Abstract
The valine at position 82 (Val 82) in the active site of the human immunodeficiency virus (HIV) protease mutates in response to therapy with the protease inhibitor ritonavir. By using the X-ray crystal structure of the complex of HIV protease and ritonavir, the potent protease inhibitor ABT-378, which has a diminished interaction with Val 82, was designed. ABT-378 potently inhibited wild-type and mutant HIV protease (Ki = 1.3 to 3.6 pM), blocked the replication of laboratory and clinical strains of HIV type 1 (50% effective concentration [EC50], 0.006 to 0.017 μM), and maintained high potency against mutant HIV selected by ritonavir in vivo (EC50, ≤0.06 μM). The metabolism of ABT-378 was strongly inhibited by ritonavir in vitro. Consequently, following concomitant oral administration of ABT-378 and ritonavir, the concentrations of ABT-378 in rat, dog, and monkey plasma exceeded the in vitro antiviral EC50 in the presence of human serum by >50-fold after 8 h. In healthy human volunteers, coadministration of a single 400-mg dose of ABT-378 with 50 mg of ritonavir enhanced the area under the concentration curve of ABT-378 in plasma by 77-fold over that observed after dosing with ABT-378 alone, and mean concentrations of ABT-378 exceeded the EC50 for >24 h. These results demonstrate the potential utility of ABT-378 as a therapeutic intervention against AIDS.
The global spread and fatal prognosis of human immunodeficiency virus (HIV) infection emphasize the urgent need for effective antiretroviral therapies. Current agents that target the HIV reverse transcriptase are limited by dose-limiting toxicities, the selection of resistant mutants (7), and the inability to adequately suppress viral replication. Inhibitors of another essential viral enzyme, HIV protease, produce a profound reduction in HIV replication and a substantial elevation in CD4 cell levels (4, 17, 24). In combination, protease and reverse transcriptase inhibitors reduce plasma HIV RNA levels to undetectable levels in many patients and significantly decrease the incidence of death and opportunistic infections (1, 3, 6). However, all of the current protease inhibitors exhibit one or more significant limitations. Many are characterized by modest oral bioavailability and a short plasma half-life, producing low trough levels and requiring frequent administration of high doses to achieve an antiviral effect in vivo. Most inhibitors are highly bound to plasma proteins, which reduces the free fraction in the blood available for penetration into infected tissue. Strict dietary restrictions and significant side effects may also compromise adherence to the treatment regimen by patients. All of these limitations can result in suboptimal, subinhibitory drug levels that allow residual viral replication and the selection of drug-resistant mutants (20). Consequently, the maintenance of concentrations in plasma in excess of those needed to completely suppress viral replication is critical for avoidance of the emergence of resistance and for durable efficacy.
We previously reported on the discovery of ritonavir (ABT-538), a potent HIV protease inhibitor with high oral bioavailability and long plasma half-life (9, 12). However, the in vitro antiviral activity of ritonavir is attenuated by 20-fold in the presence of human serum (21). Consequently, despite high concentrations in the plasma of humans (8), monotherapy with ritonavir ultimately selects for resistant HIV isolates in many patients. Sequence analysis of the HIV protease gene in patients whose HIV RNA rebounded on therapy revealed an initial mutation of the valine at position 82 (Val 82) to alanine, threonine, or phenylalanine (20). The selection of Val 82 mutants to produce HIV protease variants with reduced affinity for the inhibitor is consistent with the hydrophobic interaction between ritonavir and the isopropyl side chain of Val 82 as observed by X-ray crystallography (9). In hopes of discovering inhibitors that do not select for Val 82 mutants, we investigated a series of inhibitors that lacked this specific interaction. Here we report on the discovery of ABT-378, a potent HIV protease inhibitor that retains potency against Val 82 mutant HIV protease. Furthermore, the in vitro anti-HIV activity of ABT-378 is less affected by binding to serum proteins than is the activity of ritonavir. Thus, in the presence of human serum, ABT-378 is 10-fold more potent than ritonavir. Like most protease inhibitors, oral administration of ABT-378 to animals and humans produces only transient, low levels in plasma. Previous studies have shown that coadministration with ritonavir significantly elevates the concentrations of other protease inhibitors in plasma through inhibition of their cytochrome P-450 (CYP)-mediated metabolism (10). We report here that the concentration of ritonavir required to inhibit ABT-378 metabolism is substantially lower than that needed to inhibit the metabolism of other protease inhibitors. Consequently, ABT-378 is exquisitely sensitive to pharmacokinetic enhancement by codosing with ritonavir, producing sustained concentrations in the plasma of the rat, dog, and monkey that are >50-fold over the antiviral 50% effective concentration (EC50) in the presence of human serum. High levels of ABT-378 are also achieved in the plasma of human volunteers after coadministration with even very low doses of ritonavir. These characteristics warrant the further study of ABT-378 in combination with low-dose ritonavir as a highly potent therapy for HIV infection.
