Abstract
Background:
Testosterone prescribing for men has dramatically increased, and there have been concerns about inappropriate use and adverse events. While regulatory bodies have warned about increased risk of venous thromboembolism (VTE), published clinical data supporting an increased risk for VTE are limited.
Objective:
To conduct a systematic review of studies examining the association between testosterone therapy in men and VTE.
Methods:
Comprehensive searches of multiple databases were performed from inception through October 3rd, 2018. Randomized control trials (RCTs) and observational studies examining the association between exogenous testosterone (any route) and VTE. Study selection and data extraction were performed by two independent investigators. Random-effect model meta-analyses were used to estimate pooled odds ratios (OR) and 95% confidence intervals (CIs). Heterogeneity among studies was evaluated using the I2 statistic. Risk of bias was assessed using the Cochrane and Newcastle-Ottawa tools.
Results:
Six RCTs (n=2,236) and 5 observational studies (n=1,249,640) were included. Five RCTs were performed in men with documented hypogonadism. The observational studies included: 2 case-control studies, 2 retrospective cohorts, and 1 retrospective cohort with a nested case-control study. There was no evidence of a statistically significant association between VTE and testosterone (OR 1.41, 95%CI 0.96–2.07). Heterogeneity was high (I-squared=84.4%). The association remained nonsignificant when the analysis was stratified by study design: RCTs (2.05, 95% CI 0.78–5.39); cohort (1.06, 95% CI 0.85–1.33); and case-control (1.34, 95% CI 0.78–2.28). The overall risk of bias was moderate.
Conclusions:
The current evidence is of low certainty but does not support an association between testosterone use and VTE in men.
Keywords: testosterone, thrombosis, venous thromboembolism, hypercoagulability
Introduction:
Testosterone therapy has rapidly expanded over the past decades3–7, and there are concerns over inappropriate prescribing and adverse effects8, including venous thromboembolism (VTE). Upward trends in testosterone use are seen in numerous countries, but rates of prescribing are highest in the United States, having risen from 20.2 per 10,000 person-years in 2008 to 75.7 per 10,000 person-years in 20113. An increasing trend in prescribing has continued from 2010 to 2013 as shown by FDA data from testosterone sales9, although data from US commercial insurance claims indicate a downward trend in new testosterone users starting July 2012 and continuing through 201310.
Testosterone is indicated for the treatment of primary or secondary hypogonadism in men, but potentially inappropriate prescribing of testosterone has been demonstrated by studies that estimate that 25–50% of new-users did not have a pre-treatment serum testosterone level3,9,11. Much of the prescribing occurs in middle-aged to older men who are already at a higher risk of venous thromboembolism due to their age and comorbidities, making it difficult for clinicians to understand whether testosterone use is truly a contributor to thrombotic events, or simply coincidental. Current labeling for testosterone products in the United States warns against VTE, and the warning was expanded in 2014 to include all testosterone users rather than only patients who develop erythrocytosis12. This article will first discuss possible mechanisms by which testosterone may contribute to VTE and then systematically review the current literature to determine the association between exogenous testosterone use and VTE in men.
Proposed mechanisms of thrombosis
Erythrocytosis:
The Food and Drug Administration requires a warning in the labeling of testosterone products of VTE risk as a possible consequence of erythrocytosis, but also of increased VTE risk independent of erythrocytosis13. While testosterone therapy clearly and consistently increases hemoglobin concentrations and can lead to erythrocytosis14–18, no data have been published that show an association of testosterone-induced erythrocytosis with VTE risk. An Endocrine Society Clinical Practice Guideline recommends avoiding testosterone therapy in patients with baseline erythrocytosis (hematocrit >50%) and monitoring for a rise in hematocrit in new-users 3 and 6 months after initiation, and then annually1. Testosterone dose reduction and/or discontinuation is recommended if a patient develops erythrocytosis.
In most reports of VTE associated with testosterone use, erythrocytosis was not present or not reported19–23. Only one case report has been published about a patient taking testosterone with an otherwise unprovoked mesenteric vein thrombosis in the setting of erythrocytosis (hemoglobin 19.7g/dL)24. Several large cohort studies have specifically examined hemoglobin/hematocrit as a VTE risk factor with differing conclusions. In the Tromsø study25, men with a hemoglobin ≥15.6 g/dL had an increased risk for total VTE (HR 1.6, 95% CI 1.14–2.24) and unprovoked VTE (HR 2.20, 95% CI 1.34–3.61). Other studies have not found an association and between erythrocytosis and VTE26,27, or only found an association in women28. Erythrocytosis has been shown to increase erythrocyte aggregation and increase blood viscosity29, but whether this translates into a pro-coagulant effect is not known. Erythrocytosis in mouse models of arterial thrombosis have demonstrated a faster rate of thrombus formation and a shorter time to artery occlusion30. More research is needed to explore the role of erythrocytosis in the pathogenesis of VTE and to understand if it might lead to an increased risk for VTE in patients taking testosterone therapy.
Other mechanisms:
Testosterone is partly converted to 17β-estradiol (E2) and dihydrotestosterone (DHT) in adipose tissue and it has been speculated that the increasing E2 levels may lead to thrombosis22. Increasing doses of testosterone are associated with higher E2 levels, and older men have a higher rate of aromatization, largely due to a higher percentage of adipose tissue 31. Some randomized clinical trials have demonstrated higher levels of estradiol in subjects randomized to testosterone16,32 but others have not33. Platelet thromboxane A2 receptor density and maximum platelet aggregation response have been shown to be increased in healthy male volunteers given intramuscular testosterone34. How this might contribute to the development of VTE is unknown. It has also been proposed that previously undiagnosed inherited thrombophilia might compound the effects of testosterone20,21. Testosterone users with VTE, when compared to controls with unprovoked VTE, were more likely to have Factor V Leiden or a lupus anticoagulant35.
Methods:
Data Sources
A search of several databases from each database’s inception, any language, was conducted in Ovid Epub Ahead of Print, Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategies were designed and conducted by an experienced librarian with input from study investigators. Controlled vocabulary supplemented with keywords was used to search for relevant studies, through October 3rd, 2018. The actual search strategy is available in the appendix. Previous systematic reviews on testosterone and VTE were identified by searching PubMed and their bibliography was reviewed for possible inclusion.
Study Selection
Observational studies were eligible for inclusion if they met the following criteria: 1) cohort study or case-control study examining the association between testosterone therapy and VTE, 2) testosterone users were compared to non-users for cohort studies and subjects with VTE compared to subjects without VTE for case-control studies. All randomized control trials (RCTs) were included if VTE outcomes were reported.
Data Extraction and Quality Assessment
Study selection and data extraction were performed by two independent investigators. Unadjusted odds ratios or number of VTE events in each group, for studies reporting hazard ratios, were extracted and used for the analysis. Risk of bias was assessed in the RCTs by using the Cochrane tool 36 and in observational studies using the Newcastle-Ottawa tool37.
