Abstract
Purpose
Sex-steroid hormones are associated with postmenopausal breast cancer but potential confounding from other biological pathways is rarely considered. We estimated risk ratios for sex-steroid hormone biomarkers in relation to postmenopausal estrogen receptor (ER)-positive breast cancer, while accounting for biomarkers from insulin/insulin-like growth factor-signaling and inflammatory pathways.
Methods
This analysis included 1,208 women from a case-cohort study of postmenopausal breast cancer within the Melbourne Collaborative Cohort Study. Weighted Poisson regression with a robust variance estimator was used to estimate risk ratios (RRs) and 95% confidence intervals (CIs) of postmenopausal ER-positive breast cancer, per doubling plasma concentration of progesterone, estrogens, androgens, and sex hormone binding globulin (SHBG). Analyses included sociodemographic and lifestyle confounders, and other biomarkers identified as potential confounders.
Results
Increased risks of postmenopausal ER-positive breast cancer were observed per doubling plasma concentration of progesterone (RR: 1.22, 95% CI: 1.03 to 1.44), androstenedione (RR: 1.20, 95% CI: 0.99 to 1.45), dehydroepiandrosterone (RR: 1.15, 95% CI: 1.00 to 1.34), total testosterone (RR: 1.11, 95% CI: 0.96 to 1.29), free testosterone (RR: 1.12, 95% CI: 0.98 to 1.28), estrone (RR: 1.21, 95% CI: 0.99 to 1.48), total estradiol (RR: 1.19, 95% CI: 1.02 to 1.39) and free estradiol (RR: 1.22, 95% CI: 1.05 to 1.41). A possible decreased risk was observed for SHBG (RR: 0.83, 95% CI: 0.66 to 1.05).
Conclusion
Progesterone, estrogens and androgens likely increase postmenopausal ER-positive breast cancer risk, whereas SHBG may decrease risk. These findings strengthen the causal evidence surrounding the sex hormone-driven nature of postmenopausal breast cancer.
Keywords: breast cancer, sex-steroid hormones, progesterone, estrogens, androgens, sex hormone binding globulin
1. Background
Breast cancer is a largely hormone-driven disease and the relationships between endogenous sex-steroid hormones – especially estrogens – and postmenopausal breast cancer are thought to be well established [1–3]. A recent systematic review and meta-analysis found moderate- to high-quality evidence that higher levels of estrogens (estradiol and estrone) and androgens (testosterone and androstenedione), and lower levels of sex hormone binding globulin (SHBG), were associated with increased risks of postmenopausal breast cancer [4]. Dose-response relationships were observed for SHBG, estradiol, and estrone, with weaker evidence for androstenedione and testosterone [4]. There was also evidence to suggest that progesterone and dehydroepiandrosterone (DHEA) were not associated with breast cancer [4].
The quality of the evidence in this review was largely determined by dose-response effects and large effect sizes [4]. No extracted result had adjusted for biomarkers from other biological pathways; namely, the insulin/insulin-like growth factor (IGF)-signaling and inflammatory pathways. These pathways may confound the effect of the sex-steroid hormone pathway. For example, insulin and insulin-like growth factor-1 (IGF-1) can affect the bioavailability of estrogens and androgens via the regulation of aromatase and suppression of hepatic SHBG production [1, 5, 6]. They may also play a role in breast carcinogenesis: insulin and the IGF axis are proposed to have mitogenic and anti-apoptotic properties, and higher systemic concentrations of IGF-1 are associated with increased risks of breast cancer [1, 2, 5–8]. Further, a state of low-grade chronic inflammation – for example, in the context of physical inactivity and obesity – can foster a pro-carcinogenic environment via the overstimulation and dysregulation of immune cells, cytokines and adipokines [1, 2, 5, 6, 9]. Higher circulating levels of C-reactive protein (CRP) – a non-specific marker of chronic inflammation – are associated with increased risks of breast cancer, but the epidemiological evidence for other inflammatory markers remains uncertain [2, 10, 11]. Higher circulating levels of pro-inflammatory biomarkers including leptin, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are also associated with enhanced aromatase activity and lower circulating levels of SHBG [1, 2, 5, 6, 9, 12].
Studies that adjust for other biomarkers typically compare results with and without adjustment for other sex-steroid hormones and/or SHBG [3, 13–22]. These are often mutual or progressive adjustments to assess independence rather than confounding, on the basis that biomarkers share complex interrelationships and correlations. However, this practice can lead to overadjustment bias [23]. In addition, only a handful of studies have measured and adjusted for biomarkers from other biological pathways that may be potential confounders. One study from the Women’s Health Initiative presented results for estradiol with and without adjustment for free IGF-1 and insulin; positive associations with postmenopausal breast cancer appeared stronger with adjustment for both IGF-1 and insulin [24]. Another study from the UK Biobank presented results for testosterone with and without adjustment for SHBG and IGF-1 that were not appreciably different [25]. Further studies are needed to clarify the confounding role of other biological pathways implicated in breast carcinogenesis.
The aim of this study was to estimate risk ratios for sex-steroid hormone biomarkers in relation to postmenopausal breast cancer in a case-cohort of postmenopausal women within the Melbourne Collaborative Cohort Study (MCCS), while accounting for other biomarkers from the insulin/IGF-signaling and inflammatory pathways.
2. Methods
2.1. The Melbourne Collaborative Cohort Study
The MCCS includes 24,469 women aged 40–69 at recruitment from 1990–1994 [26]. At baseline and the second follow-up (F2, 2003–7), participants provided information about health status, lifestyle factors, sociodemographics and medical history via structured questionnaires [26]. Anthropometric and clinical measurements were performed at the study center, including the collection of blood samples [26]. At both times, plasma was stored in liquid nitrogen. Data linkages to national and state death and cancer registries – including the Victorian Cancer Registry and Australian Cancer Database – enabled vital status and cancer diagnoses to be determined prospectively [26]. The study protocol was approved by the Cancer Council Victoria Human Research Ethics Committee.
2.2. The case-cohort study
2.2.1. Initial eligibility criteria at second follow-up (2003–7)
This case-cohort study was restricted to women who attended F2. At F2, eligible women were postmenopausal, not known to be taking hormone replacement therapy (HRT), had provided a blood sample (within one year of the F2 questionnaire, if completed), had no prior invasive cancer diagnosis (except for keratinocyte cancers); at baseline, they had a body mass index (BMI) ≥ 18.5 kg/m2. Women were considered postmenopausal if they had had no menstrual periods in the past 12 months and met one of the following criteria: had experienced natural cessation of menses; had a bilateral oophorectomy; were age 55 years or older; or had had no periods in the 12 months prior to baseline and, for participants in a previous case-cohort study, measured estradiol concentration below 109 pmol/L at baseline (a threshold from that study [13, 27]). The case-cohort comprised a random sample of the 10,669 eligible women and all eligible women diagnosed with estrogen receptor (ER)-positive postmenopausal breast cancer between blood collection at F2 and 31 October 2020.
