Abstract
Platelet microbicidal proteins (PMPs), small cationic peptides released at sites of endovascular damage, kill common bloodstream pathogens in vitro. Our group previously showed that in vitro resistance of clinical staphylococcal and viridans group streptococcal bacteremic strains to PMPs correlated with the diagnosis of infective endocarditis (IE) (Wu et al., Antimicrob. Agents Chemother. 38:729–732, 1994). However, that study was limited by (i) the small number of Staphylococcus aureus isolates from IE patients, (ii) the retrospective nature of the case definitions, and (iii) the diverse geographic sources of strains. The present study evaluated the in vitro PMP susceptibility phenotype of a large number of staphylococcemic isolates (n = 60), collected at a single medical center and categorized by defined and validated clinical criteria. A significantly higher proportion of staphylococcemic strains from patients with IE was PMP resistant in vitro than the proportion of strains from patients with soft tissue sepsis (83% and 33%, respectively; P < 0.01). Moreover, the levels of PMP resistance (mean percent survival of strains after 2-h exposure to PMP in vitro) were significantly higher for isolates from patients with IE and with vascular catheter sepsis than for strains from patients with abscess sepsis (P < 0.005 and P < 0.01, respectively). These data further support the concept that bloodstream pathogens that exhibit innate or acquired PMP resistance have a survival advantage with respect to either the induction or progression of endovascular infections.
The induction and evolution of endovascular infections such as vascular catheter sepsis and infective endocarditis (IE) reflect complex interactions among the microbe, plasma proteins, and platelets (7, 9, 10). Although platelets have been traditionally viewed as facilitating the initiation and progression of endovascular infections (9, 10), recent investigations at our laboratory and others have provided a large body of data supporting the concept of a key role for platelets in host defense against such infections (2, 15–19). This host defense function of platelets has been linked to their capacity to locally secrete an array of small, cationic antimicrobial peptides under conditions that exist at sites of endovascular infections (i.e., thrombin generation [5, 6] and microbe-induced platelet aggregation [1]). This family of antimicrobial peptides, termed platelet microbicidal proteins or PMPs (20), exhibits potent microbicidal and microbiostatic activities against pathogens commonly isolated from blood cultures of patients with septicemias, including those due to coagulase-positive and coagulase-negative staphylococci, viridans group streptococci, and Candida (14, 16, 17, 18). Since the isolation of these pathogens from blood cultures is frequent (12), yet endovascular infections caused by these pathogens are relatively uncommon, PMPs likely play a major role in preventing such infections, provided the organism of interest is susceptible to the antimicrobial effects of PMPs. It follows that PMP-resistant organisms may have a distinct survival advantage at sites of endovascular damage, in terms of induction and/or propagation of endovascular infections.
Staphylococcus aureus is the prototypical endovascular pathogen. Our group previously studied S. aureus strains isolated from the blood cultures of patients with the clinical diagnosis of either IE-associated or non-IE-associated staphylococcemia, for their phenotypes for in vitro susceptibility to thrombin-induced PMP (tPMP-1) (14). These studies clearly demonstrated that those strains isolated from patients with IE were almost uniformly tPMP-1 resistant, while strains from non-IE-associated bacteremias were nearly all tPMP-1 susceptible. These data supported the notion that a tPMP-1 resistance phenotype conferred a selective survival advantage on S. aureus strains vis-à-vis their capacity to induce IE. Despite the compelling data generated in the latter study, there were several important limitations evident in the analysis. First, the total number of S. aureus strains studied was small, including only six isolates from patients with IE and 11 patients with non-IE-associated staphylococcemia. In addition, the latter bacteremias had a heterogeneous mixture of etiologies, including vascular catheter sepses, soft tissue abscesses, and pneumonias. Furthermore, these strains were collected from diverse geographic areas of the United States. Finally, the diagnostic designation of cases as either IE related or IE unrelated was made from retrospective review of medical records. To more systematically study the relationship beween the PMP susceptibility phenotypes of S. aureus in vitro and source of clinical infections, we designed the present study to include the following important features: (i) evaluation of a relatively large group of staphylococcemia-associated isolates from a single medical center for their in vitro tPMP-1 susceptibility phenotypes; (ii) utilization of well-defined and validated clinical criteria (8) to prospectively designate cases of IE; (iii) use of uniform criteria to prospectively define non-IE-associated staphylococcemias as related to either vascular catheter sepsis or soft tissue abscess; and (iv) performance of in vitro tPMP-1 susceptibility analyses by experimenters who were blinded to the clinical designation of cases.
