Abstract
In contrast to most Staphylococcus aureus isolates in which the gene for staphylococcal β-lactamase (blaZ) is plasmid borne, isolates typeable by group II bacteriophages frequently carry blaZ on the chromosome. Furthermore, the chromosomal gene encodes the type B variant of staphylococcal β-lactamase for which the nucleotide and deduced amino acid sequences have not yet been reported. To better understand β-lactamase production among phage group II staphylococci and the nature of the type B β-lactamase, we determined the type and amount of enzyme produced by 24 phage group II isolates. Of these isolates, 1 did not produce β-lactamase, 8 produced the type B enzyme, and 15 produced the type C enzyme. In all eight type B β-lactamase-producing isolates, blaZ was located on the chromosome. This was in contrast to the type C β-lactamase-producing isolates, in which blaZ was located on a 21-kb plasmid. The nucleotide sequence corresponding to the leader peptide and the N-terminal 85% of the mature exoenzyme form of type B S. aureus was determined. The deduced amino acid sequence revealed 3 residues in the leader peptide and 12 residues in the exoenzyme portion of the β-lactamase that differ from the prototypic type A β-lactamase sequence. These include the serine-to-asparagine change at residue 216 found in the kinetically similar type C enzyme, a threonine-to-lysine change at residue 128 close to the SDN loop (residues 130 to 132), and several substitutions not found in any of the other staphylococcal β-lactamases. In summary, modern isolates of S. aureus typeable by group II phages produce type B or type C staphylococcal β-lactamase. The type B gene resides on the chromosome and has a sequence that, when compared to the sequences of the other staphylococcal β-lactamases, corresponds well with its kinetic properties.
Four variants of Staphylococcus aureus β-lactamase can be distinguished by serotype (27, 28) and kinetic attributes (16, 17, 39). Although the genes encoding the type A, type C, and type D staphylococcal β-lactamases are usually located on a plasmid, the gene for the type B β-lactamase is believed to reside on the chromosome of phage group II isolates (6–9, 24, 30, 31, 33). Whereas the plasmid-borne type A, type C, and type D genes have been sequenced (4, 6, 10, 11, 38), the chromosomal type B gene has not. In this study, we characterize β-lactamase production among phage group II isolates of S. aureus and report the nucleotide and deduced amino acid sequences of the type B β-lactamase.
MATERIALS AND METHODS
Staphylococcal isolates and plasmids.
Isolates of S. aureus recovered from clinical and surveillance cultures between 1984 and 1994 were collected and frozen at −70°C in tryptic soy broth containing 10% glycerol until use. All isolates were confirmed as S. aureus by established methods including colony morphology, Gram staining characteristics, the presence of catalase activity, and the ability to coagulate rabbit serum (20). Reference isolates that produce different variants of staphylococcal β-lactamase were used as controls for β-lactamase typing and as sources for the structural genes encoding β-lactamase (blaZ). These include the following: NCTC 9789 and PS1(pS1), type A β-lactamase; 22260, type B β-lactamase; 3804(pII3804) and RN9(pII147), type C β-lactamase; and FAR10, type D β-lactamase. The phage group II isolate ST79/741 was obtained from R. R. Marples of the Central Public Health Laboratory, London, United Kingdom. The pedigrees of these isolates have been described previously (3, 10, 11, 16–19, 21, 25, 27–29, 39).
Antibiotics, chemicals, and media.
Standard powders of nitrocefin (BBL Microbiology Systems, Cockeysville, Md.), cephaloridine (Sigma Chemical Co., St. Louis, Mo.), methicillin (Bristol Laboratories, Syracuse, N.Y.), and penicillin G and cefazolin (Eli Lilly & Company, Indianapolis, Ind.) were used to prepare antimicrobial solutions for β-lactamase typing assays. Tryptic soy agar and broth were purchased from Difco Laboratories (Detroit, Mich.). Modified 1% CY broth and modified 1% CY-Tris agar with and without 0.5 μg of methicillin per ml were prepared as described previously (16, 25). Restriction endonucleases were purchased from United States Biochemical Corporation (Cleveland, Ohio). Oligonucleotide primers for PCR and for sequencing of the type B β-lactamase gene were synthesized on a Cyclone Plus Automatic DNA synthesizer (Millipore, Bedford, Mass.) by the DNA Core Facility, Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine.
β-Lactamase typing and quantitation.
