Abstract
Purpose:
This study sought to determine if disparities in insulin pump therapy among youth with type 1 diabetes (T1DM) persist despite recent increases in overall pump use rates.
Design and Methods:
All patients aged 6 months-17 years, diagnosed with T1DM, and completed 4+ outpatient diabetes visits at an academically-affiliated pediatric health care center from 2011 to 2016 were identified (n = 2131). Data were collected from existing electronic medical records and a multivariable logistic regression model was used to identify factors associated with insulin pump therapy.
Results:
Findings revealed one novel factor (patients/families whose primary language is Spanish [OR 0.47, p = 0.038] or other non-English languages [OR 0.47, p = 0.028]) and confirmed several previously known factors associated with lower insulin pump use: patients who were older (10–14 years OR 0.38, p < 0.0001; 15+ years OR 0.15, p < 0.0001), male (OR 0.80, p = 0.021), non-Hispanic black (OR 0.59, p = 0.009), American Indian/Alaska Native (OR 0.19, p = 0.023), had either government (OR 0.42, p < 0.0001) or no insurance (OR 0.52, p = 0.004) and poor glycemic control (at least one HbA1c ≥ 8.5%; OR 0.54, p < 0.0001).
Conclusion:
Significant disparities in insulin pump use in youth with T1DM persist despite known benefits associated with pump therapy and underlying causes remain unclear.
Practice Implications:
Health care providers should explore barriers to insulin pump therapy, including limited English language proficiency.
Keywords: Type 1 diabetes, Insulin pump, Health disparities, Pediatrics
Introduction
Type 1 diabetes (T1DM) is an autoimmune disease that is most frequently diagnosed in youth <18 years of age, requires complex management with exogenous insulin for life, is associated with risk for acute and chronic T1DM-related complications, and can significantly impact quality of life (Faulkner & Chang, 2007; Lawrence et al., 2012; Willi, Miller, DiMeglio, et al., 2015). Previous research has consistently shown that racial and ethnic minority youth with T1DM, those with lower levels of household income, and those who have government subsidized or no insurance have poorer glycemic control and are at elevated risk for complications compared to their counterparts with T1DM (Borschuk & Everhart, 2015; Faulkner & Chang, 2007; Lado & Lipman, 2016; Miller et al., 2015; Redondo et al., 2018; Willi et al., 2015). For example, a recent study involving 8841 participants from 31 states in the United States (US) found that even after adjusting for socioeconomic status (household income, highest parental education attained, and insurance status), non-Hispanic white youth with T1DM have significantly better glycemic control (mean HbA1c 8.4% vs. 9.6%, p < 0.001 and 8.7%, p = 0.01), monitored their blood glucose more frequently (6.1 vs. 5.4, p < 0.001 and 5.6 daily checks, p = 0.01), and were less likely to experience potentially life-threatening complications such as severe hypoglycemia (5% vs. 13%, p < 0.001 and 6%, p = 0.62 [not significant]) or diabetic ketoacidosis (DKA; 7% vs. 23%, p < 0.001 and 12%, p 0.04) than non-Hispanic black and Hispanic youth, respectively (Willi et al., 2015). Additionally, in adults who were diagnosed with T1DM as children, T1DM-related mortality rates are higher in non-Hispanic black compared to non-Hispanic white adults (p < 0.001; Secrest, Becker, Kelsey, Laporte, & Orchard, 2010).
Prior research also shows substantial disparities in insulin pump therapy among youth with T1DM despite the fact that pump use is associated with better glycemic control, fewer episodes of severe hypoglycemia, and improved quality of life (Blackman et al., 2014; Lado & Lipman, 2016; Lawrence et al., 2012; Miller et al., 2015; Misso, Egberts, Page, O'Connor, & Shaw, 2010; Paris, Imperatore, Klingensmith, et al., 2009; Sherr et al., 2016; Willi et al., 2015; Wong, Dolan, Yang, & Hood, 2015). Specifically, youth who are older, male, racial or ethnic minorities, and those with government or no insurance, lower household income, lower parental education, and one parent in the home are significantly less likely to be on insulin pump therapy (Blackman et al., 2014; Borschuk & Everhart, 2015; Faulkner & Chang, 2007; Lado & Lipman, 2016; Lin et al., 2013; Miller et al., 2015; Paris et al., 2009; Pihoker et al., 2013; Sherr et al., 2016; Valenzuela et al., 2011; Wang, Wiebe, & White, 2011; Willi et al., 2015; Wong et al., 2015). The disparities in insulin pump use are particularly concerning because the landmark Diabetes and Complications Control Trial (DCCT; The Diabetes Control and Complications Trial Research Group, 1993) established that tight glycemic control can reduce diabetes complications by up to 76% and insulin pump use is associated with better glycemic control (Blackman et al., 2014; Miller et al., 2015; Misso et al., 2010; Paris et al., 2009; Sherr et al., 2016; Willi et al., 2015; Wong et al., 2015). Evidence that established the association between insulin pump therapy and improved glycemic control in youth is based on multiple large multi-site registry and population-based studies involving 50,000+ participants from 30+ sites in the US and Europe (Blackman et al., 2014; Miller et al., 2015; Misso et al., 2010; Paris et al., 2009; Sherr et al., 2016; Willi et al., 2015; Wong et al., 2015).
