Abstract
Background
Malaria remains a significant cause of morbidity and mortality in Ethiopia with an estimated 4.2 million annual cases and 61% of the population living in areas at risk of malaria transmission. Throughout the country Plasmodium vivax and P. falciparum are co-endemic, and Duffy expression is highly heterogeneous. The public health significance of Duffy negativity in relation to P. vivax malaria in Ethiopia, however, remains unclear.
Methods
A total of 9,580 and 4,667 subjects from community and health facilities from a malaria endemic site and an epidemic-prone site in western Ethiopia were enrolled and examined for P. vivax infection and Duffy expression. Association between Duffy expression, P. vivax and P. falciparum infections were examined for samples collected from asymptomatic community volunteers and symptomatic subjects from health centers.
Results
Among the community-based cross-sectional samples, infection rate of P. vivax among the Duffy positives was 2–22 fold higher than among the Duffy negatives. Parasite positivity rate was 10–50 fold higher in Duffy positive than Duffy negatives among samples collected from the health center settings and mixed P. vivax and P. falciparum infections were significantly more common than P. vivax mono infections among Duffy negative individuals. P. vivax parasitemia measured by 18sRNA parasite gene copy number was similar between Duffy positives and Duffy negatives.
Conclusions
Duffy negativity does not offer complete protection against infection by P. vivax, and cases of P. vivax in Duffy negatives are widespread in Ethiopia, being found in asymptomatic volunteers from communities and in febrile patients from health centers. These findings offer evidence for consideration when developing control and intervention strategies in areas of endemic P. vivax and Duffy heterogeneity.
Keywords: Malaria, Duffy blood group, Plasmodium vivax, qPCR, gene copy number
Background
Despite significant progress towards malaria control in the past two decades, malaria remains a major cause of mortality and morbidity in Africa1. According to the World Health Organization, Plasmodium vivax and P. falciparum contributed to approximately 700 thousand and 230 million cases, respectively, in Africa in 20212. Current endemicity of P. vivax in Africa correlates with areas of high heterogeneity in Duffy expression3,4. The Duffy antigen receptor for chemokines (DARC), often referred to as the Fy glycoprotein, is a silent heptahelical chemokine receptor located on chromosome 1 and expressed on the surface of erythrocytes. DARC has been recognized as the binding antigen of P. vivax, and a single point mutation located in the GATA-1 transcription factor binding site of the DARC gene promoter (−67T > C) causes this receptor to not be expressed, resulting in a Duffy negative phenotype5,6. The absence of this receptor on red blood cells has been shown to confer resistance to blood-stage infection by P. vivax3, 7, 8. Despite this established dogma, cases of P. vivax are being found in confirmed Duffy negative individuals throughout different African countries9-13. Whether discovery of increasing number of P. vivax infections in Duffy negatives results from more recent research on P. vivax in Africa or from new P. vivax genetic variants, the data suggest that Duffy negativity no longer confers complete resistance to blood-stage P. vivax infection14, 15.
There is little information on the public health significance of P. vivax infection in individuals lacking the Duffy antigen in Africa. For example, how frequent are Duffy negative individuals infected with P. vivax compared to Duffy positive individuals from the same communities? How frequently does P. vivax contribute to clinical malaria among Duffy negatives compared to Duffy positives from areas of same endemicities? This population-based study aimed to address these questions in two locations with varying malaria endemicities in southwestern Ethiopia, using samples from communities and health centers. Despite significant progress towards malaria control in the past two decades, malaria remains a major cause of mortality and morbidity in Ethiopia, with an estimated annual 4.2 million annual cases2, 16. Plasmodium vivax and P. falciparum accounted for approximately 33% and 67% of all malaria cases, respectively, and it is one of only a few countries in Africa where P. vivax remains consistently endemic4.
Methods
Study Sites
Samples were collected from two study sites, Arjo-Didessa and Gambella, both located in western Ethiopia (Fig. 1) with a rainy season lasting from May to October. The Arjo-Didessa sugarcane plantation is located within the Oromia Regional State 395 km west of the Ethiopian capital Addis Ababa and the area covers most of the Arjo-Didessa sugarcane irrigation scheme. It sits at an elevation ranging from 1200m to 1500m above sea level, and comprises 15 villages in 3 districts (Jimma Arjo, Bedele District, and Dado Hana District). It contains 1 health center, 3 health posts, and 9 command posts which are smaller scale health posts located within the temporary residential areas formed by migrant workers. The sugarcane plantation was formerly the Didessa Wildlife Sanctuary before 2006 when the state owned sugarcane plantation was developed to supply the proximal sugarcane factory. It is one of the biggest sugarcane developments in the country, currently covering 5000 hectares with plans to expand to 80,000 hectares17-19. Gambella is located in the Abobo District in the Gambella Regional State, 811 km west of Addis Ababa. The area’s elevation ranges from 400-600m above sea level and as of 2019 had a population of 20,080. The main socioeconomic activity in the area is farming of cotton, maize and sorghum, or working fruit plantations to produce mango, papaya and banana. Additionally, the Alwero Dam provides fishing opportunities and employs approximately 2000 people at a large-scale rice irrigation scheme that currently spans 3,000 hectares with plans to expand to 10,000 hectares. The district comprises 19 villages containing 4 health centers and 16 health posts20, 21. These sites were chosen for the study as both areas have high levels of Duffy admixture, and continuous P. vivax endemicity11, 22.
