Introduction
The WHO Classification of Tumours aims to provide an evidence-based taxonomy for tumors of each organ system to standardize diagnostic practice worldwide and to promote translational research on cancer [1]. The 5th edition of the WHO Classification of Tumours of the Eye and Orbit (WHO Eye5) builds and improves upon the 4th edition (WHO Eye4) by furnishing a comprehensive summary of all tumors of the eye and ocular adnexa that an ophthalmic oncologist and ophthalmic pathologist may encounter [2, 3]. It employs a standardized, evidence-based approach to integrate the salient epidemiologic, ophthalmic, genetic, diagnostic, and prognostic data into a format that allows the reader to grasp the site-specific information on each tumor type and allows cross-referencing with other WHO 5th edition volumes [1, 2]. This review outlines the major general and specific changes in the WHO Eye5, which is currently available online as a beta version ahead of print [2].
Volume Contributors
The goal of the WHO Classification of Tumours is to provide an up-to-date synthesis of information on cancer, based on expert consensus review of evidence-based published data [1]. Two hundred authors and editors have participated in the production of the WHO Eye5 [2]. The revision was led by the WHO Classification of Tumours Editorial Board, composed of standing and expert members. The standing members have been nominated by pathology organizations and are the equivalent of the series editors of previous editions. The expert members have been selected on the basis of informed bibliometric analysis and advice from the standing members and are equivalent to the volume editors of previous editions. In recognition of the increasingly multidisciplinary approach to cancer diagnosis, prognostication, and management, the 5th edition authors, expert members, and editorial board included oncologists, radiologists, molecular biologists, geneticists, and pathology experts from the overlapping pathology disciplines (head and neck, dermatopathology, soft tissue pathology, and hematopathology) to address WHO Eye5-specific needs [2].
General Changes
Taxonomy
The WHO Eye5 aims to provide the most comprehensive overview of the eye and orbit tumors an ophthalmic oncologist and pathologist may encounter, with cross-reference to other volumes as necessary. Although the WHO Eye5 continues to be organized by anatomical site (e.g., conjunctiva, eyelid, uvea), its major differences with the WHO Eye4 are incorporation of tumor lineage (e.g., epithelial, adnexal, melanocytic) into the classification system, and synthesis of ocular and periocular soft tissue and bone tumors, hematolymphoid tumors, metastases, and genetic tumor predisposition syndromes into separate chapters, following the structure of the other WHO 5th edition volumes [1, 2]. In line with the 5th edition format, the WHO Eye5 content logically progresses from non-neoplastic tumor-like lesions, to benign neoplasms and, finally, to malignant neoplasms. The standardized, systematic approach is adopted to illuminate each tumor, with addition of the ICD11-codes, imaging features when relevant, macroscopic appearance, cytology, diagnostic molecular pathology, essential and desirable diagnostic criteria, and staging (Table 1).
Table 1.
WHO Eye5: subsection headings and definitions*
| Definition |
| A concise statement/description of the nature of the tumor |
| ICD-0 coding |
| ICD-11 coding |
| Related terminology |
| Synonyms, older terms, and eponymous terms are listed as either “acceptable” or “not recommended” |
| Subtypes (formerly known as “variant”) |
| A tumor variant in which one or two parameters (e.g., clinical, location, histopathological, and/or molecular) make it desirable to recognize it as being distinct from other subtypes but still related to the parent type (tumor “entity”) |
| Localization |
| Clinical features (including imaging) |
| Epidemiology |
| Etiology |
| Known risk factors and genetic factors |
| Pathogenesis |
| Explicates how the causes described in the “Etiology” subsection lead to tumor formation |
| Macroscopic appearance |
| Histopathology |
| H&E appearance and variation in pathological appearance (morphologic patterns) |
| Description of recognized histopathological subtypes |
| Diagnostic immunohistochemistry (IHC) |
| Grading (where applicable) |
| Differential diagnosis |
| Cytology (when clinically relevant) |
| Diagnostic molecular pathology |
| Analytic signature of the tumor that is diagnostically useful |
| Essential and desirable diagnostic criteria |
| Essential criteria – minimal criteria that are mandatory for diagnosis |
| Desirable diagnostic criteria – recommended criteria that are not mandatory for diagnosis |
| Staging |
| Staging system relevant to tumor, such as American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) TNM classification, Lugano, etc. |
| Prognosis and prediction |
| Includes evidence-based information on prognostic and predictive biomarkers, when available |
*Adapted from https://bboss.iarc.fr/instructions.php.
Nomenclature
The WHO Eye5 aims to use the most recent evidence-based nomenclature for classification of tumors. One major change was adoption, where possible, of the terminology proposed in 2018 by the International Society for the Study of Vascular Anomalies (ISSVA) to classify vascular tumors and malformations [4]. The definitions of tumor “type,” “subtype,” and “morphologic pattern” are implemented to classify the tumors (Table 2) [1, 2]. The preference for “subtype” over “variant” stems from the need to differentiate this concept from the homonymous genetic term “variant” to avoid potential confusion. Where appropriate, the WHO 5th edition volumes use the Human Genome Organization (HUGO) Gene Nomenclature Committee (HGNC) system for gene symbols and names (https://www.genenames.org/) [5] and the Human Genome Variation Society (HGVS) recommendations for sequence variants (http://varnomen.hgvs.org) [6]. Another notable change in the 5th edition volumes is conversion of the mitotic count from the traditional denominator, number of high-power fields, to a defined area expressed in mm2 [7].
Table 2.
WHO Eye5: definitions of tumor type, subtype, and pattern*
| Type (formerly known as “entity”) |
| Separate entity in which multiple parameters (e.g., clinical, location, histopathological, and/or molecular) differ from those of other types |
| Subtype (formerly known as “variant”) |
| A variant of a tumor type in which one or two parameters (e.g., clinical, location, histopathological, and/or molecular) make it desirable to recognize it as being distinct from other subtypes but still related to the parent type |
| Pattern |
| Morphologic or immunohistochemical pattern in a tumor, without distinct clinical, molecular, or prognostic parameters that make it desirable to be recognized as a subtype |
*Adapted from https://bboss.iarc.fr/instructions.php.
Online version
The WHO Classification of Tumours Online website, which currently features the WHO Eye4, was launched in 2019 [1]. The WHO Eye5 will replace this on the website. In addition to the text, tables, and figures, the online edition will provide a whole slide image library, which will allow a more detailed histopathologic representation of various tumors. The online format also allows easy cross-referencing of tumor entities across body sites and organ systems for non-site-specific details.
Specific Changes
Tumors of the Conjunctiva and Caruncle (Table 3)
Table 3.
WHO Eye5: tumors of the conjunctiva and caruncle [2]
| Tumor-like lesions and choristomas of the conjunctiva |
| Cysts of the conjunctiva and caruncle |
| Epithelial conjunctival inclusion cyst |
| Reactive, epithelial, and degenerative conjunctival lesions |
| Reactive epithelial hyperplasia |
| Papillary and follicular conjunctivitis |
| Pterygium and pinguecula |
| Choristomas |
| Epibulbar choristoma (dermoid; dermolipoma; complex) |
| Epibulbar osseous choristoma |
| Epithelial tumors of the conjunctiva |
| Benign epithelial tumors |
| Conjunctival squamous papilloma |
| Conjunctival oncocytoma |
| Hereditary benign intraepithelial dyskeratosis |
| Premalignant and malignant epithelial tumors of the conjunctiva |
| Conjunctival squamous intraepithelial neoplasia |
| Conjunctival squamous cell carcinoma |
| Adenosquamous carcinoma |
| Melanocytic conjunctival tumors |
| Benign melanocytic conjunctival tumors |
| Benign epithelial melanosis of the conjunctiva |
| Junctional, compound, and subepithelial naevi |
| Inflamed juvenile conjunctival nevus |
| Blue nevus of the conjunctiva |
| WNT-activated DPN |
| Combined nevus of the conjunctiva |
| Premalignant and malignant melanocytic conjunctival tumors |
| Conjunctival melanocytic intraepithelial lesions |
| Conjunctival melanoma |
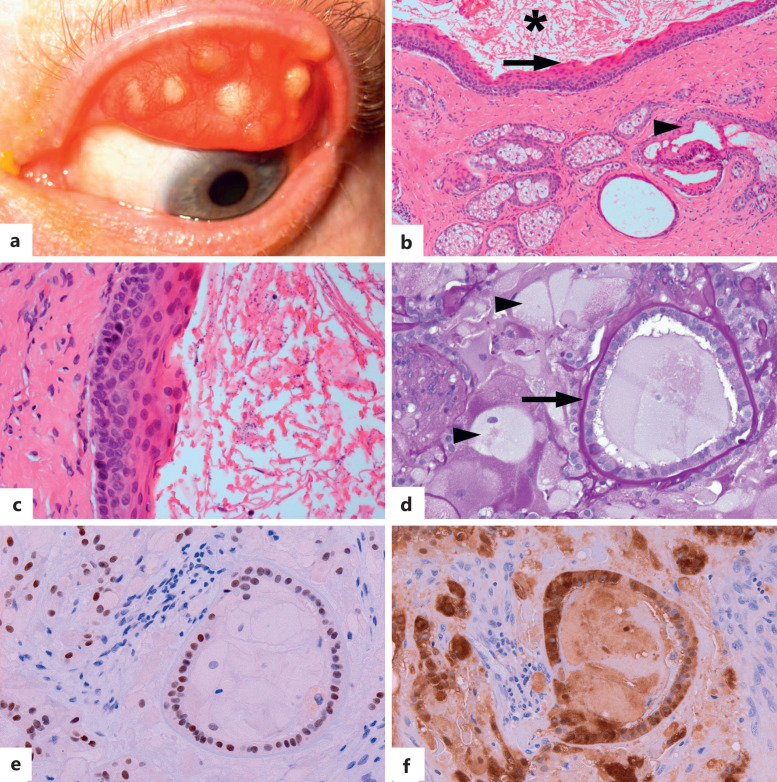
This chapter provides an update on pathogenesis and molecular genetics of melanocytic conjunctival lesions (Table 4) [2, 8–27]. Spitz nevus is removed from this chapter because of the lack of molecularly well-documented cases of this tumor in the conjunctiva. Conversely, the WNT-activated deep penetrating/plexiform melanocytoma (nevus) (DPN) – the recently agreed terminology for DPN – is added as a new well-characterized entity in the conjunctiva. This follows the 5th edition WHO Classification of the Tumours of the Skin that acknowledges that melanocytomas are genetically “intermediate” between nevus and melanoma because they carry pathogenic mutations additional to the initiating mutation that is the sole alteration in a nevus. Unlike in the skin, a WNT-activated DPN in the conjunctiva is usually a part of a combined nevus (with a common nevocellular or an inflamed juvenile nevus; shown in Fig. 1) [2, 14–17]. Another notable change is the evolving WHO classification of the conjunctival melanocytic intraepithelial lesions, based on the recent international study and on the WHO consensus editorial meeting between dermatopathologists and ophthalmic pathologists (Table 5) [2, 28, 29]. Non-neoplastic pterygium and pinguecula were added as “tumor-like lesions” to delineate them from conjunctival squamous intraepithelial neoplasia. The term adenosquamous carcinoma, introduced in the Eye4 as the preferred term for mucoepidermoid carcinoma, is now recommended to be applied only to neoplasms with bi-phenotypic differentiation comprising squamous cell carcinoma and adenocarcinoma with mucin-containing cells (shown in Fig. 2) [2, 30]. Tumors that do not form true neoplastic glands/ducts or confluent sheets of cells with intracytoplasmic mucin and, instead, show scattered or small clusters of cells with such mucin in a background of malignant squamous cells are designated as “squamous cell carcinoma with mucinous differentiation” (shown in Fig. 3) [2, 30]. Sebaceous carcinoma, covered in Tumors of the Eyelid chapter, has been removed. Soft tissue tumors, hematolymphoid neoplasms, and secondary tumors of the conjunctiva are discussed in separate chapters.
