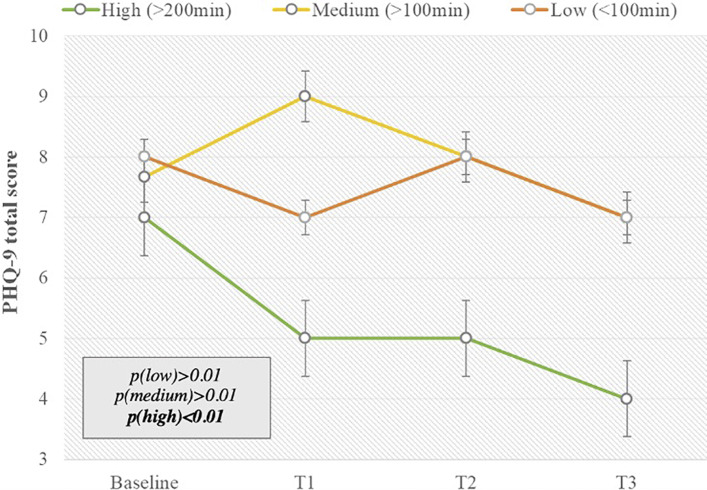
Concerning the degree of app use, the subgroup “high” used the app more than 200 min over 12 weeks, “medium” used the app between 100 and 200 min over 12 weeks, and “low” users used the app <100 min over 12 weeks. The reduction in psychological distress was seen, especially in participants who showed high app use, in comparison to medium, low, and nonusers and participants in the control group. Patients with high app usage time used PINK! Coach on average 312 min per months, which is equivalent to 11 min per day. This group contained 12 out of 33 patients in the intervention group. Effects of PINK! Coach on those patients’ psychological distress were higher than in the other usage groups.
PHQ-9 total score decreased by 3.0 score points in the “high” usage group. The levels of psychological distress in medium and low app users, nonusers, and control participants did not statistically differ after 12 weeks (Fig. 4).
Fig. 4.
Usage time in usage groups (high >200 min usage in 12 weeks; medium >100 min usage in 12 weeks; low <100 min usage in 12 weeks) versus PHQ-9 total score changes over time in the intervention group with SD and p values, with “high” n = 12, “medium” n = 6, and “low” n = 11.
App usage in the subgroup of therapy and aftercare did not differ within 12 weeks. Age did also not impact the app usage time. MBC patient used the app less intense compared to EBC patients. Effects were stronger in EBC patients than in MBC patients. Hormone receptor status and Her2 status did not impact effects or usage time.
Secondary Endpoint EORTC-QLQ-C30 Fatigue Scale
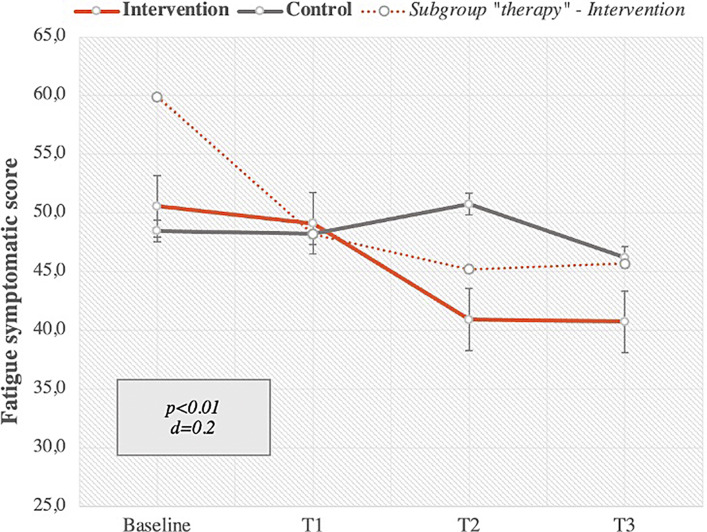
One secondary endpoint of this pilot study was a group comparison of fatigue levels in intervention and control groups after 12 weeks of app usage in intervention group. A paired samples t-test in the mITT analysis showed that patients experienced significant less fatigue symptoms after the intervention than before the intervention, with a small effect size (Cohen´s d) of d = 0.2 and a p value <0.01. On average, there was a significant mean fatigue score decrease from the baseline of 9.9 score points, which corresponds to a relative average decrease of 19.5% compared to baseline.
