Abstract
Comparative antibacterial efficacies of erythromycin, clarithromycin, and azithromycin were examined against Streptococcus pneumoniae and Haemophilus influenzae, with amoxicillin-clavulanate used as the active control. In vitro, the macrolides at twice their MICs and at concentrations achieved in humans were bacteriostatic or reduced the numbers of viable S. pneumoniae slowly, whereas amoxicillin-clavulanate showed a rapid antibacterial effect. Against H. influenzae, erythromycin, clarithromycin, and clarithromycin plus 14-hydroxy clarithromycin at twice their MICs produced a slow reduction in bacterial numbers, whereas azithromycin was bactericidal. Azithromycin at the concentrations achieved in the serum of humans was bacteriostatic, whereas erythromycin and clarithromycin were ineffective. In experimental respiratory tract infections in rats, clarithromycin (equivalent to 250 mg twice daily [b.i.d.]) and amoxicillin-clavulanate (equivalent to 500 plus 125 mg b.i.d., respectively) were highly effective against S. pneumoniae, but azithromycin (equivalent to 500 and 250 mg once daily) was significantly less effective (P < 0.01). Against H. influenzae, clarithromycin treatment (equivalent to 250 or 500 mg b.i.d.) was similar to no treatment and was significantly less effective than amoxicillin-clavulanate treatment (P < 0.01). Azithromycin demonstrated significant in vivo activity (P < 0.05) but was significantly less effective than amoxicillin-clavulanate (P < 0.05). Overall, amoxicillin-clavulanate was effective in vitro and in vivo. Clarithromycin and erythromycin were ineffective in vitro and in vivo against H. influenzae, and azithromycin (at concentrations achieved in humans) showed unreliable activity against both pathogens. These results may have clinical implications for the utility of macrolides in the empiric therapy of respiratory tract infections.
Erythromycin, a highly potent agent against gram-positive bacteria including streptococci, has a number of disadvantages including poor gastric stability, relatively poor potency against respiratory gram-negative pathogens such as Haemophilus influenzae, and a bacteriostatic mode of action. New macrolide antibiotics, including clarithromycin, and the first azalide antibiotic, azithromycin, have been developed to overcome these problems. Clarithromycin is more acid stable than erythromycin, giving enhanced and more reliable concentrations in serum (7) as well as fewer gastrointestinal side effects (22). It is also metabolized in humans to 14-hydroxy clarithromycin, which is more active in vitro against H. influenzae than the parent compound and has been found by some workers (5) to interact synergistically with the parent compound against this organism. In addition, clarithromycin has been reported to have bactericidal activity against Streptococcus pneumoniae (21). In vitro, azithromycin is more active than erythromycin against gram-negative bacteria, showing potentially useful activity against H. influenzae, but it is less potent against gram-positive organisms (15). The enhanced capability of azithromycin to penetrate cells leads to a concentration of up to 300-fold inside polymorphonuclear leukocytes and alveolar macrophages (25). Azithromycin concentrations in infected tissue have also been shown to be higher than those in noninfected tissue (26). The unusual pharmacokinetic properties of azithromycin mean that concentrations in serum and other extracellular fluids are prolonged but low (10). Both clarithromycin and azithromycin show cross-resistance with erythromycin (15, 19), however, suggesting that their introduction will not overcome the increasing incidence of resistance to erythromycin among key pathogens.
The effectiveness of azithromycin and clarithromycin against the important respiratory tract pathogens S. pneumoniae and H. influenzae was assessed in a number of in vitro and experimental animal studies and was compared with the effectiveness of erythromycin and amoxicillin-clavulanate.
MATERIALS AND METHODS
Compounds.
Clarithromycin and 14-hydroxy clarithromycin were supplied by Abbott Laboratories Ltd. (Kent, United Kingdom); clarithromycin was also extracted from commercially available tablets (Klaricid; Abbott). Azithromycin was supplied by Pfizer Laboratories (Kent, United Kingdom) or was used as the commercial preparation (Zithromax; Richborough Pharmaceuticals, Kent, United Kingdom). Erythromycin was used as the commercially available lactobionate or as the ethylsuccinate (Erythroped; Abbott). Amoxicillin trihydrate, sodium amoxicillin, and potassium clavulanate were supplied as laboratory reference standards by SmithKline Beecham Pharmaceuticals (Worthing, United Kingdom). All antibiotics were used as pure free-acid equivalents.
Bacterial strains.
The strains chosen for time-kill studies were the laboratory control strain S. pneumoniae ATCC 6303 and a typically susceptible β-lactamase-producing clinical isolate of H. influenzae, strain LH2803. The strains chosen for in vivo studies, S. pneumoniae 1629 and H. influenzae H128, also had typical antibiotic susceptibilities (Table 1) and were chosen for their virulence in animal models.
TABLE 1.
Antibiotic susceptibilities of the test organisms by agar dilution MIC determination
| Antibiotic | MIC (μg/ml)
|
|||
|---|---|---|---|---|
|
S. pneumoniae
|
H. influenzae
|
|||
| ATCC 6303 | 1629 | LH2803 | H128 | |
| Erythromycin | 0.03 | 0.03 | 4 | 2 |
| Clarithromycin | 0.06 | 0.03 | 8 | 8 |
| Azithromycin | 0.12 | 0.25 | 1.0 | 0.5 |
| Amoxicillin | 0.015 | 0.015 | 4 | 32 |
| Amoxicillin-clavulanatea | 0.015 | 0.015 | 1.0 | 0.5 |
Tested as a 2:1 ratio of amoxicillin:clavulanate and expressed as the concentration of amoxicillin.
