Abstract
Background
The ability to walk is an important factor in quality of life after stroke. Co-activation of hip adductors and knee extensors has been shown to correlate with gait impairment. We have shown previously that training with a myoelectric interface for neurorehabilitation (MINT) can reduce abnormal muscle co-activation in the arms of stroke survivors.
Methods
Here, we extend MINT conditioning to stroke survivors with leg impairment. The aim of this pilot study was to assess the safety and feasibility of using MINT to reduce abnormal co-activation between hip adductors and knee extensors and assess any effects on gait. Nine stroke survivors with moderate to severe gait impairment received six hours of MINT conditioning over six sessions, either in the laboratory or at home.
Results
MINT participants completed a mean of 159 repetitions per session without any adverse events. Further, participants learned to isolate their muscles effectively, resulting in a mean reduction of co-activation of 70% compared to baseline. Moreover, gait speed increased by a mean of 0.15 m/s, more than the minimum clinically important difference. Knee flexion angle increased substantially, and hip circumduction decreased.
Conclusion
MINT conditioning is safe, feasible at home, and enables reduction of co-activation in the leg. Further investigation of MINT’s potential to improve leg movement and function after stroke is warranted. Abnormal co-activation of hip adductors and knee extensors may contribute to impaired gait after stroke.
Trial registration
This study was registered at ClinicalTrials.gov (NCT03401762, Registered 15 January 2018, https://clinicaltrials.gov/study/NCT03401762?tab=history&a=4)
Keywords: Stroke, gait, co-activation, EMG, game-based rehabilitation, knee flexion
Background
Impaired lower limb function following stroke results in impaired walking and an increased risk of falling [1]. While some stroke survivors achieve independent walking status, about a third continue to face challenges related to lower limb coordination, gait speed, walking endurance, and balance [2,3]. Impaired movement after a stroke is often caused by a combination of weakness, spasticity, and abnormal muscle co-activation [4–7]. While many rehabilitation approaches have been developed to address weakness and spasticity, gait dysfunction can remain severe despite reduction of these components [8–10]. This suggests that abnormal muscle co-activation is a crucial contributor to gait dysfunction in many hemiparetic stroke survivors.
Stroke survivors often exhibit abnormal gait kinematics, including abnormal pelvic and leg joint motion in both the sagittal (decreased knee flexion) and frontal planes (hip hiking, circumduction) [11–13]. These abnormal kinematics are mechanically inefficient and energetically costly, which increases fatigue [14–16]. There is some evidence that hip hiking and circumduction are compensatory mechanisms to ensure toe clearance in people with stiff-knee gait [17,18]. However, when external assistance to knee flexion was applied to hemiparetic legs using an orthosis, no changes in the expression of hip hiking and circumduction was observed [13]. Further, neurotypical participants whose knee flexion was artificially restricted with an orthosis did not show compensatory circumduction [19]. These findings suggest that the abnormal kinematics may be the result of compensating for an abnormal coupling between hip adduction and knee extension, instead of compensating for reduced knee flexion [12,20]. An increase in knee extension and hip adduction at or near toe-off reduces the minimum distance between the toe and the ground, and between the foot and the contralateral leg, respectively, thus increasing the risk of tripping. To clear the ground and avoid hitting the opposite leg, patients may hip hike and circumduct [21]. While multiple abnormal coactivation patterns are seen after stroke [22–25], abnormal hip adduction/knee extension, especially at toe-off, was the dominant pattern [12]. Further, in a multiple regression model incorporating both classical impairments (decreased flexion of hip, knee, or ankle) and abnormal hip/knee coupling, abnormal hip adduction/knee extension most strongly correlated with hip hiking and most strongly predicted overground walking speed [22]. These studies were largely correlational. We have developed a system to reduce co-activation, called a myoelectric interface for neurorehabilitation (MINT), and shown that it effectively reduces co-activation between arm muscles trained [26,27] and may improve arm function [28,29]. Here, we used MINT conditioning to test the hypothesis that reducing abnormal hip adduction/knee extension co-activation would improve gait function. We found that MINT conditioning reduced abnormal co-activation and improved gait function, including gait kinematics. This implies that this abnormal co-activation pattern does indeed cause gait dysfunction after stroke and suggests that reducing it could improve gait function.
