Abstract
We studied the pharmacokinetics of intravenously and orally administered lamivudine at six dose levels ranging from 0.5 to 10 mg/kg of body weight in 52 children with human immunodeficiency virus infection. A two-compartment model with first-order elimination from the central compartment was simultaneously fitted to the serum drug concentration-time data obtained after intravenous and oral administration. The maximal concentration at the end of the 1-h intravenous infusion and the area under the concentration-time curve after oral and intravenous administration increased proportionally with the dose. The mean clearance of lamivudine (± standard deviation) in the children was 0.53 ± 0.19 liter/kg/h (229 ± 77 ml/min/m2 of body surface area), and the mean half-lives at the distribution and elimination phases were 0.23 ± 0.18 and 2.2 ± 2.1 h, respectively. Clearance was age dependent when normalized to body weight but age independent when normalized to body surface area. Lamivudine was rapidly absorbed after oral administration, and 66% ± 25% of the oral dose was absorbed. Serum lamivudine concentrations were maintained above 1 μM for ≥8 h of 24 h on the twice daily oral dosing schedule with doses of ≥2 mg/kg. The cerebrospinal fluid drug concentration measured 2 to 4 h after the dose was 12% (range, 0 to 46%) of the simultaneously measured serum drug concentration. A limited-sampling strategy was developed to estimate the area under the concentration-time curve for concentrations in serum at 2 and 6 h.
Lamivudine, the (−) enantiomer of 2′-deoxy-3′-thiacytidine, is a deoxycytidine analog that suppresses the replication of human immunodeficiency virus type 1 (HIV-1) by inhibiting viral reverse transcriptase (8). Similar to the other dideoxynucleoside antimetabolites, lamivudine is a prodrug that must be converted to its triphosphate form intracellularly before exerting its antiretroviral effects (5). Lamivudine has potent antiretroviral activity in vitro against a variety of HIV-1 isolates (including zidovudine-resistant strains) and showed a favorable toxicity profile in preclinical studies (7, 8, 13, 21, 22). Clinical trials of this agent in adults and children have demonstrated that dosages between 0.5 to 20 mg/kg of body weight divided into two daily doses are well tolerated and result in a decrease in the levels of serum HIV RNA and immune complex-dissociated (ICD) p24 antigen (16, 19). Lamivudine, in combination with zidovudine, has been approved at a dosage of 4 mg/kg twice daily for children.
The pharmacokinetic behavior of lamivudine in adults is characterized by rapid elimination, primarily by renal excretion (70%). Total clearance of lamivudine is approximately 400 ml/min (approximately 230 ml/min/m2 of body surface area), and the terminal half-life is approximately 8 h (range, 6.8 to 12.45 h) (17, 24). Lamivudine is rapidly absorbed after oral administration, and the mean fraction of the oral dose absorbed exceeds 80%. The peak concentration (Cmax) and the area under the concentration-time curve (AUC) increase in proportion to the dose (1, 19, 23). All oral formulations are considered bioequivalent for AUC and Cmax (24).
We performed a phase I and II trial of lamivudine in 90 HIV-infected children to assess safety, tolerability, and antiretroviral activity (16). In the present report, detailed pharmacokinetic studies of intravenously and orally administered lamivudine in 52 of these patients are described.
MATERIALS AND METHODS
Patients.
Between April 1992 and December 1993, 90 children were enrolled into the pediatric phase I and II trial of lamivudine (16). Fifty-two children (30 boys and 22 girls) aged 0.5 to 17.5 years (median age, 7.5 years) had pharmacokinetic samples obtained after receiving both an intravenous dose and an oral dose. Thirty-three of the children were Caucasian, 11 were African-American, 7 were Hispanic, and 1 was Asian. Thirty-two children had vertically acquired HIV, 19 were infected through transfusion of contaminated blood products, and 1 had sexually acquired HIV infection. Twelve patients had not received prior antiretroviral therapy, and the other 40 patients had been off other antiretroviral medications for at least 2 weeks prior to entering the study.
Patients were free of opportunistic infections and had normal renal function as determined by comparison of serum creatinine levels with age-adjusted normal values. The serum glutamic pyruvic transaminase level was normal (<41 U/liter) in 20 patients, ranged from 42 to 82 U/liter in 23 patients, and ranged from 83 to 205 U/liter in the remaining 9 patients. Serum bilirubin level was normal in all patients. Forty-eight patients had a total leukocyte count of at least 2,000 cells/mm3, and all had an absolute neutrophil count of >500 cells/mm3. The hemoglobin concentration was >8.0 g/dl in all patients and >10 g/dl in 37 patients. The platelet count was <100,000/mm3 in six patients but >25,000/mm3 in all patients. The median CD4 count at the time of entry into the study was 193 cells/mm3 (range, 3 to 3,229 cells/mm3), and the median serum HIV RNA level was 64,400 copies/ml (range, <100 copies/ml to 2,900,000 copies/ml).
