Abstract
Daily oral treatment with the cyclopentyl 2′-deoxyguanosine nucleoside BMS-200475 at doses ranging from 0.02 to 0.5 mg/kg of body weight for 1 to 3 months effectively reduced the level of woodchuck hepatitis virus (WHV) viremia in chronically infected woodchucks as measured by reductions in serum WHV DNA levels and endogenous hepadnaviral polymerase activity. Within 4 weeks of daily therapy with 0.5 or 0.1 mg of BMS-200475 per kg, endogenous viral polymerase levels in serum were reduced about 1,000-fold compared to pretreatment levels. Serum WHV DNA levels determined by a dot blot hybridization technique were comparably decreased in these treated animals. In the 3-month study, the sera of animals that had undetectable levels of WHV DNA by the dot blot technique were further analyzed by a highly sensitive semiquantitative PCR assay. The results indicate that BMS-200475 therapy reduced mean WHV titers by 107- to 108-fold, down to levels as low as 102 to 103 virions/ml of serum. Southern blot hybridization analysis of liver biopsy samples taken from animals during and after BMS-200475 treatment showed remarkable reductions in the levels of WHV DNA replicative intermediates and in the levels of covalently closed circular viral DNA. WHV viremia in BMS-200475-treated WHV carriers eventually returned to pretreatment levels after therapy was stopped. These results indicate that BMS-200475 should be evaluated in clinical trials for the therapy of chronic human hepatitis B virus infections.
Human hepatitis B virus (HBV), the prototype member of a small family of hepadnaviruses, is an enveloped DNA virus with a partially double-stranded 3.2-kb circular genome. HBV infection is widespread and is a major cause of human liver disease (4). While primary human HBV infections are usually self-limiting, 6 to 10% of infected adults and a higher percentage of children become chronically infected carriers who face a high incidence of sequellae such as liver cirrhosis and hepatocellular carcinoma.
Efforts to combat persistent HBV infection via antiviral chemotherapy (18, 29, 47) have focused on the viral replication cycle (36). Briefly, HBV replication uses a virally encoded polymerase with reverse transcriptase activity to convert a greater-than-genome-length 3.5-kb pregenomic RNA (pgRNA) intermediate into the characteristic double-stranded DNA genome. This process occurs inside cytoplasmic viral nucleocapsids. Most nucleocapsids bearing matured HBV DNA genomes then acquire an outer envelope coat at the endoplasmic reticulum to form virions which exit the cell via the secretory pathway. However, by mechanisms that remain to be clarified, a fraction of the newly replicated HBV genomes are recycled to the cell nucleus where they give rise to a small population of supercoiled covalently closed circular viral DNA (cccDNA) species (43). cccDNA serves as the template for the synthesis of further pgRNA transcripts (45), thus fueling additional cycles of hepadnaviral replication.
So far, a few nucleoside analogs have emerged as the most promising inhibitors of HBV replication, most notably lamivudine (3TC) and famciclovir, a prodrug of penciclovir. These compounds are active against HBV both in cultured cells (10, 20, 25) and in ongoing clinical trials (1, 9, 33). Limitations of these nucleosides, however, include rapid rebounds in HBV viremia once therapy ends and the emergence of drug-resistant mutant viruses (2, 3, 28, 30, 42). It is also not clear that nucleosides can eliminate the reservoir of long-lived cccDNA (14, 32). Thus, the need remains for additional agents for the antiviral chemotherapy of HBV.
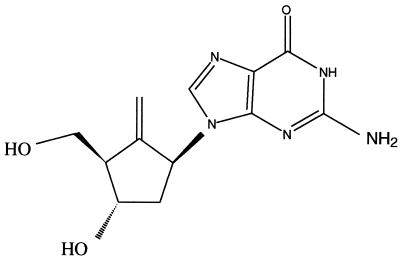
BMS-200475 (formerly SQ 34676) (5) is a cyclopentyl 2′-deoxyguanosine nucleoside analog (Fig. 1) with excellent potency and selectivity against HBV (5, 20). In the stably transfected HBV-expressing HepG2.2.15 cell line, BMS-200475 inhibits the replication of HBV with a 50% effective concentration of 3.75 nM (20). This inhibition was reversed upon withdrawal of compound and was accompanied by marked reductions in the levels of intracellular HBV DNA genomes and viral replicative intermediates, suggesting that BMS-200475 is an inhibitor of the HBV polymerase. Mechanism-of-action studies confirmed (37) that the triphosphate form of BMS-200475 directly inhibits hepadnaviral polymerases in vitro.
FIG. 1.
Chemical structure of BMS-200475.
The efficacy of BMS-200475 against hepadnavirus infection in woodchucks (Marmota monax) chronically infected with woodchuck hepatitis virus (WHV) was determined. This animal model (35, 40) of HBV infection is widely accepted and has proved to be useful for the evaluation of antiviral agents directed against HBV (7, 11, 13, 15, 19, 30, 34, 35, 39, 41). Daily oral administration of BMS-200475 at doses as low as 0.02 mg/kg of body weight markedly reduced serum WHV DNA levels in woodchucks with no evidence of toxicity at the dose levels used.
MATERIALS AND METHODS
Animals.
All experimental procedures involving woodchucks were reviewed and approved by the Animal Care and Use Committee of the Wallingford, Conn., site of the Pharmaceutical Research Institute of Bristol-Myers Squibb Co. Woodchucks chronically infected with WHV were obtained from Marmotech Inc. (Cortland, N.Y.). At weekly intervals, animals were weighed, anesthetized by intramuscular injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), and bled from the femoral vein for serum samples which were stored at −20°C. Liver biopsies were performed on animals anesthetized with ketamine-xylazine supplemented with isofluorane inhalation as needed. Liver slices of 0.5 to 1.5 cm were aseptically removed via a ventrolateral incision over the liver, snap frozen in liquid nitrogen, and stored at −70°C prior to analysis for WHV DNA (see below).
Compounds.
BMS-200475 was synthesized as described previously (5) and was prepared as a 1-mg-per-ml stock solution in pyrogen-free sterile water. 3TC was purchased from Glaxo-Wellcome (Research Triangle Park, N.C.) and was prepared similarly. For oral delivery, each drug solution was mixed with a liquid diet formulation (Liquid Woodchuck Control Diet; Dyets Inc., Bethlehem, Pa.) so as to achieve the desired dose (based on individual-animal body weights) in a final volume of 5 ml. Placebo-treated animals received liquid diet alone.
Determination of circulating WHV DNA.
The effectiveness of drug therapy was determined from the reductions in serum WHV viremia relative to pretreatment levels, as measured in serum samples collected during and after therapy. Depending on the experiment, WHV viremia was determined by one or more of three different methods: by WHV DNA dot blot hybridization (26, 27), by assay for virion-associated endogenous DNA polymerase activity (31), or by a semiquantitative PCR (23).
