Abstract
Glomerular diseases (GDs) represent the third leading cause of end-stage kidney disease (ESKD) in the US Diabetes was excluded from the CureGN Study, an NIH/NIDDK-sponsored observational cohort study of four leading primary GDs: IgA nephropathy (IgAN), membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), and minimal change disease (MCD). CureGN-Diabetes, an ancillary study to CureGN, seeks to understand how diabetes influences the diagnosis, treatment, and outcomes of GD. It is a multicenter, prospective cohort study, targeting an enrollment of 300 adults with prevalent type 1 or type 2 diabetes and MCD, FSGS, MN, or IgAN, with first kidney biopsy obtained within 5 years of enrollment in 80% (20% allowed if biopsy after 2010). CureGN and Transformative Research in DiabEtic NephropaThy (TRIDENT) provide comparator cohorts. Retrospective and prospective clinical data and patient-reported outcomes are obtained. Blood and urine specimens are collected at study visits annually. Kidney biopsy reports and digital images are obtained, and standardized pathologic evaluations performed. Light microscopy images are uploaded to the NIH pathology repository. Outcomes include relapse and remission rates, changes in proteinuria and estimated glomerular filtration rate, infections, cardiovascular events, malignancy, ESKD, and death. Multiple analytical approaches will be used leveraging the baseline and longitudinal data to compare disease presentation and progression across subgroups of interest. With 300 patients and an average of 3 years of follow-up, the study has 80% power to detect a HR of 1.4–1.8 for time to complete remission of proteinuria, a rate ratio for hospitalizations of 1.18–1.56 and difference in eGFR slope of 6.0–8.6 mL/min/year between two groups of 300 participants each. CureGN-Diabetes will enhance our understanding of diabetes as a modifying factor of the pathology and outcomes of GDs and support studies to identify disease mechanisms and improve patient outcomes in this understudied patient population.
Keywords: Glomerular disease, Diabetes, Cure Glomerulonephropathy Network, Minimal change disease, Focal segmental glomerulosclerosis, Membranous nephropathy, IgA nephropathy, IgA vasculitis
Introduction
Although individual types of glomerular diseases are rare in the general population, they jointly represent the third leading cause of end-stage kidney disease (ESKD) in the USA, accounting for approximately 10,000 incident cases of ESKD per year [1]. IgA nephropathy (including IgA vasculitis) (IgAN), membranous nephropathy (MN), focal segmental glomerulosclerosis (FSGS), and minimal change disease (MCD) are four leading primary glomerular diseases and the subject of an NIH/NIDDK-sponsored observational cohort study entitled “Cure Glomerulonephropathy Network” (CureGN) [2]. In this study, the natural histories of IgAN, MN, FSGS, and MCD are currently being mapped out alongside the establishment of biosample and digital pathology repositories. This rich resource will enable biomarker discovery and provide greater understanding of the molecular bases of these diseases, ultimately optimizing diagnosis and treatment for patients. Additionally, the prospective follow-up allows for identification of risk factors of kidney function decline as well as nonrenal complications including thromboembolic events, infection, malignancy, and cardiovascular events.
CureGN excludes people with comorbid diabetes requiring antihyperglycemic treatments at the time of kidney biopsy, a common and important comorbid condition that may impact pathogenesis and outcomes of glomerular diseases. The prevalence of diabetes has grown to approximately 11.3% of all US adults, more than a fifth of whom are unaware of their diagnosis [3]. While incidence rates of diagnosed type 2 diabetes are highest among people aged 45–64 years, the surge in obesity has also resulted in a growing incidence of diabetes and prediabetes in younger individuals, aged 18–44 years [4, 5]. Moreover, minority populations have the highest rates of diabetes, prevalent in 16.4% of African Americans, 14.7% of Hispanics, and 14.9% of Asians [3]. The prevalence of diabetes among patients with some glomerular diseases may be even higher than in the general population [6–8].
Despite the diabetes pandemic, there has not yet been a significant effort to explore diabetes as a modifying factor in the pathogenesis, natural history, and treatment of glomerular diseases. Similar gaps are notable in recent clinical trials of glomerular diseases, in which patients with diabetes have been excluded or under-enrolled [9–11]. The exclusion of diabetes from CureGN created a significant gap in this cohort’s approach to capturing an up-to-date and representative picture of glomerular diseases in the USA. The rationale for excluding people with diabetes from CureGN was rooted in the assumption, held by most nephrologists, that diabetes modifies the presentation, management, and prognosis of glomerular diseases. This hypothesis, however, has never been formally tested in a prospective fashion. Moreover, given that approximately 20% of glomerular disease patients have comorbid diabetes, this gap in knowledge creates a missed opportunity to advance precision medicine in nephrology [12] and may result in underrepresentation of minority populations who are at higher risk of diabetes. As an ancillary study to CureGN, CureGN-Diabetes is a multicenter NIH/NIDDK-funded consortium working collaboratively to address these gaps.