The type and amount of β-lactamase produced by each isolate following induction by growth on agar containing 0.5 μg of methicillin per ml were determined by using whole-cell suspensions of bacteria, as described previously (17). Initial velocity of hydrolysis assays were performed with 100 μM solutions of nitrocefin, cefazolin, and cephaloridine and a 500 μM solution of benzylpenicillin at 37°C in 1-cm cuvettes with a DU-70 recording spectrophotometer (Beckman Instruments, Fullerton, Calif.) (32, 37). Quantitative rates were corrected for small variations in the absorbance (A272) of different whole-cell preparations and are reported as micromoles (or micrograms) degraded per minute per standard cell mass (A272 = 1.0, or approximately 108 CFU). Statistical comparisons of the amount of β-lactamase activity exhibited by subgroups of phage group II isolates were determined by using the two-sample t test and computer software (Minitab Data Analysis Software, release 10.2; Minitab, Inc., State College, Pa.).
Phage typing.
Bacteriophage typing was performed with the international set of phages at the standard test dilution and 100-fold routine test dilution concentrations (35). The phages used included the following: lytic group 1, 29, 52, 52A, 79, and 80; group 2, 3A, 3C, 55, and 71; group 3, 6, 42E, 47, 53, 54, 75, 77, 83A, 84, and 85; group 5, 94 and 96; and nonallocated phages, 81 and 95.
Isolation of plasmid and chromosomal DNAs and Southern hybridization.
Large-scale isolation of plasmid DNA from S. aureus by ultracentrifugation in a cesium chloride gradient (Var lac oid Chemical Co., Inc., Bergenfield, N.J.) was performed by the methods described by Galletto et al. (12). A probe for the gene encoding type A β-lactamase, blaZ, was prepared by labeling a 1.1-kb HindIII fragment originally derived from pS1 and cloned in pVK103 as described previously (36) with [α-32P]dATP (Du Pont NEN Research Products, Boston, Mass.) by using a random primer labeling kit (United States Biochemical Corporation). This probe was used in a Southern hybridization (22) to identify blaZ in restricted plasmid and chromosomal DNAs.
Sequencing of the gene encoding type B S. aureus β-lactamase.
By using chromosomal DNA as a template, a forward primer based on the blaZ-blaR intercistronic region with the sequence 5′-AGGCTTACTATGCTCATTATTAA-3′, and a reverse primer corresponding to codons encoding the carboxy-terminal region of type A β-lactamase (4, 10, 38) with the sequence 5′-GGCGGTTTCACTTATCAACT-3′, PCR was used to amplify a 1-kb DNA fragment. This fragment was used as the template for DNA sequencing. Eleven oligonucleotide primers for sequencing were designed on the basis of the nucleotide sequences of both DNA strands of type A blaZ (4, 10, 38), and there was enough overlap to enable each nucleotide to be sequenced four to six times through about 85% of the open reading frame of the type B blaZ. Sequencing of the portion of the type B blaZ corresponding to the extreme carboxy-terminal region of the type B β-lactamase could be approached only by using upstream primers, enabling only a single DNA strand to be sequenced in this region. We did not identify any changes in the nucleotide sequence corresponding to the carboxy terminus compared to the sequences of the type A, C, and D β-lactamases (which are identical to each other); however, not every nucleotide in this region could be identified with confidence, and the results are not reported. Nucleotide sequencing was performed by the fluorescent dideoxy terminator method of cycle sequencing (23, 34) on a Perkin-Elmer Applied Biosystems (Foster City, Calif.) model 377 DNA sequencer by the Vanderbilt Cancer Center core sequencing laboratory. Nucleotide sequences were entered into the Vanderbilt VMS computer and aligned by using computer software (Fragment Assembly program of the Wisconsin Sequence Analysis Package, version 8.1; Genetics Computer Group, Madison, Wis.) to determine the consensus sequence.
Nucleotide sequence accession number.
The nucleotide sequence has been submitted to GenBank and assigned accession no. AF086644.
RESULTS
Type and amount of β-lactamase produced by phage group II isolates.
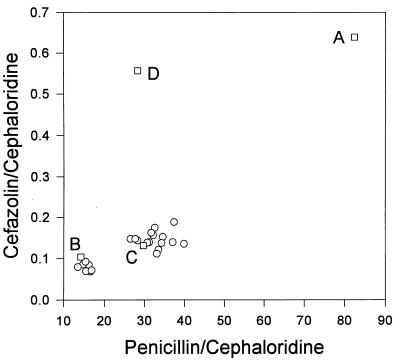
Twenty-four isolates of S. aureus typeable by group II phages were identified. Of these isolates, 1 did not produce β-lactamase, 8 produced the type B enzyme, and 15 produced the type C enzyme (Fig. 1). Two of the eight type B isolates came from Europe (22260 and ST79/741) and the other six were from cities in the United States including Nashville, Tenn.; Boston, Mass.; Montgomery, Ala.; and Salt Lake City, Utah. The 15 type C isolates came from Nashville, Tenn.; Ann Arbor, Mich.; Boston, Mass.; Montgomery, Ala.; Portland, Maine; Portland, Oreg.; and Salt Lake City, Utah.
