Abstract
Lithium is the gold standard treatment for bipolar disorder (BD). However, its mechanism of action is incompletely understood, and prediction of treatment outcomes is limited. In our previous multi-omics study of the Pharmacogenomics of Bipolar Disorder (PGBD) sample combining transcriptomic and genomic data, we found that focal adhesion, the extracellular matrix (ECM), and PI3K-Akt signaling networks were associated with response to lithium. In this study, we replicated the results of our previous study using network propagation methods in a genome-wide association study of an independent sample of 2,039 patients from the International Consortium on Lithium Genetics (ConLiGen) study. We identified functional enrichment in focal adhesion and PI3K-Akt pathways, but we did not find an association with the ECM pathway. Our results suggest that deficits in the neuronal growth cone and PI3K-Akt signaling, but not in ECM proteins, may influence response to lithium in BD.
INTRODUCTION
Bipolar disorder (BD) is a chronic psychiatric illness that presents with episodes of mania, depression, and sometimes psychosis. Globally, it is the sixth leading cause of medical disability among people from 15 to 44 years old. Patients with BD are at a higher risk of suicide than those with any other psychiatric or medical illness. Some studies report that roughly 50% of patients will attempt suicide, and up to 20% of untreated patients will complete suicide1, while treatment by lithium reduces that risk significantly2,3.Unfortunately, misdiagnosis is common and often delays an accurate treatment. Up to 70% of patients are initially misdiagnosed, usually with major depressive disorder. On average, there is a delay of 8 years before the correct diagnosis of BD is made4. During this time, patients continue to suffer, may be treated with medications that make their illness course worse, and are at risk of suicide.
Lithium is the gold standard treatment for BD5. Its mechanism of action is still not completely understood6. Many studies have investigated the neurotrophic effect of lithium. One theory posits that chronic administration of lithium inhibits glycogen synthase kinase 3 (GSK3β), a serine/threonine kinase.This leads to anti-apoptotic effects and improved cell structural stability7–10. GSK3β has also been shown to exhibit interactions with many pathways, including phosphorylation of several components of the PI3K/AKT/mTOR signaling network, as well as regulation of transcription for proteins bound to microtubules11. Another theory involves the phosphoinositol (PI) cycle. In the PI cycle, lithium inhibits inositol monophosphatase, which ultimately downregulates protein kinase C isozymes such as myristoylated alanine-rich C-kinase substrate (MARCKS). MARCKS is an actin-binding protein found in neuronal processes that is implicated in cytoskeletal restructuring. Its downregulation stabilizes the neuronal membrane and results in neurotrophic effects7,12. A more recent theory proposes that lithium alters the phosphorylation state of collapsin response mediator protein-2 (CRMP2). CRMP2 regulates cytoskeletal organization, particularly in dendritic spines13,14. Finally, a study using polygenic score modeling has indicated that the cholinergic and glutamatergic pathways may potentially serve as targets for lithium15. It is possible that lithium exerts its effects through multiple or all of these pathways. A single definitive model remains elusive, but interactions with neuronal cytoskeleton are possibly involved.
Interestingly, there is a range of responses to treatment with lithium. Previous studies have reported that 20–30% of patients with BD are excellent responders, whereas over 40% fail to demonstrate any significant clinical improvement. These patient populations have been shown to differ from each other both phenotypically and genetically16. A differential response to lithium has been previously demonstrated between induced pluripotent stem cell (iPSC) neurons derived from lithium responders and non-responders. The hyperexcitability of in vitro neurons derived from BD patients was reversed by lithium treatment, but only in those from patients who were lithium responders17. This finding is also supported by family studies, which found that the relatives of lithium responders were significantly more likely to be lithium responders as well18,19. These studies imply that patients with BD could be subcategorized based on biological differences which induce a divergent lithium response. There is a great need to better understand these differences in order to identify possible predictors of treatment response. However, dozens of previous candidate-gene association studies, genome-wide association studies (GWAS), and polygenic risk score analyses of lithium response in BD have failed to identify genetic variants with major effects. Given this pressing need to find pharmacogenetic predictors of response, more advanced methods in integrative genomic analysis are necessary16.
GWAS inherently face several limitations when used in isolation, including the challenge of genetic heterogeneity. In many disease processes with genetic associations, patients may carry diverse combinations of causal variants that impact multiple genes, creating a net effect across a particular pathway. GWAS of BD primarily detect variants of very small effect size consistent with a polygenic mode of transmission. Since each single nucleotide polymorphism (SNP) contributes only a tiny amount to the overall predisposition to BD, enormous sample sizes are required, and it can be difficult to surmise mechanisms of disease. Network approaches seek to address this biological reality by integrating GWAS results with known protein-protein interactions and other molecular networks. New causal genes may be identi ed by boosting their interactions with products of known causal genes20,21.
We have recently reported a combined analysis of transcriptomic and GWAS data from the Pharmacogenomics of Bipolar Disorder (PGBD) study22 of treatment response to lithium. After using network propagation to reprioritize candidate genes from GWAS data, we found significant overlap between both transcriptomic and GWAS results. The joint analysis yielded a 500 gene network significantly enriched in the following Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways: focal adhesion, ECM-receptor interaction, and PI3K-Akt signaling23. All three pathways play a role in axon growth and neuronal development24. Consistent with these results, post-mortem studies have found that in BD, neuronal populations may exhibit a decrease in number, size, and/or amount of dendritic spines13,25. Given that lithium may have downstream effects on these pathways, it is possible that genetic defects in focal adhesion pathways may provide both a mechanism for susceptibility to BD as well as a target for lithium treatment.