The study was approved by the National Cancer Institute’s Institutional Review Board and the Institutional Review Board of Children’s Hospital, Los Angeles, Calif. Written informed consent was obtained from the parent or legal guardian of each child.
Drug formulation and administration.
The pediatric phase I/II trial of lamivudine was an open-labeled, dose-escalation study. The trial was designed as a dose-escalation study, and patients were enrolled onto the dose level that was accruing patients at the time that they were referred for treatment. Lamivudine was manufactured and supplied by Glaxo Wellcome, Inc. (Research Triangle Park, N.C.). The intravenous formulation for pharmacokinetic studies contained 10 mg of lamivudine/ml diluted in normal saline and was infused over 1 h. Six dose levels, 0.5, 1, 2, 4, 6, and 10 mg/kg/dose, were studied in separate cohorts of patients (n = 6, 9, 12, 8, 7, and 10, respectively). A single intravenous dose of lamivudine was administered on the first day of treatment to evaluate the pharmacokinetics. Lamivudine was subsequently administered orally twice daily, and samples were obtained 48 to 72 h after the start of oral therapy. Patients fasted for at least 1 h before and 1 h after the administration of the oral dose. Patients with a body weight of <20 kg or patients who were unable to swallow capsules received either a 1- or 10-mg/ml elixir in 6% ethanol, and patients with a body weight of ≥20 kg received capsules (2.5, 10, 25, or 100 mg). The 1-mg/ml elixir was phased out as supplies of the 10-mg/ml formulation became available. Each patient received the same dose administered intravenously and orally.
Specimen collection and drug assay.
For the intravenous dose, blood samples were collected before drug administration, at the end of the 1-h infusion, and 1.5, 2, 4, 6, and 8 h after the initiation of the infusion. For the oral dose, blood samples were drawn prior to the administration and 0.5, 1, 1.5, 2, 4, 6, and 8 h after the administration. At weeks 4 and 12 of treatment, a trough blood sample was drawn 12 h after the previous oral dose, and a peak blood sample was drawn 30 min after the next dose. Cerebrospinal fluid (CSF) and, concurrently, a blood sample were obtained on day 4 and at week 12 of the oral dosing, at 2 to 4 h after the dose. After the blood samples were allowed to clot they were centrifuged, and the serum was stored at −70°C until the assays were performed. The concentration of lamivudine was measured by using a previously described high-performance liquid chromatography assay (4, 12). Samples were stored less than 1 year at less than −20° C. Lamivudine long-term stability in human serum has been demonstrated for 15 months at this temperature (11a).
Pharmacokinetic and statistical analyses.
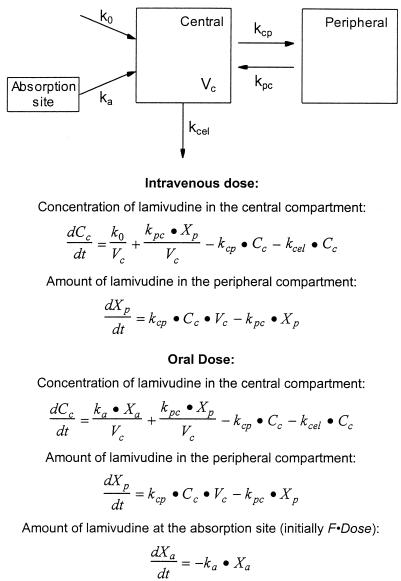
A two-compartment model with first-order elimination (Fig. 1) was fitted simultaneously to the intravenous and oral serum drug concentration-time data from each patient by using MLAB (Civilized Software, Bethesda, Md.) (15). The built-in MLAB EWT weighting function was employed for the modeling. Initially, a lag time was estimated for each patient’s oral serum drug concentration-time data by fitting the two-compartment model, which included lag time as a fitted parameter, to the oral data only if the lag time was >0 for a data set, and it was then subtracted from the time values for that data set prior to the simultaneous fittings. The fraction of the oral dose absorbed (F) was estimated from the ratio of the AUCs (by the linear trapezoidal method) after the oral and intravenous doses, and F was incorporated as a constant in the simultaneous fitting.
