Abstract
Diverse manifestations have been recognized to last for a long time in patients infected with SARS-CoV-2. However, understanding of oral sequelae after recovery from COVID-19 is relatively poor compared to that of oral symptoms in the acute phase of COVID-19 and other COVID-19 sequelae. The aim of the present study was to characterize persistent gustatory and saliva secretory dysfunctions and to speculate on their pathogenic mechanisms. Articles were retrieved by searching scientific databases with a cutoff date of September 30, 2022. The literature search indicated that ageusia/dysgeusia and xerostomia/dry mouth are reported by 1–45% of COVID-19 survivors at follow-ups of 21–365 days and by 2–40% of COVID-19 survivors at follow-ups of 28–230 days, respectively. The prevalence of gustatory sequelae partly depends on difference in ethnicity, gender, age, and disease severity of subjects. Co-occurring gustatory and saliva secretory sequelae are pathogenically related to either or both of the following: expression of SARS-CoV-2 cellular entry-relevant receptors in taste buds and salivary glands, and SARS-CoV-2 infection-induced deficiency in zinc that is essential for normality of taste perception and saliva secretion. Given the long-term oral sequelae, hospital discharge is not the end of the disease; therefore, careful attention should be continuously paid to oral conditions of post-COVID-19 patients.
Keywords: COVID-19 sequelae, Persistent oral symptom, Gustatory dysfunction, Saliva secretory dysfunction, Pathogenic mechanism
Highlights of the Study
-
•
COVID-19 oral sequelae are symptomatically characterized, and their pathogenic mechanisms are speculated.
-
•
Gustatory and saliva secretory dysfunctions persist in up to 45% of COVID-19 survivors at follow-ups of 3 weeks to 12 months.
-
•
Persistent ageusia/dysgeusia and xerostomia/dry mouth are pathogenically related to SARS-CoV-2 receptors expressed in the tongue and salivary glands and to SARS-CoV-2 infection-induced zinc deficiency.
Introduction
Since the emergence of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the disease has spread worldwide to reach over 617.7 million cases with over 6.5 million deaths as of October 1, 2022, according to the Johns Hopkins University Coronavirus Resource Center [1]. Over 3 years have passed since the first case of COVID-19 was reported; it has been well recognized that COVID-19 patients commonly present with fever, cough, dyspnea, myalgia, fatigue, myocarditis, cardiomyopathy, arrhythmia, cardiac arrest, anorexia, nausea, diarrhea, and chemosensory disorders. In addition to these clinical manifestations, several symptoms specific to oral tissues and functions are also associated with COVID-19. They include impairments of taste such as ageusia (complete taste loss) and dysgeusia (impaired taste) that is classified into severe, moderate, and mild hypogeusia (or amblygeustia), and impairments of saliva secretion such as dry mouth, xerostomia (subjective complaint of oral dryness), and hyposalivation (objective reduction of salivary flow rates) [2, 3]. While early studies intensively investigated COVID-19 symptomatology in the acute phase of the disease, there is increasing evidence that patients who have recovered from COVID-19 complain of long-term effects of COVID-19 or sequelae such as fatigue, headache, attention disorder, neurocognitive disorder (brain fog), cough, dyspnea, and hair loss [4]. In a follow-up study after 2 months from symptom onset, 87.4% of COVID-19 survivors reported at least one persistent symptom, and 55% of them had three or more sequelae [5]. However, understanding of post-COVID-19 oral symptoms is relatively poor compared with that of oral symptoms in the acute phase of COVID-19 and other COVID-19 sequelae. Although gustatory and saliva secretory dysfunctions are not life-threatening, their persistence adversely affects overall quality of life of COVID-19 survivors who have recovered from the disease. Given the expanding population of COVID-19 survivors and the ever-progressing studies on SARS-CoV-2 infection, it is essential to update our knowledge of COVID-19 oral sequelae and their pathophysiology.
There is neither generally accepted time period to follow up on COVID-19 patients nor established terminology for long-term effects of COVID-19 [6]. Greenhalgh et al. [7] defined post-acute COVID-19 as extending beyond 3 weeks from symptom onset and chronic COVID-19 as extending beyond 12 weeks. As a considerable number of COVID-19 patients recover from acute chemosensory disorders within 3 weeks, symptoms lasting longer than 3 weeks could be referred to as COVID-19 sequelae. The aim of the present bibliographic review is to characterize sequelae of gustatory and saliva secretory dysfunctions after recovery from COVID-19 and speculate on their possible pathogenic mechanisms.
Methodology
A literature search was performed in PubMed/Medline, LitCovid, and ProQuest with a cutoff date of September 30, 2022, by using the following terms or combinations thereof: “SARS-CoV-2,” “COVID-19,” “sequelae,” “persistent,” “ageusia,” “dysgeusia,” “gustatory dysfunction,” “xerostomia,” “dry mouth,” and “salivary secretion dysfunction.” Given the ever-progressing studies on SARS-CoV-2 infection and COVID-19 symptomatology, the preprint databases medRxiv and bioRxiv were also used to retrieve the most up-to-date information. The exclusion criteria were articles not published in English, studies recruiting participants who had not been diagnosed with SARS-CoV-2 infection by reverse transcription-polymerase chain reaction (RT-PCR) and/or serological test, and studies including patients with primary Sjögren’s syndrome that exerts adverse effects on saliva secretory and gustatory functions. Cited papers in the retrieved articles were further searched for additional references.
Results and Discussion
Gustatory Sequelae
Abnormalities in taste perception have been quantitatively and qualitatively assessed by using different terms. For simplicity, ageusia, dysgeusia, and hypogeusia were collectively expressed as “gustatory dysfunction” in the present study. In addition to articles cited in a previous study [8], thirty studies were newly cited to update information on gustatory dysfunctions associated with COVID-19 and characterize gustatory sequelae [5, 9–32] while comparing them with gustatory dysfunctions in the acute phase of COVID-19 [5, 9, 11, 13, 14, 19, 22, 24, 27, 32–37]. The literature search included 64 studies on persistent gustatory sequelae with a total of 23,476 COVID-19 survivors and 86 studies on the acute gustatory dysfunctions with a total of 42,759 COVID-19 patients. Although the retrieved studies may be potentially biased, a meta-analysis was not performed because of heterogeneity in the designs and data of included studies. To evaluate the study quality, risk scores were previously determined by different meta-analyses and systematic reviews [38–40]; by referring to them, the overall risk of bias of studies used in the present study is likely to be low or moderate.
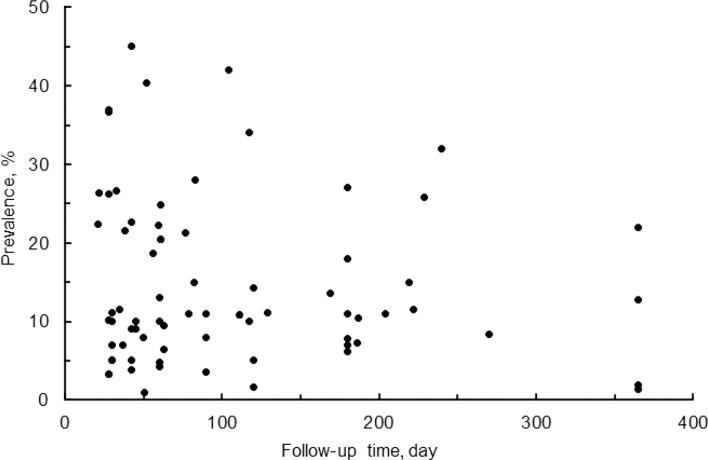
The literature search indicated that COVID-19 patients who have recovered from the disease complain of gustatory dysfunctions (ageusia, dysgeusia, and/or hypogeusia) in geographically different cohorts (Fig. 1). Gustatory dysfunctions persist with a prevalence of 1–45% in COVID-19 survivors who were followed for 21–365 days. Analysis of 8 observational studies by Moraschini et al. [41] indicated that ageusia continues in 14.1% of COVID-19 survivors at a mean 67-day follow-up. When summarizing the results of 29 different follow-up studies, gustatory dysfunctions were reported by 13.6% of COVID-19 survivors at 29.7-day follow-up, by 13.8% at 60.4-day follow-up, and by 12.7% at 181.9-day follow-up [8], suggesting that the prevalence is not significantly different between short-term and long-term gustatory sequelae. Impairments of taste are presumed to continue for at least 1 year after recovery from COVID-19 [42, 43].