An eligible tumor was defined as invasive adenocarcinoma of the breast (International Classification of Diseases, Tenth Revision [ICD-10] code C50) that was ER-positive. Tumors of unknown hormone receptor status were included as 88% of breast cancer diagnoses among eligible women of known ER status were ER-positive. ER-negative and progesterone receptor (PR)-positive cancers were also included as this tumor subtype may be misclassified and accounts for only 1–4% of diagnoses [28–31]. Unspecified adenocarcinomas and unspecified cancers were presumed to be adenocarcinomas as 99% of breast cancer diagnoses among eligible women of known morphology were adenocarcinomas.
In total, 1,412 women were selected for the case-cohort study, including 999 in the subcohort and 459 cases (46 from the subcohort) (Fig. 1). The subcohort was a random sample of eligible women (Online Resource 1).
Figure 1.
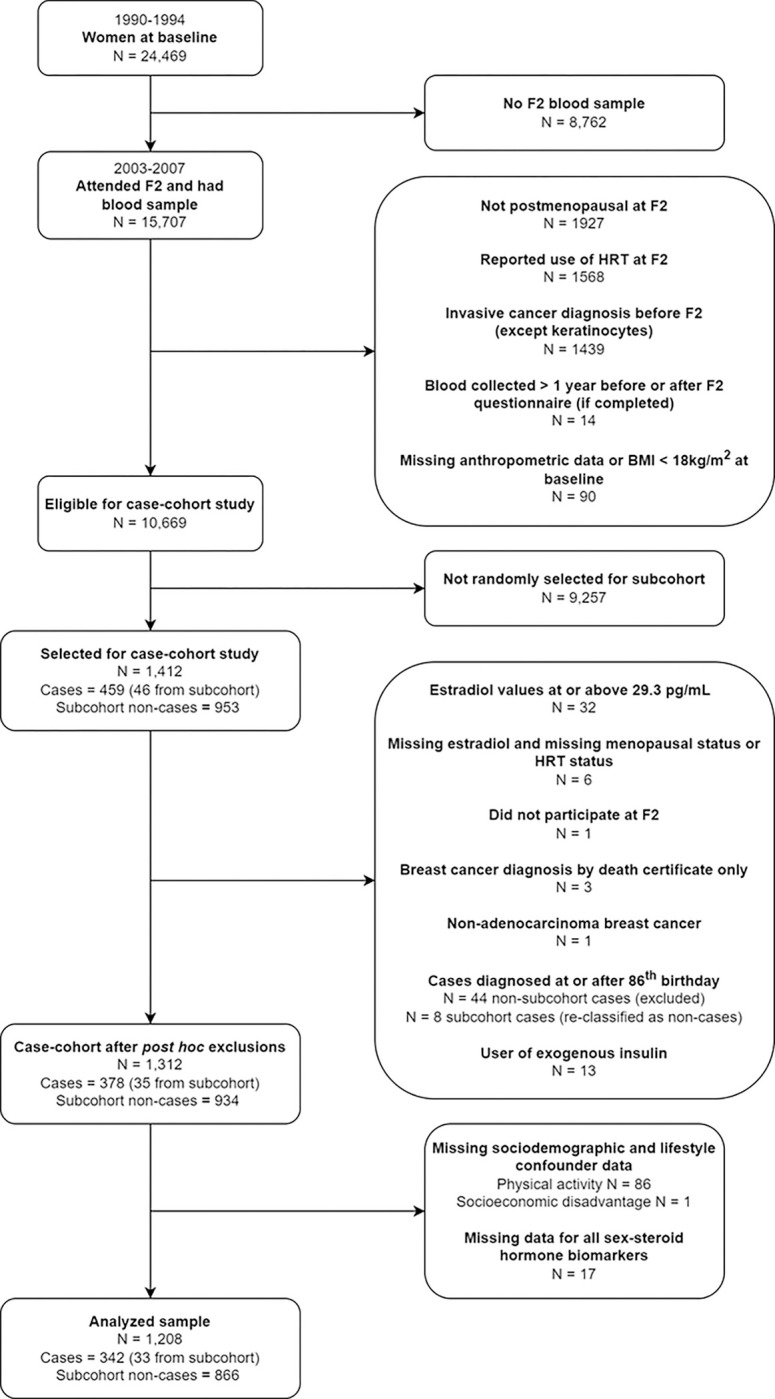
Selection of participants into the case-cohort study and analyses
N: Number. F2: Second follow up wave. HRT: Hormone replacement therapy.
2.2.2. Post hoc criteria
Of the 1,412 selected women, 286 (20%) had unknown menopausal status and/or HRT use. Eligibility was confirmed for all selected women using the distribution of measured estradiol values at F2 for naturally postmenopausal women who were not taking HRT (806, 57% of selected women). Thirty-two women with estradiol values at or above the 99th percentile of this distribution (29.3 pg/mL, equivalent to 107.6 pmol/L) were excluded, regardless of age, menopausal status, or HRT use. Menopausal status and/or HRT use could not be determined for six women missing estradiol measurements. One woman was excluded as she did not participate in F2 despite providing a blood sample.
Four cases outside the subcohort were retrospectively disqualified as cases; three diagnoses were ascertained from death certificate only and one woman was diagnosed with non-adenocarcinoma breast cancer. To minimize the impact of death as a competing risk, follow-up was chosen to end on participants’ 86th birthday (Online Resource 2). Thus, 44 cases outside the subcohort were excluded and eight cases within the subcohort were analyzed as non-cases. Thirteen users of exogenous insulin were excluded so that measured insulin concentrations were of endogenous insulin.
The total study sample after post hoc exclusions comprised 1,312 women, 969 in the subcohort and 378 cases (35 also in the subcohort) (Fig. 1).
2.3. Laboratory analysis of plasma biomarkers
Plasma samples of selected women were randomly ordered and allocated into 21 batches containing approximately equal numbers of cases. The samples were shipped on dry ice in two dispatches to the International Agency for Research on Cancer (IARC).
