Abstract
Objective
The aim of this work was to study sex differences in major bleeding risk in relation to dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) for acute coronary syndrome (ACS).
Methods and Results
The Rijnmond Collective Cardiology Research registry was designed to evaluate the application and outcomes of DAPT after ACS/PCI in the Rijnmond region in the Netherlands. Overall, 1,172 women (median age 67.5 years) and 3,087 men (median age 62.2 years) with ACS/PCI were enrolled between August 2011 and June 2013. Based on a tailored regional DAPT guideline aiming at bleeding risk minimization, 52.6% women and 66.9% men received prasugrel as first-choice P2Y12 inhibitor, in addition to aspirin. Women more frequently had contraindications for the use of prasugrel (and therefore received clopidogrel) than men (47.9 vs. 26.9%, p < 0.001). Femoral access was more common in women than in men (47.6 vs. 38.1%, p < 0.001). Women had higher incidence of major bleeding at 1 year than men (2.6 vs. 1.6%, p = 0.018). After adjustment for established bleeding risk factors, female sex was associated with over two-fold higher risk of major bleeding (adjusted hazard ratio 2.33; 95% confidence interval 1.26–4.32). This difference was apparent at discharge and appeared to be caused by access site bleedings (0.9 vs. 0.1%, p < 0.001). No sex differences were found in non-access site-related major bleeding up to 1 year.
Conclusion
Women with ACS/PCI receiving DAPT had higher major bleeding risk caused by an excess in access site bleeds, mainly in relation to the femoral approach.
Keywords: Acute coronary syndrome/non ST-segment elevation myocardia infarction, Antiplatelet therapy, Bleeding, Vascular access complications, Percutaneous coronary intervention
Highlights of the Study
-
•
This study found a two-fold higher risk of major bleeding in women, as compared to men, in subjects with acute coronary syndrome undergoing percutaneous coronary intervention and treated with dual antiplatelet therapy.
-
•
This sex difference was caused mainly by an excess femoral access site bleeds in women.
-
•
No differences in the incidence of ischemic events between women and men were found.
Introduction
The combination of aspirin and a P2Y12 receptor antagonist is the cornerstone of modern treatment of acute coronary syndrome (ACS) [1,2]. This so-called dual antiplatelet (DAPT) strategy is associated with reduced risk of coronary thrombosis after stent placement, albeit at the cost of increased risk of bleeding [3,4]. Women presenting with ACS are older and have a worse cardiovascular risk profile than men [5], which predisposes women to an increased risk of thrombotic and bleeding events following invasive therapy.
Current guidelines, mainly based on randomized controlled trials (RCTs), do not provide sex-specific recommendations on the application of antithrombotic treatment [1,2]. Previous RCTs showed no convincing evidence of sex-related differences in the efficacy and safety of currently available P2Y12 receptor antagonists [6]. Still, it should be realized that RCTs are highly selected and have a lower risk profile than real-world populations, which may limit the external validity of the RCT. Registry studies of daily life settings may provide further evidence, based on representative data with less selected patients and with a more balanced inclusion of both sexes. Against this background, we explored the prospective Rijnmond Collective Cardiology Research (CCR) registry of ACS patients in the Netherlands undergoing percutaneous coronary intervention (PCI) [7]. We studied sex disparities in bleeding complications up to 1 year after the index procedure in patients who received aspirin in combination with prasugrel or clopidogrel according to a tailored region-wide guideline, aiming at bleeding risk minimization.
Subjects and Methods
Study Design and Population
Three high-volume centers with capability for PCI and eight non-PCI hospitals in the Rijnmond region serve a population of 1.5 million. The collaborating cardiologists in the region developed a dedicated guideline for the implementation of modern DAPT in patients with non-ST-segment elevation ACS or ST-segment elevation myocardial infarction undergoing PCI, aimed at bleeding risk minimization. According to the guideline, patients were to receive aspirin and prasugrel (loading dose 60 mg, maintenance dose 10 or 5 mg daily) as first-line option for antiplatelet therapy, unless contraindicated. If any contraindications were present, clopidogrel (loading dose 600 g, maintenance dose 75 mg daily) was advised. Contraindications for prasugrel were based on results of the TIMI-TRITON 38 trial and included a history of stroke or transient ischemic attack (TIA), advanced age (≥75 years), and underweight (<60 kg) [4]. The regional guideline was intended as a working arrangement to standardize local care and practice within the context of the European Society of Cardiology (ESC) practice guideline recommendations but did not dictate treatment [8].