Fig. 1.
Prevalence of gustatory sequelae in COVID-19 survivors at different follow-ups after disease diagnosis, symptom onset, and/or hospital discharge.
It is interesting that persistent parageusia (wrong or inadequate taste sensation elicited by a taste stimulus) and phantogeusia (taste sensation in the absence of a stimulus) were observed in some follow-up studies. Parageusia and phantogeusia were reported in 23.5% and 17.6% of Italian subjects, respectively, at a mean 64-day follow-up after SARS-CoV-2-negative nasopharyngeal swabs [44]. In a British cohort, COVID-19 survivors reported phantogeusia and parageusia with prevalence of 38.3% and 14.9%, respectively, after 117–199 days from disease diagnosis, in addition to ageusia with prevalence of 34.0% [26].
Geographical or Ethnic Differences
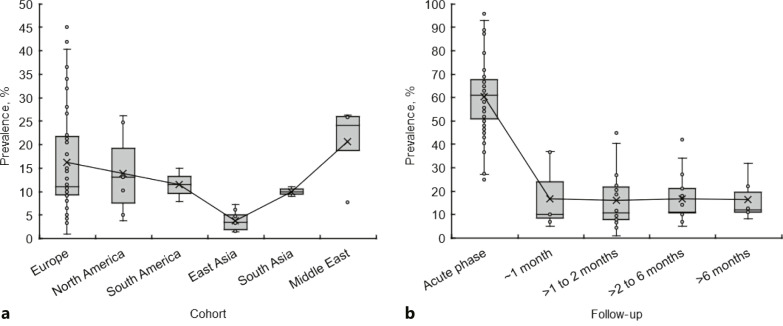
The prevalence of gustatory dysfunctions in the acute phase of COVID-19 varies depending on geographical or ethnic differences [2, 45]. As well as in the acute phase, persistent gustatory dysfunctions showed different prevalence rates in European, North American, South American, East Asian, South Asian, and Middle Eastern cohorts (Fig. 2a). Pooled prevalence (mean ± SD) of gustatory sequelae detected at follow-ups of 3 weeks to 12 months was 16.2 ± 11.01% in Europe (Italy, France, Spain, Germany, Switzerland, UK, Denmark, Belgium, Sweden, Norway, and Poland), 13.8 ± 8.8% in North America (USA and Canada), 11.5 ± 5.0% in South America (Colombia), 3.8 ± 2.0% in East Asia (China, Japan, South Korea, and Singapore), 10.0 ± 1.4% in South Asia (India), and 20.6 ± 7.5% in the Middle East (Iran, Israel, Turkey, Saudi Arabia, and Qatar). The prevalence was significantly lower in East Asia than in Europe (p < 0.005), North America (p < 0.01), South America (p < 0.01), South Asia (p < 0.01), and the Middle East (p < 0.0005), whereas there was no significant difference between European, American, South Asian, and Middle Eastern cohorts. Taste impairments are unlikely to last for a long time in COVID-19 survivors of East Asia compared to those in other areas. Geographical difference or ethnicity could be a risk factor for gustatory sequelae, which may be supported by comparisons between different cohorts. When Chinese COVID-19 patients were followed up, 3.6% of 55 patients with mild to severe disease complained of hypogeusia after 3 months from hospital discharge [20]. In a cohort of mostly Japanese subjects, 4.8% and 1.6% of 63 patients reported ongoing dysgeusia 60 and 120 days after symptom onset, respectively [46]. On the other hand, a French cohort showed that ageusia persists in 10.8% of COVID-19 survivors 110 days after hospital admission [21]. At follow-ups of 3–7 months, the prevalence of gustatory sequelae ranged from 1.6% to 7.3% in Chinese and Japanese subjects, compared to 5.0–42% in European, American, and Israeli subjects [20, 21, 23]. Andrew et al. [15] comparatively assessed oral symptoms of 114 COVID-19 patients, consisting of white (81.6%), Asian (15.8%), black/African/Caribbean (1.3%), and mixed/multiple ethnic (1.3%), 52 days after symptom onset. They found that ethnicity (being white) positively influences the recovery of taste (p = 0.022).
Fig. 2.
a Prevalence of gustatory sequelae in geographically different cohorts. b Prevalence of gustatory dysfunctions in the acute phase of COVID-19 and at different follow-ups after recovery from COVID-19.
The prevalence of gustatory dysfunctions in European populations was compared between the acute phase of COVID-19 and after recovery from COVID-19 (Fig. 2b). Pooled prevalence (mean ± SD) was 60.4 ± 14.5% for the acute phase, 16.7 ± 12.8% at follow-ups of ≤1 month, 16.0 ± 11.4% at follow-ups of >1–2 months, 16.9 ± 10.0% at follow-ups of >2–6 months, and 16.3 ± 8.2% at follow-ups of >6 months. Although the prevalence is lower in COVID-19 survivors compared with the acute phase (p < 0.001 for all follow-ups), no significant difference was observed between different follow-up time, suggesting that gustatory dysfunctions last for a significantly long time and that their duration can be over months following recovery from COVID-19. Even at 1-year follow-up, gustatory sequelae were reported by 12.7–22.0% of Italian COVID-19 survivors after symptom onset [42, 43] and by 1.4–1.9% of Chinese COVID-19 survivors after hospital discharge [29, 30].
Gender
Gender difference may influence gustatory sequelae because the prevalence of gustatory dysfunctions in the acute phase of COVID-19 is different between female and male patients [2, 3, 9, 47, 48]. Algahtani et al. [9] comparatively assessed COVID-19 symptoms in 808 Saudi Arabian subjects who had recovered from COVID-19. Ageusia persisted in 69.5% of female and 30.5% of male COVID-19 survivors, with a statistically significant difference (p = 0.0001). Females accounted for 83.7% of the subjects who presented with ageusia and/or anosmia persisting for more than 60 days after disease onset. In a Swiss cohort, ageusia persisted in 12.5% of female but 6.0% of male outpatients after 30–45 days from disease diagnosis and in 18.2% of female but 14.1% of male outpatients after 7–9 months from disease diagnosis [31]. Augustin et al. [49] followed up 958 German COVID-19 patients 1.7–6.8 months after onset of symptoms and demonstrated that the prevalence of ageusia is 13.9% in females and 7.3% in males. At 52-day follow-up, female gender negatively influenced the recovery from gustatory dysfunctions of British COVID-19 survivors [15]. Thus, being female is considered a risk factor for gustatory sequelae in COVID-19 survivors; however, some follow-up studies suggested no significant relation between gender and long-term taste alteration. Although Kim et al. [24] performed multivariate analysis of 900 South Korean COVID-19 patients, 6 months after disease diagnosis or onset of symptoms, persistent ageusia was not associated with gender and disease severity. Indian and Turkish COVID-19 survivors showed no significant difference in prevalence of gustatory dysfunctions between females and males after 2 weeks to 3 months from disease diagnosis and 2–4 weeks after hospital discharge, respectively [27, 50].