MIC determinations.
Serial twofold dilutions of antibiotic were prepared in Mueller-Hinton agar (BBL) supplemented with 5% (vol/vol) sterile defibrinated horse blood for tests with S. pneumoniae and heat-lysed sterile defibrinated horse blood for tests with H. influenzae. The agar was inoculated with 4 to 5 log10 CFU of each test organism per spot and was incubated for 18 h at 37°C. The MIC was determined as the lowest concentration of antibiotic that completely inhibited visible bacterial growth.
Time-kill studies.
Antibiotic concentrations were prepared at twice the MIC for each test organism in 20-ml volumes of Todd-Hewitt broth (Oxoid) supplemented with 5% (vol/vol) human serum for S. pneumoniae and in 20-ml volumes of Mueller-Hinton broth (BBL) supplemented with 2 μg of NAD per ml and 7.5 μg of hemin per ml for H. influenzae. The media were inoculated to give 6 to 7 log10 CFU/ml and were incubated at 37°C on an orbital shaker. Samples were taken for assessment of the numbers of viable bacteria at 0, 1, 3, 5, and 7 h. Serial 10-fold dilutions of the samples were made, and four dilutions were plated in triplicate onto nutrient agar (Lab M) supplemented with 5% (vol/vol) sterile horse blood; the horse blood was heat lysed for H. influenzae. The mean numbers of CFU were determined following 24 h of incubation at 37°C.
In vitro pharmacodynamic model.
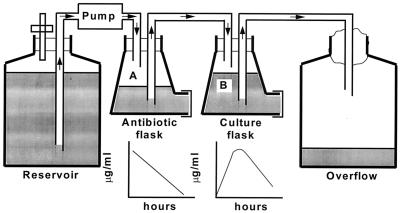
The open, one-compartment model used in the study was based on the biexponential model originally described by Grasso et al. in 1978 (13) and is shown in Fig. 1. The flow rate of the pump and the volumes in the flasks were set to simulate the elimination rate of the antibiotic with the shortest half-life (i.e., 1 h for amoxicillin and clavulanate), and the other antibiotics were supplemented at regular intervals to simulate their slower elimination from humans. The dilution rates of the bacterial cultures in the open system were therefore the same for all the test antibiotics, and the counts of viable bacteria were corrected to take this into account.
FIG. 1.
Diagrammatic representation of the in vitro pharmacodynamic model.
In the models simulating an oral dosage of clarithromycin, the concentrations of both the parent compound and the 14-hydroxy metabolite achieved in humans were mimicked. The media used were Mueller-Hinton broth (Difco) supplemented with 5% sterile, heat-treated horse serum for S. pneumoniae and brain heart infusion broth (Oxoid) supplemented with 2 μg of NAD per ml and 7.5 μg of hemin per ml for H. influenzae. Samples were removed from the culture flask at regular time points for determination of the concentration of antibiotic and the number of viable bacteria present. Viable bacterial counts were determined as in the time-kill studies, and antibiotic concentrations were assayed microbiologically.
Microbiological assays.
The macrolides were assayed by using Staphylococcus saprophyticus ATCC 9341 in blood agar base (Oxoid), which was adjusted to pH 7.8 with NaOH for azithromycin. Amoxicillin was assayed by using a commercially available Bacillus subtilis NCTC 6633 spore suspension (Difco) in nutrient agar (Lab M), and clavulanate was assayed by using Klebsiella pneumoniae NCTC 11228 in nutrient agar supplemented with 60 μg of benzylpenicillin/ml (18). H. influenzae samples containing amoxicillin were spiked with 10 μg of clavulanate/ml to prevent hydrolysis in the assay-plate wells by H. influenzae β-lactamase.
Standard solutions for the assay of plasma samples were prepared in the appropriate dilution of animal plasma (diluted in pH 7.2 phosphate-buffered saline). Samples were assayed in duplicate against standards over the concentration range of 10 to 0.078 μg/ml for the macrolides, 50 to 0.78 μg/ml for amoxicillin, and 5 to 0.078 μg/ml for clavulanate. The lowest concentration was taken as the limit of detection for the assay. The correlation coefficients for the regression lines of the standard solutions were not less than 0.997. The within-day coefficients of variation were less than 5% for erythromycin and clarithromycin, 1.1 to 7.5% for amoxicillin, 6.1 to 9.4% for clavulanate, and between 7.2 and 10.1% for azithromycin. Between-day coefficients of variation were less than 5% for erythromycin and clarithromycin, 3.5 to 8.9% for amoxicillin, 6.8 to 10.5% for azithromycin, and 6.3 to 9.8% for clavulanate.
Pharmacokinetic studies.
Compounds were administered to groups of five uninfected rats at the doses indicated in Table 2; and blood samples were taken at 5, 15, 30, 60, 90, 120, 240, and 360 min after dosing. Serum was separated by centrifugation, and antibiotic concentrations were measured by microbiological assay as described above for all compounds except amoxicillin, which was assayed by using S. saprophyticus ATCC 9341. (Within-day coefficients of variation were less than 5%, and between-day coefficients of variation were 3.7 to 6.2%. The concentration range for standards was 1 to 0.015 μg/ml.) Data were fitted with an iterative least-squares modeling program, MK-MODEL (17), and appropriate models were chosen on the basis of visual inspection and the Schwartz criterion. The areas under the concentration-time curves (AUCs) from time zero to infinity for serum were calculated by noncompartmental analysis by using the trapezoidal rule for data up to the last concentration-time point, with the remaining area to infinity calculated by dividing this point by the elimination rate constant.