FIG. 1.
Plot of the ratios of the rate of hydrolysis of cefazolin/rate of hydrolysis of cephaloridine versus rate of hydrolysis of benzylpenicillin/rate of hydrolysis of cephaloridine by whole-cell suspensions of S. aureus. Values for the reference isolates which produce the A (NCTC 9789), B (22260), C (3804), and D (FAR 10) serotypes of S. aureus β-lactamase are identified by squares. The values for the 22 β-lactamase-producing phage group II isolates excluding reference strain 22260 are represented by circles. Compared to the ratio for nitrocefin/cefazolin used in our 1990 report of whole-cell methods for the typing of staphylococcal β-lactamases (17), the use of a ratio for benzylpenicillin/cephaloridine enables easier discrimination between the type B versus type C enzyme and between the type A versus type D enzyme.
The quantitative β-lactamase activities of type B and type C β-lactamase-producing phage group II isolates were compared. Methicillin-induced whole-cell preparations of the type C isolates exhibited twice as much penicillinase activity as the type B isolates (P < 0.0001 (Table 1). Type C isolates also exhibited greater cefazolinase activity. In contrast, by using cephaloridine and nitrocefin as substrates, the induced type B and type C isolates were found to exhibit comparable quantitative β-lactamase activities. Uninduced whole-cell preparations of the type B isolates exhibited greater nitrocefin hydrolysis rates than the type C isolates (P < 0.001) but had lower ratios of the rates of induced activity/rates of uninduced activity (mean, 38- versus 83-fold increases in β-lactamase production with induction by 0.5 μg of methicillin per ml, respectively; P < 0.002).
TABLE 1.
Quantitative rates of hydrolysis of penicillin and cephalosporin substrates by phage group II isolates of S. aureus making type B and type C β-lactamases
| Substrate, preparationa | Rate of hydrolysisb
|
|
|---|---|---|
| Type B (n = 8) | Type C (n = 15) | |
| Benzylpenicillin, inducedc | 118 ± 48 | 246 ± 38 |
| Cephaloridine, induced | 7.5 ± 3.0 | 7.6 ± 1.1 |
| Cefazolin, inducedd | 0.65 ± 0.28 | 1.11 ± 0.15 |
| Nitrocefin, induced | 14.6 ± 6.5 | 14.8 ± 3.6 |
| Nitrocefin, uninducede | 0.47 ± 0.10 | 0.22 ± 0.07 |
The substrate concentrations used in these determinations were 500 μM for benzylpenicillin and 100 μM for the cephalosporins. Preparations comprised whole-cell suspensions of bacteria (17). Methicillin at a concentration of 0.5 μg/ml was used to induce β-lactamase production.
Values are means ± standard deviations and are reported as nanomoles of substrate hydrolyzed per minute per cell mass with an A272 of 1.0 (about 108 CFU).
P < 0.0001.
P < 0.002.
P < 0.001.
Location of the β-lactamase gene among phage group II isolates.
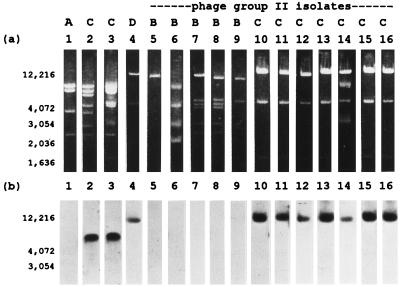
Although most β-lactamase-producing isolates of S. aureus carry the gene encoding staphylococcal β-lactamase, blaZ, on a plasmid, the failure in attempts to cure pre-1977 phage II isolates of their ability to produce β-lactamase has led some investigators to suggest that the type B blaZ is located on the chromosome (6–9, 24, 30, 31, 33). To address this issue further, ultracentrifugation in a cesium chloride gradient was used to separate the plasmid and chromosomal DNAs of all eight phage group II, type B β-lactamase-producing isolates and some representative type C β-lactamase-producing isolates. Three of the eight type B isolates were found not to contain plasmid DNA. Although the other five isolates contained plasmid DNA, Southern hybridization did not detect blaZ in any of the plasmids (Fig. 2). This was in contrast to the phage group II, type C β-lactamase-producing isolates, in which blaZ was located on a 21-kb plasmid.