The CCR study is a prospective, observational registry that is designed to evaluate the management practices and outcomes of ACS/PCI patients after the introduction of the regional DAPT guideline. During August 2011 and June 2013, all patients aged 18 years or older, who were diagnosed with non-ST-segment elevation ACS or ST-segment elevation myocardial infarction, and who underwent PCI with stent placement during the index hospitalization, were enrolled.
Data Collection
Baseline patient and procedural characteristics, concomitant antithrombotic pharmacotherapy, and in-hospital outcomes were extracted from the medical charts and entered into a secure web-based and centralized database by enrolling site personnel. After the index hospitalization, patients were routinely followed up at 1 month and 12 months at the outpatient clinic, where longitudinal information on patient treatment, effectiveness, and safety outcomes was collected and entered into the central database.
Study Endpoints
The main endpoint of the present analysis was the 1-year incidence of thrombolysis in myocardial infarction (TIMI) major bleeding episodes unrelated to coronary artery bypass grafting. This included any intracranial bleeding, clinical overt signs of hemorrhage associated with a decrease in hemoglobin of ≥5 g/dL, and fatal bleeding (bleeding that directly results in death within 7 days) [9,10]. TIMI major bleeds during the index hospitalization and at 30 days were the key secondary endpoints. We also report on (the composite of) ischemic events, including nonfatal myocardial infarction (MI), stent thrombosis, and ischemic stroke, and on (cardiac) death [9].
When a study endpoint was reached, relevant documents, including hospital discharge summaries, procedural reports, or angiographic films, were obtained. These documents were evaluated by an independent clinical event committee, which had adjudicated the endpoints reported in this study. Clinical follow-up was available for 1,111/1,172 (94.8%) women and (95.7%) men at 1 year. The follow-up of all-cause mortality, assessed using municipal civil registries, was 100% complete.
Statistical Analysis
Continuous variables are presented as both means ± standard deviation and medians with interquartile ranges. Categorical variables are presented as counts and percentages. Differences between women and men were studied by Student T tests (normal distribution) or Mann-Whitney tests (skewed distribution). Differences in categorical variables were studied by χ2 tests or Fisher’s exact tests in case any expected value was less than 5. The incidences of the study endpoints are reported as Kaplan-Meier estimates. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were removed from the study. Differences between women and men were evaluated using log-rank tests.
Multivariate Cox proportional hazards regression was applied to obtain an estimate of the relationship between sex and TIMI major bleed, adjusted for factors that have been related with bleeding in previous studies. We considered age, weight, diabetes, history of stroke or TIA, history of peripheral artery disease, access site, and estimated glomerular filtration rate (Cockcroft-Gault) as potential confounders. Further, we considered type of P2Y12 inhibitor as potential effect modifier (by including the sex* P2Y12 inhibitor interaction). A separate multivariable analysis was performed in non-access site-related TIMI major bleeding to control for oral anticoagulation with vitamin K antagonists and elected P2Y12 inhibitors (prasugrel vs. clopidogrel) at discharge. We report adjusted hazard ratios (aHRs) with corresponding 95% confidence intervals (CIs). A two-sided p value <0.05 was considered statistically significant. Analyses were performed using SPSS version 22 (SPSS, Inc., Chicago, IL, USA).
Results
Patient Characteristics
In total, 1,172 consecutive women and 3,087 men were registered. Women were on average 5.3 years older, had a higher prevalence of diabetes mellitus (20.2 vs. 15.7%, p < 0.001), hypertension (64.0 vs. 46.9%, p < 0.001), and a history of stroke or TIA (8.8 vs. 6.6%, p = 0.014), but were less often smokers (29.2 vs. 38.5%, p < 0.001) and less often had a history of MI (14.6 vs. 19.7%, p < 0.001) than men (Table 1). Fewer women than men had coronary angiography by radial access (47.3 vs. 57.0%, p < 0.001).
Table 1.