Methods
Participants and EMG Recording
The study was conducted with approval from the Institutional Review Board of Northwestern University as part of a larger study investigating MINT for improving arm function (NCT03401762), and all participants provided written informed consent before eligibility assessment. We enrolled 9 adult chronic stroke survivors who had experienced a first-time stroke at least 6 months prior and had moderate to severe gait impairment. We excluded individuals who had impairments in vision, memory, language, or concentration, received botulinum toxin on the affected leg within the previous 3 months, or were currently participating in another research study involving the leg. The aim of the study was to assess the safety and feasibility of MINT training for the leg in chronic patients with moderate to severe walking deficits. As the ultimate goal of MINT conditioning is to be usable at home, we desired to assess the safety and feasibility of both in-lab and at-home training. Four participants trained in the laboratory and were evaluated daily for 6 days (day 1 to day 6, spread over 2 weeks), while five participants trained at home on days 2–5 and trained and were evaluated in the laboratory on day 1 and day 6. The primary outcome was gait speed (measured in the 10-m walk test). Surface electromyography (EMG; recorded using Trigno sensors [Delsys, Inc.]), and leg kinematics were recorded on each evaluation day while participants performed the 10-m walk test four times. EMG signals were recorded from the following leg muscles: vastus lateralis (VL), rectus femoris (RF), vastus medialis (VM), adductor magnus (AM), biceps femoris (BF), and tensor fasciae latae (TFL). The EMG signals were digitized at 1926 Hz and bandpass filtered using a Butterworth filter (50–450 Hz, forward and backward).
Training Paradigm
Participants were asked to train with MINT for six days, 60 min per day, split into 10-min runs. On average, participants were expected to complete ~ 30 repetitions per run, so we anticipated they would perform ~ 1080 reps of total training. After completing each run, the participants were instructed to sit down and rest. MINT consists of a gaming rehabilitation system comprised of software and hardware [29]. The hardware includes a customized, wireless surface EMG system (Myomo, Inc) that amplifies, digitizes, and computes the EMG envelopes and transmits them via Bluetooth.
Each session began by performing a maximum voluntary contraction (MVC) before MINT conditioning started to calibrate the conditioning to each participant’s residual strength. Participants were instructed to maximize the activation of either RF or AM muscles in the toe-off position. The MVC and resting baseline values were used to personalize the mapping of EMG envelope to cursor movements for each participant.
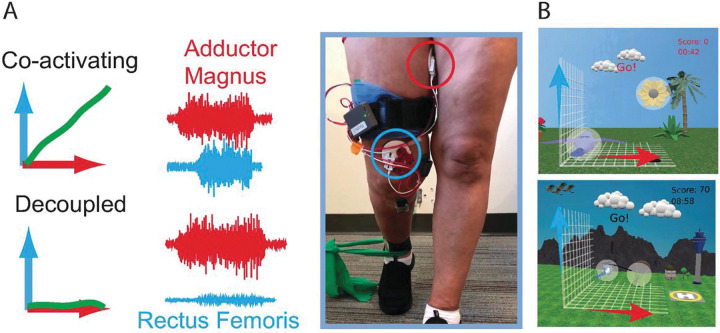
During MINT conditioning, EMG envelopes from AM and RF were mapped to orthogonal components of cursor movement, and the cursor moved as a vector sum of the two components [29] (Fig. 1). After holding the cursor in the home target (at bottom left of the screen) by relaxing both muscles, an outer target appeared toward the opposite end of the screen, and participants attempted to move the cursor into that target and hold for 0.5 s. To enhance shaping and increase participant engagement the difficulty was gradually incremented across five key factors in the following order: increasing the angular separation between the outer target and the diagonal, decreasing cursor size, decreasing target size, increasing the requisite arm muscle relaxation after each trial, and increasing the muscle activation needed to acquire the outer target. Outer targets were initially placed at a distance equivalent to 15% of the MVC from the home target. The placement of outer targets occurred at random angles within a pre-defined range, originating in proximity to the 45° diagonal between muscles (signifying high co-activation) and progressing farther away from the diagonal as the difficulty level advanced. This progression mandated enhanced muscle isolation as the difficulty level increased.