The plasma concentrations of all biomarkers were measured at the Nutrition Metabolism Branch, IARC. Plasma concentrations of sex-steroid hormones and SHBG were measured as previously described [32]. In brief, sex-steroid hormone concentrations were measured using a liquid chromatography-mass spectrometry system consisting of an ultra-high-performance liquid chromatograph (Agilent 1290, Agilent, Santa Clara, CA) and a QTRAP 5500 mass spectrometer (SCIEX, Framingham, MA). SHBG concentrations were measured by solid-phase “sandwich” enzyme-linked immunoassay (DRG International, Springfield, NJ). Interferon gamma (IFN-γ), IL-6, interleukin-8 (IL-8), interleukin-10 (IL-10), TNF-α, insulin, adiponectin, leptin, and CRP were measured by highly sensitive and highly specific electrochemiluminescent methods (Meso Scale Discovery, Rockville, MD). IGF-1 and insulin-like growth factor binding protein-3 (IGFBP-3) were measured by immunoassay methods by R&D Systems (Biotechne, Minneapolis, USA). C-peptide was measured by an enzyme-linked immunosorbent assay by ALPCO (Salem, USA). Further details are included in Online Resource 3. Three quality control samples at different concentration levels were measured in duplicate in each batch of analyses to assess the reliability of biomarker measurements. Reliability was assessed by calculating intra-assay and inter-assay coefficients of variation (CVs), as well as intra-batch and inter-batch intra-class correlation coefficients (ICCs), as described in Online Resource 4. Assay performance for estradiol and testosterone was evaluated by measuring samples created from reference standards with known concentrations. Measured values were compared with true values using validity coefficients and correlation plots, as described in Online Resource 5.
2.4. Normalization of biomarker values
Biomarker data were cleaned and normalized to correct for effects of batch, dispatch, and time since last meal (12% of study participants were not fasting at blood collection). The normalization technique was adapted from Viallon et al. [33]. Normalization models were used to estimate residual ICCs for the total proportion of variation attributable to batch for each biomarker. Methods for normalization and estimated ICCs are presented in Online Resource 6.
2.5. Calculation of free estradiol and free testosterone
Concentrations of free estradiol and free testosterone (i.e., not bound to SHBG) were calculated from normalized values of estradiol, testosterone and SHBG using the law of mass action assuming a fixed albumin concentration of 40 g/L (5.97 × 10– 4 mol/L) and the following association constants: 6 × 104 L/mol (binding of estradiol to albumin); 4 × 104 L/mol (binding of testosterone to albumin); 0.68 × 109 L/mol (binding of estradiol to SHBG); 1.6 × 109 L/mol (binding of testosterone to SHBG) [34–37].
2.6. Statistical analysis
Descriptive statistics were presented as medians and interquartile ranges (IQRs) or as frequencies and percentages, where appropriate. Weighted modified Poisson regression with a robust variance estimator was used to estimate risk ratios (RRs) and 95% confidence intervals (CIs) of postmenopausal ER-positive breast cancer, per doubling plasma concentration of progesterone, androstenedione, DHEA, total and calculated free testosterone, estrone, total and calculated free estradiol, and SHBG. Case weights were one, and weights for non-cases were the inverse of the sampling probability for non-cases [38].
Confounders including other biomarkers were identified a priori using causal diagrams informed by expert consensus and literature review. Sociodemographic and lifestyle confounders included: education; country of birth; socioeconomic disadvantage; diet at baseline (dietary intake of carotenoids and dietary intake of calcium); alcohol consumption at baseline; smoking status at baseline; adiposity at baseline; physical activity at F2; age at blood collection; and age at menopause. The identification, measurement and modelling of sociodemographic and lifestyle confounders are described in Online Resource 7. As age at menopause could only be measured for naturally postmenopausal women (821, 63% of the case-cohort after post hoc exclusions), this variable was not included in the adjustment set for the primary analyses. Sensitivity analyses were conducted, restricting to naturally postmenopausal women with a recorded age at menopause to include this variable in adjustment sets. Biomarkers that were identified as potential confounders a priori but had correlations ≥ 0.50 with the biomarker of interest were not included in the primary analysis (Online Resource 8).
The primary analyses modelled all biomarker concentrations as continuous, normalized values on the log2-scale. A one unit increase on the log2-scale represents a doubling in biomarker concentration. Analyses were repeated without adjustment for other biomarkers (where applicable). In addition, analyses that modelled concentrations of each sex-steroid hormone biomarker as quartiles corresponding to the distribution of normalized biomarker values in the subcohort were performed without adjustment for other biomarkers.
All analyses were complete-case analyses. The linearity assumption was tested for the continuous, normalized biomarker values using restricted cubic splines and Wald-tests. All statistical analyses were performed using Stata 16 (StataCorp, College Station, TX).
3. Results
Of the 1,312 women eligible after post-hoc exclusions, 87 were excluded due to missing sociodemographic and lifestyle confounder data (Fig. 1). In addition, 17 women were excluded due to missing measurements for all sex-steroid hormone biomarkers. The characteristics of the remaining 1,208 women are summarized in Table 1. Compared with non-cases, cases were more likely to be educated, have obesity, and experience the menopause at ≥ 53 years, and were less likely to be sufficiently active. The normalized concentrations of DHEA, total estradiol, free estradiol, leptin and CRP were higher, and the normalized concentration of SHBG was lower, for cases compared with non-cases. The characteristics of the 1,312 women eligible after post-hoc exclusions were not appreciably different from the 1,208 women analyzed (Online Resource 9).
Table 1.