WHV DNA dot blot hybridization analyses were performed with serum viral DNA released via alkali treatment (26, 27). Fifty-microliter aliquots of alkali-treated serum were filtered onto a 1.2-μm-pore-size nylon membrane (Biodyne Nylon 66; VWR Scientific, Philadelphia, Pa.) with a 96-well manifold filtration apparatus (The Convertible; GIBCO-BRL, Grand Island, N.Y.). The dried membrane was probed with a randomly 32P-labeled genomic WHV DNA probe (specific activity, ∼2 × 109 cpm/mg of DNA) derived from plasmid pWHV8 (American Type Culture Collection, Rockville, Md.). pWHV8 contains the entire 3.3-kb WHV genome ligated in the unique EcoRI restriction site of pBR325. Following hybridization for 16 h at 55°C and washing to remove unbound radioactive probe, signal intensities were quantitated with a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). A calibration curve was prepared by using twofold dilutions of a WHV plasmid DNA reference standard in clarified WHV-negative woodchuck serum. The lower limit of detection of WHV DNA by this method was ∼10 to 30 pg per dot. Results are given as the average of duplicate assays in picograms per milliliter.
The amount of replication-competent WHV virions present in serum samples was estimated by an endogenous polymerase assay (EPA) adapted to a 96-well assay format (37). Since WHV virions are mature, i.e., have reached the end stage of WHV DNA replication, this assay measures the synthesis of mainly second-strand DNA by the WHV polymerase (8). Concentrated WHV virions, partially freed of serum components, were prepared for this assay from ∼500-μl aliquots of thawed serum which were clarified by low-speed centrifugation (10,000 × g for 20 min at 4°C). Virions were pelleted through 25% (wt/vol) sucrose–TNE (10 mM Tris hydrochloride [pH 7.4], 160 mM NaCl, 1 mM EDTA) plus 0.75% Triton X-100 at 550,000 × g and 4°C for 1 h in a tabletop ultracentrifuge (Beckman Instruments, Palo Alto, Calif.). The virions were resuspended in TNE at 1/10 of the original serum volume and 20-μl aliquots were assayed in duplicate in a 96-well plate at 37°C for 14 h. Each assay contained (per well) 75 mM NH4Cl, 50 mM Tris-HCl (pH 7.4), 20 mM MgCl2, 0.1% Tween 20, 100 μg of tRNA per ml, 4μm (each) dGTP, dCTP, and TTP, 20 μl of 10×-concentrated WHV virions (equal to 200 μl of serum), and 1 μCi (10 nM) of [α-33P]dATP (2,000 Ci/mmol; NEN, Boston, Mass.). Following the reaction, virions bearing labeled DNA were precipitated with chilled 10% trichloroacetic acid–1% sodium pyrophosphate, collected on glass fiber filter plates (Unifilter 96; Packard, Meriden, Conn.), washed extensively with water and then ethanol, and quantified by liquid scintillation counting (TopCount; Packard) in the presence of scintillation cocktail (Microscint O; Packard). The data are presented as the percentage of incorporated dAMP in the test sample relative to that in a pretreatment sample from the same animal.
For the analysis of circulating viral genomic DNA by semiquantitative PCR, 200-μl portions of selected woodchuck sera were first treated for 1 h at 37°C with 1 U of DNase I (Boehringer Mannheim, Mannheim, Germany) per ml in the presence of 10 mM MgCl2 to remove traces of contaminating free DNA (i.e., DNA that is not packaged in virions). Virion-associated DNA was then deproteinized by treatment with 3 mg of proteinase K (Boehringer Mannheim) per ml–0.5% sodium dodecyl sulfate (SDS) for 4 to 12 h at 56°C. Following several extractions with phenol-chloroform, viral DNA was precipitated with ethanol in the presence of 0.3 M sodium acetate and 40 μg of glycogen (Boehringer Mannheim) and resuspended in 80 μl of H2O–0.1% bovine serum albumin.
Semiquantitative PCRs were performed essentially as described previously (23), with the inclusion of a Southern blot hybridization step to improve the sensitivity of detection. The WHV core region spanning nucleotides 1901 to 2351 was amplified with the forward primer 5′-GAGGAGGGCAGCATTGAT-3′ and the reverse primer 5′-ATTCTTGAACTGTATGTT-3′. Portions (15 μl) of the amplified products were electrophoresed through 2% agarose and were transferred by capillary action onto a Hybond N membrane (Amersham, Gieselweg, Germany). WHV DNA was disclosed via Southern blot hybridization (38) with a 32P-radiolabeled genomic WHV probe derived from plasmid pWHV2 (kindly provided by Stephan Menne and Michael Roggendorf, University of Essen, Essen, Germany) for 3 to 5 h at 65°C in 10 ml of RapidHyb solution (Amersham). After several high-stringency washes to remove unbound probe, the dried membranes were analyzed with a FUJIX BAS 1000 Phosphorimager and TINA software (Raytest).
A WHV reference serum sample containing approximately 7 × 1010 WHV genomes/ml was used to calibrate the PCRs and to quantitate the amount of WHV DNA (in genome equivalents per milliliter of serum) present in the original serum samples. Serial 10-fold dilutions of selected test sera and the WHV reference serum, containing from 105 to 1 WHV genome equivalents per sample, were analyzed by PCR as described above. Results for the diluted WHV reference serum were used to generate a standard WHV genome concentration curve from which the WHV DNA content of the serum samples was estimated. The values given are the means of at least three independent PCR and hybridization experiments. The detection limit of the modified PCR and hybridization analysis was estimated to be 2 × 102 to 2 × 103 WHV genome equivalents/ml of serum.
Analysis of liver DNA.
WHV DNA was prepared from infected livers as described by Yang et al. (46). Briefly, ∼200-mg portions of liver tissue were homogenized with a Dounce homogenizer in 1 ml of ice-cold 10 mM Tris-HCl–1 mM EDTA (pH 8.0) (TE buffer). For cccDNA extraction, 0.5 ml of homogenate was mixed with an equal volume of 4% SDS followed by 0.25 volumes of 2.5 M KCl to precipitate complexes of chromosomal DNA, protein, and detergent, which were removed by centrifugation (microcentrifuge, 16,000 × g, 10 min, room temperature). The supernatant was deproteinized by extraction with Tris-HCl (pH 7.5)-buffered phenol followed by phenol-chloroform-isoamyl alcohol. Nucleic acids were precipitated with 1 volume of isopropyl alcohol at room temperature and were collected by centrifugation (16,000 × g, 15 min, room temperature). The pellets were rinsed once with 70% ethanol, air dried, and resuspended in TE buffer.
Nucleocapsid-associated viral replicative intermediates were prepared from the remaining 0.5 ml of homogenate by adding Nonidet P-40 to a final concentration of 0.5%. After 30 min on ice, nuclei and cellular debris were removed by microcentrifugation (16,000 × g, 10 min). The supernatants were adjusted to 5 mM magnesium acetate and 50 μg of DNase I per ml and were then incubated at 37°C for 30 min to degrade unencapsidated DNA. The replicative intermediates were released from viral cores by incubation for 1 h at 37°C following the sequential addition of 10 mM EDTA, 1% SDS, 0.1 M NaCl, and 0.5 mg of proteinase K per ml and were then deproteinized with phenol and precipitated as described above.