Table 4.
WHO Eye5: melanocytic tumors of the conjunctiva [2]
| Conjunctival melanocytic lesion | Molecular genetic alterations | References |
|---|---|---|
| Junctional, compound, and subepithelial nevi; inflamed juvenile nevus | BRAF c.1799T>A p.V600E, NRAS mutations | [7–11] |
| Blue nevus | GNAQ mutations | [10, 12] |
| WNT-activated DPN | BRAF c.1799T>A p.V600E and CTNNB1 mutations | [13–16] |
| Melanoma | High-frequency mutations: NF1 (50–33%), BRAF (46–29%), NRAS (26–11%), ATRX (25%), TERT promoter (up to 54%), CTNNB1 (17%) Other mutations: ACSS3, ET, TP53, CKIT, TET2, CDKN2A, MAPK2, RAC1, MET, SF3B1, GNAQ, GNA1 Multiple chromosomal alterations: 6p gain, 11q gain, 6q loss, 10q loss |
[17–26] |
Fig. 1.
Combined nevocellular and WNT-activated deep penetrating/plexiform melanocytoma (nevus) (DPN) of the conjunctiva. a Circumscribed variably pigmented nodule in plica semilunaris, associated with a feeder vessel. b DPN component of the lesion is composed of lightly pigmented spindle melanocytes with abundant cytoplasm (asterisk), associated with scattered darkly pigmented melanophages (arrowhead). Mostly amelanotic nevocellular nevus (arrow) is present in the periphery. The DPN component of the lesion is positive for HMB45 (red chromogen, c), nuclear cyclin D1 (brown chromogen, d), and cytoplasmic and nuclear beta-catenin (brown chromogen, e). The nevocellular nevoid component of the lesion is negative for these markers (arrows, c–e). No proliferative activity is highlighted with Ki-67 (f) [hematoxylin-eosin (b), HMB45 (c), cyclin D1 (d), beta-catenin (e), Ki-67 (f); all figures. ×200].
Table 5.
2022 World Health Organization Classification of Conjunctival Melanocytic Intraepithelial Lesions [2]
| WHO | Acceptable alternative terminology | Increased cellularity | Histologic features | Risk of association with or progression to invasive melanoma |
|---|---|---|---|---|
| Not applicable | Benign melanosis c-MIN (grades (0–1) PAM without atypia |
No/minimal | Conjunctival hypermelanosis (increased pigment in epithelial cells without melanocytic hyperplasia or atypia). Slight or focal melanocytic hyperplasia without atypia (parabasal melanocytes with condensed round nuclei, smaller than basal epithelial cell, inconspicuous nucleoli, and inconspicuous cytoplasm) may be seen | None |
| Low-grade CMIL | PAM with mild atypia c-MIN (grades 2–4) |
Yes | Predominantly basilar melanocytic proliferation with low-grade atypia (dendritic or small to moderate size polyhedral, usually non-epithelioid melanocytes with round to irregular nuclear contours, often nuclear hyperchromasia, inconspicuous nucleoli, and inconspicuous or scant cytoplasm) | Lower |
| High-grade CMIL | PAM with moderate to severe atypia c-MIN (grade 5–10) |
Yes | More confluent basilar and significant non-basilar proliferation of melanocytes with high-grade atypia (moderate to severe), evidence of intraepithelial nested and/or pagetoid growth, and epithelioid cell cytomorphology | Higher |
| Melanoma in situ | Yes | The term melanoma in situ may be used for (1) the most atypical high-grade CMILs involving close to full thickness of the epithelium, (2) histologically obvious melanomas without documented evidence of subepithelial invasion | Highest |
CMIL, conjunctival melanocytic intraepithelial lesion.
Fig. 2.
Adenosquamous carcinoma of the conjunctiva. a Leukoplakic mass in the inferior fornix. b The invasive adenocarcinoma component is composed of epithelial cells with focal intracytoplasmic mucin (arrow) arranged in glands, in a background of desmoplastic stroma. Intracytoplasmic and luminal mucin is highlighted with Alcian blue stain (inset). c Sheets of atypical goblet cells (arrow). d Squamous cell carcinoma component is composed of irregular islands and bands of squamous cells [hematoxylin-eosin (b–d); ×100 (b), ×200 (c, d)].
Fig. 3.
Squamous cell carcinoma with mucinous differentiation. a Irregular islands and bands of invasive nonkeratinizing squamous cell carcinoma with focal intracytoplasmic mucin (b), highlighted with Alcian blue stain (c) [hematoxylin-eosin. ×50 (b), ×400 (b, c)].
Tumors of the Eyelid (Table 6)
Table 6.
WHO Eye5: tumors of the eyelid [2]
| Tumor-like lesions, hamartomas, and choristomas of the eyelid |
| Eyelid cysts |
| Epidermal inclusion cyst |
| Intratarsal cyst |
| Choristomas of the eyelid |
| Phakomatous choristoma |
| Epithelial tumors of the eyelid |
| Benign and premalignant epithelial tumors of the eyelid |
| Molluscum contagiosum |
| Verruca vulgaris |
| Squamous papilloma |
| Seborrheic keratosis |
| Inverted follicular keratosis |
| Actinic keratosis |
| Malignant epithelial tumors of the eyelid |
| Basal cell carcinoma |
| Squamous cell carcinoma |
| Merkel cell carcinoma |
| Adnexal tumors of the eyelid |
| Benign adnexal tumors of the eyelid |
| Ductal hidrocystoma |
| Sebaceous hyperplasia, adenoma, and sebaceoma |
| Syringoma |
| Mixed tumor |
| Tubular adenoma |
| Syringocystadenoma papilliferum |
| Pilomatrixoma |
| Trichilemmoma |
| Malignant adnexal tumors of the eyelid |
| Sebaceous carcinoma |
| Adenoid cystic carcinoma |
| Signet-ring cell/histiocytoid carcinoma |
| Mucinous carcinoma |
| Endocrine mucin-producing sweat gland carcinoma |
| Microcystic adnexal carcinoma |
| Apocrine adenocarcinoma |
| Melanocytic eyelid tumors |
| Benign and premalignant melanocytic eyelid tumors |
| Nevi of the eyelid |
| Spitz naevus |
| In situ cutaneous melanoma |
| Malignant melanocytic eyelid tumors |
| Invasive cutaneous melanoma |
This chapter covers common benign and malignant eyelid tumors with attention to their site-specific features. Rare adnexal tumors with predilection for the eyelid, such as sebaceous neoplasms and endocrine mucin-producing sweat gland carcinoma (shown in Fig. 4), and lesions that are unique to the eyelid, such as intratarsal keratinous cyst (shown in Fig. 5a–c) and phakomatous choristoma (shown in Fig. 5d–f), are also illuminated in this chapter [31–36]. The material is coordinated with the 5th edition WHO Classification of the Tumours of the Skin, particularly with regard to cutaneous melanocytic tumors.
Fig. 4.
Endocrine mucin-producing sweat gland carcinoma of the eyelid. a Reddish dermal-based nodule in the lower eyelid along the eyelash line. b Expansile nests of cells in cribriform arrangement. c Cuboidal neoplastic cells have ovoid, mildly pleomorphic nuclei, inconspicuous nucleoli, and moderate amounts of eosinophilic cytoplasm. d Alcian blue stain highlights intracytoplasmic and extracellular mucin. e Strong diffuse nuclear expression of estrogen receptors and insulinoma-associated protein 1 (INSM1) (f) [hematoxylin-eosin (b, c), Alcian blue (d), ER (e), INSM1 (f), ×50 (b), ×200 (c–f)].
Fig. 5.
Tarsal cyst and phakomatous choristoma. a–c Tarsal cyst. a Tan-gray subepithelial tarsal-based nodules. b The cyst is lined by stratified squamous epithelium with eosinophilic corrugated delicately keratinized cuticle (arrow) and filled with keratin (asterisk). Meibomian gland lobules with associated ducts, lined by the epithelium morphologically similar to the cyst (arrowhead), are present in the adjacent tarsus. c Higher magnification of the cyst lining and delicate lamellated luminal keratin. d–f Phakomatous choristoma. d Cataractous-appearing tissue composed of bladder cells (arrowheads) and cuboidal lens epithelium associated with a prominent periodic acid-Schiff (PAS)-positive basement membrane, reminiscent of lens capsule (arrow). The lens epithelial cells express nuclear PAX8 (e) and cytoplasmic S100 (f) [hematoxylin-eosin (b, c), PAS (d), PAX8 (e), S100 (f), ×100 (b), ×1,000 (c), ×400 (c–f)].
Tumors of the Uveal Tract (Table 7)
Table 7.
WHO Eye5: tumors of the uveal tract [2]
| Iris tumors |
| Benign lesions and hamartomas of the iris |
| Ectopic tissue |
| Implantation cyst |
| Pigmented epithelial cyst |
| Lisch nodule |
| Melanocytic tumors of the iris |
| Ocular melanocytosis |
| Melanocytic nevus |
| Iris melanoma |
| Ciliary body and choroid tumors |
| Benign melanocytic lesions of the choroid and ciliary body |
| Choroidal and ciliary body nevi |
| Uveal melanocytoma |
| BDUMP |
| Malignant melanocytic tumors of the choroid and ciliary body |
| Choroidal and ciliary body melanomas |
BDUMP, bilateral diffuse uveal melanocytic proliferation.
This chapter is limited to the non-neoplastic iris lesions and uveal melanocytic tumors and tumor-like lesions. Entities that can involve any portion of the uveal tract are described according to their dominant site. The most notable update is addition of diagnostic cytopathology for benign and malignant melanocytic tumors of the iris, ciliary body, and choroid (Table 8) [2, 37–42]. This chapter provides an update on pathogenesis of uveal melanocytoma, which harbors solitary GNAQ mutations without additional oncogenic alterations and thus distinct from cutaneous and conjunctival melanocytoma [43], and that of bilateral diffuse uveal melanocytic proliferation, which may involve autoantibodies to hepatocyte growth factor [44, 45]. Soft tissue tumors of the uveal tract, including hemangioma, peripheral nerve sheath tumors, and smooth muscle tumors are covered in chapters dedicated to these entities.
Table 8.
WHO Eye5: diagnostic cytology of melanocytic uveal tumors [2]
| Uveal melanocytic tumor | Diagnostic cytology | References |
|---|---|---|
| Conventional nevus | Paucicellular sample with predominantly small spindle to round cells with or without pigmentation and with small or absent nucleoli. The cells are immunoreactive for melanocytic markers and show rare or no cells in cycle with Ki-67 immunostaining. Molecular genetic profiling may allow differentiation from melanoma in cytologically borderline lesions | [2] |
| Melanocytoma | Large, ovoid cells with small centrally located nuclei and heavily pigmented cytoplasm. Melanin can obscure nuclear detail. The cells are immunoreactive for melanocytic markers and show rare or no cells in cycle with Ki-67 immunostaining. Molecular genetic profiling may allow differentiation from melanoma in cytologically borderline lesions | [37] |
| Melanoma | Individually dispersed, or small cohesive groups of atypical cells demonstrating spindle-shaped or rounded pleomorphic nuclei with nucleoli, coarsely hyperchromatic chromatin, and eosinophilic cytoplasm that is spindle-shaped in spindle melanoma cells and ovoid in epithelioid melanoma cells. Variable numbers of cytoplasmic finely granular melanosomes are present. Binucleation and mitotic figures can be seen. Molecular genetic profiling of cytology material plays an important role in prognostication | [38–42] |
Tumors of the Retina and Neuroepithelium (Table 9)
Table 9.