Data Synthesis and Analysis
Random-effect model meta-analyses were used to estimate pooled odds ratio (OR) and 95% confidence intervals (CIs). Heterogeneity among studies was evaluated by the I2 statistic. Forest plots and summary estimates were created for the overall analysis and stratified by study type and for men with and without a diagnosis of hypogonadism. A sensitivity analysis was performed using adjusted odds ratios. A funnel plot was created plotting the standard error of the log (OR) and the log (OR) to examine for publication bias.
Results:
Search results
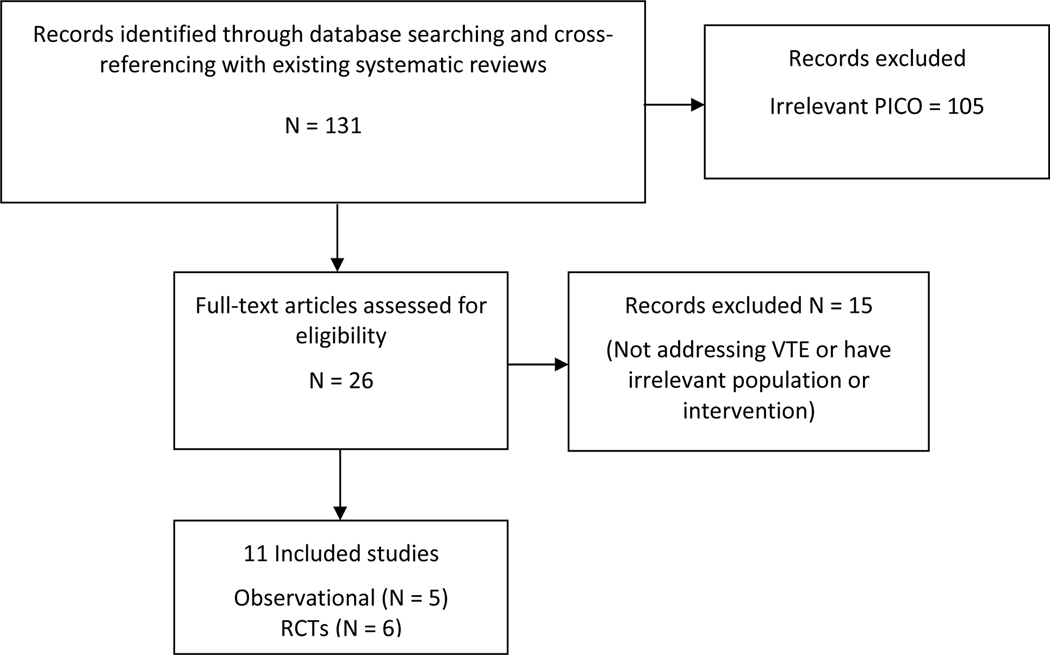
The search strategy identified 131 records, and after the title and abstract screening, 26 records underwent full-text review (Figure 1). Five observational studies2,11,38–40 and 6 RCTs16,33,41–44 met criteria for inclusion in the quantitative analysis. Two meta-analyses examining testosterone and VTE were identified45,46.
Figure 1:
PRISMA flow diagram of search results and study selection
Abbreviations: PICO= Population, Intervention, Comparison, Outcome, RCT= randomized control trial, VTE= venous thromboembolism
Description of included studies
Among the 5 observational studies, 2 were retrospective cohort studies, 2 were case-control studies, and 1 contained a retrospective cohort and a nested case-control study (Table 1). Data sources included commercial claims data, single institution academic medical center records, and governmental health data. The study by Martinez et al2 examined data from the United Kingdom; all others were conducted in patients from the United States. There were significant differences in study populations, number and type of covariates assessed, and stringency of VTE outcome definitions. All observational studies, except for the Ramasamy et al39 study, excluded patients with a history of VTE. The retrospective cohort and nested case-control study, by Li et al38, only reported associations with idiopathic VTE. For our analysis, we obtained unpublished data from the authors of this study reporting total VTE events to more closely match the definition of VTE in the other observational studies.
Table 1:
Comparison of methods and results from observational studies evaluating the association between testosterone and VTE
| Author/Year | Li, 201638 | Li, 201638 | Ramasamy, 201539 | Baillargeon, 201540 | Martinez, 20162 | Sharma, 201611 |
|---|---|---|---|---|---|---|
| Study Type | Retrospective Cohort | Nested case-control | Retrospective Cohort | Case-control | Case-control | Retrospective Cohort |
| Data Source | MarketScan | MarketScan | Single Institution Urology Practice | Clinformatics DataMart | CPRD | Veterans Administrative Corporate Data Warehouse |
| Population | Men with hypogonadism (ICD-9 or TT prescription) | Men with hypogonadism (ICD-9 or TT prescription) | Men with hypogonadism (total serum TT <300ng/dL plus three or more hypogonadal symptoms) | Men with commercial insurance | Men in the United Kingdom | Men with low serum TT on at least two occasions |
| Exclusion Criteria | History of VTE, continuous baseline insurance enrollment <12 months, age <18 years | History of VTE, continuous baseline insurance enrollment <12 months, age <18 years | Active malignancy, previous androgen deprivation therapy, TT prescription before age 65 years | Age <40 years, <12 months continuous enrollment before index date or <60 days enrollment after index date, VTE or cancer in 12 months prior to index date, hospitalized <30 days or a prescription for anticoagulant <90 days before index event | Age <20 or >89 years, <2 years up-to-standard history in CPRD before index date, previous VTE | Warfarin use, history of DVT/PE, hypercoagulable state, or cancer. |
| Exposure/ Intervention | Incident TT prescription | Current TT exposure (Rx duration +90 washout period) | Incident TT prescription | Current TT exposure (Rx duration only) | Current TT exposure (Rx duration +30 day grace period) | Incident TT prescription |
| Outcome/Case Definition | Idiopathic VTE by ICD-9 codes and review | Idiopathic VTE by ICD-9 codes and review | Thrombotic events (including VTE) by chart review | VTE identified by ICD-9 codes plus anticoagulant or IVC filter | VTE identified by ICD-9 codes plus anticoagulant prescription | VTE identified by ICD-9 codes |
| Sample Size/ Comparison | 102,650 treated and 102,650 untreated Propensity score matched |
2,785 cases with idiopathic VTE vs 11,119 controls Matched on age and index date |
153 treated men and 64 untreated men with lower urinary tract symptoms | 7,643 cases with VTE and 22,424 controls Matched (1:3) on event/index month, age, geographic region, diagnosis of hypogonadism, and diagnosis of prothrombotic disorder |
19,215 cases with VTE, 909,530 controls Matched (1:50) on year of birth, known risk factors for VTE, history of cancer, and history of pathological hypogonadism |
Normal TT on treatment (n=38,362), Low TT on treatment (n=22,191), and untreated (n=10,854) |