Figure 1. MINT paradigm.
A) Participant engaged in MINT conditioning using the wearable device. EMG signals from AM (red) and RF (blue) were mapped in orthogonal directions and vector summed to control the cursor’s movement. When muscles were co-activated, the cursor moved along a diagonal between the two directions. To encourage the participant to separate the muscle activations, targets were gradually moved progressively further away from the diagonal until they were only in the “up” or “right” positions. B) Various game skins were implemented based on participant preference to enhance enjoyment and engagement.
Seven of the participants used MINT in a standing position with toe-off. The participants used a custom-designed 45° inclined foot brace to keep their foot in the toe-off position. Two participants used MINT in a sitting position due to being easily fatigued, although they were asked to keep their foot in the toe-off position using the foot brace. We taught home group participants how to use the MINT system and place electrodes in the laboratory, and they received daily technical support (if needed) via phone or video chat from lab staff. Automated algorithms were used to monitor MVC estimates and gameplay statistics daily and alert lab staff if issues arose.
Outcome Measures
Game performance metrics (success rate, time to target) were recorded for each 10-min game run. These performance metrics were monitored remotely by lab staff using a secure cloud server to ensure participant adherence. A lab member not involved in training performed functional evaluations of the 10-m walk test (primary outcome). Participants rested for at least 20 s between walks to avoid fatigue. The baseline co-activation between hip adductors and knee extensors were computed based on the correlation coefficient between RF and AM EMGs during the 10-m walk test. Leg kinematics during gait were measured using inertial measurement unit (IMU) sensors to examine effects on hip abduction and knee flexion angles. To determine the knee angle, we positioned two IMU sensors: one over the lateral epicondyle, and the other over the tibial tuberosity. For the hip angle, an IMU sensor was placed over the anterior superior iliac spine (ASIS). Using the IMUs and the measured limb lengths to create a kinematic chain model[30] representing the lower extremity, we calculated the 3D orientation of the leg and estimated the knee flexion angle. The IMUs over the ASIS and lateral epicondyle were used similarly to estimate the hip abduction angle.
Safety
To monitor adverse events, we instructed our participants to report any incidents, such as falls while using MINT or any instances of pain or fatigue during its use. Participants were instructed to perform the MINT conditioning with a walker that was provided to them. Additionally, we advised participants to take a brief rest after each run.
Results
Nine participants (4 women, 5 men, aged 60 ± 7 (mean ± SD) years) enrolled in this study. The mean time from stroke onset at enrollment was 10 ± 6 years. Strokes were located in the right hemisphere in 5, left hemisphere in 4. During MINT conditioning, participants were operantly conditioned to reduce co-activation between adductor magnus and rectus femoris (Fig. 2A). Participants improved their MINT performance (increase in success rate and decreased time-to-target) over the 6 days (Fig. 2B, C). Participants reduced co-activation during MINT training between adductor magnus and rectus femoris by a mean of 70% compared to baseline (Fig. 2D). In total, participants completed 952 ± 287 repetitions over 6 days of training. The at-home group completed 970 ± 225 repetitions, while the in-lab group completed 938 ± 355 repetitions.
Figure 2. MINT conditioning improved game performance and decreased muscle co-activation.
A) Mean (± SEM) normalized EMG envelope in the 2 s before successful target capture (“Reward”) for all runs in days 1 (top) and 6 (bottom) for subject 1. Left plots show AM targets, right plots show RF targets (shown in insets). This participant learned to reduce activity in the non-targeted muscle by day 6. (B,C) Time-to-target and success rate (mean ± SEM) over participants improved over the course of MINT conditioning. D) Mean co-activation decreased during conditioning, especially from baseline co-activation obtained during walking.
