Abstract
Introduction
KEYNOTE-240 showed a favorable benefit/risk profile for pembrolizumab versus placebo in patients with sorafenib-treated advanced hepatocellular carcinoma (HCC); however, prespecified statistical significance criteria for overall survival (OS) and progression-free survival (PFS) superiority were not met at the final analysis. Outcomes based on an additional 18 months of follow-up are reported.
Methods
Adults with sorafenib-treated advanced HCC were randomized 2:1 to pembrolizumab 200 mg intravenously every 3 weeks or placebo. Dual primary endpoints were OS and PFS assessed per RECIST v1.1 by blinded independent central review (BICR). Secondary endpoints included objective response rate (ORR), assessed per RECIST v1.1 by BICR, and safety.
Results
413 patients were randomized (pembrolizumab, n = 278; placebo, n = 135). As of July 13, 2020, median (range) time from randomization to data cutoff was 39.6 (31.7–48.8) months for pembrolizumab and 39.8 (31.7–47.8) months for placebo. Estimated OS rates (95% CI) were 17.7% (13.4–22.5%) for pembrolizumab and 11.7% (6.8–17.9%) for placebo at 36 months. The estimated PFS rate (95% CI) for pembrolizumab was 8.9% (5.3–13.6%) and 0% for placebo at 36 months. ORR (95% CI) was 18.3% (14.0–23.4%) for pembrolizumab and 4.4% (1.6–9.4%) for placebo. Immune-mediated hepatitis events did not increase with follow-up. No viral hepatitis flare events were reported.
Conclusion
With extended follow-up, pembrolizumab continued to maintain improvement in OS and PFS and was associated with a consistent adverse event profile compared with placebo in patients with sorafenib-treated advanced HCC. Although KEYNOTE-240 did not meet prespecified statistical significance criteria at the final analysis, these results together with the antitumor activity of second-line pembrolizumab observed in KEYNOTE-224 and the statistically significant and clinically meaningful OS and PFS benefits of second-line pembrolizumab in patients from Asia observed in KEYNOTE-394 reinforce the clinical activity of pembrolizumab in previously treated patients with advanced HCC.
Keywords: Pembrolizumab, Programmed cell death protein 1, Hepatocellular carcinoma, Long-term follow-up
Introduction
Morbidity and mortality of primary liver cancer remain high globally despite improvements in detection and therapeutic management. Worldwide age-standardized incidence and mortality rates per 100,000 persons in 2020 were 9.5 and 8.7, respectively [1]. The most common histologic type of primary liver cancer is hepatocellular carcinoma (HCC), which accounts for up to 85% of all primary liver cancer cases [2]. Systemic treatment options in the first-line setting for patients with advanced stage HCC have evolved rapidly, and options now include sorafenib [3], lenvatinib [4], atezolizumab plus bevacizumab [5, 6], and durvalumab plus tremelimumab [7]. When disease progression occurs or when patients are unable to tolerate first-line treatment, second-line options include regorafenib [8], cabozantinib [9], and ramucirumab (in patients with an α-fetoprotein level ≥400 ng/mL) [10] and, in the USA, pembrolizumab [11, 12] and nivolumab with ipilimumab [13].
The programmed cell death protein 1 inhibitor pembrolizumab received accelerated regulatory approval from the US Food and Drug Administration on the basis of the global phase 2 single-arm KEYNOTE-224 study [11] in patients with sorafenib-treated advanced HCC. This study showed a promising objective response rate (ORR) of 17% at the time of the primary analysis [11] with confirmation of durability after longer term follow-up and 77% of responders with a duration of response (DOR) lasting ≥12 months [14]. Pembrolizumab also demonstrated efficacy and a manageable adverse event (AE) profile in patients with sorafenib-treated advanced HCC in the randomized, double-blind, placebo-controlled, phase 3 KEYNOTE-240 study [12]. The study did not meet prespecified statistical significance criteria for overall survival (OS) or progression-free survival (PFS) but did show a favorable benefit/risk profile for pembrolizumab. Median OS at the final analysis was 13.9 months for pembrolizumab versus 10.6 months for placebo (hazard ratio [HR], 0.781; 95% confidence interval [CI]: 0.611–0.998). At the first interim analysis when testing for PFS and ORR was prespecified, median PFS for pembrolizumab was 3.0 months versus 2.8 months for placebo (HR, 0.775; 95% CI: 0.609–0.987) and ORR was 16.9% (complete response [CR], n = 3) for pembrolizumab and 2.2% (CR, n = 0) for placebo. AEs were consistent with the known safety profile of pembrolizumab. This report describes outcomes based on an additional 18 months of follow-up since the final analysis of KEYNOTE-240, with a median follow-up of 40 months.
Methods
Study Design and Patients
KEYNOTE-240 (NCT02702401) was a double-blind, placebo-controlled, randomized phase 3 study [12]. Detailed eligibility criteria for KEYNOTE-240 have been published previously [12]. In brief, enrollment criteria included: (1) adults with a confirmed diagnosis of HCC by imaging or pathology; (2) at least 1 measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (3) documented radiographic progression after treatment with sorafenib or intolerance to sorafenib; (4) Barcelona Clinic Liver Cancer (BCLC) stage C disease or stage B disease not amenable to or refractory to locoregional therapy and not amenable to curative treatment; (5) Child-Pugh liver class A; (6) Eastern Cooperative Oncology Group performance status of 0 or 1; and (7) adequate organ function. Patients with past or ongoing hepatitis C virus (HCV) or controlled hepatitis B virus (HBV) infection were eligible if protocol-defined criteria were met. HCV infection was defined as anti-hepatitis C antibody-positive and detectable HCV RNA based on laboratory assessment, and HBV infection was defined as hepatitis B surface antigen-positive and/or detectable HBV DNA based on laboratory assessment.