While the aforementioned research has described disparities in insulin pump use among youth with T1DM and established the association with pump use and improved glycemic control, all but two studies included data from 2012 or earlier (Wong et al., 2015 did not specify years of enrollment; Blackman et al., 2014; Lin et al., 2013; Paris et al., 2009; Pihoker et al., 2013; Sherr et al., 2016; Valenzuela et al., 2011; Willi et al., 2015; Wang et al., 2011). This gap in more recent insulin pump therapy data should be addressed since overall insulin pump use has increased in recent years, which may impact known disparities (Abdullah, Pesterfield, Elleri, & Dunger, 2014; Miller et al., 2015). The purpose of the present study was to determine if disparities in insulin pump therapy among youth with T1DM persist despite recent increases in overall use rates.
Methods
Participants and Setting
This retrospective descriptive study utilized consecutive sampling to identify all patients diagnosed with T1DM and treated at an academically-affiliated pediatric quaternary health care center from September 2011–September 2016. Collectively, the large urban-based inpatient pediatric hospital and associated outpatient clinics and seven regional outpatient clinics complete >400,000 total patient visits annually. Patients were included in the study if they were 6 months −17 years old and were treated on at least one occasion at any of eight locations affiliated with the center during the study period. Prior to conducting study activities, a waiver of consent was obtained from the institutional review board of the pediatric health care center.
Data Collection
All data were collected from existing electronic medical records (EMRs) of the pediatric health care center. Data were extracted from the EMR system and de-identified by a member of the health care center's Enterprise Analytics team. Age was collected in years at the time of the first encounter of the study period and patients were defined as having T1DM if T1DM-associated primary or secondary ICD-9 (250.xx) or ICD-10 codes (E10.xx) were identified in the EMR. Additionally, because youth diagnosed with T1DM do not frequently initiate insulin pump therapy in the first year after diagnosis, we further identified patients who have completed four or more outpatient visits (approximately 1 year) as noted in the EMR for analysis of factors associated with insulin pump use (Lin et al., 2013). Because a single field that identified insulin pump use did not exist in the EMR, we used insulin pump-related orders as a proxy for insulin pump use, defined by the presence of at least one of as the following: 1) order for an insulin pump, 2) order for insulin pump supply sets, or 3) order for insulin that included either directions to be used in an insulin pump or directions for use in the case of insulin pump failure. Patients were excluded if any key variables (age, gender, race, ethnicity, language, health insurance type, HbA1c) were missing.
Data including race, ethnicity, insurance type (a proxy for household income), and glycemic control as measured by hemoglobin A1c (HbA1c) were extracted from discrete fields within the EMR. Race and ethnicity were classified as non-Hispanic white, Hispanic, non-Hispanic Black, Asian/Pacific Islander, American Indian/Alaska Native, and more than one race based on existing EMR classifications. Health insurance type was classified as commercial (private), government, and charity/self-pay (no insurance). Poor glycemic control was defined as at least one HbA1c ≥ 8.5% during the study period based on prior research (Abdullah et al., 2014) and American Diabetes Association (ADA, 2018) recommendations for target HbA1c < 7.5% for all pediatric age groups. Data were also collected for patient/family language. Language was classified as English, Spanish, or Other. For the purposes of analysis, we chose to collapse all non-English and non-Spanish languages into a single ‘Other’ category because the number of these preferred languages individually were small and collectively accounted for less than half of all non-English-speaking patients.