Prior to electrophoresis (see below) the cccDNA and replicative intermediate samples were treated with DNase-free RNase A (10 μg/ml). Portions (20 μg) of nucleic acid were electrophoresed through a 1% agarose-Tris-borate-EDTA gel and then depurinated, denatured, and transferred to a Magnagraph nylon membrane (Micron Separations Inc., Westborough, Mass.) as described by Southern (38). Immobilized WHV DNA species were disclosed with a 32P-labeled, full-length WHV genomic DNA probe (specific activity, 2 × 109 cpm/μg) prepared with a random primer kit (Life Technologies, Grand Island, N.Y.). The WHV probe was derived from plasmid pCMV82, which was a gift from Tim Block, Thomas Jefferson University, Philadelphia, Pa.
RESULTS
One-month dose-ranging study.
To establish an effective antiviral dose for BMS-200475 in the woodchuck, an initial study examined the levels of circulating WHV virions during 4 weeks of single daily per os drug treatment and 4 weeks of follow-up. Three groups of WHV carrier woodchucks were given BMS-200475 at daily doses of 0.5 (n = 5 animals), 0.1 (n = 6), and 0.02 (n = 6) mg per kg of body weight. The fourth group (n = 5) provided the placebo treatment control. The animals were monitored continuously throughout the 8-week study, and serum samples were collected weekly. The WHV virion content of each sample was determined by measuring the virion-associated polymerase activity (Fig. 2) by EPA (31). No weight loss or any other evidence of toxicity was apparent with BMS-200475 treatment at any time during the study.
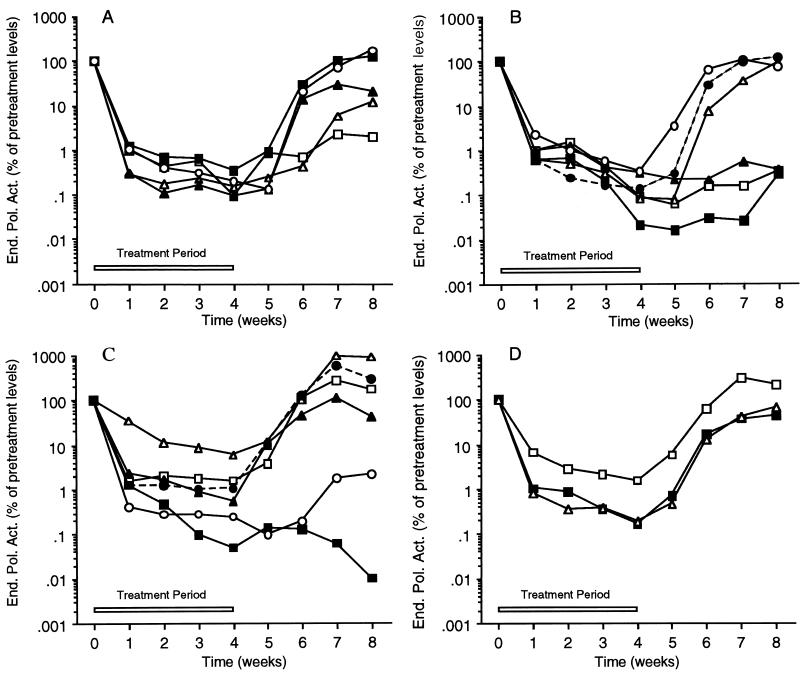
FIG. 2.
Serum WHV levels in animals treated for 1 month with BMS-200475 as measured by EPA. Data for individual animals are presented in panels A to C. Animals were treated per os with the following daily dosages of BMS-200475: 0.5 (A), 0.1 (B), and 0.02 (C) mg/kg/day. (D) Mean EPA values obtained for each of the three groups treated with BMS-200475 at 0.02 (□), 0.1 (■), and 0.5 (▵) mg/kg/day. End. Pol. Act., endogenous polymerase activity.
EPAs were performed with sera collected from the BMS-200475-treated WHV carrier woodchucks to week 4 of the follow-up period (Fig. 2). The data for individual animals are presented for the groups receiving doses of 0.5 (Fig. 2A), 0.1 (Fig. 2B), and 0.02 (Fig. 2C) mg/kg, as are the mean values for each group (Fig. 2D); for each respective animal (or group), the EPA values are presented relative to the pretreatment levels (arbitrarily set to 100%).
Virion-associated WHV polymerase activity was effectively reduced by all three BMS-200475 dose regimens, which can be compared most readily via the mean EPA values (Fig. 2D). The 0.5- and 0.1-mg/kg doses appear to be essentially equivalent, suppressing EPA activity ∼2 logs or 99% after 1 week of dosing and ∼3 logs (to about the detection limit of this method) by the end of the 4-week treatment period. The 0.02-mg/kg dose gave a lower but still respectable suppression (almost 2 logs at week 4), indicating that the therapeutic effect of BMS-200475 is titratable. All three dose groups showed significant rebounds in EPA activity within 2 weeks of the end of therapy. As expected, the EPA levels of placebo-treated control animals did not change during the 8 weeks of the study (data not shown).
Examination of the EPA profiles for individual animals revealed that the highest dose of BMS-200475 elicited a quite uniform response among the animals. With decreasing dose, the spread or variation among individual animals appeared to increase. It was also apparent that the rebound in polymerase activity following drug withdrawal was delayed in one to three animals per group.
Similar results were also obtained when the serum samples from this study were analyzed for WHV DNA by a dot blot hybridization technique (26, 27). Results of these preliminary studies (data not shown) indicated that the results from the WHV DNA dot blot assay and the EPA were quite comparable, confirming the recent finding (7) that both methods are suitable for the quantification of relative viral loads. Since the EPA method is cumbersome and required more serum, a DNA dot blot hybridization approach was used to monitor the remaining animals in the studies whose results are presented in this report.
Three-month efficacy study.
On the basis of the results of the dose-ranging study, the 0.1- and 0.02-mg/kg/day dosages of BMS-200475 were evaluated in an oral dosing study comprising 12 weeks of daily dosing and an additional 12 weeks of follow-up (Fig. 3). This study included an oral 3TC treatment group for comparison. Two groups of animals received BMS-200475 at 0.1 mg/kg/day (n = 7) or 0.02 mg/kg/day (n = 6). A third group (n = 5) received 5 mg of 3TC per kg once a day. The placebo group (n = 4) was given liquid diet without drug. Serum samples, collected at intervals of 1 to 4 weeks, were initially assayed by dot blot hybridization for serum WHV DNA. As was the case for the prior 4-week study, there were no signs of weight loss or other evidence of any toxicity in any treatment group during the entire 6-month observation period.