FIG. 2.
Agarose gel (a) and Southern hybridization with a blaZ probe (b) of EcoRI-restricted plasmid DNA from phage group II, β-lactamase-producing isolates of S. aureus. Isolates include NCTC 9789, type A control (lane 1); RN9, type C control (lane 2); 3804, type C control (lane 3); FAR10, type D control (lane 4); 22260, type B control (lane 5); ST79/741 (lane 6); DK1133 (lane 7); DK5150 (lane 8); DK6024 (lane 9); DK1041 (lane 10); DK2027 (lane 11); DK3086 (lane 12); DK5110 (lane 13); DK5154 (lane 14); DK5162 (lane 15); and DK5246 (lane 16). Electrophoresis was performed on a single gel, the photograph of which was cut and reassembled for clarity of presentation. The type of β-lactamase produced by each isolate is noted above the lane number. The positions of base pair markers are indicated. NCTC 9789 has previously been shown to carry the type A β-lactamase gene on the chromosome rather than a plasmid (3).
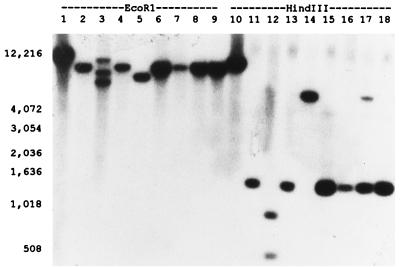
Southern hybridization of EcoRI- and HindIII-restricted chromosomal DNAs from the eight phage group II, type B β-lactamase-producing isolates identified blaZ on the chromosome of each isolate (Fig. 3). For six of these isolates including the historically serotyped reference strain 22260, blaZ appears to be on the same restriction fragment (about 10 kb with EcoRI and 1.5 kb with HindIII); however, in isolates ST79/741 and DK2034 the region of DNA around blaZ clearly differs from the regions of DNA in the other isolates because the blaZ probe hybridized with different restriction fragments.
FIG. 3.
Southern hybridization of chromosomal DNA from phage group II, type B β-lactamase-producing isolates of S. aureus using a blaZ probe. Lanes 1 to 9, EcoRI-restricted DNA; lanes 10 to 18, HindIII-restricted DNA. Isolates include NCTC 9789 (lanes 1 and 10), 22260 (lanes 2 and 11), ST79/741 (lanes 3 and 12), DK1133 (lanes 4 and 13), DK2034 (lanes 5 and 14), DK5143 (lanes 6 and 15), DK5150 (lanes 7 and 16), DK6024 (lanes 8 and 17), and DK6026 (lanes 9 and 18). The positions of base pair markers are indicated. NCTC 9789 has previously been shown to carry the type A β-lactamase gene on the chromosome rather than a plasmid (3).
Sequence of the type B β-lactamase and comparison with those of other S. aureus β-lactamases.
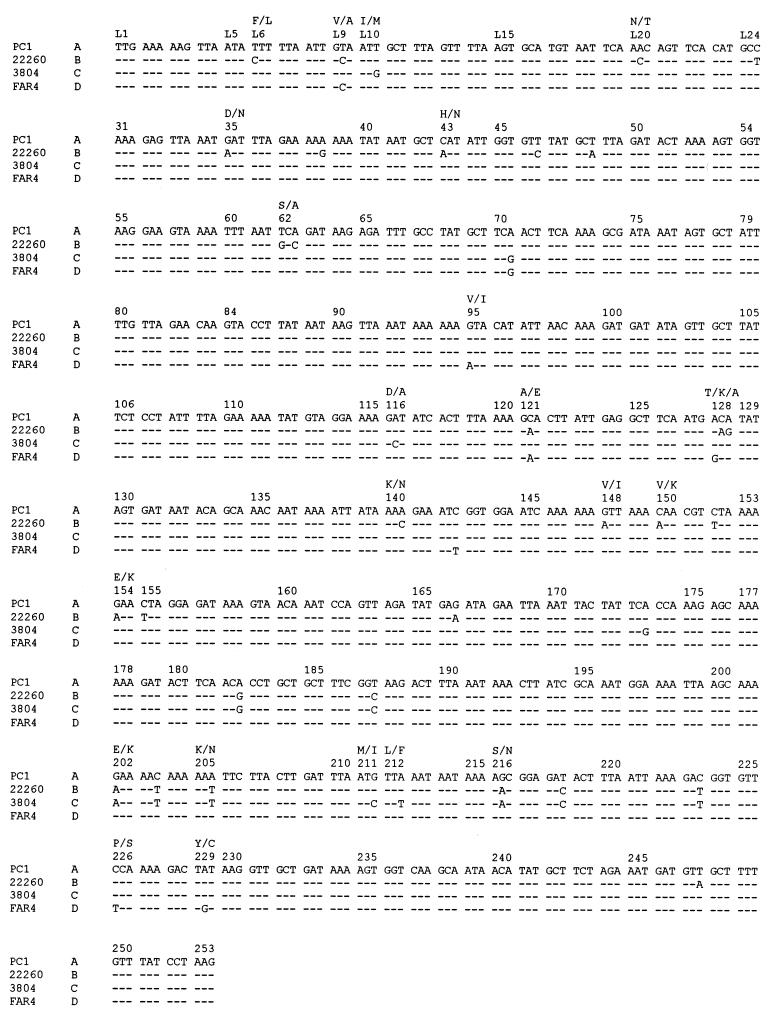
By using primers based on the DNA sequence of the type A S. aureus β-lactamase (4, 10, 38) in regions upstream of the blaZ promoter and toward the 3′ end of the open reading frame, a DNA fragment containing most of the type B blaZ was PCR amplified and sequenced. In this fashion, the nucleotide sequence corresponding to the leader peptide and the N-terminal 85% of the mature exoenzyme form of type B S. aureus was determined (Fig. 4). The deduced amino acid sequence of the type B enzyme identified 3 residues in the leader peptide and 12 residues in the exoenzyme portion of the β-lactamase that differ from the prototypic type A enzyme sequence. These include the serine-to-asparagine change at residue 216 found in the kinetically similar type C enzyme (36), a threonine-to-lysine change at residue 128 close to the SDN loop (residues 130 to 132), and several substitutions not found in any of the other β-lactamases.
FIG. 4.