WHO Eye5: tumors of the retina and neuroepithelium [2]
| Tumors of the neurosensory retina |
| Benign retinal tumors |
| Astrocytic tumors |
| Nodular and massive retinal gliosis |
| Premalignant and malignant retinal tumors |
| Retinocytoma |
| Retinoblastoma |
| Tumors of the retinal pigment epithelium (RPE) |
| Reactive and hamartomatous lesions of the RPE |
| Hamartoma of the RPE |
| Congenital hypertrophy of the RPE |
| Reactive hyperplasia of the RPE |
| Benign and malignant tumors of the RPE |
| Adenoma and adenocarcinoma of the RPE |
| Tumors of the iris and ciliary body neuroepithelium |
| Reactive and cystic lesions of the iris and ciliary body pigment epithelium |
| Reactive epithelial hyperplasia of the ciliary epithelium |
| Nodular hyperplasia of the ciliary epithelium |
| Glioneuronal hamartoma of the ciliary body |
| Benign and malignant tumors of the iris and ciliary body pigment epithelium |
| Adenoma and adenocarcinoma of the ciliary body |
| Medulloepithelioma of the ciliary body |
This chapter is devoted to the tumors and tumor-like lesions of the retina, retinal pigment epithelium, and the iris and ciliary body epithelia. Retinocytoma is classified as a premalignant tumor, considering the genetic data that these tumors carry biallelic RB1 mutations and clinical evidence that they can dedifferentiate into retinoblastoma [45, 46]. The chapter includes recent consensus protocols on screening of children at risk for retinoblastoma [47, 48] and data on MYCN-amplified, RB1 wild-type retinoblastoma (shown in Fig. 6) [49]. Novel molecular genetic methods, like liquid biopsies of tumor-derived cell-free DNA from aqueous fluid for prognostication and from plasma for prenatal testing and detection of nonocular primary malignancies in children with retinoblastoma, are discussed [50–52]. Coverage of diagnostic cytopathology for tumors of the retina and neuroepithelium is a notable addition (Table 10) [2, 53–60]. The spectrum of nodular and massive gliosis is described, nodular gliosis being the preferred term for vasoproliferative tumor [2]. True vascular lesions of the retina are covered in the chapters dedicated to vascular malformations and vascular neoplasms.
Fig. 6.
MYCN-amplified retinoblastoma. a Gross photograph of the eye enucleated for failure to respond to local therapy demonstrates calcific post-treatment regression scar (arrow) and diffuse involvement of the detached retina by viable-appearing tumor (black arrowheads). Focal choroidal invasion is present (white arrowhead). Hemorrhage and necrosis are present in subretinal space (asterisk). b Histopathology demonstrates discohesive undifferentiated retinoblastoma cells, which focally invade choroid (arrow). c Discohesive retinoblastoma cells with prominent central nucleoli and scant cytoplasm (arrow), with brisk apoptotic bodies. d Retained retinoblastoma 1 (RB1) protein expression in the tumor [hematoxylin-eosin (b, c), RB1 (d), ×100 (b), ×400 (C), ×200 (d)]. Images courtesy of Ralph C. Eagle, Jr. MD.
Table 10.
WHO Eye5: diagnostic cytology of tumors of the retina and neuroepithelium [2]
| Retinal and neuroepithelial tumor | Diagnostic cytology | References |
|---|---|---|
| Retinoblastoma | Generally, biopsy is avoided due to the risk of extraocular recurrence and metastasis. Cytological samples often have pitfalls (sampling of retinal neurons, scant material) that may be misleading. Diagnostic features of malignancy such as necrosis, mitoses, and nuclear atypia should be present when diagnosing retinoblastoma in a cytology. Consultation with an experienced ophthalmic cytopathologist is recommended if a biopsy is considered | [2] |
| Medulloepithelioma | The polymorphous nature of medulloepithelioma can reduce the sensitivity and specificity of aspiration cytology. In many reports, diagnosis is limited to a malignant round cell tumor, although more specific rosettes and focal epithelial-like fragments with cellular polarity and multilayering occasionally are observed. Immunohistochemical staining for medulloepitheliomas expresses LIN28A and PAX8 by immunohistochemistry (IHC), which may help distinguish these tumors from retinoblastoma | [53–57] |
| Adenoma and adenocarcinoma of the ciliary body epithelium (CBE) | CBE adenoma shows polygonal epithelial cells with bland round to oval nuclei and small nucleoli, with or without melanin granules. Adenocarcinoma demonstrates cohesive clusters of atypical cuboidal to large epithelioid epithelial cells with hyperchromatic, enlarged nuclei, and prominent nucleoli. The cells in adenoma and adenocarcinoma can be pigmented or non-pigmented. The presence of basement membrane material highlighted with periodic acid-Schiff (PAS) stain helps differentiate these tumors from melanoma, other stromal tumors, and metastases. PAX8 IHC is useful in distinguishing CBE adenoma and adenocarcinoma from melanocytic lesions. Adenomas and adenocarcinomas of pigmented CBE harbor BRAF c.1799T>A p.V600E mutations and lack GNAQ or GNA11 mutations (histopathology data), a profile that may favor adenoma/adenocarcinoma over melanocytic uveal neoplasm | [57–59] |
| Adenoma and adenocarcinoma of retinal pigment epithelium (RPE) | Adenocarcinoma of RPE and epithelioid melanoma cells can appear similar, and there is significant immunohistochemical overlap between RPE tumors and uveal melanoma. Documentation of PAS-positive basement material can be helpful in supporting the diagnosis of retinal pigment epithelial neoplasm. Correlation of cytologic findings with clinical and imaging findings is essential | [57], [60] |
Tumors of the Optic Disk and Optic Nerve (Table 11)
Table 11.
WHO Eye5: tumors of the optic disk and optic nerve [2]
| Malformations of the optic disk and optic nerve |
|---|
| Optic nerve choristoma |
| Primary neural tumors of the optic disk and optic nerve |
| Gliomas of the optic nerve |
| Pigment epithelial tumors of the optic disk and optic nerve |
| Medulloepithelioma of the optic disk and optic nerve |
| Meningeal tumors of the optic nerve |
| Meningioma of the optic nerve |
| Melanocytic tumors of the optic disk and optic nerve |
| Melanocytoma of the optic disk and optic nerve |
This chapter focuses on primary lesions of the optic disk and pre-chiasmal optic nerve. Optic nerve choristoma, a rare, benign, developmental/malformative lesion composed of smooth muscle and adipose tissue within and around the optic nerve, is added to this chapter [2, 61, 62] that is coordinated with the 5th edition of WHO Classification of the Tumours of the Central Nervous System [63].
Tumors of the Lacrimal Gland and Lacrimal Drainage System (Table 12)
Table 12.
WHO Eye5: tumors of the lacrimal gland and lacrimal drainage system [2]
| Tumors of the lacrimal gland |
| Epithelial tumors of the lacrimal gland |
| Cysts of the lacrimal gland |
| Dacryops cyst |
| Benign and premalignant epithelial tumors of the lacrimal gland |
| Pleomorphic adenoma (PA) of the lacrimal gland |
| Lacrimal gland oncocytoma |
| Myoepithelioma |
| Intraductal carcinoma |
| Malignant epithelial tumors of the lacrimal gland |
| Carcinoma ex PA |
| Adenoid cystic carcinoma |
| Primary ductal adenocarcinoma of the lacrimal gland |
| Secretory carcinoma |
| Mucoepidermoid carcinoma (MEC) |
| Epithelial-myoepithelial carcinoma (EMC) |
| Acinic cell carcinoma |
| Adenocarcinoma, NOS |
| Tumors of the lacrimal drainage system |
| Epithelial tumors of the lacrimal drainage system |
| Benign and premalignant epithelial tumors of the lacrimal drainage system Papillomas (squamous and inverted) |
| Malignant epithelial tumors of the lacrimal drainage system |
| Squamous cell carcinoma |
| Lymphoepithelial carcinoma |
| MEC |
| Other salivary gland-type carcinomas of the lacrimal drainage system |
| Adenocarcinoma NOS of the lacrimal drainage system |
| Melanocytic tumors of the lacrimal drainage system |
| Melanoma involving the lacrimal drainage system |
Two separate chapters provide an update regarding molecular biology and diagnosis of benign and malignant epithelial lacrimal gland (Table 13) and lacrimal drainage system tumors and include new sections on diagnostic cytopathology [64–106]. Obsolete entities, such as oncocytic carcinoma, have been removed. Tumors that are rarely encountered in the lacrimal gland or are controversial in this region, such as Warthin tumor, are also removed.
Table 13.