| Analysis | Cox proportional hazard model | Conditional stepwise logistic regression model | Logistic regression | Multivariate conditional logistic regression | Multivariate conditional logistic regression | Cox proportional hazard models and propensity score (SIPTW) |
| Covariates Included for Multivariate Model | Age, infection(s), previous VTE, obesity, cardiovascular disorders, cancer, certain medication use. | Age, infection(s), previous VTE, obesity, cardiovascular disorders, cancer, certain medication use. | None | Covariates from the Elixhauser comorbidity index not balanced between the cases and controls and prescriptions for confounding medications | Baseline erythrocytosis, pulmonary disease, diabetes, CHF, MI, PVD, stroke, and history of prothrombotic disease | Age, body mass index, diabetes, CHF, and chronic kidney disease |
| Effect Estimate for VTE | Idiopathic VTE HR 1.08 (0.911.27) *Overall VTE HR 0.93 (0.851.03) |
Idiopathic VTE OR 1.02 (0.921.13) *Overall VTE OR 1.03 (0.971.09) |
Not reported | OR 0.90 (0.73–1.12) | Overall RR 1.25 (0.94-66); ≤6 months TT: RR 1.63 (1.12–2.37); >6 months TT: RR 1.00 (0.68–1.47) |
‘NorT’ vs untreated (HR 1.10 ( 0.78–1.54) ‘LowT’ vs untreated HR 1.14 ( 0.78–1.65) |
| Strengths | •Large sample size •Controlled for multiple important variables |
•Controlled for multiple important variables •Sensitivity analyses performed on exposure definition |
•Strong definition of hypogonadism |
•Large sample size •Controlled for multiple important variables •Sensitivity analyses performed on exposure definition |
•Large sample size •Controlled for multiple important variables •Population-based study •Analysis stratified by length of TT treatment •Sensitivity analyses performed on exposure definition •Ability to capture VTE events |
•Large sample size •Strong definition of hypogonadism |
| Limitations | •Two-thirds of TT treated not matched with propensity score • “Intent-totreat” analysis may bias results towards null •Confounding by indication for TT treatment •Study performed by Eli Lilly and Co. investigators |
•Study performed by Eli Lilly and Co. investigators | •Small sample size •Selected population may not generalizable •Unadjusted logistic regression model •Confounding by indication for TT treatment •Single center study |
•Due to exclusion criteria would not include fatal VTE •Excluded subjects with hospitalization <30 days from index date •Coauthor received funding from Eli Lilly and TestoRx |
•Fewer users of TT in the United Kingdom vs. United States3 | •Use of only ICD-9 definition for VTE less specific “Intent-to-treat” analysis may bias results towards null •Confounding by indication for TT treatment •Risk for VTE for subjects without baseline TT levels unknown •Limited number of covariates •Concern for exposure misclassification |
| Selection bias | Low ROB | Low ROB | Unclear | High ROB | Low ROB | Unclear |
| Comparability | Low ROB | Low ROB | Low ROB | Low ROB | Low ROB | Low ROB |
| Outcome assessment | Low ROB | Low ROB | Low ROB | Low ROB | Low ROB | Low ROB |
Previously unpublished data obtained from the original author.
Abbreviations: CHF= congestive heart failure, CPRD = Clinical Practice Research Datalink, HR = hazard ratio, ICD = International Classification of Diseases, IVC = inferior vena cava, LowT= low testosterone level on treatment, NorT= normal testosterone level on treatment, MI= myocardial infarction, PVD= peripheral vascular disease, ROB= Risk of bias, Rx = prescription, RR = risk ratio, SIPTW = stabilized inverse probability of treatment weights, TT= testosterone, VTE= venous thromboembolism
The 6 RCTs included a total of 2,236 men (Table 2). The mean age in all RCTs was greater than 50 years and follow-up ranged from 3 to 12 months. Five trials were performed in men with documented hypogonadism16,33,42–44 (by varying definitions—see Table 2). Five trials were double-blinded16,33,41–43 and compared testosterone to placebo and one was open-label and compared testosterone to routine care44. The study in men without a diagnosis of hypogonadism41 was performed in hospitalized men with alcohol-associated liver cirrhosis and compared oral micronized free testosterone to placebo. Brock et al published two manuscripts on the same set of patients, one reporting the initial double-blind RCT with 3 months of follow up47 and the other describing an open label 6-month extension within a subset of patients44. Only the open-label study reporting the longer follow up duration was included in our analysis. Patient exclusion criteria were extensive and varied significantly between RCTs, but no study specifically excluded patients with a history of VTE or a hypercoagulable condition. The risk of bias was overall moderate in this body of evidence. Specific risks of bias indicators are reported in table 1 for observational studies and table 2 for RCTs. One conference abstract was identified that did not show an association between testosterone and VTE in a population based study from British Columbia, Canada, but due to the inclusion criteria, was not included48.