Comparison of the nucleotide sequence of the gene encoding type B β-lactamase from strain 22260 to other S. aureus β-lactamases. The sequences for the type A, C, and D β-lactamases have been reported previously (4, 6, 10, 11, 38). Differences in nucleotide sequences are shown; −, no change from the blaZ sequence of the type A gene. Amino acid substitutions in the B, C, and D enzymes compared to the type A sequence are indicated above the codons. Amino acid numbering is according to Ambler and colleagues (1, 2), including gaps at residue 58 and residues 85 to 86 (2). L1 to L24 refer to the leader peptide. Whereas the sequences of the type A, C, and D β-lactamases have been determined through the carboxy-terminal residue at Ambler position 290, reliable sequence data for the type B enzyme were obtained through residue 253.
DISCUSSION
When we first reported in 1989 that the historically serotyped A, C, and D variants of S. aureus can be identified and distinguished by the kinetics of hydrolysis of selected β-lactams (16), we did not have a serotype B-producing isolate for comparison. Therefore, and because of the historical association between phage group II isolates and serotype B as reported by Richmond (27), we selected four phage group II isolates for evaluation. However, instead of finding a single substrate hydrolysis profile among these isolates, two related but distinct substrate profiles were identified (16). Because the reference isolate 22260 which had been serotyped as type B by Lacey and Rosdahl (21) with the original typing antiserum of Richmond (27) has subsequently been obtained and used in this and some of our other studies (17, 39), we can now correlate one of these substrate profiles with that for the serotype B enzyme (Fig. 1). The other substrate profile is indistinguishable from that displayed by the historical serotype C enzyme produced by the reference isolate S. aureus 3804.
As has been reported for most β-lactamase-producing isolates of S. aureus belonging to other phage groups (6, 8, 25, 26, 31), the gene encoding the type C enzyme in phage group II isolates is located on a plasmid. In contrast, the type B gene is chromosomal, a finding suspected by earlier investigators on the basis of their inability to cure such isolates of β-lactamase production (6–9, 24, 30, 31, 33) and shown more convincingly with the Southern hybridization studies performed as part of this evaluation. Using curing experiments as an indicator of whether blaZ is located on a plasmid or the chromosome, Skov et al. (33) have reported that all phage group II isolates recovered prior to 1977 harbored blaZ on the chromosome, whereas blaZ was plasmid borne in the large majority of phage group II isolates recovered in recent years. Combined with the findings of this study and the original report of β-lactamase variants in S. aureus by Richmond in 1965 (27) in which the type C β-lactamase was found among phage group I and III isolates but not in phage group II isolates, it appears that the type B gene has been present in phage group II isolates since at least the 1960s and that the type C gene has appeared in this phage group relatively recently. Furthermore, the 21-kb plasmid on which the type C gene is carried in phage group II isolates has a distinctive endonuclease restriction pattern and appears to be found commonly among clinical isolates of S. aureus throughout the world (5, 33). All of the phage group II type C β-lactamase-producing isolates evaluated in this study contained this 21-kb plasmid. No other plasmids containing the type C blaZ have as yet been identified among phage group II strains.