FIG. 1.
Two-compartment pharmacokinetic model with differential equations describing concentration or amount of lamivudine in each compartment. Cc is the concentration of lamivudine in the central compartment at time t, Xp is the amount of drug in the peripheral compartment at time t, Xa is the amount of drug at the absorption site at time t, k0 is the rate of drug infusion for the intravenous dose, ka is the absorption rate constant for the oral dose, Vc is the volume of the central compartment, kcel is the elimination rate constant, and kcp and kpc are the rate constants for transfer of drug from the central to peripheral compartment and from the peripheral to central compartment, respectively.
The fitted model parameters (volume of the central compartment [Vc], rate constant for elimination [Kcel], and rate constants for transfer of drug from the central to peripheral compartment and from the peripheral to central compartment [kcp and kpc, respectively]) were used to calculate clearance (CL = Vc · kcel) and volume of distribution at steady state (Vdss):
 |
Half-lives were derived from the rate constants as previously described (11).
A limited sample strategy for estimating the AUC of lamivudine after an oral dose was developed by using a stepwise forward multiple regression analysis (20). The model was developed from the AUCs and the individual concentration-time datum points at the 10-mg/kg dose level. The 0.5-h time point was excluded from the analysis, because lamivudine was not detectable in these samples from some of the patients. The F test was used to determine the optimal model, which was then validated by testing it with the data from the 2-mg/kg dose level with regression analysis.
Nonparametric statistical analysis (Mann-Whitney U test) was used to assess differences in clearance when grouped by prior therapy or gender and differences in F when grouped by prior therapy or gender. A Student’s t test was used to compare the mean clearance in patients with or without concurrent therapy with trimethoprim-sulfamethoxazole (TMP/SMX). Simple regression analysis was used to assess the influence of age on clearance and bioavailability and to measure the correlation between AUC and changes in serum HIV RNA levels.
RESULTS
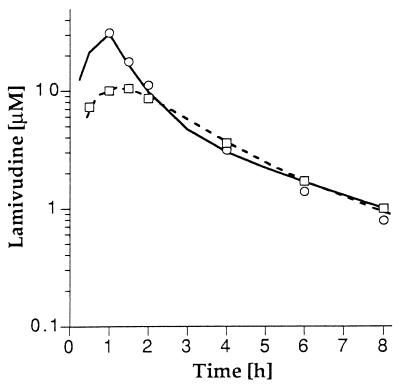
The two-compartment model (Fig. 1) was simultaneously fitted to the serum drug concentration-time data from both the intravenous and oral doses for each patient, and the fitted model parameters are listed in Table 1. Lamivudine was rapidly eliminated from serum, and the serum drug concentration-time profiles were biexponential. The mean CL of lamivudine was 0.53 ± 0.19 liter/h/kg (229 ± 77 ml/min/m2), and the mean half-lives at the distribution and elimination phases (t1/2α and t1/2β) were 0.23 ± 0.18 h and 2.2 ± 2.1 h, respectively (Table 2). The serum drug concentration-time profiles of intravenously and orally administered lamivudine for a typical patient treated at the 10-mg/kg dose level are shown in Fig. 2.
TABLE 1.
Pharmacokinetic parameters derived from fitting the two-compartment model (Fig. 1) simultaneously to the serum concentrations after intravenous administration and oral administration of lamivudine (at one of six dose levels) in 52 HIV-infected children
| Parameter | Vc (liter/kg) | kcel (h−1) | kcp (h−1) | kpc (h−1) | ka (h−1) |
|---|---|---|---|---|---|
| Geometric mean | 0.362 | 1.37 | 1.22 | 1.06 | 0.899 |
| Median | 0.395 | 1.32 | 1.55 | 1.25 | 0.850 |
| First to third quartiles | 0.292–0.505 | 0.959–1.71 | 0.808–2.68 | 0.710–2.27 | 0.535–1.27 |
| Range | 0.081–1.11 | 0.545–5.41 | 0.050–6.89 | 0.054–7.77 | 0.121–9.93 |
TABLE 2.