Table 2:
Comparison of randomized control trials
| Author/Year | Copenhagen, 198641 | Marin, 199333 | Srinivas-Shankar, 201042 | Behre, 201243 | Brock, 201644 | Snyder, 201616 |
|---|---|---|---|---|---|---|
| Study Size | N=221 | N=31 | N=274 | N=362 | N=558 | N=790 |
| Mean age | 53 years | 58 years | 74 years | 62 years | 55 years | 72 years |
| Inclusion Criteria | Hospitalized men, daily ethanol consumption >50gm for >2 years, cirrhosis diagnosed by liver biopsy within 6 months |
Men age >40 years, abdominal obesity (WHR>0.9), BMI <35, serum total testosterone <20nmol/L (577 ng/dL), stable weight | Men ≥65 years, frailty, low morning total testosterone <345 ng/dL or free T <7.2 ng/dL | Men 50–80 years old, symptomatic hypogonadism, AMS score>36, total testosterone <430 ng/dL, free testosterone < 193 ng/dL | Men ≥18, 2 total testosterone levels <300 ng/dl, symptomatic hypogonadism | Men age >65 years, serum testosterone <275 ng/dL, symptoms of hypogonadism |
| Exclusion Criteria | Malignancy, Hepatitis infection, Klinefelter’s syndrome, unable to cooperate | Prostate enlargement or elevated PSA (>3.0ug/L), diabetes mellitus, hypertension, alcohol abuse | Prostate cancer, IPSS score >21, PSA >4ng/ml, creatinine >180mmol/liter, active liver disease, moderate to severe pad, severe COPD, CHF (NYHA ≥2), angina requiring nitrates, untreated sleep apnea, major psychotic illness, certain medications, stroke, MMSE score <18, active disease of muscle or joint | BMI>35kg/m2, PSA ≥4ng/mL, IPSS≥20, prostate cancer, hematocrit >50%, prolactin >25ng/mL, metallic implants, cytochrome P450 inducing medications, psychiatric disorders, uncontrolled diabetes mellitus, uncontrolled thyroid disorder, HTN, epilepsy, severe cardiac, hepatic, or renal insufficiency | Hemoglobin A1c>11%, BMI >37kg/m2, hematocrit >50%, active cancer, PSA>4ng/mL | History of prostate cancer, high risk of prostate cancer by Prostate Cancer Risk Calculator, an IPSS >19, conditions known to cause hypogonadism, medications that alter testosterone concentration, high cardiovascular risk, severe depression, “other conditions that would affect the interpretation of the results”. |
| Intervention | Micronized-free testosterone (600mg daily) (n=134) vs. placebo (n=87) | Testosterone gel vs. DHT gel vs. placebo gel | Testosterone gel (n=130) vs. placebo gel (n=132) | Testosterone gel (n=183) vs. placebo gel (n=179) | Topical 2% testosterone (n=283) vs. observation (n=275) | Testosterone gel (n=394) vs. placebo gel (n=394) |
| Masking | Double-blind | Double-blind | Double-blind | Double-blind | Open-label | Double-blind |
| Follow Up Duration | 3 years | 9 months | 6 months | 6 months | 6 months | 12 months |
| VTE events | Testosterone = 3 Placebo = 0 | Gel testosterone = 1 Gel DHT= 0 Placebo gel = 0 |