The type B gene shares less identity with the type A, C, and D genes than these three do with each other. Including differences in the leader peptide and the 85% of the exoenzyme for which we have sequence data, the type B enzyme had 15 or 16 amino acid differences with each of the type A, type C, and type D enzymes. In contrast, the prototypic type A and C enzymes differ by 7 amino acids, the type A and D enzymes differ by 6 amino acids, and the type C and D enzymes differ by 13 amino acids. Similarly, the type B gene had more silent nucleotide differences with the other β-lactamase genes than they had among themselves. It is interesting to speculate that during the molecular evolution of staphylococcal β-lactamase, the type B gene has diverged more from the others because of its chromosomal location and/or its apparent restriction to phage group II isolates (27).
Despite the difference of 16 amino acids between the portions of the type B and C β-lactamases for which the sequences of both are known, these enzymes are kinetically very similar. We have previously reported that the serine-to-asparagine difference at residue 216 is responsible for the kinetic differences between the wild-type A and C enzymes and that replacement of this serine in the type A β-lactamase with asparagine reduces the activity of the enzyme against nitrocefin and cefazolin, conferring a type C kinetic profile (36). Accordingly, considering its kinetic similarity to the type C enzyme, the presence of an asparagine at this position in the type B enzyme was not surprising. In addition, the type B enzyme differs from the A, C, and D enzymes at amino acid 128, located just two residues from the kinetically important SDN loop at residues 130 to 132 (15). This is the same site that is responsible for the reduced activity of the type D enzyme against penicillin compared to the activity of the type A enzyme (36). Although the type B and C enzymes are kinetically similar, the type B enzyme is twofold less active against penicillin and about 40% less active against cefazolin than the type C enzyme relative to most other substrates. Lower penicillin/cephaloridine and cefazolin/cephaloridine hydrolysis ratios distinguish the type B enzyme from the type C enzyme (Fig. 1). Because studies involving site-directed mutagenesis and single amino acid substitutions have shown that a threonine-to-alanine substitution at residue 128 reduces the kcat of staphylococcal β-lactamase against penicillin and is responsible for the kinetic differences between the type A and type D enzymes (36), it is tempting to speculate that the threonine-to-lysine difference between the type C and type B β-lactamases at this same position explains the kinetic differences between these enzymes. Other amino acid differences between the type B and type C enzymes at sites close to highly conserved residues among class A β-lactamases believed to be critical for proper folding of the enzyme and/or substrate binding, as outlined by Herzberg (14) and Herzberg and Moult (13) may also contribute to the different kinetic characteristics. These include changes at residues 35 (Asp to Asn, close to residue 37), 43 (His to Asn, close to residue 45), 140 (Lys to Asn, close to residues 136 and 144), and 148 (Val to Ile, close to residue 144).
Regarding the long-standing impression that phage group II and type B β-lactamase-producing isolates of S. aureus make less β-lactamase than other staphylococcal isolates (31) and the observation by Skov et al. (33) that phage group II isolates in which blaZ is located on the chromosome exhibit only half the penicillinase activity of those in which blaZ is plasmid borne, it is noteworthy that the amount of enzyme activity detected depends upon the β-lactam used as the substrate. For example, although our findings obtained with benzylpenicillin as the substrate confirm that phage group II isolates with blaZ on their chromosomes exhibit lower β-lactamase activities than isolates in which blaZ is plasmid borne, these differences disappear when cephaloridine or nitrocefin is used to quantify β-lactamase activity (Table 1). In other words, the low penicillinase activity of the type B isolates reflects a difference in the substrate specificity of the type B and type C enzymes rather than the quantity of β-lactamase. Accordingly, and because of substrate specificity differences that are even more dramatic when the type A or type D versus type B and type C enzymes are compared, it is meaningless to compare the β-lactamase activities of different isolates of S. aureus by using a single substrate without first knowing which variant of β-lactamase the isolates produce.
In summary, modern isolates of S. aureus typeable by group II phages produce either type B or type C staphylococcal β-lactamase. The type B gene resides on the chromosome and has a sequence that, when compared to those of the other staphylococcal β-lactamases, corresponds well with its kinetic properties.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant AI 32126 from the National Institutes of Health.