Age
Occurrence of gustatory dysfunctions in the acute phase of COVID-19 is not necessarily associated with the age of patients. When comparing acute ageusia and dysgeusia, children showed significantly lower prevalence of gustatory dysfunctions than adults in French and Israeli cohorts, whereas younger patients more frequently reported taste and smell impairments in South Korean, Spanish, Italian, and Turkish cohorts [2].
Moreno-Pérez et al. [17] studied 277 Spanish patients aged 42–67.5 years with mild to severe COVID-19 10–14 weeks after discharge from hospital. The prevalence of dysgeusia/anosmia was 24.9% for patients under 65 years of age but 13.5% for patients over 65 years (p = 0.03). At 12-month follow-up of 304 Italian patients with mild to moderate COVID-19, the risk of persistence of symptoms was significantly higher in subjects aged 40–54 years than in other ages (p = 0.029) [42]. In a 195-day follow-up study of South Korean subjects, the persistence of ageusia was associated with age over 50 years (p = 0.024, compared with other years) [24]. These results suggest that the age of patients influences gustatory sequelae with a higher prevalence in relatively old age. However, a cross-sectional assessment of Indian COVID-19 patients after 2 weeks to 3 months from disease diagnosis indicated no statistically significant relation between taste alteration and age (<35 years vs. >35 years) [27]. Since old age per se is associated with alterations of gustatory perception, it remains inconclusive whether the age of COVID-19 survivors is a determinant for gustatory sequelae.
Severity of Disease
In the acute phase of COVID-19, taste is more frequently impaired in mildly symptomatic and non-severe cases than in severe to critical cases, and asymptomatic, mild, moderate, severe, and critical COVID-19 patients present with ageusia in decreasing order of prevalence [2]. With respect to gustatory sequelae associated with COVID-19, Garrigues et al. [21] comparatively assessed 120 French hospitalized patients in wards and patients in intensive care units (ICU) at 110-day follow-up after hospital admission. Although the prevalence of ageusia was 10.8% overall, ageusia persisted in 16.7% and 9.4% of ICU and ward patients, respectively. When Zhang et al. [29] and Fang et al. [30] followed up 2,433 and 1,233 Chinese subjects after 1 year from hospital discharge, they observed that taste is impaired in 2.2–2.5% of COVID-19 survivors with severe disease and 1.1–1.5% with non-severe disease. However, these comparative results were not statistically significant. In a South Korean cohort, the persistence of ageusia at least 6 months after symptom onset was not associated with moderate severity of COVID-19 [24]. Gustatory sequelae may occur independently of the severity of COVID-19.
Saliva Secretory Sequelae
Abnormalities in saliva secretion have been assessed by using different terms. For simplicity, xerostomia, dry mouth, and hyposalivation were collectively expressed as “saliva secretory dysfunction” in the present study. The present review included 7 studies [27, 29, 50–54] for saliva secretory sequelae with a total of 654 COVID-19 survivors and 13 studies [27, 34–37, 47, 48, 52, 53, 55–58] for saliva secretory dysfunctions in the acute phase of COVID-19 with a total of 2,283 patients. As the number of relevant studies and subjects was smaller compared with gustatory dysfunctions, all the retrieved articles were used to characterize symptomatically without a meta-analysis, while some of them may potentially be biased for saliva secretory sequelae. An analysis of the quality of 4 studies (including 946 participants) on COVID-19 xerostomia showed that the risk of bias is low or moderate [59].
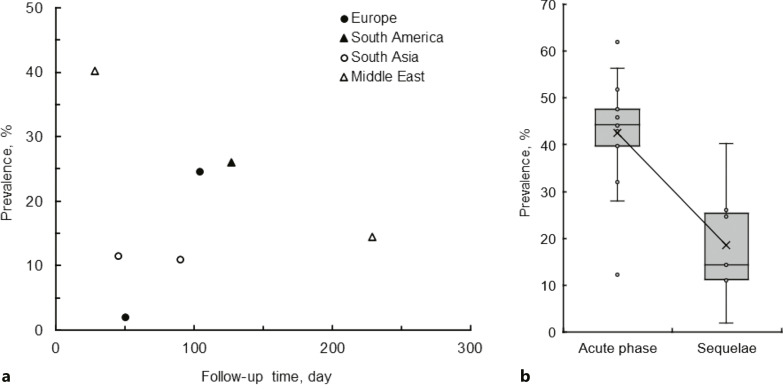
The literature search indicated that COVID-19 survivors complain of saliva secretory dysfunctions (Fig. 3a). Saliva secretory sequelae were reported by 2–40% of COVID-19 survivors followed up for 28–230 days. Although the prevalence of xerostomia and dry mouth after recovery from COVID-19 is lower than that in the acute phase of COVID-19 (mean prevalence of 18.5% vs. 42.5%, p < 0.001), it is clear that saliva secretory dysfunctions persist for a long time (Fig. 3b). Impairments of saliva secretion were observed at follow-ups of 1–2 months and >2–4 months with the prevalence of 16.2 ± 0.84% and 20.5 ± 6.8% (mean ± SD), respectively.
Fig. 3.
a Prevalence of saliva secretory sequelae in geographically different cohorts at different follow-ups after recovery from COVID-19. b Comparison of prevalence between saliva secretory dysfunctions in the acute phase of COVID-19 and saliva secretory sequelae after recovery from COVID-19.
Characterization of Saliva Secretory Sequelae
Although ethnicity (geographical difference), gender, and age are associated with xerostomia and dry mouth in non-COVID-19 populations, these factors do not necessarily determine the prevalence of saliva secretory dysfunctions in the acute phase of COVID-19 [60]. As the number of comparative studies on saliva secretory sequelae is limited, unlike COVID-19 gustatory sequelae, it is inconclusive whether the prevalence of saliva secretory sequelae correlates to geographical or ethnic difference. Biadsee et al. [52] followed up 97 Israeli subjects 7.6 months after recovery from COVID-19 and revealed that the prevalence of dry mouth is 14.4% overall, 8.2% for females (16.7% in females), and 6.2% for males (14.0% in males). However, the gender difference was not statistically significant (p = 0.905). Nor was female gender a significant determinant of persistent xerostomia in an Italian cohort (n = 122) followed up 104 days after hospital discharge [51] and in a Turkish cohort (n = 107) followed up 2–4 weeks after hospital discharge [50]. The prevalence of xerostomia 2 weeks to 3 months after disease diagnosis was found to be related neither to gender nor to age in 100 Indian subjects [27]. In 100 Colombian COVID-19 patients followed up to assess their symptoms after 7 months from symptom onset, xerostomia persisted in 25.7% of 35 ambulatory patients (with mild COVID-19), 19.5% of patients with severe COVID-19, and 37.5% of patients with critical COVID-19, but without statistically significant difference [54]. Taken together, it appears that gender, age, and disease severity are not necessarily associated with COVID-19 saliva secretory sequelae.