In this study, we aimed to replicate the results of our previous multi-omics study on a larger dataset of over 2,000 patients from the International Consortium on Lithium Genetics (ConLiGen)26. We reprioritized GWAS results using network methods to determine overlap with focal adhesion, ECM-receptor interaction, and PI3K-Akt signaling pathways.
METHODS
Summary statistics were downloaded from the NHGRI-EBI GWAS Catalog27 on 12/12/2022 for study GCST01248726. The data resulted from a GWAS of lithium response in 2,563 patients at 22 sites participating in the International Consortium on Lithium Genetics (ConLiGen). We utilized the summary statistics from a combined sample of 2,039 European ancestry individuals. In the ConLiGen study, data from over 6 million single nucleotide polymorphisms (SNPs) were tested for association with categorical and continuous retrospective ratings of lithium response using the Alda scale28,29. The Alda scale includes two scores: score A is a 0–10 retrospective rating of lifetime response, while score B captures factors reducing the confidence in score A such as lack of a documented lithium level, etc. In the ConLiGen study, under the continuous phenotype, participants were rated with the Alda A score, and individuals with a B score greater than 4 were excluded. We used the continuous rather than the dichotomous phenotype as a measure of treatment response because genome-wide significant association was detected with the continuous phenotype in the original GWAS. Quality control and statistical analysis methods are described in the original paper.
SNP, Gene, and Gene-Set Analysis
We imported the ConLiGen summary statistics into FUMA (Functional Mapping and Annotation of Genome-Wide Association Studies - https://fuma.ctglab.nl)30, a web-based platform for annotating, prioritizing, visualizing and interpreting GWAS results. We utilized the SNP2GENE function to map SNPs to genes and conduct SNP, gene-based, and gene-set analysis. We used all default settings, except for setting the maximum lead SNP p-value to 1×10e-5.
Network Analysis
We input the ConLiGen summary statistics into NAGA (Network Assisted Genomic Analysis), an online network propagation tool for pathway boosting and interpretation of genome-wide association studies21.NAGA provided a reprioritized ranked list of 19,781 genes as output. We then entered the top 500 genes with the highest final heat scores into STRING, an online database that generates mapped networks based on protein-protein interactions31. STRING additionally analyzes for overrepresentation of user-inputted gene lists in established pathways, using the hypergeometric test32. Using this function, we tested our a priori hypotheses to identify functional enrichment of the NAGA-generated top 500 gene list in the KEGG hsa04510 focal adhesion pathway, KEGG hsa04512 ECM-receptor interaction, and KEGG hsa04151 PI3K-Akt signaling pathway33. P-values were corrected for multiple testing by STRING using the Benjamini–Hochberg procedure34.
Overlap between the NAGA-generated top 500 gene list and the KEGG pathways was visualized using Cytoscape35. A hypergeometric test was conducted to test for overrepresentation of the NAGA-generated 500 gene network in the 500 gene network generated in our previous study23.
RESULTS
Demographics
The demographics of the sample can be found in the original ConLiGen study26. The study was conducted in two phases: GWAS 1 (n = 1065) and GWAS 2 (n = 1168). Sex and age were similar across both cohorts. Mean Alda scale A scores were 6.13 (SD = 3.13) and 6.52 (SD = 2.87), respectively. Mean Alda scale B scores were 1.78 (SD = 1.26) and 2.35 (SD = 1.16), respectively.
SNP, Gene, and Gene-Set Analysis
As reported in the original ConLiGen study, the only SNPs that were significant at a genome-wide significance level of 5e-08 were in linkage disequilibrium with the SNP rs74795342 on chromosome 21 (Supplemental Fig. 1). Using FUMA in our gene-wise analysis, no significant genes were found at a significance level of p < 0.05/18314 = 2.730e-6 (Supplemental Fig. 2). No gene-sets were found to be significant either, using p < 0.05 after Bonferonni correction. The most highly associated genes and gene-sets are listed in Supplemental Table 1 and Supplemental Table 2.
Network Analysis
We first tested the three a priori pathways that were significant in our previous study, which had examined an independent sample23. Using the STRING analysis function, the top 500 reprioritized gene list generated by NAGA was found to be significantly enriched in both the KEGG hsa04510 focal adhesion pathway (p = 1.74e-06) and KEGG hsa04151 PI3K-Akt signaling pathway (p = 1.90e-07). Given the goal of replication and the small number of statistical tests, this was considered as a significant replication of our previous results in an independent sample for the focal adhesion and PI3K-Akt pathways. However, the KEGG hsa04512 ECM-receptor interaction pathway was not found to be significantly enriched.
A hypergeometric test found significant overlap (p = 5.699e-07) between the 500 gene network generated by NAGA and the 500 gene network generated by network propagation analysis in our previous study23.There were 33 genes that were common to both networks.
The top 25 reprioritized genes produced by NAGA are listed in Table 1. All top 500 reprioritized NAGA genes are listed in Supplemental Table 4.
Table 1.