Characteristics of the analyzed case-cohort (N = 1,208)
| Cases N = 342 | Non-Cases N = 866 | |||
|---|---|---|---|---|
| Age at Blood Collection (Years; Median, IQR) | 66.0 | (60.0, 71.0) | 67.5 | (61.0, 73.0) |
| Dietary Calcium Intake (mg/d; Median, IQR) | 802.1 | (621.1, 1045.8) | 823.4 | (610.2, 1051.8) |
| Total Carotenoid Intake from Diet (mcg/d; Median, IQR) | 17885 | (13726, 23441) | 17274 | (13352, 23188) |
| Southern European Migrant Status (N, %) | ||||
| No | 277 | 81.0% | 696 | 80.4% |
| Yes | 65 | 19.0% | 170 | 19.6% |
| Socioeconomic Disadvantage (N, %) | ||||
| Quintile 1: Most Disadvantaged | 51 | 14.9% | 134 | 15.5% |
| Quintile 2 | 56 | 16.4% | 177 | 20.4% |
| Quintile 3 | 56 | 16.4% | 127 | 14.7% |
| Quintile 4 | 72 | 21.1% | 165 | 19.1% |
| Quintile 5: Least Disadvantaged | 107 | 31.3% | 263 | 30.4% |
| Education (N, %) | ||||
| Primary School or Some High / Technical School | 202 | 59.1% | 563 | 65.0% |
| Completed High / Technical School | 65 | 19.0% | 127 | 14.7% |
| Completed Tertiary Degree / Diploma | 75 | 21.9% | 176 | 20.3% |
| Smoking Status (N, %) | ||||
| Never Smoked | 251 | 73.4% | 637 | 73.6% |
| Ever Smoked | 91 | 26.6% | 229 | 26.4% |
| Lifetime Alcohol Consumption (N, %) | ||||
| Life Abstention | 135 | 39.5% | 318 | 36.7% |
| ≤ 19 g/d | 187 | 54.7% | 493 | 56.9% |
| 20 to 29 g/d | 12 | 3.5% | 26 | 3.0% |
| 30 to 39 g/d | 5 | 1.5% | 17 | 2.0% |
| ≥ 40 g/d | 3 | 0.9% | 12 | 1.4% |
| Body Mass Index (N, %) | ||||
| Normal (≥ 18.5 to < 25 kg/m2) | 144 | 42.1% | 374 | 43.2% |
| Overweight (≥ 25 to < 30 kg/m2) | 110 | 32.2% | 315 | 36.4% |
| Obese (≥ 30 kg/m2) | 88 | 25.7% | 177 | 20.4% |
| Physical Activitya (N, %) | ||||
| Insufficiently Active | 113 | 33.0% | 266 | 30.7% |
| Sufficiently Active | 80 | 23.4% | 245 | 28.3% |
| Highly Active | 149 | 43.6% | 355 | 41.0% |
| Age at Menopauseb (N, %) | ||||
| ≤ 48 years | 41 | 20.8% | 139 | 24.9% |
| 49–50 years | 53 | 26.9% | 144 | 25.8% |
| 51–52 years | 41 | 20.8% | 127 | 22.7% |
| ≥ 53 years | 62 | 31.5% | 149 | 26.7% |
| Normalized Biomarkers (Median, IQR) | ||||
| Sex-Steroid Hormone Pathway | ||||
| Progesterone (nmol/L) | 0.13 | (0.10, 0.19) | 0.13 | (0.10, 0.17) |
| Androstenedione (nmol/L) | 1.6 | (1.2, 2.2) | 1.5 | (1.2, 2.0) |
| DHEA (nmol/L) | 5.0 | (3.3, 7.4) | 4.5 | (2.9, 6.8) |
| Estrone (pmol/L) | 81.3 | (60.2, 114.8) | 78.7 | (58.9, 107.5) |
| SHBG (nmol/L) | 55.7 | (41.6, 79.5) | 61.9 | (45.6, 82.3) |
| Total Testosterone (nmol/L) | 0.64 | (0.45, 0.87) | 0.64 | (0.44, 0.89) |
| Total Estradiol (pmol/L) | 18.5 | (12.5, 27.7) | 16.3 | (10.9, 25.1) |
| Free Testosterone (pmol/L) | 5.5 | (3.8, 8.4) | 5.2 | (3.6, 7.5) |
| Free Estradiol (pmol/L) | 0.25 | (0.15, 0.40) | 0.20 | (0.13, 0.33) |
| Insulin/IGF-Signaling Pathway | ||||
| Insulin (pg/mL) | 298.0 | (207.5, 422.7) | 290.3 | (208.7, 438.6) |
| IGF-1 (nmol/L) | 7.8 | (6.4, 9.4) | 8.0 | (6.4, 10.0) |
| IGFBP-3 (nmol/L) | 66.2 | (58.0, 75.5) | 68.2 | (58.8, 76.8) |
| C-Peptide (ng/mL) | 2.6 | (2.1, 3.4) | 2.6 | (2.0, 3.4) |
| Inflammatory Pathway | ||||
| Leptin (pg/mL) | 16312 | (8441, 31909) | 14056 | (6387, 27578) |
| Adiponectin (ng/mL) | 25514 | (19623, 32838) | 24912 | (18922, 33098) |
| TNF-α (pg/mL) | 2.7 | (2.2, 3.3) | 2.6 | (2.2, 3.2) |
| IL-6 (pg/mL) | 0.73 | (0.55, 1.04) | 0.73 | (0.52, 1.02) |
| IL-8 (pg/mL) | 2.8 | (2.3, 3.9) | 3.0 | (2.2, 4.0) |
| IL-10 (pg/mL) | 0.25 | (0.19, 0.33) | 0.23 | (0.17, 0.32) |
| IFN-γ (pg/mL) | 5.5 | (4.1, 7.8) | 5.4 | (3.8, 8.6) |
| CRP (ng/mL) | 1633 | (804, 2936) | 1391 | (682, 3020) |
N: Number. IQR: Interquartile range. DHEA: Dehydroepiandrosterone. SHBG: Sex-hormone binding globulin. IGF: Insulin-like growth factor. IGF-1: Insulin-like growth factor-1. IGFBP-3: Insulin-like growth factor binding protein-3. TNF-α: Tumor necrosis growth factor-alpha. IL-6: Interleukin-6. IL-8: Interleukin-8. IL-10: Interleukin-10. IFN-γ: Interferon gamma. CRP: C-reactive protein. nmol/L: Nanomoles per liter. pmol/L: Picomoles per liter. ng/mL: Nanograms per milliliter. pg/mL: Picograms per milliliter. g/d: Grams per day. mg/d: Milligrams per day. mcg/d: Micrograms per day. kg/m2: Kilograms per meters squared.
Physical activity was measured as total weighted minutes of walking, moderate- and vigorous-intensity recreation- and transport-related physical activity (MVPA) per week at the second follow-up wave. Insufficiently active was defined as < 150 total weighted minutes of MVPA per week, sufficiently active was defined as 150 to ≤ 300 total weighted minutes of MVPA per week, and highly active was defined as > 300 total weighted minutes of MVPA per week.
Age at menopause was measured for naturally postmenopausal women only, when the cessation of periods for 12 months was first documented (baseline, the first follow-up wave, or the second follow-up wave).
Missing data for normalized biomarkers are as follows: 1 for progesterone; 2 for estrone; 8 for estradiol; 1 for adiponectin; 4 for CRP. Missing data for other covariates include: 452 for age at menopause (including 49 naturally postmenopausal women).
Southern European Migrant status, socioeconomic disadvantage, education, smoking status, lifetime alcohol consumption, body mass index, dietary calcium intake and total carotenoid intake from diet were measured at baseline. Biomarker concentrations, age at blood collection and physical activity were measured at the second follow-up wave.
3.1. Reliability of biomarker measurements and assay performance
The calculated overall intra-assay and inter-assay CVs were below 10% and 15% respectively for most biomarkers (Online Resource Table 4.1). The estimated intra-batch and inter-batch reliability ICCs were above 80% and 70% respectively for most biomarkers (Online Resource Table 4.2). The validity coefficients for the true and measured values of estradiol and testosterone were 0.987 and 0.997, respectively. Correlation plots are presented in Online Resource 5.