Association between Saliva Secretory and Gustatory Dysfunctions
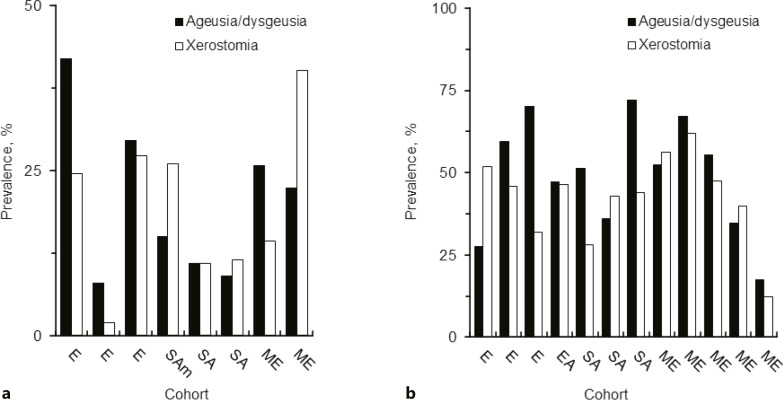
Out of retrieved articles, 7 articles dealt with not only gustatory dysfunctions but saliva secretory dysfunctions after recovery from disease, and 13 articles focused on the acute phase of COVID-19. In all these 20 studies, xerostomia was reported together with ageusia/dysgeusia by COVID-19 survivors after 28–270 days from disease diagnosis, symptom onset, or hospital discharge (Fig. 4a) and by COVID-19 patients (Fig. 4b). Studies on oral symptoms in Italian [37, 51, 53, 55], Colombian [54], Chinese [48], Indian [27, 28, 35, 36], Israeli [47, 52], Egyptian [56, 57], Turkish [50, 58], and Saudi Arabian [34] cohorts concluded that persistent saliva secretory dysfunctions can occur simultaneously with persistent gustatory dysfunctions irrespective of geographical or ethnic difference. The prevalence of xerostomia was moderately correlated with that of ageusia/dysgeusia (Fig. 5). Both oral symptoms are presumed to co-occur in the acute phase of COVID-19 and persist after recovery from COVID-19.
Fig. 4.
a Prevalence of gustatory and saliva secretory sequelae after recovery from COVID-19. b Prevalence of gustatory and saliva secretory dysfunctions in the acute phase of COVID-19 in different cohorts. E, Europe; SAm, South America; SA, South Asia; ME, Middle East.
Fig. 5.
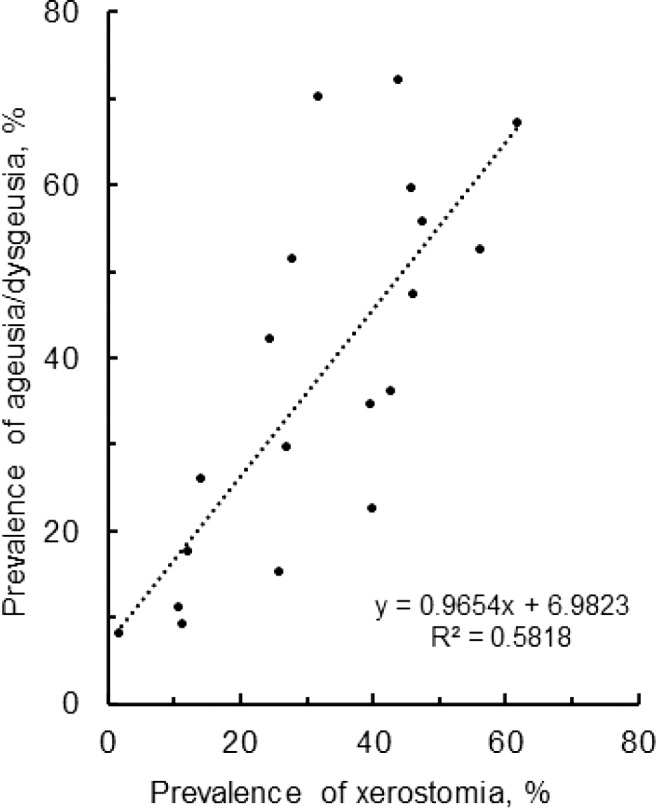
Relation in prevalence between saliva secretory and gustatory dysfunctions.
Possible Pathogenic Mechanisms
COVID-19 patients present with gustatory and saliva secretory dysfunctions, both of which last for a long time in COVID-19 survivors, giving insights into the pathogenic mechanisms common to co-occurring oral symptoms.
ACE2 Responsible for Cellular Binding of SARS-CoV-2
Spike protein, one of four major structural proteins encoded by the SARS-CoV-2 genome, plays a primary role in recognition of the host cell receptor and cellular entry of the virus. SARS-CoV-2 binds to cellular receptor angiotensin-converting enzyme 2 (ACE2) through its spike protein, followed by the fusion between viral and cellular membranes that is mediated by cellular protein convertase Furin and transmembrane serine protease 2 (TMPRSS2) [61]. The spike protein consists of two subunits: S1 responsible for ACE2 binding and S2 responsible for membrane fusion. The S1 subunit interacts with the ACE2 receptor on host cells, and subsequently, the spike protein is cleaved by Furin at the S1/S2 site to dissociate the S1 subunit. After the S1 dissociation, TMPRSS2 cleavage at the S2’ site of the S2 subunit causes fusion between the viral envelope and the host cell membrane, allowing SARS-CoV-2 to enter host cells.
Xu et al. [62] explored public genomic databases and suggested that ACE2 is expressed in human oral tissues, especially in dorsal tongue. Sakaguchi et al. [63] performed RT-PCR analysis and immunochemical staining of human pathological samples collected from non-COVID-19 patients. They demonstrated that ACE2, Furin, and TMPRSS2 are abundantly present not only in taste receptor cell-containing taste buds and taste bud-locating papillae but also in ductal, acinar, and myoepithelial cells of salivary glands. ACE2 and TMPRSS2 are co-localized in human parotid, submandibular, sublingual, and minor salivary glands, and concentrated in their acini and ducts [3]. Matuck et al. [64] conducted ultrasound-guided postmortem biopsies in COVID-19 fatal cases and immunohistochemically found that ACE2 and TMPRSS are expressed in the ductal epithelium and serous acinar cells of the parotid and submandibular glands and minor salivary glands. Their ultrastructural investigation showed that spherical viral particles consistent in size and shape with Coronaviridae family exist in the ductal lining cell cytoplasm, acinar cells, and ductal lumen of the submandibular and parotid glands. SARS-CoV-2 infection of major and minor salivary glands was also confirmed by using autopsy tissues from COVID-19 patients. Given the expression of viral cellular entry-relevant biofactors and the viral detection in oral tissues, SARS-CoV-2 is able to target the oral cavity. The cytopathic activities of SARS-CoV-2 induce the functional disorders of target cells [65]. Taste buds and salivary glands expressing the viral cellular receptor ACE2 are attacked by SARS-CoV-2 to receive collateral damage, which adversely affects taste perception and salivary secretion. Damaged taste bud cells require weeks to proliferate and recover their functions, and the turnover of saliva-producing acinar cells ranges from 50 to 125 days. Thus, we can speculate that gustatory and saliva secretory dysfunctions may persist after recovery from COVID-19.
Genes encoding biofactors relevant to the viral cellular entry vary depending on ethnicity as comparative genetic analyses indicated that ACE2 and TMPRSS2 are expressed with significant differences between Asian and European populations [2], being consistent with the ethnic characteristics of COVID-19 gustatory sequelae. Since ACE2 and TMPRSS2 expression is not different between females and males, gender differences in prevalence of gustatory sequelae could be due to those in taste responsiveness and the number of taste buds.
TRPV1 Interacting with SARS-CoV-2
Transient receptor potential (TRP) channels are expressed in various tissues infected with SARS-CoV-2 [66]. Among the TRP superfamily, transient receptor potential vanilloid 1 (TRPV1) contains the ankyrin repeat domains, while the spike protein of SARS-CoV-2 contains two ankyrin binding motifs. TRPV1 expression is activated by infection with several viruses, including respiratory rhinovirus and syncytial virus, measles virus, herpes simplex virus, hepatitis C virus, etc. [67]. It is presumed that TRPV1 is responsible for cellular binding and infection of SARS-CoV-2, therefore participates in symptoms, susceptibility, and pathogenesis of COVID-19.