Methods
Organizational Structure of the Consortium
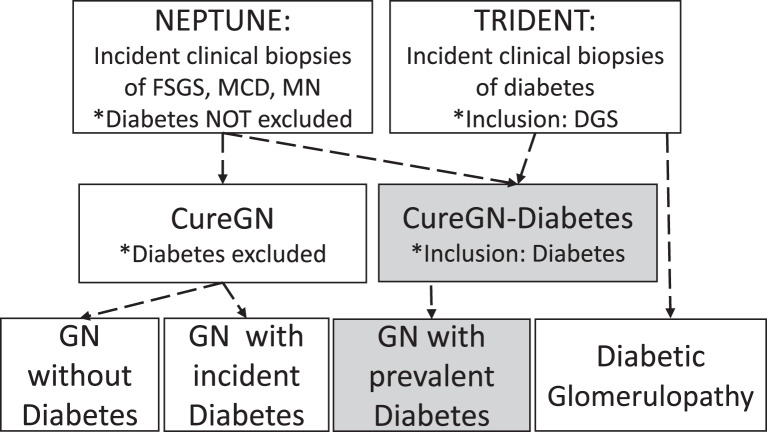
The CureGN-Diabetes consortium has 3 cores: (i) clinical (9 sites; coordinated at the University of North Carolina, Columbia University and the University of Pennsylvania), (ii) pathology (coordinated at Cedars- Sinai Medical Center), and (iii) data coordination (University of North Carolina, Arbor Research Collaborative for Health and the University of Michigan). A unique feature of CureGN-Diabetes is that it leverages data from CureGN and the NIH/NIDDK funded NEPhrotic syndrome sTUdy NEtwork (NEPTUNE) as well as an industry-academia collaboration, the Transformative Research In DiabEtic NephropaThy (TRIDENT) study (shown in Fig. 1). NEPTUNE focuses on three of the CureGN cohort diseases, FSGS, MCD, and MN, and included patients with diabetes at the time of biopsy, providing a subset of participants who can be added to our analyses [13]. TRIDENT is a prospective observational study of patients with diabetic glomerulosclerosis (DGS) identified on clinical biopsy, aimed at elucidation of pathogenetic mechanisms and clinical and pathologic features predicting rapid decline in eGFR [14]. CureGN, NEPTUNE, and TRIDENT each have captured broad data sets, collected biorepository specimens, and generated a digital pathology repository (DPR) that can be used in the analysis of CureGN-Diabetes data sets.
Fig. 1.
Schematic representation of the study cohorts with glomerular disease and/or diabetes that feed into and/or provide comparator populations to the CureGN-Diabetes cohort. DGS, diabetic glomerulosclerosis; FSGS, focal and segmental glomerulosclerosis; GN, glomerulonephritis; IgA, IgA nephropathy and IgA vasculitis; MN, membranous nephropathy.
Objectives
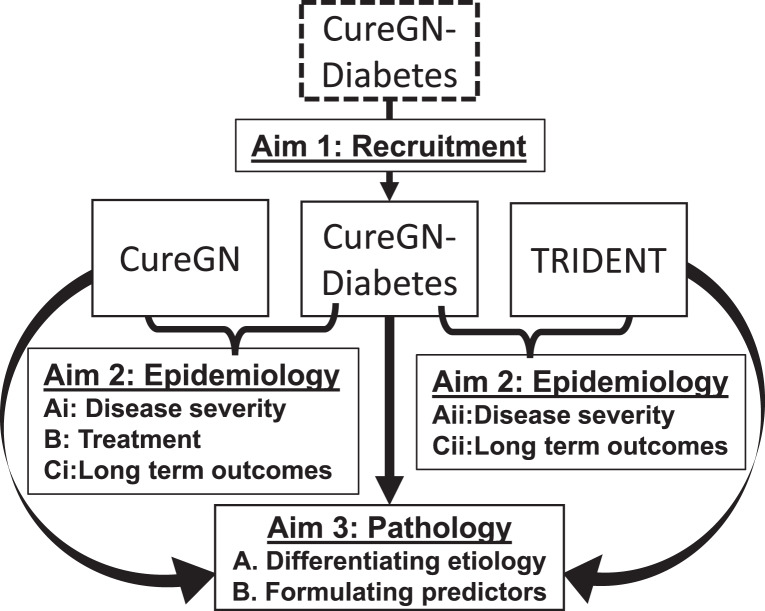
The primary objectives of CureGN-Diabetes are to understand how diabetes affects the presentation, histopathology, treatment, and outcomes of glomerular diseases, as shown in Figure 2. The study aims to recruit and longitudinally follow a multiethnic cohort of 300 adult participants with diabetes and biopsy-documented IgAN, FSGS, MN, or MCD with or without concurrent DGS, with data collection as detailed below. The data will be used to assess the impact of diabetes on glomerular disease by comparing baseline clinical and pathologic characteristics including kidney disease chronicity/severity at presentation, use of immunomodulatory therapy for glomerular disease, and long-term kidney, cardiovascular, thromboembolic, and infectious outcomes of participants in CureGN-Diabetes versus those with glomerular disease alone (CureGN) and DGS alone (TRIDENT). Pathologic feature definitions used in CureGN-Diabetes will be harmonized with those in CureGN and TRIDENT, and the former two will share a parallel database and DPR framework. This will provide ideal cohorts for comparing pathologic lesions in DGS and primary glomerular disease to determine which lesions overlap or are specific to DGS and the cohort glomerular diseases, to identify individual and patterns of pathologic lesions that have diagnostic value and determine morphologic predictors of clinical outcome in primary glomerular diseases versus primary glomerular disease with clinical diabetes and with or without DGS.
Fig. 2.
Aims for the CureGN-Diabetes study.