3.2. Risk ratios per doubling of biomarker concentration
For the primary analyses, increased risks of postmenopausal ER-positive breast cancer were observed per doubling plasma concentration of progesterone (RR: 1.22, 95% CI: 1.03 to 1.44), androstenedione (RR: 1.20, 95% CI: 0.99 to 1.45), DHEA (RR: 1.15, 95% CI: 1.00 to 1.34), total testosterone (RR: 1.11, 95% CI: 0.96 to 1.29), calculated free testosterone (RR: 1.12, 95% CI: 0.98 to 1.28), estrone (RR: 1.21, 95% CI: 0.99 to 1.48), total estradiol (RR: 1.19, 95% CI: 1.02 to 1.39) and calculated free estradiol (RR: 1.22, 95% CI: 1.05 to 1.41) (Table 2). A decreased risk was suggested for SHBG (RR: 0.83, 95% CI: 0.66 to 1.05).
Table 2.
Risk ratios for postmenopausal estrogen receptor-positive breast cancer per doubling of biomarker concentration
| Biomarker (per doubling concentration) | Cases | Subcohort Non-Cases | Risk Ratio | 95% CI |
|---|---|---|---|---|
| Progesterone (nmol/L) | ||||
| Primary analysis | 342 | 865 | 1.22 | (1.03, 1.44) |
| Androstenedione (nmol/L) | ||||
| Primary analysis | 342 | 866 | 1.20 | (0.99, 1.45) |
| DHEA (nmol/L) | ||||
| Primary analysis | 342 | 866 | 1.15 | (1.00, 1.34) |
| Total Testosterone (nmol/L) | ||||
| Primary analysis (adjusted for SHBG) | 342 | 866 | 1.11 | (0.96, 1.29) |
| Not adjusted for other biomarkers | 342 | 866 | 1.10 | (0.95, 1.27) |
| Free Testosterone (nmol/L) | ||||
| Primary analysis | 342 | 866 | 1.12 | (0.98, 1.28) |
| Estrone (pmol/L) | ||||
| Primary analysis (adjusted for adiponectin, leptin, TNF-α, IL-6, insulin, IGF-1 and SHBG) | 342 | 863 | 1.21 | (0.99, 1.48) |
| Not adjusted for other biomarkers | 342 | 864 | 1.20 | (0.98, 1.45) |
| Total Estradiol (pmol/L) | ||||
| Primary analysis (adjusted for adiponectin, leptin, TNF-α, IL-6, insulin, IGF-1 and SHBG) | 341 | 858 | 1.19 | (1.02, 1.39) |
| Not adjusted for other biomarkers | 341 | 859 | 1.20 | (1.04, 1.38) |
| Free Estradiol (pmol/L) | ||||
| Primary analysis (adjusted for adiponectin, leptin, TNF-α, IL-6, insulin and IGF-1) | 341 | 858 | 1.22 | (1.05, 1.41) |
| Not adjusted for other biomarkers | 341 | 859 | 1.18 | (1.03, 1.35) |
| SHBG (nmol/L) | ||||
| Primary analysis (adjusted for adiponectin, leptin, insulin and IGF-1) | 342 | 865 | 0.83 | (0.66, 1.05) |
| Not adjusted for other biomarkers | 342 | 866 | 0.90 | (0.73, 1.11) |
CI: Confidence interval. DHEA: Dehydroepiandrosterone. SHBG: Sex hormone binding globulin. IGF-1: Insulin-like growth factor-1. IL-6: Interleukin-6. TNF-α: Tumor necrosis factor-alpha. nmol/L: Nanomoles per liter. pmol/L: Picomoles per liter.
The results of the primary analyses were adjusted for sociodemographic and lifestyle confounders (education, socioeconomic disadvantage, Southern European Migrant status, dietary intake of carotenoids at baseline, dietary intake of calcium at baseline, lifestyle alcohol consumption at baseline, smoking status at baseline, adiposity at baseline, physical activity at the second follow-up wave and age at blood collection) and other biomarkers identified as potential confounders, where applicable (Online Resource 8).
Results did not appreciably differ in analyses without adjustment for other biomarkers (Table 2), except that the inverse association for SHBG was somewhat weaker (RR: 0.90, 95% CI: 0.73 to 1.11). For the sensitivity analyses in the subset of naturally postmenopausal women with a recorded age at menopause (Online Resource 10), the point estimates for RR were closer to the null for progesterone (RR: 1.11, 95% CI: 0.90 to 1.36) and androstenedione (RR: 1.08, 95% CI: 0.85 to 1.39), and further away from the null for estrone (RR: 1.30, 95% CI: 0.99 to 1.69), total estradiol (RR: 1.29, 95% CI: 1.04 to 1.58) and calculated free estradiol (RR: 1.31, 95% CI: 1.08 to 1.60). Results with and without adjustment for age at menopause were similar, whereas the point estimates for RR without adjustment for other biomarkers were closer to the null for estrone, free estradiol and SHBG (Online Resource 10).
3.3. Risk ratios for quartiles of biomarker concentration
The highest versus lowest levels of biomarker concentrations were associated with increased risks of postmenopausal ER-positive breast cancer for progesterone (RR: 1.56, 95% CI: 1.09 to 2.24), androstenedione (RR: 1.39, 95% CI: 0.97 to 2.00), DHEA (RR: 1.55, 95% CI: 1.06 to 2.25), total estradiol (RR: 1.49, 95% CI: 1.01 to 2.19) and calculated free estradiol (RR: 1.47, 95% CI: 0.99 to 2.17) (Table 3). RRs were suggestive of monotonic increases for DHEA, estrone and total estradiol. In contrast, the positive relationship between calculated free estradiol and postmenopausal ER-positive breast cancer plateaued at the third-highest plasma concentration compared to the lowest.
Table 3.