WHO Eye5: epithelial tumors of the lacrimal gland [2]
| Epithelial tumor of the lacrimal gland/ Definition |
Molecular genetic alterations/ Diagnostic molecular pathology |
Immunohistochemistry (IHC) | Essential and desirable diagnostic criteria | Ref |
|---|---|---|---|---|
|
Pleomorphic adenoma (PA)
Benign lacrimal gland neoplasm consisting of a mixture of epithelial, myoepithelial, and mesenchymal-appearing components |
Recurrent translocations of PLAG1 on 8q12 PLAG1 translocation t(5; 8) (p13; q12) is highly specific for PA HMGA2 translocation on 12q14 less frequent Diagnostic molecular pathology Usually not required |
Epithelial cells: CK7+, CAM5.2+ Myoepithelial cells: p40+, p63+, SMA+, calponin+ Ki67 low PLAG1 (nuclear)+ GFAP +/− |
Essential: admixture of bilayered ducts, myoepithelial cells, and chondromyxoid/fibrous stroma in the absence of invasion and malignant cytomorphological features Desirable:PLAG1 or HMGA2 alteration (by IHC or molecular methods) in select cases |
[64–69, 73] |
|
Carcinoma ex PA
Malignant lacrimal gland neoplasm that shows presence of an epithelial and/or myoepithelial malignancy arising in association with primary or recurrent PA |
PLAG1 and HMGA2 rearrangements identical to PA Sequential loss of heterozygosity of 8q, 12q, 17p Alterations in 12q genes (HMGIC, HMGA2, MDM2) Copy number alterations in 9p and 22q, resulting in activation of NFIB and PDGFB Activation of IL6/JAK/STAT3 Diagnostic molecular pathologyPLAG1 or HMGA2 rearrangements serve as markers of pre-existing PA, differentiating from de novo lacrimal gland carcinomas |
Carcinoma areas: p53+, c-MYC+, Ki-67 high PA component: PLAG1+ and HMGA2+ Androgen receptor (AR)+ in carcinoma component may indicate lacrimal duct carcinoma differentiation |
Essential: histological areas compatible with PA or history of previous PA in the same site; presence of an epithelial and/or myoepithelial malignant component | [70–74] |
|
Adenoid cystic carcinoma
Malignant lacrimal gland neoplasm composed of epithelial and myoepithelial cells (EMCs) arranged in tubular, cribriform, and solid patterns associated with a basophilic matrix and reduplicated basement membrane material, frequently accompanied by MYB::NFIB rearrangements |
t(6; 9) (q22-23; p23-24) (up to 50%) results in MYB::NFIB fusion and activation of the MYB oncogene and associated target genes such as KIT and BCL2 which are involved in cell growth, apoptosis, transcription, and cell cycle regulation Loss of 6q, 12q, and 17p Gains of 19q, 8q, and 11q Aberrant NOTCH signaling KRAS, NRAS, Met mutations Diagnostic molecular pathology MYB rearrangement in select cases |
MYB+ (although less sensitive, may be used as a surrogate for MYB rearrangement in select cases) KIT+ p53 overexpression Mean Ki67 index 30% |
Essential: a malignant biphasic tumor composed of proliferating myoepithelial cells and luminal ductal cells Desirable: perineural invasion. Positive MYB, KIT IHC, or MYB rearrangement in challenging cases |
[66, 75–79, 96] |
|
Primary ductal adenocarcinoma of the lacrimal gland
High-grade adenocarcinoma and a counterpart of salivary duct carcinoma, resembling invasive ductal carcinoma of the breast |
Unknown, although at least a subset of cases show HER2 overexpression by IHC and/or HER2(ERBB2) gene amplification similar to salivary duct carcinoma Diagnostic molecular pathology HER2 amplification in select cases |
AE1/AE3+, CK7+, CK19+, EMA+, AR+, GCDFP-15+, HER-2/neu+, p53+, cyclin D1+ Usually SMA−, p63−, calponin−, ER−, PR−, PSA− Ki-67 up to 70% |
Essential: an invasive adenocarcinoma with ductal differentiation, arising de novo (not arising from a benign tumor such as PA) in the lacrimal gland Desirable: tumor comedonecrosis |
[80–84, 96] |
|
Secretory carcinoma
Rare monophasic lacrimal gland carcinoma characterized by a rearrangement of the ETV6 gene resulting in ETV6::NTRK3 fusion in most cases |
Lacrimal gland: ETV6 rearrangement resulting in ETV6::NTRK3 fusion Salivary glands: alternative ETV6 fusion partners include RET, MET, MAML3 Diagnostic molecular pathology ETV6 gene rearrangement can be demonstrated by breaking apart FISH. Detection of NTRK3 fusion by RT-PCR or RNA sequencing may be predictive of response to NTRK inhibitors for unresectable or metastatic cases |
S100+, SOX10+, mammaglobin+, CK18+, Cam5.2+, GCDFP-15+, NTRK+ (or pan-TRK+) CK5−, p63−, DOG-1, AR− Ki-67 < 5% |
Essential: monophasic tumor with vacuolated secretory material, lacking cytoplasmic granules; IHC positivity for S100 protein, mammaglobin, and NTRK (or Pan-TRK) and negativity for p40/p63, and CK5 Desirable:ETV6 and/or NTRK3 gene rearrangement |
[85–96] |
|
Mucoepidermoid carcinoma (MEC)
Malignant lacrimal gland neoplasm characterized by squamous (epidermoid) cells, mucous cells, columnar cells, and intermediate cells |
CTRC1::MAML2 gene fusion is present in majority lacrimal gland MEC and is considered a key oncogenic driver leading to EGFR upregulation RAS/PIK3 mutations in poorly differentiated tumors involved in EGFR upregulation Diagnostic molecular pathology MAML2 rearrangement/CTRC1::MAML2 fusion in select cases |
P63+, p40+ S100−, SOX10− |
Essential: squamous cells, intermediate cells, and mucous cells in varying proportions Lower grade tumors: cystic, mucous cell rich, and well circumscribed Higher grade tumors: solid, higher proportion of squamous and intermediate cells Desirable:MAML2 rearrangement/CRTC1::MAML2 fusion |
[96–98] |
|
EMC
Low-grade malignant biphasic tumor of the lacrimal gland characterized by tubular structures, usually composed of tightly coupled inner ductal and prominent outer myoepithelial cells |
HRAS mutations, most commonly at the codon 61 Diagnostic molecular pathology Assessment of HRAS mutations is useful for diagnosing EMC and excluding its mimics; MYB fusion by molecular methods/IHC might help in differentiation from adenoid cystic carcinoma and hybrid tumors |
Inner cells: panCK+, CK7+ Outer myoepithelial cells: p63+, SMA+, calponin+, S100+ Diffuse membranous and cytoplasmic RAS Q61R expression, almost always restricted to myoepithelial cells in 65% of EMCs |
Essential: usually multinodular invasive growth; at least partly with a dual arrangement of inner ductal cells and outer prominent, and usually clear, myoepithelial cells | [99–103] |
|
Acinic cell carcinoma
Rare malignant lacrimal gland neoplasm displaying predominantly serous acinar differentiation |
Salivary gland tumors: recurrent rearrangement [t(4; 9) (q13; q31)] leads to NR4A3 upregulation Diagnostic molecular pathology NR4A subfamily rearrangements by molecular or IHC methods in challenging cases |
NR4A3+ or NR4A2+ DOG1+, SOX10+ p63−, S100− Mammaglobin−, pan−TRK− |
Essential: tumor cells with acinar differentiation; zymogen granules in focal areas Desirable: nuclear staining for NR4A3/NOR-1 or NR4A2/Nuur1 Molecular demonstration of NR4A3 rearrangement |
[104–106] |
Tumors of the Orbit (Table 14)
Table 14.
WHO Eye5: tumors of the orbit [2]
| Teratomas, hamartomas, and choristomas |
| Choristomas and cysts |
| Orbital dermoid cysts and other orbital cysts |
| Vascular malformations |
| Cavernous vascular malformation |
| Combined lymphatic-venous malformation of the orbit |
| Orbital venous malformation |
| Arteriovenous malformation |
| Hamartomas |
| Orbital glial heterotopia |
| Mesenchymal hamartoma of the orbit |
| Germ cell tumors |
| Teratoma |
| Yolk sac tumor |
| Melanocytic tumors of the orbit |
| Primary orbital melanoma |
This new chapter, which focuses primarily on orbital teratomas, choristomas, and hamartomas, adopts, where possible, ISSVA terminology to classify vascular malformations of the eye and orbit [4]. Cavernous vascular malformation (cavernous hemangioma), combined lymphatic-venous malformation (lymphangioma), and orbital venous malformation (orbital varix) are discussed, with an update on their molecular genetics and pathogenesis [2]. Vascular neoplasms are covered in the chapter dedicated to soft tissue tumors.
Hematolymphoid Tumors (Table 15)
Table 15.
WHO Eye5: hematolymphoid tumors [2]
| Reactive lymphoid lesions |
| Reactive lymphoid lesions |
| Reactive lymphoid hyperplasia |
| IgG4-related disease |
| Intraocular hematolymphoid proliferations |
| Primary choroidal lymphoma (PCL) |
| Primary vitreoretinal large cell B-cell lymphoma (PVR-LCBL) |
| Ocular adnexal lymphomas |
| B-cell lymphomas |
| Extranodal marginal zone lymphoma |
| Follicular lymphoma |
| Mantle cell lymphoma |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| Diffuse large B-cell lymphoma |
| Plasmacytoma |
| T/NK cell lymphomas |
| CD30-positive lymphoproliferative disorders |
| T-cell lymphomas |
| Extranodal lymphoma of mature NK or T-cell lineage |
| Myeloid tumors |
| Extramedullary myeloid sarcoma |
| Histiocytic and dendritic cell tumors |
| Juvenile xanthogranuloma |
| Rosai-Dorfman disease |
| Erdheim-Chester disease |
| Langerhans cell tumors |
| Xanthelasma |
| Adult orbital xanthogranulomatous disease |
In this chapter, reactive lesions of the eye and orbit, and intraocular and ocular adnexal hematolymphoid proliferations are presented separately. The chapter provides an update on the molecular genetics, diagnostic cytopathology, diagnostic molecular pathology, and essential and desirable diagnostic criteria of intraocular lymphomas (shown in Fig. 7–9; Table 16) [107–129]. Ocular adnexal lymphomas, myeloid neoplasms, and histiocytic and dendritic cell neoplasms are comprehensively discussed, emphasizing site-specific characteristics and the most up-to-date immunophenotypic, molecular genetic, and prognostic features [2, 130, 131]. The material is coordinated with the 5th edition of WHO Classification of the Hematolymphoid Tumours [132].
Fig. 7.
Primary choroidal lymphoma (PCL), clinical and imaging findings. a Creamy choroidal infiltrates. b Optical coherence tomography demonstrates thickened choroid with an undulating hyperreflective anterior border (arrow) and obscuration of underlying choroidal vasculature from an infiltrative process.
Fig. 9.
Primary vitreoretinal large B-cell lymphoma (PVR-LCBL), clinical and cytopathologic findings (subretinal fine needle aspiration biopsy and vitrectomy). a Punctate and nodular subretinal pigment epithelial (RPE) deposits (arrow), geographic placoid gray subretinal deposits focally associated with hemorrhage (black arrowhead), and areas of RPE atrophy. b Optical coherence tomography highlights subretinal (arrowhead) and sub-RPE (arrow) deposits. c Large cells with irregular nuclear contours, prominent nucleoli, and scant to moderate amounts of cytoplasm. d Foci of granulomatous inflammation (paraneoplastic granulomatous vitritis). e The large atypical cells and the apoptotic debris are positive for CD20 (e) and co-express bcl6 (f) and interferon regulatory factor 4 (IRF4/MUM1) (g). CD10 is negative (h). Ki-67 proliferative index is brisk (i). Targeted MYD88 mutation studies detected MYD88 p.L265P mutation [hematoxylin-eosin (c, d), CD20 (e), bcl6 (f), MUM1 (g), CD10 (h), Ki-67 (i), ×630 (c), ×400 (d–i)].
Table 16.
