Abstract
Background
Chronic pain after breast cancer surgery affects up to 60% of patients. Evidence supports the fact that pain outwith the surgical site is a significant issue. This systematic review and meta-analysis sought to evaluate the prevalence of non-surgical site pain (NSSP) in women after breast cancer surgery at 6 months post-operatively.
Methods
Adult women with a confirmed breast cancer diagnosis who had undergone breast cancer surgery were identified. The outcome pursued was pain outwith the surgical site measured on either NRS/VRS or VAS rating scale. CENTRAL, Embase, PubMed, MEDLINE, CINAHL, PsycInfo, Web of Science, and Scopus were searched to identify studies that examined NSSP after breast cancer surgery at 6 months. Data were gathered via pre-piloted Excel forms and analysed both quantitively and qualitatively. Meta-analysis was carried out using a random-effects model to assess risk difference with 95% confidence interval (CI).
Results
A total of sixteen studies were identified for inclusion. Eleven studies failed to provide sufficient data and consequently were analysed qualitatively. Five studies were adequate for quantitative analysis, including a total of 995 patients. Meta-analysis identified a risk difference of 18% (95% CI: 5–31%) between patients who had breast cancer surgery and a reference, however, this is low-quality evidence.
Conclusion
This review has highlighted that breast cancer surgery increases the risk of pain outwith the surgical site postoperatively. It was additionally identified that NSSP data are often gathered in research yet rarely presented in results or highlighted as a primary outcome. As the quality of evidence was low, research specifying NSSP as a primary outcome is required to provide more certainty.
Keywords: Quality of life, Pain
Introduction
With the advancement in breast cancer diagnostics and treatment options, prognosis is improving, with a 5-year overall survival of 85% [1]. This is positive news for breast cancer patients; however, this increased longevity brings in survivorship issues, most notably the side effects of therapies [2]. Chronic post-surgical pain, for example, is a significant adverse effect with an estimated prevalence of 21.8% in breast cancer survivors in a large systematic review [3]. In 2003, Reitman et al. [4] highlighted in their systematic review that pain and weakness post breast cancer surgery have significant effects on quality of life.
Novel research has begun to categorise the types of pain that are recurrently creating issues for women post breast cancer surgery. Non-surgical site pain (NSSP), such as myalgia, neuralgia, and headaches, have been highlighted by Forget et al. [5] in their paper categorising chronic pain in high-risk breast cancer patients. They evaluated chronic pain at 12 months and 24 months post-surgery, as the definition of chronic pain is pain 3 months post-surgery [6]. This showed that musculoskeletal pain was the most prevalent amongst their population, a finding contrary to the current body of evidence surrounding post-treatment pain for breast cancer therapy as it was always reported mainly in the site of the “breast area” [3]. Conversely, a large Danish study identified that along with pain in the breast region, pain in the axilla, the side of the body, and the arm (on the surgical side) were typically described. Therefore, the finding of Forget et al. [7] that musculoskeletal pain was the most common pain condition, could be considered either as discordant or as reflecting an insufficiently studied problem. The aim of this review is to assess the prevalence of NSSP post-breast cancer surgery at 6 months.
Methods
This review was carried out according to the guidance of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Cochrane Handbook for Systematic Reviews of Interventions (v6.1) (Cochrane Handbook for Systematic Reviews of Interventions, 2021). A protocol for this review was developed in accordance with the PRISMA recommendations and is registered at PROSPERO (CRD: 42021233905): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021233905.
Eligibility Criteria
Eligible studies adhere to the PICO criteria set out in the introduction. Population (P): Human adult females with a breast cancer diagnosis from any setting under secondary care. Intervention (I): Breast cancer surgical treatment with curative intent including both mastectomy and breast-conserving therapy and no exclusion based upon adjuvant therapy. Control (C): Not applicable to this review as assessing the prevalence of pain in one population. It is not possible to achieve a control for breast cancer surgery as the control would be null treatment and would not be best practice. Outcome (O): NSSP graded on a measurable pain scale as advised by IMMPACT recommendations, such as numerical rating scale (NRS), verbal rating scale, and visual analogue scale (VAS). The criteria for exclusion: language other than English, abstracts, editorials, studies involving paediatric patients, studies involving terminal cancer, and duplicate populations.
Searching Methods
Search Sources
The search strategy comprised bibliographic database searching and subsequent searching of citation databases, to ensure the opportunity to identify a wide breadth of papers. Eight databases were used for the literature search: Cochrane Central Register of Controlled Trials (CENTRAL), Embase, PubMed, MEDLINE, CINAHL, PsycInfo, Web of Science (WoS), and Scopus. Databases were searched using Boolean operators and controlled vocabulary terms, including Medical Subject Headings for PubMed, MEDLINE, and Cochrane (search strategies found in online suppl. Appendix; for all online suppl. material, see https://doi.org/10.1159/000531621). The use of index terms was employed for Scopus. To supplement these searches, the use of free text terms was utilised to ensure that all uncommon terminologies and differing spellings would be included. The use of the explode (exp) function was used to ensure inclusion of derivatives of the above search terms.
Supplementary Searches
The search of Scopus and WoS served as supplementary searches in this review. These publicly available citation databases of peer-reviewed literature were used for automated searching. Hand-searching was performed by one investigator in which conference abstracts and reference lists of relevant studies were manually screened.
Search Strategy
Table 1 shows an example of one full search strategy. This example was the strategy utilised in the CENTRAL database.
Table 1.