A subgroup analysis of patients in therapy also showed a significant reduction of psychological distress after 12 weeks as well as a higher value of fatigue symptoms at baseline. Patients in therapy showed a decrease of 14.2 score points in fatigue symptoms, which corresponds to a relative average decrease of 23.7%. Figure 5 shows the fatigue score changes over 12 weeks in intervention and control groups as well as in the subgroup of patients undergoing chemotherapy.
Fig. 5.
Fatigue symptoms measured with EORTC-QLQ-C30 fatigue scale from baseline to T3 (after 12 weeks) – intervention versus control group and subgroup “intervention in therapy” with SD, p value of intervention group and Cohen’s d effect size, intervention group n = 33, control group n = 20, subgroup therapy n = 24.
Concerning the degree of fatigue symptoms at baseline, the subgroup “high” had a fatigue score from 66.8 to 100, the subgroup “medium” had a fatigue score from 33.4 to 66.7, and the subgroup “low” had a fatigue score from 0 to 33.3 at baseline.
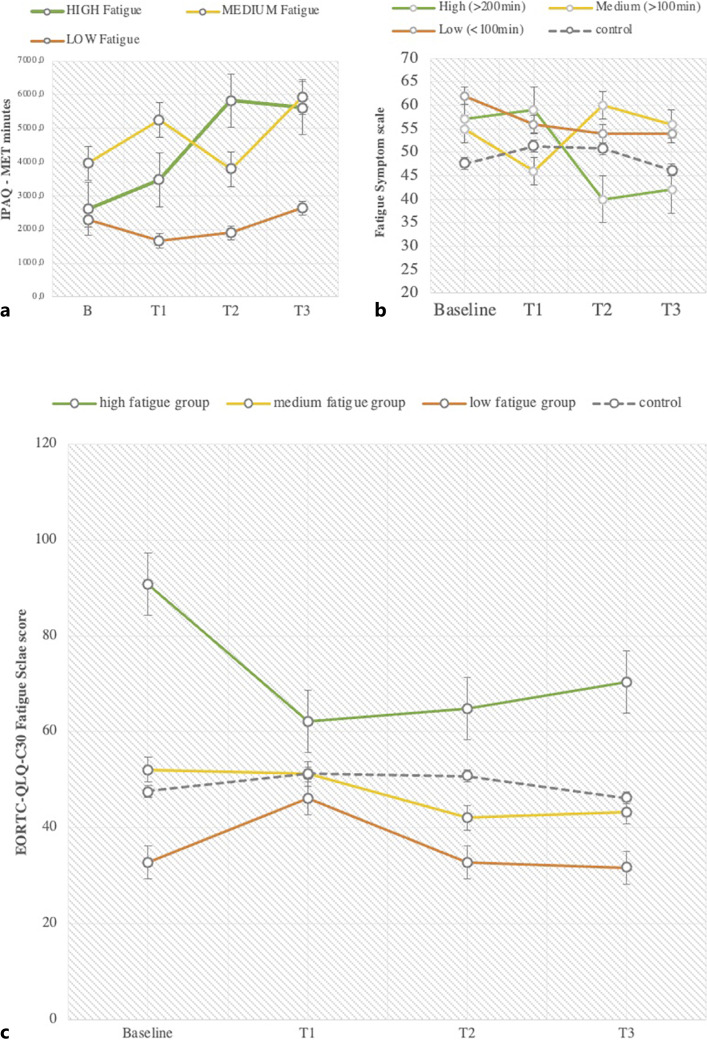
Patients who started with strong (high) fatigue symptoms at baseline also started with a low physical activity level (IPAQ MET minutes per week <600) but increased their activity level by 3,005 MET minutes per month, which corresponds to 751 MET minutes per week and a total value of 1,405 MET minutes per week. According to the IPAQ scoring manual, this number of MET minutes per week is equivalent to a medium activity level (medium activity level means 600–1,500 MET minutes per week). Patients who started at a medium fatigue symptom level at baseline also started with a low activity level and increased their activity level by 2,027 MET minutes per month which corresponds to 506 MET minutes per week and a total value of 996 MET minutes per week. According to the IPAQ scoring manual, this is equivalent to a medium activity level. Patients with low fatigue symptoms started with a medium activity level (821 MET minutes per week) at baseline and increased their activity by 526 MET minutes per month, which is equivalent to 131 MET minutes per week and a total score of 689 MET minutes per week. According to the IPAQ scoring manual, this value corresponds to a medium activity level. This is shown in Figure 6a [22].