Figure 1.
Map showing location of both study sites; Arjo and Gambella, in western Ethiopia. Includes locations of study clusters, health facilities, and major towns in the regions.
Blood sample collection
Finger prick blood samples were collected throughout both study sites from asymptomatic community members during cross-sectional surveys and febrile patients at health centers. From each individual, a total of 3 blood spots, equaling ~ 50ul, was pressed to Whatman 3MM filter paper for storage and transportation. For community collections all residents willing to participate were included in the study and provided signed informed consent and/or assent for minors under 18 years old. At the time of sample collection, for both clinical and community samples, the age and sex of participants were recorded when possible. Dried blood spots were transported to the University of California Irvine and stored at 4C.
DNA Extraction and qPCR
Parasite DNA was extracted from dried blood spots (DBS) using a standardized saponin/chelex method23. DNA was eluted to ~ 200ul molecular grade water stored at 4C in the short term or −20C for long term storage. Plasmodium species-specific primers and probes were used to amplify the 18sRNA gene using a previously described protocol with modification24. Real time PCR was conducted at a total volume of 12ul containing; 6μl ThermoFisher FastAdvanced MM (2X), 0.5μl of each species-specific probe, 0.4μl of each forward and reverse species-specific primer, and 2μl parasite DNA. Reaction conditions were set as follows: 50C for 2 min, and 45 cycles of 95C for 2 min, 95C for 3 seconds, 60C for 30 seconds and run on a QuantStudio 3 Real-Time PCR System.
Duffy Sequencing
An approximately ~ 600-bp fragment of the human DARC gene encompassing the – 33rd nucleotide position located in the GATA-1 box of the promoter region was amplified sequenced following established protocols to assess Duffy expression22, 25, 26. Specifically, the total volume for amplification was a 20ul reaction mixture containing; 10μl DreamTaq Green PCR MM (2X), 0.3μl of each forward and reverse primer, and 2μl genomic DNA. Thermocycling conditions were set at; 94C for 2 minutes, 35 cycles of 94C for 30 seconds, 61 for 30 seconds, and 65 for 40 seconds followed by a 2-minute extension at 65C. Five microliters of PCR product were run on a 1.5% agarose gel to confirm amplification. PCR product which had successful amplification was cleaned was cleaned enzymatically to remove remaining primers and dNTPs; 2μl SAP and 0.2μl XO1 per was added to PCR product and cleaned via the following thermocycling conditions; 37C for 15 minutes, 80C for 15 minutes, and then held at a 4C extension. Sanger sequencing was conducted by Retrogen Inc. using forward primers and chromatogram results were visually analyzed via Chromas for a T◊C mutation at the 33rd nucleotide position indicating Duffy negativity. Only samples positive for P. vivax were sequenced for Duffy expression.
Data Analysis
Malaria prevalence was calculated for both study settings at each study site separately. Overall Plasmodium prevalence was compared between study sites for community and clinical collections via the Chi-Square test for independence. Given that only P. vivax positive samples were sequenced for Duffy expression, rates of Duffy negativity in the population was not directly assessed for this study, the overall rate of P. vivax in Duffy negative and Duffy positive individuals was calculated by dividing the number of P. vivax infections by the expected number of Duffy negative and positive individuals at each site. Expected Duffy negative and positive populations were calculated by multiplying the total number of samples by the Duffy negativity rate in Arjo (43.6%) and Gambella (45.9%) as determined in a previously published study27. The ratio of mixed (Pv + Pf) to mono (Pv) infections was determined for each study setting, community and health facility, for both Duffy negatives and Duffy positives. Comparisons of the rate of P. vivax in Duffy negatives to Duffy positives, and the and the ratio of mixed to mono infections for Duffy negatives and positives were made via Fisher’s Exact test for both community and health facility collected samples. Parasite Gene Copy Number (GCN) was calculated from qPCR Cycle threshold (Ct) values via standard curve to estimate parasite density. Log10 transformed GCN was compared between community and health facility settings for both P. vivax and P. falciparum via two-sample t-test, and between Duffy negatives and Duffy positives for both settings via Fisher’s Exact test.