NAGA Top 25 Gene List
| NAGA | FUMA Gene-wise Analysis | ||||
|---|---|---|---|---|---|
| Gene | Input Heat | Final Heat | Rank | Rank | P-value |
| UBC | 0 | 37.88310418 | 1 | 4335 | .22849 |
| GNB1 | 2.624306658 | 21.36254772 | 2 | 12993 | .69873 |
| PRKACB | 6.770605441 | 19.12717575 | 3 | 5778 | .30963 |
| GNAL | 0 | 18.71021909 | 4 | 16294 | .88169 |
| GNGT1 | 0 | 18.61047924 | 5 | 12956 | .69712 |
| REEP1 | 0 | 17.83396241 | 6 | 13325 | .71728 |
| ARRB1 | 7.336233161 | 17.55148794 | 7 | 12430 | .66845 |
| RTP2 | 0 | 17.50819164 | 8 | 11202 | .60084 |
| RTP1 | 0 | 17.50727661 | 9 | 13284 | .71484 |
| PRKACA | 0 | 15.7274719 | 10 | 5506 | .29380 |
| ARRB2 | 0 | 15.43877781 | 11 | 14216 | .76615 |
| PRKACG | 0 | 15.38554785 | 12 | 10721 | .57518 |
| GRK2 | 0 | 15.30495263 | 13 | * | * |
| GNG13 | 0 | 14.28098978 | 14 | 9444 | .50737 |
| GNG7 | 0 | 14.26968321 | 15 | 2636 | .13696 |
| GRK3 | 0 | 13.36097413 | 16 | * | * |
| TAF1 | 0 | 10.51679188 | 17 | * | * |
| APP | 0 | 9.571290241 | 18 | 11903 | .63889 |
| JUN | 5.596453897 | 9.123060759 | 19 | 6091 | .32547 |
| HNF4A | 0 | 7.372223527 | 20 | 13583 | .73157 |
| ELAVL1 | 0 | 7.080313649 | 21 | 2194 | .1154 |
| C1orf94 | 14.45796462 | 7.001838181 | 22 | 9155 | .49203 |
| CSMD2 | 14.45796462 | 6.383962772 | 23 | 36 | .0016402 |
| KCNJ5 | 11.85176049 | 6.256678752 | 24 | 13972 | .75419 |
| INS | 3.272540349 | 5.764244643 | 25 | 10662 | .57147 |
Data does not exist as gene was not evaluated in FUMA
After testing the three a priori hypotheses based on previous results, we tested the top 500 NAGA gene list for enrichment in all pathways in STRING. The top 10 KEGG pathways found to be most strongly enriched are found in Supplemental Table 3. These include cancer and growth pathways (such as Pathways in Cancer, Estrogen Signaling Pathway, Ras Signaling Pathway) as well as the dopaminergic synapse pathway.
We additionally used the STRING analysis function to test for functional enrichment of the top 100, 200, 300, and 400 reprioritized gene lists generated by NAGA in all three a priori KEGG pathways. The results agreed with the primary analysis, since all gene lists were significantly enriched in the KEGG hsa04510 focal adhesion pathway and KEGG hsa04151 PI3K-Akt signaling pathways at a level of p < .05. Only the top 100 reprioritized gene list was found to be significantly enriched in the KEGG hsa04512 ECM-receptor interaction pathway (p = .0050) inconsistent with a robust result. (Supplemental Table 5).
DISCUSSION
In this study, we attempted to replicate our previous results which were from an independent sample23. We used network methods via NAGA to reprioritize GWAS results from the ConLiGen study on lithium response and used STRING to test three a priori network hypotheses: KEGG focal adhesion, ECM-receptor interaction and PI3K-Akt signaling. Two of these three networks, KEGG focal adhesion and PI3K-Akt signaling, were enriched in our top 500 reprioritized genes. However, we did not find significant enrichment for the ECM-receptor interaction pathway in the 500 gene network. Besides this pathway, we were otherwise able to replicate the results of our previous paper in a larger, independent sample of patients with BD. We found highly significant overlap between the top 500 gene network generated by NAGA in this study and the 500 gene network generated in the previous study, providing further evidence for replication.
Focal adhesions are points of contact between cells and proteins in the ECM. The formation of cell-ECM adhesion structures is initiated by cell surface integrins and driven by local actin polymerization. These structures function to not only mediate cell attachment to ECM, but also mediate transmembrane signaling. Integrin-ECM ligand binding can induce a number of downstream changes affecting cell shape, growth, and proliferation36. In neurons, specifically, the actin cytoskeleton of growth cones interacts with the ECM to guide axon development and extension24,37.
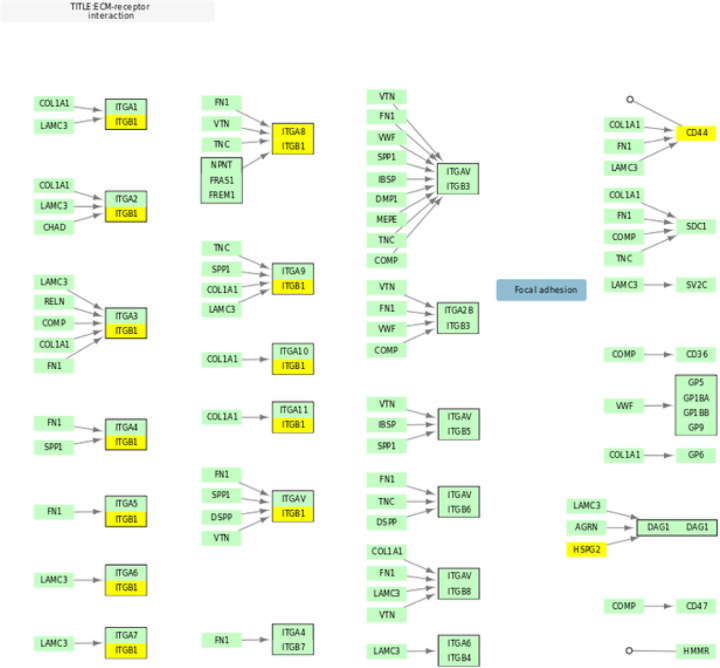
We had originally hypothesized that genetic deficits in focal adhesion, ECM, and PI3K-Akt pathways may impair axonal growth in neurons and determine response to lithium. Though one integrin protein was included in our top 500 genes, in general ECM proteins did not overlap with the top 500 gene list (Supplemental Table 4)(Fig. 2), and the pathway was not significant. This result is inconsistent with our previous study. However, it may suggest the possibility that the deficits influencing lithium response may be inherent to the growth cone rather than components of the ECM. This is supported by a number of studies, which have shown that lithium prevents collapse and induces growth of growth cones38–40.