Data Analysis
Descriptive statistics including means, standard deviations and proportions were calculated for all independent variables. A multivariable logistic regression model was then used to identify factors associated with insulin pump use. For each independent variable analyzed in the model, all other variables were held constant to determine the effect of each variable independently. Reference groups for each variable were chosen based on interpretability. Independent variables included in the models were selected a priori based on previous research (Blackman et al., 2014; Borschuk & Everhart, 2015; Faulkner & Chang, 2007; Lado & Lipman, 2016; Lin et al., 2013; Miller et al., 2015; Paris et al., 2009; Pihoker et al., 2013; Sherr et al., 2016; Wang et al., 2011; Willi et al., 2015; Wong et al., 2015) and results were reported as odds ratios (OR) with estimated 95% confidence intervals (CI). Significance testing was done at the α = 0.05 level. SAS 9.4 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
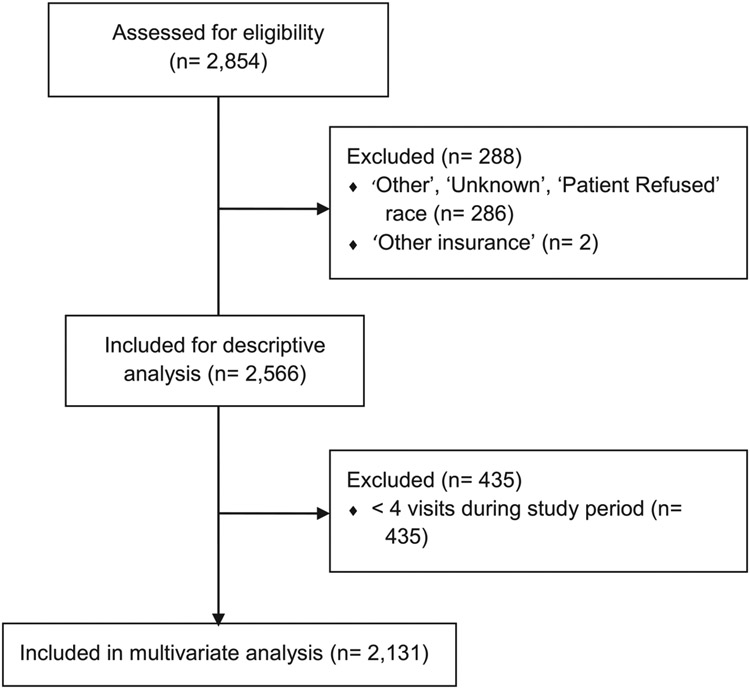
The initial sample identified 2854 patients seen during the study period. After review, 286 were excluded for having ‘Other’, ‘Refused’, or ‘Unknown’ race and/or ethnicity and two were excluded for having ‘other insurance’. The final sample size was 2566 for all patients and 2131 for patients who completed 4+ outpatient visits (see Fig. 1). Mean age at initial encounter was 11.4 years (standard deviation [SD], 4.3), and the majority of patients were male (52.5%), non-Hispanic white (77.6%), spoke English as their primary language (94.8%), and had commercial/private health insurance (70.1%; see Table 1). The socio-demographic variables were similar for patients who completed ≥4 visits and for all patients diagnosed with T1DM during the study period, although the proportion of patients with at least one HbA1c ≥ 8.5% was 6.6% higher in those who completed ≥4 outpatient visits.
Fig. 1.
Inclusion diagram.
Table 1.
Demographic characteristics and glycemic control for all pediatric patients (0–17 years) treated for type 1 diabetes and for patients with 4+ visits.
| All patients n (%) |
4+ visits n (%) |
Pump use, 4+ visits n (%) |
|
|---|---|---|---|
| N | 2566 | 2131 | 1316 (61.8) |
| Age (years), mean (SD) | 11.7 (4.6) | 11.4 (4.3) | 10.3 (4.2) |
| 0–4 years | 188 (7.3) | 139 (6.5) | 118 (8.9) |
| 5–9 years | 693 (27.0) | 603 (28.3) | 469 (35.6) |
| 10–14 years | 987 (38.5) | 890 (41.8) | 534 (40.5) |
| 15+ years | 697 (27.2) | 499 (23.4) | 195 (14.8) |
| Sex | |||
| Male | 1364 (53.2) | 1119 (52.5) | 670 (50.9) |
| Female | 1202 (46.8) | 1012 (47.5) | 647 (49.1) |
| Race and ethnicity | |||
| Non-Hispanic White | 1999 (77.9) | 1654 (77.6) | 1077 (81.8) |
| Hispanic | 222 (8.7) | 190 (8.9) | 86 (6.5) |
| Non-Hispanic Black | 173 (6.7) | 138 (6.5) | 63 (4.8) |
| >1 Race | 86 (3.4) | 74 (3.5) | 49 (3.7) |
| Asian or Pacific Islander | 72 (2.8) | 62 (2.9) | 39 (2.9) |
| American Indian or Alaskan Native | 14 (0.5) | 13 (0.6) | 3 (0.2) |
| Language | |||
| English | 2440 (95.1) | 2020 (94.8) | 1281 (97.3) |
| Spanish | 71 (2.8) | 61 (2.9) | 18 (1.4) |
| Other | 55 (2.1) | 50 (2.3) | 18 (1.4) |