Results
Prevalence of PV Across Study Sites, Collection Method and Duffy Expression
A total of 14,247 dried blood spots were collected from two study sites in southwestern Ethiopia (Fig. 1) from February 2018 to December 2021. Asymptomatic community collections were made via cross-sectional surveys conducted during the spring and late-fall of each year and making up 9,580 of the total dried blood spots. The remaining 4,667 samples were symptomatic infections collected from health clinics and facilities in the regions via passive case detection (PCD). In total 344 DBS were positive for only P. vivax, 937 for only P. falciparum and 35 samples exhibited a mixed infection being positive for both P. vivax and P. falciparum (Table 1). A total of 7,519 of these DBS were collected from Arjo; 5,454 from cross-sectional surveys and 2,065 from passive case detection. In Gambella 6,728 samples were collected in total; 4,126 were collected from the community during cross-sectional surveys and 2,602 via passive case detection (Table 1). Overall, P. vivax and P. falciparum infection rate was significantly higher in Gambella than in Arjo (P < 0.001 for both species).
Table 1.
PCR Prevalence of Plasmodium vivax (Pv) and P. falciparum (Pf) infections among community-based asymptomatic sampling and sample positivity among febrile patients detected by passive case surveillance from health centers in two sites in Ethiopia.
| Settings | Site | Samples (n) |
Total Plasmodium infections |
Mixed Pv and Pf infections |
Pv mono infections |
Pf mono infections |
P- value* |
|---|---|---|---|---|---|---|---|
| Community | Arjo | 5454 | 19 (0.35%) | 0 | 3 (0.06%) | 16 (0.29%) | < 0.05 |
| Gambella | 4126 | 424 (10.28%) | 8 (0.19%) | 133 (3.22%) | 283 (6.86%) | < 0.001 | |
| Total | 9580 | 443 (4.62%) | 8 (0.08%) | 136 (1.42%) | 299 (3.12%) | ||
| Health Center | Arjo | 2065 | 313 (15.16%) | 9 (4.36%) | 114 (5.52%) | 190 (9.20%) | < 0.001 |
| Gambella | 2602 | 560 (21.52%) | 18 (0.69%) | 94 (3.61%) | 448 (17.22%) | < 0.001 | |
| Total | 4667 | 873 (18.79%) | 27 (0.57%) | 208 (4.46%) | 638 (13.67%) |
Fishers exact test comparison between Pv and Pf mono infection rate
Duffy genotyping was performed only on P. vivax mono infections and mixed P. vivax and P. falciparum infections across all study sites and collection methods (Table 2). Of the 379 P. vivax positive and mixed-species infections, 345 were successfully sequenced at the T33C promoter of the GATA-1 transcription factor. Among the community-based cross-sectional samples, infection rate of P. vivax among the Duffy negatives and positives was low and similar in Arjo, but significantly higher infection rate was found in Gambella among Duffy positives than Duffy negatives (5.6% vs. 0.26%, P < 0.001; Table 2). Similarly, sample positivity rate was more than 10–50 fold higher in Duffy positive than Duffy negatives in both sites among samples collected from the health center settings (Table 2), suggesting a much reduced P. vivax burden among Duffy negative people in febrile patients.
Table 2.
Rate of Plasmodium vivax (Pv) infections among Duffy negative and Duffy positive individuals in both community-based asymptomatic and passive case surveillance from health centers at two study sites in Ethiopia.
| Setting | Site | Total Samples |
Expected Duffy Negatives |
Expected Duffy Positives |
Total Pv Infections |
Rate of Pv in Duffy Negatives |
Rate of Pv in Duffy Positives |
P- value* |
|---|---|---|---|---|---|---|---|---|
| Community | Arjo | 5454 | 2525 | 2929 | 3 | 0.04% (1/2525) | 0.07% (2/2929) | > 0.05 |
| Gambella | 4126 | 1894 | 2232 | 130 | 0.26% (5/1894) | 5.60% (125/2232) | < 0.001 | |
| Health Center | Arjo | 2065 | 956 | 1109 | 120 | 0.21% (2/956) | 10.64% (118/1109) | < 0.001 |
| Gambella | 2602 | 1194 | 1408 | 92 | 0.59% (7/1194) | 6.04% (85/1408) | < 0.001 |
P-value calculated via Fishers Exact Test for comparing Rate of Pv in Duffy Negatives to Duffy Positives
Interestingly, we found that a considerably large proportion of malaria infections were mixed species infection. Among the community-based samples, eight out of 133 (6.0%) malaria infections were mixed species and 26 out of 212 (12.3%) samples were mixed infections from the health center settings (Table 3). Among the Duffy negatives, P. vivax was found more frequently found in the form of mixed-species infection than mono infections, whereas mono P. vivax infections were far more common in Duffy positives. In the community asymptomatic samples, the ratio of mixed species infection to P. vivax mono infection was 0.5 among Duffy negatives, but this ratio was reduced to 0.05 in Duffy positives (P < 0.05; Table 3). In febrile samples from health centers the ratio of mixed species infection to P. vivax mono infection was 3.5 among Duffy negatives, far greater than the ratio observed in Duffy positive (0.10; P < 0.001). This data strongly suggests that in Duffy negative individuals P. vivax is more frequently found in mixed infections compared to P. vivax only mono infections.