Fig. 6.
a Physical activity levels (MET minutes of IPAQ questionnaire over 4 weeks) from baseline to T3 (after 12 weeks) in 3 fatigue intensity groups: “high fatigue,” “medium fatigue,” “low fatigue” and control at baseline with “high” n = 12, “medium” n = 6, and “low” n = 11. b Fatigue symptom changes in usage groups: “high,” “medium,” and ”low” from baseline to T3. c Fatigue changes from baseline to T3 in 3 fatigue intensity groups with “high” n = 12, “medium” n = 6, and “low” n = 11.
Concerning the degree of app use, the subgroup “high” used the app more than 200 min over 12 weeks, “medium” used the app between 100 and 200 min over 12 weeks, and “low” users used the app <100 min over 12 weeks. Patients with overall high app usage showed the highest reduction in fatigue symptoms after 12 weeks. Medium and low app usage groups showed a small decrease of fatigue symptoms after 12 weeks. App usage group “high” reduced fatigue symptoms by 15 score points, app usage group “medium” increased fatigue symptoms by 1 score point, and app usage group “low” showed a reduction of fatigue by 8 score points. According to the EORTC-QLQ-C30 scoring manual, a change of 15 score points is equivalent to a moderate change [27]. This is shown in Figure 6b.
Figure 6c shows the data pattern of fatigue symptoms within fatigue intensity groups at baseline. The subgroup of” high” fatigue symptoms at baseline showed a reduction of 20.4 score points after 12 weeks, which corresponds to a large effect according to the scoring manual. The subgroup with “medium” fatigue symptoms showed a decrease of 8.8 score points and the subgroup of “low” symptoms decreased by 1.1 score points after 12 weeks.
Discussion
This waiting‐list randomized controlled pilot study primarily examined whether psychological distress, one of the main side effects of breast cancer diagnosis, treatment, and aftercare, can be improved by an app-based coaching. Psychological distress and fatigue were effectively reduced with the self‐management app PINK! for breast cancer patients and survivors. Our data showed that having 12‐week access to the PINK! app and its individual coaching significantly improved levels of psychological distress and fatigue symptoms on average. Physical activity level could also be increased within 12 weeks of usage. These effects were found, especially among those participants who used the app to a high degree, which was in practice related to ≥200 min of app use in 12 weeks. On average, patients in the usage group “high” used PINK! 312 min per month, which corresponds to 10.4 min per day. Nevertheless, more research on potential moderating factors is needed, and the present results suggest that the self‐management apps like PINK! could be beneficial for patients with breast cancer in any stage of disease and therapy (therapy, aftercare).
As this was a pilot study, the number of patients is too small to allow a definite statement about the effectiveness of PINK!. Nonetheless, these are initial promising results that led us to plan and perform a multicentric randomized-controlled waiting-list trial in German breast cancer centers with a specified study design and a higher number of patients.
We demonstrated that a digital intervention such as PINK! shows independent large effect sizes in the reduction of psychological distress (d = 0.8) and a small effect size regarding a reduction of fatigue symptoms. The effects on psychological distress are comparable to face-to-face therapy [28]. However, it is important to note that the population was heterogeneous in terms of therapy status (in initial therapy or in aftercare), tumor stage, and biology as all breast cancer patients were included in this pilot study. Statements about effects on specific groups of breast cancer patients cannot be made. Still, with digital self‐management interventions, a higher number of patients and survivors can be reached and helped simultaneously at lower costs. At the same time, it offers immediate, individualized help and self-determined use for patients.
Furthermore, the data indicated an association between app usage and fatigue reduction as well as between app usage and increase of physical activity level, which also leads to fatigue reduction. Running a waiting-list RCT has the disadvantage that it might artificially inflate intervention effect estimates since participants in the control group could have been influenced by the design to literally “wait‐to‐change” and thus do not improve during study duration. Nevertheless, as the control group data also changed, this seems not to be the case [29].