Pharmacokinetic parameters for lamivudine administered intravenously and orally at doses ranging from 0.5 to 10 mg/kg in 52 HIV-infected childrena
| Dose level (mg/kg) | n | AUCiv (μM · h) | CL (liter/h/kg) | Vdss (liter/kg) | t1/2α (h) | t1/2β (h) | AUCpo (μM · h) | F (%) |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 6 | 5.74 ± 3.60 | 0.496 ± 0.242 | 1.04 ± 0.59 | 0.192 ± 0.062 | 2.24 ± 1.06 | 2.84 ± 1.04 | 58 ± 28 |
| 1 | 9 | 8.63 ± 1.93 | 0.533 ± 0.133 | 1.03 ± 1.07 | 0.221 ± 0.143 | 2.23 ± 2.16 | 6.45 ± 2.19 | 74 ± 18 |
| 2 | 12 | 20.6 ± 12.5 | 0.537 ± 0.234 | 0.975 ± 0.552 | 0.256 ± 0.173 | 2.16 ± 1.48 | 11.2 ± 5.2 | 63 ± 28 |
| 4 | 8 | 35.7 ± 13.7 | 0.536 ± 0.146 | 0.843 ± 0.171 | 0.338 ± 0.315 | 2.07 ± 1.47 | 22.5 ± 10.2 | 66 ± 28 |
| 6 | 7 | 55.7 ± 24.8 | 0.546 ± 0.206 | 1.03 ± 0.41 | 0.233 ± 0.145 | 3.36 ± 4.45 | 37.2 ± 20.7 | 69 ± 31 |
| 10 | 10 | 98.8 ± 35.3 | 0.528 ± 0.189 | 0.925 ± 0.333 | 0.144 ± 0.112 | 1.67 ± 0.66 | 61.8 ± 35.8 | 66 ± 26 |
| Mean ± SD | 0.531 ± 0.186 | 0.967 ± 0.571 | 0.231 ± 0.179 | 2.23 ± 2.09 | 66 ± 25 |
AUCiv, area under the serum concentration-time curve for the intravenous dose; Vdss, volume of distribution at steady state; AUCpo, area under the serum concentration-time curve for the oral dose; F, bioavailability of the oral dose.
FIG. 2.
Serum lamivudine concentrations after intravenous (○) and oral (□) doses of 10 mg/kg in a single patient. Points represent the measured serum concentrations, and lines represent the model-predicted concentrations for intravenous (—) and oral (- - - ) administration from simultaneous fitting of the intravenous and oral drug concentration-time data.
The absorption of oral lamivudine was rapid, with a median absorption rate constant of 0.85 h−1 (range, 0.12 to 9.9 h−1) and a median time to peak of 1.5 h (range, 0.5 to 4 h). Lamivudine was also well absorbed: the mean F was 0.66 ± 0.25. Peak and trough (12 h) serum concentrations were stimulated from the model parameters for a 4-mg/kg dose. The peak serum concentration was predicted to be 5.1 μM at 1.2 h, with the concentration dropping to 0.069 μM at 12 h.
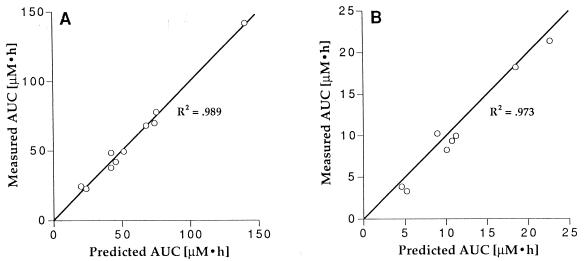
The AUC increased in proportion to the dose for both the intravenous and oral routes of administration (Fig. 3), and the clearance and fraction of the oral dose absorbed were equivalent at all dose levels studied, indicating that the absorption and elimination of lamivudine are not dose dependent over the dosage range from 0.5 to 10 mg/kg/dose.
FIG. 3.
Correlation between dose of lamivudine and AUC for intravenous (A) and oral (B) administration over a dosage range from 0.5 to 10 mg/kg. Points and bars represent the mean and SD at each dose level. The line was constructed by normalizing the AUCs at all dose levels to a dose of 10 mg/kg, taking the mean of the normalized AUCs, and drawing a line from the origin through the mean, normalized AUC.
The clearance and bioavailability of lamivudine were similar in treatment-naive (n = 12) and previously treated (n = 40) patients. The mean CL (± standard deviation [SD]) in the treatment-naive group was 226 ± 79 ml/min/m2, and it was 234 ± 74 ml/min/m2 for the previously treated group (P = 0.86); the mean (±SD) F values in the treatment-naive and previously treated patients were 0.69 ± 0.19 and 0.65 ± 0.27, respectively (P = 0.69). There were no gender differences in CL (228 ± 75 ml/min/m2 in males versus 230 ± 81 ml/min/m2 in females; P = 0.88) and F (0.65 ± 0.25 in males versus 0.67 ± 0.26 in females; P = 0.75).