There is evidence that TRPV1 is expressed in the oral cavity and plays important roles in oral functions and various pathophysiological conditions [68]. TRPV1 immunoreactivity is distributed in the taste papillae and palate of rats [69], and TRPV1 is present in the tongue and palatal epithelia of rats [70]. Marincsák et al. [71] confirmed the expression of TRPV1 in the basal layers of the human tongue epithelium. Such TRPV1 expression correlates with the sensation of sour and salty taste [72, 73]. Immunohistochemical and RT-PCR experiments on rats also indicated the localization of TRPV1 in myoepithelial, acinar, and ductal cells of the submandibular, sublingual, and parotid glands [74]. Ding et al. [75] revealed that TRPV1 is expressed in human submandibular glands and localized in their serous acinar and ductal cells. By interacting with TRPV1 in the tongue and salivary glands, SARS-CoV-2 could affect their functions through its cytopathic effects to impair taste and secretion of saliva.
Zinc Deficiency Associated with SARS-CoV-2 Infection
Zinc is essential for the physiology of taste buds and taste stimulus-transmitting nerves and also for the regeneration and maintenance of taste cells [76, 77]. Zinc is present at high levels in taste bud membranes [78] and is also localized in the membrane surfaces, granules, and vesicles of the glandular epithelial cells and in the pits of the myoepithelial cells in submandibular glands [79]. Zinc deficiency has a negative impact on vallate papillae; it leads to a decrease in the number and size of taste buds [80] and also taste sensitivity and saliva secretion [81]. When cells and tissues become deficient in zinc for some pathological reason, their physiological functions are significantly perturbed; therefore, zinc homeostasis is tightly controlled at a cellular level by the zinc-binding proteins zinc metalloenzyme, zinc metallothionein, and zinc transporter. Zinc metalloenzyme carbonic anhydrases bind to cellular zinc with high affinity and require zinc for their activity. Yagi et al. [82] discovered that isozyme carbonic anhydrase VI is localized in human tongue taste buds and salivary glands and participates in the growth, development, and maintenance of taste buds and fungiform taste papillae. Among zinc metallothioneins, isoform metallothionein-3 was found in rat taste buds [83] and in human salivary glands [84]. In addition, zinc transporters were identified in human salivary glands [85]. These zinc-binding proteins are involved in in vivo zinc homeostasis to modulate taste perception and saliva secretion.
Different cohorts showed that serum zinc concentrations (mean ± SD) were 64.8 ± 7.5 μg/dL in COVID-19 patients and 88.3 ± 14.0 μg/dL in healthy subjects [8]. Viral infection induces hypozincemia by redistributing zinc from the blood to and accumulating zinc in the liver at the expense of zinc in other tissues as observed in sepsis and infectious systemic inflammation [77]. The resulting zinc deficiency in peripheral tissues affects or damages the cells that require zinc to maintain their normal functions. Comparative studies have suggested that serum zinc concentrations are significantly decreased in COVID-19 patients and that hypozincemia persists after recovery from COVID-19. Yasui et al. [86] studied Japanese COVID-19 patients who had been admitted to ICU and finally discharged from hospital after treatment; these patients had serum zinc concentrations below or near the zinc deficiency cutoff concentration for 4 weeks after disease onset. Zinc deficiency-induced effects on gustatory and saliva secretory functions could persist in COVID-19 survivors, causing oral sequelae of ageusia/dysgeusia and xerostomia/dry mouth.
Supporting Hypothetical Evidence
Compared with Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants, Omicron (B.1.1.529) undergoes more mutations, with at least 32 mutations in the spike protein. Although the Omicron variant shares several mutations with previous variants, the receptor-binding domain (RBD) in Omicron’s spike protein has 15 mutations. Such a mutation profile not only increases infectivity but characterizes clinical symptoms of the Omicron variant. It is becoming evident that patients infected with the Omicron variant complain of ageusia and dysgeusia with a relatively low frequency compared with other SARS-CoV-2 strains and variants [87, 88]. Xerostomia is very unlikely to occur frequently in patients infected with the Omicron variant as only one case was reported in the Omicron infection [89].
Wu et al. [90] measured affinity constants of the RBDs of different SARS-CoV-2 isolates for human ACE2. The affinity constant was 6.01 ± 3.02 × 107, 26.91 ± 0.46 × 107, and 0.37 ± 4.66 × 107 L/mol for the wild type, the Delta variant, and the Omicron variant, respectively, indicating that binding of the Omicron variant to the ACE2 receptor is significantly weaker than that of other SARS-CoV-2 strains. When comparing the binding affinity for human ACE2, a dissociation constant (KD) was 31.4, 25.1, 24.6, 5.4, 13.8, and 11.0 nm for Omicron, Delta, prototype, Alpha, Beta, and Gamma spike RBD, respectively [91], and 19.5, 10.0, 22.1, 6.7, and 25.1 nm for Omicron sub-variant BA.1, BA.2, BA.3, Alpha, and Delta spike RBD, respectively [92]. Molecular dynamic simulation indicated a stronger interaction of the Omicron variant with human ACE2 than the Wuhan wild type but much weaker than the Delta variant [93]. Jawaid et al. [94] revealed that the Omicron variant exhibits weakened ACE2 binding compared with the Delta variant. In a comparative assessment by Du et al. [95], the Omicron variant also showed weaker membrane fusogenicity than Delta, Lambda, and Mu variants. It could be speculated that affinity of the Omicron variant for the cellular entry-relevant biofactors present in tongue taste buds and salivary glands may be lower than that of other SARS-CoV-2 strains and previous variants of concern, resulting in less impact of the Omicron variant on taste perception and saliva secretion to lower the prevalence of oral sequelae in COVID-19 survivors.
Turkish patients with asymptomatic, mild, moderate, and severe COVID-19 had serum zinc concentrations of 64.9 ± 12.4 µg/dL, 60.1 ± 18.1 µg/dL, 56.9 ± 22.1 µg/dL, and 56.5 ± 18.1 µg/dL, respectively, which were lower than 87.3 ± 33.5 µg/dL seen in healthy subjects [96]. Hypozincemia was detected in 4%, 6%, and 9% of Russian patients with mild, moderate, and severe COVID-19, respectively [97]. In an Iranian cohort study, serum zinc concentrations were 94.2 ± 26.0 µg/dL for COVID-19 patients who died, 98.8 ± 30.5 µg/dL for COVID-19 patients who were admitted to ICU, and 118.8 ± 34.4 µg/dL for COVID-19 patients who survived after admission to non-ICU [98]. Taken together, the decreasing degree of serum zinc concentrations is considered to depend on the severity of COVID-19. Many COVID-19 cases due to the Omicron variant are asymptomatic or mild [99]. A comparative study by Christie [100] demonstrated that the risk of hospitalization is lower in Omicron cases compared with Delta cases. Sigal [101] reported that COVID-19 during the Omicron wave is milder at a population level than COVID-19 during Beta and Delta waves. Since most cases of Omicron infection are asymptomatic or mildly severe, the Omicron variant is unlikely to induce severe zinc deficiency. Relatively mild zinc deficiency would decrease the prevalence of gustatory and saliva secretory dysfunctions in the acute phase of COVID-19 and after recovery from COVID-19.