Patients were randomly assigned 2:1 to receive pembrolizumab 200 mg or saline placebo by intravenous infusion once every 3 weeks. Treatment was continued until disease progression, unacceptable toxicity, withdrawal from the study, or receipt of 35 doses of study treatment (approximately 2 years). Best supportive care was provided to patients in both treatment arms at the discretion of the investigator per local treatment practices. Treatment allocation was stratified by geographic region (Asia without Japan vs. non-Asia with Japan), macrovascular invasion (yes vs. no), and α-fetoprotein level (<200 ng/mL vs. ≥200 ng/mL).
The study protocol and all amendments were approved by the relevant Ethics Committee or Institutional Review Board at each participating center, and the study was conducted in accordance with standards of Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent prior to enrollment. The study protocol and the name of each Ethics Committee/Institutional Review Board at each participating center including approval numbers are shown in the Online Supplemental Materials.
Assessments and Endpoints
Disease progression and tumor response were assessed using computed tomography or magnetic resonance imaging. Initial tumor imaging was conducted within 3 weeks of randomization, and tumor imaging during the study was conducted 6 weeks after randomization, with subsequent imaging conducted every 6 weeks until disease progression, start of the new anticancer treatment, withdrawal of consent, death, or notification by the sponsor, whichever occurred first. Response was assessed per RECIST v1.1 by blinded independent central review (BICR). Patients who discontinued treatment underwent imaging assessment at the time of discontinuation. Patients who discontinued treatment without documented disease progression were monitored until starting new anticancer therapy, disease progression, or the end of study, whichever occurred first. AEs were monitored throughout the study and for 30 days following the end of treatment or before the start of new anticancer therapy, whichever occurred first. Serious AEs were monitored for 90 days following the end of treatment or 30 days after the end of treatment if the patient started new anticancer therapy, whichever occurred first. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0.
The primary efficacy endpoints were PFS per RECIST v1.1 by BICR and OS. Secondary endpoints were ORR, DOR, disease control rate (DCR), and time to progression (TTP), all assessed per RECIST v1.1 by BICR, and safety and tolerability.
Statistical Analysis
OS, PFS, TTP, and DOR were estimated using the Kaplan-Meier method. HR and 95% CI are based on the Cox regression model with Efron’s method for handling ties, with treatment as a covariate stratified by the same stratification factors for randomization, with small strata collapsed as prespecified in the statistical analysis plan [12]. Efficacy was assessed in the intention-to-treat (ITT) population, defined as all randomly assigned patients, and safety was assessed in the as-treated population, defined as all randomly assigned patients who received ≥1 dose of treatment.
An exploratory post hoc landmark analysis was conducted to evaluate the association between objective response and OS in the ITT population among patients receiving pembrolizumab. The landmark method [15, 16] was used to evaluate OS after the landmark time by objective response at landmark time points of 6, 12, and 18 weeks after randomization for patients in the pembrolizumab arm. Landmark times were determined by the tumor imaging assessment scheduled at every 6 weeks plus a 7-day assessment window. Responders at each landmark time point were defined as patients with any BICR assessment of CR or partial response (PR) before the landmark time (specifically, before the end of the respective scheduled visit window); all other patients were defined as nonresponders. Confirmation of response on the subsequent scan was not required for this analysis. Patients who died before a landmark time point were excluded from that landmark time point analysis. OS after the landmark was estimated using the Kaplan-Meier method, and the HR for survival after the landmark for the responders versus nonresponders at the landmark and its 95% CI were calculated from the Cox proportional hazard regression model with Efron’s method for handling ties, with responder status by the landmark time as a single covariate. The data cutoff for this analysis was July 13, 2020.
Results
Patients
A total of 413 patients were enrolled in KEYNOTE-240. Of these, 278 were randomly assigned to pembrolizumab and 135 to placebo. Baseline demographic and disease characteristics have been previously published [12]. Briefly, in the pembrolizumab and placebo arms the median age was 67 years and 65 years, 81.3% and 83.0% were male, 63.7% and 63.7% had a Child-Pugh liver score of A5, and 79.9% and 78.5% had BCLC stage C disease, respectively.
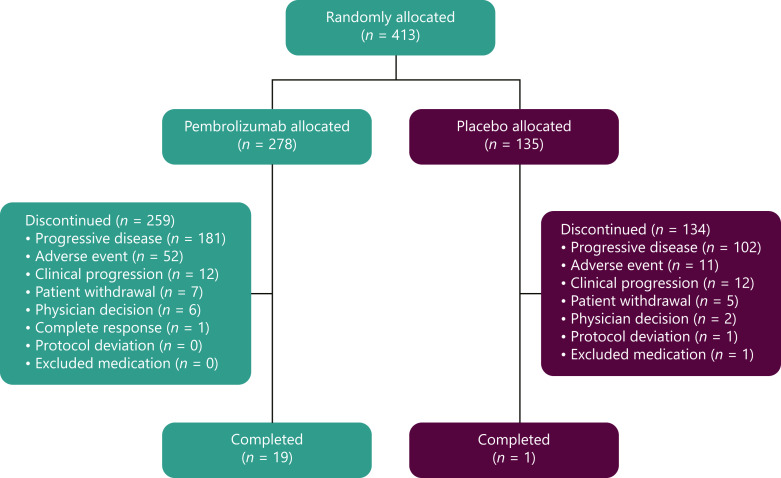
As of July 13, 2020, the median (range) time from randomization to data cutoff was 39.6 (31.7–48.8) months for pembrolizumab and 39.8 (31.7–47.8) months for placebo among patients in the ITT population. Treatment was discontinued in 259 patients (93.2%) in the pembrolizumab arm and 134 patients (99.3%) in the placebo arm, mostly because of disease progression (pembrolizumab, n = 181, 65.1%; placebo, n = 102, 75.6%; Fig. 1). Among treated patients, median (range) duration of treatment exposure was 3.48 (0.03–37.1) months for pembrolizumab and 2.83 (0.03–24.2) months for placebo.