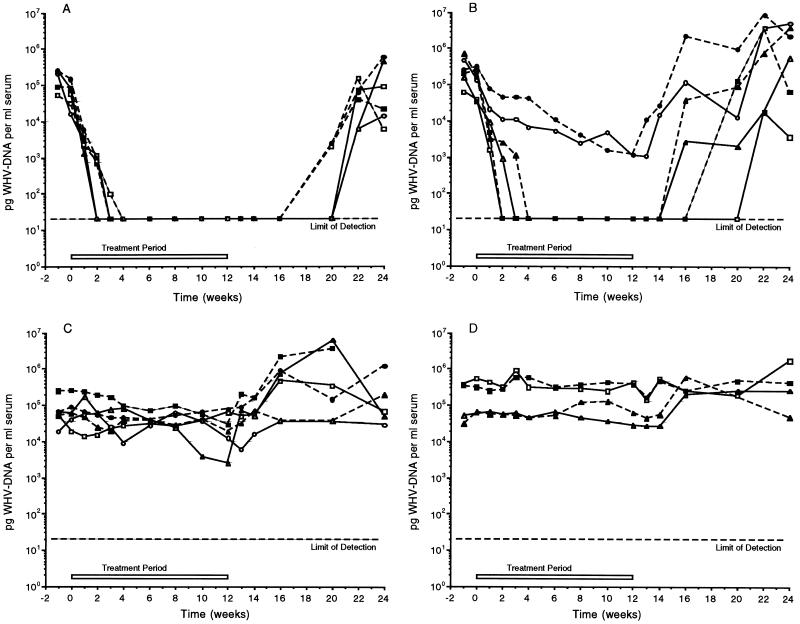
FIG. 3.
Serum WHV DNA levels following daily oral administration of BMS-200475 or 3TC for 3 months. Shown are WHV DNA dot blot data. The treatment regimens and the individual animals treated were as follows: (A) 0.1 mg of BMS-200475/kg per day; animals 94-053 (□), 94-141 (■), 94-313 (▵), 95-153 (▴), 94-146 (○), and 95-321 (•); (B) 0.02 mg of BMS-200475/kg per day; animals 94-163 (□), 95-341 (■), 95-204 (▵), 95-323 (▴), 95-422 (○), and 95-012 (•); (C) 5 mg of 3TC/kg per day; animals 95-093 (□), 95-181 (▴), 95-182 (▵), 95-423 (○), 95-411 (■), and 95-414 (▵); (D) placebo-treated animals; animals 95-111 (□), 94-144 (▴), 95-152 (▵), and 94-423 (○). See text for further details.
In the group receiving BMS-200475 at a dosage of 0.1 mg/kg/day (Fig. 3A), the sera of all seven animals became WHV DNA negative by dot blot analysis within 2 to 4 weeks of treatment and remained negative for the duration of treatment. The WHV DNA titers in the sera of most animals were reduced approximately 1,000-fold before falling to the detection limit of the assay, which was estimated to be ∼2 × 106 genomes per ml. Interestingly, viremia continued to be undetectable by the dot blot assay for at least 8 weeks beyond the end of therapy (to week 20) in all six animals. Three animals became detectably viremic 8 weeks posttreatment (i.e., at week 20), while the remaining three animals were not positive for WHV DNA until week 22. At the conclusion of the study, the WHV viremia in three animals was still below pretreatment levels.
Consistent with the results of the prior 4-week study, BMS-200475 at 0.02 mg/kg/day was also effective in reducing viremia (Fig. 3B), although this dosage was less effective than the higher dosage. After 2 to 4 weeks of therapy, four of the six animals in this group became negative for viral DNA, reflecting a >1,000-fold suppression in circulating virus. These four animals remained negative for WHV DNA for significant time periods after the completion of drug treatment at week 12 of the study. Viral DNA first became detectable at week 16 in two animals, at week 18 in another animal, and at week 22 in the final animal. The two treated animals that failed to become negative for WHV DNA did exhibit progressive declines in serum WHV DNA levels of approximately 100-fold by the end of therapy. The onset of viral DNA rebound was rapid for these animals. By the end of the study only one of the six animals in the 0.02-mg/kg group had a viral DNA load that appeared to be significantly (∼1 log) below pretreatment levels.
Unexpectedly, in light of its success in human clinical trials at comparable doses (9, 33), 3TC administered at 5 mg/kg daily had essentially no depressive effect on serum WHV DNA levels (Fig. 3C). Several animals appeared to exhibit elevated WHV DNA levels after 3TC therapy was halted. The apparent inefficacy of 3TC therapy is discussed later in this report. As expected, the WHV DNA levels in the placebo-treated control animals were unchanged over the 6-month trial period (Fig. 3D).
It was apparent that BMS-200475 was highly effective in reducing circulating WHV DNA levels in WHV carriers as measured by DNA dot blot analysis. The 0.1-mg/kg/day oral dosage rendered 100% of the test animals negative for viral DNA during the 12 weeks of therapy, while the 0.02-mg/kg dose achieved the same result in four of six animals. At both doses, the absence of detectable viremia was sustained for up to 10 weeks postdosing in many of the animals.
PCR analysis of samples from the 3-month study.
As noted above, 11 of the 13 animals receiving BMS-200475 became WHV DNA negative within the first 2 to 4 weeks of therapy, as judged by DNA dot blot assay. For most of these animals WHV titers fell some 3 or even 4 logs to reach the lower limit of detection of the dot blot method (about 2 × 106 genomes per ml). To address whether BMS-200475 therapy could reduce WHV viremia levels to below this level in the 12-week dosing study, a semiquantitative PCR method (23) with a Southern hybridization readout that significantly increases sensitivity (detection limit, about 2 × 102 to 2 × 103 genome equivalents per ml) was used. PCR analyses were performed with selected WHV DNA dot blot-negative sera from the 12-week study described above. Each analysis was verified and quantified by means of a calibration curve derived from a reference standard sample of known WHV virion titer (see Materials and Methods). To ensure that the DNA dot blot and PCR methods gave comparable estimates for the DNA content of a given sample, extensive comparative studies were conducted with some of the weakly viremic samples from the 12-week study described above. The two methods were found to be in good agreement, provided that the serum samples were treated with DNase I prior to the PCR assay. This treatment presumably eliminates contaminating trace amounts of “naked” WHV DNA which can generate artifactually high DNA estimates via PCR.
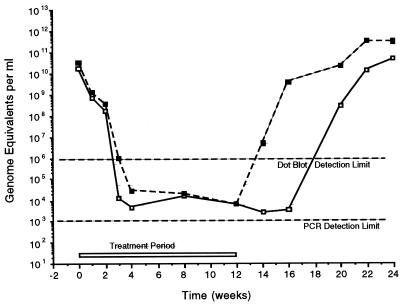
Figure 4 shows the serum WHV DNA profile that emerged when the data obtained by the dot blot approach (see above) were combined with the PCR data for selected dot blot-negative sera. To simplify the comparison between the groups receiving BMS-200475 at 0.1 and 0.02 mg/kg, only the mean DNA values (in WHV genome equivalents per milliliter of serum) are presented. It should be noted that the data for the two animals from the group receiving 0.02 mg of BMS-200475 per kg and whose circulating WHV DNA was not suppressed to the lower limit of the dot blot assay were not included in this comparison. Thus, the results for the 0.02-mg/kg group in Fig. 4 suggest that this dose is more effective than it really was.
FIG. 4.