(These data were presented in part at the American Society for Microbiology Conference on Hemostasis, Microbes, and the Vascular System, Galveston, Tex., 1 April 1998, and at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [1a].)
MATERIALS AND METHODS
S. aureus strains.
Blood culture isolates were identified as S. aureus by subculturing on sheep blood agar and then performing the Staphaurex test (Murex Diagnostics, Norcross, Ga.) and Gram staining. Antimicrobial susceptibility testing was performed by disk diffusion according to the National Committee for Clinical Laboratory Standards criteria. Methicillin resistance was confirmed by using an agar screening plate containing 4% NaCl and 6 μg of oxacillin/ml. Isolates were frozen (−70°C) from the time of identification until they were subcultured on sheep blood agar for in vitro analysis of their tPMP-1 susceptibility phenotypes.
Patient selection.
From September 1994 to June 1997, daily reports were received from the microbiology laboratory about all patients at Duke University Medical Center with one or more blood cultures positive for S. aureus and clinical evidence of infection. The clinical charts of these patients were then reviewed within 36 h of the detection of the bacteremia to confirm the presence of a clinical infection. Exclusion criteria included S. aureus bacteremia in patients who were outpatients or less than 18 years of age, as well as in those with polymicrobial infection, those with neutropenia (leukocyte count less than 1.0 × 109/liter), or those who died before the return of positive blood cultures. To maintain the statistical assumption of independence of observations, only the initial episode of bacteremia for each patient was included in the study.
Clinical definitions.
Each eligible patient was prospectively evaluated for the potential source of the bacteremia. Three categories of S. aureus bacteremia were identified in this study. Thus, specific clinical criteria were utilized to designate the patients as having either IE-related or IE-unrelated staphylococcemia (8) (see below). For the latter group of staphylococcemias, only patients with either vascular catheter sepsis or soft tissue abscess sepsis were analyzed. This restriction in the definition of IE-unrelated staphylococcemia cases to be included in our analyses was designed to provide two comparator groups for the IE-associated bacteremias, one representative of nonvalvular endovascular infections (i.e., catheter sepsis) and one representative of staphylococcemias originating from outside the vascular system (i.e., soft tissue abscesses). The three diagnostic groups of patients with S. aureus bacteremia were designed to be mutually exclusive, with no patient exhibiting clinical or laboratory evidence of more than one of the above three infectious disease syndromes.
A patient was considered to have intravascular catheter-associated S. aureus bacteremia if the following three criteria were fulfilled: (i) inflammation was present around the catheter insertion site, (ii) a catheter tip culture was positive for S. aureus, and (iii) no other site of infection was evident (11). A patient was considered to have soft tissue abscess-associated bacteremia if (i) clinical signs of local soft tissue infection antedated the bacteremia, (ii) culture of the soft tissue site was positive for S. aureus, and (iii) no other source for the bacteremia was evident. A patient was considered to have IE if the Duke criteria for definite IE were fulfilled (8) (see Results, below).
Preparation of tPMP-1.
tPMP-1 was prepared as previously described (18). In brief, blood from New Zealand White rabbits was collected into siliconized tubes containing citrate anticoagulant. Anticoagulated whole blood was then centrifuged (75 × g, 10 min) to produce a platelet-rich plasma supernatant containing <1% leukocyte contamination. Platelets were pelleted by centrifugation (1,000 × g, 10 min) of the upper two-thirds of the platelet-rich plasma supernatant, and the resulting platelet pellet was washed twice in a solution of Tyrode’s salts (Sigma Chemical Co., St. Louis, Mo.) and resuspended in Eagle’s minimal essential medium (Irvine Scientific, Santa Ana, Calif.). Preparations rich in tPMP-1 were subsequently produced from washed platelet suspensions (108/ml) by stimulation with bovine thrombin (1 U/ml) (Sigma Chemical Co.) at 37°C for 20 min in the presence of 0.2 M CaCl2. Residual platelet material was then removed by centrifugation, and the tPMP-1-rich supernatant was recovered. Prior acid urea gel electrophoreses and reversed-phase high-performance liquid chromatography studies have confirmed that this preparation contains tPMP-1 as the predominant antimicrobial peptide (21).