Participants did not report any adverse events from MINT conditioning. Further, participants’ gait speed on the 10-m walk test increased by 0.15 m/s (p = 0.006, paired t-test) from day 1 baseline (prior to training) to after training on day 6 (Fig. 3A). This value was higher than the minimum clinically important difference (MCID) of 0.1 m/s. Both participant groups (those that trained at home and in lab) improved walking speed after training (Fig. 3B). In addition, while walking, participants’ knee flexion angle significantly increased 13° from pre-training day 1 baseline (p = 0.03, paired t-test) and hip circumduction showed a non-significant decreasing trend (p = 0.24, paired t-test) of 7° from pre-training day 1 baseline (Fig. 3C, D).
Figure 3. Functional outcomes of MINT conditioning.
A) Gait speed significantly improved by a (mean ± SE) of 0.15 ±0.04 m/s across all participants, more than the MCID. B) Both in-lab and home training led to improvements in walking speed. Each point represents the speed change from day 1 pre-training baseline sessions. C) Knee flexion increased by 13° and (D) hip abduction showed a decreasing trend by 7° from baseline over all participants (gray). Each point shows the after-MINT training knee or hip angle.
We analyzed whether stroke participants with more severe impairment engaged less in using the MINT device in terms of the number of repetitions, and if the severity of impairment affected the amount of repetitions that participants completed. We sorted the participants into two groups based on their walking speed: limited community ambulators with a gait speed of 0.4 to 0.8 m/s (n = 6), and full community ambulators with a gait speed between 0.8 and 1.2 m/s (n = 3)[31]. Full community ambulators completed (810 ± 380) repetitions over the 6-day period, while limited community ambulators completed (1023 ± 233) repetitions (Fig. 4A, p = 0.32, unpaired t-test). Additionally, there was no significant difference observed in the co-activation between the RF and AM muscles in the two groups during training (Fig. 4B, p = 0.95, one-way ANOVA).
Figure 4. Effects of impairment severity and responder status on repetitions and abnormal co-activation.
A) Total number of repetitions vs. baseline speed for each participant (square). Horizontal dashed line represents the theoretical expected number of repetitions over 6 days and vertical dashed line divides participants into limited and full community ambulators. B) Co-activation (R) between RF and AM during 6-day MINT conditioning for full and limited community ambulators. C) Number of repetitions vs. gait speed change (day 1 to day 6) due to training. Horizontal dashed line is the same as in A; vertical dashed line shows MCID of 0.1 m/s used to divide participants to responders and non-responders. D) Co-activation between RF and AM for responder and non-responder groups.
To investigate whether training intensity influenced functional improvement, we divided the participants into “responders” (those who improved by at least the MCID) and “non-responders” (those who did not improve by greater than or equal to the MCID). Responders completed 954 ± 225 total repetitions over 6 days, while non-responders completed 950 ± 390 repetitions (Fig. 4C, p = 0.68, unpaired t-test). Figure 4D compares co-activation during training for responders and non-responders. Responders had greater reduction in co-activation throughout the 6-day training. Notably, there was a significant difference observed in the co-activation between RF and AM between responder and non-responder participants (p = 0022, one-way ANOVA).
Discussion
In this study, we investigated the safety, feasibility, and impact of MINT conditioning on abnormal co-activation between AM and RF and walking function in chronic stroke survivors. Participants trained with MINT safely, with no adverse events even when training at home while standing. MINT conditioning is feasible—as evidenced by a high number of repetitions, improved performance, and reduced co-activation —both in the lab and at home. After just six days of MINT conditioning, participants improved walking function significantly and by more than the MCID. This was true of both in-lab and at-home use of MINT. This preliminary causal evidence suggests that abnormal co-activation between hip adductors and knee extensors does indeed contribute to gait dysfunction after stroke [12]. To the best of our knowledge, there are no previous rehabilitation studies designed specifically to counteract abnormal co-activation in the leg after stroke. In particular, there exist none that address hip adductor-knee extensor co-activation. The results here, though uncontrolled, suggest that therapies addressing this issue may help gait function, as well as arm function. They further suggest that MINT conditioning warrants further study of efficacy in a longer, larger, randomized controlled trial. MINT can provide an enjoyable, and ultimately affordable, game-based solution for at-home rehabilitation, which encourages participants to engage in high doses of training at home. Its new mechanism of action reduces abnormal co-activation, which is not typically addressed in conventional therapies. The ability to train at home is advantageous, as it could enable higher dosage and greater penetration into underserved communities.