Mean serum WHV DNA levels following daily oral administration of BMS-200475 for 3 months. Data reflect combined results of dot blot and PCR analyses of serum samples from the 10 woodchucks that responded to BMS-200475 treatment with undetectable levels of WHV DNA as determined by the dot blot assay. Not included are data for two animals receiving 0.02 mg of BMS-200475 per kg/day, in which reductions in WHV DNA levels were about 2 log units. ■, 0.02 mg/kg per day; □, 0.1 mg/kg per day. See text for further details.
With the caveat given above, the composite profile reveals that both dosing regimens gave broadly comparable results during the treatment phase. During the first 4 weeks of therapy, mean viral DNA titers plunged sharply from the pretreatment values of 2 × 1010 to 3 × 1010 to about 3 × 104 or even 2 × 103 to 3 × 103 genome equivalents per ml of serum. These levels did not appear to decrease much further during the remaining 8 weeks of treatment. The biggest distinction between the two cohorts came in the rebound phase, during which mean virus levels remained suppressed for 2 weeks longer in the 0.1-mg/kg group than in the 0.02-mg/kg group.
From this PCR analysis it can be concluded that BMS-200475 therapy reduced WHV viremia by 107- to 108-fold from the pretreatment baseline level down to levels that are fully 103- to 104-fold below those measurable by the dot blot assay. Although the WHV titers were reduced to below 2 × 102 genome equivalents per ml for extended periods in certain individual animals, this PCR-negative status was transient. In no instance did 12 weeks of BMS-200475 therapy eradicate WHV viremia.
BMS-200475 reduces liver WHV DNA species.
Since BMS-200475 therapy dramatically decreased the levels of circulating viral DNA, it was determined whether the levels of WHV DNA species present in infected hepatocytes were similarly reduced. Cells infected with hepadnaviruses contain two categories of extrachromosomal viral DNA species (6, 36, 43, 46). The nucleus carries unencapsidated cccDNA supercoils which act as the template for the production of pgRNA transcripts, while the cytoplasm harbors a smear of nucleocapsid-associated hepadnaviral replicative intermediates that result from the reverse transcription of pgRNA in hepatocytes. This smear includes circular and linear double-stranded WHV DNA species, as well as shorter single-stranded DNA species.
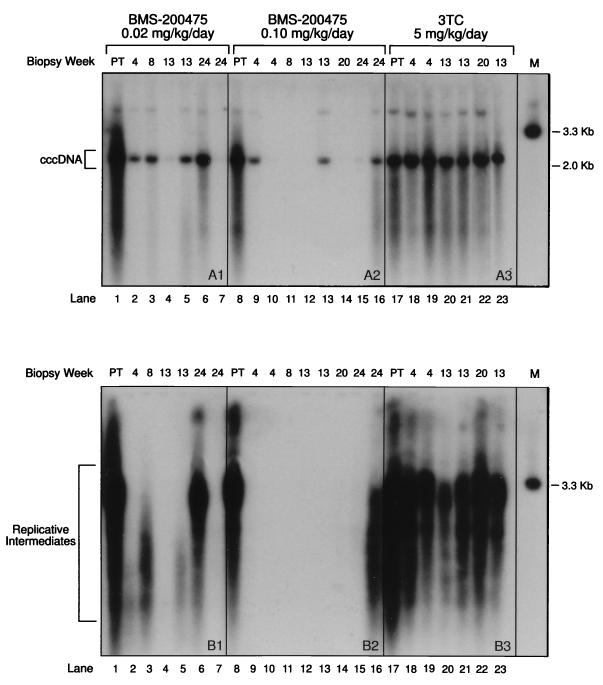
The effect of drug treatment on WHV cccDNA and DNA replicative intermediates was assessed with wedge liver biopsy specimens obtained from woodchucks from the 12-week study described above. The animals were biopsied at roughly 4-week intervals during the entire 12-week treatment and 12-week followup periods. Because animal welfare considerations did not allow for repeated biopsy of individual animals, liver samples were collected from different animals over the course of the study. The resulting data thus reflect the efficacy of the drug with time throughout the cohort of treated animals. Half of each liver sample was processed to obtain cccDNA, while the remaining half was processed to isolate replicative intermediates (see Materials and Methods). Agarose gel electrophoresis and Southern hybridization with a radiolabeled double-stranded WHV genomic DNA probe were used to disclose the cccDNA (Fig. 5A1 to A3) species (which migrates with a size of ∼2 kb) and the corresponding smear of replicative species (Fig. 5B1 to B3).
By comparison with the pretreatment samples (lanes 1, 8, and 17), BMS-200475 dosages of 0.02 (Fig. 5A1 and B1) and 0.1 (Fig. 5A2 and B2) mg/kg/day markedly reduced the levels of both intrahepatic WHV DNA species in woodchucks. In contrast, in the group receiving 3TC at 5 mg/kg/day, little or no suppression of WHV cccDNA was shown (Fig. 5A3) and only a modest overall decrease in replicative intermediates was found (Fig. 5B3). The decrease in replicative intermediates was most marked for one animal at 13 weeks, shortly posttherapy. Overall, these results accord well with those of the serum DNA analyses described above and indicate that BMS-200475 is a more effective anti-WHV agent than 3TC at the doses tested.
Closer inspection of the results of therapy with BMS-200475 at 0.1 mg/kg/day reveals that the replicative intermediates became essentially undetectable in the majority of woodchucks (Fig. 5B2) by treatment week 4 and that their levels recovered only marginally by week 20 or even week 24 (lanes 13 to 15), 8 to 10 weeks beyond the end of dosing. Remarkably, cccDNA (Fig. 5A2) was also undetectable in four animals from this group at anywhere from 4 to 20 weeks (lanes 10 to 12 and 14). However, two animals showed only modest reductions in cccDNA at weeks 4 and 13 (lanes 9 and 13) respectively), suggesting that individual animals may vary significantly in their response to low doses of drug. In the two animals sampled at 24 weeks, the trace to moderate levels of cccDNA observed (lanes 15 and 16, respectively) are suggestive of the onset of viral rebounds.
The dosing regimen with BMS-200475 at 0.02 mg/kg/day clearly reduced the levels of both cccDNA (Fig. 5A1) and the replicative intermediates (Fig. 5B1). However, the reductions were less dramatic and there were more animal-to-animal variations compared to those found among animals in the higher-dose group. Thus, only two of six livers showed substantial reductions in the levels of both cccDNA and the corresponding replicative intermediates (lanes 4 and 7). Some of the livers showed evidence of short replicative DNA species (Fig. 5B1), presumably representing prematurely terminated WHV DNA chains, which were not seen in the livers of animals receiving the higher drug dose. This finding accords with prior studies (20, 37) in suggesting that BMS-200475 treatment leads to the premature termination of HBV DNA chains. By week 24, one animal had rebounded fully (lane 6), but another animal (lane 7) remained essentially negative for both cccDNA and replicative intermediates.