Study Population
CureGN-Diabetes aims to recruit a multiethnic cohort of 300 patients with clinical diabetes mellitus present prior to diagnosis of one of the four cohort CureGN diseases (FSGS, MCD, MN, IgAN) with or without DGS. The protocol and data collection mirror the parent CureGN Study, with additional detailed pathologic scoring for diabetic lesions. Inclusion and exclusion criteria for this ancillary cohort largely overlap with those of the parent CureGN Study [2]. Inclusion criteria include a diagnosis of MCD, FSGS, MN, or IgAN on first diagnostic kidney biopsy within 5 years of enrollment in at least 80% of participants. A sample (≤20%) will be allowed back to the year of CureGN initial enrollment (2010) to capture a sample with longer follow-up in order to align with the parent CureGN Study for comparison. Inclusion also requires access to first kidney biopsy report and pathology materials for enrollment. Overlapping exclusion criteria are ESKD (defined as dialysis or transplant) at the time of enrollment, diagnoses of active hepatitis B or C infection, HIV infection, or systemic lupus erythematosus at the time of first diagnostic kidney biopsy, active malignancy (except for nonmelanoma skin cancer), and institutionalized patients. Clinical inclusion criteria that differ from the parent CureGN study are type 1 or type 2 diabetes requiring antihyperglycemic medication treatment at the time of biopsy (or DGS on kidney pathology) and age ≥18 years at the time of study enrollment. Other forms of diabetes are excluded (e.g., maturity-onset diabetes of the young, cystic fibrosis, liver, or pancreatic diseases). Classification of clinical diabetes is based on prior clinic notes and laboratory data or, in cases where it is unclear, at the discretion of the site investigator. Steroid-induced diabetes is only allowed if diabetes persisted long after (>6 months) the cessation of steroids, and for steroid administration was for a diagnosis other than glomerular disease. We surveyed several pediatric nephrology sites to determine the frequency of comorbid diabetes in CureGN screenings, and its rarity (<3%) in this population would greatly increase cost and effort with minimal scientific gain, resulting in exclusion of those <18 years of age. Pathology inclusion and exclusion criteria vary by disease and largely mirror those of CureGN with additional criteria for DGS shown in Table 1. For this study, a diagnosis of FSGS or MCD cannot be made in the setting of nodular DGS due to overlapping histologic features of these entities and the inability to distinguish between diabetic injury and a podocytopathy. For similar reasons, MCD with concomitant class 1 or class 2 DGS also requires extensive podocyte foot process effacement and rapid clinical onset of nephrotic syndrome defined as (i) sudden onset (within 3 months of biopsy) edema and hypoalbuminemia (albumin <3.0 g/dL) and (ii) UPCR >3 g/g from a baseline ACR <500 mg/g or UPCR <1 g/g.
Table 1.
CureGN-Diabetes pathology inclusion and exclusion criteria, stratified by glomerular disease subtype
| Disease cohort | Adequacy | Light microscopy | Immunofluorescence | Electron microscopy | Exclusions | Clinical criteria* |
|---|---|---|---|---|---|---|
| MCD | ≥10 glomeruli | Unremarkable glomeruli. Mesangial hypercellularity, global glomerulosclerosis, arteriosclerosis, thick GBMs, diffuse DGS allowed | <1+ glomerular IgG, IgA. Any staining for IgM allowed | Extensive foot process effacement. No immune-type deposits (except in C1qN) | Findings indicative of other disease, nodular DGS. | If thick GBMs or diffuse DGS, there must be sudden onset (within 3 mos of biopsy) of edema, low serum albumin (<3.0 g/dL), and a UPCR >3 g/g from a baseline ACR <500 mg/g or UPCR <1 g/g in the past 2 years |
| ≤1+ C3, C1q | ||||||
| >1+ C1q reflexes to C1q nephropathy | ||||||
| FSGS | ≥5 glomeruli. ≥1 glomerulus with any variant of segmental sclerosis | Mesangial hypercellularity, thick GBMs, diffuse DGS, arteriosclerosis allowed | <1+ glomerular IgG, IgA | Only small segmental mesangial immune-type deposits (except in C1qN) | Findings indicative of other disease, nodular DGS | |
| ≤1+ C3, C1q, IgM (areas of sclerosis excluded) | ||||||
| >1+ C1q reflexes to C1qN | ||||||
| C1qN | See above for MCD and FSGS | Fulfills criteria for MCD or FSGS. global glomerulosclerosis, mild mesangial hypercellularity, diffuse DGS, arteriosclerosis allowed | >1+ glomerular C1q with/without any IgG, IgA, IgM, C3 | Mesangial with/without scattered subendothelial, subepithelial immune-type deposits. No evidence of lupus nephritis | Findings indicative of other disease, nodular DGS. | |
| IgAN | ≥5 glomeruli | Normal glomeruli, all Oxford IgA lesions, any DGS allowed | ≥1+ IgA dominant or co-dominant diffuse mesangial staining | Segmental thin GBMs and segmental subendothelial or subepithelial immune-type deposits allowed | Findings indicative of other disease | |
| MN | ≥5 glomeruli | Glomerular capillaries normal or thickened, with or without “spikes.” Segmental sclerosis, <10% crescents, mesangial hypercellularity, mild focal segmental endocapillary hypercellularity, any DGS, arteriosclerosis allowed | Granular capillary wall IgG with/without IgA, IgM, C1q, C3 | Subepithelial/intramembranous Immune-type deposits (Ehrenreich/Churg stage I, II, III, and/or IV). No evidence of lupus nephritis | Findings indicative of other disease |
C1qN, C1q nephropathy; DGS, diabetic glomerulosclerosis; GBM, glomerular basement membrane; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; MCD, minimal change disease; MN, membranous nephropathy.
*Clinical criteria are required for a diagnosis of MCD in the setting of thick GBMs or diffuse DGS to ensure diagnostic certainty for enrollment due to the possibility of severe podocyte foot process effacement associated with diabetic injury to the GBMs.