Conclusions
A significant number of COVID-19 survivors complain of ageusia/dysgeusia and xerostomia/dry mouth even after 7 months to 1 year after recovery from the disease. Co-occurring gustatory and saliva secretory sequelae are pathogenically related to either or both of (i) expression of SARS-CoV-2 cellular entry-relevant receptors in taste buds and salivary glands and (ii) SARS-CoV-2 infection-induced deficiency in zinc that is essential to maintain normality of taste perception and saliva secretion. Although oral symptoms associated with COVID-19 are not life-threatening, their long-term persistence gives a strong negative impact on overall quality of life. Given the possibility that gustatory and saliva secretory dysfunctions persist after recovery from COVID-19, hospital discharge is not the end of COVID-19. Careful attention should be continuously paid to oral conditions of post-COVID-19 patients.
Statement of Ethics
Not applicable as this study includes neither human nor animal experiments.
Conflict of Interest Statement
The author has no conflicts of interest to declare.
Funding Sources
This study was supported by JSPS KAKENHI Grant No. 20K10152.
Author Contributions
Hironori Tsuchiya designed and conducted this study, prepared the manuscript, and approved the final manuscript.
Funding Statement
This study was supported by JSPS KAKENHI Grant No. 20K10152.
References
- 1.Johns Hopkins University Coronavirus Resource Center Website. Available from: https://coronavirus.jhu.edu/map.html (Accessed at 20:37, October 1, 2022).
- 2. Tsuchiya H. Oral symptoms associated with COVID-19 and their pathogenic mechanisms: a literature review. Dent J. 2021;9(3):32. 10.3390/dj9030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27(5):892–903. 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group . Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–5. 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18(5):2621. 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026. 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 8. Tsuchiya H. Gustatory and saliva secretory dysfunctions in COVID-19 patients with zinc deficiency. Life. 2022;12(3):353. 10.3390/life12030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Algahtani SN, Alzarroug AF, Alghamdi HK, Algahtani HK, Alsywina NB, Bin Abdulrahman KA. Investigation on the factors associated with the persistence of anosmia and ageusia in Saudi COVID-19 patients. Int J Environ Res Public Health. 2022;19(3):1047. 10.3390/ijerph19031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le Bon SD, Payen L, Prunier L, Steffens Y, Horoi M, Vaira LA, et al. Making scents of loss of taste in COVID-19: is self-reported loss of taste due to olfactory dysfunction? A prospective study using psychophysical testing. Int Forum Allergy Rhinol. 2021;11(10):1504–7. 10.1002/alr.22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Bon SD, Pisarski N, Verbeke J, Prunier L, Cavelier G, Thill MP, et al. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 2021;278(1):101–8. 10.1007/s00405-020-06267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cellai M, O’Keefe JB. Characterization of prolonged COVID-19 symptoms in an outpatient telemedicine clinic. Open Forum Infect Dis. 2020;7(10):ofaa420. 10.1093/ofid/ofaa420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daher A, Balfanz P, Cornelissen C, Müller A, Bergs I, Marx N, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosales-Castillo A, García de Los Ríos C, Mediavilla García JD. Persistent symptoms after acute COVID-19 infection: importance of follow-up. Med Clin. 2021;156(1):35–6. 10.1016/j.medcle.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrews PJ, Pendolino AL, Ottaviano G, Scarpa B, Grant J, Gaudioso P, et al. Olfactory and taste dysfunction among mild-to-moderate symptomatic COVID-19 positive health care workers: an international survey. Laryngoscope Investig Otolaryngol. 2020;5(6):1019–28. 10.1002/lio2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Monti G, Leggieri C, Fominskiy E, Scandroglio AM, Colombo S, Tozzi M, et al. Two-months quality of life of COVID-19 invasively ventilated survivors; an Italian single-center study. Acta Anaesthesiol Scand. 2021;65(7):912–20. 10.1111/aas.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moreno-Pérez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–83. 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horvath L, Lim JWJ, Taylor JW, Saief T, Stuart R, Rimmer J, et al. Smell and taste loss in COVID-19 patients: assessment outcomes in a Victorian population. Acta Otolaryngol. 2021;141(3):299–302. 10.1080/00016489.2020.1855366. [DOI] [PubMed] [Google Scholar]
- 19. Sampaio Rocha-Filho PA, Albuquerque PM, Carvalho LCLS, Dandara Pereira Gama M, Magalhães JE. Headache, anosmia, ageusia and other neurological symptoms in COVID-19: a cross-sectional study. J Headache Pain. 2022;23(1):2. 10.1186/s10194-021-01367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garrigues E, Janvier P, Kherabi Y, Le Bot A, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81(6):e4–6. 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stavem K, Ghanima W, Olsen MK, Gilboe HM, Einvik G. Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2021;76(4):405–7. 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boari GEM, Bonetti S, Braglia-Orlandini F, Chiarini G, Faustini C, Bianco G, et al. Short-term consequences of SARS-CoV-2-related pneumonia: a follow up study. High Blood Press Cardiovasc Prev. 2021;28(4):373–81. 10.1007/s40292-021-00454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim Y, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. Significance and associated factors of long-term sequelae in patients after acute COVID-19 infection in Korea. Infect Chemother. 2021;53(3):463–76. 10.3947/ic.2021.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hintschich CA, Fischer R, Hummel T, Wenzel JJ, Bohr C, Vielsmeier V. Persisting olfactory dysfunction in post-COVID-19 is associated with gustatory impairment: results from chemosensitive testing eight months after the acute infection. PLoS One. 2022;17(3):e0265686. 10.1371/journal.pone.0265686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khatri Y. Alterations in taste and smell associated with SARS-CoV-2: an exploratory study investigating food consumption and subsequent behavioural changes for those suffering from post-acute sequelae of COVID-19. J Nutr Sci. 2022;11:e14. 10.1017/jns.2022.19. [DOI] [Google Scholar]
- 27. Muthyam AK, Reddy MP, Kulkarni S, Srilatha A, Sahithi K, Satyanarayana D. Oral manifestations in COVID-19 patients: an observational study. J Family Med Prim Care. 2022;11(3):1000–5. 10.4103/jfmpc.jfmpc_1264_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verma H, Shah J, Akhilesh K, Shukla B. Patients’ perspective about speech, swallowing and hearing status post-SARS-CoV-2 (COVID-19) recovery: E-survey. Eur Arch Otorhinolaryngol. 2022;279(5):2523–32. 10.1007/s00405-021-07217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang X, Wang F, Shen Y, Zhang X, Cen Y, Wang B, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4(9):e2127403. 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fang X, Ming C, Cen Y, Lin H, Zhan K, Yang S, et al. Post-sequelae one year after hospital discharge among older COVID-19 patients: a multi-center prospective cohort study. J Infect. 2022;84(2):179–86. 10.1016/j.jinf.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nehme M, Braillard O, Chappuis F, Courvoisier DS, Guessous I; CoviCare Study Team . Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med. 2021;174(9):1252–60. 10.7326/M21-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wanga V, Chevinsky JR, Dimitrov LV, Gerdes ME, Whitfield GP, Bonacci RA, et al. Long-term symptoms among adults tested for SARS-CoV-2 – United States, January 2020–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(36):1235–41. 