TABLE 2.
Dose levels used in the rat and serum AUC values compared with those achieved in humans following conventional oral dosage
| Antibiotic | Regimen | Rat
|
Human
|
Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | t1/2 (h)a | Cmax (mg · liter−1) | AUC (mg · h · liter−1) | Dose (mg) | AUC (mg · h · liter−1) | |||
| Erythromycin | t.i.d.b | 100 | 1.1 ± 0.15 | 1.90 ± 0.23 | 3.50 ± 0.41 | 500 | 3.6 | 30 |
| Clarithromycin | b.i.d. | 20 | 1.7 ± 0.28 | 3.04 ± 0.37 | 7.94 ± 0.42 | 250 | 6.3 | 11 |
| Clarithromycin | b.i.d. | 40c | 15.88 | 500 | 15.4 | 11 | ||
| Azithromycin | o.d. | 20 | 2.87 ± 0.34 | 1.02 ± 0.07 | 4.1 ± 0.25 | 500 | 3.4 | 10 |
| Amoxicillin | b.i.d. | 200 | 0.75 ± 0.11 | 28.5 ± 5.3 | 25.2 ± 3.15 | 500 | 22.5 | 18 |
| Clavulanate | b.i.d. | 50 | 0.68 ± 0.12 | 4.03 ± 0.34 | 4.91 ± 2.86 | 125 | 5.9 | 18 |
t1/2, half-life.
t.i.d., three times daily.
Data extrapolated from a 20-mg/kg dose.
Experimental respiratory infection in rats.
Experimental respiratory infection was induced in specific-pathogen-free male Sprague-Dawley CD rats weighing 140 to 160 g (Charles River, Manston, United Kingdom) as described in a previous report (28), except that the animals were not rendered neutropenic. Briefly, the bacterial inoculum was prepared from overnight broth cultures in Todd-Hewitt broth (Oxoid) for S. pneumoniae 1629 and in nutrient broth (Oxoid) plus 5% (vol/vol) Fildes extract (Oxoid) for H. influenzae. A 10-fold dilution was prepared in molten nutrient agar (Oxoid), maintained at 40°C, immediately prior to infection. Animals were anesthetized by separate intramuscular injections of fentanyl fluanisone (Hypnorm; Janssen Pharmaceuticals, Oxfordshire, United Kingdom) and diazepam (Valium; Roche Products, Hertfordshire, United Kingdom) and were infected by intrabronchial instillation of a 50-μl inoculum (approximately 5 × 105 CFU) by means of nonsurgical intubation. Antibacterial agents (or water for untreated animals) were administered orally by gavage to groups of 8 to 10 animals, commencing 24 h postinfection and continuing for 2.5 (for S. pneumoniae) or 3 (for H. influenzae) days. The antibiotic doses used were chosen to approximate the AUC values measured in the serum of humans following therapeutic oral dosing (Table 2). At approximately 18 h after the cessation of therapy, the animals were killed and the lungs were excised and homogenized in 1 ml of isotonic saline to enumerate viable bacterial numbers.
Data are presented as means ± standard deviations.
Statistical analysis.
Statistical comparison of the data was done by the Student t test.
RESULTS
Time-kill studies.
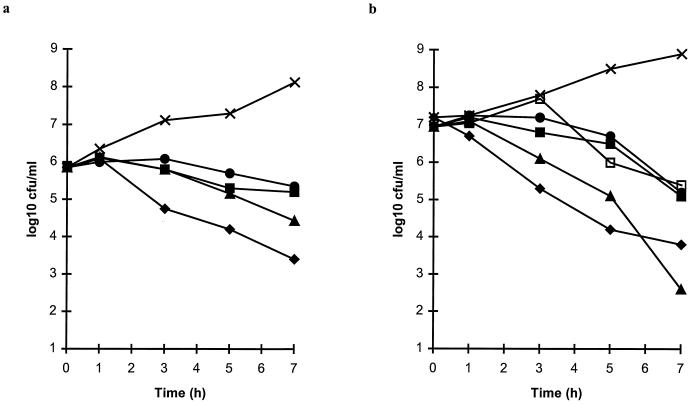
When tested at twice the MIC for S. pneumoniae ATCC 6303 (Fig. 2a), the control compound, amoxicillin-clavulanate (0.03 and 0.015 μg/ml, respectively), reduced the numbers of viable bacteria by at least 99.5% over 7 h. Erythromycin (0.06 μg/ml) and clarithromycin (0.12 μg/ml) caused a slow diminution in the numbers of viable streptococci, with less than a 10-fold decrease by 7 h. Azithromycin (0.25 μg/ml) was more effective than the other macrolides but was less active than amoxicillin-clavulanate.
FIG. 2.
Bactericidal activities of erythromycin (•), clarithromycin (■), clarithromycin plus 14-hydroxy clarithromycin (1:1) (□), azithromycin (▴), and amoxicillin-clavulanate (⧫) at twice the MICs compared with the results for untreated control cultures (×) of S. pneumoniae ATCC 6303 (a) and H. influenzae LH2803 (b).