Search strategy for CENTRAL database
| Search number | Search terms | Results |
|---|---|---|
| 1 | MeSH descriptor (breast neoplasm) explode all trees OR (breast cancer): ti,ab,kw OR (breast neoplasm):ti,ab,kw | 37,845 |
| 2 | MeSH description (Mastectomy) explode all trees OR (lymph node excision): ti,ab, kw OR (“sentinel node biopsy”):ti,ab, kw OR (“sentinel lymph node dissection”):ti,ab, kw OR (wide local excision):ti,ab,kw | 3,274 |
| 3 | MeSH descriptor: (Chronic Pain) explode all trees OR MeSH descriptor: (Pain, Postoperative) explode all trees OR MeSH descriptor: (Arthralgia) explode all trees OR MeSH descriptor: (Myalgia) explode all trees OR MeSH descriptor: (Pain Measurement) explode all trees OR MeSH descriptor: (Pain Measurement) explode all trees OR (arthralgia):ti,ab, kw OR (myalgia):ti,ab, kw OR (neuralgia):ti,ab, kw OR (“post-operative pain”):ti,ab,kw | 97,219 |
| 4 | MeSH descriptor: (Postoperative Period) explode all trees OR (after surgery): ti,ab, kw OR (following surgery):ti,ab, kw OR (“post-operative”):ti,ab, kw OR (“post-surgery”):ti,ab,kw | 153,039 |
| 5 | #1 AND #2 AND #3 AND #4 | 188 |
MeSH, Medical Subject Headings.
Inclusion Screening
Papers were uploaded to the reference manager Rayyan [8]. Two independent researchers (GB and PF) assessed the papers’ suitability for inclusion. Duplicates were manually removed and the remaining papers were screened via title, abstract, and full-text review. Any disagreements between reviewers were resolved with a third party (YAM).
Data Collection Process
Pre-piloted Excel forms were used to extract data. As not all the included studies incorporated the desired data, the piloted forms included qualitative data sections involving reasons for not including the specific NSSP data and quantitative sections for those studies which provided data. The characteristics collected can be seen in Table 2. The findings sought included the method of data collection, where NSSP was included in the paper, how NSSP was published, and data values relating to NSSP. As a significant proportion (75%, N = 12) of the included studies did not publish the required data, these authors were contacted via email to access the missing data. If the author did not respond within the first 5 days, a second aide-mémoire email was sent. If primary authors had still failed to respond, a secondary email address was sought for each paper in question and the initial email was forwarded to that address.
Table 2.
Patient characteristics, intervention, primary outcome, and follow-up time
| First author | SIGN quality rating | Year | Location | Study design | Sample size | Mean age, years | Intervention | Primary outcome | Follow-up period |
|---|---|---|---|---|---|---|---|---|---|
| Albi-Feldzer [9] | High quality (++) | 2013 | France | RCT | 236 | 56 | Ropivacaine would infiltration | Pain brief pain inventory score at 3 months | Up to 12 months post-operatively |
| Amr [10] | Low quality (0) | 2010 | Egypt | RCT | 150 | 44 | Perioperative administration of Venlafaxine or Gabapentin | Acute pain VAS | Up to 6 months post-operatively |
| Andersen [11] | Acceptable (+) | 2015 | Denmark | Observational (prospective cohort) | 545 | 61 (median) | Breast cancer surgery for stage I and II disease | Predictive factors for pain experienced 1 year after surgery | Up to 12 months post-operatively |
| Baudic [12] | Acceptable (+) | 2016 | France | Observational (prospective) | 100 | 55 | Mastectomy or lumpectomy with axillary node dissection | Effect of alexithymia and emotional repression on the development of post-operative pain | Up to 12 months post-operatively |
| Divella [13] | Acceptable (+) | 2020 | Italy | Observational | 218 | 55 | Conservative breast surgery including excisional biopsy and nipple sparing mastectomy | Describe incidence of chronic pain after breast cancer surgery | Up to 6 months post-operatively |
| Fujii [14] | Acceptable (+) | 2019 | Japan | RCT | 80 | 58.15 | Pectoral nerve block versus serratus plane block | Rate of pain worse than mild at six post-operative months | Up to 6 months post-operatively |
| Habib [15] | Acceptable (+) | 2019 | USA | Observational | 124 | 58 | Elective breast cancer surgery | Maximum acute pain score in the first 72 h | Up to 12 months post-operatively |
| Kang [16] | High quality (++) | 2020 | Republic of Korea | RCT | 185 | 50.25 | Intraoperative low-dose ketamine | Incidence of post-surgical site pain at 3 months after surgery | Up to 6 months post-operatively |
| Karki [17] | Acceptable (+) | 2005 | Finland | Observational | 110 | 58.1 | Elective breast cancer surgery | Describe the impairments of upper body and limb functions and structures among breast cancer patients | Up to 12 months post-operatively |
| Karmakar [18] | Acceptable (+) | 2014 | Hong Kong | RCT | 180 | 52.66 | Thoracic paravertebral block | Reduction in the incidence of chronic pain after a modified radical mastectomy when compared with general anaesthesia | Up to 6 months post-operatively |
| Kendall [19] | Acceptable (+) | 2018 | USA | RCT | 148 | 49.5 | Intraoperative systemic lidocaine | Frequency of chronic post-surgical pain | Up to 6 months post-operatively |
| Lötsch [20] | High quality (++) | 2018 | Finland | Observational | 1,000 | 56.7 | Breast-conserving surgery or mastectomy and sentinel node biopsy and/or axillary clearance | Identify parameters that predict the persistence of significant pain via supervised machine learning | Up to 36 months post-operatively |
| Okamoto [21] | Acceptable (+) | 2018 | Japan | Observational | 123 | 59 | Breast cancer surgery under general anaesthesia | Compare the predictive accuracy outcomes of the acute pain trajectory and pain intensity at 1 day after the surgery for pain prevalence at 6 months after the surgery | Up to 6 months post-operatively |
| Qian [22] | Acceptable (+) | 2019 | China | RCT | 184 | 46.6 | Pre-operative ultrasound-guided multilevel paravertebral blocks | Whether the use of ultrasound-guided multilevel paravertebral blocks with ropivacaine reduced the incidence of postmastectomy chronic pain 3 months after surgery | Up to 6 months post-operatively |
| Spivey [23] | Acceptable (+) | 2018 | USA | Observational | 216 | 55.6 | Breast surgery with or without known malignancy | Chronic pain at 6 months, collected using the breast cancer pain questionnaire first developed by Gartner et al. | Up to 6 months post-operatively |
| Tasmuth [24] | Acceptable (+) | 1996 | Finland | Observational | 93 | 58 (median) | Breast-conserving surgery or mastectomy and/or axillary clearance | How pain and other symptoms develop during the first year after two different types of surgery (breast conservative or not) and how these symptoms are related to the psychological state of the patients | Up to 12 months post-operatively |
RCT, randomized controlled trial.