10.15585/mmwr.mm7036a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto K, Fujiya Y, Kuronuma K, Ogasawara N, Ohkuni T, Yokota SI, et al. Self-reported smell and taste disorders in patients with COVID-19: a Japanese single-center study. In Vivo. 2022;36(2):918–24. 10.21873/invivo.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Binmadi NO, Aljohani S, Alsharif MT, Almazrooa SA, Sindi AM. Oral manifestations of COVID-19: a cross-sectional study of their prevalence and association with disease severity. J Clin Med. 2022;11(15):4461. 10.3390/jcm11154461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ganesan A, Kumar S, Kaur A, Chaudhry K, Kumar P, Dutt N, et al. Oral manifestations of COVID-19 infection: an analytical cross-sectional study. J Maxillofac Oral Surg. 2022;21(4):1326–35. 10.1007/s12663-021-01679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chawla J, Y N, Bakshi SS, Kalidoss VK, Yadav S, Polineni S, et al. Oral manifestations associated with COVID-19 disease: an observational cross sectional study. J Oral Biol Craniofac Res. 2022;12(2):279–83. 10.1016/j.jobcr.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferdeghini C, Mirabelli L, Bianco E, Hari S, Maddalone M. Oral manifestations in hospitalized COVID patients. World J Dent. 2022;13(5):434–40. 10.5005/jp-journals-10015-2082. [DOI] [Google Scholar]
- 38. Kim JW, Han SC, Jo HD, Cho SW, Kim JY. Regional and chronological variation of chemosensory dysfunction in COVID-19: a meta-analysis. J Korean Med Sci. 2021;36(4):e40. 10.3346/jkms.2021.36.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boscutti A, Delvecchio G, Pigoni A, Cereda G, Ciappolino V, Bellani M, et al. Olfactory and gustatory dysfunctions in SARS-CoV-2 infection: a systematic review. Brain Behav Immun Health. 2021;15:100268. 10.1016/j.bbih.2021.100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu D, Wang VY, Chen YH, Ku CH, Wang PC. The prevalence of olfactory and gustatory dysfunction in covid-19 – a systematic review. Auris Nasus Larynx. 2022;49(2):165–75. 10.1016/j.anl.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moraschini V, Reis D, Sacco R, Calasans-Maia MD. Prevalence of anosmia and ageusia symptoms among long-term effects of COVID-19. Oral Dis. 2022;28(Suppl 2):2533–7. 10.1111/odi.13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Antonucci P, Capriotti V, et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur Arch Otorhinolaryngol. 2022;279(1):515–20. 10.1007/s00405-021-06839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boscolo-Rizzo P, Guida F, Polesel J, Marcuzzo AV, Capriotti V, D’Alessandro A, et al. Sequelae in adults at 12 months after mild-to-moderate coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2021;11(12):1685–8. 10.1002/alr.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ercoli T, Masala C, Pinna I, Orofino G, Solla P, Rocchi L, et al. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol Sci. 2021;42(12):4921–6. 10.1007/s10072-021-05611-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cirillo N, Bizzoca ME, Lo Muzio E, Cazzolla AP, Lo Muzio L. Gustatory dysfunction in COVID-19 patients: a rapid systematic review on 27,687 cases. Acta Odontol Scand. 2021;79(6):418–25. 10.1080/00016357.2020.1869828. [DOI] [PubMed] [Google Scholar]
- 46. Miyazato Y, Morioka S, Tsuzuki S, Akashi M, Osanai Y, Tanaka K, et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7(11):ofaa507. 10.1093/ofid/ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Biadsee A, Biadsee A, Kassem F, Dagan O, Masarwa S, Ormianer Z. Olfactory and oral manifestations of COVID-19: sex-related symptoms – a potential pathway to early diagnosis. Otolaryngol Head Neck Surg. 2020;163(4):722–8. 10.1177/0194599820934380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, et al. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif. 2020;53(12):e12923. 10.1111/cpr.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Omezli MM, Torul D. Evaluation of the xerostomia, taste and smell impairments after Covid-19. Med Oral Patol Oral Cir Bucal. 2021;26(5):e568–75. 10.4317/medoral.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gherlone EF, Polizzi E, Tetè G, De Lorenzo R, Magnaghi C, Rovere Querini P, et al. Frequent and persistent salivary gland ectasia and oral disease after COVID-19. J Dent Res. 2021;100(5):464–71. 10.1177/0022034521997112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biadsee A, Dagan O, Ormianer Z, Kassem F, Masarwa S, Biadsee A. Eight-month follow-up of olfactory and gustatory dysfunctions in recovered COVID-19 patients. Am J Otolaryngol. 2021;42(4):103065. 10.1016/j.amjoto.2021.103065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Freni F, Meduri A, Gazia F, Nicastro V, Galletti C, Aragona P, et al. Symptomatology in head and neck district in coronavirus disease (COVID-19): a possible neuroinvasive action of SARS-CoV-2. Am J Otolaryngol. 2020;41(5):102612. 10.1016/j.amjoto.2020.102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Anaya JM, Rojas M, Salinas ML, Rodríguez Y, Roa G, Lozano M, et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev. 2021;20(11):102947. 10.1016/j.autrev.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fantozzi PJ, Pampena E, Di Vanna D, Pellegrino E, Corbi D, Mammucari S, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am J Otolaryngol. 2020;41(6):102721. 10.1016/j.amjoto.2020.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. AbuBakr N, Salem ZA, Kamel AHM. Oral manifestations in mild-to-moderate cases of COVID-19 viral infection in the adult population. Dent Med Probl. 2021;58(1):7–15. 10.17219/dmp/130814. [DOI] [PubMed] [Google Scholar]
- 57. El Kady DM, Gomaa EA, Abdella WS, Ashraf Hussien R, Abd ElAziz RH, Khater AGA. Oral manifestations of COVID-19 patients: an online survey of the Egyptian population. Clin Exp Dent Res. 2021;7(5):852–60. 10.1002/cre2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tuter G, Yerebakan M, Celik B, Kara G. Oral manifestations in SARS-CoV-2 infection. Med Oral Patol Oral Cir Bucal. 2022;27(4):e330–9. 10.4317/medoral.25259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aragoneses J, Suárez A, Algar J, Rodríguez C, López-Valverde N, Aragoneses JM. Oral manifestations of COVID-19: updated systematic review with meta-analysis. Front Med. 2021;8:726753. 10.3389/fmed.2021.726753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsuchiya H. Characterization and pathogenic speculation of xerostomia associated with COVID-19: a narrative review. Dent J. 2021;9(11):130. 10.3390/dj9110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sakaguchi W, Kubota N, Shimizu T, Saruta J, Fuchida S, Kawata A, et al. Existence of SARS-CoV-2 entry molecules in the oral cavity. Int J Mol Sci. 2020;21(17):6000. 10.3390/ijms21176000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Matuck BF, Dolhnikoff M, Duarte-Neto AN, Maia G, Gomes SC, Sendyk DI, et al. Salivary glands are a target for SARS-CoV-2: a source for saliva contamination. J Pathol. 2021;254(3):239–43. 10.1002/path.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nguyen HT, Zhang S, Wang Q, Anang S, Wang J, Ding H, et al. Spike glycoprotein and host cell determinants of SARS-CoV-2 entry and cytopathic effects. J Virol. 2021;95(5):e02304–20. 10.1128/JVI.02304-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jaffal SM, Abbas MA. TRP channels in COVID-19 disease: potential targets for prevention and treatment. Chem Biol Interact. 2021;345:109567. 10.1016/j.cbi.2021.109567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liviero F, Campisi M, Mason P, Pavanello S. Transient receptor potential vanilloid subtype 1: potential role in infection, susceptibility, symptoms and treatment of COVID-19. Front Med. 2021;8:753819. 10.3389/fmed.2021.753819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takahashi N, Tsuzuno T, Mineo S, Yamada-Hara M, Aoki-Nonaka Y, Tabeta K. Epithelial TRPV1 channels: expression, function, and pathogenicity in the oral cavity. J Oral Biosci. 2020;62(3):235–41. 10.1016/j.job.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 69. Kido MA, Muroya H, Yamaza T, Terada Y, Tanaka T. Vanilloid receptor expression in the rat tongue and palate. J Dent Res. 2003;82(5):393–7. 10.1177/154405910308200513. [DOI] [PubMed] [Google Scholar]