Patient baseline characteristics according to sex
| Women (n = 1,172) | Men (n = 3,087) | p value | |
|---|---|---|---|
| Risk factors and cardiovascular history | |||
| Age, years | 67.5 (12.1), 68.0 (59.0–77.0) | 62.2 (12.0), 62.0 (53.0–71.0) | <0.001 |
| Age ≥75 years | 384 (32.8) | 531 (17.2) | <0.001 |
| Weight, kga | 73.9 (13.9), 72.0 (65.0–80.0) | 86.2 (15.0), 84.0 (77.0–94.0) | <0.001 |
| Weight <60 kg | 100/908 (11.0) | 35/2,438 (1.4) | <0.001 |
| Diabetes mellitus | 236/1,166 (20.2) | 481/3,057 (15.7) | <0.001 |
| Hypertension | 743/1,161 (64.0) | 1,420/3,029 (46.9) | <0.001 |
| Hypercholesterolemia | 431/1,143 (37.7) | 1,128/3,006 (37.5) | 0.91 |
| Current smoking | 332/1,138 (29.2) | 1,150/2,984 (38.5) | <0.001 |
| Family history of CAD | 462/1,083 (42.7) | 1,290/2,870 (44.9) | 0.20 |
| Prior MI | 170/1,163 (14.6) | 604/3,060 (19.7) | <0.001 |
| Prior PCI | 179/1,166 (15.4) | 584/3,071 (19.0) | 0.006 |
| Prior CABG | 53/1,167 (4.5) | 186/3,075 (6.0) | 0.057 |
| Prior CVA or TIA | 103/1,164 (8.8) | 204/3,070 (6.6) | 0.014 |
| PAD | 93/1,160 (8.0) | 212/3,060 (6.9) | 0.22 |
| History of heart failure | 38/1,161 (3.3) | 124/3,063 (4.0) | 0.24 |
| eGFR, mL/minb | 116.6 (51.6), 107.7 (81.6–142.5) | 101.4 (40.1), 97.9 (74.8–123.1) | <0.001 |
| Procedure | |||
| Access site | |||
| Radial access | 521/1,102 (47.3) | 1,682/2,950 (57.0) | <0.001 |
| Femoral access | 525/1,102 (47.6) | 1,125/2,950 (38.1) | |
| Other | 56/1,102 (5.1) | 143/2,950 (4.8) | |
| Multivessel PCI | 166/1,118 (14.8) | 463/2,965 (15.6) | 0.55 |
| Hospitalization | |||
| Time admission – PCI, days | 1.8 (3.6), 0.0 (0.0–3.0) | 1.7 (7.6), 0.0 (0.0–2.0) | 0.076 |
| Time admission – discharge, days | 5.8 (7.0), 4.0 (3.0–7.0) | 5.3 (8.7), 4.0 (3.0–6.0) | 0.012 |
| Discharge diagnosis | |||
| STEMI | 245/1,172 (20.9) | 580/3,087 (18.8) | 0.19 |
| NSTEMI | 436/1,172 (37.2) | 1,134/3,087 (36.7) | |
| Unstable angina | 491/1,172 (41.9) | 1,373/3,087 (44.5) | |
Continuous variables are presented as both means ± standard deviation (SD) and medians with interquartile ranges (IQR). Categorical variables are presented as counts and percentages.
CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CVA, cerebrovascular accident; TIA, transient ischemic accident; PAD, peripheral artery disease; mL, milliliter; eGFR, estimated glomerular filtration rate (calculated with Cockcroft-Gault formula); (N)STEMI, (non) ST elevation myocardial infarction.
aData available for 908 women and 3,346 men.
bData available for 852 women and 2,291 men.
Antiplatelet Therapy
The prescription of DAPT was comparable at discharge in women and men and remained similar up to 1 year (Table 2). Women more frequently had contraindications for the use of prasugrel than men (47.9 vs. 26.9%, p < 0.001), and, consequently, women less often received prasugrel (52.6 vs. 66.9%, p < 0.001). Among the patients without contraindications, fewer women than men received prasugrel (74.0% vs. 79.4%, p = 0.008), whereas among those with contraindications, a similar percentage of women and men were nevertheless treated with prasugrel (23.2 vs. 23.8, p = 0.82).
Table 2.