In summary, the findings from the studies examining WHV DNA in the livers further confirm the antiviral efficacy of BMS-200475 in the animal model of WHV infection. As was seen from the analysis of circulating viral DNA, the higher dosage of BMS-200475 (0.1 mg/kg/day) appeared more effective in reducing liver WHV DNA markers. However, both doses markedly reduced the levels of WHV cccDNA and replicative intermediates within as little as 4 weeks of therapy. In some animals, this suppression was still maintained at up to 12 weeks beyond the cessation of therapy. While the number of animals was too small to permit one to draw clear conclusions about the temporal order of these reductions, it should be noted that replicative intermediates were found only in animals with detectable cccDNA, whereas cccDNA was clearly present in some animals with no observable replicative intermediates.
DISCUSSION
The results of this study confirm and extend the results of prior reports on the in vitro efficacy of the carbocyclic guanosine nucleoside analog BMS-200475 against HBV (5, 20, 37) by demonstrating that BMS-200475 is a very potent inhibitor of WHV replication in vivo in woodchucks chronically infected with WHV.
BMS-200475 was given orally to woodchucks at 0.02, 0.1, and 0.5 mg/kg/day in an initial 1-month dose-ranging study and at the two lower dosages in a subsequent 3-month study. Virus levels in serum were monitored by measuring circulating WHV DNA levels either by DNA dot blot hybridization (detection limit, about 2 × 106 genomes/ml), by semiquantitative PCR (detection limit, 2 × 102 to 2 × 103 genomes/ml), or by an EPA which measures the polymerase activity of virions. DNA samples from biopsied livers in the 3-month studies were also analyzed for WHV DNA species (see below). With respect to dosage, the dosages of BMS-200475 of 0.5 and 0.1 mg/kg/day appeared to be essentially equivalent in the 1-month study, as judged from dot blot assays and EPAs. In the 3-month dosing study, the 0.1-mg/kg/day dosage was associated with (i) a 2-week delay in viral rebounds posttherapy, (ii) a more consistent suppression of liver WHV DNA markers (see below), and (iii) maximal suppression of WHV viremia in 100% of the animals tested. However, the 0.02-mg dose was almost equally efficacious in all except two animals, in which there were only modest (∼2 log) antiviral suppressive effects. These differences suggest possible variations in the responses of individual animals to low levels of BMS-200475.
Taken together, the findings of these two studies (particularly the 12-week study) provide a striking picture of the efficacy of BMS-200475 in the woodchuck, which is essentially summarized in Fig. 4. Within 2 to 4 weeks of BMS-200475 therapy, serum WHV DNA levels were reduced on the order of 1 × 107-fold from pretreatment levels of typically 2 × 1010 or 3 × 1010 genomes/ml to final mean titers of ∼2 × 103 genomes/ml. In the 3-month study, comparable reductions occurred in six of six animals receiving the 0.1-mg/kg dose and in four of six animals receiving the 0.02-mg/kg dose. Beyond 4 weeks, continued therapy did not appear to cause significant further decreases in titer. Once therapy ended, viral titers rebounded, albeit with a delay that was dose dependent. In some individual animals, viremia continued to be suppressed for up to 2 to 4 weeks beyond the end of therapy, as measured by PCR, or as long as 10 weeks, as judged by dot blot assays.
Along with the strongly suppressive effect of BMS-200475 on virus levels in serum, there were marked reductions in two hepatocyte-associated WHV DNA species: cytoplasmic viral replicative intermediates and nuclear cccDNA. Replicative intermediates were essentially eliminated within 4 weeks of dosing with 0.1 mg of BMS-200475 per kg. Only traces of truncated WHV intermediates were seen in sporadic liver samples from the 0.02-mg/kg-dose group, a finding that reinforces the suggestion from prior in vitro studies (20, 37) that BMS-200475 acts as a nonobligate chain terminator of hepadnaviral polymerases.
More remarkable, however, was the fact that BMS-200475 treatment significantly reduced nuclear cccDNA levels in some, although not all, animals, with the higher dose being more effective in this regard. cccDNA, which is normally present at 10 to 50 copies per infected hepatocyte (29, 43, 45), serves to sustain chronic HBV infection. It is widely viewed as being refractory to nucleoside therapy and hence could pose a major barrier to the eradication of HBV infection (14, 29, 32). Opposing this view, however, is evidence suggesting that cccDNA is not particularly stable in cultured cells (6) as well as intriguing data supporting the existence of a T-lymphocyte-mediated, intracellular inactivation mechanism that can effectively “cure” hepatocytes of HBV without killing the cell (16). Other studies show that cccDNA is rapidly eliminated under certain circumstances, e.g., at the end of an acute hepadnaviral infection (21, 22), or following treatment with the nucleoside carbocyclic 2′-deoxyguanosine in the DHBV model (14). While curative and/or antiviral mechanisms could account for this loss of cccDNA, it has been proposed (14) that the actual mechanism may be an acceleration of hepatocyte turnover mediated either by the host immune system or, in the case of carbocyclic 2′-deoxyguanosine, by its liver cell toxicity. The possibility that the cccDNA clearance mediated by BMS-200475 in this study may be due to toxicity-related liver cell turnover has not been conclusively excluded; however, it is considered unlikely. In contrast to the overt toxicity seen with carbocyclic 2′-deoxyguanosine (14), woodchucks treated with BMS-200475 showed no frank evidence of toxicity in these studies, and the lowest dose (0.02 mg/kg) tested corresponds to only ∼70 μg of compound in a typical animal. Pending further studies to address the mechanism(s) by which BMS-200475 is active against cccDNA, it is suggested that this effect is explained by the profound suppression of WHV replication due to BMS-200475. This would in turn block new cccDNA synthesis, which depends on active replication. The resultant decline in cccDNA presumably reflects its intrinsic instability.
To address how BMS-200475 compares in efficacy against a nucleoside with proven activity against HBV in cultured cells (10, 20, 25) and in humans (9, 33), the 12-week treatment study included 3TC given orally at 5 mg/kg/day. Despite a preliminary report suggesting that 3TC is active against WHV in woodchucks at this dosage (24), 3TC was essentially ineffective in the current study. Two recent studies from other groups confirm that 3TC and its derivatives are in fact only weakly active in this model. Mason et al. (30) found that woodchucks had to be dosed with 200 mg of 3TC per kg/day for extended periods to attain 2- to 3-log reductions in WHV titers. Even during 3TC treatment, WHV titers were elevated, with the detection of drug-resistant mutants in the virus population. In studies with (−)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine [(−)-FTC], a compound structurally similar to 3TC, woodchucks were treated intraperitoneally twice a day for 4 weeks with 30- or 20-mg/kg doses (7). The 30-mg/kg dose of (−)-FTC (total dosage, 60 mg/kg/day) gave only 20- to 150-fold reductions in serum WHV DNA levels, while a dosage of 40 mg/kg/day caused only 6- to 49-fold suppressive effects. These decreases were transient and were accompanied by only modest reductions in WHV DNA levels in liver biopsy specimens. On the basis of the results of the present study, BMS-200475 would appear to be a far more effective anti-WHV agent than (−)-FTC in the woodchuck, even when it is given at a 3,000-fold lower dose [0.02 mg of BMS-200475 per kg versus 60 mg of (−)-FTC per kg]. The low efficacy of 3TC in woodchucks may be explained by the poor phosphorylation of certain pyrimidine nucleosides in rodents, reflecting the distinctive substrate specificities of rodent nucleoside kinases (17).