We thank Gary A. Hancock and J. Michael Miller of the Centers for Disease Control and Prevention for performing the phage typing assays. We thank Pat McGraw and Hiriam Gates for technical assistance.
REFERENCES
- 1.Ambler R P. Amino-acid sequence of the Staphylococcus aureus penicillinase. Biochem J. 1975;151:197–218. doi: 10.1042/bj1510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambler R P, Coulson A F W, Frère J M, Ghuysen J M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A beta-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asheshov E H. The genetics of penicillinase production in Staphylococcus aureus strain PS80. J Gen Microbiol. 1969;59:289–301. doi: 10.1099/00221287-59-3-289. [DOI] [PubMed] [Google Scholar]
- 4.Chan P T. Nucleotide sequence of the Staphylococcus aureus PC1 β-lactamase gene. Nucleic Acids Res. 1986;14:5940. doi: 10.1093/nar/14.14.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doebbeling B N, Pfaller M A, Hollis R J, Boyken L D, Pignatari A C, Herwaldt L A, Wenzel R P. Restriction endonuclease analysis of Staphylococcus aureus plasmid DNA from three continents. Eur J Clin Microbiol Infect Dis. 1992;11:4–8. doi: 10.1007/BF01971263. [DOI] [PubMed] [Google Scholar]
- 6.Dyke K, Gregory P. Resistance to β-lactam antibiotics: resistance mediated by β-lactamases. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 139–157. [Google Scholar]
- 7.Dyke K G H, Parker M T, Richmond M H. Penicillinase production and metal-ion resistance in Staphylococcus aureus cultures isolated from hospital patients. J Med Microbiol. 1970;3:125–136. doi: 10.1099/00222615-3-1-125. [DOI] [PubMed] [Google Scholar]
- 8.Dyke K G H. Beta-lactamases of Staphylococcus aureus. In: Hamilton-Miller J M T, Smith J T, editors. Beta-lactamases. London, United Kingdom: Academic Press; 1979. pp. 291–310. [Google Scholar]
- 9.Dyke K G H, Noble W C. Plasmids of phage-group II Staphylococcus aureus. J Med Microbiol. 1984;17:325–334. doi: 10.1099/00222615-17-3-325. [DOI] [PubMed] [Google Scholar]
- 10.East A K, Dyke K G H. Cloning and sequence determination of six Staphylococcus aureus beta-lactamases and their expression in Escherichia coli and Staphylococcus aureus. J Gen Microbiol. 1989;135:1001–1015. doi: 10.1099/00221287-135-4-1001. [DOI] [PubMed] [Google Scholar]
- 11.East A K, Curnock S P, Dyke K G H. Change of a single amino acid in the leader peptide of a staphylococcal beta-lactamase prevents the appearance of the enzyme in the medium. FEMS Microbiol Lett. 1990;69:249–254. doi: 10.1016/0378-1097(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 12.Galletto D W, Johnston J L, Archer G L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987;31:1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzberg O, Moult J. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 Å resolution. Science. 1987;236:694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- 14.Herzberg O. Refined crystal structure of β-lactamase from Staphylococcus aureus PC1 at 2.0 Å resolution. J Mol Biol. 1991;217:701–719. doi: 10.1016/0022-2836(91)90527-d. [DOI] [PubMed] [Google Scholar]
- 15.Jacob F, Joris B, Lepage S, Dusart J, Frère J M. Role of the conserved amino acids of the ’SDN’ loop (Ser130, Asp131 and Asn132) in a class A beta-lactamase studied by site-directed mutagenesis. Biochem J. 1990;271:399–406. doi: 10.1042/bj2710399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kernodle D S, Stratton C W, McMurray L W, Chipley J R, McGraw P A. Differentiation of beta-lactamase variants of Staphylococcus aureus by substrate hydrolysis profiles. J Infect Dis. 1989;159:103–108. doi: 10.1093/infdis/159.1.103. [DOI] [PubMed] [Google Scholar]
- 17.Kernodle D S, McGraw P A, Stratton C W, Kaiser A B. Use of extracts versus whole-cell bacterial suspensions in the identification of Staphylococcus aureus beta-lactamase variants. Antimicrob Agents Chemother. 1990;34:420–425. doi: 10.1128/aac.34.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kernodle D S, Zygmunt D J, McGraw P A, Chipley J R. Purification of Staphylococcus aureus beta-lactamases by using sequential cation-exchange and affinity chromatography. Antimicrob Agents Chemother. 1990;34:2177–2183. doi: 10.1128/aac.34.11.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kernodle D S, Classen D C, Burke J P, Kaiser A B. Failure of cephalosporins to prevent Staphylococcus aureus surgical wound infections. JAMA. 1990;263:961–966. [PubMed] [Google Scholar]