Medication according to sex
| Women (n = 1,172) | Men (n = 3,087) | p value | |
|---|---|---|---|
| Medication peri-/postprocedural | |||
| Aspirin | 950/1,118 (85.0) | 2,619/2,965 (88.3) | 0.004 |
| P2Y12 inhibitor | 967/1,118 (86.5) | 2,647/2,965 (89.3) | 0.012 |
| Clopidogrel | 439/1,118 (39.3) | 915/2,965 (30.9) | <0.001 |
| Prasugrel | 528/1,118 (47.2) | 1,732/2,965 (58.4) | <0.001 |
| GP IIb/IIIa inhibitor | 119/1,118 (10.6) | 468/2,965 (15.8) | <0.001 |
| LMWH | 39/1,118 (3.5) | 72/2,965 (2.4) | 0.063 |
| Medication at discharge | |||
| Aspirin | 1,072/1,172 (91.5) | 2,857/3,087 (92.5) | 0.24 |
| P2Y12 inhibitor | 1,126/1,172 (96.1) | 2,985/3,087 (96.7) | 0.32 |
| Clopidogrel | 508/1,172 (43.3) | 912/3,087 (29.5) | <0.001 |
| Prasugrel | 617/1,172 (52.6) | 2,065/3,087 (66.9) | <0.001 |
| Prasugrel dose | |||
| 5 mg | 80/617 (13.0) | 102/2,065 (4.9) | <0.001 |
| 10 mg | 537/617 (87.0) | 1,963/2,065 (95.1) | |
| Ticagrelor | 1/1,172 (0.1) | 8/3,087 (0.3) | 0.27 |
| Vitamin K antagonist | 111/1,172 (9.5) | 248/3,087 (8.0) | 0.13 |
| Triple therapya | 59/1,172 (5.0) | 134/3,087 (4.3) | 0.33 |
| Medication 1-year follow-up | |||
| Aspirin | 927/1,057 (87.7) | 2,420/2,739 (88.4) | 0.58 |
| P2Y12 inhibitor | 844/1,057 (79.8) | 2,220/2,739 (81.1) | 0.40 |
| Clopidogrel | 399/1,057 (37.7) | 694/2,739 (25.3) | <0.001 |
| Prasugrel | 443/1,057 (41.9) | 1,514/2,739 (55.3) | <0.001 |
| Prasugrel dose | |||
| 5 mg | 47/443 (10.6) | 59/1,514 (4.0) | <0.001 |
| 10 mg | 396/443 (89.4) | 1,455/1,514 (96.1) | |
| Ticagrelor | 2/1,057 (0.2) | 12/2,739 (0.4) | 0.26 |
| Vitamin K antagonist | 114/1,057 (10.8) | 261/2,739 (9.5) | 0.25 |
| Triple therapya | 32/1,057 (3.0) | 63/2,739 (2.3) | 0.20 |
Categorical variables are presented as counts and percentages.
GP, glycoprotein; LMWH, low-molecular-weight heparin; mg, milligram.
aThe combination of aspirin, a P2Y12 inhibitor, and vitamin K antagonist.
TIMI Major Bleeding
A larger percentage of women experienced TIMI major bleeding events than men (2.6 vs. 1.6%, p = 0.018) up to 1-year follow-up. This difference was apparent at discharge and was mainly driven by access site bleedings (0.9 vs. 0.1%, p < 0.001) (Table 3; Fig. 1). In the period around the intervention, patients with a TIMI major bleeding were more often treated with clopidogrel (43.0 vs. 33.0%, p = 0.060) and LMWH (6.3 vs. 2.6%, p = 0.046), but less often with prasugrel (40.5 vs. 55.6%, p = 0.017). Also, they less often received aspirin (78.5 vs. 92.5%, p < 0.001) and any P2Y12 inhibitor (87.3 vs. 96.7%, p < 0.001) at discharge but more often vitamin K antagonists (17.7 vs. 8.3%, p = 0.003). The use of prasugrel, despite contraindications at discharge, led to a higher absolute number of bleeding events up to 1 year in women (6.2%) than in men (1.2%), although this difference did not reach statistical significance (p = 0.15). In patients without contraindications, women treated with prasugrel had more bleedings than men (2.0 vs. 0.8%, p < 0.001). We found a significant sex*P2Y12Type interaction (p value for interaction = 0.002) in multivariate analysis. Women treated with prasugrel experienced relatively more often a major bleeding within 1-year follow-up than men treated with prasugrel.
Table 3.
TIMI major bleeding and secondary outcomes according to sex
| Women (n = 1,172) | Men (n = 3,087) | p valuea | |
|---|---|---|---|
| Outcome at discharge | |||
| TIMI major bleeding | 16 (1.4) | 21 (0.7) | 0.057 |
| Bleeding class | |||
| Access site bleeding | 7 (0.6) | 3 (0.1) | 0.006 |
| GI bleeding | 2 (0.2) | 5 (0.2) | 0.98 |
| Intracranial bleeding | 0 (0.0) | 3 (0.1) | 0.27 |
| Other bleeding | 7 (0.6) | 10 (0.3) | 0.25 |
| Combined ischemic endpointb | 17 (1.5) | 32 (1.0) | 0.56 |
| MI | 5 (0.4) | 17 (0.6) | 0.48 |
| CV death | 26 (2.2) | 79 (2.6) | 0.30 |
| Death | 31 (2.6) | 87 (2.8) | 0.47 |
| Outcome at 30 days | |||
| TIMI major bleeding | 19 (1.6) | 30 (1.0) | 0.075 |
| Bleeding class | |||
| Access site bleeding | 8 (0.7) | 4 (0.1) | 0.002 |
| GI bleeding | 3 (0.3) | 9 (0.3) | 0.85 |
| Intracranial bleeding | 1 (0.1) | 6 (0.2) | 0.43 |
| Other bleeding | 7 (0.6) | 11 (0.4) | 0.28 |
| Combined ischemic endpointb | 20 (1.7) | 46 (1.5) | 0.61 |
| MI | 6 (0.5) | 23 (0.7) | 0.41 |
| CV death | 29 (2.5) | 88 (2.9) | 0.51 |
| Death | 35 (3.0) | 96 (3.1) | 0.84 |
| Outcome at 1 year | |||
| TIMI major bleeding | 31 (2.6) | 48 (1.6) | 0.018 |
| Bleeding class | |||
| Access site bleeding | 11 (0.9) | 4 (0.1) | <0.001 |
| GI bleeding | 5 (0.4) | 16 (0.5) | 0.70 |
| Intracranial bleeding | 3 (0.3) | 13 (0.4) | 0.43 |
| Other bleedingc | 12 (1.0) | 15 (0.5) | 0.048 |
| Combined ischemic endpointb | 39 (3.3) | 91 (2.9) | 0.52 |
| MI | 13 (1.1) | 55 (1.8) | 0.12 |
| CV death | 44 (3.8) | 106 (3.4) | 0.62 |
| Death | 61 (5.2) | 132 (4.3) | 0.20 |
Categorical variables are presented as counts and percentages.