The clearance of lamivudine has been reported to be delayed by the concurrent administration of TMP/SMX (17). The CL values for lamivudine in our trial were 222 ± 74 ml/min/m2 for 29 children receiving TMP/SMX prophylaxis and 236 ± 81 ml/min/m2 in the remaining 23 children not receiving TMP/SMX (P = 0.53); however, the exact timing of TMP/SMX administration in relation to performance of lamivudine pharmacokinetics was not standardized.
We also assessed the relationship between age and clearance normalized to body weight (liter/kg/h) and body surface area (ml/min/m2) (Fig. 4). There was a significant correlation (P = 0.0022) between age and clearance when clearance was normalized to body weight, whereas clearance normalized to body surface area was age independent. There was no correlation between age and F.
FIG. 4.
Correlation between age and lamivudine clearance normalized to body weight (A) and body surface area (B). The equations for the regression lines shown in panels A and B are CL (liter/kg/h) = 0.68 − 0.018 · age (r = 0.425; P = 0.0022) and CL (ml/min/m2) = 242 − 1.7 · age (r = 0.096; P = 0.50), respectively.
Although children receiving ≥4 mg/kg per dose of lamivudine tended to have a slightly better virologic response as measured by a decrease in serum HIV RNA levels (16), no relationship was demonstrated between AUC and change in RNA level from baseline (r2 was 0.007 for data at 4 weeks, 0.013 for those at 12 weeks, and 0.003 for those at 24 weeks).
The lamivudine concentration was measured in 68 CSF samples collected from 44 patients (Table 3). Lamivudine was not detectable (<0.1 μM) in three of the CSF samples from two patients treated at the lowest dose level (0.5 mg/kg) and 1 patient treated at the 1-mg/kg dose level. CSF lamivudine concentration increased proportionally with the dose, and the median CSF to serum ratio was 0.12 (range, 0.00 to 0.46). However, these ratios may not accurately reflect total drug exposure in CSF relative to serum because they were not obtained at steady state.
TABLE 3.
Concentration of lamivudine in CSFa
| Dose level (mg/kg) | No. of patients | No. of CSF samples | Concn of lamivudine in CSF (μM)b | % CSF drug concn to serum drug concnc |
|---|---|---|---|---|
| 0.5 | 5 | 8 | 0.07 (UD–0.12) | 13 (0–22) |
| 1 | 7 | 10 | 0.12 (UD–0.16) | 17 (0–46) |
| 2 | 8 | 13 | 0.23 (0.08–0.35) | 10 (6–21) |
| 4 | 9 | 14 | 0.31 (0.06–1.44) | 11 (3–31) |
| 6 | 9 | 14 | 0.59 (0.06–1.99) | 12 (3–31) |
| 10 | 6 | 9 | 0.99 (0.32–2.23) | 10 (6–22) |
The concentration was measured in 68 CSF samples from 44 patients.
Expressed as median (range). UD, undetectable.
Derived after dividing the CSF drug concentration by the simultaneously measured serum drug concentration.
A limited-sampling strategy was developed for oral lamivudine by using a stepwise forward multiple regression analysis of the AUCs and individual serum drug concentrations for 10 patients studied at the 10-mg/kg oral dose level. The concentrations in serum at 2 and 6 h after the oral dose were the most predictive (Fig. 5). The AUC can be estimated from data for these two samples by using the following equation:
 |
where C2h is the concentration in serum at 2 h, C6h is the concentration at 6 h, and dose is the dose level (mg/kg/dose). The limited-sampling strategy was validated in eight patients treated at the 2-mg/kg dose level (Fig. 5), yielding an excellent correlation between the predicted AUC and measured AUC (r2 = 0.973). The slope from the regression analysis of the measured and predicted AUCs was 0.97 (expected slope, 1.0), and the mean predictive error was 18% (the coefficient of variation [CV] for the measured AUCs at the 2-mg/kg dose level was 60%).
FIG. 5.
Limited-sampling strategy for oral lamivudine. The model was developed by stepwise regression analysis of data for patients treated at the 10-mg/kg dose level (A) and validated with data for patients treated at the 2-mg/kg dose level (B). The equation for estimating the AUC from the serum concentrations at 2 (C2h) and 6 (C6h) h after the dose is AUC = 2.51 · C2h + 6.46 · C6h + 0.97 · dose. The graphs plot the AUC predicted from the limited-sampling model versus the actual AUC derived from the entire set of measured serum drug concentrations by the trapezoidal method. The line is the line of unity.