Importantly, MINT was used as much for stroke survivors with limited community ambulation as those with community ambulation. The innovation of MINT conditioning lies in providing a wearable (and ultimately affordable) rehabilitation option that specifically targets abnormal co-activation in the leg. All participants demonstrated a high level of engagement, and limited community ambulators performed the expected number of repetitions over the six-day period. This finding suggests that MINT conditioning was motivating for more severely impaired individuals. Further, even limited community ambulators participants could learn to reduce abnormal co-activation (Fig. 4B). This aligns with motor learning studies indicating that unilateral stroke does not impair the acquisition of motor skills [32,33]. It also aligns with our prior MINT conditioning studies in the arm [28,29], in which even those with severe arm impairments could use and benefit from MINT. This population, often excluded from clinical trials, typically is most in need of new therapies.
We also investigated the relationship of training dose and responder status with co-activation. Both responders and non-responders achieved a high number of repetitions, with no significant difference between them. In contrast the learning curve of responders was significantly lower in co-activation than non-responders (Fig. 4D). This suggests that learning to reduce abnormal co-activation was an important factor in explaining the improvement observed in responders. The rapid change in the learning curve (days 1–2) supports the notion that participants quickly adapted to using MINT to decouple these muscles (Fig. 2).
It is not clear what specifically causes abnormal hip adductor/knee extensor co-activation. In the arm, it has been proposed that abnormal co-activation results from reduced availability of the corticospinal tract (CST), leading to a compensatory reliance on other tracts, particularly the corticoreticulospinal tract. While this may be the cause for abnormal hip adductor/knee extensor co-activation as well, some findings also indicate that could be attributed to changes in the polysynaptic spinal reflexes [34]. In addition to this abnormal co-activation pattern, others have been reported [12,23]. Thus, it is possible that MINT conditioning could help other abnormal co-activation patterns in the leg as well. Although there is substantial evidence suggesting that MINT improves movement by changing the co-activation patterns of only the targeted muscles [27], the specific locations of plastic changes in the brain or spine from this training remain unclear and are a subject for future investigations.
Our study had some limitations. While the number of participants was relatively small, we were still able to observe significant improvements in both walking function and knee kinematics. Without a control group, we cannot definitively attribute the observed improvements to the MINT intervention and rule out the possibility that any type of training could lead to similar outcomes. Nevertheless, the fact that responders were able to decouple abnormal hip adductor and knee extensor muscles to a greater extent than non-responders support the likelihood that MINT conditioning’s ability to reduce co-activation is important for improving arm function. The fact that significant effects were seen after just 6 days of training was remarkable and encouraging. The optimal dose remains to be determined. Finally, the current design of the MINT device may pose challenges for users. Its cumbersome nature could potentially limit its usability and acceptance among stroke survivors. Addressing this issue and developing a more user-friendly design is important for future iterations of the device. Despite these limitations, our study provides valuable insights into the potential benefits of MINT for improving walking function in stroke survivors with abnormal co-activation. Further investigation of MINT conditioning in the leg in a randomized, controlled trial of longer duration is thus warranted based on this pilot study.
Conclusion
This study suggests that MINT conditioning led to a significant reduction in abnormal co-activation and improved walking function and kinematics. This suggests that abnormal co-activation contributes to gait impairment after stroke, and that reducing this co-activation may improve function. Overall, our study contributes to the understanding of gait dysfunction after stroke and highlights the potential of MINT conditioning as a wearable approach to improve walking function in stroke survivors.