Figure 2. Overlap Between KEGG ECM-receptor interaction and Top 500 Genes.
KEGG hsa04512 pathway for ECM-receptor interaction adapted to illustrate gene overlap. Genes in yellow overlap with the 500 gene NAGA network.
Previously, neurons derived from induced pluripotent stem cells of patients with BD have been shown to exhibit hyperexcitability in vitro. This hyperexcitable phenotype was rescued by lithium only in neurons derived from lithium good responders17. Elevated neuroactivity in BD may induce vulnerability in neurons through impairment of focal adhesion pathways. Chronic elevation of neuroactivity has been shown to dramatically reduce surface expression of integrin β1 in animal models, leading to axonal and dendritic degeneration and eventually cell death41.
Unsurprisingly, neurons in patients with BD have been shown to be present with smaller size, fewer numbers, and more limited branching. We had previously proposed that in lithium responders, this deficit is caused by deficits in focal adhesion and is rescued by lithium treatment. Furthermore, we proposed that in patients who are not lithium responders, focal adhesion is not dysregulated, and lithium is unable to address the relevant impairments42–44. Our results in this study are consistent with this hypothesis.
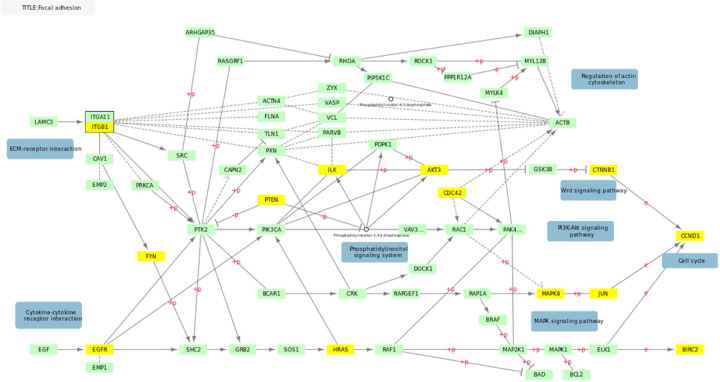
After testing our three a priori hypotheses, we conducted exploratory analyses using network methods. We listed the top 10 most significant KEGG pathways that were associated by STRING with the NAGA generated gene-list in Supplemental Table 3. These pathways are mostly cancer pathways associated with cell growth and proliferation or pathways of addiction and other dopamine-related processes.Dopamine neurotransmission has previously been associated with response to lithium treatment in BD45.Genes in associated cancer pathways show some overlap with focal adhesion as well, which suggests the possibility of shared mechanisms. (Fig. 1).
Figure 1. Overlap Between KEGG Focal adhesion and Top 500 Genes.
KEGG hsa04510 pathway for focal adhesion adapted to illustrate gene overlap. Genes in yellow overlap with the 500 gene NAGA network.
Limitations of our study include the relatively small sample size (N = 2,039) and the generalizability of the data-set, given that all participants were of European descent. Additionally, data was collected retrospectively. As a result, outcomes may be less accurate in determining response phenotypes46 which can blur our findings due to false negatives.
This study also demonstrates the utility of network propagation methods, which can add power to GWAS with limited sample sizes. These methods are beneficial in identifying which genes and gene-sets are of interest to a disease process, but future research is still indicated for con rmation20,21.
In summary, we replicated our previous results reinforcing that genetic deficits in focal adhesion and PI3K-Akt signaling are associated with lithium response in BD patients. We hypothesize, as before, that malformed axonal growth cones result in shorter and less branched axons and susceptibility to BD in a subpopulation of patients who are lithium responders. This is also consistent with the idea that response to lithium results from a disease mechanism distinct from that of lithium non-responders. Furthermore, we propose that lithium rescues disrupted neuronal growth and axon extension processes by addressing deficits in focal adhesion. A better understanding of the pathophysiology of BD and lithium treatment may lead to the future development of drugs similar to lithium, as well as possible clinical predictors for treatment response.