Erythromycin (8 μg/ml) and clarithromycin (16 μg/ml) caused almost 99% reductions in the numbers of viable H. influenzae LH2803 when the drugs were tested at twice their MICs (Fig. 2b), but no difference was seen between the activities of these compounds and that of a 1:1 combination of clarithromycin and 14-hydroxy clarithromycin (8 μg/ml each). In contrast, azithromycin at 2 μg/ml (twice the MIC) showed a good bactericidal effect against H. influenzae LH2803. Although this effect was not initially as rapid as that seen with amoxicillin-clavulanate tested at the same concentration, by 7 h it was as extensive as that seen with amoxicillin-clavulanate.
In vitro pharmacodynamic model.
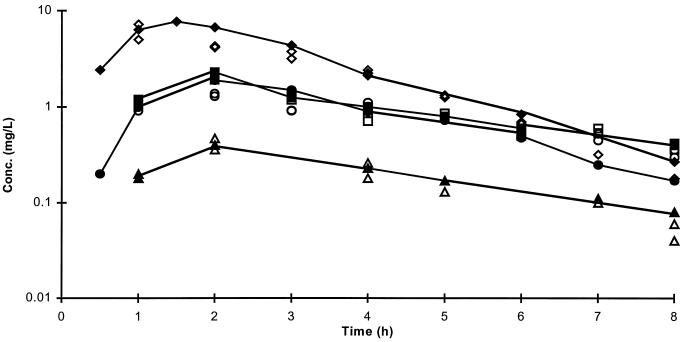
The concentrations of antibiotic simulated in the in vitro pharmacodynamic model are presented in Fig. 3. Microbiological assay results confirmed that the concentrations in the culture flasks were close to those achieved in humans. The viable bacterial counts were corrected for the dilution rate in the culture, which explains the apparently high (10 log10 CFU/ml) bacterial counts in some of the cultures.
FIG. 3.
Concentrations of antibiotic achieved in the serum of humans following the administration of oral doses of 250 mg of erythromycin (•) (21), 250 mg of clarithromycin (clarithromycin plus 14-hydroxy clarithromycin) (■) (9), 500 mg of azithromycin (▴) (11), and 500 mg of amoxicillin (⧫) (18) and concentrations achieved in the pharmacokinetic model used to simulate these data (open symbols).
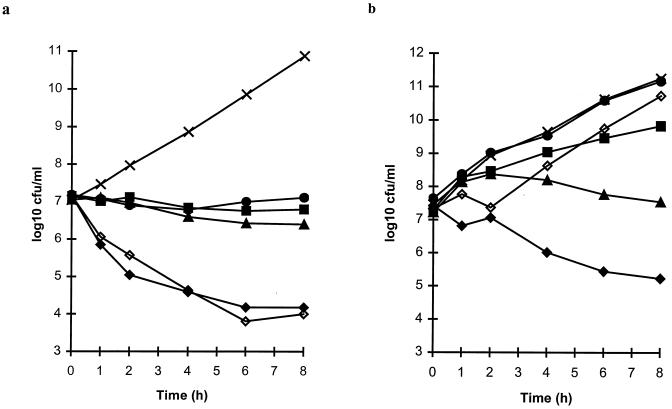
All of the macrolides showed a bacteriostatic effect against S. pneumoniae ATCC 6303 when tested at concentrations achieved in the serum of humans following the administration of oral doses of 250 mg of erythromycin, 250 mg of clarithromycin, and 500 mg of azithromycin (Fig. 4a). The model simulating the concentrations of clarithromycin in serum also contained concentrations of the 14-hydroxy metabolite measured in humans following the administration of an oral dose of 250 mg of clarithromycin, so the activity of this compound was included in the effect that was observed. The control compounds amoxicillin-clavulanate (oral doses of 500 plus 125 mg, respectively) and amoxicillin alone (500 mg) were rapidly bactericidal against S. pneumoniae ATCC 6303, causing at least 99.9% decreases in the numbers of viable bacteria by 6 h.
FIG. 4.
Bactericidal activities of simulated concentrations achieved in the serum of humans following the administration of oral doses of 250 mg of erythromycin (•), 250 mg of clarithromycin (■), 500 mg of azithromycin (▴), 500 and 125 mg of amoxicillin and clavulanate, respectively (⧫), and 500 mg of amoxicillin (◊) compared with the results for untreated control cultures (×) of S. pneumoniae ATCC 6303 (a) and H. influenzae LH2803 (b).
Against H. influenzae LH2803, the maximum concentrations of erythromycin in serum (Cmax; 1.9 μg/ml [19]) were ineffective, with the erythromycin-treated culture (erythromycin MIC, 4 μg/ml) growing as rapidly as the untreated control culture (Fig. 4b). Similarly, the simulated concentrations of clarithromycin in serum (serum Cmax, 1.5 μg of clarithromycin per ml plus 0.8 μg of 14-hydroxy clarithromycin per ml [7]) showed little activity against H. influenzae LH2803 (MICs, 8 and 4 μg/ml for the parent compound and the metabolite, respectively). Azithromycin, although initially ineffective, slowly reduced the numbers of viable bacteria from 2 h, at which time the concentration in serum peaked at 0.4 μg/ml (10). At 8 h after dosing, however, the numbers of viable bacteria had grown to the level of the starting inoculum (Fig. 4b). Amoxicillin showed an initial inhibitory effect, which was not unexpected, since the Cmax of 7.7 μg/ml was above the MIC of 4 μg/ml for this strain, but the culture regrew rapidly after 2 h. Microbiological assay results revealed that the concentration of active amoxicillin in this β-lactamase-producing culture had fallen below the limit of detection (0.1 μg/ml) by 2 h (data not shown). In contrast, the concentrations of amoxicillin in the culture of H. influenzae LH2803 treated with amoxicillin-clavulanate were close to the concentrations achieved in humans for the whole 8-h period (Fig. 3) and consequently produced a good antibacterial effect, with a 99% reduction in the numbers of viable bacteria.