Table 3.
Distribution of Duffy phenotypes across Plasmodium vivax (Pv) and Mixed (Pv and Pf) infections in both community-based asymptomatic and passive case surveillance via health centers from two study sites in Ethiopia
| Setting | Infection | n | Duffy negative |
Duffy positive |
Ratio of mixed species infection to P. vivax only infections |
Fisher’s exact test |
|
|---|---|---|---|---|---|---|---|
| Duffy negative | Duffy positive | ||||||
| Community | Pv | 125 | 4 | 121 | 0.5 | 0.05 | P < 0.05 |
| Pv + Pf | 8 | 2 | 6 | ||||
| Health Center | Pv | 186 | 2 | 184 | 3.5 | 0.10 | P < 0.001 |
| Pv + Pf | 26 | 7 | 19 | ||||
P. vivax Parasitemia in Community and Clinical Samples and Across Duffy Expressions
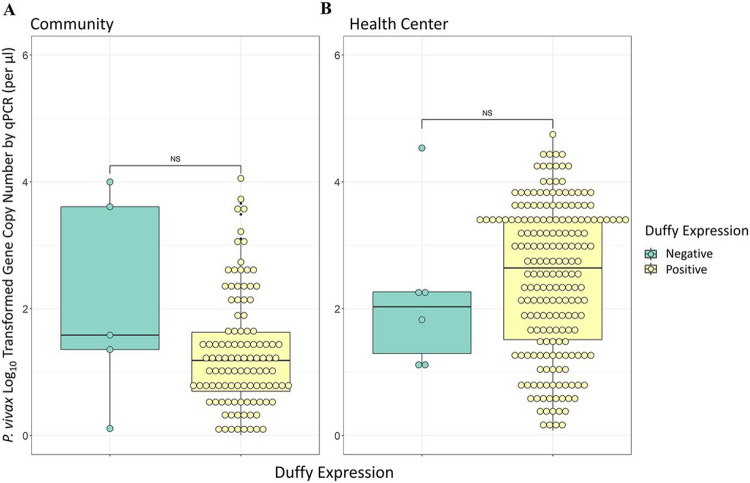
Analyses of qPCR data revealed significant differences in the parasitemia between cross-sectional samples without clinical symptoms and clinical samples collected during passive case detection from health centers for both P. vivax and P. falciparum. In both P. vivax and P. falciparum infections parasitemia was significantly higher in samples collected via passive case detection than via cross-sectional survey (P < 0.001, Fig. 2). Symptomatic P. vivax infections showed a geometric mean gene copy number (GCN) of 2.03 parasites/μl, which was significantly higher than the asymptomatic P. vivax infections, which had a geometric mean of 0.94 parasites/μl (P < 0.001, Fig. 2). Similarly, symptomatic P. falciparum infections exhibited a geometric mean of 1.67 parasites/μl, which was significantly higher than the asymptomatic P. falciparum infections which had a mean of 0.90 parasites/μl (P < 0.001). Community Duffy-negative and Duffy-positive samples exhibited a similar parasitemia, with a GCN of 1.28 and 0.93 parasites/μl respectively (P > 0.05, Fig. 3). Similarly, PCD Duffy-negative and Duffy positive samples showed a mean GCN of 1.93 and 2.07 parasites/μl respectively (P > 0.05, Fig. 3). These data do not include four Duffy negative samples as their gene copy numbers fell just outside of our standard curve based cutoff range. Given the substantial differences in sample sizes between Duffy-negatives and Duffy-positives it is possible that the lack of significance observed here is indeed due to a small samples size of Duffy negatives.
Figure 2. Log-transformed parasite gene copy number of community and clinical samples.
Violin box-plot of the log-transformed parasite Gene copy number (GCN) of (A) Plasmodium vivax and (B) Plasmodium falciparum by qPCR for individuals of all ages. These samples were collected both in the community via cross-sectional surveys and at health centers via Passive Case Detection (PCD) at two Ethiopian field sites; Arjo and Gambella. The central box represents the interquartile range with the median shown as the center line in the box. P-values (above) are calculated via two-sample T.test (***, P < 0.001).
Figure 3. Box plots of the log-transformed parasite gene copy number for Duffy negative and Duffy positive individuals.