DISCUSSION
Lamivudine disposition after intravenous and oral administration in children with HIV infection was well described by a two-compartment pharmacokinetic model with first-order absorption and elimination. The drug was rapidly eliminated (t1/2β = 2.2 h) and had excellent oral bioavailability (Tmax = 1.5 h; F = 0.66). Over the 20-fold dosage range (0.5 to 10 mg/kg), the clearance and absorption of lamivudine were dose independent, such that drug exposure (AUC) increased in proportion to dose for both the intravenous and oral routes.
The every-12-h dosing interval of lamivudine is considerably longer than the half-life of the parent prodrug, and trough concentrations of the parent drug in serum are well below 1 μM, the concentration that inhibits viral replication by 50% in vitro. However, the intracellular half-life of the active, triphosphate form of the drug is 10.5 to 15.5 h in vitro in phytohemagglutinin-stimulated, HIV-infected peripheral blood lymphocytes. If prolonged intracellular retention of drug in triphosphate form also occurs in vivo, we would expect a prolonged terminal elimination phase of parent drug in serum as the drug is slowly degraded to its nucleoside form and released from cells. However, the concentrations of trough samples, drawn 12 h after the last dose, were below the limit of detection of the assay at all dose levels including the highest dose level. The fact that a prolonged terminal phase was not detected in our study could be because the amount of drug in triphosphate form is small and slow release of the parent drug from this compartment results in a serum drug concentration that is below the level of detection of the assay used in this study.
The extent of absorption (F = 0.66) and degree of variability (CV = 38%) after oral lamivudine administration in children is comparable to that observed with zidovudine (F = 0.68; CV = 37%) (3). In contrast, the absorption of didanosine in children is more limited (F = 0.19) and more variable (CV = 90%) (2).
Lamivudine, like zalcitabine (dideoxycytidine), is eliminated primarily by renal excretion (14), whereas cytidine and other cytidine analogs (e.g., cytarabine) are rapidly eliminated by deamination to uridine or uridine analogs. The deaminated form of lamivudine was not detected in the sera of patients treated in our trial, indicating that lamivudine is not a substrate for cytidine deaminase. The pharmacokinetic behavior of lamivudine in children is similar to that of zalcitabine (6, 18), which had a clearance of 150 ml/min/m2, a t1/2 of 0.8 to 1.4 h, and oral bioavailability of 0.54.
The pharmacokinetics of lamivudine in children (CL = 229 ml/min/m2; t1/2 = 2.2 h) is similar to that reported in adults (CL = 230 ml/min/m2; t1/2 = 2.5 h) (23, 24). In two other adult studies, in which pharmacokinetic sampling was performed at 24 and 32 h after the dose, a longer estimated terminal t1/2 of approximately 8 h (range, 6.8 to 12.45 h) was reported (17, 24). However, lamivudine was not measurable 12 h after the dose in our pediatric patients who were treated at comparable dose levels. It is therefore not likely that a prolonged terminal elimination phase would have been detected even with a longer sampling schedule. The mean absolute bioavailability of lamivudine in adults (F = 0.86) (24) appears to be slightly higher than that in children. We also evaluated the relationship of age to pharmacokinetic parameters within the pediatric population and found a significant correlation between age and clearance when clearance was normalized to body weight, whereas clearance normalized to body surface area was age independent. Lamivudine dose in this trial was based on body weight rather than body surface area. However, lamivudine is eliminated primarily by renal excretion (14), and renal blood flow and glomerular filtration rate after the neonatal period are more closely related to body surface area than to body weight (10). Given the safety profile for lamivudine and lack of a clear relationship between dose or AUC and response, the currently recommended dosing in milligrams per kilogram appears to be adequate for the majority of pediatric patients. Further dosing adjustment based on body surface area or body weight may be required for patients less than 1 year of age once an adequate number of these patients have been studied.
The CSF penetration of lamivudine in children was limited. The percent CSF to serum drug concentration 2 to 4 h after a dose was 12%, which is similar to the percentage of 8% that we previously reported for a nonhuman primate model in which multiple ventricular CSF samples were obtained after an intravenous dose and the ratio (percent) was derived from the AUCs in CSF and plasma (4). Although the value of single time-point measurements is limited, the concordance of the preclinical data with the clinical data is reassuring. The CSF penetration of pyrimidine nucleoside analogs appears to be a function of the nucleobase rather than the degree of lipophilicity. The thymidine analogs zidovudine and dideoxythymidine had percents CSF to plasma of 21% and 30%, respectively, whereas the percentages for the cytidine analogs azidocytidine and dideoxycytidine were only 1 and 3, respectively (9). The CSF lamivudine penetration appears to exceed the penetration of the dideoxycytidine compounds. Encephalopathy was an exclusion criterion for our pediatric trial of lamivudine; thus, we were unable to evaluate the relationship between CSF lamivudine concentrations and improvement in neuropsychometric test results.