TIMI, thrombolysis in myocardial infarction; GI, gastrointestinal; CV, cardiovascular.
aKaplan-Meier estimates, log-rank (Mantel-Cox).
bComposite of myocardial infarction, stent thrombosis, and ischemic stroke.
cOther bleedings included pericardial effusion (n = 9), posttraumatic bleeding (including post-resuscitation [n = 3]), postoperative bleeding (n = 2), epistaxis (n = 1), spleen bleeding (n = 1), bleeding due to dislocation of wire (n = 1), or bleedings with unknown origin (n = 10).
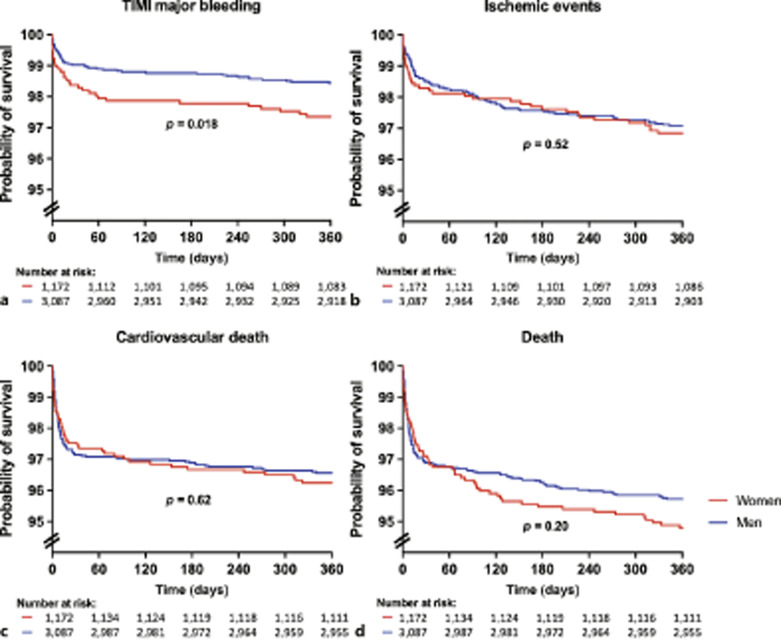
Fig. 1.
Outcome at 1-year follow-up according to sex. Kaplan-Meier event-free survival estimates for various endpoints in women (red) and men (blue). p values are based on log-rank tests.
Besides sex, advanced age, current smoking, a previous history of stroke, or peripheral artery disease, a lower estimated glomerular filtration rate and coronary angiography by femoral approach instead of radial approach were associated with TIMI major bleed in our dataset. After adjustment for these potential confounders, women had more than two-fold higher risk of TIMI major bleeding at 1-year follow-up than men (aHR 2.33 and 95% CI: 1.26–4.32, p = 0.007). However, an increased risk in women was not observed for non-access site-related TIMI major bleeding (aHR 1.70, 95% CI: 0.83–3.48, p = 0.139).
Other Endpoints
We found no differences between women and men in the incidence of ischemic events, neither at discharge (1.5 vs. 1.0%, p = 0.56) nor at 1-year follow-up (3.3 vs. 2.9%, p = 0.52). All-cause mortality was 2.6 versus 2.8% (p = 0.47) in women and men, respectively, and remained similar (5.2 vs. 4.3%, p = 0.20) at 1-year follow-up.