Fig. 1.
Patient disposition.
Efficacy
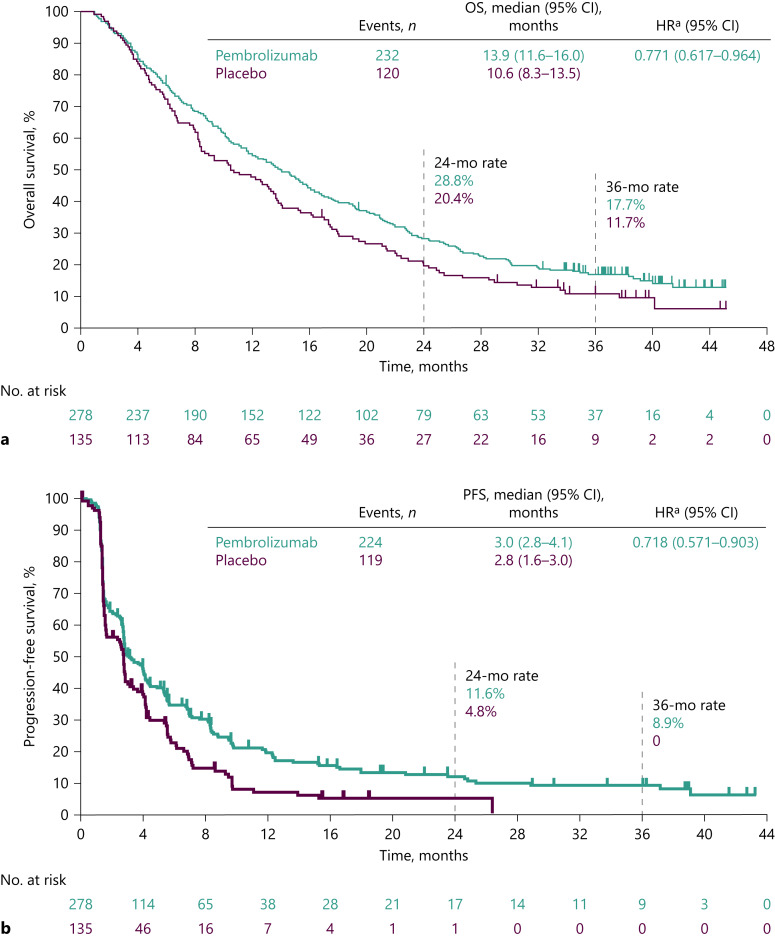
As of the data cutoff, 232 (83.5%) of 278 patients in the pembrolizumab arm and 120 (88.9%) of 135 patients in the placebo arm had died; median (95% CI) OS was 13.9 (11.6–16.0) months for pembrolizumab and 10.6 (8.3–13.5) months for placebo (HR for death, 0.771; 95% CI: 0.617–0.964; Fig. 2a). The estimated OS rates (95% CI) were 28.8% (23.6–34.2) for pembrolizumab and 20.4% (14.1–27.6) for placebo at 24 months, and 17.7% (13.4–22.5) and 11.7% (6.8–17.9), respectively, at 36 months.
Fig. 2.
OS and PFS (ITT). OS (a) and PFS (b) per RECIST v1.1 by BICR. a Based on the Cox regression model with Efron’s method for handling ties, with treatment as a covariate stratified by the same stratification factors for randomization, with small strata collapsed as prespecified in the statistical analysis plan. BICR, blinded independent central review; CI, confidence interval; HR, hazard ratio; ITT, intention to treat; PFS, progression-free survival; OS, overall survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
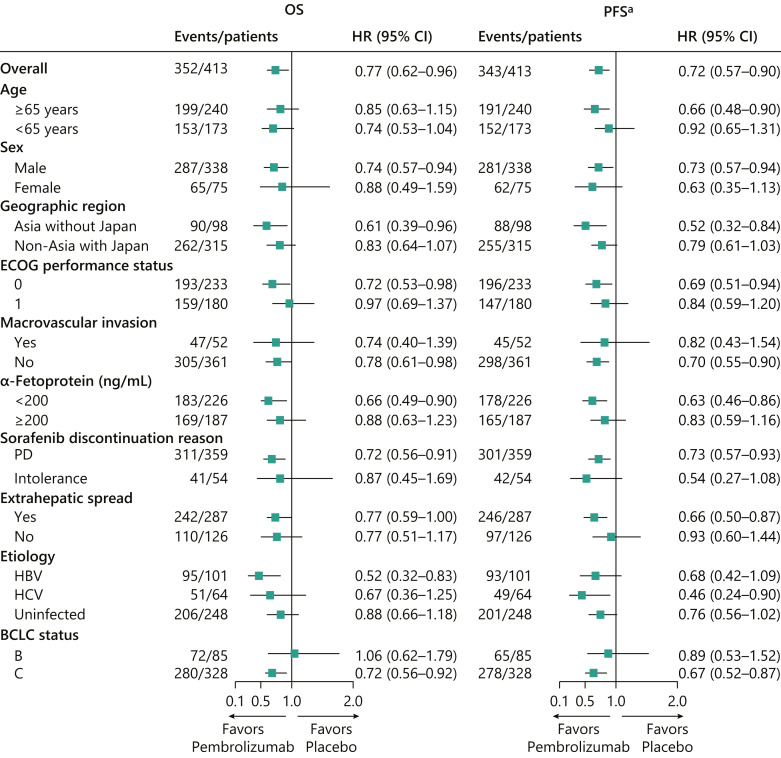
With extended follow-up, the median (95% CI) PFS was 3.0 (2.8–4.1) months for pembrolizumab and 2.8 (1.6–3.0) months for placebo (HR for progressive disease [PD] or death, 0.718; 95% CI: 0.571–0.903; Fig. 2b). The estimated PFS rates (95% CI) were 11.6% (7.7–16.5) for pembrolizumab and 4.8% (1.8–10.0) for placebo at 24 months, and 8.9% (5.3–13.6) and 0% (not applicable [NA]–NA), respectively, at 36 months. The median (95% CI) TTP was 3.8 (2.8–4.4) months for pembrolizumab and 2.8 (1.6–2.9) months for placebo (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000529636). OS and PFS across subgroups based on baseline demographic and clinical characteristics are shown in Figure 3.