Our data indicate that supportive care in the form of digital lifestyle interventions is worth to be routinely implemented in breast cancer care. Aside from that, lifestyle interventions may be suitable for patients who seek a low-threshold treatment that is easy to integrate into daily life activities and routines, as one can follow the program when and wherever preferred. As almost all breast cancer patients are using the Internet and 67.3% were interested in assistance via the Internet regarding health-related topics [30]. Studies also showed a high usage of Internet-related technologies among physicians and breast cancer patients. This indicates that the use of eHealth for advanced and individualized support in breast cancer care could be a promising addition in therapy management. Such technology-based interventions have the potential to enhance adherence and compliance in therapy among cancer patients [31].
The currently ongoing multicenter randomized controlled study on the app was initiated to confirm the previously presented results. The PINK! main study design was specified regarding the patient groups, inclusion and exclusion criteria, and the definition of “therapy” and “aftercare.” This multicentric study will also explore the mechanisms behind the app in order to investigate which specific functionalities of the app were used to influence study endpoints.
PINK! is the first app-based and lifestyle coaching DiGA (German: Digitale Gesundheitsanwendung) for breast cancer patients in Germany. Nevertheless, many app- and web-based offers or programs were designed within the last years all over the world for lifestyle coaching of breast cancer patients and survivors during therapy and in rehabilitation or aftercare. Studies [32] show that lifestyle coaching programs can improve general health, bodily pain, vitality, and global physical and mental health significantly. Also, the physical activity level and adherence can be improved [33] in addition to cancer-related fatigue [34]. Digital app- or web-based interventions exist in various categories and high variation in attributes, recommendation of usage time, and provision of content. Nevertheless, there is a high demand for supportive care during and after therapy, and digital intervention is accepted by most of the patients [35]. Studies with other coaching apps indicate that cancer patients were interested in having a straightforward app for monitoring symptoms and daily goal setting. They also suggest that cancer patients are looking for a digital, personalized, simple, guiding, encouraging, and trustworthy solution for being coached during therapy and aftercare [36]. Looking at other digital interventions for breast cancer patients worldwide to improve psychological distress and quality of life, CANKADO PRO-React Onco [37, 38], Kaiku Health [39], Attune [40], and Optimune [41] also focus on breast cancer patients and their psychological well-being, symptom monitoring, and supportive care needs. Those digital interventions can address unmet needs of patients in regard to self-care, lifestyle, and symptom management, which leads to higher adherence, educated, and empowered patients that self-manage their disease and treatment. This in turn improves the quality of care, access to new treatment options, safety of medications, health outcomes such as side effects and quality of life, and in the end the medical costs [42]. Other studies show that empowering breast cancer patients to increase their physical activity level has potential to improve survival and to decrease the risk of mortality from breast cancer by up to 40%. A reduction in sex hormone levels, insulin resistance, and inflammation have been examined to explain those associations [43]. Further research with digital therapeutics for breast cancer patients should take study endpoints such as overall survival, mortality, and pCR into account in order to improve study outcomes and therefore the quality of personalized digital therapeutics such as PINK!. However, the PINK! Coach app is one of only two certified apps in oncology that have been approved as a digital health application. The barriers regarding the evidence of effectiveness for digital health application reimbursement in Germany are high. Demonstrating scientific evidence of such a digital intervention and communication of these results in the oncology community including patients’ feedback is important. It can be recognized that digital interventions like the PINK app can provide relevant benefits and improvement of quality of life independent of time and distance compared to individuals who would otherwise have limited access to supportive care.
Furthermore, economic analyses are increasingly demonstrating the benefits of additional interventions such as apps or patients with cancer. Its effects on survival need to be investigated in further trials.
Acknowledgments
The authors gratefully acknowledge the help of the following people who have assisted in the evaluation of this pilot study: all participants of this pilot study, the entire PINK! team, Christine zu Eulenburg, and Prof. Dr. Hans-Peter Zenner.
Statement of Ethics
The PINK app pilot study has been approved by the Medical Ethical Committee of the LMU University of Munich, Germany, on August 19, 2021 (Reference number: 21-0757). The trial has been registered on October 6, 2021 on the DRKS Trial Registry (DRKS00025811). All patients in this study signed a written informed consent to participate in this study, which are archived at LMU Breast Cancer Center.