Bioactivity of tPMP preparations.
Bioactivity of tPMP-1 preparations was determined by previously described methods (18). In brief, bioassays were performed with Bacillus subtilis (ATCC 6633), a highly tPMP-1-susceptible indicator organism (18). To determine tPMP-1 bioactivity, B. subtilis at an inoculum concentration of 2 × 104 CFU/ml was added to microtiter wells containing a range of dilutions of the tPMP-1-rich preparation to achieve a final inoculum concentration of 2 × 103 CFU/ml per well and final range of tPMP dilutions from 1:1 to 1:1,024 (final well volume, 200 μl). After 30 min of incubation at 37°C, a 20-μl aliquot was removed from each well, diluted in phosphate-buffered saline containing 0.01% (wt/vol) sodium polyanetholesulfonate (Sigma Chemical Co.) to inhibit further PMP-induced bacterial killing, and quantitatively cultured on 6.6% sheep blood agar. tPMP-1 bioactivity (U/ml) was defined as the inverse of the highest tPMP dilution which retained 95% lethality against B. subtilis within the 30-min assay period (17). The specific activity of each tPMP-1 preparation was approximately 20 U/μg of protein (∼2 μg/ml) as determined by spectrophotometry (OD220).
In vitro assay for tPMP-1 susceptibility phenotype.
The tPMP-1 susceptibility of the staphylococcemic strains in this study was determined by exposing 2 × 103 bacterial cells to 2 μg of tPMP/ml for 2 h at 37°C in a microtiter well assay system, as previously described (18). Three independent runs on separate days were performed. As in prior studies, the breakpoint for in vitro resistance of gram-positive bacteria to tPMP-1 was defined as 50% survival after 2 h of exposure (14, 18). Of importance, this resistance breakpoint in vitro correlates with the capacity of microbial strains to survive and proliferate within cardiac vegetations in vivo in experimental streptococcal or staphylococcal endocarditis models relative to that of tPMP-1-susceptible strains (2, 3, 4). B. subtilis (ATCC 6633) was used as a tPMP-1-susceptible control. Isogenic S. aureus strains ISP479C and ISP479R, which exhibit stable in vitro tPMP-1 susceptibility and resistance, respectively (4), were used as additional control strains for these assays. Exposure of the tPMP-1-susceptible strain, ISP479C, to tPMP-1 routinely resulted in <10% survival in the 2-h in vitro assay; exposure of the tPMP-1-resistant strain, ISP479R, to tPMP-1 routinely yielded >80% survival in this assay.
Statistical analyses.
The proportions of staphylococcemic isolates in the three clinical groups which were susceptible or resistant to tPMP-1 in vitro were compared by the Fisher exact test (with Bonferroni’s correction for multiple comparisons). The mean percent survival values after exposure to tPMP-1 in vitro among the isolates from the three patient groups were compared by the Kruskal-Wallis test, with the Tukey post hoc correction for multiple comparisons. A probability (P) value of ≤0.05 was considered significant.
RESULTS
Patient selection and categorization.
A total of 390 consecutive adult in-patients with S. aureus bacteremia were prospectively evaluated, and their clinical information was entered into a database. A representative subset of 60 of these patients with either bona fide IE, intravascular catheter sepsis, or soft tissue abscess sepsis was selected for subsequent analysis of their strains for in vitro tPMP-1 susceptibility phenotyping. Twenty-one (35%) of these 60 patients had intravascular catheter-associated staphylococcemia, 21 patients (35%) had soft tissue-associated staphylococcemia, and 18 patients (30%) had definite IE identified by the Duke criteria. Twenty (33.3%) of the 60 patients were infected with methicillin-resistant S. aureus strains.