Study Procedures, Data, and Sample Collection and Storage
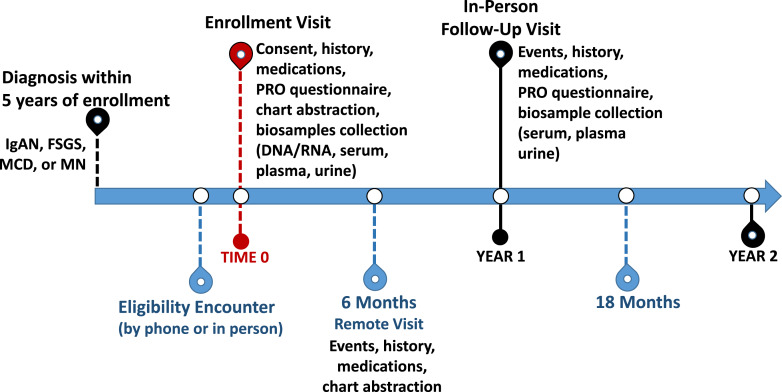
Visit types and schedule are similar to the parent CureGN Study [2], with participant contact made every 6 months via rotating in-person and remote visits as shown in Figure 3. After consent, the kidney biopsy report is reviewed by a CureGN-Diabetes pathologist to ensure pathology eligibility criteria, report quality and deidentification assurance, and for cohort assignment. If needed, de-identified light microscopic (LM) slides and digital EMs are obtained via the data coordinating center and sent to one of the study pathologists for review and, if necessary, a process for second opinion review and adjudication is in place.
Fig. 3.
Timeline for study visits in CureGN-Diabetes. FSGS, focal and segmental glomerulosclerosis; GN, glomerulonephritis; IgA, IgA nephropathy and IgA vasculitis; MN, membranous nephropathy; PRO, patient-reported outcomes.
There is an annual in-person follow-up visit starting in year 2 with vital signs, patient-reported outcomes (PRO), and biospecimens collected. A remote visit occurs annually, via either online questionnaires or telephone interviews. Remote visits are aimed at assessing major clinical events (interval hospitalizations, cardiovascular events, thromboembolic events, and ESKD) and promoting participant retention via contact updates and consistent interaction with study staff. Chart abstraction occurs with every follow-up visit (remote and in-person). Medical records are reviewed for comorbidities/interval diagnoses, medications, hospitalizations (discharge diagnoses), imaging studies, and laboratories (including blood chemistries, hemoglobin A1c, hematology, coagulation, rheumatology/autoimmune and infection serologies, and urine studies). PROs are obtained at all in-person visits using the Patient-Reported Outcomes Measurement Information System (PROMIS), which has been validated and used in the parent CureGN and other glomerular disease studies [15–17].
Participants who develop ESKD and undergo kidney transplantation after study enrollment continue to follow the same visit schedule; those on dialysis, however, have a single in-person visit followed by remote visits only (if they are later transplanted, they will revert to the original visit schedule). Continued in-person visits for transplant patients are to assess for recurrence of glomerular disease.
Biosample collections include DNA, RNA, serum and plasma, and a first-morning urine collection for storage. Serum, plasma, and urine are processed on site at each individual recruitment center and stored in −80°C freezers until they can be batched and sent to the UNC Kidney Center (UNCKC) biorepository. Samples are stored at the UNCKC until the end of the funding period when they will be transferred to the NIDDK biorepository at study closeout, available for utilization in future ancillary studies.
CureGN-Diabetes data storage is in GNLink, a configurable web-based electronic data capture system developed by Arbor Research to support unique protocols in observational research, including the parent CureGN study. It includes tools for study management, reporting and data validations, suppressions, and real-time queries that support the highest data quality possible for research and analysis [18]. The application can display multiple cohorts at a site (CureGN and CureGN-Diabetes), allowing for seamless integration of data from ancillary protocols and ease of use by site study coordinators.
Digital Pathology Repository
All enrolled patients have de-identified pathology reports and digital EMs uploaded to the CureGN DPR. LM slides are sent to a central scanning facility, scanned at 20X into WSIs and stored in the DPR. Patients with prevalent diabetes and MN, FSGS, or MCD enrolled in the Neptune study also may be included in the study. For such patients, pathology materials are available in the Neptune DPR. A QC process will occur to ensure appropriate deidentification of materials and image quality.
Pathology Scoring
WSIs and EMs in the DPR are scored for 62 LM and 16 EM individual morphologic features with IF findings obtained from the pathology reports. These include features scored in the CureGN and TRIDENT studies in addition to more granular unconventional features specific for or often observed in DGS (Table 2). Data are captured in a CureGN-Diabetes case report form in GNLink. Lesions are scored as present or absent, or severity indicated semiquantitatively (online suppl. Table S1; for all online suppl. material, see https://doi.org/10.1159/000531679) [2, 19]. Each case is assigned a Renal Pathology Society diabetes class [20]. There are initial training webinars with all study pathologists to ensure consistency in lesion identification with a lesion dictionary available. The first 5 biopsies are scored by all pathologists and results analyzed to ensure good or better reproducibility using Gwet’s AC1 [21]. If needed, additional training webinars will be carried out. Overall, 10% of the biopsies are scored by two randomly selected pathologists to ensure reliability of study data. A process is in place for second opinions and adjudication of lesions, if necessary.
Table 2.