| Insurance | |||
| Commercial | 1798 (70.1) | 1505 (70.6) | 1029 (78.2) |
| Government | 659 (25.7) | 525 (24.6) | 240 (18.2) |
| Charity/self-pay | 109 (4.2) | 101 (4.7) | 48 (3.7) |
| Mean A1c (SD) | 8.9 (1.8) | 9.0 (1.8) | 8.7 (1.6) |
| At least one HbA1c ≥ 8.5 | |||
| No | 988 (38.5) | 679 (31.9) | 503 (38.2) |
| Yes | 1578 (61.5) | 1452 (68.1) | 814 (61.8) |
Multivariable logistic regression analysis revealed several statistically significant factors that were associated with insulin pump use (see Table 2). Older patients (vs. 5–9 years, n = 139; 10–14 years, n = 890, OR 0.38, 95% CI [0.30, 0.49], p < 0.0001; 15+ years, n = 499, OR 0.15, 95% CI [0.11, 0.20], p < 0.0001) and male patients (n = 1119) (vs. female, n = 1012, OR 0.80, 95% CI [0.66, 0.97], p = 0.021) were less likely to use an insulin pump. Compared to non-Hispanic white youth (n = 1654), non-Hispanic Black youth (n = 138; OR 0.59, 95% CI [0.36, 0.88], p = 0.009) and American Indian/Alaska Native youth (n = 13) were significantly less likely to be on insulin pump therapy (OR 0.19, 95% CI [0.05, 0.79], p = 0.023). Compared to those whose primary language is English (n = 2020), patients/families whose primary language is Spanish (n = 61; OR 0.47, 95% CI [0.23, 0.96], p = 0.038) or other non-English language (n = 50; OR 0.47, 95% CI [0.24, 0.92], p = 0.028) were about half as likely to be on insulin pump therapy. Patients with either government (n = 525; OR 0.42, 95% CI [0.33, 0.54], p < 0.0001) or no insurance (n = 101; charity/self-pay, OR 0.52, 95% CI [0.33, 0.81], p = 0.004) were also about half as likely to be on insulin pump therapy compared to patients with commercial/private health insurance (n = 1505). Finally, patients with at least one HbA1c ≥ 8.5% (n = 1452) were less likely to use an insulin pump compared to patients with better glycemic control (n = 679; OR 0.54, 95% CI [0.44, 0.68], p < 0.0001).
Table 2.
Multivariable model for insulin pump usage in pediatric patients with 4+ visits.
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Age group | |||
| 5–9 years (Reference) | – | – | – |
| 0–4 years | 1.57 | 0.92, 2.68 | 0.098 |
| 10–14 years | 0.38 | 0.30, 0.49 | <0.0001*** |
| 15+ years | 0.15 | 0.11, 0.20 | <0.0001*** |
| Sex | |||
| Female (Reference) | – | – | – |
| Male | 0.80 | 0.66, 0.97 | 0.021* |
| Race and ethnicity | |||
| Non-Hispanic White (Reference) | – | – | – |
| Hispanic | 0.71 | 0.47, 1.06 | 0.091 |
| Non-Hispanic Black | 0.59 | 0.36, 0.88 | 0.009** |
| >1 Race | 1.05 | 0.62, 1.80 | 0.853 |
| Asian/Pacific Islander | 1.14 | 0.61, 2.11 | 0.687 |
| American Indian or Alaskan Native | 0.19 | 0.05, 0.79 | 0.023* |
| Language | |||
| English (Reference) | – | – | – |
| Spanish | 0.47 | 0.23, 0.96 | 0.038* |
| Other | 0.47 | 0.24, 0.92 | 0.028* |
| Insurance | |||
| Commercial (Reference) | – | – | – |
| Government | 0.42 | 0.33, 0.54 | <0.0001*** |
| Charity/self-pay | 0.52 | 0.33, 0.81 | 0.004** |
| At least one A1C ≥ 8.5 | |||
| No (Reference) | – | – | – |
| Yes | 0.54 | 0.44, 0.68 | <0.0001*** |
Note. Results significant at the following levels: * = p < 0.05; ** = p < 0.01; *** = p < 0.0001.