Critical Appraisal: Risk of Bias
Quality of the studies was guided by the Scottish Intercollegiate Guidelines Network (SIGN) cohort and RCT checklists [25], accessible in online supplementary Appendix B. The synthesised findings will be measured using both the GRADE and GRADE-CERQual frameworks.
Summary of Measures
Primary data measures sought were risk differences between the included quantitative studies against a reference. As no control group was available due to patient characteristics, a reference was generated, representing a population in which there was a prevalence of NSSP at 6 months post-operative of zero persons.
Synthesis of Results
Due to variations in papers, two separate synthesis methods were utilised. Any NSSP data that were available were applied to a meta-analysis to seek the risk difference (with 95% confidence interval [CI]) via a generic inverse variance model. Data were combined using random-effects model as the suspected heterogeneity will be above 50%. The I2 statistic was used to measure heterogeneity. Tau2 was obtained to measure the extent of variance between the effects observed in different studies. The remaining studies were subject to a qualitative analysis and were synthesised using the GRADE-CERQual framework.
Results
Study Selection
Inclusion Screening
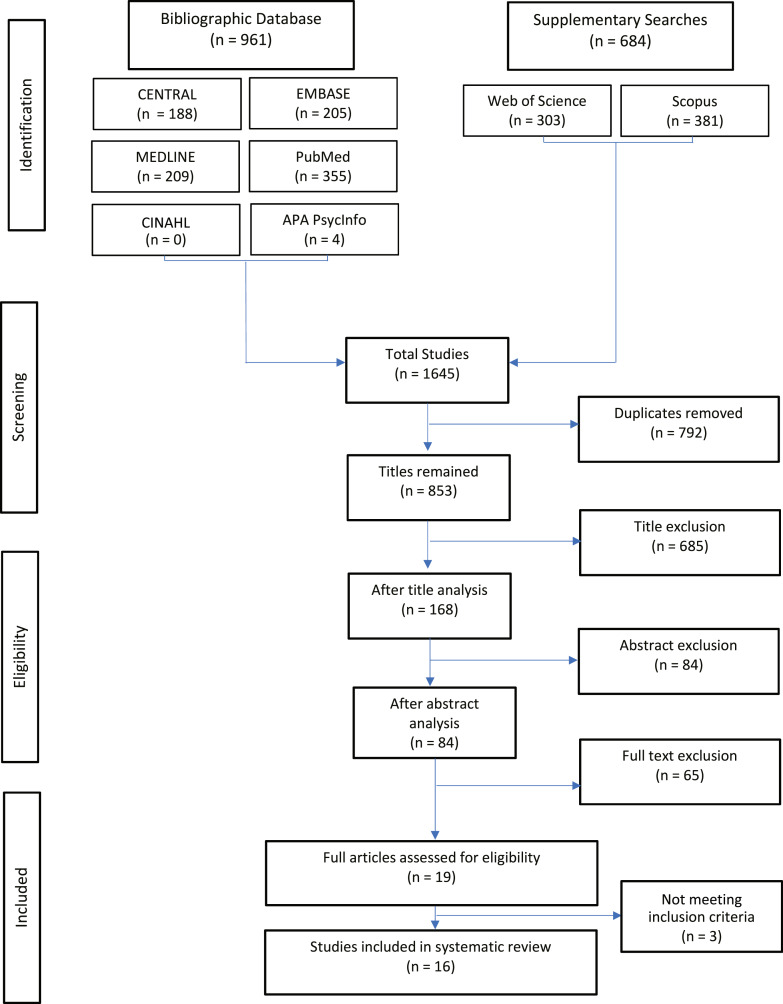
The search strategy was carried out over February 1 and February 2, 2021. From CENTRAL, PubMed, Embase, Scopus, WoS, CINAHL, PsycInfo, and MEDLINE, a total of 1,645 papers were identified. 792 duplicates were terminated. Two members of the review team individually and independently assessed the eligibility of papers for inclusion. Titles and abstracts were retrieved by RAYYAN and both independent investigators screened based on the aforementioned inclusion/exclusion criteria. 685 papers were excluded on basis of title search. 168 papers were screened via reading of abstracts and 84 papers were subsequently excluded. Papers were mostly excluded for failing to meet the follow-up period criteria, or their proposed questionnaires were not specific to NSSP. A final 19 papers were included after full-text reading. However, on further examination, 3 of the papers were not suitable for inclusion (not meeting inclusion criteria) and were therefore excluded. A total of 16 papers were included in this systematic review. The search was carried out according to the PRISMA flow chart as seen below, Figure 1.
Fig. 1.
Search strategy with results according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Study Characteristics
Seven of the sixteen papers were randomized control trials (RCTs), and the remaining were observational cohort studies. Each study was critically appraised according to the SIGN guidelines and the GRADE criteria. A total of 3,692 patients were included within the sixteen studies. A significant heterogeneity was noted within the studies, primarily as some were RCTs while the greater proportion were prospective observational studies. All the RCTs were investigating pharmacological interventions to alleviate the burden of chronic postoperative pain after breast cancer surgery. There was a significant variety within the outcomes of the studies, although it is notable that the bulk of the studies investigated the incidence of chronic pain in the breast area within the first-year post breast cancer surgery. Additional outcomes were not assessed.
Population
Patients included were those with clinically proven breast cancer diagnosis with variable prognosis and management pathways. The studies were carried out in numerous centres within an array of countries. All patients recruited were over the age of 18. When available, the mean ages of the included patients are shown in Table 2.
Intervention
All studies examined patients who had undergone either mastectomy or wide local excision. Patients who are subject to these surgeries will likely undergo either sentinel node biopsy or axillary node clearance; these interventions were examined in some of the studies and were not a basis for exclusion. Additionally, the papers included patients who had undergone adjuvant therapies such as endocrine therapies, radiotherapy (RT), and chemotherapy.