Discussion
In this real-world registry of PCI-treated ACS patients who received APT according to a tailored region-wide guideline, and with the aspirin/P2Y12 inhibitor combination as a cornerstone, women experienced more access site TIMI major bleedings than men. However, no sex differences in bleedings were seen after the procedure until 1-year follow-up, despite differences in bleeding risk factors. Thus, while applying our tailored bleeding risk minimization strategy, the prolonged use of DAPT appears equally safe in women and men. Importantly, no differences were observed in ischemia-related endpoints between women and men, whereas event rates were low overall.
Previous registries and RCTs report incidences of major bleeding across the broad spectrum of ACS, which range from 1.8 to 6.1% [4, 11–15]. Hence, the observed incidence of major bleeding in our region (according to the TIMI definition) was at the lower end of the spectrum. The Rotterdam/Rijnmond APT guideline aimed at intensive platelet inhibition by the P2Y12 inhibitor prasugrel in patients with a supposedly low bleeding risk, and a less intensive inhibition (by clopidogrel) in their counterparts with high-risk features, as revealed by the TIMI-TRITON 38 investigators [4]. Apparently, that strategy of selective prescription, which was also observed in recent registries [16,17] although not in relation to a specific guideline, has been successful.
Despite the low overall incidence, women experienced more bleeds than men. This observation is in agreement with other studies (registries as well as clinical trials) in patients with ACS [11, 18, 19] and has been explained by their higher age and prevalence of other risk factors for bleeding. However, that explanation is not fully sufficient, as we still revealed sex as a risk determinant after adjustment for these factors. In this respect, it is relevant to note that the excess in bleeding complications in women was driven by access site bleeds. Indeed, higher numbers of access site bleedings in women than in men have been reported repeatedly [20,21]. A study by Pandie et al. [22] showed that major vascular complications were significantly reduced by the radial approach compared with the femoral approach, regardless of whether PCI was performed. This favorable effect was observed in both genders and was even more prominent in women [22]. However, the radial arteries of females are smaller and more vulnerable to spasm, which adds to operative difficulty and may undermine the efficacy of transradial PCI. As a result, the transradial approach is paradoxically low in women. Also, in our registry, women were more often treated via the femoral approach than men, probably because of failed access or because the operator feared arterial spasm and wanted to avoid access site crossover; the exact reason was not documented [23]. Nevertheless, our findings suggest that radial access must be considered the first option even in women, if technically possible. Recent guidelines also recommend radial access over femoral access for coronary angiography and PCI if performed by an experienced radial operator [1,2].
Inappropriate dosing of antithrombotic drugs is a risk factor for bleeding. Alexander et al. [24] demonstrated that women – who generally have lower body volume – are more likely to receive excess doses than men. If dosage reduction is possible without nullifying the anti-ischemic effect, bleeding risk complications around the procedure in women might be prevented. Currently, there are no randomized trials that implemented sex-based dosage recommendations. The tailored CCR guideline recommended clopidogrel or low-dose prasugrel (5 mg) in patients with body weight <60 kg. We found no relation between body weight and bleeding risk, neither in men nor in women. Apparently, the weight criterion is appropriate and needs no modification.
Despite the absence of contraindications, women were treated less often with the more potent prasugrel. This is in line with findings of the START ANTIPLATELET registry which also showed that P2Y12 inhibitor choice is influenced by sex [25]. The reason for the lower prescription in our study is unclear. A higher bleeding risk in women in relation to P2Y12 inhibitor treatment has been reported earlier in a meta-analysis by Lau et al. [26], and one might speculate therefore that medical doctors are somewhat reluctant to prescribe DAPT in women. On the other hand, it is equally important to protect women from ischemic complications, and the same meta-analysis described comparable protective effects of P2Y12 inhibitors with regard to ischemic events in women and men. Careful selection of women might lead to lower ischemic risks in women than in men when using more potent P2Y12 inhibitors, without the risk of increased bleeding. This hypothesis cannot be confirmed with currently available studies as many studies have several limitations for a fair comparison. A sex-specific analysis of the ISAR-REACT 5 trial showed a superior efficacy of prasugrel with respect to MI and a composite endpoint of ischemic events in men than in women [27]. However, these differences were explained by the underrepresentation of women in the study and the lower number of women who underwent PCI because of non-obstructive CAD and resulting in a lower prescription of prasugrel at discharge. Other studies also found lower risk reduction percentages for clopidogrel and prasugrel in women compared to men but should be carefully interpreted in te light of limitations in these studies [28,29].