Fig. 3.
Analysis of OS and PFS across predefined subgroups. aPFS assessed per RECIST v1.1 by BICR. BCLC, Barcelona Clinic Liver Cancer status; BICR, blinded independent central review; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; OS, overall survival; PD, progressive disease; PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.
The ORR (95% CI) was 18.3% (14.0–23.4) for pembrolizumab and 4.4% (1.6–9.4) for placebo (Table 1). The DCR (95% CI) was 62.2% (56.2–68.0) for pembrolizumab and 53.3% (44.6–62.0) for placebo. Among responders in the pembrolizumab arm, a best overall response of CR was observed in 10 patients and PR in 41 patients. A total of 122 patients had stable disease, and 91 patients had PD in the pembrolizumab arm. Among responders in the placebo arm, a best overall response of PR was observed in 6 patients; there was no CR. A total of 66 patients had stable disease, and 57 patients had PD in the placebo arm. Median (range) time to response was 2.7 (1.2–16.9) months for pembrolizumab and 2.9 (1.1–6.9) months for placebo. With extended follow-up, 53.7% of responders in the pembrolizumab arm and 50.0% of responders in the placebo arm had DOR ≥12 months, and the median (range) DOR was 13.9 (1.5+ to 41.9+) months for pembrolizumab and 15.2 (2.8–21.9) months for placebo.
Table 1.
Response in ITT population assessed per RECIST v1.1 by BICR
| Pembrolizumab (n = 278) | Placebo (n = 135) | |
|---|---|---|
| Objective response rate (CR + PR), n % | 51 (18.3) | 6 (4.4) |
| Disease control rate (CR + PR + SD), n (%) | 173 (62.2) | 72 (53.3) |
| Best overall response, n (%)a | ||
| CR | 10 (3.6) | 0 |
| PR | 41 (14.7) | 6 (4.4) |
| SD | 122 (43.9) | 66 (48.9) |
| PD | 91 (32.7) | 57 (42.2) |
| Not evaluable | 6 (2.2) | 3 (2.2) |
| Not assessableb | 8 (2.9) | 3 (2.2) |
| TTR, median (range), months | 2.7 (1.2–16.9) | 2.9 (1.1–6.9) |
| DOR,c median (range), months | 13.9 (1.5+ to 41.9+) | 15.2 (2.8–21.9) |
| DOR ≥12 months, %d | 53.7 | 50.0 |
BICR, blinded independent central review; CR, complete response; DOR, duration of response; ITT, intention to treat; PD, progressive disease; PR, partial response; RECIST v1.1, Response Evaluation Criteria in Solid Tumors, version 1.1; SD, stable disease; TTR, time to response.
aConfirmed best response assessed per RECIST v1.1 by BICR.
bPatients with baseline assessment by investigator or central radiology but without post-baseline assessment on the data cutoff date, including discontinuation or death before first post-baseline assessment.
cAssessed in responders who had a best overall response as confirmed complete response or partial response by product-limit (Kaplan-Meier) method for censored data.
dFrom product-limit (Kaplan-Meier) method for censored data for patients with confirmed response. “+” indicates that there is no progressive disease by the time of the last disease assessment. Data cutoff: July 13, 2020.
In the landmark analysis, OS after landmark time was longer for responders compared with nonresponders at each landmark time point. At week 6, there were 21 responders and 248 nonresponders. The median OS after landmark time was not reached (NR; 95% CI: 18.2–NR) in responders and 12.1 months (95% CI: 9.9–14.1) in nonresponders (online suppl. Fig. 2). The HR for OS after landmark time for responders versus nonresponders at week 6 was 0.27 (95% CI: 0.14–0.53). At week 12, there were 37 responders and 218 nonresponders. The median OS after landmark time was 26.9 months (95% CI: 17.8–NR) in responders and 10.8 months (95% CI: 8.5–12.7) in nonresponders (online suppl. Fig. 3). The HR for OS after landmark time for responders versus nonresponders at week 12 was 0.36 (95% CI: 0.23–0.57). At week 18, there were 41 responders and 191 nonresponders. The median OS after landmark time was 30.8 months (95% CI: 16.5–NR) in responders and 10.9 months (95% CI: 9.0–13.2) in nonresponders (online suppl. Fig. 4). The HR for OS after landmark time for responders versus nonresponders at week 18 was 0.34 (95% CI: 0.21–0.53).