The RCTs employed varying interventions. Some looked at intraoperative interventions including ropivacaine wound infiltration [9], intraoperative low-dose ketamine [16], and intraoperative systemic lidocaine [19]. Amr et al. [10] examined the use of pre-operative venlafaxine or gabapentin. Blocks also have been studied, with Fujii et al. [14] comparing the use of a pectoral nerve block versus a serratus nerve block, while Karmakar et al. [18] examined thoracic paravertebral block intraoperatively, while Qian et al. [22] inspected the effect of pre-operative ultrasound-guided multilevel blocks. Each study utilised differing follow-up periods. Six of the studies followed patients for a total of 12 months [9, 11, 12, 15, 17, 24], nine of the studies followed up patients for 6 months [10, 13, 14, 16, 18, 19, 21–23], while only one had a 36 month follow up [20].
Control
Seven studies are RCTs and describe control groups [9, 10, 14, 16, 18, 19, 22], with four using a placebo control [9, 10, 16, 19], two assigned their control group to normal surgery, therefore the absence of intervention was their control [18, 22] and one failed to include a control group which raises concerns about the design and quality of the study [14].
Outcome
The primary outcomes of each of the sixteen studies can be found in the paper characteristics table, Table 2. The principal outcome that was sought was pain outwith the surgical site. Studies were included on the basis that the authors were likely to have the data in question, due to mentioning these data in methods or discussion, or the data would have been gathered as part of the questionnaire that was used. These factors were identified and collated into the table of characteristics.
Risk of Bias within Studies
Quality of Study Assessment
SIGN study quality checklists were utilised. Bias was reviewed by a single author. Quality assessment included selection, performance, attrition, detection, and reporting bias to provide a comprehensive understanding of the bias affecting the studies (online suppl. Appendix B).
Results of Individual Studies
Provision of Data
Of the sixteen studies included in the review, a total of four papers presented their NSSP findings within the published article. One author (Habib et al. [15]) provided data via email correspondence. There was substantial heterogeneity as to which site of pain was collected and whether NRS, verbal rating scale, or VAS was utilised. Andersen et al. [11] collected the number of patients with arm pain at 6 months. The question was binary to consider the presence or absence of pain, finding a proportion of 5% (n = 25). Habib et al. [11] collected both the arm and body pain prevalence together with the NRS for each site. A proportion of 13% (n = 13) suffered from either arm or body, with n = 8 reporting arm pain and n = 8 reporting body pain. Kärki et al. [17] provided the most detailed assessment of NSSP, detailing the site as upper limb (11.5%, n = 11) or neck and shoulder pain (38.5%, n = 37). Kärki et al. [17] reported the highest proportion of patients suffering pain post breast cancer surgery. Okamoto et al. [21] assessed the binary question of the presence of NSSP in both the arms (13.8% n = 17), back (7.3% n = 8), and other (9.8% n = 12). Tasmuth et al. [24] collected arm pain at 6 months for mastectomy (23%, n = 12) and breast-conserving surgery patients (35%, n = 14), whilst also providing a pain rating via NRS, resulting in the second highest proportion of pain at 6 months.
Overall Pain Prevalence
There were 995 patients included in the analysis of pain prevalence. Of the five included studies, 118 patients reported a form of NSSP (either arm, neck/shoulder, body) at 6 months post-operatively giving an overall proportion of 11.8%. Using a generic inverse variance model, the mean proportion of patients reporting NSSP at 6 months was 19% with 95% CI [1.55%, 36.44%]. This large variation within CIs highlights the heterogeneity within the results. Pain prevalence was further analysed within meta-analysis. Both Andersen et al. [11] and Tasmuth et al. [24] collected baseline pain prevalence information. Andersen et al. [11] reported an increase in reported arm pain from 1.8% to 5%, no 95% CI was available. Tasmuth et al. [24] similarly reported increases in arm pain prevalence: post-mastectomy reported pain rose from 10% to 23%, while in the breast-conserving surgery the rise was from 10% to 35% (no 95% CI was available).
Pain Severity
Pain rating was collected via NRS by Tasmuth et al. [24] and Habib et al. [15] Habib et al. identified a mean arm NRS rating of 3.63 with 95% CI [2, 5.23] and a mean body NRS rating of 3.38 with 95% CI [2.39, 4.37]. Tasmuth et al. [24] collected a mean arm NRS rating post mastectomy of 5.0 with 95% CI [2.0, 7.0] and a mean arm NRS rating post resection of 4.4 with 95% CI [1.4, 5.3]. Kärki et al. [17] gathered VAS scores for both neck/shoulder ache (27 mm) and upper limb ache (17 mm), however, 95% CI was not specified. Andersen et al. [11] and Okamoto et al. [21] did not collect pain rating scores.
Qualitative Results
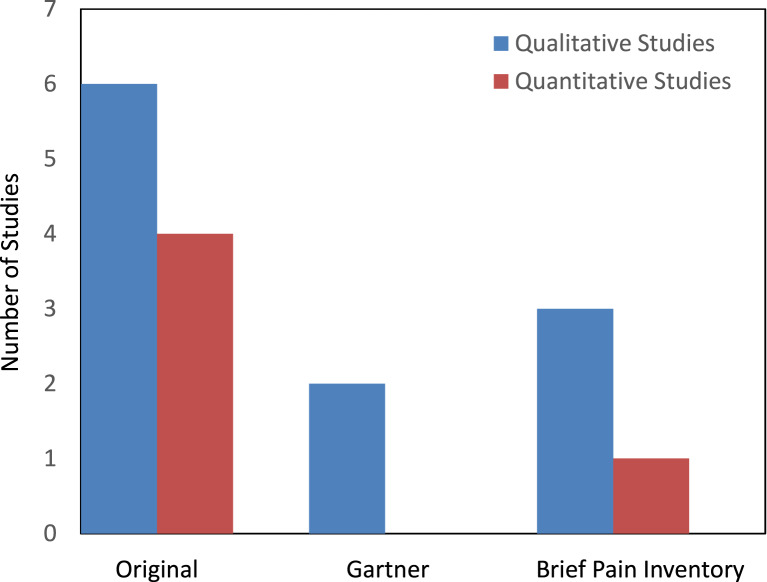
Among sixteen studies, nine mentioned NSSP as being collected within the methods section but failed to include these within the results section. Two studies mentioned NSSP as being collected within the methods section and additionally referred to these results in the discussion, however, did not publish data within the results section. One study did not comment on NSSP collection at any point within the study but utilised a questionnaire which collected the required information. Four studies included the NSSP data within the results section. Figure 2 outlines the type of questionnaire utilised by each study. It can be deduced that the most popular method of data collection was the original questionnaires that the authors had penned.