Box plot of the log-transformed gene copy number for Plasmodium vivax for Duffy negative and Duffy positive individuals of all ages in both (A) the community via cross-sectional survey and (B) health centers via Passive Case Detection. Box plots represent the interquartile range with the median expressed as the center line. All individual data points are shown via open circles, P-values were calculated via Fisher’s Exact test (NS, non-significant).
Discussion
This study sought to examine P. vivax malaria burden in Duffy negative individuals at two field sites with similar proportion of Duffy negativity, but different malaria endemicities in southwest Ethiopia. We found, firstly, that P. vivax posed a significant health burden at both sites, but was far more prevalent in the community in Gambella than in Arjo where infection prevalence was over 50 times higher. In febrile patients P. vivax was found more often in Arjo than in Gambella; however, this difference was much less drastic than in the community with Arjo exhibiting only 1.5 times more P. vivax clinical infections than Gambella. Across both sites and collection settings P. vivax was found far less frequently in Duffy negatives than Duffy positives. In the community Duffy positives had approximately 2 and 22-fold greater infection rate of P. vivax than Duffy negatives at Arjo and Gambella, respectively. In febrile patients and samples collected from health facilities this trend was even more apparent; in Arjo and Gambella Duffy positives exhibited a 51 and 10-fold greater positivity rate of P. vivax infections, respectively, than Duffy negatives. The variations in rate of infection were highly significant for samples from health centers at both sites, but only significant for community samples from Gambella. The lack of significance in Arjo community samples could potentially be due to the small sample size as only three P. vivax infections were found in the community in Arjo. These strongly suggest that P. vivax infections, despite being commonly found in Duffy negative individuals, are still predominantly occurring in Duffy positive people. Despite the significant variations in rate of P. vivax infection between Duffy expressions, we did not observe significant differences in parasitemia between Duffy negatives and Duffy positives in either the community or health centers. Perhaps most interestingly this study highlights a pattern of mixed versus mono infections related to Duffy negativity. By calculating the ratio of mixed to mono P. vivax infections among Duffy negative and positive individuals we found that Duffy negatives exhibited a 10 and 35-fold greater ratio of mixed to mono infections than Duffy positives in both the community and clinical settings, respectively. Therefore, for Duffy negatives, P. vivax is predominantly found in mixed infections more than mono infections.
Collectively our findings build on previous work documenting P. vivax infections in Duffy negative individuals in numerous African countries28, 29 including Cameroon30, Madagascar9, Angola and Equatorial Guinea31, Kenya32, Ethiopia4. These studies are consistent with our findings and support the conclusion that Duffy negative individuals are not completely resistant to infection by P. vivax, yet still have a greatly reduced prevalence of P. vivax infections compared to Duffy positive individuals. Our data show that despite no significant variation in parasitemia between Duffy positives and Duffy negatives, several P. vivax infections from Duffy negatives exhibited relatively high levels of parasitemia potentially implying that these parasites readily infect and adapt to Duffy negativity, allowing for greater erythrocyte invasion. Despite this, several studies have ample evidence that parasitemia of P. vivax is greatly reduced in Duffy negatives, supporting the hypothesis that parasite infectivity to the human erythrocyte, though not completely inhibited, is indeed reduced in the absence of the Duffy antigen33, 34. Several prior studies have also observed that P. vivax infections within Duffy negative individuals are frequently mixed infections, yet these data are limited in that they are predominantly descriptive and do not explore these mixed infections in detail nor compare their prevalence between Duffy negatives and positives9, 35, 36. Thus this current study stands out in its efforts to systematically evaluate the prevalence of mixed infections in individuals with Duffy negative status as compared to those with Duffy positive status. Our findings thus shed light on the noteworthy phenomenon that P. vivax infections in Duffy negatives frequently encompass mixed-species infections, especially when compared to Duffy positives.
Since the level of P. vivax exposure remained consistent among both Duffy positive and Duffy negative individuals across our distinct study locations, the observed diminished burden of P. vivax in Duffy negative individuals underscores that while Duffy negativity does not confer absolute resistance to P. vivax infection, it does exert a significant inhibitory effect on infection establishment. The mechanism behind P. vivax infections of Duffy negatives remains highly elusive, however, several studies have highlighted potential invasion mechanism adaptations of P. vivax that may circumvent Duffy-based infection inhibition and allow for infection on a lesser scale. One of the most well studied of these potential adaptations is the P. vivax Duffy binding protein 1 (PvDBPI) copy number expansion. Several different studies have clearly shown that PvDBP gene amplification both facilitated binding to alternative lower affinity receptors in Duffy negatives, and also suggested that the binding affinity of DARC with high copies of PvDBP could be much higher than with single-copy PvDBP parasites14, 37-40, providing a potential selective pressure towards gene duplication and thus increased infectivity. Two additional ligands, P. vivax glycosylphosphatidylinositol-anchored micronemal antigen (PvGAMA) and P. vivax merozoite surface protein-1 paralog (PvMSP1P), were recently found capable of binding to both Duffy positive and negative red blood cells, suggesting possible involvement in Duffy-independent invasion pathways41.