MATERIALS AND METHODS
Details of the chemical synthesis of ABT-378 will be published elsewhere; prior to publication, they may be obtained from H.L.S.
HIV protease inhibition.
Inhibition of the activity of recombinant wild-type and mutant HIV type 1 (HIV-1) proteases was measured by a continuous fluorometric assay (18) with the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS (Bachem) as described previously (23). The apparent Ki was estimated by nonlinear regression by the equation for tightly binding inhibitors (5).
Antiviral assay.
MT4 cells and wild-type virus stocks were obtained through the AIDS Research and Reference Reagent Program, AIDS Program, National Institute of Allergy and Infectious Diseases. Mutant viral molecular clones were constructed as described previously (20). For drug susceptibility assays, viruses were propagated in CEM cells and titers were determined in MT4 cells. Inhibition of viral replication and compound cytotoxicity were determined in parallel in MT4 cells by a standard colorimetric assay by the method of Pauwels et al. (22).
Molecular modeling studies.
A three-dimensional model of ABT-378 bound to the active site of HIV-1 protease was created as follows. The crystal structure of ritonavir bound to HIV-1 protease (9) was modified to possess the chemical structure of ABT-378. The valinyl-cyclic urea bond within the S2 subsite was manually rotated until a match of H-bond donors and acceptors with residues Asp 29 or Asp 30 was achieved. The 2,6-dimethylphenoxy unit was docked into the S2′ subsite so as to fill the available volume. Energy minimization of ABT-378 within a constrained active site provided a final model of the complex. The DISCOVER CVFF force field within the INSIGHT-II modeling software was used to carry out the energy minimization.
Pharmacokinetic analysis.
Ritonavir and ABT-378 were coformulated as a solution in a mixture of ethanol-propylene glycol-D5W with appropriate equivalents of methanesulfonic acid at concentrations of 5 mg/ml for each component. Sprague-Dawley-derived rats (males; weight, 0.25 to 0.35 kg; n = 4) or cynomolgus monkeys (weight, 3 to 4 kg; n = 3) received a dose of 10 mg/kg of body weight by oral gavage with and without an equal ritonavir dose. Beagle dogs (males and females; weight, 8 to 12 kg; n = 3) received a 5-mg/kg dose with and without an equal ritonavir dose. Additional studies explored the effect of dose and dose ratio on the pharmacokinetics of ABT-378 and ritonavir. By using a constant 2-ml/kg dose volume, doses of ABT-378 and ritonavir were administered to groups of three to four rats (see Table 4). Plasma samples, obtained as a function of time after dosing (for rats, 10 time points over 8 h; for dogs and monkeys, 12 time points over 12 h), were extracted into mixtures of ethyl acetate and hexane, concentrated, and analyzed by reversed-phase high-pressure liquid chromatography (HPLC) with an internal standard (17a). The drug concentration in each plasma sample was calculated by least-squares linear regression analysis (unweighted) of the peak area ratio (parent/internal standard) of the spiked plasma standards versus concentration. The maximum concentration in plasma (Cmax) and the time to reach Cmax (Tmax) were read directly from the observed plasma concentration-time data. The area under the plasma concentration-time curve was calculated by using the linear trapezoidal rule over a single dosing interval. As part of a single rising-dose study, healthy human volunteers (males and females; fasted; n = 14) were given four 100-mg capsules of ABT-378 with a single 50-mg capsule of the semisolid formulation of ritonavir (n = 10) or placebo (n = 4).
TABLE 4.