While the CCR registry was successful in monitoring the application of an APT treatment guideline in a broad range of practices within the Rotterdam/Rijnmond region, in the Netherlands, the registry has some limitations with respect to the current analysis. First, although encouraging from patient’s point of view, the low bleeding rates resulted in insufficient statistical power to control the sex-bleeding relation for a broad range of clinicopathological variables. Consequently, residual confounding might be present. Second, cause and timing of bleeding are important components of the TIMI definition but in practice can be rather vague. For example, we found some cases with a decrease in hemoglobin but without clinically overt signs. Third, we have insufficient data to analyze the effect of inappropriate dosing of antithrombotic drugs in our study. This data might not accurately reflect contemporary practice in other countries, as this registry was conducted in 2011–2013 in only one country, and the practice has likely changed over time (e.g., use of anticoagulants and invasive management) and is different across countries.
Conclusion
The higher TIMI major bleeding risk in women with ACS/PCI receiving aspirin/P2Y12 inhibitor-based DAPT was largely caused by an excess in access site bleeds. Women were more often treated via the femoral artery, and bleeds are thus more common. Hence, our registry confirms that the access site in women with ACS who need PCI under DAPT should be carefully considered. Our registry also showed that, if implemented according to a tailored strategy, prolonged DAPT will result in similarly low rates of ischemic and bleeding events in women and men.
Acknowledgements
The authors acknowledge the staff at all hospitals participating in the CCR collaboration.
Statement of Ethics
Patients were not subjected to acts or be imposed to any mode of behavior, other than their regular treatment. Therefore, according to Dutch law, written informed consent was not required. This study was conducted according to the Privacy Policy of the Erasmus MC and according to the Erasmus MC regulations for the appropriate use of data in patient-oriented research and is approved by the Regional Ethics Committee (reference # MEC-2010-417).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the Eli-Lilly Nederland B.V. and Daiichi Sankyo Nederland B.V. Other than providing financial support, the sponsors were not involved in study design, study management, or data interpretation.
Author Contributions
Robert-Jan van Geuns, Marc van der Linden, Pieter Smits, Arie de Vries, Tuncay Yetgin, and Eric Boersma designed the original CCR study. Monique ten Haaf analyzed and interpreted the data and drafted the manuscript. Eric Boersma analyzed and interpreted the data. Pieter Doevendans and Yolande Appelman interpreted the data. All authors read, reviewed, and approved the final manuscript.
Funding Statement
This work was supported by the Eli-Lilly Nederland B.V. and Daiichi Sankyo Nederland B.V. Other than providing financial support, the sponsors were not involved in study design, study management, or data interpretation.
Data Availability Statement
The data that support the findings of this study are available on request from E. Boersma in the Erasmus MC.
References
- 1. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367. [DOI] [PubMed] [Google Scholar]
- 2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 3. James SK, Roe MT, Cannon CP, Cornel JH, Horrow J, Husted S, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ. 2011;342:d3527. 10.1136/bmj.d3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15. 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 5. Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, et al. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302(8):874–82. 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schreuder MM, Badal R, Boersma E, Kavousi M, Roos-Hesselink J, Versmissen J, et al. Efficacy and safety of high potent P2Y(12) inhibitors Prasugrel and Ticagrelor in patients with coronary heart disease treated with dual antiplatelet therapy: a sex-specific systematic review and meta-analysis. J Am Heart Assoc. 2020;9(4):e014457. 10.1161/JAHA.119.014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yetgin T, van der Linden MMJM, de Vries AG, Smits PC, van Mechelen R, Yap SC, et al. Current discharge management of acute coronary syndromes: data from the Rijnmond Collective Cardiology Research (CCR) study. Neth Heart J. 2014;22(1):20–7. 10.1007/s12471-013-0484-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yetgin T, van der Linden MMJM, de Vries AG, Smits PC, Boersma E, van Geuns RJM, et al. Adoption of prasugrel into routine practice: rationale and design of the Rijnmond Collective Cardiology Research (CCR) study in percutaneous coronary intervention for acute coronary syndromes. Neth Heart J. 2014;22(2):55–61. 10.1007/s12471-013-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet. 2009;373(9665):723–31. 10.1016/S0140-6736(09)60441-4. [DOI] [PubMed] [Google Scholar]