Testosterone = 1 Placebo = 0 | Testosterone = 1 Placebo = 0 | Testosterone = 2 Observation = 0 | Testosterone = 3 Placebo = 2 |
| Random sequence generation | Low ROB | Unclear | Low ROB | Low ROB | Low Risk | Low ROB |
| Allocation concealment | Low ROB | Unclear | Low ROB | Unclear | Unclear | Low ROB |
| Blinding of participants and personnel | Low ROB | Low ROB | Low ROB | Low ROB | High ROB | Low ROB |
| Blinding of outcome assessment | Low ROB | Unclear | Low ROB | Low ROB | Low ROB | Low ROB |
Abbreviations: AMS = Aging Males Symptoms, BMI = body mass index, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease, DHT = dihydrotestosterone, HTN= hypertension, IPSS = International Prostate Symptom Score, MMSE = Mini Mental Status Examination, Prostate Symptom Score, PSA = prostate antigen, ROB = risk of bias, WHR = waist-hip ratio
Meta-analysis results
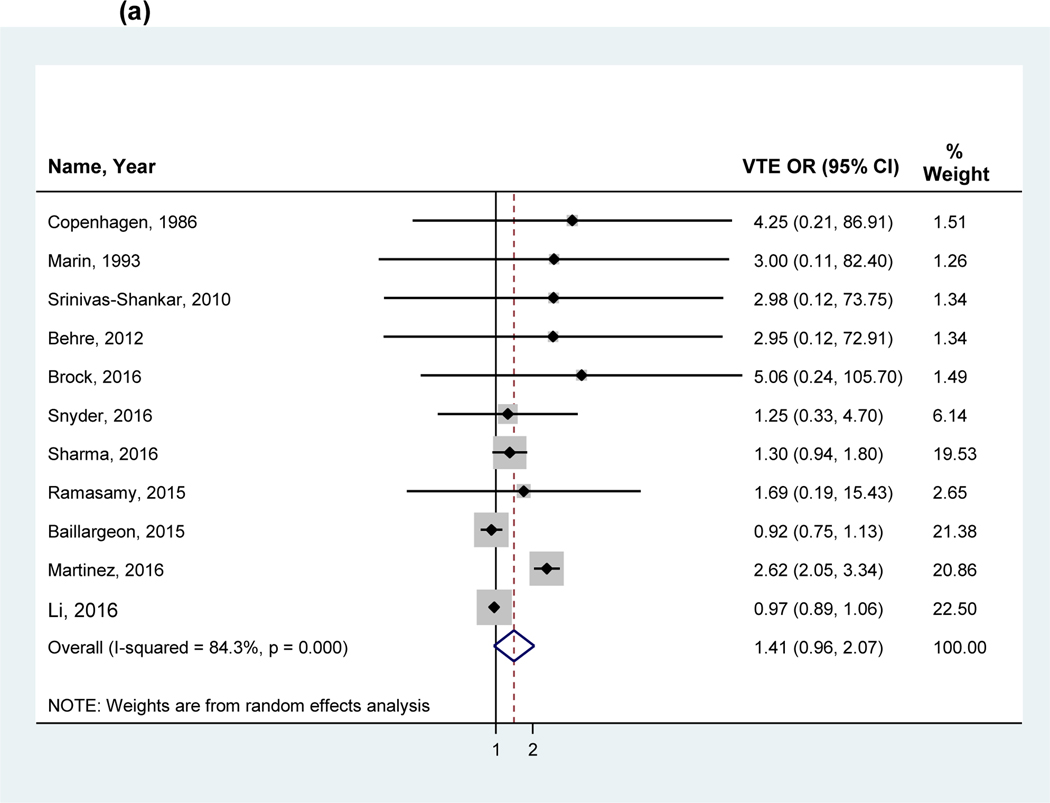
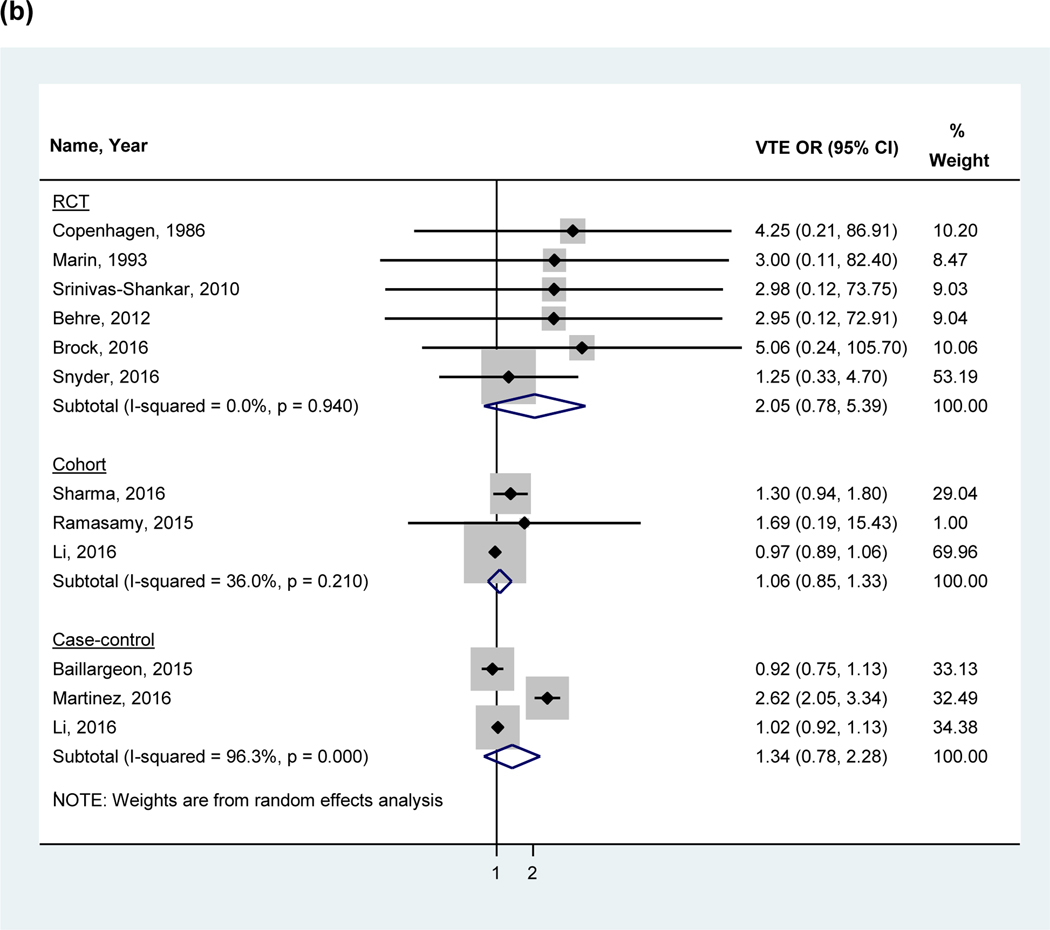
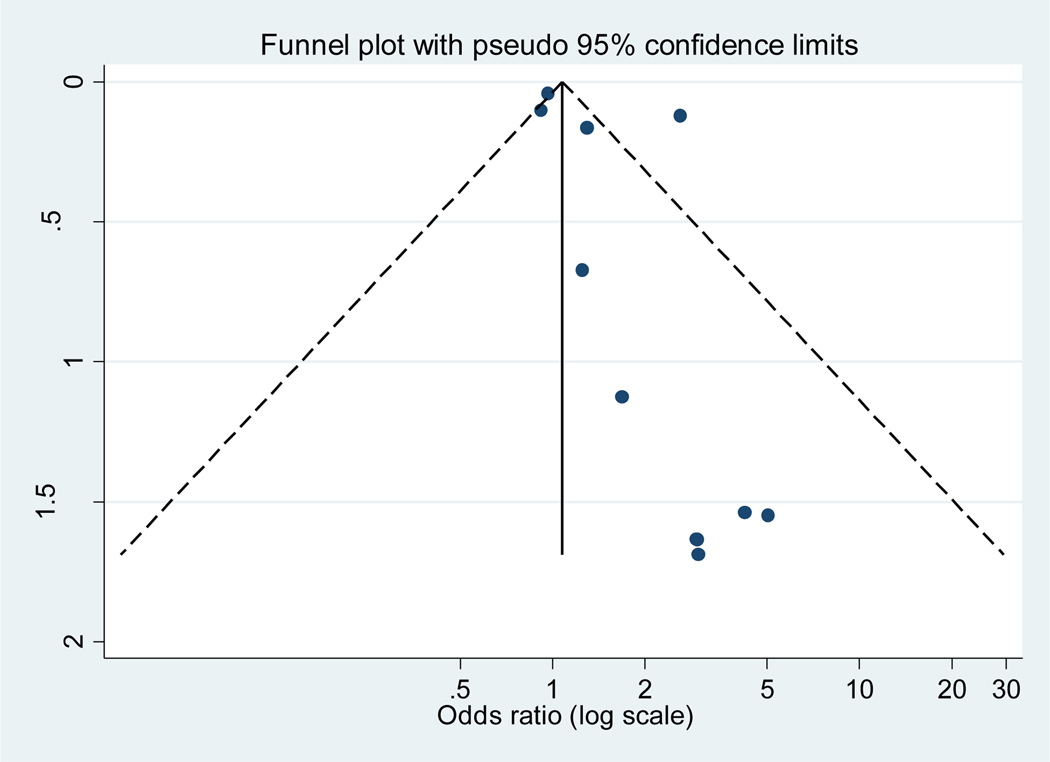
The overall pooled OR in a random effects model including all studies found no statistically significant association between VTE and testosterone use (OR 1.41, 95%CI 0.96–2.07, I2 = 84.4%; Figure 2a). The analyses were also stratified by study design: RCTs (2.05, 95%CI 0.78–5.39), observational studies (cohort: 1.06, 95%CI 0.85–1.33 and case-control studies; 1.34, 95%CI 0.78–2.28; Figure 2b). A sensitivity analysis performed using the adjusted odds ratio for studies performing multivariate adjustment also showed no significant association (OR 1:00, 95% CI: 0.93 to 1.08). The funnel plot analysis (Figure 3) demonstrated asymmetry.
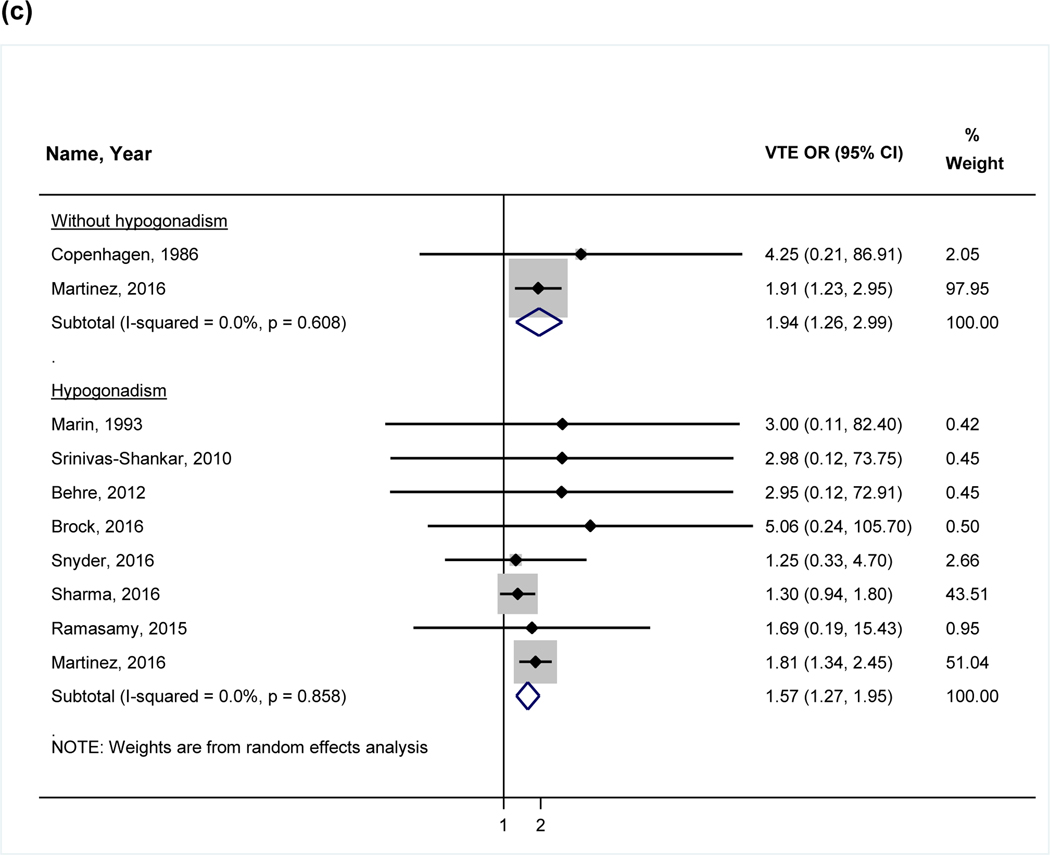
Figure 2:
Forest plot of the individual and pooled OR’s for venous thromboembolism (a) overall analysis (b) stratified by study design (c) stratified by hypogonadism
Note: Studies by Li et al38 and Baillargeon et al40 excluded from the stratified analysis for hypogonadism (c) due to the inclusion of a mixed patient population without stratification by hypogonadism. Abbreviations: VTE= venous thromboembolism, RCT= randomized control trial
Figure 3:
Funnel plot analysis
In recognizing that testosterone may be prescribed for conditions other than hypogonadism in men, we performed an additional analysis stratified by hypogonadism based on the individual definition of hypogonadism from each study (Figure 2c). The studies by Li et al and Baillargeon et al could not be included because stratified VTE outcomes were not reported and were not obtainable from the authors. In this analysis, testosterone was associated with VTE both in men with and without a diagnosis of hypogonadism (OR 1.57, 95% CI 1.27–1.95 vs. OR 1.94, 95% CI 1.26–2.99). There was not a difference between these two groups (p=0.39), suggesting no significant interaction (i.e., effect modification) between hypogonadism and VTE risk.