To date, no nucleosides with efficacies approaching that of BMS-200475 appear to have been reported on in the published studies of antiviral agents in woodchucks (11, 13, 15, 19, 30, 34, 39, 41). While efficacy has been seen with 2’-fluorinated arabinosyl-pyrimidine nucleosides (15, 41), there was also manifest toxicity marked by anorexia and death. In the current study with BMS-200475, no signs of toxicity were noted in woodchucks treated with this nucleoside for up to 12 weeks.
In the woodchuck model of HBV infection, oral BMS-200475 therapy can lead to a rapid ∼7-log suppression of viremia, with reductions to about 103 genome equivalents/ml. It is further possible that much of this circulating DNA reflects pseudovirions, i.e., uninfectious virions harboring drug-arrested DNA molecules, as reported in studies with acyclovir treatment (39). Suppression of viremia was sustained during dosing and was accompanied by marked reductions in the levels of WHV DNA species originating in the liver, including cccDNA. Nevertheless, since human immunodeficiency virus infection (12, 44) may survive even prolonged, highly active suppression of viremia, it remains to be seen whether BMS-200475 or indeed any nucleoside analog can ultimately eliminate chronic HBV infection. Further studies that will address this question are under way. In the meantime, the present findings make a strong case for assessing the efficacy of BMS-200475 against chronic human HBV infection in clinical trials.
FIG. 5.
Analysis of liver WHV DNA species. Nucleic acid samples enriched for either viral cccDNA (A) or replicative intermediates (B) were prepared from liver biopsy samples. The nucleic acids were electrophoresed in parallel through 1% agarose and analyzed by Southern hybridization. See text for further details. The dosing regimens and individual animals represented in the study were as follows: (A1 and B1) BMS-200475 (0.02 mg/kg/day); animals 95-422 (lanes 1 and 5), 95-341 (lanes 2 and 4), 95-012 (3 and 6), and 95-163 (lane 7); (A2 and B2) BMS-200475 (0.1 mg/kg/day); animals 94-313 (lane 8), 94-141 (lanes 9 and 12), 95-053 (lanes 10, 14, and 15), 95-253 (lane 11), and 95-321 (lanes 13 and 16); (A3 and B3) 3TC (5 mg/kg/day); animals 95-414 (lanes 17 and 23), 95-423 (lane 18, 21, and 22), and 95-181 (lanes 19 and 20). The biopsy times (in weeks) are indicated above the autoradiogram (PT, pretreatment). The positions of a 3.3-kb WHV DNA marker (lane M) and a 2.0-kb DNA size marker are indicated at the right of the panels; the cccDNA and replicative intermediate species are indicated at the left.
ACKNOWLEDGMENTS
We thank B. C. Tennant (Cornell University, Ithaca, N.Y.) for help and discussions concerning all aspects of the woodchuck animal model and Stefan Schmehl, Michael Kann, and Wolfram Gerlich (University of Giessen, Giessen, Germany) for performing the PCR analyses under contract.
REFERENCES
- 1.Bacon T H. Famciclovir, from the bench to the patient—a comprehensive review of preclinical data. Int J Antimicrob Agents. 1996;7:119–134. doi: 10.1016/0924-8579(96)00303-2. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomeusz A, Locarnini S. Mutations in the hepatitis B virus polymerase gene that are associated with resistance to famciclovir and lamivudine. Int Antivir News. 1997;5:123–124. [Google Scholar]
- 3.Bartholomew M M, Jansen R W, Jeffers L J, Reddy K R, Johnson L C, Bunzendahl H, Condreay L D, Tzakis A G, Schiff E R, Brown N A. Hepatitis-B-virus resistance to lamivudine given for recurrent infection after orthotopic liver transplantation. Lancet. 1997;349:20–22. doi: 10.1016/S0140-6736(96)02266-0. [DOI] [PubMed] [Google Scholar]
- 4.Beasley R P, Hwang L Y. Overview on the epidemiology of hepatocellular carcinoma. In: Hollinger F B, Lemon S M, Margolis M, editors. Viral hepatitis and liver disease. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 532–535. [Google Scholar]
- 5.Bisacchi G S, Chao S T, Bachard C, Daris J P, Innaimo S F, Jacobs J A, Kocy O, Lapointe P, Martel A, Merchant Z, Slusarchyk W A, Sundeen J E, Young M G, Colonno R J, Zahler R. BMS-200475, a novel carbocyclic 2′-deoxyguanosine analog with potent and selective anti-hepatitis B virus activity in vitro. Bioorg Med Chem Lett. 1997;7:127–132. [Google Scholar]
- 6.Civitico G M, Locarnini S A. The half-life of duck hepatitis B virus supercoiled DNA in congenitally infected primary hepatocyte cultures. Virology. 1994;203:81–89. doi: 10.1006/viro.1994.1457. [DOI] [PubMed] [Google Scholar]
- 7.Cullen J M, Smith S L, Davis M G, Dunn S E, Botteron C, Cecchi A, Linsey D, Linzey D, Frick L, Paff M T, Goulding A, Biron K. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 1997;41:2076–2082. doi: 10.1128/aac.41.10.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis M G, Wilson J E, VanDraanen N A, Miller W H, Freeman G A, Daluge S M, Boyd F L, Aulabaugh A E, Painter G R, Boone L R. DNA polymerase activity of hepatitis B virus particles: differential inhibition by l-enantiomers of nucleotide analogs. Antivir Res. 1996;30:133–145. doi: 10.1016/0166-3542(96)00938-2. [DOI] [PubMed] [Google Scholar]
- 9.Dienstag J L, Perillo R P, Schiff E R, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 10.Doong S-L, Tsai C-H, Schinazi R F, Liotta D C, Cheng Y C. Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enriquez P M, Jung C, Josephson L, Tennant B C. Conjugation of adenine arabinoside 5′-monophosphate to arabinogalactan: synthesis, characterization, and antiviral activity. Bioconjugate Chem. 1995;6:195–202. doi: 10.1021/bc00032a007. [DOI] [PubMed] [Google Scholar]
- 12.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 13.Fiume L, Di Stefano G, Busi C, Mattioli A, Rapicetta M, Giuseppetti R, Ciccaglione A R, Argenti C. Inhibition of woodchuck hepatitis virus replication by adenine arabinoside monophosphate coupled to lactosaminated poly-l-lysine and administered by intramuscular route. Hepatology. 1995;22:1072–1077. doi: 10.1016/0270-9139(95)90611-8. [DOI] [PubMed] [Google Scholar]
- 14.Fourel I, Cullen J M, Saputelli J, Aldrich C E, Schaffer P, Averett D, Pugh J, Mason W S. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J Virol. 1994;68:8321–8330. doi: 10.1128/jvi.68.12.8321-8330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fourel I, Hantz O, Watanabe K A, Jacquet C, Chomel B, Fox J J, Trepo C. Inhibitory effects of 2′-fluorinated arabinosyl-pyrimidine nucleosides on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob Agents Chemother. 1990;34:473–475. doi: 10.1128/aac.34.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 17.Habteyesus A, Nordenskjöld A, Bohman C, Eriksson S. Deoxynucleoside phosphorylating enzymes in monkey and human tissues show great similarities, while mouse deoxycytidine kinase has a different substrate specificity. Biochem Pharmacol. 1991;42:1829–1836. doi: 10.1016/0006-2952(91)90522-7. [DOI] [PubMed] [Google Scholar]