It warrants mention that in the present study is limited in that Duffy expression (negative vs. positive) was inferred based on genotype data of the T33C point mutation in the promoter region of the GATA-1 transcription factor binding site of the Duffy antigen receptor for chemokines (DARC) gene, which is known to alter erythroid expression and eliminate Duffy antigen expression on the red blood cell surface25, 26, 42. However, the direct antigen expression (phenotype) was not assessed. It is therefore not impossible for a genotypically categorized Duffy negative individual to potentially express Duffy receptors in some quantity, and the P. vivax strains infecting Duffy negatives in this study may be utilizing such an expression in invasion, despite determined genotypic negativity. Additionally, we did not assess the prevalence or burden of P. falciparum across Duffy negatives and positives as Duffy expression is not known to be associated with P. falciparum infection.
Understanding the distribution of P. vivax in Africa and exploring the significance of Duffy expression continues to be a challenging and intricate endeavor. Given the low parasitemia often associated with P. vivax infections of Duffy negative individuals, microscopy and RDTs are often not sensitive enough to detect infection, hindering their diagnosis and study in the field. Indeed, corresponding microscopy data from this area accounted for only approximately 70%, of all qPCR confirmed P. vivax positive infections17, highlighting the need for more sensitive molecular detection tools in the field. This has significant implications for malaria elimination on the continent as a high proportion of P. vivax cases are likely being overlooked by traditional diagnostic methods and thus going unaddressed in intervention and elimination efforts. It is clear through the current work that Duffy negativity is not a definitive barrier to infection, mixed infections are common in Duffy negatives, and that not only does P. vivax transmission remain widespread in Ethiopia, but asymptomatic community infections make up a significant portion of P. vivax cases resulting in a large undetected parasite reservoir that may greatly complicate and hinder interventions and elimination efforts. This information is vital to informing control and intervention strategies in areas of Sub-Saharan Africa with endemic P. vivax and areas of high Duffy heterogeneity.
Acknowledgements
The authors sincerely thank the field teams and staff at Jimma University in Ethiopia who were involved in this project. We also acknowledge the local healthcare.
Funding
This study is funded by the National Institutes of Health (5 F31 AI161887-02, U19 AI129326, D43 TW001505, and R01 AI162947). These funders were not involved in study design or development, data collection and analysis, publication process or manuscript preparation.
Footnotes
Ethical Approval
Ethical and scientific approval and clearance was obtained from the institutional scientific and ethical review boards of the University of California, Irvine USA and Jimma University, Ethiopia. Written informed consent/assent for participation in the study was obtained from all participants and/or parents/guardians (for minors under the age of 18).
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests The authors declare that they have no competing interests.
Contributor Information
Lauren Bradley, University of California Irvine.
Delenasaw Yewhalaw, Jimma University.
Elizabeth Hemming-Schroeder, Colorado State University.
Brook Jeang, University of California Irvine.
Ming-Chieh Lee, University of California Irvine.
Endalew Zemene, Jimma University.
Teshome Degefa, Jimma University.
Eugenia Lo, Drexel University.
Christopher King, Case Western Reserve University.
James Kazura, Case Western Reserve University.
Guiyun Yan, University of California Irvine.