Discussion
This systematic review is the most comprehensive literature review on this topic and the meta-analysis including both RCTs and observational studies provide the best evidence available on the association between exogenous testosterone use in men and the risk for VTE. In the overall pooled OR of the 11 included studies, we did not find a significant association between testosterone and VTE. Results remained nonsignificant when using adjusted odds ratios. Two previous meta-analyses have examined VTE risk associated with testosterone use in RCTs. Xu et al45, examining only three RCTs, found an OR of 5.94 (95% CI 1.00–35.3)49. A more recent meta-analysis by Corona et al46 screened 2904 RCTs, and in the 6 studies included16,33,41–44, found that testosterone was associated with an OR of 1.9 for VTE (95% CI 0.75–5.17), but the results were not statistically significant. Our search for RCTs ultimately identified the same studies, and our results were similar (2.05, 95% CI 0.78–5.39).
Testosterone-associated VTE may be a consequence of poorly selected candidates such as men without hypogonadism or with significant comorbidities. The use of testosterone in patients without hypogonadism is an important population to study the potential risks of therapy. Only one RCT identified in our systematic literature review evaluated this patient population. In the Copenhagen study41, hospitalized men with alcoholic cirrhosis were randomized to micronized testosterone or placebo and when combined with patients without a diagnosis of hypogonadism from the Martinez et al study, a statistically significant association between testosterone and VTE was identified. In this stratified analysis, there was also a significantly increased OR for VTE in men with hypogonadism and the test for interaction did not demonstrate a significant difference in the association between the groups. This finding is discrepant from our overall analysis, which did not find a statistically significant association. Two of the observational studies could not be included in this additional analysis due to insufficient data, and therefore the significance of these findings compared to the overall analysis is unclear. It does demonstrate the consequences of a limited data pool and suggests that additional large studies could significantly influence the balance of the association. Notably, removal of studies that did not stratify their results by hypogonadism significantly reduced the heterogeneity between the remaining studies, indicating that confounding by this variable may have been present. The discrepancy between the overall analysis and stratified analyses also suggests the presence of other important confounders.
It is important to realize that even if no statistical difference in VTE has been identified in the meta-analysis of RCTs, the analyzed RCTs would not be able to detect significant differences in VTE given the limited number of patients studied. The available data is currently inadequate and should not be interpreted as “negative,” and in fact is potentially consistent with an increased risk. Assuming a baseline rate of VTE of approximately 30 per 10,000 person-years (for men 60–64 years old)50,51, identifying a significant risk ratio of 1.5 (RR=1.5) would require 15,613 subjects per group (testosterone and placebo). Thus, the currently available randomized studies may simply be underpowered to detect an increased VTE risk in testosterone users. Clinically, if a statistically significant VTE risk with testosterone were demonstrated in an adequately powered study, an RR of 1.5 – while possibly considered a “mild” VTE risk – could be clinically meaningful, as it would translate to one additional VTE event for every 400 men treated [number needed to harm (NNH)=400]. Oral contraceptive therapy in younger women, by point of comparison, is associated with a RR of 4.17 for VTE52, and a NNH of 1,048.
In general, subjects in RCTs tend to be healthier than average due to extensive exclusion criteria, have higher medication adherence rates, and have more frequent evaluations than those receiving routine care in observational studies and one might suspect lower rates of VTE in these trials. Well-designed observational studies could provide useful information on real-world outcomes, especially when data from RCTs is limited. Important differences in patient populations between RCTs and observational studies were observed in this review and important confounding variables were not uniformly assessed. One important difference between observational studies and RCTs was that observational studies largely excluded patients with a history of VTE. Another potential difference between randomized and observational studies is medication adherence. If testosterone treatment discontinuation is high in clinical practice, extended follow-up of patients in retrospective cohort studies who discontinued testosterone, but continue to contribute exposed person time, would potentially dilute the adverse events occurring in the continually exposed group (assuming adverse events are not late sequelae of treatment). Data does suggest that only 17% of new-users of testosterone continuously use testosterone for one full year, while 23% discontinue after the first prescription and 18% discontinue after the second prescription53. This could contribute to differences between randomized control trials and observational studies.
Route of administration
The route of testosterone administration has also been investigated regarding thrombotic risk because of inherent differences in pharmacokinetics. Intramuscular injection use is associated with higher peak and lower trough plasma drug concentrations, while transdermal gel and patch testosterone formulations provide more consistent daily levels. The testosterone market in the United States and the United Kingdom has been rapidly shifting toward gel formulations and away from injection and patch use3. No randomized control trial in our systematic review evaluated patients treated with intramuscular testosterone. Oral testosterone is infrequently prescribed in clinical practice but one RCT included in our review did use it.
One study has specifically compared the risk of VTE by route of testosterone administration (gel, patch, injection) in a new-user retrospective cohort54. Using multiple data sources (MarketScan, Medicare, Clinical Practice Research Datalink (CPRD)), the investigators identified 544,115 testosterone initiators and evaluated cardiovascular events (including VTE) for up to one year. Although an increase in cardiovascular and cerebrovascular events was found in injection users compared to gel users, no increased risk was found for VTE (HR 0.92, 95% CI 0.76–1.11). This finding is also consistent with four studies identified in this review2,11,38,40 that also did not find an association between VTE and any specific route of testosterone administration.