Safety
All-cause AEs occurred in 270 (96.8%) of 279 treated patients in the pembrolizumab arm and 122 (91.0%) of 134 treated patients in the placebo arm (grade 3 or 4: pembrolizumab, n = 149 [53.4%]; placebo, n = 62 [46.3%] Table 2). The most frequent (≥15% of patients) of these are shown in Table 2. All-cause AEs leading to discontinuation occurred in 52 (18.6%) patients in the pembrolizumab arm and 13 (9.7%) patients in the placebo arm. The most frequent AEs leading to discontinuation were ascites (pembrolizumab, n = 13 [4.7%]; placebo, n = 3 [2.2%]), increased aspartate aminotransferase (pembrolizumab, n = 5 [1.8%]; placebo, n = 1 [0.7%]), increased blood bilirubin (pembrolizumab, n = 4 [1.4%]; placebo, n = 2 [1.5%]), and esophageal varices hemorrhage (pembrolizumab, n = 4 [1.4%]; placebo, n = 0). To ensure patient safety, stringent discontinuation criteria were applied per protocol. For instance, any clinically detectable ascites required permanent discontinuation from treatment. All-cause AEs leading to death occurred in 7 (2.5%) patients in the pembrolizumab arm and 4 (3.0%) patients in the placebo arm.
Table 2.
AEs in treated patients
| Pembrolizumab (n = 279) | Placebo (n = 134) | |||
|---|---|---|---|---|
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| Any AE (all-cause) | 270 (96.8) | 149 (53.4) | 122 (91.0) | 62 (46.3) |
| AEs occurring in ≥10% of patients in either treatment arm (all-cause)a | ||||
| Increased aspartate aminotransferase | 67 (24.0) | 38 (13.6) | 22 (16.4) | 10 (7.5) |
| Increased blood bilirubin | 55 (19.7) | 21 (7.5) | 17 (12.7) | 8 (6.0) |
| Fatigue | 54 (19.4) | 7 (2.5)a | 32 (23.9) | 2 (1.5)a |
| Pruritus | 53 (19.0) | 1 (0.4)a | 17 (12.7) | 0 |
| Increased alanine aminotransferase | 51 (18.3) | 19 (6.8) | 13 (9.7) | 4 (3.0) |
| Decreased appetite | 51 (18.3) | 3 (1.1)a | 21 (15.7) | 0 |
| Diarrhea | 51 (18.3) | 4 (1.4)a | 21 (15.7) | 3 (2.2)a |
| Abdominal pain | 42 (15.1) | 4 (1.4)a | 9 (6.7) | 0 |
| Rash | 35 (12.5) | 5 (1.8)a | 7 (5.2) | 0 |
| Nausea | 34 (12.2) | 2 (0.7)a | 21 (15.7) | 1 (0.7)a |
| Peripheral edema | 32 (11.5) | 0 | 17 (12.7) | 0 |
| Ascites | 31 (11.1) | 22 (7.9) | 14 (10.4) | 8 (6.0) |
| Constipation | 30 (10.8) | 1 (0.4)a | 15 (11.2) | 0 |
| Back pain | 29 (10.4) | 5 (1.8)a | 14 (10.4) | 0 |
| Pyrexia | 29 (10.4) | 2 (0.7)a | 15 (11.2) | 0 |
| Anemia | 28 (10.0) | 11 (3.9) | 14 (10.4) | 12 (9.0) |
| Asthenia | 26 (9.3) | 0 | 15 (11.2) | 0 |
| Cough | 24 (8.6) | 0 | 24 (17.9) | 0 |
| Arthralgia | 22 (7.9) | 1 (0.4)a | 14 (10.4) | 1 (0.7)a |
| Dyspnea | 20 (7.2) | 0 | 15 (11.2) | 2 (1.5)a |
| Immune-mediated AEs (all-cause)b,c | ||||
| Any | 50 (17.9) | 20 (7.2) | 11 (8.2) | 1 (0.7) |
| Hypothyroidism | 14 (5.0) | 1 (0.4) | 7 (5.2) | 0 |
| Pneumonitis | 10 (3.6) | 4 (1.4) | 1 (0.7) | 0 |
| Hyperthyroidism | 8 (2.9) | 0 | 0 | 0 |
| Severe skin reaction | 8 (2.9) | 6 (2.2) | 0 | 0 |
| Hepatitis | 5 (1.8) | 4 (1.4) | 0 | 0 |
| Colitis | 4 (1.4) | 2 (0.7) | 2 (1.5) | 0 |
| Infusion reaction | 3 (1.1) | 0 | 0 | 0 |
| Adrenal insufficiency | 2 (0.7) | 0 | 0 | 0 |
| Hypophysitis | 2 (0.7) | 1 (0.4) | 0 | 0 |
| Myositis | 2 (0.7) | 1 (0.4) | 0 | 0 |
| Myasthenic syndrome | 1 (0.4) | 0 | 0 | 0 |
| Thyroiditis | 1 (0.4) | 0 | 0 | 0 |
| Type 1 diabetes mellitus | 1 (0.4) | 1 (0.4) | 1 (0.7) | 1 (0.7) |
AE, adverse event. aGrade 3 AEs only.
bListed in decreasing frequency in the pembrolizumab arm.
cImmune-mediated AEs and infusion reactions were based on a list of terms prepared by the sponsor and included AEs regardless of attribution to study treatment by the investigator.