Fig. 2.
Questionnaire type utilised between the differing studies.
Each study was analysed to identify why they did not provide the data. Responses from email were also gathered to assess the reasoning. Two authors provided reasons for not including the data in question. Kendall et al. [19] collected the NRS values of NSSP at baseline but did not collect at 6 months. Their aim was to assess the overall pain and surgical site pain of the patients and therefore felt it redundant to collect the NSSP data. Okamoto et al. [21] collected the presence of NSSP pain but only in a binary manner and did not collect a pain rating scale as it was not beneficial to their primary outcomes.
Synthesis of Results
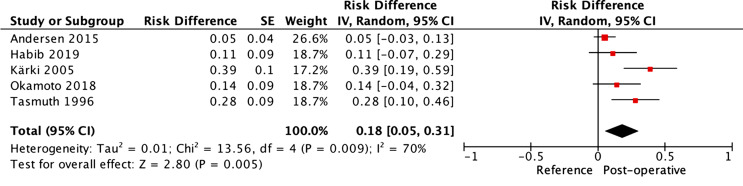
A generic inverse variant method was used to assess the risk difference between a reference and the included population. As described above, a reference was used to compare the included cohorts in terms of the prevalence of NSSP at 6 months post-operative. Random effects were employed as a noteworthy heterogeneity (>50%) was expected between the studies. It was additionally considered a priori that the clinical heterogeneity between study designs precluded the use of any other model than random effects. The resulting forest plot can be seen in Figure 3.
Fig. 3.
Forest plot of non-surgical site pain (NSSP) at 6 months post-operative.
The I2 statistic confirms the hypothesis that the results between the included studies were heterogenous, with a result of 70%. The overall risk difference highlights that compared with the reference, breast cancer surgery creates a risk difference of 18% with 95% CI [5%, 31%]. The Z statistic reports a p value <0.05 meaning that the summary finding is statistically significant. The implications of this are that undergoing breast cancer surgery is associated with a significant risk of NSSP at 6 months post-surgery compared with not undergoing breast cancer surgery. A significant caution should be given with these results as the comparison is made with a reference and it would therefore not be recommended to guide clinical decision-making.
Table 3 summarises the main quantitative findings and the quality assessment in accordance with GRADE framework [26]. As a great proportion of studies lacked the required data, the findings of these papers are summarised in a GRADE-CERQual summary of findings table, Table 4, which assesses them qualitatively [27]. This qualitative table summarises the findings discussed earlier and provides a confidence rating for each result.
Table 3.
GRADE summary of quantitative findings table
| Quality assessment | Summary of findings | |||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence of NSSP at 6 months post-operative | ||||||||
| participants, n (studies); Follow up | risk of bias | inconsistency | indirectness | imprecision | publication bias | overall quality of evidence | risk difference (95% CI) | anticipated absolute effects |
| 995 (5 studies); 6 months | No serious limitations, do not downgrade | Do not downgrade, inconsistency due to observational nature of studies and variety of interventions | Do not downgrade, minor indirect comparisons present (arm vs. body vs. neck/shoulder) | Not applicable to the reference | Do not downgrade, low suspicion of publication bias | ⊕⊕○○ LOW Due to observational nature of studies and minor levels of inconsistency and indirectness | 18% (5%, 31%) | 18 more per 100 (from 5 more to 31 more) |
Table 4.
Qualitative analysis via GRADE-CERQual framework
| Summary of review finding | Studies contributing to the review finding | CERQual assessment of confidence in the evidence | Explanation of CERQual assessment |
|---|---|---|---|
| 1. Non-surgical site pain is mentioned within methods as being collected, is considered during discussion but data are not presented within study | Karmarkar [18], Amr [10] | Low confidence | Moderate concerns surrounding the methodological limitations of one of the studies along with minor concerns regarding relevance of data to primary outcome |
| 2. Non-surgical site pain is mentioned within methods, collected but not presented within study | Spivey [23], Lötsch [20], Kendall [19], Kang [16], Habib [15], Fujii [14], Divella [13], Baudic [12], Albi-Feldzer [9] | Moderate confidence | Minor concerns regarding the methodology and relevance of data |
| 3. Authors utilise a questionnaire which contains non-surgical site pain data but not presented within study | Qian [22] | Moderate confidence | Minor concerns regarding the methodology and relevance of data |
| 4. Non-surgical site pain is not included due to not being relevant to study outcomes | Andersen [11] | Moderate confidence | Minor concerns regarding the methodology and relevance of data |
| 5. Non-surgical site pain data are not published within the paper but were provided by author on request | Habib [15] | Moderate confidence | Minor concerns regarding the methodology and relevance of data |
| 6. Non-surgical site pain data are provided within the study | Tasmuth [24], Okamoto [21], Kärki [17], Andersen [11] | Moderate confidence | Minor concerns regarding the methodology and relevance of data |
Risk of Bias across Studies
To assess the risk of bias across studies, both the GRADE criteria and GRADE-CERQual framework were used to allow clinicians to evaluate how trustworthy the findings are. These judgements on bias were assessed in accordance with the SIGN guideline checklists for RCT/observational studies [25]. These assessments of confidence can be found in each summary of the findings tables above.