Features of diabetic glomerulosclerosis to be scored in CureGN-Diabetes
| Light microscopy | Electron microscopy | |
|---|---|---|
| glomerular compartment | vascular compartments | |
| Glomerulomegaly, largest glomerulus diameter | Interstitial eosinophils, plasma cells, neutrophils | Loss of glomerular endothelial cell fenestrae |
| Periglomerular fibrosis | Interstitial foam cells | Capillary wall cellular interposition |
| Extracapillary hypercellularity | Tubular cell protein droplets | GBM duplication |
| Insudates, lipid, foam cells | Thickened TBMs | Abnormal GBM texture |
| Capsular drops | TBM double contours | GBM thickness |
| Mesangiolysis, microaneurysms | Afferent and efferent arteriolar hyalinosis | Mesangial matrix fibrillosis |
| Capillary double contours | Nodular arteriolar hyalinosis | Podocyte microvillous transformation |
| Urinary space collagen | ||
| Severity and extent of diffuse mesangial matrix expansion | ||
| Small and large mesangial matrix nodules | ||
| Matrix nodule characteristics | ||
Data Sharing
Data from CureGN-Diabetes will be available for collaboration and use by consortium and ancillary study investigators via a data sharing and analytic platform supported by the tranSMART Foundation (transmartfoundation.org). TranSMART is a password-protected open-source web-based software platform containing curated, clinical, laboratory, and pathology data, which is updated at regular intervals. Other data sets (genomic, proteomic, etc.) that are generated will be added for data analysis. Platform analytic tools can be used to identify cohorts of interest, explore data element associations, or develop descriptive statistics, promoting scientific collaboration and advancement in glomerular disease in diabetes.
Clinical Statistical Analysis
We will compare clinical characteristics including demographics, physical exam, comorbidities, disease severity indicators (i.e., eGFR, UPCR, and hematuria) at time of kidney biopsy across CureGN-Diabetes, CureGN, and TRIDENT to assess differences in disease presentation between glomerular disease, diabetic kidney disease (DKD), and the combination. Comparisons will be stratified by duration of diabetes and current glucose control. We will also compare treatment regimens after biopsy to assess whether patients with diabetes are treated differently as compared to participants in CureGN who do not have this comorbidity. Both kidney and non-kidney longitudinal outcomes will be assessed using different modeling approaches. For example, time-to-event models for 40% decline in eGFR, remission of proteinuria, ESKD, cardiovascular events and death, mixed models of eGFR slope and Poisson models for disease relapses, infections, and hospitalization rates. The primary exposure of interest will be presence of diabetes with glomerular disease compared to either condition alone.
Pathology Statistical Analysis
Upon completion of pathology scoring for CureGN-Diabetes, kidney biopsy pathologic lesions identified in CureGN-Diabetes, CureGN, and TRIDENT will be compared using a tiered approach to match for primary glomerular disease and diabetes-glomerular class [20]. Appropriate descriptive statistics and group comparison tests will be used to describe differences in prevalence and severity of pathologic lesions across the three cohorts (CureGN-Diabetes vs. CureGN vs. TRIDENT) and how these lesions vary according to demographic, glomerular disease diagnosis, and clinical characteristics. Additionally, unsupervised cluster analysis will be used to identify sets of patients sharing common pathologic profiles which may cross typical diagnostic categories. These pathology feature-driven clusters will then be compared to the current clinical diagnostic categories to assess overlap and individual features which drive cluster membership.
Sample Size and Power Calculation
We calculated the minimum detectable difference in baseline eGFR between the current adult CureGN sample and the proposed CureGN-Diabetes cohort, using nQueryadvisor sample size analysis and t tests for different potential sample sizes and standard deviations. Assuming a CureGN-Diabetes sample of 300, minimum detectable difference for eGFR between the full cohorts and glomerular disease strata-specific cohorts are shown in online supplementary Table S2A and B, respectively, and are consistent with retrospective studies of patients with glomerular disease [22–26]. For comparison of pathology features between CureGN and CureGN-Diabetes, assuming a total sample size of 600 (300 with and without diabetes), we have 80% power at a significance level of 0.05 to detect a 10% difference (20% vs. 30%) in prevalence of a common pathologic feature and 99% power to detect a 10% difference (5% vs. 15%) in prevalence of a rarer pathologic feature. With respect to longitudinal kidney outcomes, we have 80% power at a significance level of 0.05 to detect a minimum hazard ratio for time to loss of 50% eGFR from baseline of 2.0–6.2 based on a presumed event rate of 0.04–0.15 per person year for comparing two subgroups of 150 participants each with average follow-up of 3 years. While participants will be followed indefinitely, a timeline of 3 years was chosen for power analyses given the 5 year funding duration. The minimum detectable hazard ratio for time to complete remission is 1.4–1.8, based on an event rate of 0.2–0.7 events/person-year and 1.5–1.7 for time to hospitalization or ER visit based on an event rate of 0.25–0.45 events/person-year. The mean detectable difference in eGFR slope (change/year) is 1.17–2.85 based on a mean person-specific root mean squared error of the subgroups ranging 13.5–22.6 (online suppl. Table S3). Greater power would be observed if comparing the full CureGN-Diabetes cohort compared to CureGN or Trident or if higher event rates are observed in participants with diabetes.
Discussion
DKD is often “presumed,” as the vast majority of patients with diabetes and CKD do not undergo kidney biopsy. Recognition of this fact has led to a change in terminology from DKD to diabetes and CKD, unless a kidney biopsy has been performed showing DGS, in which case the term “DKD” is appropriate [27]. However, there is growing awareness of the prevalence of non-DKD (NDKD) with or without concomitant DGS in patients with diabetes. Specifically, a large proportion of NDKD found on kidney biopsies is glomerular diseases [28, 29]. This knowledge draws attention to the possibility of undiagnosed, potentially reversible lesions in this patient population. The CureGN-Diabetes Study is the first prospective cohort designed to specifically examine this high-risk population.