Conflict of Interest Statement
The authors declare the following conflicts of interest: Josefine Wolff has currently a working contract with PINK! gegen Brustkrebs GmbH, Prof. Dr. Pia Wülfing is the CEO of PINK! gegen Brustkrebs GmbH. Prof. Dr. Nadia Harbeck and PD Dr. Rachel Würstlein (Breast Center, Department of Gynecology and Obstetrics, and Comprehensive Cancer Center Munich) received funding for the subsequent multicentric proof of efficacy RCT with PINK! Coach. Dr. Martin Smollich and Prof. Dr. Freerk Baumann received consulting fees from PINK! gegen Brustkrebs GmbH.
Funding Sources
This research was initiated by Breast Center, Department of Gynecology and Obstetrics, and Comprehensive Cancer Center Munich, Ludwig-Maximilians-University, University Hospital to investigate the PINK! app and offer it to their patients. LMU was the sponsor of this study.
Author Contributions
Josefine Wolff and PD. Dr. Rachel Würstlein planned and conducted the study and evaluated the data. Prof. Dr. Nadia Harbeck was the scientific advisor. Prof. Dr. Pia Wülfing, Dr. Martin Smollich, and Dr. Freerk Baumann developed the PINK! app and provided the entire content of the App. Alexander Koenig, Brigitte Ehrl, and Jana Damsch helped finding patients to include in the study.
Funding Statement
This research was initiated by Breast Center, Department of Gynecology and Obstetrics, and Comprehensive Cancer Center Munich, Ludwig-Maximilians-University, University Hospital to investigate the PINK! app and offer it to their patients. LMU was the sponsor of this study.
Data Availability Statement
The data that support the findings of this study are not available.
References
- 1. Harbeck N, Gnant M. Breast cancer. Lancet. 2017 Mar 18;389(10074):1134–50 Epub 2016 Nov 17. 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 2. Voigt V, Neufeld F, Kaste J, Bühner M, Sckopke P, Wuerstlein R, et al. Clinically assessed posttraumatic stress in patients with breast cancer during the first year after diagnosis in the prospective, longitudinal, controlled COGNICARES study. Psychooncology. 2017 Jan;26(1):74–80 Epub 2016 Feb 22. 10.1002/pon.4102. [DOI] [PubMed] [Google Scholar]
- 3. Modi ND, Danell NO, Perry RNA, Abuhelwa AY, Rathod A, Badaoui S, et al. Patient-reported outcomes predict survival and adverse events following anticancer treatment initiation in advanced HER2-positive breast cancer. ESMO Open. 2022 Jun;7(3):100475 Epub 2022 Apr 28. 10.1016/j.esmoop.2022.100475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou K, Bellanger M, Le Lann S, Robert M, Frenel JS, Campone M. The predictive value of patient-reported outcomes on the impact of breast cancer treatment-related quality of life. Front Oncol. 2022 Oct 14;12:925534. 10.3389/fonc.2022.925534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Cicco P, Catani MV, Gasperi V, Sibilano M, Quaglietta M, Savini I. Nutrition and breast cancer: a literature review on prevention, treatment and recurrence. Nutrients. 2019 Jul 3;11(7):1514. 10.3390/nu11071514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedenreich CM, Morielli AR, Lategan I, Ryder-Burbidge C, Yang L. Physical activity and breast cancer survival-epidemiologic evidence and potential biologic mechanisms. Curr Nutr Rep. 2022 Dec;11(4):717–41 Epub 2022 Aug 11. 10.1007/s13668-022-00431-2. [DOI] [PubMed] [Google Scholar]
- 7. Aguiñaga S, Ehlers DK, Cosman J, Severson J, Kramer AF, McAuley E. Effects of physical activity on psychological well-being outcomes in breast cancer survivors from prediagnosis to posttreatment survivorship. Psychooncology. 2018 Aug;27(8):1987–94 Epub 2018 Jun 1. 10.1002/pon.4755. [DOI] [PubMed] [Google Scholar]
- 8. Cillessen L, Johannsen M, Speckens AEM, Zachariae R. Mindfulness: based interventions for psychological and physical health outcomes in cancer patients and survivors: a systematic review and meta-analysis of randomized controlled trials. Psychooncology. 2019;28(12):2257–69. 10.1002/pon.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matis J, Svetlak M, Slezackova A, Svoboda M, Sumec R. Mindfulness-based programs for cancer patients via eHealth and mHealth: a systematic review and synthesis of quantitative research (preprint). J Med Internet Res. 2020;22:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nissen ER, O’Connor M, Kaldo V, Højris I, Borre M, Zachariae R, et al. Internet-delivered mindfulness-based cognitive therapy for anxiety and depression in cancer survivors: a randomized controlled trial. Psychooncology. 2020;29(1):68–75. 10.1002/pon.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Høeg BL, Frederiksen MH, Andersen EAW, Saltbæk L, Friberg AS, Karlsen RV, et al. Is the health literacy of informal caregivers associated with the psychological outcomes of breast cancer survivors? J Cancer Surviv. 2021 Oct;15(5):729–37 Epub 2020 Nov 9. 10.1007/s11764-020-00964-x. [DOI] [PubMed] [Google Scholar]
- 12. Arbeitsgruppe Gynäkologische Onkologie (AGO) . Guidelines Breast Version 2022.1D, Gesundheitskompetenz und Kommunikation.