Side effects thought to be related to the study drug were uncommon and consisted of elevated serum transaminase levels, hyperactivity, and pancreatitis (16). All the cases of pancreatitis occurred during the expansion phase of the study, and pharmacokinetic parameter values were not available for the affected patients.
The limited-sampling strategy developed in this study allows for accurate estimation of the AUC of lamivudine from two serum samples, drawn 2 and 6 h after an oral dose. The AUC is a better measure of drug exposure than are the peak and trough concentrations given the short half-life and long dosing interval of lamivudine. Results from this trial do not support a role for therapeutic drug monitoring in the management of children receiving lamivudine, because we failed to identify a relationship between pharmacokinetic parameters and measures of toxicity (16) and response. However, the limited-sampling strategy presented here would be useful for larger scale studies of patients treated at a single dose level to define therapeutic and toxic levels.
REFERENCES
- 1.Angel J B, Hussey E K, Hall S T, Donn K H, Morris D M, McCormack J P, Montaner J S G, Ruedy J. Pharmacokinetics of 3TC (GR109714X) administered with and without food to HIV-infected patients. Drug Investig. 1993;6:70–74. [Google Scholar]
- 2.Balis F M, Pizzo P A, Butler K M, Hawkins M E, Brouwers P, Husson R N, Jacobsen F, Blaney S M, Gress J, Jarosinski P, Poplack D G. Clinical pharmacology of 2′,3′-dideoxyinosine in human immunodeficiency virus-infected children. J Infect Dis. 1992;165:99–104. doi: 10.1093/infdis/165.1.99. [DOI] [PubMed] [Google Scholar]
- 3.Balis F M, Pizzo P A, Eddy J, Wilfert C, McKinney R, Scott G, Murphy R F, Jarosinski P F, Falloon J, Poplack D G. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989;114:880–884. doi: 10.1016/s0022-3476(89)80158-1. [DOI] [PubMed] [Google Scholar]
- 4.Blaney S M, Daniel M J, Harker A J, Godwin K, Balis F M. Pharmacokinetics of lamivudine and BCH-189 in plasma and cerebrospinal fluid of nonhuman primates. Antimicrob Agents Chemother. 1995;39:2779–2782. doi: 10.1128/aac.39.12.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cammack N, Rouse P, Marr C L, Reid P J, Boehme R E, Coates J A, Penn C R, Cameron J M. Cellular metabolism of (−) enantiomeric 2′-deoxy-3′-thiacytidine. Biochem Pharmacol. 1992;43:2059–2064. doi: 10.1016/0006-2952(92)90162-c. [DOI] [PubMed] [Google Scholar]
- 6.Chadwick E G, Nazareno L A, Nieuwenhuis T J, Massarella J W, de Dennis S R, Williams K, Yogev R. Phase I evaluation of zalcitabine administered to human immunodeficiency virus-infected children. J Infect Dis. 1995;172:1475–1479. doi: 10.1093/infdis/172.6.1475. [DOI] [PubMed] [Google Scholar]
- 7.Chen C H, Cheng Y C. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J Biol Chem. 1989;264:11934–11937. [PubMed] [Google Scholar]
- 8.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C A, Rouse P L, Viner K C, Cameron J M. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins J M, Klecker R W, Kelley J A, Roth J S, McCully C L, Balis F M, Poplack D G. Pyrimidine dideoxyribonucleosides: selectivity of penetration into cerebrospinal fluid. J Pharmacol Exp Ther. 1988;245:466–470. [PubMed] [Google Scholar]
- 10.Crawford J D, Terry M E, Rourke G M. Simplification of drug dosage calculated by application of the surface area principle. Pediatrics. 1950;5:783–789. [PubMed] [Google Scholar]
- 11.Gibaldi M, Perrier D. Estimation of areas. In: Swarbrick J, editor. Pharmacokinetics. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 445–449. [Google Scholar]
- 11a.Glaxo Wellcome. Personal communication (Amy Keller).