Pharmacokinetics of ABT-378 administered in combination with ritonavira
| Species | Dose (mg/kg)
|
Ritonavir
|
ABT-378
|
n | Cmax/EC50b | Ct/EC50b | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABT-378 | RTV | Cmax (μg/ml) | Tmax (h) | AUC0–t (μg · h/ml) | Cmax (μg/ml) | Tmax (h) | AUC0–t (μg · h/ml) | ||||
| Rat | 10 | 0 | 0.96 | 0.8 | 1.92 | 4 | 15.0 | ||||
| 10 | 10 | 1.13 | 2.5 | 4.83 | 3.68 | 7.3 | 23.41 | 3 | 57.5 | 55.1 | |
| 10 | 5 | 0.15 | 0.6 | 0.38 | 2.11 | 4.5 | 12.29 | 4 | 33.0 | 20.5 | |
| 10 | 1 | 0.00 | 0.00 | 1.19 | 1.0 | 4.37 | 3 | 18.6 | 2.1 | ||
| 5 | 5 | 0.12 | 0.8 | 0.22 | 1.55 | 6.0 | 9.28 | 4 | 24.2 | 24.0 | |
| 5 | 2.5 | 0.00 | 0.00 | 1.14 | 4.1 | 5.26 | 4 | 17.8 | 7.4 | ||
| 5 | 1 | 0.00 | 0.00 | 1.31 | 1.2 | 5.72 | 4 | 20.5 | 6.1 | ||
| 3 | 3 | 0.06 | 0.8 | 0.03 | 0.91 | 5.0 | 4.20 | 4 | 14.2 | 5.4 | |
| 2.5 | 5 | 0.13 | 2.8 | 0.41 | 1.67 | 6.0 | 10.32 | 4 | 26.1 | 22.6 | |
| Monkey | 10 | 0 | 0.00 | 0.00 | 3 | ||||||
| 10 | 10 | 1.48 | 3.3 | 5.01 | 3.06 | 3.3 | 14.72 | 3 | 47.8 | 4.2 | |
| Dog | 5 | 0 | 0.00 | 0.00 | 3 | ||||||
| 5 | 5 | 1.32 | 0.8 | 2.19 | 2.50 | 1.3 | 11.26 | 3 | 39.1 | 0.70 | |
Data are provided as mean values. AUC0–t, area under the concentration-time curve from time zero to time t; Ct, concentration in plasma derived from the last sampling time point in each pharmacokinetic study (8 h for rats and 12 h for monkeys and dogs); RTV, ritonavir.
EC50 for anti-HIV-1IIIB activity in MT4 cells in the presence of 50% HS plus 10% fetal calf serum; the EC50 of ABT-378 was 0.064 μg/ml.
Metabolism in vitro.
Human liver microsomes were prepared as reported previously (14). Inhibition of in vitro metabolism by ritonavir was performed as described previously (10). Briefly, ABT-378 (25 μM) was coincubated in pH 7.4 phosphate buffer with various concentrations of ritonavir, 1 mg of liver microsomal protein/ml, and an NADPH-generating system containing the following: MgCl2 (15 mM), NADP+ (4.0 mM), glucose-6-phosphate (10 mM), and glucose-6-phosphate dehydrogenase (2.0 U/ml). The sample workup included stopping the reaction with 2 volumes of acetonitrile, evaporation of protein-free supernatant under nitrogen, and reconstitution of the residue in mobile phase for HPLC analysis. The disappearance of parent ABT-378 was quantitated by reversed-phase HPLC. The 50% inhibitory concentrations (IC50s) were calculated by the graphical method.
RESULTS
In the process of identifying an expanded-spectrum HIV protease inhibitor, we modified aspects of our program to address the shortcomings of existing inhibitors. First, the in vitro HIV assay was modified to include 50% human serum (HS) to assess the effect of serum binding on the antiviral potency (21). Second, the pharmacokinetic properties of new analogs were evaluated both singly and following coadministration with ritonavir in order to identify compounds that could achieve and maintain high concentrations in plasma. Finally, to design inhibitors that maintained activity against resistant HIV isolates, we focused our efforts on structures that minimized the interaction with the Val 82 side chain within the enzyme active site.
Design and in vitro activity of ABT-378.