Risk ratios for postmenopausal estrogen receptor-positive breast cancer, by quartiles of biomarker concentrations
| Quartilesa of Normalized Biomarker Concentrations | Cases | Subcohort Non-Cases | Risk Ratio | 95% CI |
|---|---|---|---|---|
| Progesterone | ||||
| Quartile 1 | 72 | 208 | Ref | Ref |
| Quartile 2 | 92 | 218 | 1.25 | (0.87, 1.81) |
| Quartile 3 | 67 | 220 | 0.96 | (0.65, 1.41) |
| Quartile 4 | 111 | 219 | 1.56 | (1.09, 2.24) |
| Androstenedione | ||||
| Quartile 1 | 72 | 214 | Ref | Ref |
| Quartile 2 | 97 | 214 | 1.32 | (0.92, 1.90) |
| Quartile 3 | 69 | 219 | 0.92 | (0.63, 1.35) |
| Quartile 4 | 104 | 219 | 1.39 | (0.97, 2.00) |
| DHEA | ||||
| Quartile 1 | 65 | 216 | Ref | Ref |
| Quartile 2 | 75 | 207 | 1.19 | (0.82, 1.74) |
| Quartile 3 | 94 | 224 | 1.38 | (0.94, 2.00) |
| Quartile 4 | 108 | 219 | 1.55 | (1.06, 2.25) |
| Total Testosterone | ||||
| Quartile 1 | 80 | 211 | Ref | Ref |
| Quartile 2 | 85 | 216 | 1.04 | (0.73, 1.49) |
| Quartile 3 | 98 | 223 | 1.16 | (0.82, 1.65) |
| Quartile 4 | 79 | 216 | 1.04 | (0.72, 1.50) |
| Free Testosterone | ||||
| Quartile 1 | 75 | 218 | Ref | Ref |
| Quartile 2 | 78 | 203 | 1.08 | (0.75, 1.55) |
| Quartile 3 | 83 | 225 | 1.02 | (0.71, 1.47) |
| Quartile 3 | 106 | 220 | 1.26 | (0.89, 1.80) |
| Estrone | ||||
| Quartile 1 | 79 | 207 | Ref | Ref |
| Quartile 2 | 78 | 214 | 0.95 | (0.66, 1.37) |
| Quartile 3 | 86 | 223 | 1.03 | (0.72, 1.48) |
| Quartile 4 | 99 | 220 | 1.13 | (0.78, 1.64) |
| Total Estradiol | ||||
| Quartile 1 | 64 | 215 | Ref | Ref |
| Quartile 2 | 81 | 212 | 1.27 | (0.87, 1.85) |
| Quartile 3 | 93 | 218 | 1.41 | (0.97, 2.05) |
| Quartile 4 | 103 | 214 | 1.49 | (1.01, 2.19) |
| Free Estradiol | ||||
| Quartile 1 | 67 | 216 | Ref | Ref |
| Quartile 2 | 64 | 214 | 0.95 | (0.64, 1.41) |
| Quartile 3 | 101 | 213 | 1.46 | (1.01, 2.12) |
| Quartile 4 | 109 | 216 | 1.47 | (0.99, 2.17) |
| SHBG | ||||
| Quartile 1 | 112 | 215 | Ref | Ref |
| Quartile 2 | 83 | 213 | 0.79 | (0.56, 1.10) |
| Quartile 3 | 72 | 224 | 0.67 | (0.47, 0.95) |
| Quartile 4 | 75 | 214 | 0.83 | (0.57, 1.21) |
CI: Confidence interval. Ref: Reference category. DHEA: Dehydroepiandrosterone. SHBG: Sex-hormone binding globulin.
Quartiles based on the distribution of normalized biomarker values in the subcohort. Minimum, median and maximum values for each quartile are presented in Online Resource 11.
Results were adjusted for sociodemographic and lifestyle confounders (education, socioeconomic disadvantage, Southern European Migrant status, dietary intake of carotenoids at baseline, dietary intake of calcium at baseline, lifestyle alcohol consumption at baseline, smoking status at baseline, adiposity at baseline, physical activity at the second follow-up wave and age at blood collection).
4. Discussion
Higher plasma concentrations of progesterone, estrogens and androgens, and decreasing plasma concentration of SHBG, were associated with increased risks of postmenopausal ER-positive breast cancer in this case-cohort of postmenopausal women. Similar results were obtained with and without control for other biomarkers that were identified as potential confounders, suggesting that confounding by the insulin/IGF-signaling and inflammatory pathways was minimal. The exception was SHBG; a somewhat stronger inverse relationship was observed with adjustment for adiponectin, leptin, insulin and IGF-1. Results of the sensitivity analyses in the subset of naturally postmenopausal women with a recorded age at menopause were not sensitive to adjustment for age at menopause. Rather, the deviations observed from the primary analyses could be explained by reduced precision in the subsample, or differences between women who were naturally postmenopausal (with a known age at menopause) and women who were assumed to be postmenopausal for other reasons.
A strength of our study was that careful consideration was given to biomarkers from the insulin/IGF-signaling and inflammatory pathways that may confound the associations between biomarkers of the sex-steroid hormone pathway and risk of postmenopausal ER-positive breast cancer. Biomarkers that may be potential confounders were identified a priori using a causal diagram that was informed by literature review and expert opinion. Causal diagrams can minimize the pitfalls of other confounder selection methods, including overadjustment bias [23, 39, 40]. However, residual confounding may remain if our assumptions are inaccurate or if important confounders have not been identified or correctly measured [39, 40]. Depicting the true complexity of biomarker interrelationships and their role in breast carcinogenesis is challenging. The current body of causal knowledge is limited, and we could not account for bidirectional relationships as the biomarkers had only been measured at one point in time. Thus, we assumed what the net direction of the effects of the measured biomarkers would be in a relatively older cohort of postmenopausal women in our causal diagram. Our assumptions can be refined with the advancement of causal knowledge over time, ideally in studies that measure biomarkers at multiple points in time.
The validity of our results depends upon the extent to which the measurements of the chosen biomarkers accurately represent the biological components of the inflammation, insulin/IGF-signaling and sex-steroid hormone pathways implicated in breast carcinogenesis. A major strength of our study was the use of a highly sensitive liquid chromatography-mass spectrometry method to measure the plasma concentrations of sex-steroid hormones in postmenopausal women with high precision and accuracy. We were able to demonstrate the validity of this method using reference standards for estradiol and testosterone. The measured and true values of estradiol and testosterone were highly correlated. Further, intra-assay and inter-assay CVs, as well as intra-batch and inter-batch ICCs, calculated from quality control samples indicated that the biomarker measurements were reliable, with few exceptions that may be attributable to batch and dispatch effects (Online Resource 4). We adopted a novel analysis approach to correct for batch effects, dispatch effects and time since last meal, whilst retaining meaningful biological variation in the biomarker measurements [33]. Further, we measured the plasma concentrations of a breadth of biomarkers selected through expert consultation and literature review. However, plasma concentrations of biomarkers measured at only one point in time will not be perfect proxies of complex and time-varying biological processes that may operate at cellular and systemic levels.