- 13. Di Nardo P, Lisanti C, Garutti M, Buriolla S, Alberti M, Mazzeo R, et al. Chemotherapy in patients with early breast cancer: clinical overview and management of long-term side effects. Expert Opin Drug Saf. 2022 Nov;21(11):1341–55 Epub 2022 Dec 5. 10.1080/14740338.2022.2151584. [DOI] [PubMed] [Google Scholar]
- 14. Piet J, Würtzen H, Zachariae R. The effect of mindfulness-based therapy on symptoms of anxiety and depression in adult cancer patients and survivors: a systematic review and Meta-analysis. J Consult Clin Psychol. 2012;80(6):1007–20. 10.1037/a0028329. [DOI] [PubMed] [Google Scholar]
- 15. Zernicke KA, Campbell TS, Speca M, McCabe-Ruff K, Flowers S, Carlson LE. A randomized wait-list controlled trial of feasibility and efficacy of an online mindfulness-based cancer recovery program: the eTherapy for cancer applying mindfulness trial. Psychosom Med. 2014;76(4):257–67. 10.1097/PSY.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 16. Berger AM, Mooney K, Alvarez PA, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13(8):10121039. 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bower JE. Cancer-related fatigue: mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galiano-Castillo N, Ariza-García A, Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, Arroyo-Morales M. Depressed mood in breast cancer survivors: associations with physical activity, cancer-related fatigue, quality of life, and fitness level. Eur J Oncol Nurs. 2014 Apr;18(2):206–10 Epub 2013 Nov 5. 10.1016/j.ejon.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 19. Pagola I, Morales JS, Alejo LB, Barcelo O, Montil M, Oliván J, et al. Concurrent exercise interventions in breast cancer survivors with cancer-related fatigue. Int J Sports Med. 2020 Oct;41(11):790–7 Epub 2020 Jun 29. 10.1055/a-1147-1513. [DOI] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22. Meh K, Jurak G, Soriæ M, Rocha P, Sember V. Validity and reliability of IPAQ-SF and GPAQ for assessing sedentary behaviour in adults in the European union: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021 Apr 26;18(9):4602. 10.3390/ijerph18094602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schenker N, Taylor J. Partially parametric techniques for multiple imputation. Stat Data Anal. 1996;22(4):425–46. 10.1016/0167-9473(95)00057-7. [DOI] [Google Scholar]
- 24. Heitjan D, Little R. Multiple imputation for the fatal accident reporting system. Appl Stat. 1991;40(1):13–29. 10.2307/2347902. [DOI] [Google Scholar]
- 25. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 26. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Effect sizes based on correlations Introduction to Meta-Analysis. 2009. p. 21–32. 10.1002/9780470743386. [DOI] [Google Scholar]
- 27. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D. Bottomley A, on behalf of the EORTC quality of life group the EORTC QLQ-C30 scoring manual. 3rd ed. European organisation for research and treatment of cancer. Brussels; 2001. Published by. [Google Scholar]
- 28. Compen F, Bisseling E, Schellekens M, Donders R, Carlson L, van der Lee M, et al. Face-to-face and internet-based mindfulness-based cognitive therapy compared with treatment as usual in reducing psychological distress in patients with cancer: a multicenter randomized controlled trial. J Clin Oncol. 2018;36(23):2413–21. 10.1200/JCO.2017.76.5669. [DOI] [PubMed] [Google Scholar]
- 29. Cunningham JA, Kypri K, McCambridge J. Exploratory randomized controlled trial evaluating the impact of a waiting list control design. [DOI] [PMC free article] [PubMed]