Discussion
This study sought to determine whether disparities in insulin pump provision persist in light of increased overall pump use in recent years (Abdullah et al., 2014; Miller et al., 2015). Retrospective EMR data from 2011 to 2016 do show an increase in overall pump use (61.8%) compared to earlier registry and population-based data (use rates ranged from 22.0% in the early-mid 2000s to 56.3% in 2012; Blackman et al., 2014; Paris et al., 2009) and are similar to multi-site registry data collected in 2013–14 (Miller et al., 2015; Willi et al., 2015). The present study identified one new factor that is significantly associated with lower insulin pump use rates not previously identified: patients/families with non-English preferred languages. Results also confirmed several other factors previously associated with lower rates of insulin pump use: those who are older (> 10 years), male, racial and ethnic minorities, and have no or government sponsored insurance. Insulin pump use was also significantly associated with better glycemic control (HbA1c levels <8.5%). While overall pump use has increased across all groups, the disparities between sociodemographic groups remain. For example, national (Lin et al., 2013; Paris et al., 2009; Pihoker et al., 2013; Willi et al., 2015) and international (Sherr et al., 2016) studies similarly found that older age and male sex were associated with lower rates of insulin pump use. Prior studies that explored race and ethnicity in insulin pump therapy among youth have also uniformly concluded that non-Hispanic black and other racial and ethnic minority youth were significantly less likely to use an insulin pump compared with non-Hispanic white youth (Borschuk & Everhart, 2015; Lin et al., 2013; Miller et al., 2015; Paris et al., 2009; Pihoker et al., 2013; Sherr et al., 2016; Wang et al., 2011; Willi et al., 2015; Wong et al., 2015). In fact, in their study of nearly 9000 youth from 60 clinical sites in the US, Willi et al. (2015) found that even at the highest household income levels (≥ $100,000/per year) non-Hispanic white youth were still significantly more likely than non-Hispanic black youth (73% vs. 45%, respectively; p < 0.001) to use an insulin pump. The approximately 20% gap in overall pump use rates between non-Hispanic white youth and non-Hispanic black youth in this study (65.1% of non-Hispanic white youth vs. 45.7% of non-Hispanic black youth) has remained unchanged since 2009 (Paris et al.; 26.3% of non-Hispanic white youth vs. 5.3% of non-Hispanic black youth), but is far lower than the gap identified by Willi et al. (2015; 61% of non-Hispanic white youth vs. 26% of non-Hispanic black youth). As in the present study, having no or government health insurance has also been significantly associated with lower rates of insulin pump use in previous research (Lin et al., 2013; Pihoker et al., 2013; Paris et al., 2009; Wong et al., 2015). Finally, better glycemic control as evidenced by lower HbA1c levels has been widely associated with insulin pump use compared to multiple daily insulin injections (Blackman et al., 2014; Borschuk & Everhart, 2015; Faulkner & Chang, 2007; Paris et al., 2009; Pihoker et al., 2013; Sherr et al., 2016; Wong et al., 2015).
Patients/families whose primary language is English were significantly more likely to use an insulin pump than those who primarily speak non-English languages in the current study. To our knowledge, patient/family language has not been explored in previous research involving insulin pump use in youth with T1DM. While data on factors such as household income, parental education, family structure, and T1DM duration were not available in the present study because of retrospective EMR data collection, it is notable that previous research has established an association between insulin pump use and higher levels of household income (Blackman et al., 2014; Borschuk & Everhart, 2015; Lin et al., 2013; Paris et al., 2009; Pihoker et al., 2013; Shulman et al., 2017; Willi et al., 2015), higher levels of parental education (Blackman et al., 2014; Paris et al., 2009; Pihoker et al., 2013), having 2 parents in the home (Wong et al., 2015), and longer T1DM duration (Blackman et al., 2014; Paris et al., 2009).
The present study adds to the body of evidence that has identified sociodemographic variables associated with insulin pump use in youth with T1DM but identifying the factors that contribute to these and other health disparities is complex, inter-related, hard to measure, and includes both patient/family and provider factors (Blair, Steiner, & Havranek, 2011; FitzGerald & Hurst, 2017; Hall et al., 2015; Institute of Medicine [IOM], 2002; The Joint Commission, 2016). Although the research remains equivocal, significant evidence suggests that provider factors such as implicit (or unconscious) bias also contribute to persistent health disparities based on a number of identities (ability, age, gender identity, sexual orientation, level of income/education, language, immigration status, housing status, race/ethnicity, weight, and others) and across multiple health care settings (e.g. cardiology, emergency medicine, pediatrics, oncology, mental health, primary care, surgery) (FitzGerald & Hurst, 2017; Hall et al., 2015; IOM, 2002; The Joint Commission, 2016; Willi et al., 2015). A recent systematic review (Hall et al., 2015) showed that provider implicit bias is “significantly related to patient-provider interactions, treatment decisions, treatment adherence, and patient health outcomes”. In the context of disparities in insulin pump use, the fact that statistically significant variation in use rates exist between clinical sites in several large US and European studies suggest that provider factors do play a role (Blackman et al., 2014; Lin et al., 2013; Paris et al., 2009; Pihoker et al., 2013; Sherr et al., 2016; Shulman et al., 2017). Additionally, among non-pump users in the study by Commissariat et al. (2017), patients/families with higher annual household income (≥$75,000; p = 0.02) and those with longer T1DM duration (p = 0.02) were significantly more likely to be recommended pump therapy by their providers.