Of the 21 patients with intravascular catheter-associated S. aureus bacteremia, 5 (23.8%) had an infected tunneled catheter, 10 (47.6%) had an infected central catheter, and 6 (28.6%) had an infected peripheral vascular catheter. All 21 patients underwent echocardiography (16 transesophageal and 5 transthoracic echocardiography procedures) to detect any evidence of vegetative IE; none was found. All patients received intravenous antibiotic therapy for 14 days or less, and all but one were followed up for 12 weeks after their initial bacteremia to detect any late development of IE after discharge from the hospital. Twenty of the 21 patients had no evidence of recurrent infection at the time of follow-up. One patient died from a malignancy 10 weeks after the initial bacteremia but had no evidence of recurrent bacteremia at the time of death.
Twenty-one patients had S. aureus bacteremia as a consequence of a soft tissue abscess. Of these patients, eight patients (four of whom had diabetes mellitus) had a foot or leg ulcer, four patients had cellulitis, three patients had an infected decubitus ulcer, and six patients had soft tissue abscesses of other sites. Twelve (57%) of these 21 patients underwent echocardiography (11 transesophageal and 1 transthoracic echocardiography procedure); none had evidence of IE.
Eighteen patients had definite IE as assessed by the Duke criteria (8). In 17 of the 18 patients, the diagnosis was based on fulfillment of two major criteria (persistently positive blood cultures and echocardiographic evidence of vegetative IE). One patient was designated as having definite IE based upon the presence of one major criterion (persistently positive blood cultures) and three minor criteria (fever, recent intravenous drug abuse, and clinical evidence of embolic events).
Distribution of tPMP-1 susceptibility among bacteremic S. aureus strains.
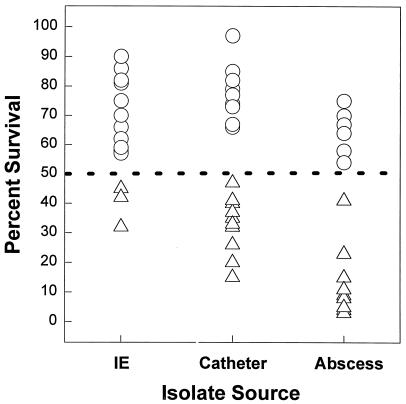
As shown in Fig. 1 (scattergram) and Table 1, the majority of staphylococcemic strains from patients with either vascular catheter sepsis (52%) or IE (83%) exhibited tPMP-1 resistance in vitro, in contrast to only 33% of strains from patients with soft tissue abscess-related staphylococcemias (P < 0.01; IE-associated strains versus abscess-related strains).
FIG. 1.
Scattergram of distributions of percent survival values for individual staphylococcemia-associated strains following 2-h exposure to tPMP-1 in vitro. Three groups include strains from patients with infective endocarditis (IE), vascular catheter sepsis (Catheter), and soft tissue abscess sepsis (Abscess). Triangles represent isolates that are susceptible in vitro to tPMP-1; circles represent isolates that are resistant in vitro to tPMP-1. See the text for discussion of the 50% breakpoint for defining susceptibility or resistance to tPMP-1 in vitro.
TABLE 1.
In vitro resistance to tPMP-1 among clinical bacteremia-derived isolates of S. aureusa
| Group | No. of tPMP-1 susceptible strains | No. of tPMP-1 resistant strains | % Resistant strains | P value vs abscess group | Mean % survival | P value vs abscess group |
|---|---|---|---|---|---|---|
| Abscess | 14 | 7 | 33 | 27 ± 27 | ||
| Catheter | 10 | 11 | 52 | 57 ± 26 | <0.01 | |
| IE | 3 | 15 | 83 | <0.01 | 67 ± 16 | <0.005 |
See text for criteria.
Quantitative survival of staphylococcemic strains following tPMP-1 exposure.
As seen in Table 1, the mean percent survival values for both the IE-associated strains and the vascular catheter sepsis-related strains following a 2-h in vitro exposure to tPMP-1 were significantly greater than that observed for the abscess-related strains (P < 0.005 and P < 0.01, respectively). There was no apparent correlation between the presence of the methicillin resistance phenotype (n = 20 isolates) and the presence or level of tPMP-1 resistance in vitro.