In vivo studies.
The results of treatment of experimental respiratory tract infections in rats are presented in Fig. 5, 6, and 7.
FIG. 5.
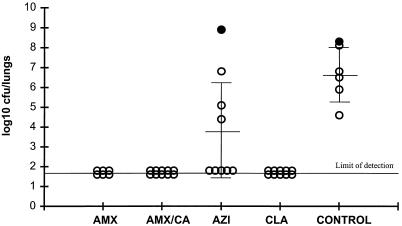
Efficacies of amoxicillin (AMX) given at 200 mg/kg b.i.d., amoxicillin-clavulanate (AMX/CA) given at 200 plus 50 mg/kg, respectively, b.i.d., azithromycin (AZI) given at 20 and then 10 mg/kg o.d., and clarithromycin (CLA) given at 20 mg/kg b.i.d. against respiratory tract infection in rats caused by S. pneumoniae 1629. Each circle represents an animal. Closed circles represent animals that died before the end of the study.
FIG. 6.
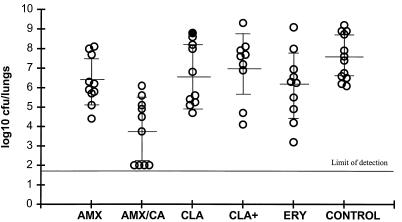
Efficacies of amoxicillin (AMX) given at 200 mg/kg b.i.d., amoxicillin-clavulanate (AMX/CA) given at 200 plus 50 mg/kg, respectively, b.i.d., clarithromycin (CLA) given at 20 mg/kg b.i.d., clarithromycin (CLA+) given at 40 mg/kg, and erythromycin (ERY) given at 100 mg/kg against respiratory tract infection in rats caused by H. influenzae H128. Each circle represents an animal. The closed circle represents an animal that died before the end of the study.
FIG. 7.
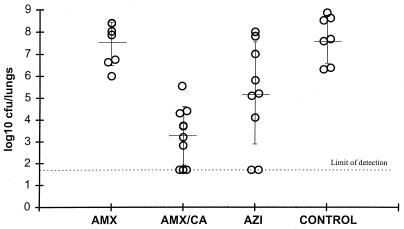
Efficacies of amoxicillin (AMX) given at 200 mg/kg b.i.d., amoxicillin-clavulanate (AMX/CA) given at 200 plus 50 mg/kg, respectively, b.i.d., and azithromycin (AZI) given at 20 and then 10 mg/kg o.d. against respiratory tract infection in rats caused by H. influenzae H128. Each circle represents an animal.
Against S. pneumoniae 1629 (a typical penicillin-susceptible, macrolide-susceptible strain), amoxicillin, amoxicillin-clavulanate, and clarithromycin were all highly effective (Fig. 5) (P < 0.01). Bacterial numbers in the lungs of the treated animals were reduced to below the limit of detection (<1.7 log10 CFU) compared with those in the lungs of animals in the untreated control group (mean, 6.6 ± 1.3 log10 CFU/lungs). In contrast, azithromycin (given at 20 mg/kg of body weight once daily [o.d.] on day 1 and at 10 mg/kg o.d. thereafter) was significantly less active than the other three treatments (P < 0.01), with a mean of 3.7 ± 2.5 log10 CFU of S. pneumoniae cultured from the lungs at 96 h.
Two studies have compared the efficacies of these agents against H. influenzae H128. In the first study, mean bacterial numbers in untreated control animals were 7.6 ± 1.1 log10 CFU/lungs (Fig. 6). Amoxicillin given at 200 mg/kg twice daily (b.i.d.) (mean, 6.3 ± 1.2 log10 CFU/lungs) was considerably less effective (P < 0.01) than amoxicillin-clavulanate (200 plus 50 mg/kg, respectively), which reduced the numbers of viable bacteria to 3.7 ± 1.7 log10 CFU/lungs. The bacterial numbers obtained following the administration of 100 mg of erythromycin per kg three times daily (6.1 ± 1.7 log10 CFU/lungs) and 20 mg of clarithromycin per kg b.i.d. (6.6 ± 1.6 log10 CFU/lungs) were significantly (P < 0.01) higher than those seen following treatment with amoxicillin-clavulanate, and this was also true with a higher dosage of clarithromycin, 40 mg/kg b.i.d. (mean, 7.0 ± 1.7 log10 CFU/lungs). In fact, both dosages of clarithromycin produced an effect which was not significantly different (P > 0.05) from that observed in the untreated control group.