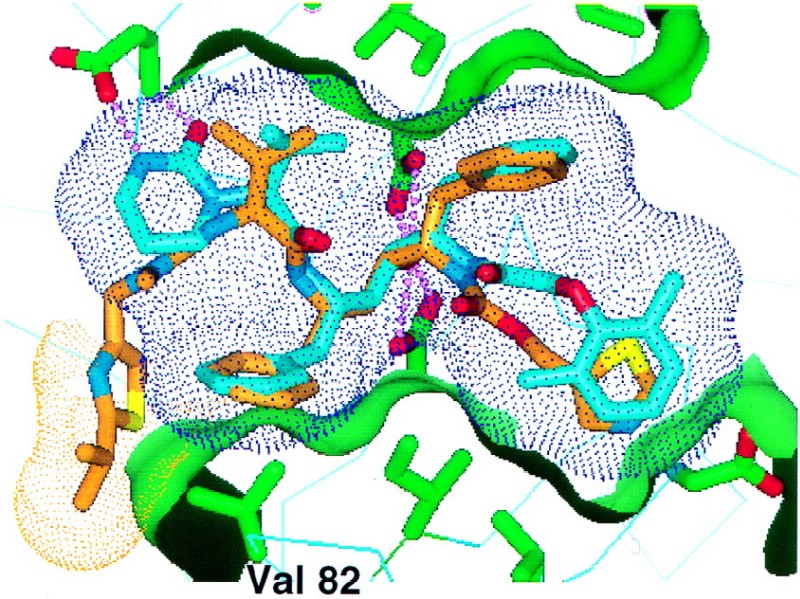
The X-ray crystal structure of the complex of HIV-1 protease with ritonavir reveals a hydrophobic interaction between the isopropyl side chain of Val 82 of the enzyme and the isopropyl substituent projecting from the 2 position of the P3 thiazolyl group of ritonavir (Fig. 1). Modeling studies suggested that the binding of ritonavir to Val 82 mutants might be compromised by loss of this optimized (11) interaction. Indeed, the Ki of ritonavir for recombinant HIV protease containing the V82A, V82F, and V82T mutations was 12- to 52-fold higher than that for wild-type protease (Table 1). To identify an inhibitor whose activity was less dependent on an interaction with Val 82, we began by eliminating the P3 isopropylthiazolyl group of ritonavir. The resulting inhibitors were evaluated both biochemically against wild-type HIV protease and virologically against wild-type HIV in the presence and absence of 50% HS (Table 2). The analog A-155704, which lacked the P3 group of ritonavir, displayed a significant loss of affinity for wild-type HIV protease and a 30-fold loss of anti-HIV activity compared to the anti-HIV activity of ritonavir. However, the activity of A-155704 was only marginally attenuated by serum binding, and in the presence of 50% HS, only a fourfold difference in the activities of the two compounds was observed. Conformation constraint provided the cyclic urea A-155564, which displayed improved inhibitory potency and maintained a relatively low level of protein binding. Finally, replacement of the P2′ (thiazolyl)methoxycarbonyl moiety with the known P2′ dimethylphenoxyacetyl group (25) produced ABT-378, which inhibited 93% of wild-type HIV protease activity at 0.5 nM and bound with a Ki of 1.3 pM. A high (>105-fold) specificity for HIV protease over those of the mammalian aspartic proteinases renin, cathepsin D, and cathepsin E was observed (data not shown). In MT4 cells, the EC50s of ABT-378 in the absence and presence of 50% HS were 17 ± 4 and 102 ± 44 nM, respectively (mean ± standard deviation of 10 triplicate determinations). In a direct comparison, the EC50s of ritonavir were 58 ± 14 and 1,044 ± 306 nM, respectively. Thus, in the presence of 50% HS, the anti-HIV activity of ABT-378 was 10-fold greater than that of ritonavir. ABT-378 was also highly potent against primary HIV cultured in peripheral blood mononuclear cells (in the absence of HS), with an EC50 of 6.5 nM (mean for six isolates; range, 4 to 11 nM).
FIG. 1.