References
- 1.Girum T, Shumbej T, Shewangizaw M: Burden of malaria in Ethiopia, 2000-2016: findings from the Global Health Estimates 2016. Trop Dis Travel Med Vaccines 2019, 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Malaria Report. World Health Organization 2022, Geneva [Google Scholar]
- 3.Twohig KA, Pfeffer DA, Baird JK, Price RN, Zimmerman PA, Hay SI, Gething PW, Battle KE, Howes RE: Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis 2019, 13:e0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ketema T, Bacha K, Getahun K, Portillo HAD, Bassat Q: Plasmodium vivax epidemiology in Ethiopia 2000-2020: A systematic review and meta-analysis. PLoS Negl Trop Dis 2021, 15:e0009781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langhi DM Jr., Bordin JO: Duffy blood group and malaria. Hematology 2006, 11:389–398. [DOI] [PubMed] [Google Scholar]
- 6.Hoher G, Fiegenbaum M, Almeida S: Molecular basis of the Duffy blood group system. Blood Transfus 2018, 16:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis H. Miller SJM, Clyde David F., McGinnis Mary H.: The resistance factor to Plasmodium vivax in Blacks: The Duffy Blood Group Genotype, FyFy. The New England Journal of Medicine 1976, 295. [DOI] [PubMed] [Google Scholar]
- 8.Howes RE, Patil AP, Piel FB, Nyangiri OA, Kabaria CW, Gething PW, Zimmerman PA, Barnadas C, Beall CM, Gebremedhin A, et al. : The global distribution of the Duffy blood group. Nat Commun 2011, 2:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard D, Barnadas C, Bouchier C, Henry-Halldin C, Gray LR, Ratsimbasoa A, Thonier V, Carod JF, Domarle O, Colin Y, et al. : Plasmodium vivax clinical malaria is commonly observed in Duffy-negative Malagasy people. Proc Natl Acad Sci U S A 2010, 107:5967–5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wurtz NL K. M.; Bogreau H.; Pradines B.; Rogier C.; Mohamed Salem Boukhary A. O.; Hafid J. E.; Ould Ahmedou Salem M. S.; Trape J; Basco L. K.; Briolant S.: Vivax malaria in Maritania includes infection of a Duffy-negative individual. Malaria Journal 2011, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woldearegai TG, Kremsner PG, Kun JF, Mordmuller B: Plasmodium vivax malaria in Duffy-negative individuals from Ethiopia. Trans R Soc Trop Med Hyg 2013, 107:328–331. [DOI] [PubMed] [Google Scholar]
- 12.Abdelraheem MH, Albsheer MM, Mohamed HS, Amin M, Mahdi Abdel Hamid M: Transmission of Plasmodium vivax in Duffy-negative individuals in central Sudan. Trans R Soc Trop Med Hyg 2016, 110:258–260. [DOI] [PubMed] [Google Scholar]
- 13.Russo G, Faggioni G, Paganotti GM, Djeunang Dongho GB, Pomponi A, De Santis R, Tebano G, Mbida M, Sanou Sobze M, Vullo V, et al. : Molecular evidence of Plasmodium vivax infection in Duffy negative symptomatic individuals from Dschang, West Cameroon. Malar J 2017, 16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunalan K, Niangaly A, Thera MA, Doumbo OK, Miller LH: Plasmodium vivax Infections of Duffy-Negative Erythrocytes: Historically Undetected or a Recent Adaptation? Trends Parasitol 2018, 34:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilairatana P, Masangkay FR, Kotepui KU, De Jesus Milanez G, Kotepui M: Prevalence and risk of Plasmodium vivax infection among Duffy-negative individuals: a systematic review and meta-analysis. Sci Rep 2022, 12:3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dawit G Ayele TTZHGM: Prevalence and risk factors of malaria in Ethiopia. Malaria Journal 2012, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawaria D, Getachew H, Zhou G, Demissew A, Habitamu K, Raya B, Lee MC, Yewhalaw D, Yan G: Ten years malaria trend at Arjo-Didessa sugar development site and its vicinity, Southwest Ethiopia: a retrospective study. Malar J 2019, 18:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang A-L, Lee M-C, Zhou G, Zhong D, Hawaria D, Kibret S, Yewhalaw D, Sanders BF, Yan G, Hsu K: Predicting distribution of malaria vector larval habitats in Ethiopia by integrating distributed hydrologic modeling with remotely sensed data. Scientific Reports 2021, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demissew A, Hawaria D, Kibret S, Animut A, Tsegaye A, Lee MC, Yan G, Yewhalaw D: Impact of sugarcane irrigation on malaria vector Anopheles mosquito fauna, abundance and seasonality in Arjo-Didessa, Ethiopia. Malar J 2020, 19:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taffese HSH-S E.; Koepfli C.; Tesfaye G.; Lee M. C.; Kazura J.; Yan G.; Zhou G.: Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infectious Diseases of Poverty 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haileselassie W, Parker DM, Taye B, David RE, Zemene E, Lee MC, Zhong D, Zhou G, Alemu T, Tadele G, et al. : Burden of malaria, impact of interventions and climate variability in Western Ethiopia: an area with large irrigation based farming. BMC Public Health 2022, 22:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo E, Yewhalaw D, Zhong D, Zemene E, Degefa T, Tushune K, Ha M, Lee MC, James AA, Yan G: Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malar J 2015, 14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bereczy SM A.; Gil J. P.; Farnert A: Short Report: Rapid DNA Extraction from archive blood spots on filter paper for genotyping plasmodium falciparum. Am J Trop Med Hyg 2005, 72:249–251. [PubMed] [Google Scholar]