In a second study with H. influenzae H128, the efficacies of amoxicillin and amoxicillin-clavulanate were compared with that of azithromycin (Fig. 7). Amoxicillin administered at 200 mg/kg b.i.d. was ineffective in reducing the numbers of viable organisms in the lungs (7.7 ± 0.96 log10 CFU/lungs), and bacterial numbers were not significantly different (P > 0.05) from those in the lungs of untreated control animals (8.0 ± 1.05 log10 CFU/lungs). Azithromycin (given at 20 mg/kg on day 1 followed by 10 mg/kg o.d.) reduced the bacterial numbers significantly compared with those in nontreated control animals (5.28 ± 2.25 log10 CFU/lungs; P < 0.05). In contrast, amoxicillin-clavulanate (given at 200 plus 50 mg/kg, respectively, b.i.d.) significantly reduced the bacterial numbers in the lungs (3.66 ± 1.4 log10 CFU/lungs) compared with the numbers in the lungs of control animals and animals in all other treatment groups (P < 0.05).
DISCUSSION
The aim of these studies was to investigate parameters other than tolerability in order to differentiate the two new macrolides, clarithromycin and azithromycin, from erythromycin and to investigate their potential utility in the eradication of the two key respiratory pathogens, S. pneumoniae and H. influenzae. In these studies amoxicillin-clavulanate was included for comparison.
Clarithromycin was essentially bacteriostatic against S. pneumoniae in our studies, which is in contrast to the bactericidal activity reported by other workers (21). Despite the bacteriostatic mode of action, clarithromycin was effective against S. pneumoniae in an experimental respiratory tract infection in rats. These data therefore indicate that clarithromycin may have good clinical efficacy when it is used as treatment against macrolide-susceptible S. pneumoniae infections. This has previously been seen with erythromycin and could be due to the clearance of the organisms by the defense system in an immunocompetent host. Nevertheless, antibiotics which inhibit rather than kill the infecting organism may allow a recurrence of infection or spread between individuals (2) and may encourage the selection of resistance.
Against H. influenzae, clarithromycin and erythromycin at twice their MICs both showed a slow diminution in viable bacterial numbers, and this was not improved for a 1:1 combination of clarithromycin plus 14-hydroxy clarithromycin. This was in contrast to the synergistic interactions reported between clarithromycin and its 14-hydroxy metabolite against H. influenzae (5) but is in agreement with the findings of other workers, who found no greater than an additive effect (24).
Neither clarithromycin nor erythromycin is highly potent in vitro against H. influenzae, with MICs higher than the Cmaxs achieved in the serum of humans following oral administration of conventional 250- or 500-mg doses. Consequently, neither of these compounds prevented the growth of H. influenzae in the in vitro pharmacodynamic model, despite the presence of concentrations of 14-hydroxy clarithromycin in serum in the model simulating those achieved after the administration of an oral dose of clarithromycin. These findings were confirmed by the in vivo studies: the effect of clarithromycin at dosages equivalent to both 250 and 500 mg b.i.d. was not significantly different from that seen from no treatment. Erythromycin was also significantly less effective than the active control, amoxicillin-clavulanate. Both strains of H. influenzae produced β-lactamase and were therefore amoxicillin resistant, but they had typical susceptibility to clarithromycin (MIC, 8 μg/ml). Therefore, they would be classified as susceptible to clarithromycin by the current National Committee for Clinical Laboratory Standards breakpoints.
Clinical trials with the recommended empiric dose of clarithromycin, 250 mg b.i.d., have also shown it to be less effective against lower respiratory tract infections attributed to H. influenzae than comparator compounds (14). The time for which the concentrations in serum are above the MIC is the most important pharmacokinetic parameter for determining the efficacies of clarithromycin and erythromycin (3). Because this value is zero for both compounds (4) against H. influenzae, these results are not unexpected. Moreover, the concentrations of clarithromycin (2.5 μg/ml) and 14-hydroxy clarithromycin (1.3 μg/ml) achieved in the middle-ear fluid of children with secretory otitis media following 5 days of oral treatment with 7.5 mg of pediatric suspension per kg b.i.d. (29) did not reach the MICs for H. influenzae (14), suggesting unreliable efficacy against otitis media infections caused by H. influenzae. This was supported by data from the clinical study (29), in which only 50% of H. influenzae infections were eradicated following treatment with clarithromycin, whereas 100% of S. pneumoniae infections were eradicated.
Azithromycin demonstrated marginally better in vitro bactericidal activity than erythromycin and clarithromycin against S. pneumoniae when the drugs were used at twice their MICs, but azithromycin was bacteriostatic at concentrations achieved in the serum of humans. The lower potency of azithromycin compared with those of erythromycin and clarithromycin against S. pneumoniae and its low levels in serum meant that the AUC:MIC ratio (considered to be the important pharmacokinetic parameter for determination of the efficacies of azalides [3]) was 20. It is thought that an AUC:MIC ratio of at least 50 is required to achieve bacterial stasis in immunocompetent animals treated with azithromycin (9), and this may explain the poor activity of azithromycin observed against the rat respiratory tract infection caused by S. pneumoniae. The dose administered to the rats gave serum AUC values equivalent to those seen in humans following the administration of an oral dose of 500 mg, and the concentrations of azithromycin achieved in the lungs of rats following administration of this dose (27) are reported to be higher than those achieved in the lungs of humans following oral administration of 500 mg (10), so the poor efficacy observed in rats is likely to reflect the clinical situation.