Modeled overlay of ABT-378 (blue) and ritonavir (brown) in the active site of HIV protease (green). The conformations of HIV protease and ritonavir are derived from the X-ray crystal structure of the HIV protease-ritonavir complex (21a). The conformation of ABT-378 was modeled as described in the Materials and Methods section. A partial surface of the protein is shown in green to highlight the active-site pockets. A surface is shown on ABT-378 (blue dots) to illustrate how the inhibitor efficiently fills the active site. A surface is shown for ritonavir (brown dots) over the P3 isopropylthiazolyl group, which interacts with the side chain of Val 82 (labeled).
TABLE 1.
Activity of ABT-378 and ritonavir against wild-type and mutant HIV proteases
| Protease |
Ki (pM)a
|
|
|---|---|---|
| ABT-378 | Ritonavir | |
| Wild type | 1.3 | 10 |
| V82A | 4.9 | 120 |
| V82F | 3.7 | 520 |
| V82T | 3.6 | 300 |
Values represent the mean of at least three determinations.
TABLE 2.
Activities of HIV protease inhibitors leading to the identification of ABT-378
| Protease inhibitor | Structurea | % Protease inhibition with drug at 0.5 nMb | EC50 (μM) in the presence of the followingc:
|
|
|---|---|---|---|---|
| 0% HS | 50% HS | |||
| Ritonavir | 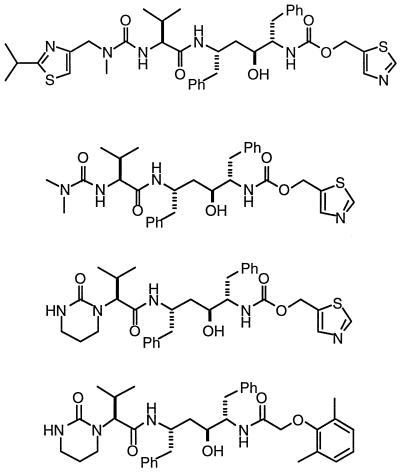 |
79 | 0.06 | 1.04 |
| A-155704 | 49 | 1.8 | 4.2 | |
| A-155564 | 79 | 0.15 | 0.64 | |
| ABT-378 | 93 | 0.017 | 0.10 | |
Ph, phenyl.
Values for percent inhibition of protease represent those from single-point assays.
The EC50s of A-155704 and A-155564 represent the means of a single triplicate assay. The EC50s of ritonavir and ABT-378 represent the means of 10 triplicate determinations.
We also examined the activity of ABT-378 against mutant HIV protease and primary HIV from patients whose viral RNA rebounded on ritonavir monotherapy with mutations in HIV protease (20). Against the V82A, V82F, and V82T mutant proteases, the level of binding of ABT-378 declined by less than fourfold compared to that for the wild-type protease (Table 1). Similarly, HIV with multiple mutations was significantly less resistant to ABT-378 than to ritonavir (Table 3). Against HIV isolates from three patients, the EC50s of ritonavir were 28-, 41-, and 17-fold higher than those against the corresponding baseline viruses. In contrast, although the activity of ABT-378 declined significantly against the multiply mutated strains compared to its activity against the baseline strains, the extent of the decline (6-, 13-, and 9-fold, respectively) was substantially less than that observed with ritonavir. Upon comparison of nine patient HIV isolates containing three or more mutations (data not shown), the relative resistance to ritonavir was three times greater than the relative resistance to ABT-378. Furthermore, the mean EC50 of ABT-378 against those isolates was 10-fold lower than that of ritonavir and in most cases was similar to or only slightly higher than the EC50 of ritonavir against the baseline (wild-type) isolates.
TABLE 3.
In vitro activities of ABT-378 and ritonavir against patient HIV isolates containing mutations conferring resistance to ritonavir
| Patient no. | Resistance mutations in sequence | EC50 (nM)a
|
|
|---|---|---|---|
| ABT-378 | Ritonavir | ||
| 129 | Baseline | 5 | 24 |
| V82T, I54V, A71A/V, M36M/I, K20K/R | 29 | 680 | |
| 131 | Baseline | 4 | 18 |
| V82A, I54I/V, M36I, K20K/R | 52 | 730 | |
| 224 | Baseline | 6 | 29 |
| V82S, I54V/M, M36M/I, K20K/R, L33L/F | 52 | 496 | |
EC50s represent the means of two or more determinations.
Model of ABT-378 in the HIV protease active site.