- 10. Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374(9683):29–38. 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 11. Moscucci M, Fox KAA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (GRACE). Eur Heart J. 2003;24(20):1815–23. 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 12. Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, et al. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122(21):2131–41. 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- 13. Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374(9696):1149–59. 10.1016/S0140-6736(09)61484-7. [DOI] [PubMed] [Google Scholar]
- 14. Mosca L, Grundy SM, Judelson D, King K, Limacher M, Oparil S, et al. Guide to preventive Cardiology for women.AHA/ACC scientific statement consensus panel statement. Circulation. 1999;99(18):2480–4. 10.1161/01.cir.99.18.2480. [DOI] [PubMed] [Google Scholar]
- 15. Husted S, James SK, Bach RG, Becker RC, Budaj A, Heras M, et al. The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective, randomized, PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2014;35(23):1541–50. 10.1093/eurheartj/ehu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damman P, Varenhorst C, Koul S, Eriksson P, Erlinge D, Lagerqvist B, et al. Treatment patterns and outcomes in patients undergoing percutaneous coronary intervention treated with prasugrel or clopidogrel (from the Swedish Coronary Angiography and Angioplasty Registry [SCAAR]). Am J Cardiol. 2014;113(1):64–9. 10.1016/j.amjcard.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 17. Chandrasekhar J, Baber U, Sartori S, Faggioni M, Aquino M, Kini A, et al. Sex-related differences in outcomes among men and women under 55 years of age with acute coronary syndrome undergoing percutaneous coronary intervention: results from the PROMETHEUS Study. Catheter Cardiovasc Interv; 2016. [DOI] [PubMed] [Google Scholar]
- 18. Hess CN, McCoy LA, Duggirala HJ, Tavris DR, O’Callaghan K, Douglas PS, et al. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. J Am Heart Assoc. 2014;3(1):e000523. 10.1161/JAHA.113.000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, et al. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel: thrombolysis in myocardial infarction 38 (TRITON-TIMI 38). Circulation. 2011;123(23):2681–9. 10.1161/CIRCULATIONAHA.110.002683. [DOI] [PubMed] [Google Scholar]
- 20. Ndrepepa G, Schulz S, Neumann FJ, Byrne RA, Hoppmann P, Cassese S, et al. Bleeding after percutaneous coronary intervention in women and men matched for age, body mass index, and type of antithrombotic therapy. Am Heart J. 2013;166(3):534–40. 10.1016/j.ahj.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 21. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377(9775):1409–20. 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 22. Pandie S, Mehta SR, Cantor WJ, Cheema AN, Gao P, Madan M, et al. Radial versus femoral access for coronary angiography/intervention in women with acute coronary syndromes: insights from the RIVAL Trial (Radial vs. femorAL access for coronary intervention). JACC Cardiovasc Interv. 2015;8(4):505–12. 10.1016/j.jcin.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 23. Rao SV, Hess CN, Barham B, Aberle LH, Anstrom KJ, Patel TB, et al. A registry-based randomized trial comparing radial and femoral approaches in women undergoing percutaneous coronary intervention: the SAFE-PCI for Women (Study of Access Site for Enhancement of PCI for Women) trial. JACC Cardiovasc Interv. 2014;7(8):857–67. 10.1016/j.jcin.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 24. Alexander KP, Chen AY, Newby LK, Schwartz JB, Redberg RF, Hochman JS, et al. Sex differences in major bleeding with glycoprotein IIb/IIIa inhibitors: results from the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines) initiative. Circulation. 2006;114(13):1380–7. 10.1161/CIRCULATIONAHA.106.620815. [DOI] [PubMed] [Google Scholar]
- 25. Cirillo P, Di Serafino L, Patti G, Antonucci E, Calabrò P, Gresele P, et al. Gender-related differences in antiplatelet therapy and impact on 1-Year clinical outcome in patients presenting with ACS: the START ANTIPLATELET Registry. Angiology. 2019;70(3):257–63. 10.1177/0003319718783866. [DOI] [PubMed] [Google Scholar]
- 26. Lau ES, Braunwald E, Murphy SA, Wiviott SD, Bonaca MP, Husted S, et al. Potent P2Y(12) inhibitors in men versus women: a collaborative meta-analysis of randomized trials. J Am Coll Cardiol. 2017;69(12):1549–59. 10.1016/j.jacc.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 27. Gewalt S, Lahu S, Ndrepepa G, Pellegrini C, Bernlochner I, Neumann FJ, et al. Efficacy and safety of ticagrelor versus prasugrel in women and men with acute coronary syndrome: a pre-specified, sex-specific analysis of the ISAR-REACT 5 trial. J Atheroscler Thromb. 2022;29:747–61. 10.5551/jat.62776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown O, Rossington J, Buchanan GL, Patti G, Hoye A. Is there sex-related outcome difference according to oral P2Y12 in patients with acute coronary syndromes? A systematic review and meta-analysis of 107,126 patients. Curr Vasc Pharmacol. 2019;17(2):191–203. 10.2174/1570161116666180123092054. [DOI] [PubMed] [Google Scholar]
- 29. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345(7):494–502. 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from E. Boersma in the Erasmus MC.