A review of native kidney biopsies at Columbia University for evidence of NDKD in people with diabetes estimated that 23.5% of patients with native kidney biopsies carry a diagnosis of diabetes, roughly double the prevalence of diabetes in US adults. Of those patients, 37% had biopsy evidence of DGS alone, 36% had NDKD alone, and 27% had concomitant DGS and NDKD [12]. A retrospective examination of the Southern California Permanente Medical Group database for renal biopsies performed between 1995 and 2005 found a larger proportion of patients with NDKD alone (53.2%) or DGS alone (27.5%) and fewer with both DKD and NDKD (19.3%) [30]. Both studies cited FSGS as the most common pathology in the NDKD alone group at 22% and 21%, respectively, followed by hypertensive nephrosclerosis, acute tubular necrosis, IgAN, and MN. Acute tubular necrosis was the most common diagnosis in the DKD plus NDKD group in the Columbia cohort, whereas IgAN was most common in the California cohort [12, 31]. This may reflect bias in biopsy practice, as proteinuria without kidney injury or hematuria may be assumed to be secondary to DKD. It has been estimated that 40–60% of ESKD in patients with type 2 diabetes is caused by nondiabetic primary kidney diseases, though NDKD causes only 2–3% of renal disease in people with type 1 diabetes [29]. A Canadian retrospective cohort study of IgAN, FSGS, MN, and MCD cited a prevalence of diabetes of 16.5%, with the highest prevalence in the FSGS stratum (25.4%) and the lowest in the IgAN stratum (9.5%) [8]. It is not clear if or how FSGS secondary to diabetes was excluded from the FSGS cohort, so the diabetes prevalence estimate may be particularly high.
Patients with diabetes and glomerular disease represent a sizable patient population that, unfortunately, has been excluded from or under-enrolled in many recent clinical trials investigating management of glomerular diseases. The MENTOR trial, a randomized control trial published in 2019 that found rituximab to be non-inferior to cyclosporine in inducing complete or partial remission of MN, excluded patients with type 1 and type 2 diabetes [32]. The DUET trial, a 2018 trial proving the efficacy and safety of sparsentan in FSGS patients only included well-controlled type 2 diabetes and did not stratify subgroups by diabetes status [33]. Similarly, the STOP-IgAN trial, a 2015 randomized control trial comparing immunosuppressive therapy to supportive care in IgAN, excluded patients with “other chronic renal diseases” without explicit mention of diabetes status [34]. The TESTING trial, a 2017 randomized control trial comparing methylprednisolone to placebo for management of IgAN, did not exclude diabetes, yet there was only 1 patient with diabetes in the treatment group (0.7%) and just three in the control group (2.4%) [9]. The exclusion of diabetes from these trials is predicated on the assumption that the presence of diabetes alters the natural history of primary glomerular diseases. Conversely, studies of chronic kidney disease, such as the Chronic Renal Insufficiency Cohort (CRIC) study, may not include patients with glomerular disease as CRIC excluded those on immunosuppression for active glomerulonephritis [35]. The CureGN study, which aims to characterize the clinical and histopathological presentation and long-term outcomes of MCD, FSGS, IgAN, and MN, also excludes patients with a history of diabetes at the time of first biopsy.
An analysis of people with diabetes and either FSGS or MN who were excluded from CureGN due to their glycemic status, but who otherwise would have been eligible for participation, compared these patients to an age-matched control group from CureGN and found that kidney function at presentation was comparable in all groups [36]. However, participants with diabetes in both disease groups had higher levels of proteinuria than controls, though only the MN group achieved statistical significance. Patients with concurrent DGS and MN on biopsy have been shown to have reduced eGFR (48.0 vs. 70.8 mL/min/1.73 m2) and greater tubulointerstitial fibrosis (25.3% vs. 6.3%) compared to those with MN alone, though the same has not been seen with FSGS [36]. A study of MN from China found that both eGFR and proteinuria at the time of kidney biopsy were worse in the setting of comorbid diabetes, regardless of the presence of DGS on pathology [37].
The presence of concurrent diabetes or histologic DGS has been associated with a higher rate of progression to ESKD in FSGS and MN [36, 37]. These data align with a 2011 retrospective study by Chang et al. [38] showing significantly worse cumulative renal survival in patients with DGS alone versus either NDKD alone or concomitant DGS and NDKD, suggesting potential prognostic value of biopsy in cases with clinical suspicion for NDKD. Furthermore, those authors noted that nearly half of the patients with NDKD were treated with immunosuppression, mostly prednisolone, with a 67.6% complete or partial remission rate of both proteinuria and kidney failure [38]. These findings suggest there is a significant proportion of people with diabetes and NDKD for whom biopsy may not only aid in the diagnosis and prognosis but also change disease management.
Diabetes increases the risk for infection and cardiovascular events, but there are few data addressing if diabetes has an additive or multiplicative interaction with glomerular disease and/or its treatment on these comorbidities. In a retrospective analysis of patients with MN, 42 of 206 had concurrent diabetes, with a higher incidence rate of infectious complications over 1 year (28.6% vs. 15.2% (p < 0.05), but there was no difference in venous thromboembolism (4.9% vs. 3.7%, p = 0.7) [37]. In a large retrospective of 1,912 patients with glomerular disease, 315 (16.5%) had a diagnosis of diabetes at the time of biopsy. Diabetes increased the risk of major adverse cardiovascular event with an unadjusted subdistribution hazard ratio of 3.1 (95% CI: 2.4–4.2), and a multivariable subdistribution hazard ratio of 1.5 (95% CI: 1.1–2.0) when including for both traditional and disease-specific risk factors [8]. A retrospective analysis from Southeast Asia, with 601 glomerular disease patients from Southeast Asia (153 with diabetes; 448 without diabetes), also found that diabetes at the time of biopsy increased the risk for cardiovascular-related hospitalization (HR 2.69; 95% CI: 1.21–5.98) [39].