WHO Eye5: intraocular hematolymphoid proliferations [2]
| Primary choroidal lymphoma (PCL) | Primary vitreoretinal large cell B-cell lymphoma (PVR-LCBL) | Ref | |
|---|---|---|---|
| Definition | PCL is an extranodal marginal zone B-cell lymphoma (EMZL) of the uveal tract without evidence of systemic lymphoma | PVR-LCBL is the most common high-grade lymphoma that arises in the eye and is frequently accompanied by involvement of the central nervous system (CNS) | [2] |
| Pathogenesis | Morphological, immunohistological, and genetic alterations are similar to those of EMZL at other locations Clonal expansion of post-germinal center (GC) B cells t(11; 18) (q21; q21) MYD88 mutations Dysregulation of the NF-κB, BCR, and PI3K signaling pathways |
PVR-LCBL cells demonstrate similarity to LCBL of immunoprivileged sites (IP-LCBL) Genetic alterations that enable immune escape and downregulation of specific immune reactions Features corresponding to mature GC-exit B cells, which have undergone a prolonged GC reaction with evidence of ongoing somatic hypermutation in their rearranged immunoglobulin (IG) genes Rearrangement in the IGHV4-34 gene (55%) Concordant MYD88 (L265P) and CD79B mutations (likely early events) The genomic signature resembles the C5/MCD/MYD88 signature found in diffuse large B-cell lymphoma NOS Other mutations: PIM1 (71%), IGLL5 (52%), TBL1XR1 (48%), and ETV6 (45%) Translocations in IG and BCL6 genes Frequent genetic imbalances, particularly 9p21/CDKN2A deletions (75%) |
[107–122, 126, 127, 129] |
| Histopathology (enucleation, tissue biopsy) | Diffuse infiltration of choroid by small-to-medium lymphocytes with mildly irregular nuclear contours, small-to-inconspicuous nucleoli, typically with expansion of small centrocyte-like lymphocytes, and varying numbers of plasmacytoid and monocytoid B cells Lymphoid follicles with GCs variably colonized by neoplastic cells The infiltrate may extend to ciliary body, iris and/or sub-conjunctival tissue, or even through Bruch’s membrane |
Large lymphocytes with irregular nuclear contours and prominent nucleoli, with brisk apoptosis/necrosis in the vitreous, neural retina, and/or beneath the RPE The highly characteristic sub-RPE infiltrates (of variable thickness) consist of neoplastic B cells accumulating anterior to the Bruch membrane There is perivascular accumulation of atypical lymphocytes, often associated with occlusion of retinal vessels |
[107, 108, 123–125, 127] |
| Diagnostic cytopathology | Monomorphous population of small atypical B cells | Large atypical B cells in a background of apoptosis, necrosis, small T cells, and macrophages | [125, 127] |
| Immunohistochemistry (IHC) and flow cytometry | Neoplastic cells are CD20+, CD79a+, PAX5+, BCL2+, IgM+, CD43+/−, MUM1+, CD5−, CD23−, CCND1−, CD10−, frequent light chain restriction, with a low Ki67 index | Neoplastic cells are CD20+, CD79a+, PAX5+, BCL2+, BCL6+, MUM1/IRF4+, IgM+, CD10− and have a high Ki67 index | [107] [121] |
| Diagnostic molecular pathology | Clonality analysis (IGH and/or IGK PCR) demonstrates clonal B-cell population | Clonality analysis of IGH genes demonstrates clonal B-cell population In rare cases where a PVR T-cell lymphoma is suspected, clonality analysis of the T-cell receptor (TCR) gene Mutational analysis: MYD88 and CD79B hotspot mutations (particularly useful in low cellularity cases) |
[108, 111, 116–119, 123, 125, 128] |
| Essential diagnostic criteria | Diffuse infiltrate of small- to medium-sized lymphoid cells in the uveal tissue Expression of B lineage markers Exclusion of systemic lymphoma with secondary ocular involvement |
Large B-cell lymphoma primarily confined to the vitreous or retina at presentation, with or without concurrent CNS lymphoma Exclusion of secondary involvement by a systemic DBCL (after clinicopathological correlation) |
[2] |
| Desirable diagnostic criteria | Demonstration of light chain restriction or clonal IG gene rearrangement | Post-GC B-cell phenotype (CD20+; CD79a+; MUM1+; BCL6+; CD10−) Demonstration of a high Ki67 growth fraction Absence of Epstein-Barr virus Demonstration of clonal B-cell population or a MYD88 and/or CD79B hotspot mutations in cases in which histology is not definitive (e.g., corticosteroid-mitigated PVR-LCBL) and paucicellular cytology) |
[2] |
Fig. 8.
Primary choroidal lymphoma (PCL), cytopathologic findings, fine needle aspiration biopsy. a Monomorphic small blue cells in a background of hemorrhage. Neoplastic cells express CD20 (b), bcl2 (c), and interferon regulatory factor 4 (IRF4/MUM1) (d). Neoplastic cells are negative for CD5, which highlights T cells (e) and CD10 (f). Overexpression of kappa light chain (g) over lambda (h). The Ki-67 labels rare nuclei (i) [hematoxylin-eosin (a), CD20 (b), bcl2 (c), MUM1 (d), CD5 (e), CD10 (f), kappa (g), lambda (h), ki-67 (i); all figures. ×400].
Soft Tissue and Bone Tumors (Table 17)
Table 17.
WHO Eye5: soft tissue and bone tumors [2]
| Soft tissue tumors |
| Fibrous, fibroblastic tumors |
| Conjunctival stromal tumor and ocular surface fibroma |
| Fibrous histiocytoma |
| Nodular and proliferative fasciitis, proliferative myositis |
| Solitary fibrous tumor |
| Inflammatory myofibroblastic tumor |
| Dermatofibrosarcoma protuberans |
| Angiomyxoma |
| Adipocytic tumors |
| Benign lipomatous lesions |
| Malignant adipocytic tumors |
| Skeletal muscle tumors |
| Rhabdomyosarcoma family |
| Smooth muscle tumors |
| Leiomyoma |
| Mesectodermal leiomyoma |
| Leiomyosarcoma |
| Peripheral nerve sheath tumors |
| Schwannoma |
| Neurofibroma |
| Solitary circumscribed neuroma |
| Granular cell tumor |
| Malignant peripheral nerve sheath tumor |
| Vascular tumors |
| Masson tumor |
| Infantile hemangioma |
| Lobular capillary hemangioma |
| Lymphangiectasia |
| Epithelioid hemangioma |
| Hemangioblastoma |
| Angiosarcoma |
| Perivascular tumors |
| Glomus tumor |
| Myopericytoma, including myofibroma |
| Tumors of uncertain derivation |
| PEComa |
| Epithelioid sarcoma |
| Synovial sarcoma |
| Alveolar soft part sarcoma |
| Tumors of orbital bone |
| Osteogenic tumors of orbital bone |
| Osteoma |
| Osteoblastoma |
| Osteosarcoma |
| Chondrogenic tumors of orbital bone |
| Soft tissue chondroma |
| Mesenchymal chondrosarcoma |
| Fibrous, fibroblastic tumors of orbital bone |
| Fibrous dysplasia |
| Vascular tumors of orbital bone |
| Hemangioma of bone |
| Epithelioid hemangioendothelioma |
| Angiosarcoma of bone |
| Osteoclast-rich giant cell tumors of orbital bone |
| Aneurysmal bone cyst |
| Giant cell tumor of bone |
| Other tumors of orbital bone |
| Ewing sarcoma |
This chapter provides salient clinical, radiologic, etiological, pathogenic, diagnostic, and prognostic information on each ocular and ocular adnexal soft tissue tumor, with an emphasis on site-specific characteristics. Vascular lesions classified as benign neoplasms of blood vessels, in accordance with the ISSVA nomenclature [4], such as infantile hemangioma and lobular capillary hemangioma, are covered. Vascular malformations are discussed in the chapter dedicated to the tumors of the orbit. The material is coordinated with the 5th edition of WHO Classification of the Soft Tissue and Bone Tumours [133].
Metastases to the Eye and Surrounding Structures
This chapter combines information from the prior edition on secondary and metastatic tumors to various ocular tissues into a separate section, Metastases to Intra-Ocular Structures [2]. Analogously, metastases to ocular adnexa are covered in a new section, Metastases to Ocular Adnexal Structures [2].
Genetic Tumor Predisposition Syndromes
This chapter synthesizes the most up-to-date clinical, epidemiologic, genetic, diagnostic, and prognostic information on the genetic tumor predisposition syndromes of particular relevance to the ophthalmologist: the retinoblastoma syndrome, BAP1 tumor predisposition syndrome, von Hippel-Lindau syndrome, Sturge-Weber syndrome, neurofibromatosis type 1, Goldenhar syndrome, and Muir-Torre syndrome [2]. The information is coordinated with the upcoming new WHO Classification of Tumours volume, on genetic tumor syndromes, which will be last of the 14 vol making up the 5th edition.
Conclusion
In conclusion, the WHO Eye5 provides an up-to-date comprehensive taxonomy for the range of tumors occurring in the eye and orbit with an emphasis on their site-specific features. The information presented is standardized across the 5th edition volumes. This systematic approach not only facilitates cross-referencing the information presented with other volumes within the classification, but also highlights the remaining gaps in knowledge. As such, the classification will not only serve as a valuable reference for a practicing ophthalmic oncologist and pathologist, but also drive future research bridging various oncology and pathology disciplines. It is hoped that the information conveyed in this overview will help to distill the major changes in the latest edition and will facilitate more detailed study of the volume.
Acknowledgments
The authors acknowledge the International Agency for Research on Cancer (IARC) staff, IARC editorial board, and authors, who have directly and indirectly contributed to the WHO Eye5 edition and other 5th edition WHO volumes, on whose behalf this paper has been prepared.
Statement of Ethics
Participants provided written informed consent for the publication of their details and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding received.
Author Contributions
Study design and data acquisition (T.M., H.E.G., I.A.C., G.G.-L., T.T.K., S.E.C., H.S.M., C.G.E., R.M.V., S.H., A.J.G., A.A.R., B.E., M.J.J., V.A.W., and J.C.H.), manuscript drafting (T.M.), and manuscript review (T.M., H.E.G., I.A.C., G.G.-L., T.M., H.E.G., I.A.C., G.G.-L., T.T.K., S.E.C., H.S.M., C.G.E., R.M.V., S.H., A.J.G., A.A.R., B.E., M.J.J., V.A.W., and J.C.H.). The content of this article represents the personal views of the authors and does not represent the views of the authors’ employers and associated institutions. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Funding Statement
No funding received.
References
- 1. International Agency for Research on Cancer [Internet]. Lyon: WHO Classification of Tumours Online. Available from: https://tumourclassification.iarc.who.int (accessed August 28, 2022). [Google Scholar]
- 2. WHO classification of tumours editorial board. Eye tumours [Internet; beta version ahead of print]. WHO classification of tumours series. 5th ed.Lyon (France): International Agency for Research on Cancer; 2023. vol. 13 [cited]. Available from: https://tumourclassification.iarc.who.int/chapters/??. [Google Scholar]
- 3. Grossniklaus HE, Eberhart CG, Kivelä TT. WHO classifiction of tumours of the eye. 4th ed.Lyon: International Agency for Research on Cancer; 2018. [Google Scholar]
- 4. International Society for the Study of Vascular Anomalies [Internet] . 2018 ISSVA classification of vascular anomalies [cited 2018, may]. Available from: issva.org/classification.