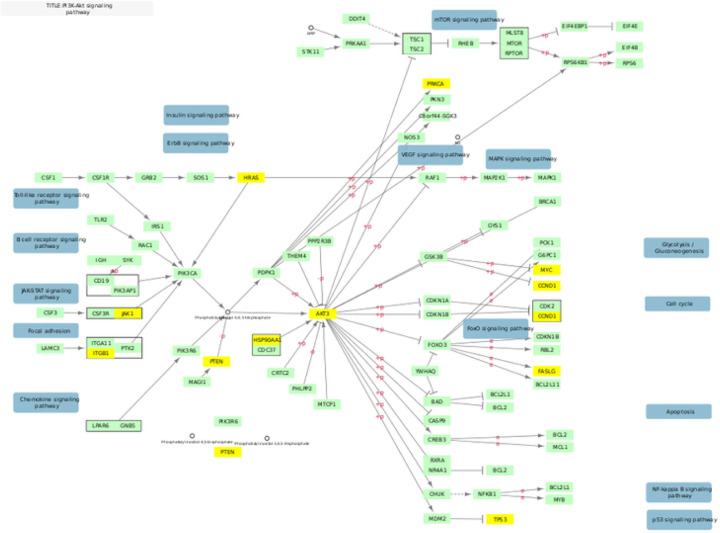
Figure 3. Overlap Between KEGG PI3k-Akt and Top 500 Genes.
KEGG hsa04151 pathway for PI3k-Akt signaling adapted to illustrate gene overlap. Genes in yellow overlap with the 500 gene NAGA network.
Table 2.
Functional Enrichment of NAGA Top 500 Gene List in Focal Adhesion, ECM, and PI3K Akt Pathways
| Pathway | P-value | Number of genes overlapped |
|---|---|---|
| KEGG Focal Adhesion | 1.74e-06* | 21 of 198 |
| KEGG ECM-receptor interaction | .1494 | 5 of 88 |
| KEGG PI3k-Akt | 1.90e-07* | 31 of 350 |
All p-values corrected for multiple testing using the Benjamini–Hochberg procedure.
Significant at p < .05
ACKNOWLEDGEMENTS
We thank the subjects who participated in the original study, without whom this work would not be possible. Additionally, JRK was supported by the NIMH (U01 MH92758), and AHO was supported by the UCSD SOM Summer Research Training Program. AT Amare received the 2019-2021 National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Grant from the Brain & Behaviour Research Foundation (BBRF) and is currently supported by the National Health and Medical Research Council (NHMRC) Emerging Leadership (EL1) Investigator Grant (APP2008000). This work was in part funded by the Deutsche Forschungsgemeinschaft (DFG; grant no RI 908/7-1; grant FOR2107, RI 908/11-1 to Marcella Rietschel, Michael Bauer, and Thomas G Schulze, NO 246/10-1 to MMN) and the Intramural Research Program of the National Institute of Mental Health (ZIA-MH00284311; NCT00001174). The genotyping was in part funded by the German Federal Ministry of Education and Research (BMBF) through the Integrated Network IntegraMent (Integrated Understanding of Causes and Mechanisms in Mental Disorders), under the auspices of the e:Med Programme (grants awarded to TGS, MR, and MMN). OG, AP, TSt, MB, AR, and TGS received support from the German Federal Ministry of Education and Research (BMBF) within the framework of the BipolLife network. MMN received support from the Alfried Krupp von Bohlen und Halbach-Stiftung. Franziska Degenhardt received support from the BONFOR Programme of the University of Bonn, Germany. EZR received funding from the Land Steiermark as principal investigator. MS received funds from the Swedish Research Council, Swedish Brain Foundation and funds from Karolinska Institutet and Karolinska University Hospital. Some data and biomaterials were collected as part of eleven projects (Study 40) that participated in the National Institute of Mental Health (NIMH) Bipolar Disorder Genetics Initiative. From 2003-07, the principal investigators and co-investigators were: Indiana University, Indianapolis, IN, R01 MH59545 (John Nurnberger, Marvin J Miller, Elizabeth S Bowman, N Leela Rau, P Ryan Moe, Nalini Samavedy, Rif El-Mallakh [University of Louisville], Husseini Manji [Johnson and Johnson], Debra A Glitz [Wayne State University], Eric T Meyer [Oxford University, UK], Carrie Smiley, Tatiana Foroud, Leah Flury, Danielle M Dick [Virginia Commonwealth University], Howard Edenberg); Washington University, St Louis, MO, R01 MH059534 (John Rice, Theodore Reich, Allison Goate, Laura Bierut [K02 DA21237]); Johns Hopkins University, Baltimore, R01 MH59533 (Melvin McInnis, J Raymond DePaulo Jr, Dean F MacKinnon, Francis M Mondimore, James B Potash, Peter P Zandi, Dimitrios Avramopoulos, Jennifer Payne); University of Pennsylvania, PA, R01 MH59553 (Wade Berrettini); University of California at San Francisco, CA, R01 MH60068 (William Byerley, Sophia Vinogradov); University of Iowa, IA, R01 MH059548 (William Coryell, Raymond Crowe); University of Chicago, IL, R01 MH59535 (Elliot Gershon, Judith Badner, Francis McMahon, Chunyu Liu, Alan Sanders, Maria Caserta, Steven Dinwiddie, Tu Nguyen, Donna Harakal); University of California at San Diego, CA, R01 MH59567 (John Kelsoe, Rebecca McKinney); Rush University, IL, R01 MH059556 (William Scheftner, Howard M Kravitz, Diana Marta, Annette Vaughn-Brown, Laurie Bederow); and NIMH Intramural Research Program, Bethesda, 1Z01MH002810-01 (Francis J McMahon, Layla Kassem, PhD, Sevilla Detera-Wadleigh, Lisa Austin, Dennis L Murphy [Howard University], William B Lawson, Evarista Nwulia, Maria Hipolito). This work was supported by the NIH grants P50CA89392 from the National Cancer Institute and 5K02DA021237 from the National Institute of Drug Abuse. The Canadian part of the study was supported by a grant #166098 from the Canadian Institutes of Health Research, by CIHR under the frame of ERAPerMed (grant PLOT-BD), and Genome Canada grant RP3 to MAl. We wish to thank Joanne Petite and Giselle Kraus for assistance with data collection. Collection and phenotyping of the Australian UNSW sample, by PBM, PRS, JMF, and