Acknowledgements
We thank our participants for their valuable time and support in contributing to this study. Additionally, we express our appreciation to the members of our research team who were involved in this research study including Prashanth Prakash and Na-Teng Hung.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by National Institutes of Health Grants R01NS099210, R01NS112942 and R01AR069176.
Abbreviations
- EMG:
Electromyogram
- MINT:
Myoelectric interface for neurorehabilitation
- VL:
vastus lateralis
- RF:
rectus femoris
- VS:
vastus medialis
- AM:
adductor magnus
- BF:
biceps femoris
- TFL:
tensor fasciae latae
- MVC:
Maximum voluntary contraction
- IMU:
Inertial measurement unit
- ASIS:
Anterior superior iliac spine
- MCID:
Minimum clinically important difference
Footnotes
Competing interests
The authors declare that they do not have any conflicts of interest concerning the research, authorship, and/or publication of this article
Ethics approval and consent to participate
The study protocol was approved by the Northwestern University Institutional Review Board, and each participant gave written informed consent prior to eligibility assessment.
Contributor Information
Abed Khorasani, Northwestern University.
Joel Hulsizer, Northwestern University.
Vivek Paul, Northwestern University.
Cynthia Gorski, Northwestern University.
Yasin Y. Dhaher, University of Texas Southwestern Medical Center
Marc W. Slutzky, Northwestern University
Availability of data and materials
The data used in this study may be made available by the corresponding author upon a reasonable request.
References
- 1.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol. 2004;3:528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster A, Young J. Incidence and consequences offalls due to stroke: a systematic inquiry. BMJ. 1995;311:83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keenan MA, Perry J, Jordan C. Factors affecting balance and ambulation following stroke. Clin Orthop. 1984;165–71. [PubMed] [Google Scholar]
- 4.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech. 1999;14:125–35. [DOI] [PubMed] [Google Scholar]
- 5.Nadeau S, Arsenault AB, Gravel D, Bourbonnais D. Analysis of the clinical factors determining natural and maximal gait speeds in adults with a Stroke. Am J Phys Med Rehabil. 1999;78:123. [DOI] [PubMed] [Google Scholar]
- 6.Adams RW, Gandevia SC, Skuse NF. The distribution of muscle weakness in upper motoneuron lesions affecting the lower limb. Brain. 1990;113:1459–76. [DOI] [PubMed] [Google Scholar]
- 7.Kaji R, Osako Y, Suyama K, Maeda T, Uechi Y, Iwasaki M. Botulinum toxin type A in post-stroke lower limb spasticity: a multicenter, double-blind, placebo-controlled trial. J Neurol. 2010;257:1330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley N, Murie-Fernandez M, Speechley M, Salter K, Sequeira K, Teasell R. Does the treatment of spastic equinovarus deformity following stroke with botulinum toxin increase gait velocity? A systematic review and meta-analysis. Eur J Neurol. 2010;17:1419–27. [DOI] [PubMed] [Google Scholar]
- 9.Hesse S, Krajnik J, Luecke D, Jahnke M t., Gregoric M, Mauritz K h. Ankle Muscle Activity Before and After Botulinum Toxin Therapy for Lower Limb Extensor Spasticity in Chronic Hemiparetic Patients. Stroke. 1996;27:455–60. [DOI] [PubMed] [Google Scholar]
- 10.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev. 2004;41:293–312. [DOI] [PubMed] [Google Scholar]
- 11.Kerrigan DC, Frates EP, Rogan S, Riley PO. Hip Hiking and Circumduction: Quantitative Definitions. Am J Phys Med Rehabil. 2000;79:247. [DOI] [PubMed] [Google Scholar]
- 12.Hayes Cruz T, Dhaher YY. Evidence of Abnormal Lower-Limb Torque Coupling After Stroke. Stroke. 2008;39:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulzer JS, Gordon KE, Dhaher YY, Peshkin MA, Patton JL. Preswing Knee Flexion Assistance Is Coupled With Hip Abduction in People With Stiff-Knee Gait After Stroke. Stroke. 2010;41:1709–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing The Cost of Transport and Increasing Walking Distance After Stroke: A Randomized Controlled Trial on Fast Locomotor Training Combined With Functional Electrical Stimulation. Neurorehabil Neural Repair. 2016;30:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiozzo E, Youbi M, Dave K, Perez-Pinzon M, Rundek T, Sacco RL, et al. Aerobic, Resistance, and Cognitive Exercise Training Poststroke. Stroke. 2015;46:2012–6. [DOI] [PubMed] [Google Scholar]