- 5. Bruford EA, Braschi B, Denny P, Jones TEM, Seal RL, Tweedie S. Guidelines for human gene nomenclature. Nat Genet. 2020 Aug;52(8):754–8. 10.1038/s41588-020-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016 Jun;37(6):564–9. 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 7. Cree IA, Tan PH, Travis WD, Wesseling P, Yagi Y, White VA, et al. Counting mitoses: SI(ze) matters!. Mod Pathol. 2021 Sep;34(9):1651–7. 10.1038/s41379-021-00825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Price HN. Congenital melanocytic nevi: update in genetics and management. Curr Opin Pediatr. 2016 Aug;28(4):476–82. 10.1097/MOP.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 9. Roh MR, Eliades P, Gupta S, Tsao H. Genetics of melanocytic nevi. Pigment Cell Melanoma Res. 2015 Nov;28(6):661–72. 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003 Jan;33(1):19–20. 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 11. Francis JH, Grossniklaus HE, Habib LA, Marr B, Abramson DH, Busam KJ, et al. BRAF, NRAS, and GNAQ mutations in conjunctival melanocytic nevi. Invest Ophthalmol Vis Sci. 2018 Jan;59(1):117–21. 10.1167/iovs.17-22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldenberg-Cohen N, Cohen Y, Rosenbaum E, Herscovici Z, Chowers I, Weinberger D, et al. T1799A BRAF mutations in conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2005 Sep;46(9):3027–30. 10.1167/iovs.04-1449. [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez-H Leon A, Chavez Y, Kamil ZS, Ghazarian D, Krema H. Diagnosis of the origin of an epibulbar melanocytic tumor with molecular genomics. Ophthalmic Genet. 2022 Aug;43(4):518–521. [DOI] [PubMed] [Google Scholar]
- 14. Yeh I, Lang UE, Durieux E, Tee MK, Jorapur A, Shain AH, et al. Combined activation of MAP kinase pathway and β-catenin signaling cause deep penetrating nevi. Nat Commun. 2017 Sep 21;8(1):644. 10.1038/s41467-017-00758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Šekoranja D, Vergot K, Hawlina G, Pižem J. Combined deep penetrating nevi of the conjunctiva are relatively common lesions characterised by BRAFV600E mutation and activation of the beta catenin pathway: a clinicopathological analysis of 34 lesions. Br J Ophthalmol. 2020 Jul;104(7):1016–21. 10.1136/bjophthalmol-2019-314807. [DOI] [PubMed] [Google Scholar]
- 16. van Ipenburg JA, Damman J, Paridaens D, Verdijk RM. Histopathological and molecular features of a conjunctival caruncular deep penetrating nevus. Ocul Oncol Pathol. 2020 Aug;6(4):293–6. 10.1159/000504966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eiger-Moscovich M, Eagle RC, Milman T, Acid-Schiff P. β-Catenin and periodic acid-schiff distinguish granular cell nevus from deep penetrating nevus. Arch Pathol Lab Med. 2021 Dec 1;145(12):1475–6. 10.5858/arpa.2021-0301-LE. [DOI] [PubMed] [Google Scholar]
- 18. Larsen AC, Dahl C, Dahmcke CM, Lade-Keller J, Siersma VD, Toft PB, et al. BRAF mutations in conjunctival melanoma: investigation of incidence, clinicopathological features, prognosis and paired premalignant lesions. Acta Ophthalmol. 2016 Aug;94(5):463–70. 10.1111/aos.13007. [DOI] [PubMed] [Google Scholar]
- 19. Griewank KG, Westekemper H, Schilling B, Livingstone E, Schimming T, Sucker A, et al. Conjunctival melanomas harbor BRAF and NRAS mutations–response. Clin Cancer Res. 2013 Nov 15;19(22):6331–2. 10.1158/1078-0432.CCR-13-2368. [DOI] [PubMed] [Google Scholar]
- 20. Scholz SL, Cosgarea I, Süßkind D, Murali R, Möller I, Reis H, et al. NF1 mutations in conjunctival melanoma. Br J Cancer. 2018 May;118(9):1243–7. 10.1038/s41416-018-0046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gardrat S, Houy A, Brooks K, Cassoux N, Barnhill R, Dayot S, et al. Definition of biologically distinct groups of conjunctival melanomas according to etiological factors and implications for precision medicine. Cancers. 2021 Jul 30;13(15):3836. 10.3390/cancers13153836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Poppelen NM, van Ipenburg JA, van den Bosch Q, Vaarwater J, Brands T, Eussen B, et al. Molecular genetics of conjunctival melanoma and prognostic value of TERT promoter mutation analysis. Int J Mol Sci. 2021 May 28;22(11):5784. 10.3390/ijms22115784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lally SE, Milman T, Orloff M, Dalvin LA, Eberhart CG, Heaphy CM, et al. Mutational landscape and outcomes of conjunctival melanoma in 101 patients. Ophthalmology. 2022 Jun;129(6):679–93. 10.1016/j.ophtha.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 24. Kenawy N, Kalirai H, Sacco JJ, Lake SL, Heegaard S, Larsen AC, et al. Conjunctival melanoma copy number alterations and correlation with mutation status, tumor features, and clinical outcome. Pigment Cell Melanoma Res. 2019 Jul;32(4):564–75. 10.1111/pcmr.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mudhar HS, Smith K, Talley P, Whitworth A, Atkey N, Rennie IG. Fluorescence In Situ Hybridisation (FISH) in histologically challenging conjunctival melanocytic lesions. Br J Ophthalmol. 2013 Jan;97(1):40–6. 10.1136/bjophthalmol-2012-302261. [DOI] [PubMed] [Google Scholar]
- 26. Milman T, Zhang Q, Ang S, Elder D, Ida CM, Salomao DR, et al. Conjunctival nevi and melanoma: multiparametric immunohistochemical analysis, including p16, SOX10, HMB45, and Ki-67. Hum Pathol. 2020 Sep;103:107–19. 10.1016/j.humpath.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 27. María Moral R, Monteagudo C, Muriel J, Moreno L, María Peiró A. Fluorescent In Situ Hybridization (FISH): a useful diagnostic tool for childhood conjunctival melanoma. Eur J Ophthalmol. 2021 Jul 9;32(6):NP13–9. 10.1177/11206721211030775. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28. Milman T, Eiger-Moscovich M, Henry RK, Folberg R, Coupland SE, Grossniklaus HE, et al. Validation of the newly proposed World Health Organization classification system for conjunctival melanocytic intraepithelial lesions: a comparison with the C-MIN and PAM classification schemes. Am J Ophthalmol. 2021 Mar;223:60–74. 10.1016/j.ajo.2020.10.020. [DOI] [PubMed] [Google Scholar]
- 29. Damato B, Coupland SE. Conjunctival melanoma and melanosis: a reappraisal of terminology, classification and staging. Clin Exp Ophthalmol. 2008 Nov;36(8):786–95. 10.1111/j.1442-9071.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- 30. Mudhar HS, Milman T, Zhang PJL, Shields CL, Eagle RC, Lally SE, et al. Conjunctival “mucoepidermoid carcinoma” revisited: a revision of terminology, based on morphologic, immunohistochemical and molecular findings of 14 cases, and the 2018 WHO Classification of Tumours of the Eye. Mod Pathol. 2020 Jul;33(7):1242–55. 10.1038/s41379-020-0456-9. [DOI] [PubMed] [Google Scholar]
- 31. Agni M, Raven ML, Bowen RC, Laver NV, Chevez-Barrios P, Milman T, et al. An update on endocrine mucin-producing sweat gland carcinoma: clinicopathologic study of 63 cases and comparative analysis. Am J Surg Pathol. 2020 Aug;44(8):1005–16. 10.1097/PAS.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yonekawa Y, Jakobiec FA, Zakka FR, Fay A. Sebaceoma of the eyelid. Ophthalmology. 2012 Dec;119(12):2645.e1–4. 10.1016/j.ophtha.2012.07.054. [DOI] [PubMed] [Google Scholar]
- 33. Misago N, Mihara I, Ansai S, Narisawa Y. Sebaceoma and related neoplasms with sebaceous differentiation: a clinicopathologic study of 30 cases. Am J Dermatopathol. 2002 Aug;24(4):294–304. 10.1097/00000372-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 34. Tetzlaff MT, Singh RR, Seviour EG, Curry JL, Hudgens CW, Bell D, et al. Next-generation sequencing identifies high frequency of mutations in potentially clinically actionable genes in sebaceous carcinoma. J Pathol. 2016 Sep;240(1):84–95. 10.1002/path.4759. [DOI] [PubMed] [Google Scholar]
- 35. Peterson C, Moore R, Hicks JL, Morsberger LA, De Marzo AM, Zou Y, et al. NGS analysis confirms common TP53 and RB1 mutations, and suggests MYC amplification in ocular adnexal sebaceous carcinomas. Int J Mol Sci. 2021 Aug 6;22(16):8454. 10.3390/ijms22168454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tetzlaff MT, Curry JL, Ning J, Sagiv O, Kandl TL, Peng B, et al. Distinct biological types of ocular adnexal sebaceous carcinoma: HPV-driven and virus-negative tumors arise through nonoverlapping molecular-genetic alterations. Clin Cancer Res. 2019 Feb 15;25(4):1280–90. 10.1158/1078-0432.CCR-18-1688. [DOI] [PubMed] [Google Scholar]
- 37. Saro F, Clua A, Esteva E, Carreras A, Adán A, Lerma E. Cytologic diagnosis of ocular melanocytoma: a case report. Acta Cytol. 2008 Jan-Feb;52(1):87–90. 10.1159/000325440. [DOI] [PubMed] [Google Scholar]
- 38. Grossniklaus HE. Fine-needle aspiration biopsy of the iris. Arch Ophthalmol. 1992 Jul;110(7):969–76. 10.1001/archopht.1992.01080190075033. [DOI] [PubMed] [Google Scholar]
- 39. Char DH, Kemlitz AE, Miller T, Crawford JB. Iris ring melanoma: fine needle biopsy. Br J Ophthalmol. 2006 Apr;90(4):420–2. 10.1136/bjo.2005.088294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hussain RN, Kalirai H, Groenewald C, Kacperek A, Errington RD, Coupland SE, et al. Prognostic biopsy of choroidal melanoma after proton beam radiation therapy. Ophthalmology. 2016 Oct;123(10):2264–5. 10.1016/j.ophtha.2016.05.028. [DOI] [PubMed] [Google Scholar]
- 41. Jager MJ, Shields CL, Cebulla CM, Abdel-Rahman MH, Grossniklaus HE, Stern MH, et al. Uveal melanoma. Nat Rev Dis Primers. 2020 Apr 9;6(1):24. 10.1038/s41572-020-0158-0. [DOI] [PubMed] [Google Scholar]
- 42. Abbott DW, Simons K, Giorgadze T. Choroidal melanoma diagnosed by aspiration cytology: a case report with cyto-histologic correlation and review of the literature. Ann Diagn Pathol. 2019 Oct;42:39–41. 10.1016/j.anndiagpath.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 43. Solomon DA, Ramani B, Eiger-Moscovich M, Milman T, Uludag G, Crawford JB, et al. Iris and ciliary body melanocytomas are defined by solitary GNAQ mutation without additional oncogenic alterations. Ophthalmology. 2022 Dec;129(12):1429–39. 10.1016/j.ophtha.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 44. Niffenegger JH, Soltero A, Niffenegger JS, Yang S, Adamus G. Prevalence of Hepatocyte Growth Factor and autoantibodies to α-HGF as a new etiology for bilateral diffuse uveal melanocytic proliferation masquerading as neovascular age-related macular degeneration. J Clin Exp Ophthalmol. 2018;9(4):740. 10.4172/2155-9570.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mudhar HS, Bata BM, Quhill H, Milman T, Salvi SM, Perivascular P. Uveal melanoma and paraneoplastic perivascular dermal melanocytic proliferation in the setting of bilateral diffuse uveal melanocytic proliferation: the potential role of the hepatocyte growth factor/c-met Axis in their pathogenesis. Ocul Oncol Pathol. 2021 Dec;7(6):418–27. 10.1159/000519177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dimaras H, Khetan V, Halliday W, Orlic M, Prigoda NL, Piovesan B, et al. Loss of RB1 induces non-proliferative retinoma: increasing genomic instability correlates with progression to retinoblastoma. Hum Mol Genet. 2008 May 15;17(10):1363–72. 10.1093/hmg/ddn024. [DOI] [PubMed] [Google Scholar]