Our findings were generally consistent with previous studies, including a recent systematic review by Drummond et al. [4], a previous case-cohort study conducted at baseline (1990–1994) within the MCCS [13], and a pooled analysis of nine prospective studies examining the relationship between endogenous sex-steroid hormones and postmenopausal breast cancer [3]. A notable finding was the estimated risk ratio per doubling plasma concentration of progesterone; we observed the largest increased risk of postmenopausal ER-positive breast cancer for this biomarker (RR: 1.22, 95% CI: 1.03 to 1.44) compared to any other measured biomarker from the sex-steroid hormone pathway. Previous studies have either not measured endogenous progesterone or have drawn inconclusive results regarding its relationship with breast cancer after the menopause, largely due to insufficient assay sensitivity and low circulating levels in postmenopausal women [41]. Our result is in support of a recent study by Trabert at al. [42], which also used a highly sensitive liquid chromatography-mass spectrometry method and found increased risks of postmenopausal breast cancer per standard deviation increase in circulating endogenous progesterone levels (hazard ratio for invasive breast cancers: 1.24, 95% CI: 1.07 to 1.43). Trabert et al. [42] also present evidence for effect modification: reduced risks of postmenopausal breast cancer were observed with higher levels of progesterone among women in the lowest quintile of circulating estradiol (< 6.30 pg/mL), while increased risks were observed among women in the higher quintiles (≥ 6.30 pg/mL). Collectively, these results may challenge the plausibility of our a priori assumption that progesterone does not have a direct effect on postmenopausal ER-positive breast cancer (depicted by no direct arrow from progesterone to postmenopausal breast cancer in our causal diagram, Online Resource Fig. 8.1). This assumption was based on the systematic review by Drummond et al. [4], which found moderate quality evidence of no association between progesterone and breast cancer risk (albeit in both pre- and postmenopausal women combined). The implication of this assumption is that we should interpret the risk ratio for progesterone as an indirect effect, possibly driven by its role as a precursor of androgens and estrogens in steroidogenesis. This finding – in addition to concerns over the sensitivity of progesterone measurements in early studies, as well as studies demonstrating paracrine effects of progesterone via neighboring PR-positive cells [41] – warrants future studies including mediation analyses to determine what dictates the effect of progesterone on postmenopausal ER-positive breast cancer.
Our study confirms the causal role that sex-steroid hormones and SHBG play in the etiology of postmenopausal ER-positive breast cancer. We strengthen the causal evidence by demonstrating that potential confounding from other biological pathways implicated in breast carcinogenesis is likely non-substantial. Of note, two recent systematic reviews found insufficient evidence to establish a causal link between the inflammation and insulin/IGF-signaling pathways and breast cancer [8, 11]. Future research could examine whether adjustment for biomarkers from other biological pathways is more important for pre-menopausal breast cancer or ER-negative postmenopausal breast cancer. In addition, time-varying confounding could be examined in future studies that measure biomarkers at multiple points in time.
Acknowledgements
The authors thank Audrey Gicquiau for the measurement of the sex-steroid hormones and sex hormone binding globulin, and Anne-Sophie Navionis for the measurement of the biomarkers of the insulin\insulin-like growth factor-signaling and inflammatory pathways. The authors also thank the participants of the Melbourne Collaborative Cohort Study.
Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database.
Funding
Melbourne Collaborative Cohort Study (MCCS) cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further augmented by Australian National Health and Medical Research Council grants 209057, 396414 and 1074383 and by infrastructure provided by Cancer Council Victoria. Funding for IIG_2018_1730 was obtained from World Cancer Research Fund (WCRF UK), as part of the World Cancer Research Fund International grant programme. The reference standards for estradiol and testosterone were purchased and analyzed using funds from a NIH grant (NIH R01 CA207369) held by Dr Sue Hankinson at University of Massachusetts. Frances EM Albers and Makayla WC Low are each supported by a Research Training Program Scholarship from the Australian Government and the University of Melbourne. Makayla WC Lou is further supported by a scholarship from the Macau Special Administrative Region Government Higher Education Fund (Governo da Região Administrativa Especial de Macau Fundo do Ensino Superior).
Footnotes
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Ethics approval
The study protocol was approved by the Cancer Council Victoria Human Research Ethics Committee (Approval Number: CCV IEC 9001).
Consent to participate
All participants of the MCCS gave written informed consent.
Contributor Information
Frances EM Albers, University of Melbourne.
Makayla WC Lou, University of Melbourne.
S Ghazaleh Dashti, Murdoch Children’s Research Institute.
Christopher TV Swain, University of Melbourne.
Sabina Rinaldi, International Agency for Research on Cancer.
Vivian Viallon, International Agency for Research on Cancer.
Amalia Karahalios, University of Melbourne.
Kristy A Brown, University of Kansas Medical Center.
Marc J Gunter, Imperial College London.
Roger L Milne, Cancer Council Victoria.
Dallas R English, Cancer Council Victoria.
Brigid M Lynch, Cancer Council Victoria.
Data availability
The dataset generated for the current study is not publicly available due to compliance with participant informed consent and human research ethics committee approvals, but can be requested by contacting pedigree@cancervic.org.au.
References
- 1.Gérard C, Brown KA. (2018) Obesity and breast cancer - Role of estrogens and the molecular underpinnings of aromatase regulation in breast adipose tissue. Molecular and cellular endocrinology. 466: 15–30. 10.1016/j.mce.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 2.(2018) Absence of Excess Body Fatness. IARC Handbooks of Cancer Prevention. Lyon, France: IARC. [Google Scholar]
- 3.Key T, Appleby P, Barnes I, Reeves G. (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 94: 606–16. 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 4.Drummond AE, Swain CTV, Brown KA, et al. (2022) Linking Physical Activity to Breast Cancer via Sex Steroid Hormones, Part 2: The Effect of Sex Steroid Hormones on Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 31: 28–37. 10.1158/1055-9965.Epi-21-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedenreich CM, Ryder-Burbidge C, McNeil J. (2021) Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol Oncol. 15: 790–800. 10.1002/1878-0261.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lynch BM, Leitzmann MF. (2017) An evaluation of the evidence relating to physical inactivity, sedentary behavior, and cancer incidence and mortality. Current Epidemiology Reports. 4: 221–31. 10.1007/s40471-017-0119-7. [DOI] [Google Scholar]