The good level of activity observed against H. influenzae with twice the MIC of azithromycin for H. influenzae was consistent with previously reported data (12). At concentrations simulating those achieved in the serum of humans, however, azithromycin showed no activity for the first 2 h. Once the Cmax had been reached, slow antibacterial activity was observed, even though the Cmax (0.4 μg/ml) did not reach the MIC for this strain in agar (1.0 μg/ml) and even though the AUC:MIC ratio was only 2.4. In the experimental respiratory tract infection in the rat, azithromycin showed efficacy against H. influenzae but was not as effective (when given at a dosage approximating 500 mg on the first day, followed by 250 mg o.d., in humans) as amoxicillin-clavulanate (given at dosages approximating 500 plus 125 mg, respectively, b.i.d. in humans).
These data indicate that the high intracellular concentrations of azithromycin achieved both in rats (27) and in humans (10) do not translate into good in vivo efficacy against S. pneumoniae and H. influenzae in these models. Although a number of clinical trials show equivalent efficacies of azithromycin and various comparator agents (16, 23, 31), there are also data that suggest a relatively poor response. The data presented here are consistent with those reported by Davies et al. (8) from an open clinical study of acute exacerbation of chronic bronchitis in which azithromycin failed to eradicate H. influenzae. More recently, Beghi et al. (1) reported data from a comparative clinical trial of acute exacerbation of chronic bronchitis. Those investigators found a 32% treatment failure with azithromycin at 500 mg o.d. and only a 1% treatment failure with amoxicillin-clavulanate at 875 plus 125 mg, respectively, b.i.d. The bacteriological failure rate in that study was 50% for H. influenzae infections and 30% for S. pneumoniae infections among patients treated with azithromycin and 0% for infections caused by both pathogens in the amoxicillin-clavulanate-treated group. In addition, Dagan et al. (6) have reported high bacteriological failure rates in patients with acute otitis media caused by H. influenzae following treatment with azithromycin.
In conclusion, clarithromycin showed no advantage over erythromycin in terms of either antibacterial activity or enhanced in vivo efficacy, even when it was combined with the 14-hydroxy metabolite, against S. pneumoniae or H. influenzae. The superior potency of azithromycin at twice the MIC compared with the potencies of erythromycin and clarithromycin and the antibacterial activity of azithromycin against H. influenzae were confirmed. Azithromycin was not as effective as clarithromycin against S. pneumoniae in vivo or as effective as amoxicillin-clavulanate in any of the studies against S. pneumoniae and H. influenzae. An agent with a greater potential for efficacy against both key pathogens, S. pneumoniae and H. influenzae, such as amoxicillin-clavulanate, may therefore be the preferred choice for empiric therapy of respiratory tract infections.
ACKNOWLEDGMENTS
We thank Joanna Bryant and Helen Fairclough for technical assistance.
REFERENCES
- 1.Beghi G, Berni F, Carratu L, Casalini A, Consigli G, D’Antò M, Giogia V, Molino A, Paizis G, Vaghi A. Efficacy and tolerability of azithromycin versus amoxicillin/clavulanic acid in acute purulent exacerbation of chronic bronchitis. J Chemother. 1995;7:146–152. doi: 10.1179/joc.1995.7.2.146. [DOI] [PubMed] [Google Scholar]
- 2.Craig W A. Antimicrobial resistance issues of the future. Diagn Microbiol Infect Dis. 1996;25:213–217. doi: 10.1016/s0732-8893(96)00162-9. [DOI] [PubMed] [Google Scholar]
- 3.Craig W A. The future—can we learn from the past? Diagn Microbiol Infect Dis. 1997;27:49–53. doi: 10.1016/s0732-8893(97)00022-9. [DOI] [PubMed] [Google Scholar]
- 4.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Dabernat, H., C. Delmas, M. Seguy, J. B. Fourtillan, J. Girault, and M. B. Lareng. 1991. The activity of clarithromycin and its 14-hydroxy metabolite against Haemophilus influenzae, determined by in-vitro and serum bactericidal tests. J. Antimicrob. Chemother. 27(Suppl. A):19–30. [DOI] [PubMed]
- 6.Dagan R, Leibovitz E, Jacobs M, Fliss D, Lieberman A, Yagupsky P. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Bacteriologic response to acute otitis media (AOM) caused by Haemophilus influenzae (HI) treated with azithromycin (AZ), abstr. K102; p. 345. [Google Scholar]
- 7.Davey, P. G. 1991. The pharmacokinetics of clarithromycin and its 14-OH metabolite. J. Hosp. Infect. 19(Suppl. A):29–37. [DOI] [PubMed]
- 8.Davies B I, Maesen F P V, Gubbelmans R. Azithromycin (CP-62,993) in acute exacerbations of chronic bronchitis: an open clinical, microbiological and pharmacokinetic study. J Antimicrob Chemother. 1989;23:743–751. doi: 10.1093/jac/23.5.743. [DOI] [PubMed] [Google Scholar]
- 9.Drusano, G. L., and W. A. Craig. 1997. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J. Chemother. 9(Suppl. 3):38–44. [PubMed]