There are several strengths of this study including its geographical diversity, collection of data prospectively and unique comparison groups in the CureGN and Trident studies. However, there are some limitations of the CureGN-Diabetes study. Inclusion of some biopsies back to 2015 may hinder collection of data at the time of biopsy. Sample sizes within some glomerular disease subtypes will be small, reducing statistical power, particularly when considering the variability of treatment regimens. The kidney biopsies are locally processed and interpreted leading to variability in slide and EM image quality and potentially to representation of findings on the electron microscopy images. Furthermore, we excluded people with FSGS or MCD with concurrent nodular glomerulosclerosis to retain confidence that glomerular disease apart from DGS alone was being sampled, potentially reducing generalizability. Lastly, this study only includes people who have undergone clinical biopsy, and thus there is inherent bias in the population.
“Personalized” or “precision” medicine addresses the heterogeneity of diseases and their interactions with individual-level characteristics. Understanding diabetes as a key modifying factor in the natural history of glomerular disease is essential to providing precision care for patients with IgA, FSGS, MCD, and MN and concomitant diabetes, many of whom are in under-represented communities. Participants in CureGN-Diabetes will provide samples for and have access to the same precision medicine opportunities as participants in the parent study, including whole-genome sequencing, deposition of sequence data in public repositories, sharing of data and bio-samples with outside investigators, and return of clinically relevant genetic results. The materials in the CureGN and CureGN-Diabetes DPRs will be a rich resource for future use of computational pathology and machine learning strategies to identify novel diagnostic and prognostic pathology features. This study will broaden the generalizability of the CureGN Study and provide new knowledge of diabetes-glomerular disease interfaces to allow a better understanding of and improving patient care in this patient population.
Acknowledgments
We are grateful for the efforts of all CureGN-Diabetes participants, investigators, and staff.
Statement of Ethics
The study protocol used a single IRB via the Ethical & Independent Review Services, approval number 21121-01, and was approved by all participating institutions’ committee on human research. All participants in the CureGN-Diabetes study have given their written informed consent.
Conflict of Interest Statement
None of the authors have a COI related to this study. A.K.M. has received research support from Alexion, Bayer, Boehringer-Ingelheim, Calliditas, Duke Clinical Research Institute, and Pfizer; consulting fees from Bayer; and royalties from UpToDate. L.H.M. receives research support from Boehringer-Ingelheim and Travere Therapeutics; consulting fees from Reata Pharmaceuticals, Chinook Therapeutics, Travere Therapeutics, and Calliditas Therapeutics.
Funding Sources
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK126959. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
A.K.M., A.S.B., L.H.M., G.C., and C.C.N. wrote, edited, and approved the final version of the manuscript. J.C.J., S.A., D.G., S.K., J.K., L-P.L., K.M., A.O., M.P., D.R., N.S., K.S., B.S., and S.W. provided substantive edits and approved the final version of the manuscript.
Funding Statement
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK126959. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data Availability Statement
Data from CureGN-Diabetes will be available for collaboration and use by consortium and ancillary study investigators via a data sharing and analytic platform supported by the tranSMART Foundation (transmartfoundation.org). Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 Suppl 1):A6–7. 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 2. Mariani LH, Bomback AS, Canetta PA, Flessner MF, Helmuth M, Hladunewich MA, et al. CureGN study rationale, design, and methods: establishing a large prospective observational study of glomerular disease. Am J Kidney Dis. 2019;73(2):218–29. 10.1053/j.ajkd.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.<national-diabetes-statistics-report.pdf>.
- 4. Nichols GA, Schroeder EB, Karter AJ, Gregg EW, Desai J, Lawrence JM, et al. Trends in diabetes incidence among 7 million insured adults, 2006-2011: the SUPREME-DM project. Am J Epidemiol. 2015;181(1):32–9. 10.1093/aje/kwu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005-2016. JAMA Pediatr. 2020;174(2):e194498. 10.1001/jamapediatrics.2019.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casey JI, Heeter BJ, Klyshevich KA. Impaired response of lymphocytes of diabetic subjects to antigen of Staphylococcus aureus. J Infect Dis. 1977;136(4):495–501. 10.1093/infdis/136.4.495. [DOI] [PubMed] [Google Scholar]
- 7. Nasr SH, Markowitz GS, Whelan JD, Albanese JJ, Rosen RM, Fein DA, et al. IgA-dominant acute poststaphylococcal glomerulonephritis complicating diabetic nephropathy. Hum Pathol. 2003;34(12):1235–41. 10.1016/s0046-8177(03)00424-6. [DOI] [PubMed] [Google Scholar]
- 8. Canney M, Gunning HM, Zheng Y, Rose C, Jauhal A, Hur SA, et al. The risk of cardiovascular events in individuals with primary glomerular diseases. Am J Kidney Dis. 2022;80(6):740–50. 10.1053/j.ajkd.2022.04.005. [DOI] [PubMed] [Google Scholar]