Discussion
Key Findings
Based on the studies included in the meta-analysis, it can be proposed that undergoing breast cancer surgery, including both mastectomy and wide local excision, increases the risk of NSSP at 6 months post-operatively, with a risk difference of 18% (95% CI: 5%, 31%). However, these findings are based on a comparison against a theoretical reference, suggesting the need for a high level of caution when interpreting these results. The findings are consistent with recent observational studies which report NSSP rates of 37% and 40%, respectively [5, 28]. Nonetheless, these studies are not systematic reviews, and their results should be judged accordingly. Taken together, the results of recent studies and this systematic review challenge the standard view that post-surgical pain is significantly prevalent at the surgical site [3].
An interesting finding from two of the studies providing quantitative data is that the prevalence of NSSP increased from baseline (pre-surgery) to post-operative at 6 months. Andersen et al. [11] and Tasmuth et al. [24] reported increases of 1.8%–5% and 10%–23%, respectively. This suggests that breast cancer surgery increases the risk of NSSP at 6 months, though it is disappointing to be unable to evaluate baseline rates of NSSP in all included studies. Carrying out this analysis would provide specific evidence to guide clinical judgement. It was due to a lack of these precise data that the reference had to be created and used as the control in the meta-analysis.
Analysis of Findings
All five studies included in the meta-analysis were observational in nature and therefore of low quality according to the GRADE framework, limiting the certainty of the meta-analysis [26]. Qualitative assessment of the eleven remaining studies provided an insight into the method of information gathering and reasoning for non-inclusion of NSSP figures.
Qualitative analysis identified that 68% of the articles stated the collection of NSSP, but importantly failed to include these results in the published paper. Qian et al. [22] did not mention the collection of NSSP but rather included the data collection as the questionnaire utilised included it. This proved a significant challenge to the question of this review, as adequate data were unavailable. Articles primarily sought the rate of chronic pain in the breast post-surgically. The reasons for not providing these data were only obtained from two authors due to a lack of correspondence from the outstanding authors. The primary reasons for non-inclusion of data were that the authors were assessing the level of chronic post-operative pain in the breast area and sites outwith this were not relevant to their investigation. Due to the lack of correspondence, it is difficult to attribute certainty to the reasons of non-inclusion. To provide a thorough evaluation of these studies, the type of questionnaire used was analysed. In both studies that provided quantitative data and those that did not, the most used questionnaires were those that were created by the research teams themselves, likely so that they could tailor the questions specifically. The Brief Pain Inventory form was the second most common questionnaire type utilised. This questionnaire allows the patient to self-report pain, the site of pain, and intensity of pain along with how pain interferes with life [29]. The questionnaire used the least was that created by Gartner et al. [28]. This questionnaire makes use of dichotomous answers evaluating the severity and frequency of pain in four main sites, the breast area, the axilla, the arm, and the surgical side of body. Interestingly, the two studies which employed the Gartner questionnaire did not include the pain figures for the arm or for the side of the body. This emphasises the requirement for new research specifically evaluating pain outwith the surgical site.
These findings clarify that whilst most studies collected information on NSSP, they felt it not imperative to analyse these findings. The variation between reported pain prevalence is another factor identifying the need for further research: Kärki et al. [17] reported a pain prevalence of 39% and Tasmuth et al. [24] reported a prevalence of 28%, significantly higher than Andersen et al. [11], who notably had the largest sample population, reporting only a prevalence of 5%.
A restraint of this study is the time frame set out: 6 months. A consequence of this is that this review is subject to reporting bias; certain valuable observational studies not carried out within the set time frame are unable to be included. This is exemplified in the research carried out by Hamood et al. [30] and Fuzier et al. [31]. It was highlighted by Hamood et al. [30] with the unignorable pain prevalence rates of 46.6% in the arm and 28.9% in the side of the body at 3 months post-operatively in their 2018 cross sectional study. Fuzier et al. [31] carried out a cohort study that examined chronic pain after wide local excision surgery. They identified 10% of patients with ipsilateral arm pain and interestingly 61% of patients with chronic pain had a neuropathic component to their pain.
Contextualising NSSP
Both pain in the arm and shoulder are commonly credited to breast cancer surgery. Arm pain is a frequent side effect of axillary lymph node clearance, and this can be attributed to the removal of the intercostobrachial nerve [32]. Impaired range of shoulder movement, pain, muscle loss, and lymphoedema are also common adverse effects of treatment [7]. Lee et al. [33] found 67% of women will experience shoulder or arm symptoms up to 3 years after surgery. The cause of these shoulder symptoms is multifactorial in nature, but a few key risk factors have been identified: adjuvant therapy, axillary surgery, and pre-operative shoulder problems [34].
One reason there is such a variability within the reporting of NSSP is that the adjuvant therapies have their own long-term sequelae, and it can be difficult to differentiate between these and the adverse effects of the surgery itself. For example, aromatase inhibitors used for postmenopausal patients with oestrogen receptor-positive breast cancer are often the cause of side effects such as bone loss and arthralgia [35]. Chemotherapy-induced peripheral neuropathy also commonly affects women [36]. Adjuvant RT is associated with both long- and short-term toxicities, such as breast oedema, fibrosis, and pain. Smith and Wu explain that RT is included in the multifactorial picture which generates pain after breast cancer surgery [37]. This variability in NSSP reporting ascertains the requirement for further classification of the causes of chronic post-operative pain. Alongside this, interventions restricted to a biomedical approach to pain have limited effectiveness compared to multidisciplinary biopsychosocial care. This means, at least indirectly, that the complexity of pain also encompasses psychosocial aspects, which should always be taken into account in assessment and management [38].