- 12.Harker A J, Evans G L, Hawley A E, Morris D M. High-performance liquid chromatographic assay for 2′-deoxy-3′-thiacytidine in human serum. J Chromatogr Biomed Appl. 1994;657:227–232. doi: 10.1016/0378-4347(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 13.Hart G J, Orr D C, Penn C R, Figueiredo H T, Gray N M, Boehme R E, Cameron J M. Effects of (−)-2′-deoxy-3′-thiacytidine 5′-triphosphate on human immunodeficiency virus reverse transcriptase and mammalian DNA polymerases alpha, beta, and gamma. Antimicrob Agents Chemother. 1992;36:1688–1694. doi: 10.1128/aac.36.8.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heald A E, Hsyu P H, Yuen G J, Robinson P, Mydlow P, Bartlett J A. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob Agents Chemother. 1996;40:1514–1519. doi: 10.1128/aac.40.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knott G D. MLAB—a mathematical modeling tool. Comput Programs Biomed. 1979;10:261–280. doi: 10.1016/0010-468x(79)90075-8. [DOI] [PubMed] [Google Scholar]
- 16.Lewis L L, Venzon D, Church J, Farley M, Wheeler S, Keller A, Rubin M, Yuen G, Mueller B, Sloas S, Wood L, Balis F, Shearer G M, Brouwers E, Goldsmith J, Pizzo P A NCI Pediatric Branch HIV Working Group. Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. J Infect Dis. 1996;174:16–25. doi: 10.1093/infdis/174.1.16. [DOI] [PubMed] [Google Scholar]
- 17.Moore K H P, Yuen G J, Raasch R H, Eron J J, Martin D, Mydlow P K, Hussey E K. Pharmacokinetics of lamivudine administered alone and with trimethoprim-sulfamethoxazole. Clin Pharmacol Ther. 1996;59:550–558. doi: 10.1016/S0009-9236(96)90183-6. [DOI] [PubMed] [Google Scholar]
- 18.Pizzo P A, Butler K, Balis F, Brouwers E, Hawkins M, Eddy J, Einloth M, Falloon J, Husson R, Jarosinski P, Meer J, Moss H, Poplack D G, Santacroce S, Wiener L, Wolters P. Dideoxycytidine alone and in an alternating schedule with zidovudine in children with symptomatic human immunodeficiency virus infection. J Pediatr. 1990;117:799–808. doi: 10.1016/s0022-3476(05)83348-7. [DOI] [PubMed] [Google Scholar]
- 19.Pluda J M, Cooley T P, Montaner J S G, Shay L E, Reinhalter N E, Warthan S N, Ruedy J, Hirst H M, Vicary C A, Quinn J B, Yuen G J, Wainberg M A, Rubin M, Yarchoan R. A phase I/II study of 2′-deoxy-3′-thiacytidine in patients with advanced human immunodeficiency virus infection. J Infect Dis. 1995;171:1438–1447. doi: 10.1093/infdis/171.6.1438. [DOI] [PubMed] [Google Scholar]
- 20.Ratain M J, Vogelzang N J. Limited sampling model for vinblastine pharmacokinetics. Cancer Treat Rep. 1987;71:935–939. [PubMed] [Google Scholar]
- 21.Sommadossi J P, Schinazi R F, Chu C K, Xie M Y. Comparison of cytotoxicity of the (−)- and (+)-enantiomer of 2′,3′-dideoxy-3′-thiacytidine in normal human bone marrow progenitor cells. Biochem Pharmacol. 1992;44:1921–1925. doi: 10.1016/0006-2952(92)90093-x. [DOI] [PubMed] [Google Scholar]
- 22.Soudeyns H, Yao X I, Gao Q, Belleau B, Kraus J L, Nguyen-Ba N, Spira B, Wainberg M A. Anti-human immunodeficiency virus type 1 activity and in vitro toxicity of 2′-deoxy-3′-thiacytidine (BCH-189), a novel heterocyclic nucleoside analog. Antimicrob Agents Chemother. 1991;35:1386–1390. doi: 10.1128/aac.35.7.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Leeuwen R, Lange J M A, Hussey E K, Donn K H, Hall S T, Harker A J, Jonker P, Danner S A. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS. 1992;6:1471–1475. doi: 10.1097/00002030-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Yuen G J, Morris D M, Mydlow P K, Haidar S, Hall S T, Hussey E K. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35:1174–1180. doi: 10.1002/j.1552-4604.1995.tb04043.x. [DOI] [PubMed] [Google Scholar]