DISCUSSION
An increasing body of data attests to the importance of the platelet as a key cell in host defense against the induction and propagation of endovascular infections (2, 10, 15–17). This function has been attributed principally to the capacity of platelets to release one or more antimicrobial peptides at endovascular sites which are damaged either by specific disease states (e.g., rheumatic carditis) or following microbial colonization. This array of peptides has been termed PMPs (21). In this paradigm, damaged endothelium and subendothelial stroma elaborate tissue factor, which catalytically converts prothrombin to thrombin and initiates potent local procoagulant activity. Thrombin itself is also a potent agonist for platelet release of PMPs, particularly tPMP-1 (18, 21). tPMP-1 has a broad range of effects against pathogens commonly isolated from the bloodstream of patients with clinical infections, including those with the staphylococci, viridans group streptococci, and Candida (12, 14, 16). These effects include microbicidal and postexposure growth inhibitory properties and anti-platelet adherence and aggregation effects (18, 20). Thus, it is reasonable to hypothesize that microbial pathogens which are intrinsically resistant to PMPs would have a distinct survival advantage in terms of colonization and/or proliferation at sites of endovascular damage. In this regard, members of our group have previously shown, using the experimental rabbit IE model, that microbial pathogens which are tPMP-1 resistant in vitro have a superior ability to proliferate within cardiac vegetations and to hematogenously disseminate to and proliferate within extracardiac target organs (e.g., the kidney and spleen) (2, 3, 4).
In an earlier study, we evaluated bacteremic isolates from patients with staphylococcal or viridans group streptococcal infections of either IE-related or IE-unrelated etiology for their profiles of in vitro susceptibility to tPMP-1 (14). That investigation demonstrated a correlation between the clinical diagnosis of IE and tPMP-1 resistance in vitro. However, as pointed out above, the overall number of isolates studied was small (particularly for S. aureus-related IE), the clinical designation of the patients was made retrospectively without utilization of strict case definitions, and the in vitro assays were performed without blinding as to the clinical categorization of the samples. In order to address these issues, we evaluated a large group of staphylococcemic patients, recently treated at a single medical center (Duke University), who were prospectively classified into IE, vascular catheter sepsis, and soft tissue abscess groups by strict case definitions (8).
The data in the present study are quite consistent with our prior observations; in vitro tPMP-1 resistance correlates with a significant propensity to cause endovascular infections in vivo, especially in the case of IE. The mechanism(s) by which this effect occurs pathogenetically remains incompletely characterized. However, it is known from data emanating from our laboratory and others that the major impact of tPMP-1 resistance on an organism’s survival advantage eventuates after its colonization at sites of endovascular damage. Thus, several studies using the experimental IE model have shown that both tPMP-1-susceptible and tPMP-1-resistant strains colonize sterile cardiac vegetations with equal efficiency (2, 3, 4). One potential explanation for the relationship of in vitro tPMP-1 resistance with an increased propensity to cause endovascular infections might be that bacteremic strains represent a heterotypic population, including clones which are both tPMP-1 susceptible and tPMP-1 resistant (similar to methicillin-resistant staphylococci). In this model, under the influence of local tPMP-1 secretion at sites of endovascular colonization, the tPMP-1-resistant subpopulation would emerge and proliferate. However, experimental data from our laboratory and others (2, 4, 19) tend to refute this concept, in that it has been confirmed that tPMP-1-susceptible strains which colonize experimental IE vegetations retain their susceptible phenotype for as long as 6 days in vivo (4, 19).
The present study tested the in vitro susceptibility profile of human S. aureus bacteremic isolates, utilizing tPMP-1 isolated from rabbit platelets. Tang et al. (13a) and Krijgsveld et al. (10a) have previously shown that thrombin-stimulated human platelets also release antimicrobial peptides analogous to tPMP-1 (13a). When tested against a panel of microbial isolates (Candida, Escherichia coli, B. subtilis, and S. aureus), the human thrombin-induced peptides exhibited in vitro potencies and activities similar to those of rabbit tPMP-1 (13a, 21).