As NSSP analysis was not the primary outcome for any of the included papers, it was not possible to analyse adequately the differing prevalence of NSSP between types of breast cancer surgery. Though, we can refer to studies provided by other researchers to gain an understanding. Reitman et al. [4] performed a systematic review investigating late morbidity and furthermore clarified that axillary lymph node dissection is more readily associated with upper limb morbidity than sentinel lymph node biopsy, likely due to the sparing of the intercostal brachial nerve. Furthermore, women who undergo mastectomy are at a greater risk of post-operative shoulder complications than those who undergo breast-conserving therapy (OR 5.7, 95% CI: 1.03–31.2) [33]. Keeping in mind that women that need mastectomy are more likely to have more advanced disease and more likely to need axillary node clearance. These findings add to the understanding that the types of breast cancer management can alter the adverse effects experienced by women and may justify specific pre-, intra-, and post-operative approaches [39]. This is indirectly supported by the fact that physical therapies and techniques can improve specific postoperative conditions, including pain and upper limb function [40].
The heterogeneity within this review provides both a positive and negative effect. Firstly, the heterogeneity renders comparisons between the studies challenging. It also means the results of the meta-analysis are less impactful. Most studies within the meta-analysis did not distinguish between NSSP after varying adjuvant therapies. This further highlights the variability of treatments that breast cancer sufferers are managed with. A positive of the significant heterogeneity is the increased incorporation of differing surgeries; however, with such a small sample of papers, it is difficult to ascertain conclusive results.
Strengths and Limitations
This is an independent systematic review without any conflicts of interest, therein minimising additional bias. The narrow outcome of interest allows for more meaningful and direct correlations to be made. This review had numerous issues. A total of sixteen studies were identified for inclusion, but only five studies were included in quantitative analysis of primary outcome. The results between the included studies were heterogenous. In relation to the availability of data, only a small proportion of studies published the required data. And, among the selected studies, a majority did not have NSSP as their primary objective, significantly increasing the risk of bias. After correspondence, only two authors were able to produce the required data. This small sample of papers included in meta-analysis meant that multivariable analysis was unable to be carried out. Another limitation of this study is that often the only available pain information was the ipsilateral arm. Ideally, this review had hoped to investigate other sites of pain than the commonly found areas, such as back pain and headaches. Additionally, the rates of pain severity would have been assessed if the data had been provided. Ideally, a comparison would also have been made between the baseline prevalence of NSSP and the 6-month prevalence of NSSP. Regarding pain severity, only mean pain scores were reported and not pain classifications, which may add interesting information. Future studies may also include risk factors for NSSP. Altogether, the quality of evidence for the primary outcome being low, the clinical significance of these findings remains uncertain, even if further research can be recommended.
Conclusion
Breast cancer surgery increases the risk of suffering NSSP at 6 months post-operatively. This review highlighted the gaps in the body and quality of evidence surrounding NSSP. Primarily that NSSP is not the outcome commonly sought and that the information is often collected on a secondary basis, but rarely discussed or presented within research.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
Patrice Forget received fees from Grunenthal for an educational activity not related to any product. The other authors have no conflict of interest.
Funding Sources
No external support.
Author Contributions
George Burton and Patrice Forget designed the work. George Burton, Yazan Masannat, and Patrice Forget contributed to data collection, analyses, interpretation, and discussions. George Burton drafted the manuscript, critically reviewed by all the authors, all approving the final version.
Funding Statement
No external support.
Data Availability Statement
All data are included in the report.
Supplementary Material
Supplementary Material
References
- 1. Di Girolamo C, Walters S, Benitez Majano S, Rachet B, Coleman MP, Njagi EN, et al. Characteristics of patients with missing information on stage: a population-based study of patients diagnosed with colon, lung or breast cancer in England in 2013. BMC Cancer. 2018;18(1):492. 10.1186/s12885-018-4417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sprod LK, Janelsins MC, Palesh OG, Carroll JK, Heckler CE, Peppone LJ, et al. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J Cancer Surviv. 2012;6(2):146–54. 10.1007/s11764-011-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang K, Yee C, Tam S, Drost L, Chan S, Zaki P, et al. Prevalence of pain in patients with breast cancer post-treatment: a systematic review. Breast. 2018;42:113–27. 10.1016/j.breast.2018.08.105. [DOI] [PubMed] [Google Scholar]
- 4. Rietman JS, Dijkstra PU, Hoekstra HJ, Eisma WH, Szabo BG, Groothoff JW, et al. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol. 2003;29(3):229–38. 10.1053/ejso.2002.1403. [DOI] [PubMed] [Google Scholar]
- 5. Forget P, Sitter TM, Hollick RJ, Dixon D, van Maanen A, Dekleermaker A, et al. The KG: characterization of preoperative, postsurgical, acute and chronic pain in high risk breast cancer patients. J Clin Med. 2020;9(12):3831. 10.3390/jcm9123831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986, 3:S1–226. [PubMed] [Google Scholar]
- 7. Mejdahl MK, Andersen KG, Gartner R, Kroman N, Kehlet H. Persistent pain and sensory disturbances after treatment for breast cancer: six year nationwide follow-up study. BMJ. 2013;346:f1865. 10.1136/bmj.f1865. [DOI] [PubMed] [Google Scholar]
- 8. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albi-Feldzer A, Mouret-Fourme E, Hamouda S, Motamed C, Dubois PY, Jouanneau L, et al. A double-blind randomized trial of wound and intercostal space infiltration with ropivacaine during breast cancer surgery: effects on chronic postoperative pain. Anesthesiology. 2013;118(2):318–26. 10.1097/ALN.0b013e31827d88d8. [DOI] [PubMed] [Google Scholar]
- 10. Amr YM, Yousef AA. Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain. 2010;26(5):381–5. 10.1097/AJP.0b013e3181cb406e. [DOI] [PubMed] [Google Scholar]