- 47. Eagle RC Jr. High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Arch Pathol Lab Med. 2009 Aug;133(8):1203–9. 10.5858/133.8.1203. [DOI] [PubMed] [Google Scholar]
- 48. Skalet AH, Gombos DS, Gallie BL, Kim JW, Shields CL, Marr BP, et al. Screening children at risk for retinoblastoma: consensus report from the American association of ophthalmic oncologists and pathologists. Ophthalmology. 2018 Mar;125(3):453–8. 10.1016/j.ophtha.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 49. Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. 2013 Apr;14(4):327–34. 10.1016/S1470-2045(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 50. Kim ME, Polski A, Xu L, Prabakar RK, Peng CC, Reid MW, et al. Comprehensive somatic copy number analysis using aqueous humor liquid biopsy for retinoblastoma. Cancers. 2021 Jul 3;13(13):3340. 10.3390/cancers13133340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gerrish A, Jenkinson H, Cole T. The impact of cell-free DNA analysis on the management of retinoblastoma. Cancers. 2021 Mar 29;13(7):1570. 10.3390/cancers13071570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abramson DH. Cell free DNA (cfDNA) in the blood of retinoblastoma patients the robert M. Ellsworth lecture. Ophthalmic Genet. 2022 Apr 5;43(6):731–735. 10.1080/13816810.2021.2004433. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh G, Gupta R, Kakkar A, Iyer VK, Kashyap S, Bakhshi S, et al. Fine needle aspiration cytology of metastatic ocular medulloepithelioma. Cytopathology. 2011 Oct;22(5):343–5. 10.1111/j.1365-2303.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 54. Mahdjoubi A, Cassoux N, Levy-Gabriel C, Desjardins L, Klos J, Caly M, et al. Adult ocular medulloepithelioma diagnosed by transscleral fine needle aspiration: a case report. Diagn Cytopathol. 2017 Jun;45(6):561–4. 10.1002/dc.23694. [DOI] [PubMed] [Google Scholar]
- 55. Saunders T, Margo CE. Intraocular medulloepithelioma. Arch Pathol Lab Med. 2012;136(2):212–6. 10.5858/arpa.2010-0669-RS. [DOI] [PubMed] [Google Scholar]
- 56. Babu N, Dey P. Medulloepithelioma of ciliary body diagnosed by fine needle aspiration cytology. Cytopathology. 2003 Apr;14(2):93–4. 10.1046/j.1365-2303.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 57. Mudhar HS, Milman T, Eagle RC Jr, Sanderson T, Pheasey L, Paine S, et al. Usefulness of PAX8 immunohistochemistry in adult intraocular tumor diagnosis. Ophthalmology. 2021 May;128(5):765–78. 10.1016/j.ophtha.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 58. Char DH, Miller TR, Crawford JB. Cytopathologic diagnosis of benign lesions simulating choroidal melanomas. Trans Am Ophthalmol Soc 1991;112(1):235–44; discussion 70–75. 10.1016/s0002-9394(14)76216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mori T, Sukeda A, Sekine S, Shibata S, Ryo E, Okano H, et al. SOX10 expression as well as BRAF and GNAQ/11 mutations distinguish pigmented ciliary epithelium neoplasms from uveal melanomas. Invest Ophthalmol Vis Sci. 2017;58(12):5445–51. 10.1167/iovs.17-22362. [DOI] [PubMed] [Google Scholar]
- 60. Shields JA, Eagle RC Jr, Dutton J, Ehya H, Shields CL. Adenocarcinoma of the retinal pigment epithelium: clinicopathologic correlation with paradoxical immunohistochemical findings. JAMA Ophthalmol. 2014 Oct;132(10):1249–52. 10.1001/jamaophthalmol.2014.2369. [DOI] [PubMed] [Google Scholar]
- 61. Benson JC, Giannini C, Cohen Cohen S, Van Gompel J, Kim DK, Port J, et al. Optic nerve choristoma mimicking a neurenteric cyst. AJNR Am J Neuroradiol. 2021 Jan;42(2):228–32. 10.3174/ajnr.A6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harrison W, Pittman P, Cummings T. Optic nerve choristoma. Saudi J Ophthalmol. 2018 Jan-Mar;32(1):90–1. 10.1016/j.sjopt.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. WHO Classification of Tumours Editorial Board . World Health organization classification of tumours of the central nervous system. 5th ed.Lyon: International Agency for Research on Cancer; 2021. [Google Scholar]
- 64. Andreasen S, von Holstein SL, Homøe P, Heegaard S. Recurrent rearrangements of the PLAG1 and HMGA2 genes in lacrimal gland pleomorphic adenoma and carcinoma ex pleomorphic adenoma. Acta Ophthalmol. 2018 Nov;96(7):e768–71. 10.1111/aos.13667. [DOI] [PubMed] [Google Scholar]
- 65. Harrison W, Pittman P, Cummings T. Pleomorphic adenoma of the lacrimal gland: a review with updates on malignant transformation and molecular genetics. Saudi J Ophthalmol. 2018 Jan-Mar;32(1):13–6. 10.1016/j.sjopt.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mendoza PR, Jakobiec FA, Krane JF. Immunohistochemical features of lacrimal gland epithelial tumors. Am J Ophthalmol. 2013 Dec;156(6):1147–58. e1. 10.1016/j.ajo.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 67. Jakobiec FA, Stagner AM, Eagle RC Jr, Lally SE, Krane JF. Unusual pleomorphic adenoma of the lacrimal Gland: immunohistochemical demonstration of PLAG1 and HMGA2 oncoproteins. Surv Ophthalmol. 2017 Mar-Apr;62(2):219–26. 10.1016/j.survophthal.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 68. Shah SS, Chandan VS, Wilbur DC, Khurana KK. Glial fibrillary acidic protein and CD57 immunolocalization in cell block preparations is a useful adjunct in the diagnosis of pleomorphic adenoma. Arch Pathol Lab Med. 2007 Sep;131(9):1373–7. 10.5858/2007-131-1373-GFAPAC. [DOI] [PubMed] [Google Scholar]
- 69. Curran AE, Allen CM, Beck FM, Damm DD, Murrah VA. Distinctive pattern of glial fibrillary acidic protein immunoreactivity useful in distinguishing fragmented pleomorphic adenoma, canalicular adenoma and polymorphous low grade adenocarcinoma of minor salivary glands. Head Neck Pathol. 2007 Sep;1(1):27–32. 10.1007/s12105-007-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tom A, Bell D, Ford JR, Debnam JM, Guo Y, Frank SJ, et al. Malignant mixed tumor (carcinoma ex pleomorphic adenoma) of the lacrimal gland. Ophthalmic Plast Reconstr Surg. 2020 Sep;36(5):497–502. 10.1097/IOP.0000000000001625. [DOI] [PubMed] [Google Scholar]
- 71. Neerukonda VK, Carruth B, Estopinal MDV. Invasive carcinoma ex-pleomorphic adenoma of the lacrimal gland with a cystadenocarcinoma component: a case report and review of the literature. Case Rep Pathol. 2020;2020:6482837. 10.1155/2020/6482837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Andreasen S, Heegaard S, Grauslund M, Homøe P. The interleukin-6/Janus kinase/STAT3 pathway in pleomorphic adenoma and carcinoma ex pleomorphic adenoma of the lacrimal gland. Acta Ophthalmol. 2016 Dec;94(8):798–804. 10.1111/aos.13122. [DOI] [PubMed] [Google Scholar]
- 73. Katabi N, Xu B, Jungbluth AA, Zhang L, Shao SY, Lane J, et al. PLAG1 immunohistochemistry is a sensitive marker for pleomorphic adenoma: a comparative study with PLAG1 genetic abnormalities. Histopathology. 2018 Jan;72(2):285–93. 10.1111/his.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang P, Tang LJ, Gao HH, Zhang WX, Lin JX, Yang HS. Immunohistochemical features of carcinoma ex pleomorphic adenoma and pleomorphic adenoma in the lacrimal gland. Int J Ophthalmol. 2019 Aug 18;12(8):1238–42. 10.18240/ijo.2019.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. von Holstein SL, Fehr A, Persson M, Therkildsen MH, Prause JU, Heegaard S, et al. Adenoid cystic carcinoma of the lacrimal gland: MYB gene activation, genomic imbalances, and clinical characteristics. Ophthalmology. 2013 Oct;120(10):2130–8. 10.1016/j.ophtha.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 76. Chen TY, Keeney MG, Chintakuntlawar AV, Knutson DL, Kloft-Nelson S, Greipp PT, et al. Adenoid cystic carcinoma of the lacrimal gland is frequently characterized by MYB rearrangement. Eye. 2017 May;31(5):720–5. 10.1038/eye.2016.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sant DW, Tao W, Field MG, Pelaez D, Jin K, Capobianco A, et al. Whole exome sequencing of lacrimal gland adenoid cystic carcinoma. Invest Ophthalmol Vis Sci. 2017 May 1;58(6):BIO240–6. 10.1167/iovs.16-21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bell D, Sniegowski MC, Wani K, Prieto V, Esmaeli B. Mutational landscape of lacrimal gland carcinomas and implications for treatment. Head Neck. 2016 Apr;38(Suppl 1):E724–9. 10.1002/hed.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Duarte AF, Alpuim Costa D, Caçador N, Boavida AM, Afonso AM, Vilares M, et al. Adenoid cystic carcinoma of the palpebral lobe of the lacrimal gland - case report and literature review. Orbit. 2022 Oct;41(5):605–10. 10.1080/01676830.2021.1901293. [DOI] [PubMed] [Google Scholar]
- 80. Andreasen S, Grauslund M, Heegaard S. Lacrimal gland ductal carcinomas: clinical, Morphological and Genetic characterization and implications for targeted treatment. Acta Ophthalmol. 2017 May;95(3):299–306. 10.1111/aos.13310. [DOI] [PubMed] [Google Scholar]
- 81. Kubota T, Moritani S, Ichihara S. Clinicopathologic and immunohistochemical features of primary ductal adenocarcinoma of lacrimal gland: five new cases and review of literature. Graefes Arch Clin Exp Ophthalmol. 2013 Aug;251(8):2071–6. 10.1007/s00417-013-2350-3. [DOI] [PubMed] [Google Scholar]
- 82. Zhu MM, Cui HG, Teng XD. GCDFP-15, AR, and Her-2 as biomarkers for primary ductal adenocarcinoma of the lacrimal gland: a Chinese case and literature review. Onco Targets Ther. 2015 May 11;8:1017–24. 10.2147/OTT.S82168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. See TRO, Stålhammar G, Tang T, Manusow JS, Jordan DR, Nerad JA, et al. Primary ductal adenocarcinoma of the lacrimal gland: a review and report of five cases. Surv Ophthalmol. 2020 May-Jun;65(3):371–80. 10.1016/j.survophthal.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tripathy D, Agarwal S, Biala A, Rath S, Mittal R. Primary De novo ductal adenocarcinoma of the lacrimal gland. Ann Diagn Pathol. 2021 Feb;50:151651. 10.1016/j.anndiagpath.2020.151651. [DOI] [PubMed] [Google Scholar]
- 85. Hyrcza MD, Andreasen S, Melchior LC, Tucker T, Heegaard S, White VA. Primary secretory carcinoma of the lacrimal gland: report of a new entity. Am J Ophthalmol. 2018 Sep;193:178–83. 10.1016/j.ajo.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 86. Bortz JG, Zhang PJL, Eagle RC Jr, Yong JJ, Milman T. Secretory carcinoma of the lacrimal gland: a rare case report. Ophthalmic Plast Reconstr Surg. 2018 Sep;34(5):e154–7. 10.1097/IOP.0000000000001173. [DOI] [PubMed] [Google Scholar]
- 87. Bao Y, Li J, Zhu Y. Mammary analog secretory carcinoma with ETV6 rearrangement arising in the conjunctiva and eyelid. Am J Dermatopathol. 2018 Jul;40(7):531–5. 10.1097/DAD.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 88. Armijos PO, Uhlenhake E, Milman T. Secretory carcinoma of the eyelid arising in an adnexal gland. Ophthalmology. 2022 Jun 23;129(10):1218–6420. 10.1016/j.ophtha.2022.02.024. [DOI] [PubMed] [Google Scholar]