However, health disparities are multifaceted and are also influenced by patient/family factors and preferences. For example, in the present study those with commercial/private insurance (a proxy for higher levels of income) may have greater exposure/use of technology and therefore may be more receptive to pump use (Commissariat et al., 2017). Logistical barriers (e.g. transportation, time requirements, child care) likely dissuade some patients/families from attending trainings that must be completed prior to the start of insulin pump therapy. While we were unable to identify evidence of this in the literature, there is significant evidence that describes logistical barriers for other non-essential clinic visits such as those required for research participation (Aristizabal et al., 2015; Buseh, Stevens, Millon-Underwood, Kelber, & Townsend, 2017; George, Duran, & Norris, 2014). Insulin pump use may also be influenced by perceived barriers to use among parents. The top five perceived barriers to pump use among parents of young children (<7 years old) with T1DM in a recent registry study surprisingly did not include financial concerns but instead focused on other concerns: 1) wearing the pump would be uncomfortable, 2) would interfere with sports/activities, 3) difficulty of use of set/tubing, 4) increased risk of hypoglycemia, and 5) insertion site reactions (Commissariat et al., 2017).
A requisite first step to addressing health disparities is to describe the disparities, which this study and prior research have done in the context of insulin pump use among youth with T1DM. Additional research in this area is now necessary to explore the various factors contributing to these disparities. For example, a study that collects data from patients/families regarding if/when they were offered insulin pump therapy and reasons they accepted or declined therapy would greatly add to knowledge in this area. This will allow providers to identify and address perceived or actual barriers to insulin pump therapy. Given the substantial evidence indicating that provider factors also play a role in disparities, further research to test interventions that may reduce provider impact is also needed. van Ryn et al. (2011) recommend that nurses and other healthcare providers practice 1) perspective-taking, 2) emotional regulation, and 3) partnership-building to minimize the potential effect of implicit bias on health care and outcomes, but to our knowledge the efficacy of employing these strategies have yet to be tested.
Practice Implications
It is essential that members of the diabetes care team be aware of continuing disparities in pediatric insulin pump use based on sociodemographic factors. Factors that are significantly associated with lower rates of insulin pump use include being older (≥10 years old), identifying as a racial or ethnic minority or male, having primary languages other than English, and having government insurance even though government-sponsored health plans cover insulin pump therapy. We did not explore potential causes of disparities in insulin pump therapy orders in the present study; however, prior evidence suggests that health care provider/staff bias contributes to persistent health disparities based on race, ethnicity and other socio-demographic factors (FitzGerald & Hurst, 2017; Hall et al., 2015; IOM, 2002; The Joint Commission, 2016; Willi et al., 2015). The issue of provider bias is particularly salient with regard to the present study because we collected data on insulin pump-related orders from diabetes providers as a proxy for insulin pump use. The overall quality of diabetes care can be improved and the effects of implicit biases can potentially be mitigated if nurses and other health care providers practice 1) perspective-taking, 2) emotional regulation, and 3) partnership-building (van Ryn et al., 2011). Perceived or actual patient/family barriers and preferences also likely play a role in insulin pump use rates (Commissariat et al., 2017). Nurses are particularly well-positioned to 1) provide education for patients/families regarding the benefits of insulin pump therapy, 2) help navigate logistical challenges that patients/families might encounter, and 3) advocate for patients/families within the diabetes care team.
Limitations
While the use of retrospective data is cost-effective and relatively easy to access, this method of data collection is subject to missing data and restricted by existing classifications and available data in the EMR (e.g. unable to account for date of T1DM diagnosis or combining Asian and Pacific Islander in one race category; Peabody, Luck, Glassman, Dresselhaus, & Lee, 2000). Thus, we were unable to account for other known factors that are associated with pump use, such as parental education or household income (we used health insurance type as a proxy for income). To avoid bias from imputed data, we excluded patients with missing data (n = 288). However, given this was a substantial number of patients, excluding them could potentially impact study findings. This is especially true in instances where samples sizes were quite small (e.g. American Indian/Alaska Native patients, n = 13). Another important limitation is the inability to identify if patients/families were offered insulin pump therapy but declined this option. Patients/families may decline insulin pump therapy for personal reasons as noted above and it is likely that logistical barriers, such as transportation, time away from work, and/or child care may hinder the ability for some families to complete required trainings prior to pump therapy initiation. Finally, given the retrospective study design we were unable to identify patients who may have discontinued insulin pump therapy during the study period; however, existing literature suggests this is an uncommon occurrence (Shulman et al., 2017; Wong et al., 2015).