- 24.Veron V, Simon S, Carme B: Multiplex real-time PCR detection of P. falciparum, P. vivax and P. malariae in human blood samples. Exp Parasitol 2009, 121:346–351. [DOI] [PubMed] [Google Scholar]
- 25.King CL, Adams JH, Xianli J, Grimberg BT, McHenry AM, Greenberg LJ, Siddiqui A, Howes RE, da Silva-Nunes M, Ferreira MU, Zimmerman PA: Fy(a)/Fy(b) antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria. Proc Natl Acad Sci U S A 2011, 108:20113–20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo E, Hostetler JB, Yewhalaw D, Pearson RD, Hamid MMA, Gunalan K, Kepple D, Ford A, Janies DA, Rayner JC, et al. : Frequent expansion of Plasmodium vivax Duffy Binding Protein in Ethiopia and its epidemiological significance. PLoS Negl Trop Dis 2019, 13:e0007222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.al Be: DETERMINATION OF PLASMODIUM VIVAX AND PLASMODIUM FALCIPARUM MALARIA EXPOSURE IN TWO ETHIOPIAN COMMUNITIES AND ITS RELATIONSHIP TO DUFFY EXPRESSION American Journal of Tropical Medicine and Hygiene 2023, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman PA: Plasmodium vivax Infection in Duffy-Negative People in Africa. Am J Trop Med Hyg 2017, 97:636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo E, Russo G, Pestana K, Kepple D, Abagero BR, Dongho GBD, Gunalan K, Miller LH, Hamid MMA, Yewhalaw D, Paganotti GM: Contrasting epidemiology and genetic variation of Plasmodium vivax infecting Duffy-negative individuals across Africa. Int J Infect Dis 2021, 108:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngassa Mbenda HG, Das A: Molecular evidence of Plasmodium vivax mono and mixed malaria parasite infections in Duffy-negative native Cameroonians. PLoS One 2014, 9:e103262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendes C, Dias F, Figueiredo J, Mora VG, Cano J, de Sousa B, do Rosario VE, Benito A, Berzosa P, Arez AP: Duffy negative antigen is no longer a barrier to Plasmodium vivax–molecular evidences from the African West Coast (Angola and Equatorial Guinea). PLoS Negl Trop Dis 2011, 5:e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan: Evidence for Transmission of Plasmodium vivax amond a Duffy Antigen Negative Population in Western Kenya. American Journal of Tropical Medicine and Hygiene 2006, 74. [PubMed] [Google Scholar]
- 33.Albsheer MMA, Pestana K, Ahmed S, Elfaki M, Gamil E, Ahmed SM, Ibrahim ME, Musa AM, Lo E, Hamid MMA: Distribution of Duffy Phenotypes among Plasmodium vivax Infections in Sudan. Genes (Basel) 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abate A, Bouyssou I, Mabilotte S, Doderer-Lang C, Dembele L, Menard D, Golassa L: Vivax malaria in Duffy-negative patients shows invariably low asexual parasitaemia: implication towards malaria control in Ethiopia. Malar J 2022, 21:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oboh MA, Badiane AS, Ntadom G, Ndiaye YD, Diongue K, Diallo MA, Ndiaye D: Molecular identification of Plasmodium species responsible for malaria reveals Plasmodium vivax isolates in Duffy negative individuals from southwestern Nigeria. Malar J 2018, 17:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oboh MA, Singh US, Ndiaye D, Badiane AS, Ali NA, Bharti PK, Das A: Presence of additional Plasmodium vivax malaria in Duffy negative individuals from Southwestern Nigeria. Malar J 2020, 19:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menard D, Chan ER, Benedet C, Ratsimbasoa A, Kim S, Chim P, Do C, Witkowski B, Durand R, Thellier M, et al. : Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis 2013, 7:e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunalan K, Lo E, Hostetler JB, Yewhalaw D, Mu J, Neafsey DE, Yan G, Miller LH: Role of Plasmodium vivax Duffy-binding protein 1 in invasion of Duffy-null Africans. Proc Natl Acad Sci U S A 2016, 113:6271–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson RD, Amato R, Auburn S, Miotto O, Almagro-Garcia J, Amaratunga C, Suon S, Mao S, Noviyanti R, Trimarsanto H, et al. : Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet 2016, 48:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hostetler JB, Lo E, Kanjee U, Amaratunga C, Suon S, Sreng S, Mao S, Yewhalaw D, Mascarenhas A, Kwiatkowski DP, et al. : Independent Origin and Global Distribution of Distinct Plasmodium vivax Duffy Binding Protein Gene Duplications. PLoS Negl Trop Dis 2016, 10:e0005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popovici J, Roesch C, Rougeron V: The enigmatic mechanisms by which Plasmodium vivax infects Duffy-negative individuals. PLoS Pathog 2020, 16:e1008258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tournamille C, Colin Y, Cartron JP, Van Kim C: Disruption of a GATA motif in the Duffy gene promoter abolishes erythroid gene expression in Duffy–negative individuals. Nature Genetics 1995, 10:224–228. [DOI] [PubMed] [Google Scholar]