- 18.Honkoop P, deMan R A. Clinical aspects of nucleoside analogues for chronic hepatitis B. Int Antivir News. 1995;3:78–80. [Google Scholar]
- 19.Ikeda N, Kaneko S, Shimoda A, Inagaki Y, Unoura M, Okada M, Yonekawa Y, Takashashi K, Kobayashi K. Efficiency of oxetanocin-G, a novel nucleoside against the woodchuck hepatitis virus. J Antimicrob Chemother. 1994;33:83–89. doi: 10.1093/jac/33.1.83. [DOI] [PubMed] [Google Scholar]
- 20.Innaimo S F, Seifer M, Bisacchi G S, Standring D N, Zahler R, Colonno R J. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997;41:1444–1448. doi: 10.1128/aac.41.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jilbert A R, Wu T-T, England J M, Hall D L M, Carp N Z, O’Connell A P, Mason W S. Rapid resolution of duck hepatitis B virus occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajino K, Jilbert A R, Saputelli J, Aldrich C E, Cullen J, Mason W S. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kann M, Bischof A, Gerlich W H. In vitro model for the nuclear transport of the hepadnavirus genome. J Virol. 1997;71:1310–1316. doi: 10.1128/jvi.71.2.1310-1316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korba B E, Baldwin B, Cote P, Schinazi R, Gangemi D, Gerin J L, Tennant B C. Effectiveness of combination therapies with 3TC, famciclovir and alpha interferon against woodchuck hepatitis virus replication in chronically-infected woodchucks: model for potential anti-HBV treatments. Antivir Res. 1997;34:A52. [Google Scholar]
- 25.Korba B E, Boyd M R. Penciclovir is a selective inhibitor of hepatitis B virus replication in cultured human hepatoblastoma cells. Antimicrob Agents Chemother. 1996;40:1282–1284. doi: 10.1128/aac.40.5.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korba B E, Gerin J L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antivir Res. 1992;19:55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- 27.Korba B E, Milman G. A cell culture assay for compounds which inhibit hepatitis B virus replication. Antivir Res. 1991;15:217–228. doi: 10.1016/0166-3542(91)90068-3. [DOI] [PubMed] [Google Scholar]
- 28.Ling R, Mutimer D, Ahmed M, Boxall E H, Elias E, Dusheiko G M, Harrison T J. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 29.Locarnini S A, Civitico G M, Newbold J E. Hepatitis B: new approaches for antiviral chemotherapy. Antivir Chem Chemother. 1996;7:53–64. [Google Scholar]
- 30.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C F, Miller D S, Tennant B, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 31.Miller R, Marion P, Robinson W. Hepatitis B viral DNA-RNA hybrid molecules in particles from infected liver are converted to viral DNA molecules during an endogenous DNA polymerase reaction. Virology. 1984;139:64–72. doi: 10.1016/0042-6822(84)90330-1. [DOI] [PubMed] [Google Scholar]
- 32.Moraleda G, Saputelli J, Aldrich C E, Averett D, Condreay L, Mason W S. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nevens F, Main J, Honkoop P, Tyrrell D L, Barber J, Sullivan M T, Fevery J, De Man R A, Thomas H C. Lamivudine therapy for chronic hepatitis B: a six-month randomized dose-ranging study. Gastroenterology. 1997;113:1258–1263. doi: 10.1053/gast.1997.v113.pm9322520. [DOI] [PubMed] [Google Scholar]
- 34.Ponzetto A, Fiume L, Forzani B, Song S Y, Busi C, Mattioli A, Spinelli C, Marinelli M, Smedile A, Chiaberge E, Bonino F, Gervasi G B, Rapicetta M, Verme G. Adenine arabinoside monophosphate and acyclovir monophosphate coupled to lactosaminated albumin reduce woodchuck hepatitis virus viremia at doses lower than do the unconjugated drugs. Hepatology. 1991;14:16–24. doi: 10.1002/hep.1840140104. [DOI] [PubMed] [Google Scholar]
- 35.Roggendorf M, Tolle T K. The woodchuck: an animal model for hepatitis B virus infection in man. Intervirology. 1995;38:100–112. doi: 10.1159/000150418. [DOI] [PubMed] [Google Scholar]
- 36.Seeger C, Summers J, Mason W S. Viral DNA synthesis. Curr Top Microbiol Immunol. 1991;168:41–59. doi: 10.1007/978-3-642-76015-0_3. [DOI] [PubMed] [Google Scholar]
- 37.Seifer M, Hamatake R K, Colonno R J, Standring D N. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob Agents Chemother. 1998;42:3200–3208. doi: 10.1128/aac.42.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 39.Tencza M G, Newbold J E. Heterogeneous response for mammalian hepadnavirus infection to acyclovir: drug arrested intermediates of minus strand viral DNA are enveloped and secreted from infected cells as virion-like particles. J Med Virol. 1997;51:6–16. [PubMed] [Google Scholar]
- 40.Tennant B C, Gerin J L. The woodchuck model of hepatitis B virus infection. In: Arias I M, Boyer J L, Fausto N, Jakoby W B, Schachter D A, Shafritz D A, editors. The liver: biology and pathobiology. New York, N.Y: Raven Press, Ltd.; 1994. pp. 1455–1466. [Google Scholar]
- 41.Tennant B C, Baldwin B H, Graham L A, Ascenzi M A, Hornbuckle W E, Rowland P H, Tochkov I A, Yeager A E, Erb H N, Colacino J M, Lopez C, Engelhardt J A, Bowsher R R, Richardson F C, Lewis W, Cote P J, Korba B E, Gerin J L. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;28:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]
- 42.Tipples G A, Ma M M, Fisher K P, Bain V G, Kneteman N M, Tyrrell D L. Mutations in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;3:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 43.Tuttleman J S, Pourcel C, Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus infected cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 44.Wong J K, Hezareh M, Günthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 45.Wu T-T, Coates L, Aldrich C E, Summers J, Mason W S. In hepatocytes infected with duck hepatitis B virus, the template for RNA synthesis is amplified by an intracellular conversion pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 46.Yang W, Mason W S, Summers J. Covalently closed circular viral DNA formed from two types of linear DNA in woodchuck hepatitis B virus-infected livers. J Virol. 1996;70:4567–4575. doi: 10.1128/jvi.70.7.4567-4575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zoulim F. Improving hepatitis B virus therapy—new inhibitors of reverse transcriptase. Int Antivir News. 1997;5:110–112. [Google Scholar]