In order to understand the improved binding affinity of ABT-378 toward both wild-type and mutant proteases, we constructed a model of the inhibitor in the active site of HIV protease based on the crystal structure of ritonavir (9). As expected, the P3 region of ABT-378 displayed minimal interaction with the side chain of Val 82 (Fig. 1). Instead, the model suggested two energetically feasible binding modes that differed in the orientation of the cyclic urea moiety in ABT-378 and that use hydrogen bonding interactions between the cyclic urea and either Asp 30 or Asp 29 in the S2 subsite of the HIV protease active site. In the first model, the urea carbonyl was located ca. 2.8 Å from the backbone NH of Asp 30, while the urea NH pointed toward and was located ca. 3.0 Å from the carboxylate oxygen of the Asp 30 side chain after energy minimization (Fig. 1). This orientation allowed an intramolecular hydrophobic interaction between the trimethylene portion of the cyclic urea and the P1 benzyl group of ABT-378 but prevented hydrogen bonding to Asp 29. Alternatively, a 40 to 60° rotation around the exocyclic carbon-nitrogen bond repositioned the cyclic urea so that the NH of Asp 29 was located within hydrogen bonding distance of the urea carbonyl (data not shown). This alternative binding orientation did not allow hydrogen bonding to Asp 30, decreased the degree of van der Waal contact with the P1 benzyl group, and introduced a close contact with the Gly 48 carbonyl oxygen. Protein X-ray crystallographic experiments are under way to delineate the detailed interactions of the cyclic urea unit with Asp 29 and/or Asp 30.
Pharmacokinetic properties of ABT-378.
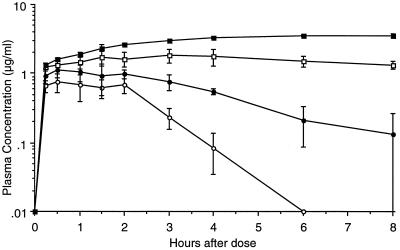
To initially characterize the pharmacokinetic properties of ABT-378, we administered ABT-378 orally (10 mg/kg) and intravenously (5 mg/kg) to rats. After oral dosing, the Cmax was 0.8 μg/ml and the calculated oral bioavailability in rats was 25%. By 6 h, the levels in plasma had declined below the level of quantitation (0.01 μg/ml). In contrast, coadministration of ABT-378 with ritonavir (10 mg/kg each) (10) produced sustained concentrations of ABT-378 in excess of 3 μg/ml with low variability (Fig. 2). The area under the plasma concentration-time curve (AUC) from 0 to 8 h for ABT-378 was elevated 14-fold by ritonavir coadministration. Importantly, the plasma ABT-378 levels were steady at the last time point (8 h), when concentrations of ritonavir were declining. Plasma ritonavir levels were not significantly affected by codosing with ABT-378 (data not shown). We also examined the pharmacokinetic profile of ABT-378 after oral administration to monkeys (10 mg/kg) and dogs (5 mg/kg). No quantifiable levels were detected in the plasma of either species. In contrast, plasma ABT-378 levels reached 3.1 μg/ml and declined slowly following codosing of ABT-378 and ritonavir (10 mg/kg each) in monkeys. Even after 12 h, concentrations more than fourfold over the HS-adjusted EC50 were observed (Table 4). The elevation of ABT-378 levels by ritonavir coadministration was even more significant in dogs. After codosing of 5 mg/kg each, plasma ABT-378 levels remained stable at ca. 2.5 μg/ml (64-fold over the EC50) for >12 h, representing a >350-fold enhancement in AUC.
FIG. 2.
Mean ± standard error of the mean plasma ABT-378 levels after oral dosing with 10 mg/kg singly and in combination with various doses of ritonavir in rats. Open circles, dosed singly; closed circles, codosed with 1 mg of ritonavir per kg; open squares, codosed with 5 mg of ritonavir per kg; closed squares, codosed with 10 mg of ritonavir per kg.