- 9. Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA. 2017;318(5):432–42. 10.1001/jama.2017.9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, et al. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol. 2013;8(3):399–406. 10.2215/CJN.06100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rojas-Rivera J, Fernandez-Juarez G, Ortiz A, Hofstra J, Gesualdo L, Tesar V, et al. A European multicentre and open-label controlled randomized trial to evaluate the efficacy of Sequential treatment with TAcrolimus-Rituximab versus steroids plus cyclophosphamide in patients with primary MEmbranous Nephropathy: the STARMEN study. Clin Kidney J. 2015;8(5):503–10. 10.1093/ckj/sfv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, et al. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8(10):1718–24. 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PXK, Barisoni L, et al. Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int. 2013;83(4):749–56. 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Townsend RR, Guarnieri P, Argyropoulos C, Blady S, Boustany-Kari CM, Devalaraja-Narashimha K, et al. Rationale and design of the transformative research in diabetic nephropathy (TRIDENT) study. Kidney Int. 2020;97(1):10–3. 10.1016/j.kint.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 15. Krissberg JR, Helmuth ME, Almaani S, Cai Y, Cattran D, Chatterjee D, et al. Racial-ethnic differences in health-related quality of life among adults and children with glomerular disease. Glomerular Dis. 2021;1(3):105–17. 10.1159/000516832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Troost JP, Waldo A, Carlozzi NE, Murphy S, Modersitzki F, Trachtman H, et al. The longitudinal relationship between patient-reported outcomes and clinical characteristics among patients with focal segmental glomerulosclerosis in the Nephrotic Syndrome Study Network. Clin Kidney J. 2020;13(4):597–606. 10.1093/ckj/sfz092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murphy SL, Mahan JD, Troost JP, Srivastava T, Kogon AJ, Cai Y, et al. Longitudinal changes in health-related quality of life in primary glomerular disease: results from the CureGN study. Kidney Int Rep. 2020;5(10):1679–89. 10.1016/j.ekir.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gillespie BW, Laurin L-P, Zinsser D, Lafayette R, Marasa M, Wenderfer SE, et al. Improving data quality in observational research studies: report of the Cure Glomerulonephropathy (CureGN) network. Contemp Clin Trials Commun. 2021;22:100749. 10.1016/j.conctc.2021.100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palmer MB, Abedini A, Jackson C, Blady S, Chatterjee S, Sullivan KM, et al. The role of glomerular epithelial injury in kidney function decline in patients with diabetic kidney disease in the TRIDENT cohort. Kidney Int Rep. 2021;6(4):1066–80. 10.1016/j.ekir.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21(4):556–63. 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 21. Zee J, Hodgin JB, Mariani LH, Gaut JP, Palmer MB, Bagnasco SM, et al. Reproducibility and feasibility of strategies for morphologic assessment of renal biopsies using the nephrotic syndrome study network digital pathology scoring system. Arch Pathol Lab Med. 2018;142(5):613–25. 10.5858/arpa.2017-0181-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group . Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16(4):1061–8. 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 23. Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry Group . Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66(3):1199–205. 10.1111/j.1523-1755.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 24. Moranne O, Watier L, Rossert J, Stengel B; GN-Progress Study Group . Primary glomerulonephritis: an update on renal survival and determinants of progression. Qjm. 2008;101(3):215–24. 10.1093/qjmed/hcm142. [DOI] [PubMed] [Google Scholar]
- 25. Hladunewich MA, Troyanov S, Calafati J, Cattran DC; Metropolitan Toronto Glomerulonephritis Registry . The natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol. 2009;4(9):1417–22. 10.2215/CJN.01330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geddes CC, Rauta V, Gronhagen-Riska C, Bartosik LP, Jardine AG, Ibels LS, et al. A tricontinental view of IgA nephropathy. Nephrol Dial Transpl. 2003;18(8):1541–8. 10.1093/ndt/gfg207. [DOI] [PubMed] [Google Scholar]
- 27. Oliva-Damaso N, Mora-Gutiérrez JM, Bomback AS. Glomerular diseases in diabetic patients: implications for diagnosis and management. J Clin Med. 2021;10(9):1855. 10.3390/jcm10091855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Erdogmus S, Kiremitci S, Celebi ZK, Akturk S, Duman N, Ates K, et al. Non-diabetic kidney disease in type 2 diabetic patients: prevalence, clinical predictors and outcomes. Kidney Blood Press Res. 2017;42(5):886–93. 10.1159/000484538. [DOI] [PubMed] [Google Scholar]
- 29. Prakash J. Non-diabetic renal disease (NDRD) in patients with type 2 diabetes mellitus (type 2 DM). J Assoc Physicians India. 2013;61(3):194–9. [PubMed] [Google Scholar]
- 30. Mou S, Wang Q, Liu J, Che X, Zhang M, Cao L, et al. Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;87(3):354–9. 10.1016/j.diabres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 31. Pham TT, Sim JJ, Kujubu DA, Liu ILA, Kumar VA. Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol. 2007;27(3):322–8. 10.1159/000102598. [DOI] [PubMed] [Google Scholar]
- 32. Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 33. Trachtman H, Nelson P, Adler S, Campbell KN, Chaudhuri A, Derebail VK, et al. DUET: a phase 2 study evaluating the efficacy and safety of sparsentan in patients with FSGS. J Am Soc Nephrol. 2018;29(11):2745–54. 10.1681/ASN.2018010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373(23):2225–36. 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 35. Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The chronic renal insufficiency cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–53. 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 36. Freeman NS, Canetta PA, Bomback AS. Glomerular diseases in patients with diabetes mellitus: an underappreciated epidemic. Kidney. 2020;1(3):220–2. 10.34067/KID.0000792019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie Z, Li Z, Dong W, Chen Y, Li R, Wu Y, et al. The impact of coexisting diabetes mellitus on clinical outcomes in patients with idiopathic membranous nephropathy: a retrospective observational study. BMC Nephrol. 2020;21(1):224. 10.1186/s12882-020-01878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang TI, Park JT, Kim JK, Kim SJ, Oh HJ, Yoo DE, et al. Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract. 2011;92(2):198–204. 10.1016/j.diabres.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 39. Lim CC, Choo JCJ, Tan HZ, Mok IYJ, Chin YM, Chan CM, et al. Changes in metabolic parameters and adverse kidney and cardiovascular events during glomerulonephritis and renal vasculitis treatment in patients with and without diabetes mellitus. Kidney Res Clin Pract. 2021;40(2):250–62. 10.23876/j.krcp.20.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from CureGN-Diabetes will be available for collaboration and use by consortium and ancillary study investigators via a data sharing and analytic platform supported by the tranSMART Foundation (transmartfoundation.org). Further inquiries can be directed to the corresponding author.