AW, was funded by an Australian NHMRC Program Grant (No. 1037196). The collection of the Barcelona sample was supported by the Centro de Investigación en Red de Salud Mental (CIBERSAM) IDIBAPS (grant numbers PI080247, PI1200906, PI12/00018), and Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2021SGR1358 and 2014SGR398). J-MA and AD were supported by the Swiss National Science Foundation (grant number 32003B_125469 and NCCR Synapsy). DAC was supported by a Medical Research Council Clinician Scientist Fellowship Award (MR/L006642/1). LF was supported by the Swedish Research Council (grant no 523-2011-3807).M. Grigoroiu-Serbanescu was supported by UEFISCDI, Romania, grant no 89/2012 and grant no 203/2021. P-HK was funded by the Taiwan Ministry of Science and Technology (grant no MST 99-2314-B-002-140-MY3 and 102-2314-B-002-117-MY3). CALJ was funded by the “Estrategia de Sostenibilidad 2014-2015” program of the University of Antioquia. TN was supported by the Ministry of Health of the Czech Republic (grant no IGA NT13891). JBP was supported by the Reuben Stoltzfus Bipolar Research Fund and with SKT received funding from the James Wah Fund and Project MATCH. TGS and UH received support from the Dr-Lisa-Oehler-Foundation (Kassel, Germany). This study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, MD. Genotyping for part of the Swedish sample was funded by the Stanley Center for Psychiatric Research at the Broad Institute.
CONFLICT OF INTERESTS
MAd has received a grant from Servier, speaker’s fees from Servier, Lundbeck, Aristo, Parexel, Gilead, ViiV, Deutsche Bank, MSD, and MyTomorrows, plus a non-financial support from Lundbeck. KA has received speaker’s fees from Taisho Toyama Pharmaceutical. MAl is funded by a grant of the Canadian Institutes of Health Research. MB has received speaker’s fees from AstraZeneca, Pfizer, Lilly, Lundbeck, GlaxoSmithKline, Servier, and Ferrer Internacional. BÉ received non-financial support from Labex Biopsy and Fondation Fondamental. RH received grants and speaker honoraria from Dainippon Sumitomo Pharma and Novartis plus speaker honoraria from Eli Lilly Japan, GlaxoSmithKline, Hisamitsu Pharmaceutical, Janssen Pharmaceutical, Nippon Zoki Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Pfizer, and the Yoshitomiyakuhin Corporation. TK received a grant from Takeda Pharmaceutical and fees from Kyowa Hakko Kirin, Eli Lilly Japan, Otsuka Pharmaceutical, GlaxoSmithKline, Taisho Toyama Pharmaceutical, Dainippon Sumitomo Pharma, Meiji Seika Pharma, PfizerJapan, Mochida Pharmaceutical, Shionogi & Co, Janssen Pharmaceutical, Yoshitomiyakuhin Corporation, Agilent Technologies, Astellas Pharma, and Wako Pure Chemical Industries. IK received grants and fees from Dainippon Sumitomo Pharma, Eisai, Eli Lilly, GlaxoSmithKline, Kyowa Hakko Kirin, Meiji Seika Pharma, MSD, Novartis, Otsuka, Ono Pharmaceutical, Pfizer, Tanabe Mitsubishi Pharma, Takeda Pharmaceutical, Shionogi, and Yoshitomi Pharmaceutical; he received grants from AbbVie GK, Asahi Kasei Pharma, Boehringer Ingelheim, Chugai Pharmaceutical, and Daiichi Sankyo and fees from Astellas Pharma and Janssen Pharmaceutical. MJM served as unpaid consultant for Pathway Genomic (San Diego, USA). SLM received a grant and fees from Naurex and Shire, further grants from Alkermes, Cephalon, Forest, Marriott Foundation, Orexigen Therapeutics, and Takeda Pharmaceutical, he further has served on the advisory boards for Bracket, Hoffmann-La Roche, MedAvante, Sunovion and received fees from Novo Nordisk. RHP received personal fees from RID Ventures, Genomind LLC, Healthrageous, P zer, Perfect Health, Proteus, and Psybrain. PRS received a grant from NHMRC. TGS received a grant and fees from Roche Pharmaceuticals. TSt received personal fees from Servier, Lundbeck, and Bristol-Myers Squibb. MM has received grants from Lundbeck and Angelini. EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, AbbVie, Adamed, Angelini, Biogen, Biohaven, Boehringer-Ingelheim, Celon Pharma, Compass, Dainippon Sumitomo Pharma, Ethypharm, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, HMNC, Idorsia, Janssen, Lundbeck, Medincell, Merck, Novartis, Orion Corporation, Organon, Otsuka, Roche, Rovi, Sage, Sanofi-Aventis, Sunovion, Takeda, and Viatris, outside the submitted work. SRC has participated in advisory and educational boards and received speaker’s fees from Janssen-Cilag, Lundbeck, Otsuka, and Servier; research funding from Janssen-Cilag, Lundbeck, Otsuka, and Gilead; and data sharing from Viatris Australia. ML has received lecture honoraria from Lundbeck pharmaceuticals. All above listed interests are outside of the submitted work. All other authors declare no competing interests.