In summary, the present study further supports the concept that tPMP resistance is an important S. aureus virulence factor for the induction and/or propagation of endovascular infections in vivo. Whether the same can be said for coagulase-negative staphylococci, viridans group streptococci, and other endovascular pathogens is a question that requires additional investigations.
ACKNOWLEDGMENTS
This study was supported in part by the following grants from the National Institutes of Health: AI-39001 (to M.R.Y.) and AI-39108 (to M.R.Y. and A.S.B.). M.R.Y. was also supported by a grant-in-aid from the American Heart Association (National Center; 95-03-2620). V.G.F. was supported by a Health Services Research and Development Fellowship from the Veterans Administration Medical Center, Durham, N.C.
REFERENCES
- 1.Azizi N, Li C, Shen A J, Bayer A S, Yeaman M R. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Staphylococcus aureus elicits release of platelet microbicidal proteins in vitro, abstr. G54; p. 153. [Google Scholar]
- 1a.Cheng D, Bayer A S, Yeaman M R, Corey G R, McClelland R S, Harrell E, Fowler V G. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. In vitro resistance to thrombin-induced platelet microbicidal protein (tPMP) among Staphylococcus aureus bacteremic (SAB) isolates correlates with endovascular infection source, abstr. B-20; p. 50. [Google Scholar]
- 2.Dankert J, van der Werff J, Zaat S A J, Joldersma W, Klein D, Hess J. Involvement of bacterial factors from thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect Immun. 1995;63:663–671. doi: 10.1128/iai.63.2.663-671.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhawan V K, Bayer A S, Yeaman M R. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect Immun. 1998;66:3476–3479. doi: 10.1128/iai.66.7.3476-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake T A, Pang M. Effects of interleukin-1, lipopolysaccharide, and streptococci on procoagulant activity of cultured human cardiac valve endothelial and stromal cells. Infect Immun. 1989;57:507–512. doi: 10.1128/iai.57.2.507-512.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 7.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 8.Durack D T, Lukes A S, Bright D K. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 9.Herzberg M, MacFarlane G D, Gong K E, Armstrong N N, Witt A R, Erickson P R, Meyer M W. The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect Immun. 1992;60:4809–4818. doi: 10.1128/iai.60.11.4809-4818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzberg M C, Gong K, MacFarlane G D, Erickson P R, Soberay A H, Kresbach P H, Manjula G, Schilling K, Bowen W H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990;58:512–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Krijgsveld J, Zaat S A J, Dankert J. Abstracts of the 96th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1996. Purification of antimicrobial proteins from human blood platelets, abstr. B-364; p. 217. [Google Scholar]
- 11.Libman H, Arbeit R D. Complications associated with Staphylococcus aureus bacteremia. Arch Intern Med. 1984;144:541–545. [PubMed] [Google Scholar]
- 12.Pittet D, Wenzel R P. Nosocomial bloodstream infections—secular trends in rates, mortality and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. doi: 10.1001/archinte.155.11.1177. [DOI] [PubMed] [Google Scholar]
- 13.Sullam P M, Frank U, Tauber M G, Yeaman M R, Bayer A S, Chambers H F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993;168:910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- 13a.Tang Y O, Yeaman M R, Selsted M E. Purification, characterization and antimicrobial properties of peptides released from thrombin-induced human platelets. Blood. 1995;86:910a. [Google Scholar]
- 14.Wu T, Yeaman M R, Bayer A S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob Agents Chemother. 1994;38:729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 16.Yeaman M R, Ibrahim A, Filler S G, Bayer A S, Edwards J E, Jr, Ghannoum M A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeaman M R, Soldan S S, Ghannoum M A, Edwards J E J, Jr, Filler S G, Bayer A S. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect Immun. 1996;64:1379–1384. doi: 10.1128/iai.64.4.1379-1384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeaman M R, Sullam P M, Dazin P F, Bayer A S. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureus adherence to platelets in vitro. Infect Immun. 1994;62:3416–3423. doi: 10.1128/iai.62.8.3416-3423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman M R, Shen A, Tang Y, Bayer A S, Selsted M A. Isolation and antimicrobial activity of microbicidal proteins from rabbit platelets. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]