- 10.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73–82. [DOI] [PubMed]
- 11.Fraschini, F., F. Scaglioni, G. Pintucci, G. Maccarinelli, S. Dugnani, and G. Demartini. 1991. The diffusion of clarithromycin and roxithromycin into nasal mucosa, tonsil and lung in humans. J. Antimicrob. Chemother. 27(Suppl. A):61–65. [DOI] [PubMed]
- 12.Goldstein, F. W., M. F. Emirian, A. Coutrot, and J. F. Acar. 1990. Bacteriostatic and bactericidal activity of azithromycin against Haemophilus influenzae. J. Antimicrob. Chemother. 25(Suppl. A):25–28. [DOI] [PubMed]
- 13.Grasso S, Meinardi G, DeCarneri I, Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother. 1978;13:570–576. doi: 10.1128/aac.13.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy D J, Guay D R P, Jones R N. Clarithromycin, a unique macrolide. A pharmacokinetic, microbiological and clinical overview. Diagn Microbiol Infect Dis. 1992;15:39–53. doi: 10.1016/0732-8893(92)90055-x. [DOI] [PubMed] [Google Scholar]
- 15.Hardy D J, Hensey D M, Beyer J M, Vojtko C, McDonald E J, Fernandes P B. Comparative in vitro activities of new 14-, 15-, and 16-membered macrolides. Antimicrob Agents Chemother. 1988;32:1710–1719. doi: 10.1128/aac.32.11.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoepleman, A. I. M., A. P. Sips, J. L. M. van Helmond, P. W. C. van Barneveld, A. J. Neve, M. Zwinkles, M. Rozenberg-Arska, and J. Verhoef. 1993. A single-blind comparison of three-day azithromycin and ten-day co-amoxiclav treatment of acute lower respiratory tract infections. J. Antimicrob. Chemother. 31(Suppl. E):147–152. [DOI] [PubMed]
- 17.Holford N. MK-MODEL, a quantitative modelling system for pharmacologists, version 5. Cambridge, United Kingdom: Elsevier-Biosoft; 1994. [Google Scholar]
- 18.Jackson D, Cooper D L, Horton R, Langley P F, Staniforth D H, Sutton J A. Absorption, pharmacokinetic and metabolic studies with Augmentin. In: Croydon E A P, Michel M F, editors. Augmentin: clavulanate-potentiated amoxycillin, Proceedings of the European Symposium. Amsterdam, The Netherlands: Excerpta Medica; 1983. pp. 83–101. [Google Scholar]
- 19.Jacobs, M. 1997. Respiratory tract infections: epidemiology and surveillance. J. Chemother. 9(Suppl. 3):10–17. [PubMed]
- 20.Josefsson K, Bergan T, Magni L. Dose-related pharmacokinetics after oral administration of a new formulation of erythromycin base. Br J Clin Pharmacol. 1982;13:685–691. doi: 10.1111/j.1365-2125.1982.tb01437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loza E, Matinez Beltran J, Baquero F, Leon A, Canton R, Garijo B the Spanish Collaborative Group. Comparative in vitro activity of clarithromycin. Eur J Clin Microbiol Infect Dis. 1992;11:856–866. doi: 10.1007/BF01960892. [DOI] [PubMed] [Google Scholar]
- 22.Neu, H. C. 1991. The development of macrolides: clarithromycin in perspective. J. Antimicrob. Chemother. 27(Suppl. A):1–9. [DOI] [PubMed]
- 23.O’Doherty, B. 1996. An open comparative study of azithromycin versus cefaclor in treatment of patients with upper respiratory tract infections. J. Antimicrob. Chemother. 37(Suppl. C):71–81. [DOI] [PubMed]
- 24.Olsson-Liljequist, B., and B.-M. Hoffman. 1991. In vitro activity of clarithromycin combined with its 14-hydroxy metabolite A-62671 against Haemophilus influenzae. J. Antimicrob. Chemother. 27(Suppl. A):11–17. [DOI] [PubMed]
- 25.Pantiex, G., B. Guillaumond, R. Harf, A. Desbos, V. Sapin, M. Leclerq, and M. Perrin-Fayolle. 1993. In-vitro concentration of azithromycin in human phagocytic cells. J. Antimicrob. Chemother. 31(Suppl. E):1–4. [DOI] [PubMed]
- 26.Retsema, J. A., J. M. Bergeron, D. Girard, W. B. Millisen, and A. E. Girard. 1993. Preferential concentration of azithromycin in an infected mouse thigh model. J. Antimicrob. Chemother. 31(Suppl. E):5–16. [DOI] [PubMed]
- 27.Shepard, R. M., and F. C. Falkner. 1990. Pharmacokinetics of azithromycin in rats and dogs. J. Antimicrob. Chemother. 25(Suppl. A):49–60. [DOI] [PubMed]
- 28.Smith G M, Abbott K H. Development of experimental respiratory infections in neutropenic rats with either penicillin-resistant Streptococcus pneumoniae or β-lactamase-producing Haemophilus influenzae. Antimicrob Agents Chemother. 1994;38:608–610. doi: 10.1128/aac.38.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundberg L, Cederberg A. Penetration of clarithromycin and its 14-hydroxy metabolite into middle ear effusion in children with secretory otitis media. J Antimicrob Chemother. 1994;33:299–307. doi: 10.1093/jac/33.2.299. [DOI] [PubMed] [Google Scholar]
- 30.Thompson P J, Burgess K R, Martin G E. Influence of food on absorption of erythromycin ethyl succinate. Antimicrob Agents Chemother. 1980;12:157–162. doi: 10.1128/aac.18.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachariah, J. 1996. A randomised, comparative study to evaluate the efficacy and tolerability of a 3-day course of azithromycin versus a 10-day course of co-amoxiclav as treatment of adult patients with lower respiratory tract infections. J. Antimicrob. Chemother. 37(Suppl):103–113. [DOI] [PubMed]