A total of 171 (61.3%) patients in the pembrolizumab arm and 65 (48.5%) patients in the placebo arm experienced treatment-related AEs of any grade, with most low grade in severity (grade 3 or 4: pembrolizumab, n = 54 [19.4%]; placebo, n = 10 [7.5%]; online suppl. Table 1). The most frequent (≥10% of patients) treatment-related AEs were pruritus (pembrolizumab, n = 39 [14.0%]; placebo, n = 7 [5.2%]) and fatigue (pembrolizumab, n = 28 [10.0%]; placebo, n = 19 [14.2%]). A total of 19 (6.8%) patients in the pembrolizumab arm and 1 (0.7%) patient in the placebo arm discontinued due to treatment-related AEs. The most frequent treatment-related AEs leading to discontinuation in the pembrolizumab arm were increased alanine aminotransferase, increased aspartate aminotransferase, increased blood bilirubin, immune-mediated hepatitis, and interstitial lung disease (n = 2 [0.7%] each). The only treatment-related AE that led to discontinuation in the placebo arm was anemia. In the follow-up period, there were no treatment-related AEs that led to death. The only treatment-related AE that led to death remained the malignant neoplasm progression reported at the time of the final analysis in the pembrolizumab arm.
All-cause immune-mediated AEs based on a list of terms prespecified by the sponsor regardless of attribution to study treatment by the investigator occurred in 50 (17.9%) patients in the pembrolizumab arm and 11 (8.2%) patients in the placebo arm, with most low grade in severity (grade 3 or 4: pembrolizumab, n = 20 [7.2%]; placebo, n = 1 [0.7%]; Table 2). The most common immune-mediated AEs were hypothyroidism and pneumonitis. Few immune-mediated AEs led to discontinuation (pembrolizumab, n = 10 [3.6%]; placebo, n = 0). There were 23 patients (8.2%) in the pembrolizumab arm and 1 patient (0.7%) in the placebo arm who received steroids for possible immune-mediated AEs. Immune-mediated hepatitis events occurred infrequently (pembrolizumab, n = 10 [3.6%]; placebo, n = 0). No viral hepatitis flare events were reported.
Discussion
This analysis of KEYNOTE-240 evaluated longer term efficacy and safety of pembrolizumab in patients with advanced HCC compared with placebo. After an additional 18 months of follow-up since the final analysis and a median follow-up of 40 months overall, pembrolizumab continued to demonstrate a favorable benefit/risk profile in patients with sorafenib-treated advanced HCC. Although prespecified statistical significance criteria for OS or PFS superiority compared with placebo were not met at the final analysis, the HRs for OS and PFS continued to favor pembrolizumab, and results for OS and PFS remained generally consistent across predefined subgroups based on baseline demographics and clinical characteristics with longer term follow-up. Compared with other globally available therapies for HCC in the second-line treatment setting, higher response rates continued to be observed with pembrolizumab compared with placebo (ORR, 18.3% vs. 4.4%), with the number of patients achieving a CR increasing from 6 patients in the final analysis to 10 patients in this longer term analysis. Durable responses were observed in the pembrolizumab arm, with 53.7% of responders in the pembrolizumab arm continuing to respond for ≥12 months, and some patients having a DOR of ∼3.5 years that was ongoing at the data cutoff. Post hoc landmark analysis of the association between objective response and OS showed that patients who achieved an objective response (CR or PR) with pembrolizumab at 6, 12, or 18 weeks had longer survival after that time than patients who did not achieve a response by that time. The safety profile of pembrolizumab remained consistent over time. Additionally, the frequency of immune-mediated hepatitis events did not increase with additional follow-up. These data support the favorable benefit/risk profile of pembrolizumab in patients with sorafenib-treated advanced HCC.
The clinical improvements observed in the current analysis are comparable in magnitude to those observed in longer term follow-up of the phase 2 KEYNOTE-224 study which was conducted in a similar population of patients [14]. After a median follow-up of 31.2 months, pembrolizumab showed durable antitumor activity with an ORR of 18.3% and a DCR of 61.5% [14], which is consistent with that observed in the current analysis (ORR, 18.3%; DCR, 62.2%). Survival benefit was also comparable in both updated analyses. In KEYNOTE-224, the median OS was 13.2 months and the median PFS was 4.9 months for pembrolizumab, and in KEYNOTE-240 the median OS was 13.9 months and the median PFS was 3.0 months for pembrolizumab [14]. Furthermore, after extended follow-up, the safety profile in both updated analyses was similar, and no HCV or HBV viral flare events were observed. Recently, KEYNOTE-394 (ClinicalTrials.gov, NCT03062358), which evaluated the efficacy and safety of pembrolizumab plus best supportive care compared with placebo plus best supportive care in previously treated patients with advanced HCC in Asian countries, met its primary endpoint of OS [17]. Statistically significant and clinical meaningful improvement in OS (median, 14.6 vs. 13.0 months; HR for death, 0.79; 95% CI: 0.63–0.99; p = 0.0180) and secondary endpoints of PFS (median, 2.6 vs. 2.3 months; HR for progression or death, 0.74 [95% CI: 0.60–0.92]; p = 0.0032) and ORR were observed with pembrolizumab compared with placebo (ORR, 12.7% [95% CI: 9.1–17.0] versus 1.3% [95% CI: 0.2–4.6]; estimated treatment difference, 11.4% [95% CI: 6.7–16.0]; p < 0.0001) [17]. The AE profile remained consistent with previous reports. KEYNOTE-394 provides additional support for the generalizability of the data to patients receiving second-line pembrolizumab for advanced HCC worldwide. Lastly, a meta-analysis of pooled patient data from the KEYNOTE-240 and KEYNOTE-394 studies showed improvement in OS, PFS, and ORR with pembrolizumab versus placebo in the second-line treatment of advanced HCC [18]. Similar outcomes were observed across major subgroups including HBV viral etiology, BCLC stage, age, and region. These data expand on findings from the KEYNOTE-240 and KEYNOTE-394 studies individually and provide additional evidence of the benefit of second-line pembrolizumab for the treatment of advanced HCC in a global population.