Footnotes
Supplementary Files
Contributor Information
John Kelsoe, University of California San Diego.
Anna Ou, University of California San Diego.
Kazufumi Akiyama, Department of Biological Psychiatry and Neuroscience, Dokkyo Medical University.
Nirmala Akula, National Institutes of Health, US Dept of Health & Human Services.
Martin Alda, Dalhousie University.
Azmeraw T. Amare, University of Adelaide, AUSTRALIA
Raffaella Ardau, Hospital University Agency of Cagliari.
Bárbara Arias, Facultat de Biologia and Institut de Biomedicina (IBUB), Universitat de Barcelona, CIBERSAM.
Jean-Michel Aubry, Geneva University Hospitals.
Michael Bauer, University Hospital Carl Gustav Carus.
Bernhard Baune, University of Münster.
Joanna Biernacka, Mayo Clinic.
Pablo Cervantes, McGill University Health Centre.
Hsi-Chung Chen, 3Department of Psychiatry, National Taiwan University Hospital, Taipei, Taiwan 4Department of Psychiatry, Center of Sleep Disorders, National Taiwan University Hospital, Taipei, Taiwan.
Sven Cichon, Mayo Clinic.
Scott Clark, University of Adelaide.
Cristiana Cruceanu, Max Plank Institute for Psychiatry.
Piotr Czerski, Poznan University of Medical Sciences.
Alexandre Dayer, University of Geneva.
Franziska Degenhardt, University of Essen.
J. Raymond DePaulo, Johns Hopkins University.
Peter Falkai, University Hospital LMU.
Andreas J. Forstner, University of Bonn, School of Medicine & University Hospital Bonn
Louise Frisen, School of Medicine & University Hospital Bonn.
Mark Frye, Mayo Clinic.
Janice Fullerton, Neuroscience Research Australia.
Maria Grigoroiu-Serbanescu, Alexandru Obregia Clinical Psychiatric Hospital.
Ryota Hashimoto, National Center of Neurology and Psychiatry.
Urs Heilbronner, Institute of Psychiatric Phenomics and Genomics, University Hospital, LMU Munich.
Per Hoffmann, Institute of Human Genetics.
Liping Hou, National Institute of Mental Health Intramural Research Program, National Institutes of Health.
Stéphane Jamain, Univ Paris Est Creteil, INSERM, IMRB.
Layla Kassem, School of Medicine & University Hospital Bonn.
Tadafumi Kato, Juntendo University Graduate School of Medicine.
Po-Hsiu kuo, College of Public Health, National Taiwan University, Taipei, Taiwan.
Ichiro Kusumi, Hokkaido University Graduate School of Medicine.
Mikael Landén, Gothenburg University.
Catharina Lavebratt, Karolinska Institutet.
Mario Maj, University of Naples, Italy.
Mirko Manchia, Dalhousie University.
Cynthia Marie-Claire, INSERM UMR-S 1144.
Lina Martinsson, Karolinska Institutet.
Manuel Mattheisen, Johns Hopkins University.
Susan McElroy, Lindner Center.
Francis McMahon, National Institute of Mental Health Intramural Research Program; National Institutes of Health.
Philip Mitchell, University of New South Wales.
Marina Mitjans, Max Planck Institute of Experimental Medicine, Göttingen, Germany.
Francis Mondimore, Neuroscience Research Australia.
Palmiero Monteleone, University of Salerno, University of Naples SUN.
Caroline Nievergelt, University of California, San Diego.
Markus Nöthen, School of Medicine & University Hospital Bonn.
Tomas Novak, National Institute of Mental Health, Klecany.
Urban Osby, Karolinska Institutet.
Norio Ozaki, Nagoya University.
Sergi Papiol, University Hospital LMU.
Roy Perlis, Massachusetts General Hospital.
Andrea Pfennig, University Hospital Carl Gustav Carus, TU Dresden.
Andreas Reif, University Hospital Frankfurt, Germany.
Marcella Rietschel, University of Mannheim.
Guy Rouleau, McGill University.
Janusz K. Rybakowski, Poznan University of Medical Sciences
Martin Schalling, Karolinska Institutet.
Peter Schofield, Neuroscience Research Australia.
Klaus Oliver Schubert, University of Adelaide.
Thomas Schulze, University of Munich.
Giovanni Severino, University of Cagliari.
Alessio Squassina, Universita degli Studi Di Cagliari.
Thomas Stamm, Charité - Universitätsmedizin Berlin, Campus Charité Mitte.
Alfonso Tortorella, Department of Psychiatry, University of Perugia, Perugia, Italy.
Gustavo Turecki, Douglas Institute, Department of Psychiatry, McGill University.
Eduard Vieta, Hospital Clinic of Barcelona.
Stephanie Witt, University Medical Centre Mannheim.
Naomi Wray, University of Queensland.
Peter Zandi, Johns Hopkins University.
Maria Del Zompo, University of Cagliari.