- 11. Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156(12):2413–22. 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 12. Baudic S, Jayr C, Albi-Feldzer A, Fermanian J, Masselin-Dubois A, Bouhassira D, et al. Effect of alexithymia and emotional repression on postsurgical pain in women with breast cancer: a prospective longitudinal 12-month study. J Pain. 2016;17(1):90–100. 10.1016/j.jpain.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 13. Divella M, Vetrugno L, Bertozzi S, Seriau L, Cedolini C, Bove T. Patient-reported pain and other symptoms among breast cancer survivors: prevalence and risk factors. Tumori. 2020;106(6):480–90. 10.1177/0300891620908930. [DOI] [PubMed] [Google Scholar]
- 14. Fujii T, Shibata Y, Akane A, Aoki W, Sekiguchi A, Takahashi K, et al. A randomised controlled trial of pectoral nerve-2 (PECS 2) block vs. serratus plane block for chronic pain after mastectomy. Anaesthesia. 2019;74(12):1558–62. 10.1111/anae.14856. [DOI] [PubMed] [Google Scholar]
- 15. Habib AS, Kertai MD, Cooter M, Greenup RA, Hwang S. Risk factors for severe acute pain and persistent pain after surgery for breast cancer: a prospective observational study. Reg Anesth Pain Med. 2019;44(2):192–9. 10.1136/rapm-2018-000040. [DOI] [PubMed] [Google Scholar]
- 16. Cho AR, Cho AR, Kim KH, Lee EA, Lee HJ, Kwon JY, et al. Effects of intraoperative low-dose ketamine on persistent postsurgical pain after breast cancer surgery: a prospective, randomized, controlled, double-blind study. Pain Physician. 2020;1;23(1;1):37–47. 10.36076/ppj.2020/23/37. [DOI] [PubMed] [Google Scholar]
- 17. Karki A, Simonen R, Malkia E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180–8. 10.1080/16501970410024181. [DOI] [PubMed] [Google Scholar]
- 18. Karmakar MK, Samy W, Li JW, Lee A, Chan WC, Chen PP, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Reg Anesth Pain Med. 2014;39(4):289–98. 10.1097/AAP.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 19. Kendall MC, McCarthy RJ, Panaro S, Goodwin E, Bialek JM, Nader A, et al. The effect of intraoperative systemic lidocaine on postoperative persistent pain using initiative on methods, measurement, and pain assessment in clinical trials criteria assessment following breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Pain Pract. 2018;18(3):350–9. 10.1111/papr.12611. [DOI] [PubMed] [Google Scholar]
- 20. Lotsch J, Sipila R, Tasmuth T, Kringel D, Estlander AM, Meretoja T, et al. Machine-learning-derived classifier predicts absence of persistent pain after breast cancer surgery with high accuracy. Breast Cancer Res Treat. 2018;171(2):399–411. 10.1007/s10549-018-4841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okamoto A, Yamasaki M, Yokota I, Mori M, Matsuda M, Yamaguchi Y, et al. Classification of acute pain trajectory after breast cancer surgery identifies patients at risk for persistent pain: a prospective observational study. J Pain Res. 2018;11:2197–206. 10.2147/JPR.S171680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian B, Fu S, Yao Y, Lin D, Huang L. Preoperative ultrasound-guided multilevel paravertebral blocks reduce the incidence of postmastectomy chronic pain: a double-blind, placebo-controlled randomized trial. J Pain Res. 2019;12:597–603. 10.2147/JPR.S190201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spivey TL, Gutowski ED, Zinboonyahgoon N, King TA, Dominici L, Edwards RR, et al. Chronic pain after breast surgery: a prospective, observational study. Ann Surg Oncol. 2018;25(10):2917–24. 10.1245/s10434-018-6644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74(12):2024–31. 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Methodology checklist 1: systematic reviews and meta-analyses. https://www.sign.ac.uk/what-we-do/methodology/checklists/. [Google Scholar]
- 26. Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 27. Lewin S, Booth A, Glenton C, Munthe-Kaas H, Rashidian A, Wainwright M, et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: introduction to the series. Implement Sci. 2018;13(Suppl 1):2. 10.1186/s13012-017-0688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gartner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985–92. 10.1001/jama.2009.1568. [DOI] [PubMed] [Google Scholar]
- 29. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 30. Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Chronic pain and other symptoms among breast cancer survivors: prevalence, predictors, and effects on quality of life. Breast Cancer Res Treat. 2018;167(1):157–69. 10.1007/s10549-017-4485-0. [DOI] [PubMed] [Google Scholar]
- 31. Fuzier R, Puel F, Izard P, Sommet A, Pierre S. Prospective cohort study assessing chronic pain in patients following minor surgery for breast cancer. J Anesth. 2017;31(2):246–54. 10.1007/s00540-016-2288-9. [DOI] [PubMed] [Google Scholar]
- 32. Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;2013:CD008307. 10.1002/14651858.CD008307.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat. 2008;110(1):19–37. 10.1007/s10549-007-9710-9. [DOI] [PubMed] [Google Scholar]
- 34. Bruce J, Thornton AJ, Powell R, Johnston M, Wells M, Heys SD, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: a population-based cohort study. Pain. 2014;155(2):232–43. 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 35. Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L. Aromatase inhibitors-induced musculoskeletal disorders: current knowledge on clinical and molecular aspects. Int J Mol Sci. 2020;21(16):5625. 10.3390/ijms21165625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fehrenbacher JC. Chemotherapy-induced peripheral neuropathy. Prog Mol Biol Transl Sci. 2015;131:471–508. 10.1016/bs.pmbts.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 37. Smith HS, Wu SX. Persistent pain after breast cancer treatment. Ann Palliat Med. 2012;1(3):182–94. 10.3978/j.issn.2224-5820.2012.10.13. [DOI] [PubMed] [Google Scholar]
- 38. De Groef A, Penen F, Dams L, Van der Gucht E, Nijs J, Meeus M. Best-evidence rehabilitation for chronic pain Part 2: pain during and after cancer treatment. J Clin Med. 2019 Jul 5;8(7):979. 10.3390/jcm8070979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tait RC, Zoberi K, Ferguson M, Levenhagen K, Luebbert RA, Rowland K, et al. Persistent post-mastectomy pain: risk factors and current approaches to treatment. J Pain. 2018 Dec;19(12):1367–83. 10.1016/j.jpain.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippi L, de Sire A, Losco L, Mezian K, Folli A, Ivanova M, et al. Axillary web syndrome in breast cancer women: what is the optimal rehabilitation strategy after surgery? A systematic review. J Clin Med. 2022 Jul 1;11(13):3839. 10.3390/jcm11133839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the report.