In the second-line treatment setting, the tyrosine kinase inhibitors regorafenib [8] and cabozantinib [9] and the VEGF receptor 2 inhibitor ramucirumab (in patients with an α-fetoprotein level ≥400 ng/mL) [10] met their primary endpoints in phase 3 studies showing OS superiority compared with placebo; however, the AE profile, specifically risk of bleeding, thrombosis, and proteinuria may make these agents unsuitable for certain patients with HCC. Furthermore, nivolumab monotherapy which was initially granted accelerated approval on the basis of encouraging antitumor activity in the phase 1/2 CheckMate 040 study [19] did not achieve prespecified statistical significance criteria for its primary endpoint of OS superiority compared with sorafenib in the confirmatory phase 3 CheckMate 459 study [20] and was subsequently withdrawn for the second-line treatment setting in the USA [21], although it remains in use in combination with ipilimumab. Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks for a total of 4 doses followed by monotherapy with nivolumab 240 mg/kg every 2 weeks (cohort A) showed efficacy with longer term follow-up in a phase 1/2 study, although the proportion of patients with treatment-related AEs was high (any: 94%; grade 3 or 4: 55%) and immune-mediated AEs requiring immune-modulating medication, most often systemic steroids, were frequent [22]. In the current study, the proportion of patients who received steroids for potential immune-mediated AEs was low and remained consistent over time. Therefore, pembrolizumab monotherapy may be a suitable alternative for patients unable to tolerate other potential options in this treatment setting.
A limitation of this analysis is that the KEYNOTE-240 study included patients with well-preserved liver function, and the results may not be generalizable to patients with more advanced disease. Despite this limitation, the data show continuous efficacy with numeric improvement in OS and PFS over time compared with placebo. Additionally, the AE profile remained consistent with the previous report for the final analysis.
Conclusion
This analysis of longer term follow-up of KEYNOTE-240 shows that efficacy is maintained over time with pembrolizumab compared with placebo in patients who have advanced HCC. Additionally, the AE profile is consistent over time, and AEs are manageable. In summary, these data, together with results from KEYNOTE-224 and KEYNOTE-394, support the clinical activity and potential global use of pembrolizumab in sorafenib-treated patients with advanced HCC.
Acknowledgments
We thank the patients and their families and caregivers for participating in this trial, and all investigators and site personnel. We also thank Olga Kuznetsova, PhD; Scot Ebbinghaus, MD; Ken Hatogai, MD, PhD; and Amos Odeleye-Ajakaye (of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA) for their contributions to this analysis and development of the manuscript. Medical writing and editorial assistance were provided by Lauren D’Angelo, PhD, of ApotheCom (Yardley, PA, USA).
Statement of Ethics
The study protocol and all amendments were approved by the relevant Ethics Committee or Institutional Review Board at each participating center, and the study was conducted in accordance with standards of Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent prior to enrollment. The study protocol and the name of each Ethics Committee/Institutional Review Board at each participating center including approval numbers are shown in the Online Supplemental Materials.
Conflict of Interest Statement
P. Merle reports receiving grants from Ipsen and other financial or non-financial interests for advisory board participation from Roche, AstraZeneca, Eisai, MSD, Ipsen, Eli Lilly, and Bayer. M. Kudo reports receiving support for the present manuscript from MSD, grants or contracts from Gilead Sciences, Taiho, Sumitomo Dainippon Pharma, Takeda, Otsuka, EA Pharma, AbbVie, and Eisai, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Eli Lilly, Bayer, Eisai, Chugai, Takeda, and MSD. J. Edeline reports no conflicts of interest. M. Bouattour reports receiving consulting fees from Roche, Eisai, AstraZeneca, Bayer, Ipsen, Sirtex Medical, Servier, MSD, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Roche, AstraZeneca, Bayer, Ipsen, Sirtex Medical, and Servier, and support for attending meetings and/or travel from Roche, AstraZeneca, Bayer, and Sirtex Medical. A-L. Cheng reports receiving consulting fees from Eisai, Ono Pharmaceutical, Ipsen Innovation, Bayer Healthcare, and Merck Sharp and Dohme, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from F. Hoffmann-La Roche Ltd., Chugai Pharmaceutical, Bayer Yakuhin, Ltd., Novartis, Eisai, Ono Pharmaceutical, and Amgen Taiwan, support for attending meetings and/or travel from Bayer Yakuhin, Ltd., Eisai, and IQVIA, and participation on a data safety monitoring board or advisory board for Novotech. S.L. Chan reports receiving grants or contracts from Bayer, Ipsen, and Sirtex, consulting fees from AstraZeneca, Eisai, Ipsen, and MSD, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AstraZeneca, Eisai, Roche, and MSD. T. Yau reports no conflicts of interest. M. Garrido reports receiving payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from MSD, Bristol Myers Squibb, Roche, Pfizer, and Merck, payment for expert testimony from Bristol Myers Squibb, and support for attending meetings and/or travel from MSD. J. Knox reports receiving a grant for an investigator-initiated trial from Merck, consulting fees from Merck, Eisai, and AstraZeneca, and payment for expert testimony from Incyte and AstraZeneca. B. Daniele reports receiving payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Eisai, Ipsen, Eli Lilly, Bayer, MSD, Roche, Amgen, Merck Serono, and AstraZeneca, support for attending meetings and/or travel from AstraZeneca, Sanofi, Celgene, and Bristol Myers Squibb, and