- 70. Wang B, Danjo A, Kajiya H, Okabe K, Kido MA. Oral epithelial cells are activated via TRP channels. J Dent Res. 2011;90(2):163–7. 10.1177/0022034510385459. [DOI] [PubMed] [Google Scholar]
- 71. Marincsák R, Tóth BI, Czifra G, Márton I, Rédl P, Tar I, et al. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral Dis. 2009;15(5):328–35. 10.1111/j.1601-0825.2009.01526.x. [DOI] [PubMed] [Google Scholar]
- 72. Liu L, Simon SA. Acidic stimuli activates two distinct pathways in taste receptor cells from rat fungiform papillae. Brain Res. 2001;923(1–2):58–70. 10.1016/s0006-8993(01)03190-0. [DOI] [PubMed] [Google Scholar]
- 73. Ishimaru Y, Matsunami H. Transient receptor potential (TRP) channels and taste sensation. J Dent Res. 2009;88(3):212–8. 10.1177/0022034508330212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sobhan U, Sato M, Shinomiya T, Okubo M, Tsumura M, Muramatsu T, et al. Immunolocalization and distribution of functional temperature-sensitive TRP channels in salivary glands. Cell Tissue Res. 2013;354(2):507–19. 10.1007/s00441-013-1691-x. [DOI] [PubMed] [Google Scholar]
- 75. Ding QW, Zhang Y, Wang Y, Wang YN, Zhang L, Ding C, et al. Functional vanilloid receptor-1 in human submandibular glands. J Dent Res. 2010;89(7):711–6. 10.1177/0022034510366841. [DOI] [PubMed] [Google Scholar]
- 76. Kambe T, Tsuji T, Hashimoto A, Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev. 2015;95(3):749–84. 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 77. Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, et al. Zinc and respiratory tract infections: perspectives for COVID-19 (Review). Int J Mol Med. 2020;46(1):17–26. 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Law JS, Nelson N, Henkin RI. Zinc localization in taste bud membranes. Biol Trace Elem Res. 1983;5(3):219–24. 10.1007/BF02916625. [DOI] [PubMed] [Google Scholar]
- 79. Ishii K, Sato M, Akita M, Tomita H. Localization of zinc in the rat submandibular gland and the effect of its deficiency on salivary secretion. Ann Otol Rhinol Laryngol. 1999;108(3):300–8. 10.1177/000348949910800315. [DOI] [PubMed] [Google Scholar]
- 80. Chou HC, Chien CL, Huang HL, Lu KS. Effects of zinc deficiency on the vallate papillae and taste buds in rats. J Formos Med Assoc. 2001;100(5):326–35. [PubMed] [Google Scholar]
- 81. Wessels I, Rolles B, Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yagi T, Asakawa A, Ueda H, Ikeda S, Miyawaki S, Inui A. The role of zinc in the treatment of taste disorders. Recent Pat Food Nutr Agric. 2013;5(1):44–51. 10.2174/2212798411305010007. [DOI] [PubMed] [Google Scholar]
- 83. Hozumi I, Suzuki JS, Kanazawa H, Hara A, Saio M, Inuzuka T, et al. Metallothionein-3 is expressed in the brain and various peripheral organs of the rat. Neurosci Lett. 2008;438(1):54–8. 10.1016/j.neulet.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 84. Irie Y, Mori F, Keung WM, Mizushima Y, Wakabayashi K. Expression of neuronal growth inhibitory factor (metallothionein-III) in the salivary gland. Physiol Res. 2004;53(6):719–23. 10.33549/physiolres.930529. [DOI] [PubMed] [Google Scholar]
- 85. Yang J, Zhang Y, Cui X, Yao W, Yu X, Cen P, et al. Gene profile identifies zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr Mol Med. 2013;13(3):401–9. 10.2174/1566524011313030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yasui Y, Yasui H, Suzuki K, Saitou T, Yamamoto Y, Ishizaka T, et al. Analysis of the predictive factors for a critical illness of COVID-19 during treatment – relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis. 2020;100:230–6. 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pyar KP, Su KK, Wunna K, Aung ZNH, Maung NL, Kyaw AP, et al. Initial presenting symptoms and severity of SARS-CoV-2 Wild type, the Delta variant and the Omicron variant infected cases in early fourth wave of epidemics in Myanmar. J Med Res Health Sci. 2022;5(1):1765–9. [Google Scholar]
- 88. Piersiala K, Kakabas L, Bruckova A, Starkhammar M, Cardell LO. Acute odynophagia: a new symptom of COVID-19 during the SARS-CoV-2 Omicron variant wave in Sweden. J Intern Med. 2022;292(1):154–61. 10.1111/joim.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jia H, Wang H, Cao L, Lai Z, Cheng Z, Chen Q, et al. Genetic analysis of a SARS-CoV-2 Omicron variant from a Chinese traveller returning from overseas. Emerg Microbes Infect. 2022;11(1):306–9. 10.1080/22221751.2022.2025747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu L, Zhou L, Mo M, Liu T, Wu C, Gong C, et al. SARS-CoV-2 Omicron RBD shows weaker binding affinity than the currently dominant Delta variant to human ACE2. Signal Transduct Target Ther. 2022;7(1):8. 10.1038/s41392-021-00863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Han P, Li L, Liu S, Wang Q, Zhang D, Xu Z, et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185(4):630–40.e10. 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li L, Liao H, Meng Y, Li W, Han P, Liu K, et al. Structural basis of human ACE2 higher binding affinity to currently circulating Omicron SARS-CoV-2 sub-variants BA.2 and BA.1.1. Cell. 2022;185(16):2952–60.e10. 10.1016/j.cell.2022.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Santra D, Maiti S. Molecular dynamic simulation suggests stronger interaction of Omicron-spike with ACE2 than wild but weaker than Delta SARS-CoV-2 can be blocked by engineered S1-RBD fraction. Struct Chem. 2022;33(5):1755–69. 10.1007/s11224-022-02022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jawaid MZ, Baidya A, Mahboubi-Ardakani R, Davis RL, Cox DL. Simulation of the omicron variant of SARS-CoV-2 shows broad antibody escape, weakened ACE2 binding, and modest increase in furin binding. bioRxiv. 2021. 10.1101/2021.12.14.472704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Du X, Tang H, Gao L, Wu Z, Meng F, Yan R, et al. Omicron adopts a different strategy from Delta and other variants to adapt to host. Signal Transduct Target Ther. 2022;7(1):45. 10.1038/s41392-022-00903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kocak OF, Ozgeris FB, Parlak E, Kadıoglu Y, Yuce N, Yaman ME, et al. Evaluation of serum trace element levels and biochemical parameters of COVID-19 patients according to disease severity. Biol Trace Elem Res. 2022;200(7):3138–46. 10.1007/s12011-021-02946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Skalny AV, Timashev PS, Aschner M, Aaseth J, Chernova LN, Belyaev VE, et al. Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites. 2021;11(4):244. 10.3390/metabo11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shakeri H, Azimian A, Ghasemzadeh-Moghaddam H, Safdari M, Haresabadi M, Daneshmand T, et al. Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID-19. J Med Virol. 2022;94(1):141–6. 10.1002/jmv.27277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94(6):2376–83. 10.1002/jmv.27643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Christie B. Covid-19: early studies give hope omicron is milder than other variants. BMJ. 2021;375:n3144. 10.1136/bmj.n3144. [DOI] [PubMed] [Google Scholar]
- 101. Sigal A. Milder disease with Omicron: is it the virus or the pre-existing immunity? Nat Rev Immunol. 2022;22(2):69–71. 10.1038/s41577-022-00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]