References
- 1.Dome P., Rihmer Z. & Gonda X. Suicide Risk in Bipolar Disorder: A Brief Review. Medicina 55, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cipriani A., Hawton K., Stockton S. & Geddes J. R. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 346, f3646 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Plans L. et al. Association between completed suicide and bipolar disorder: A systematic review of the literature. J. Affect. Disord. 242, 111–122 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Sajatovic M. Bipolar disorder: disease burden. Am. J. Manag. Care 11, S80–4 (2005). [PubMed] [Google Scholar]
- 5.Rybakowski J. K. Lithium. Eur. Neuropsychopharmacol. 57, 86–87 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Kato T. Mechanisms of action of anti-bipolar drugs. Eur. Neuropsychopharmacol. 59, 23–25 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Machado-Vieira R., Manji H. K. & Zarate C. A. Jr. The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 11 Suppl 2, 92–109 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freland L. & Beaulieu J.-M. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front. Mol. Neurosci. 5, 14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jope R. S. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 24, 441–443 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Mishra H. K. et al. Contributions of circadian clock genes to cell survival in broblast models of lithium-responsive bipolar disorder. Eur. Neuropsychopharmacol. 74, 1–14 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Hermida M. A., Dinesh Kumar J. & Leslie N. R. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv. Biol. Regul. 65, 5–15 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Watson D. G. & Lenox R. H. Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J. Neurochem. 67, 767–777 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Tobe B. T. D. et al. Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 114, E4462–E4471 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao W.-N. et al. Discovery of suppressors of CRMP2 phosphorylation reveals compounds that mimic the behavioral effects of lithium on amphetamine-induced hyperlocomotion. Transl. Psychiatry 10, 76 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amare A. T. et al. Association of polygenic score and the involvement of cholinergic and glutamatergic pathways with lithium treatment response in patients with bipolar disorder. Mol. Psychiatry (2023) doi: 10.1038/s41380-023-02149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papiol S., Schulze T. G. & Heilbronner U. Lithium response in bipolar disorder: Genetics, genomics, and beyond. Neurosci. Lett. 785, 136786 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Mertens J. et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527, 95–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grof P. et al. Is response to prophylactic lithium a familial trait? J. Clin. Psychiatry 63, 942–947 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Cruceanu C., Alda M. & Turecki G. Lithium: a key to the genetics of bipolar disorder. Genome Med. 1, 79 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiserson M. D. M., Eldridge J. V., Ramachandran S. & Raphael B. J. Network analysis of GWAS data. Curr. Opin. Genet. Dev. 23, 602–610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlin D. E. et al. A Fast and Flexible Framework for Network-Assisted Genomic Association. iScience 16, 155–161 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oedegaard K. J. et al. The Pharmacogenomics of Bipolar Disorder study (PGBD): identi cation of genes for lithium response in a prospective sample. BMC Psychiatry 16, 129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niemsiri V. et al. Focal adhesion is associated with lithium response in bipolar disorder: evidence from a network-based multi-omics analysis. Mol. Psychiatry (2023) doi: 10.1038/s41380-022-01909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Short C. A., Suarez-Zayas E. A. & Gomez T. M. Cell adhesion and invasion mechanisms that guide developing axons. Curr. Opin. Neurobiol. 39, 77–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maletic V. & Raison C. Integrated neurobiology of bipolar disorder. Front. Psychiatry 5, 98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou L. et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome-wide association study. Lancet 387, 1085–1093 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sollis E. et al. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. 51, D977–D985 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manchia M. et al. Assessment of Response to Lithium Maintenance Treatment in Bipolar Disorder: A Consortium on Lithium Genetics (ConLiGen) Report. PLoS One 8, e65636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marie-Claire C., Courtin C., Bellivier F., Scott J. & Étain B. Methylomic Biomarkers of Lithium Response in Bipolar Disorder: A Proof of Transferability Study. Pharmaceuticals 15, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K., Taskesen E., van Bochoven A. & Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szklarczyk D. et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 51, D638–D646 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szklarczyk D. et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 49, D605–D612 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M. & Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y. & Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995). [Google Scholar]
- 35.Shannon P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C. Focal adhesion: a focal point in current cell biology and molecular medicine. Cell Adh. Migr. 1, 13–18 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Omotade O. F., Pollitt S. L. & Zheng J. Q. Actin-based growth cone motility and guidance. Mol. Cell. Neurosci. 84, 4–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams R. S. B., Cheng L., Mudge A. W. & Harwood A. J. A common mechanism of action for three mood-stabilizing drugs. Nature 417, 292–295 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Shah S. M., Patel C. H., Feng A. S. & Kollmar R. Lithium alters the morphology of neurites regenerating from cultured adult spiral ganglion neurons. Hear. Res. 304, 137–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen R. & Gordon-Weeks P. R. Inhibition of glycogen synthase kinase 3β in sensory neurons in culture alters lopodia dynamics and microtubule distribution in growth cones. Mol. Cell. Neurosci. 23, 626–637 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Murase S. Impaired Focal Adhesion Kinase-Grb2 Interaction during Elevated Activity in Hippocampal Neurons. Int. J. Mol. Sci. 16, 15659–15669 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gigante A. D. et al. Morphometric post-mortem studies in bipolar disorder: possible association with oxidative stress and apoptosis. Int. J. Neuropsychopharmacol. 14, 1075–1089 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Konradi C. et al. Hippocampal interneurons in bipolar disorder. Arch. Gen. Psychiatry 68, 340–350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajkowska G. Cell pathology in bipolar disorder. Bipolar Disord. 4, 105–116 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Mohamadian M. et al. Mood and behavior regulation: interaction of lithium and dopaminergic system. Naunyn. Schmiedebergs. Arch. Pharmacol. (2023) doi: 10.1007/s00210-023-02437-1. [DOI] [PubMed] [Google Scholar]
- 46.Talari K. & Goyal M. Retrospective studies - utility and caveats. J. R. Coll. Physicians Edinb. 50, 398–402 (2020). [DOI] [PubMed] [Google Scholar]