participating in a data safety monitoring board or advisory board for Sanofi. V. Breder reports receiving consulting fees from Eisai, Novartis, and Bayer, honoraria for lectures, presentations, and educational events from Bristol Myers Squibb, Roche, Eisai, Ipsen, and Bayer, and support for attending meetings and/or travel from Bristol Myers Squibb, Ipsen, and Roche. H. Y. Lim reports receiving payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Roche, and participation on a data safety monitoring board or advisory board for Bayer, Eisai, and Roche. S. Ogasawara reports receiving grants or contracts from Bayer, AstraZeneca, Eli Lilly, Eisai, and Chugai, consulting fees from Chugai and AstraZeneca, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bayer, AstraZeneca, Eli Lilly, Eisai, Chugai, and MSD. S. Cattan reports no conflicts of interest. Y. Chao reports no conflicts of interest. A.B. Siegel is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. I. M-Forero is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. Z. Wei was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA at the time of the analysis. C-C. Liu is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and has stock in Merck & Co., Inc., Rahway, NJ, USA. R. S. Finn reports receiving support for the present manuscript from Merck, consulting fees and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from AstraZeneca, Bayer, Bristol Myers Squibb, CStone, Eisai, Exelixis, Eli Lilly, Pfizer, Merck, and Roche/Genentech, payment for expert testimony from Bayer AS, grants paid to his institution from Merck, Eisai, Pfizer, Bristol Myers Squibb, Roche/Genentech, Eli Lilly, Adaptimmune, and Bayer, stock/stock options from CStone, and reports participating on a data safety monitoring board or advisory board from AstraZeneca and Hengrui.
Funding Sources
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck and Co., Inc., Rahway, NJ, USA. Medical writing and editorial assistance were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author Contributions
All the authors approved the final version of the manuscript, contributed significantly to the work, and agreed to be accountable for all aspects of it. Conception of the work: A-L.C., S.L.C., T.Y., J.K., A.B.S, and R.S.F. Design of the work: R.S.F., A.B.S., A-L.C., S.L.C., T.Y., and J.K. Acquisition of data for the work: P.M., V.B., R.S.F., S.C., M.K., J.E., A-L.C., S.L.C., T.Y., M.G., J.K., H.Y.L., and Y.C. Analysis of data for the work: I.M.F., R.S.F., A.B.S., C.C.L., Z.W., T.Y., M.G., J.K., and H.Y.L. Interpretation of data for the work: I.M.F., P.M., V.B., R.S.F., A.B.S., Z.W., M.K., M.B., T.Y., M.G., J.K., B.D., H.Y.L., S.O., and Y.C. Drafting of the work: A.B.S., J.K., and H.Y.L. Critical revision of the work for important intellectual content: I.M.F., P.M., V.B., R.S.F., A.B.S., M.B., S.C., M.K., J.E., A-L.C., S.L.C., T.Y., J.K., B.D., H.Y.L., S.O., Y.C., Z.W., and C-C.L.
Funding Statement
This study was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck and Co., Inc., Rahway, NJ, USA. Medical writing and editorial assistance were funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Supplementary Material
References
- 1. International Agency for Research on Cancer . GLOBOCAN 2020. 2021 [cited 2021 02/03/2021]; Available from: https://gco.iarc.fr/today/home.
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–905. 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–73. 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 7. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. 10.1056/evidoa2100070. [DOI] [PubMed] [Google Scholar]
- 8. Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 9. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–96. 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 11. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52. 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 12. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- 13. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kudo M, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer DH, et al. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer. 2022;167:1–12. 10.1016/j.ejca.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 15. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 16. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24):3913–5. 10.1200/JCO.2008.16.1000. [DOI] [PubMed] [Google Scholar]
- 17. Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, et al. Pembrolizumab versus placebo as second-line therapy in patients from asia with advanced hepatocellular carcinoma: a randomized, double-blind, phase III trial. J Clin Oncol. 2023;41(7):1434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finn RS, Gu K, Chen X, Merle P, Lee KH, Bouattour M, et al. Abstract CT222: pembrolizumab (pembro) for previously treated advanced hepatocellular carcinoma (aHCC): meta-analysis of the phase 3 KEYNOTE-240 and KEYNOTE-394 studies. Cancer Res. 2022;82(12_Suppl ment):CT222. 10.1158/1538-7445.am2022-ct222. [DOI] [Google Scholar]
- 19. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. 10.1016/S1470-2045(21)00604-5. [DOI] [PubMed] [Google Scholar]
- 21. Bristol Myers Squibb Statement on Opdivo® (nivolumab) Monotherapy Post-Sorafenib Hepatocellular Carcinoma U.S. Indication: Princeton, NJ, USA: Bristol: Myers Squibb Company. 2021. Available from:https://news.bms.com/news/corporate-financial/2021/Bristol-Myers-Squibb-Statement-on-Opdivo-nivolumab-Monotherapy-Post-Sorafenib-Hepatocellular-Carcinoma-U.S.-Indication/default.aspx. [Google Scholar]
- 22. El-Khoueiry AB, Yau T, Kang YK, Kim TY, Santoro A, Sangro B, et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): long-term results from CheckMate 040. J Clin Oncol. 2021;39(3_Suppl l):269. 10.1200/jco.2021.39.3_suppl.269.33275488 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the USA and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.