- 16.Peterson CL, Hall AL, Kautz SA, Neptune RR. Pre-swing deficits in forward propulsion, swing initiation and power generation by individual muscles during hemiparetic walking. J Biomech. 2010;43:2348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanhope VA, Knarr BA, Reisman DS, Higginson JS. Frontal plane compensatory strategies associated with self-selected walking speed in individuals post-stroke. Clin Biomech. 2014;29:518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reissman ME, Gordon KE, Dhaher YY. Manipulating post-stroke gait: Exploiting aberrant kinematics. J Biomech. 2018;67:129–36. [DOI] [PubMed] [Google Scholar]
- 19.Akbas T, Prajapati S, Ziemnicki D, Tamma P, Gross S, Sulzer J. Hip circumduction is not a compensation for reduced knee flexion angle during gait. J Biomech. 2019;87:150–6. [DOI] [PubMed] [Google Scholar]
- 20.Neckel ND, Blonien N, Nichols D, Hidler J. Abnormal joint torque patterns exhibited by chronic stroke subjects while walking with a prescribed physiological gait pattern. J NeuroEngineering Rehabil. 2008;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz TH, Dhaher YY. Impact of ankle-foot-orthosis on frontal plane behaviors post-stroke. Gait Posture. 2009;30:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: Multi-factorial associations. J Biomech. 2009;42:1673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol. 2010;103:844–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan AQ, Dhaher YY. Evaluation of lower limb cross planar kinetic connectivity signatures post-stroke. J Biomech. 2014;47:949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen JL, Kautz SA, Neptune RR. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech Bristol Avon. 2013;28:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright ZA, Rymer WZ, Slutzky MW. Reducing Abnormal Muscle Coactivation After Stroke Using a Myoelectric-Computer Interface: A Pilot Study. Neurorehabil Neural Repair. 2014;28:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo G, Kishta A, Mugler E, Slutzky MW, Roh J. Myoelectric interface training enables targeted reduction in abnormal muscle co-activation. J NeuroEngineering Rehabil. 2022;19:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mugler EM, Tomic G, Singh A, Hameed S, Lindberg EW, Gaide J, et al. Myoelectric Computer Interface Training for Reducing Co-Activation and Enhancing Arm Movement in Chronic Stroke Survivors: A Randomized Trial. Neurorehabil Neural Repair. 2019;33:284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung N-T, Paul V, Prakash P, Kovach T, Tacy G, Tomic G, et al. Wearable myoelectric interface enables high-dose, home-based training in severely impaired chronic stroke survivors. Ann Clin Transl Neurol. 2021;8:1895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie JZ, Nie JW, Hung N-T, Cotton RJ, Slutzky MW. Portable, open-source solutions for estimating wrist position during reaching in people with stroke. Sci Rep. 2021;11:22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fulk GD, He Y, Boyne P, Dunning K. Predicting home and community walking activity poststroke. Stroke. 2017;48:406–11. [DOI] [PubMed] [Google Scholar]
- 32.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84. [DOI] [PubMed] [Google Scholar]
- 33.Winstein CJ, Merians AS, Sullivan KJ. Motor learning after unilateral brain damage. Neuropsychologia. 1999;37:975–87. [DOI] [PubMed] [Google Scholar]
- 34.Finley JM, Perreault EJ, Dhaher YY. Stretch reflex coupling between the hip and knee: implications for impaired gait following stroke. Exp Brain Res. 2008;188:529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study may be made available by the corresponding author upon a reasonable request.