- 30. Drewes C, Kirkovits T, Schiltz D, Schinkoethe T, Haidinger R, Goldmann-Posch U, et al. EHealth acceptance and new media preferences for therapy assistance among breast cancer patients. JMIR Cancer. 2016 Sep 14;2(2):e13. 10.2196/cancer.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirkovits T, Schinkoethe T, Drewes C, Gehring C, Bauerfeind I, Harbeck N, et al. eHealth in modern patient-caregiver communication: high rate of acceptance among physicians for additional support of breast cancer patients during long-term therapy. JMIR Cancer. 2016 Sep 19;2(2):e14. 10.2196/cancer.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seib C, Anderson D, McGuire A, Porter-Steele J, McDonald N, Balaam S, et al. Improving health-related quality of life in women with breast, blood, and gynaecological Cancer with an eHealth-enabled 12-week lifestyle intervention: the women’s wellness after Cancer program randomised controlled trial. BMC Cancer. 2022 Jul 8;22(1):747. 10.1186/s12885-022-09797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Signorelli GR, Monteiro-Guerra F, Rivera-Romero O, Núñez-Benjumea FJ, Fernández-Luque L. Breast cancer physical activity mobile intervention: early findings from a user experience and acceptability mixed methods study. JMIR Form Res. 2022 Jun 22;6(6):e32354. 10.2196/32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nápoles AM, Santoyo-Olsson J, Chacón L, Stewart AL, Dixit N, Ortiz C. Feasibility of a mobile phone app and telephone coaching survivorship care planning program among Spanish-speaking breast cancer survivors. JMIR Cancer. 2019 Jul 9;5(2):e13543. 10.2196/13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beenhakker L, Witteveen A, Wijlens KAE, Siemerink EJM, van der Lee ML, Bode C, et al. Patient preference attributes in eHealth interventions for cancer-related fatigue: a scoping review. Eur J Cancer Care 2022 Nov;31(6):e13754 Epub 2022 Nov 16. 10.1111/ecc.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monteiro-Guerra F, Signorelli GR, Rivera-Romero O, Dorronzoro-Zubiete E, Caulfield B. Breast cancer survivors’ perspectives on motivational and personalization strategies in mobile app-based physical activity coaching interventions: qualitative study. JMIR Mhealth Uhealth. 2020 Sep 21;8(9):e18867. 10.2196/18867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Degenhardt T, Harbeck N, Fasching PA, Wuerstlein R, Lüftner D, Kates RE, et al. Documentation patterns and impact on observed side effects of the CANKADO ehealth application: an exploratory analysis of the PreCycle trial. J Clin Oncol. 2020;38(15_Suppl l):2083. 10.1200/jco.2020.38.15_suppl.2083. [DOI] [Google Scholar]
- 38. Harbeck N, Wuerstlein R, Schinkoethe T. Improved patient management using EHealth tools: potential and pitfalls. Breast Cancer Manag. 2015;4(1):1–5. 10.2217/bmt.14.53. [DOI] [Google Scholar]
- 39. Denis F, Yossi S, Septans AL, Charron A, Voog E, Dupuis O, et al. Improving survival in patients treated for a lung cancer using self-evaluated symptoms reported through a web application. Am J Clin Oncol. 2017 Oct;40(5):464–9. 10.1097/COC.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 40. Penedo FJ, Fox RS, Oswald LB, Moreno PI, Boland CL. Technology-based psychosocial intervention to improve quality of life and reduce symptom burden in men with advanced prostate cancer: results from a randomized controlled trial. Int J Behav Med. 2020 Oct;27(5):490–505. 10.1007/s12529-019-09839-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holtdirk F, Mehnert A, Weiss M, Mayer J, Meyer B, Bröde P. Results of the Optimune trial: a randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. PLoS One. 2021 May 7;16(5):e0251276. 10.1371/journal.pone.0251276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gussoni G, Ravot E, Zecchina M. Digital therapeutics in Oncology: findings, barriers and prospects. A narrative review. Ann Res Oncol. 2022. [Google Scholar]
- 43. Hayes SC, Steele ML, Spence RR, Gordon L, Battistutta D, Bashford J, et al. Exercise following breast cancer: exploratory survival analyses of two randomised, controlled trials. Breast Cancer Res Treat. 2018 Jan;167(2):505–14 Epub 2017 Oct 23. 10.1007/s10549-017-4541-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not available.