Conclusion
Despite the fact that insulin pump therapy can improve glycemic control, decrease episodes of severe hypoglycemia, and improve quality of life, disparities in insulin pump use based on sociodemographic factors continue to exist in youth with T1DM. These disparities persist even with overall increases in pump use rates (Abdullah et al., 2014; Miller et al., 2015). The present study identified a novel factor significantly associated with lower insulin pump use: patients/families who have primary languages other than English. The study also adds to the existing body of comparative research showing that youth who are older (≥10 years old), identify as a racial or ethnic minority or male, and have government or no insurance are significantly less likely to use an insulin pump. Multiple factors likely contribute to these and other health care disparities including actual or perceived barriers to insulin pump use by patients/families and provider factors such as implicit bias (FitzGerald & Hurst, 2017; Hall et al., 2015; The Joint Commission, 2016; Willi et al., 2015). Future research is needed to both understand the factors contributing to the disparities in insulin pump use in youth with T1DM and to develop interventions to reduce these disparities in an effort to improve health equity and outcomes.
Acknowledgments
Funding: This research was supported by the Institute for Translational Health Sciences Rising Stars Program (UL1TR000423, NIH/NCATS/CTSA).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest.
References
- Abdullah N, Pesterfield C, Elleri D, & Dunger DB (2014). Management of insulin pump therapy in children with type 1 diabetes. Archives of Disease in Childhood. Education and Practice Edition, 99(6), 214–220. 10.1136/archdischild-2013-304501. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2018). Children and adolescents: Standards of medical care in diabetes—2018. Diabetes Care, 41(Supplement 1), S126–S136. 10.2337/dc18-S012. [DOI] [PubMed] [Google Scholar]
- Aristizabal P, Singer J, Cooper R, Wells KJ, Nodora J, Milburn M, … Martinez ME (2015). Participation in pediatric oncology research protocols: Racial/ethnic, language and age-based disparities. Journal of Pediatric Blood Cancer, 62(8), 1337–1344. 10.1002/pbc.25472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackman SM, Raghinaru D, Adi S, Simmons JH, Ebner-Lyon L, … DiMeglio LA (2014). Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatric Diabetes, 15(8), 564–572. 10.1111/pedi.12121. [DOI] [PubMed] [Google Scholar]
- Blair IV, Steiner JF, & Havranek EP (2011). Unconscious (implicit) bias and health disparities: Where do we go from here? The Permanente Journal, 15(2), 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borschuk AP, & Everhart RS (2015). Health disparities among youth with type 1 diabetes: A systematic review of the current literature. Families, Systems & Health, 33(3), 297–313. 10.1037/fsh0000134. [DOI] [PubMed] [Google Scholar]
- Buseh AG, Stevens PE, Millon-Underwood S, Kelber ST, & Townsend L (2017). Embracing an “African Ethos” to facilitate African immigrants participation in medical genetics and genomics research. Nursing Outlook, 65(1), 9–17. 10.1016/j.outlook.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Commissariat PV, Boyle CT, Miller KM, Mantravadi MG, DeSalvo DJ, … Anderson BJ (2017). Insulin pump use in young children with type 1 diabetes: Sociodemographic factors and parent-reported barriers. Diabetes Technology & Therapeutics, 19(6), 363–369. 10.1089/dia.2016.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner MS, & Chang LI (2007). Family influence on self-care, quality of life, and metabolic control in school-age children and adolescents with type 1 diabetes. Journal of Pediatric Nursing, 22, 59–68. 10.1016/j.pedn.2006.02.008. [DOI] [PubMed] [Google Scholar]
- FitzGerald C, & Hurst S (2017). Implicit bias in healthcare professionals: A systematic review. BMC Medical Ethics, 18(1), 19. 10.1186/s12910-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Duran N, & Norris K (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health, 104(2), e16–e31. 10.2105/ajph.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, … Coyne-Beasley T (2015). Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: A systematic review. American Journal of Public Health, 105(12), e60–e76. 10.2105/AJPH.2015.302903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (2002). Unequal treatment: Confronting racial and ethnic disparities in health care. Retrieved from http://www.nationalacademies.org/hmd/Reports/2002/Unequal-Treatment-Confronting-Racial-and-Ethnic-Disparities-in-Health-Care.aspx.