Limitations
Thousands of randomized control trials have been performed with various formulations of testosterone, but unfortunately, most have not specifically reported VTE outcomes. High heterogeneity was seen in the overall pooled OR, limiting the interpretation of the summary estimate. The safety data for randomized trials evaluating testosterone is limited to relatively short-term follow up (up to 12 months) and no RCTs included use of intramuscular injections of testosterone. Among the observational studies, differences in study design, covariates assessed, ability to control for confounding, varying lengths of follow up, and different criteria to assess VTE outcomes significantly limit definitive conclusions on the association between testosterone and VTE. The funnel plot demonstrated significant asymmetry which may represent publication bias, but the test is not reliable when the number of studies is small or when heterogeneity is present. Asymmetry could also indicate selective outcome or analysis reporting, poor methodologies, or true heterogeneity among the studies included.
The study by Martinez et al2 did find an increased risk of VTE when examining outcomes after an initial six months of treatment (RR 1.63, 95% CI 1.12–2.37), but not in the overall follow up data, potentially indicating a healthy user bias for more long-term users. We were not able to perform additional sensitivity analyses regarding duration of follow up. Erythrocytosis as it relates to VTE was not reported or not considered in most of the studies included in the analysis; therefore it remains unclear to what extent testosterone-induced erythrocytosis may be associated with VTE. Varying definitions of hypogonadism between studies could reduce the ability to determine differences between these groups in the stratified analysis. Confounding by indication is a major limitation of retrospective cohort studies that compare patients treated, versus not treated with testosterone. Additionally, the analyses performed as “intent-to-treat”, although ideal for preventing biased treatment effect measures, may bias safety data towards the null. No consensus exists on how to best manage patients with VTE occurring while taking testosterone55; therefore, we propose an approach based on the available evidence and observations from clinical practice (Figure 4).
Figure 4:
Conclusion
This systematic review and meta-analysis did not show a significant association between testosterone use and VTE in men. The analysis highlights the scarcity of high-quality research on this topic, preventing any definitive conclusions. Testosterone therapy remains a very active area of research and we urge all future clinical trials to specifically report VTE as an outcome. Additional observational studies will be critical to fully evaluate the risk of testosterone outside of clinical trials and these should focus on new-users of testosterone to identify time-varying hazards, capture early events, reduce healthy user bias, and correctly time covariate assessment. If an increase in VTE with testosterone is demonstrated in future studies, we must understand what groups are at the highest risk and if there are clinically apparent mediators of VTE that can be modified to minimize the risk.
Supplementary Material
Highlights:
The Food and Drug Administration warns against VTE in testosterone users.
6 RCTs and 5 observational studies examining testosterone and VTE are reviewed.
No significant association was found between testosterone and VTE.
Limited data from RCTs and heterogeneity in observational studies limit conclusions.
We conclude our review with 8 summarizing clinical management considerations.
Acknowledgements
Prior presentations:
Data from this manuscript was presented at The International Society of Thrombosis and Haemostasis conference in Dublin, Ireland in July, 2018.
Disclosure statement:
JBL is an employee of RTI International, an independent, non-profit research organization that performs contact work on behalf of government agencies and pharmaceutical companies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. [DOI] [PubMed] [Google Scholar]
- 2.Martinez C, Suissa S, Rietbrock S, et al. Testosterone treatment and risk of venous thromboembolism: population based case-control study. BMJ. 2016;355:i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99(3):835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gan EH, Pattman S, Pearce SHS, Quinton R. A UK epidemic of testosterone prescribing, 2001–2010. Clinical Endocrinology. 2013;79(4):564–570. [DOI] [PubMed] [Google Scholar]
- 5.Handelsman DJ. Pharmacoepidemiology of testosterone prescribing in Australia, 1992–2010. The Medical journal of Australia. 2012;196(10):642–645. [DOI] [PubMed] [Google Scholar]
- 6.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in Androgen Prescribing in the United States, 2001 to 2011. Jama Intern Medicine. 2013;173(15):1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TJ, Shores MM, Fox AE, et al. Recent trends in testosterone testing, low testosterone levels, and testosterone treatment among Veterans. Andrology. 2015;3(2):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen CP, Hirsch MS, Moeny D, Kaul S, Mohamoud M, Joffe HV. Testosterone and “Age-Related Hypogonadism” — FDA Concerns. New Engl J Medicine. 2015;373(8):689–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch MS NC, Joffe HV. https://wayback.archive-it.org/7993/20170405210613/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ReproductiveHealthDrug sAdvisoryCommittee/UCM412536.pdf. Accessed 3/30/2018. [Google Scholar]
- 10.Layton JB, Kim Y, Alexander GC, Emery SL. Association Between Direct-to-Consumer Advertising and Testosterone Testing and Initiation in the United States, 2009–2013. JAMA. 2017;317(11):1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma R, Oni OA, Chen G, et al. Association Between Testosterone Replacement Therapy and the Incidence of DVT and Pulmonary Embolism A Retrospective Cohort Study of the Veterans Administration Database. Chest. 2016;150(3):563–571. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. Testosterone Products: FDA/CDER Statement - Risk of Venous Blood Clots https://wayback.archive-it.org/7993/20170112164224/ http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm402054.htm. Accessed 3/30/2018. [Google Scholar]
- 13.FDA adding general warning to testosterone products about potential for venous blood clots. 6/19/2014; https://wayback.archive-it.org/7993/20171102213348/https://www.fda.gov/Drugs/DrugSafety/ucm401746.htm. Accessed 3/30/2018. [Google Scholar]
- 14.Fernández-Balsells M, Murad MH, Lane M, et al. Adverse Effects of Testosterone Therapy in Adult Men: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metabolism. 2010;95(6):2560–2575. [DOI] [PubMed] [Google Scholar]
- 15.Roy CN, Snyder PJ, Stephens-Shields AJ, et al. Association of Testosterone Levels With Anemia in Older Men: A Controlled Clinical Trial. JAMA Intern Med. 2017;177(4):480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of Testosterone Treatment in Older Men. New Engl J Medicine. 2016;374(7):611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and Polycythemia Secondary to Testosterone Replacement Therapy in the Aging Male. Sex Med Rev. 2015;3(2):101–112. [DOI] [PubMed] [Google Scholar]