- 89. Ito Y, Ishibashi K, Masaki A, Fujii K, Fujiyoshi Y, Hattori H, et al. Mammary analogue secretory carcinoma of salivary glands: a clinicopathologic and molecular study including 2 cases harboring ETV6-X fusion. Am J Surg Pathol. 2015 May;39(5):602–10. 10.1097/PAS.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 90. Skálová A, Vanecek T, Simpson RH, Laco J, Majewska H, Baneckova M, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6-NTRK3 fusion transcript by standard RT-PCR: report of 4 cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016 Jan;40(1):3–13. 10.1097/PAS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 91. Skalova A, Vanecek T, Martinek P, Weinreb I, Stevens TM, Simpson RHW, et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation: report of 10 cases. Am J Surg Pathol. 2018 Feb;42(2):234–46. 10.1097/PAS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 92. Rooper LM, Karantanos T, Ning Y, Bishop JA, Gordon SW, Kang H. Salivary secretory carcinoma with a novel ETV6-MET fusion: expanding the molecular spectrum of a recently described entity. Am J Surg Pathol. 2018 Aug;42(8):1121–6. 10.1097/PAS.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 93. Guilmette J, Dias-Santagata D, Nosé V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019 Jan;83:50–8. 10.1016/j.humpath.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 94. Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol. 2016 May;27(5):920–6. 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019 Nov;30(Suppl 8):viii23–30. 10.1093/annonc/mdz282. [DOI] [PubMed] [Google Scholar]
- 96. Skálová A, Stenman G, Simpson RHW, Hellquist H, Slouka D, Svoboda T, et al. The role of molecular testing in the differential diagnosis of salivary gland carcinomas. Am J Surg Pathol. 2018 Feb;42(2):e11–27. 10.1097/PAS.0000000000000980. [DOI] [PubMed] [Google Scholar]
- 97. Chen Z, Ni W, Li JL, Lin S, Zhou X, Sun Y, et al. The CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight. 2021 Apr 8;6(7):e139497. 10.1172/jci.insight.139497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Morita M, Murase T, Okumura Y, Ueda K, Sakamoto Y, Masaki A, et al. Clinicopathological significance of EGFR pathway gene mutations and CRTC1/3-MAML2 fusions in salivary gland mucoepidermoid carcinoma. Histopathology. 2020 Jun;76(7):1013–22. 10.1111/his.14100. [DOI] [PubMed] [Google Scholar]
- 99. Urano M, Nakaguro M, Yamamoto Y, Hirai H, Tanigawa M, Saigusa N, et al. Diagnostic significance of HRAS mutations in epithelial-myoepithelial carcinomas exhibiting a broad histopathologic spectrum. Am J Surg Pathol. 2019 Jul;43(7):984–94. 10.1097/PAS.0000000000001258. [DOI] [PubMed] [Google Scholar]
- 100. Nakaguro M, Nagao T. Epithelial-Myoepithelial carcinoma. Surg Pathol Clin. 2021 Mar;14(1):97–109. 10.1016/j.path.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 101. Bishop JA, Westra WH. MYB translocation status in salivary gland epithelial-myoepithelial carcinoma: evaluation of classic, variant, and hybrid forms. Am J Surg Pathol. 2018 Mar;42(3):319–25. 10.1097/PAS.0000000000000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007 Jan;31(1):44–57. 10.1097/01.pas.0000213314.74423.d8. [DOI] [PubMed] [Google Scholar]
- 103. Nakaguro M, Tanigawa M, Hirai H, Yamamoto Y, Urano M, Takahashi RH, et al. The diagnostic utility of RAS Q61R mutation-specific immunohistochemistry in epithelial-myoepithelial carcinoma. Am J Surg Pathol. 2021 Jul 1;45(7):885–94. 10.1097/PAS.0000000000001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Haller F, Bieg M, Will R, Körner C, Weichenhan D, Bott A, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat Commun. 2019 Jan 21;10(1):368. 10.1038/s41467-018-08069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Haller F, Skálová A, Ihrler S, Märkl B, Bieg M, Moskalev EA, et al. Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am J Surg Pathol. 2019 Sep;43(9):1264–72. 10.1097/PAS.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 106. Wong KS, Mariño-Enriquez A, Hornick JL, Jo VY. NR4A3 immunohistochemistry reliably discriminates acinic cell carcinoma from mimics. Head Neck Pathol. 2021 Jun;15(2):425–32. 10.1007/s12105-020-01213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Coupland SE, Foss HD, Hidayat AA, Cockerham GC, Hummel M, Stein H. Extranodal marginal zone B cell lymphomas of the uvea: an analysis of 13 cases. J Pathol. 2002 Jul;197(3):333–40. 10.1002/path.1130. [DOI] [PubMed] [Google Scholar]
- 108. Coupland SE, Damato B. Understanding intraocular lymphomas. Clin Exp Ophthalmol. 2008 Aug;36(6):564–78. 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 109. Martinez-Lopez A, Curiel-Olmo S, Mollejo M, Cereceda L, Martinez N, Montes-Moreno S, et al. MYD88 (L265P) somatic mutation in marginal zone B-cell lymphoma. Am J Surg Pathol. 2015 May;39(5):644–51. 10.1097/PAS.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 110. Lue JK, O’Connor OA, Bertoni F. Targeting pathogenic mechanisms in marginal zone lymphoma: from concepts and beyond. Ann Lymphoma. 2020 Sep 30;4:7. 10.21037/aol-20-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Coupland SE, Hummel M, Müller HH, Stein H. Molecular analysis of immunoglobulin genes in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005 Oct;46(10):3507–14. 10.1167/iovs.05-0401. [DOI] [PubMed] [Google Scholar]
- 112. Belhouachi N, Xochelli A, Boudjoghra M, Lesty C, Cassoux N, Fardeau C, et al. Primary vitreoretinal lymphomas display a remarkably restricted immunoglobulin gene repertoire. Blood Adv. 2020 Apr 14;4(7):1357–66. 10.1182/bloodadvances.2019000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bonzheim I, Sander P, Salmerón-Villalobos J, Süsskind D, Szurman P, Gekeler F, et al. The molecular hallmarks of primary and secondary vitreoretinal lymphoma. Blood Adv. 2022 Mar 8;6(5):1598–607. 10.1182/bloodadvances.2021004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Riemersma SA, Jordanova ES, Schop RF, Philippo K, Looijenga LH, Schuuring E, et al. Extensive genetic alterations of the HLA region, including homozygous deletions of HLA class II genes in B-cell lymphomas arising in immune-privileged sites. Blood. 2000 Nov 15;96(10):3569–77. 10.1182/blood.v96.10.3569. [DOI] [PubMed] [Google Scholar]
- 115. Booman M, Douwes J, Glas AM, Riemersma SA, Jordanova ES, Kok K, et al. Mechanisms and effects of loss of human leukocyte antigen class II expression in immune-privileged site-associated B-cell lymphoma. Clin Cancer Res. 2006 May 1;12(9):2698–705. 10.1158/1078-0432.CCR-05-2617. [DOI] [PubMed] [Google Scholar]
- 116. Pulido JS, Salomao DR, Frederick LA, Viswanatha DS. MyD-88 L265P mutations are present in some cases of vitreoretinal lymphoma. Retina. 2015 Apr;35(4):624–7. 10.1097/IAE.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 117. Bonzheim I, Giese S, Deuter C, Süsskind D, Zierhut M, Waizel M, et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: a valuable tool to improve diagnostic yield of vitreous aspirates. Blood. 2015 Jul 2;126(1):76–9. 10.1182/blood-2015-01-620518. [DOI] [PubMed] [Google Scholar]
- 118. Hiemcke-Jiwa LS, Ten Dam-van Loon NH, Leguit RJ, Nierkens S, Ossewaarde-van Norel J, de Boer JH, et al. Potential diagnosis of vitreoretinal lymphoma by detection of MYD88 mutation in aqueous humor with ultrasensitive droplet digital polymerase chain reaction. JAMA Ophthalmol. 2018 Oct 1;136(10):1098–104. 10.1001/jamaophthalmol.2018.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yonese I, Takase H, Yoshimori M, Onozawa E, Tsuzura A, Miki T, et al. CD79B mutations in primary vitreoretinal lymphoma: diagnostic and prognostic potential. Eur J Haematol. 2019 Feb;102(2):191–6. 10.1111/ejh.13191. [DOI] [PubMed] [Google Scholar]
- 120. Lee J, Kim B, Lee H, Park H, Ho Byeon S, Choi JR, et al. Whole exome sequencing identifies mutational signatures of vitreoretinal lymphoma. Haematologica. 2020 Sep 1;105(9):e458–60. 10.3324/haematol.2019.233783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lacy SE, Barrans SL, Beer PA, Painter D, Smith AG, Roman E, et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: a Haematological Malignancy Research Network report. Blood. 2020 May 14;135(20):1759–71. 10.1182/blood.2019003535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Balikov DA, Hu K, Liu CJ, Betz BL, Chinnaiyan AM, Devisetty LV, et al. Comparative molecular analysis of primary central nervous system lymphomas and matched vitreoretinal lymphomas by vitreous liquid biopsy. Int J Mol Sci. 2021 Sep 16;22(18):9992. 10.3390/ijms22189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Coupland SE, Joussen A, Anastassiou G, Stein H. Diagnosis of a primary uveal extranodal marginal zone B-cell lymphoma by chorioretinal biopsy: case report. Graefes Arch Clin Exp Ophthalmol. 2005 May;243(5):482–6. 10.1007/s00417-004-1050-4. [DOI] [PubMed] [Google Scholar]
- 124. Mashayekhi A, Shukla SY, Shields JA, Shields CL. Choroidal lymphoma: clinical features and association with systemic lymphoma. Ophthalmology. 2014 Jan;121(1):342–51. 10.1016/j.ophtha.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 125. Fend F, Ferreri AJ, Coupland SE. How we diagnose and treat vitreoretinal lymphoma. Br J Haematol. 2016 Jun;173(5):680–92. 10.1111/bjh.14025. [DOI] [PubMed] [Google Scholar]
- 126. Arai A, Takase H, Yoshimori M, Yamamoto K, Mochizuki M, Miura O. Gene expression profiling of primary vitreoretinal lymphoma. Cancer Sci. 2020 Apr;111(4):1417–21. 10.1111/cas.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tan WJ, Wang MM, Ricciardi-Castagnoli P, Chan ASY, Lim TS, Diagnostics M. Cytologic and molecular diagnostics for vitreoretinal lymphoma: current approaches and emerging single-cell analyses. Front Mol Biosci. 2020;7:611017. 10.3389/fmolb.2020.611017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tanaka R, Kaburaki T, Taoka K, Karakawa A, Tsuji H, Nishikawa M, et al. More accurate diagnosis of vitreoretinal lymphoma using a combination of diagnostic test results: a prospective observational study. Ocul Immunol Inflamm. 2021 Apr 1;30(6):1354–60. 10.1080/09273948.2021.1873394. [DOI] [PubMed] [Google Scholar]
- 129. Wang X, Su W, Gao Y, Feng Y, Wang X, Chen X, et al. A pilot study of the use of dynamic analysis of cell-free DNA from aqueous humor and vitreous fluid for the diagnosis and treatment monitoring of vitreoretinal lymphomas. Haematologica. 2022 Sep 1;107(9):2154–62. 10.3324/haematol.2021.279908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health organization classification of haematolymphoid tumours: myeloid and histiocytic/Dendritic Neoplasms. Leukemia. 2022 Jul;36(7):1703–19. 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBd O, Berti E, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022 Jul;36(7):1720–48. 10.1038/s41375-022-01620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. WHO Classification of Tumours Editorial Board . World Health organization classification of hematolymphoid tumors. 5th ed. Lyon: International Agency for Research on Cancer; in press. [Google Scholar]
- 133. WHO Classification of Tumours Editorial Board . World Health organization classification of soft tissue and bone tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2020. [Google Scholar]