- Lado JJ, & Lipman TH (2016). Racial and ethnic disparities in the incidence, treatment, and outcomes of youth with type 1 diabetes. Endocrinology and Metabolism Clinics of North America, 45(2), 453–461. 10.1016/j.ecl.2016.01.002 2016. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Yi-Frazier JP, Black MH, Anderson A, Hood K, … Seid M (2012). Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. Journal of Pediatrics, 161(2), 201–7.e2. 10.1016/j.jpeds.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Connor CG, Ruedy KJ, Beck RW, Kollman C, … Wood JR (2013). Race, socioeconomic status, and treatment center are associated with insulin pump therapy in youth in the first year following diagnosis of type 1 diabetes. Diabetes Technology & Therapeutics, 15(11), 929–934. 10.1089/dia.2013.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose S, … Tamborlane WV (2015). Current state of type 1 diabetes treatment in the U.S.: Updated data from the T1D Exchange clinic registry. Diabetes Care, 38(6), 971–978. 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- Misso ML, Egberts KJ, Page M, O'Connor D, & Shaw J (2010). Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database of Systematic Reviews, 1, CD005103. 10.1002/14651858.CD005103.pub2. [DOI] [PubMed] [Google Scholar]
- Paris CA, Imperatore G, Klingensmith G, et al. (2009). Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: The SEARCH for Diabetes in Youth study. The Journal of Pediatrics, 155(2), 183–189 e181 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Peabody JW, Luck J, Glassman P, Dresselhaus TR, & Lee M (2000). Comparison of vignettes, standardized patients, and chart abstraction: A prospective validation study of 3 methods for measuring quality. Journal of the American Medical Association, 283(13), 1715–1722. [DOI] [PubMed] [Google Scholar]
- Pihoker C, Badaru A, Anderson A, Morgan T, Dolan L, & Klingensmith GJ (2013). Insulin regimens and clinical outcomes in a type 1 diabetes cohort: The SEARCH for Diabetes in Youth study. Diabetes Care, 36(1), 27–33. 10.2337/dc12-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo MJ, Libman I, Cheng P, Kollman C, Tosur M, … Clements M, … for the Pediatric Diabetes Consortium (2018). Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care, 41(5), 1017–1024. 10.2337/dc17-2335. [DOI] [PubMed] [Google Scholar]
- van Ryn M, Burgess DJ, Dovidio JF, Phelan SM, Saha S, … Perry S (2011). The impact of racism on clinical cognition, behavior, and clinical decision-making. Du Bois Review, 8(1), 199–218. 10.1017/S1742058X11000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secrest AM, Becker DJ, Kelsey SF, Laporte RE, & Orchard TJ (2010). Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes, 59(12), 3216–3222. 10.2337/db10-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr JL, Hermann JM, Campbell F, Foster NC, Hofer SE, … Warner JT (2016). Use of insulin pump therapy in children and adolescents with type 1 diabetes and its impact on metabolic control: Comparison of results from three large, transatlantic paediatric registries. Diabetoiogia, 59(1), 87–91. 10.1007/s00125-015-3790-6. [DOI] [PubMed] [Google Scholar]
- Shulman R, Stukel TA, Miller FA, Newman A, Daneman D, & Guttmann A (2017). Insulin pump use and discontinuation in children and teens: A population-based cohort study in Ontario, Canada. Pediatric Diabetes, 18(1), 33–44. 10.1111/pedi.12353. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control and Complications Trial Research Group (1993). The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine, 329(14), 977–986. [DOI] [PubMed] [Google Scholar]
- The Joint Commission. Quick Safety (2016). Implicit bias in health care. Quick safety Retrieved from https://www.jointcommission.org/assets/1/23/Quick_Safety_Issue_23_Apr_2016.pdf. [Google Scholar]
- Valenzuela JM, La Greca AM, Hsin O, et al. (2011). Prescribed regimen intensity in diverse youth with type 1 diabetes: Role of family and provider perceptions. Pediatric Diabetes, 12(8), 696–703. 10.1111/j.1399-5448.2011.00766.x. [DOI] [PubMed] [Google Scholar]
- Wang JT, Wiebe DJ, & White PC (2011). Developmental trajectories of metabolic control among White, Black, and Hispanic youth with type 1 diabetes. The Journal of Pediatrics, 159, 571–576. 10.1016/j.jpeds.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Willi SM, Miller KM, DiMeglio LA, et al. (Mar 2015). Racial-ethnic disparities in management and outcomes among children with type 1 diabetes. Pediatrics, 135(3), 424–434. 10.1542/peds.2014-177425687140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JC, Dolan LM, Yang TT, & Hood KK (2015). Insulin pump use and glycemic control in adolescents with type 1 diabetes: Predictors of change in method of insulin delivery across two years. Pediatric Diabetes, 16(8), 592–599. 10.1111/pedi.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]