- 18.Calof OM, Singh AB, Lee ML, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–1457. [DOI] [PubMed] [Google Scholar]
- 19.Freedman J, Glueck CJ, Prince M, Riaz R, Wang P. Testosterone, thrombophilia, thrombosis. Transl Res. 2015;165(5):537–548. [DOI] [PubMed] [Google Scholar]
- 20.Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone Therapy, Thrombophilia–Hypofibrinolysis, and Hospitalization for Deep Venous Thrombosis-Pulmonary Embolus. Clin Appl Thrombosis Hemostasis. 2013;20(3):244–249. [DOI] [PubMed] [Google Scholar]
- 21.Glueck CJ, Wang P. Testosterone therapy, thrombosis, thrombophilia, cardiovascular events. Metabolis. 2014;63(8):989–994. [DOI] [PubMed] [Google Scholar]
- 22.Glueck CJ, Goldenberg N, Budhani S, et al. Thrombotic events after starting exogenous testosterone in men with previously undiagnosed familial thrombophilia. Transl Res. 2011;158(4):225–234. [DOI] [PubMed] [Google Scholar]
- 23.Glueck CJ, Lee K, Prince M, Jetty V, Shah P, Wang P. Four Thrombotic Events Over 5 Years, Two Pulmonary Emboli and Two Deep Venous Thrombosis, When Testosterone-HCG Therapy Was Continued Despite Concurrent Anticoagulation in a 55-Year-Old Man With Lupus Anticoagulant. J Investigative Medicine High Impact Case Reports. 2016;4(3):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz H, Popov E, Bray N, Berman B. Mesenteric vein thrombosis caused by secondary polycythaemia from AndroGel. BMJ Case Rep. 2014;2014:bcr2014206023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The Tromso study. Haematologica. 2010;95(2):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Archives of internal medicine. 2002;162(10):1182–1189. [DOI] [PubMed] [Google Scholar]
- 27.Nadeem O, Gui J, Ornstein DL. Prevalence of Venous Thromboembolism in Patients With Secondary Polycythemia. Clin Appl Thromb-Hem. 2013;19(4):363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eischer L, Tscholl V, Heinze G, Traby L, Kyrle PA, Eichinger S. Hematocrit and the risk of recurrent venous thrombosis: a prospective cohort study. PLoS One. 2012;7(6):e38705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. European heart journal. 1999;20(5):344–353. [DOI] [PubMed] [Google Scholar]
- 30.Walton BL, Lehmann M, Skorczewski T, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129(18):2537–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshman KM, Kaplan B, Travison TG, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010;95(8):3955–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sih R, Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–1667. [DOI] [PubMed] [Google Scholar]
- 33.Mårin P, Holmtäng S, Gustafsson C, et al. Androgen Treatment of Abdominally Obese Men. Obesity Research. 1993;1(4):245–251. [DOI] [PubMed] [Google Scholar]
- 34.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91(11):2742–2747. [DOI] [PubMed] [Google Scholar]
- 35.Glueck CJ, Goldenberg N, Wang P. Thromboembolism peaking 3 months after starting testosterone therapy: testosterone-thrombophilia interactions. J Investig Med. 2018;66(4):733–738. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. 2011; http://training.cochrane.org/handbook. Accessed 3/30/2018. [Google Scholar]
- 37.Wells G, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2004; http://www.ohri.ca/programs/ clinical_epidemiology/oxford.asp. Accessed 3/30/2018. [Google Scholar]
- 38.Li H, Benoit K, Wang W, Motsko S. Association between Use of Exogenous Testosterone Therapy and Risk of Venous Thrombotic Events among Exogenous Testosterone Treated and Untreated Men with Hypogonadism. J Urol. 2016;195(4):1065–1072. [DOI] [PubMed] [Google Scholar]
- 39.Ramasamy R, Scovell J, Mederos M, Ren R, Jain L, Lipshultz L. Association between testosterone supplementation therapy and thrombotic events in elderly men. Urology. 2015;86(2):283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baillargeon J, Urban RJ, Morgentaler A, et al. Risk of Venous Thromboembolism in Men Receiving Testosterone Therapy. Mayo Clin Proc. 2015;90(8):1038–1045. [DOI] [PubMed] [Google Scholar]
- 41.Copenhagen. Testosterone treatment of men with alcoholic cirrhosis: A double-blind study. Hepatology. 1986;6(5):807–813. [PubMed] [Google Scholar]
- 42.Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of Testosterone on Muscle Strength, Physical Function, Body Composition, and Quality of Life in Intermediate-Frail and Frail Elderly Men: A Randomized, Double-Blind, Placebo-Controlled Study. The Journal of Clinical Endocrinology & Metabolism. 2010;95(2):639–650. [DOI] [PubMed] [Google Scholar]
- 43.Behre HM, Tammela TLJ, Arver S, et al. A randomized, double-blind, placebo-controlled trial of testosterone gel on body composition and health-related quality-of-life in men with hypogonadal to low-normal levels of serum testosterone and symptoms of androgen deficiency over 6 months with 12 months open-label follow-up. Aging Male. 2012;15(4):198–207. [DOI] [PubMed] [Google Scholar]
- 44.Brock G, Heiselman D, Knorr J, Ni X, Kinchen K. 9-Month Efficacy and Safety Study of Testosterone Solution 2% for Sex Drive and Energy in Hypogonadal Men. J Urol. 2016;196(5):1509–1515. [DOI] [PubMed] [Google Scholar]
- 45.Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corona G, Dicuio M, Rastrelli G, et al. Testosterone treatment and cardiovascular and venous thromboembolism risk: what is ‘new’? Journal of Investigative Medicine. 2017;65:964–973. [DOI] [PubMed] [Google Scholar]
- 47.Brock G, Heiselman D, Maggi M, et al. Effect of Testosterone Solution 2% on Testosterone Concentration, Sex Drive and Energy in Hypogonadal Men: Results of a Placebo Controlled Study. The Journal of Urology. 2016;195(3):699–705. [DOI] [PubMed] [Google Scholar]
- 48.Flannigan R, Locke J, Tavakoli H, et al. Pulmonary emboli in the setting of testosterone therapy. a nested case control study among 40,081 testosterone users. Journal of Urology. 2018;199 (4 Supplement 1):e1022. [Google Scholar]
- 49.Xu L, Schooling CM. Differential risks in men and women for first and recurrent venous thrombosis: the role of genes and environment: comment. J Thromb Haemost. 2015;13(5):884–886. [DOI] [PubMed] [Google Scholar]
- 50.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. Journal of thrombosis and thrombolysis. 2016;41(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. [DOI] [PubMed] [Google Scholar]
- 52.Lidegaard O, Lokkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Layton JB, Li D, Sharpless JL, Stürmer T, Brookhart MA. Patterns of Testosterone Persistence and Switching Among Commercially-Insured Men in the United States. Pharmacoepidemiology & Drug Safety. 2015;24(S1):499. [Google Scholar]
- 54.Layton JB, Meier CR, Sharpless JL, Sturmer T, Jick SS, Brookhart MA. Comparative Safety of Testosterone Dosage Forms. JAMA Intern Med. 2015;175(7):1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moll S. Testosterone Therapy: Risk Factor for Venous Thromboembolism? The Hematologist: ASH News and Reports. 2017;14(2). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.