- 20.Kloos W E, Jorgensen J H. Staphylococci. In: Lennette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C: American Society for Microbiology; 1985. pp. 143–153. [Google Scholar]
- 21.Lacey R W, Rosdahl V T. An unusual “penicillinase plasmid” of Staphylococcus aureus: evidence for its transfer under natural conditions. J Med Microbiol. 1974;7:1–9. doi: 10.1099/00222615-7-1-1. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.McCombie W R, Heiner C, Kelly J M, Fitzgerald M G, Gocayne J D. Rapid and reliable fluorescent cycle sequencing of double stranded templates. DNA Sequence. 1992;2:289–296. doi: 10.3109/10425179209030961. [DOI] [PubMed] [Google Scholar]
- 24.Meijers J A, Stobberingh E E. Chromosomal penicillin resistance in Staphylococcus aureus strains of phage group II. Antonie Leeuwenhoek. 1980;46:577–586. doi: 10.1007/BF00394013. [DOI] [PubMed] [Google Scholar]
- 25.Novick R P. Analysis of transduction of mutations affecting penicillinase formation in Staphylococcus aureus. J Gen Microbiol. 1963;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- 26.Novick R P, Richmond M H. Nature and interactions of the genetic elements governing penicillinase synthesis in Staphylococcus aureus. J Bacteriol. 1965;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richmond M H. Wild type variants of exopenicillinase from Staphylococcus aureus. Biochem J. 1965;94:584–593. doi: 10.1042/bj0940584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosdahl V T. Naturally occurring constitutive beta-lactamase of novel serotype in Staphylococcus aureus. J Gen Microbiol. 1973;77:229–231. doi: 10.1099/00221287-77-1-229. [DOI] [PubMed] [Google Scholar]
- 29.Rosdahl V T, Rosendal K. Correlation of penicillinase production with phage type and susceptibility to antibiotics and heavy metals in Staphylococcus aureus. J Med Microbiol. 1983;16:391–399. doi: 10.1099/00222615-16-4-391. [DOI] [PubMed] [Google Scholar]
- 30.Rosdahl V T. Localization of the penicillinase gene in naturally occurring Staphylococcus aureus strains. Acta Pathol Microbiol Immunol Scand Sect B. 1985;93:383–388. doi: 10.1111/j.1699-0463.1985.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 31.Rosdahl V T. Penicillinase production in Staphylococcus aureus strains of clinical importance. Dan Med Bull. 1986;33:175–184. [PubMed] [Google Scholar]
- 32.Ross G W, Chanter K V, Harris A M, Kirby S M, Marshall M J, O’Callaghan C H. Comparison of assay techniques for beta-lactamase activity. Anal Biochem. 1974;54:9–16. doi: 10.1016/0003-2697(73)90241-8. [DOI] [PubMed] [Google Scholar]
- 33.Skov R L, Williams T J, Pallesen L, Rosdahl V T, Espersen F. Beta-lactamase production and genetic location in Staphylococcus aureus: introduction of a β-lactamase plasmid in strains of phage group II. J Hosp Infect. 1995;30:111–124. doi: 10.1016/0195-6701(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 34.Smith L M, Sander J Z, Kaiser R J, Hughes P, Dodd D, Connel C R, Heiner C, Kent S B, Hood L E. Fluorescence detection in automated DNA sequence analysis. Nature. 1986;321:674–679. doi: 10.1038/321674a0. [DOI] [PubMed] [Google Scholar]
- 35.Smith P B. Bacteriophage typing of Staphylococcus aureus. In: Cohn J O, editor. The staphylococci. New York, N.Y: John Wiley & Sons, Inc.; 1972. pp. 431–441. [Google Scholar]
- 36.Voladri R K R, Tummuru M K R, Kernodle D S. Structure-function relationships among wild-type variants of Staphylococcus aureus β-lactamase: importance of amino acids 128 and 216. J Bacteriol. 1996;178:7248–7253. doi: 10.1128/jb.178.24.7248-7253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waley S G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974;139:789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P, Novick R P. Nucleotide sequence and expression of the beta-lactamase gene from Staphylococcus aureus plasmid pI258 in Escherichia coli, Bacillus subtilis, and Staphylococcus aureus. J Bacteriol. 1987;169:1763–1766. doi: 10.1128/jb.169.4.1763-1766.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zygmunt D J, Stratton C W, Kernodle D S. Characterization of four β-lactamases produced by Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:440–445. doi: 10.1128/aac.36.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]