- 7.Key TJ, Appleby PN, Reeves GK, Roddam AW. (2010) Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. The Lancet. Oncology. 11: 530–42. 10.1016/s1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond AE, Swain CTV, Milne RL, et al. (2022) Linking Physical Activity to Breast Cancer Risk via the Insulin/Insulin-like Growth Factor Signaling System, Part 2: The Effect of Insulin/Insulin-like Growth Factor Signaling on Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 31: 2116–25. 10.1158/1055-9965.Epi-22-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. (2019) Obesity and Breast Cancer: Role of Leptin. Frontiers in oncology. 9: 596. 10.3389/fonc.2019.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan DS, Bandera EV, Greenwood DC, Norat T. (2015) Circulating C-Reactive Protein and Breast Cancer Risk-Systematic Literature Review and Meta-analysis of Prospective Cohort Studies. Cancer Epidemiol Biomarkers Prev. 24: 1439–49. 10.1158/1055-9965.Epi-15-0324. [DOI] [PubMed] [Google Scholar]
- 11.Lou MWC, Drummond AE, Swain CTV, et al. (2023) Linking Physical Activity to Breast Cancer via Inflammation, Part 2: The Effect of Inflammation on Breast Cancer Risk. Cancer Epidemiol Biomarkers Prev. 32: 597–605. 10.1158/1055-9965.Epi-22-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM. (2015) Novel insights in SHBG regulation and clinical implications. Trends in endocrinology and metabolism: TEM. 26: 376–83. 10.1016/j.tem.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Baglietto L, Severi G, English DR, et al. (2010) Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev. 19: 492–502. 10.1158/1055-9965.Epi-09-0532. [DOI] [PubMed] [Google Scholar]
- 14.Kaaks R, Rinaldi S, Key TJ, et al. (2005) Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocrine-related cancer. 12: 1071–82. 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 15.Tworoger SS, Zhang X, Eliassen AH, et al. (2014) Inclusion of endogenous hormone levels in risk prediction models of postmenopausal breast cancer. J Clin Oncol. 32: 3111–7. 10.1200/jco.2014.56.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Tworoger SS, Eliassen AH, Hankinson SE. (2013) Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat. 137: 883–92. 10.1007/s10549-012-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeleniuch-Jacquotte A, Bruning PF, Bonfrer JM, et al. (1997) Relation of serum levels of testosterone and dehydroepiandrosterone sulfate to risk of breast cancer in postmenopausal women. Am J Epidemiol. 145: 1030–8. 10.1093/oxfordjournals.aje.a009059. [DOI] [PubMed] [Google Scholar]
- 18.Zeleniuch-Jacquotte A, Shore RE, Koenig KL, et al. (2004) Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 90: 153–9. 10.1038/sj.bjc.6601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieri S, Krogh V, Bolelli G, et al. (2009) Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 18: 169–76. 10.1158/1055-9965.Epi-08-0808. [DOI] [PubMed] [Google Scholar]
- 20.Berrino F, Muti P, Micheli A, et al. (1996) Serum sex hormone levels after menopause and subsequent breast cancer. J Natl Cancer Inst. 88: 291–6. 10.1093/jnci/88.5.291. [DOI] [PubMed] [Google Scholar]
- 21.Fourkala EO, Zaikin A, Burnell M, et al. (2012) Association of serum sex steroid receptor bioactivity and sex steroid hormones with breast cancer risk in postmenopausal women. Endocrine-related cancer. 19: 137–47. 10.1530/erc-11-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas HV, Key TJ, Allen DS, et al. (1997) A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br J Cancer. 76: 401–5. 10.1038/bjc.1997.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schisterman EF, Cole SR, Platt RW. (2009) Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 20: 488–95. 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunter MJ, Hoover DR, Yu H, et al. (2009) Insulin, insulin-like growth factor-I, and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 101: 48–60. 10.1093/jnci/djn415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tin Tin S, Reeves GK, Key TJ. (2021) Endogenous hormones and risk of invasive breast cancer in pre- and post-menopausal women: findings from the UK Biobank. Br J Cancer. 125: 126–34. 10.1038/s41416-021-01392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milne RL, Fletcher AS, MacInnis RJ, et al. (2017) Cohort profile: The Melbourne Collaborative Cohort Study (Health 2020). Int J Epidemiol. 46: 1757–i. 10.1093/ije/dyx085. [DOI] [PubMed] [Google Scholar]
- 27.Baglietto L, English DR, Hopper JL, et al. (2009) Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat. 115: 171–9. 10.1007/s10549-008-0069-3. [DOI] [PubMed] [Google Scholar]
- 28.Hefti MM, Hu R, Knoblauch NW, et al. (2013) Estrogen receptor negative/progesterone receptor positive breast cancer is not a reproducible subtype. Breast Cancer Res. 15: R68. 10.1186/bcr3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivotto IA, Truong PT, Speers CH, et al. (2004) Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol. 22: 1769–70. 10.1200/jco.2004.99.251. [DOI] [PubMed] [Google Scholar]
- 30.De Maeyer L, Van Limbergen E, De Nys K, et al. (2008) Does estrogen receptor negative/progesterone receptor positive breast carcinoma exist? J Clin Oncol. 26: 335–6; author reply 6–8. 10.1200/jco.2007.14.8411. [DOI] [PubMed] [Google Scholar]
- 31.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. (2005) Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 123: 21–7. . [DOI] [PubMed] [Google Scholar]
- 32.Mori N, Keski-Rahkonen P, Gicquiau A, et al. (2021) Endogenous Circulating Sex Hormone Concentrations and Colon Cancer Risk in Postmenopausal Women: A Prospective Study and Meta-Analysis. JNCI Cancer Spectr. 5: 10.1093/jncics/pkab084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viallon V, His M, Rinaldi S, et al. (2021) A new pipeline for the normalization and pooling of metabolomics data. Metabolites. 11: 631. 10.3390/metabo11090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endogenous Hormones and Breast Cancer Collaborative Group. (2003) Free Estradiol and Breast Cancer Risk in Postmenopausal Women: Comparison of Measured and Calculated Values. Cancer Epidemiology, Biomarkers & Prevention. 12: 1457–61. [PubMed] [Google Scholar]
- 35.Dunn JF, Nisula BC, Rodbard D. (1981) Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab. 53: 58–68. 10.1210/jcem-53-1-58. [DOI] [PubMed] [Google Scholar]
- 36.Rinaldi S, Geay A, Déchaud H, et al. (2002) Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 11: 1065–71. [PubMed] [Google Scholar]
- 37.Södergård R, Bäckström T, Shanbhag V, Carstensen H. (1982) Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 16: 801–10. 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 38.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. (2000) Exposure stratified case-cohort designs. Lifetime Data Anal. 6: 39–58. 10.1023/a:1009661900674. [DOI] [PubMed] [Google Scholar]
- 39.VanderWeele TJ. (2019) Principles of confounder selection. Eur J Epidemiol. 34: 211–9. 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernán MA, Robins JM. (2023) Causal Inference: What If. Boca Raton: Chapman & Hall/CRC. [Google Scholar]
- 41.Trabert B, Sherman ME, Kannan N, Stanczyk FZ. (2020) Progesterone and Breast Cancer. Endocrine reviews. 41: 320–44. 10.1210/endrev/bnz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trabert B, Bauer DC, Buist DSM, et al. (2020) Association of Circulating Progesterone With Breast Cancer Risk Among Postmenopausal Women. JAMA Netw Open. 3: e203645. 10.1001/jamanetworkopen.2020.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated for the current study is not publicly available due to compliance with participant informed consent and human research ethics committee approvals, but can be requested by contacting pedigree@cancervic.org.au.