A more extensive characterization of the pharmacokinetic relationship between ABT-378 and ritonavir following oral dosing in rats is shown in Table 4. AUC values derived from a 10-mg/kg dose of ABT-378 were enhanced more than twofold when it was coadministered with as little as 1 mg of ritonavir per kg, a dose which provided no quantifiable plasma ritonavir concentrations. Greater than 10-fold increases in AUC were obtained with lower ABT-378-to-ritonavir ratios. Importantly, the plasma ABT-378 concentrations at the end of the study were very similar to those noted 1 to 2 h after dosing, with concentrations being >20-fold higher than the in vitro EC50 in MT4 cells measured in the presence of 50% HS (Fig. 2). The ABT-378 AUC from 0 to 8 h after coadministration with ritonavir (10 mg/kg each) was more than twice that of saquinavir observed previously under the same dosing conditions (10). Furthermore, the concentration of ABT-378 after 8 h (3.53 μg/ml) was nearly fourfold higher than the corresponding level of saquinavir.
In vitro inhibition of ABT-378 metabolism by ritonavir.
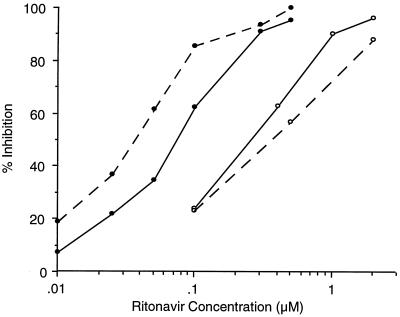
Previously, the pharmacokinetic enhancement of other protease inhibitors by ritonavir was shown to be a consequence of the inhibition of the metabolism of those agents by ritonavir (10). To understand the degree of the pharmacokinetic interaction between ABT-378 and ritonavir, we studied the inhibition of the metabolism of ABT-378 by ritonavir in vitro using rat and human liver microsomal preparations (10). In both species, ABT-378 was metabolized almost exclusively by the 3A4 isozyme of CYP (13b). In vitro, the metabolism of ABT-378 was inhibited by very low concentrations of ritonavir, with IC50s of 0.036 and 0.073 μM in rat and human liver microsomes, respectively (Fig. 3). In contrast, the concentrations of ritonavir required to inhibit the metabolism of the same concentration of saquinavir were >10- and 3.4-fold higher in rat and human liver microsomes, respectively (10).
FIG. 3.
Inhibition of the metabolism of ABT-378 and saquinavir by ritonavir in rat and human liver microsomes. Values represent the means of triplicate determinations. Dashed lines, rat microsomes; solid lines, human microsomes; filled circles, ABT-378; open circles, saquinavir.
Enhancement of plasma ABT-378 levels in humans.
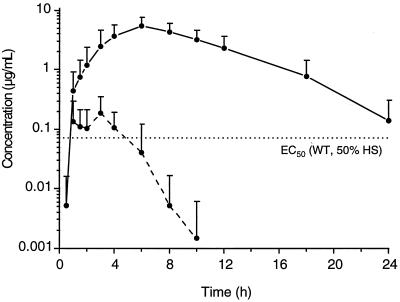
The pharmacokinetic interactions of ABT-378 and ritonavir are under evaluation in humans. As part of a single rising-dose study (16), healthy volunteers were given either a single 400-mg dose of ABT-378 or a 400-mg dose of ABT-378 combined with a 50-mg dose of ritonavir (Fig. 4). After dosing singly, the mean levels of ABT-378 in plasma only briefly exceeded 0.1 μg/ml and declined to <0.01 μg/ml by 8 h. In contrast, coadministration of ABT-378 with a small amount of ritonavir produced elevated (Cmax = 5.5 ± 2.0 μg/ml) and sustained levels of ABT-378 so that the mean concentrations exceeded 0.1 μg/ml even after 24 h. Comparison of the AUC from 0 to 24 h for ABT-378 indicated an enhancement in drug exposure of 77-fold by coadministration of the 50-mg dose of ritonavir.
FIG. 4.
Mean ± SD plasma ABT-378 levels in healthy human volunteers following administration of a single 400-mg dose. Dashed line, ABT-378 dosed singly; solid line, ABT-378 dosed with 50 mg of ritonavir; dotted line, EC50 of ABT-378 against wild type (WT) HIV in vitro.
DISCUSSION
Although current protease inhibitors, in combination with other antiretroviral agents, profoundly suppress HIV replication, the decline in viral load following initial therapy is not durable in a significant percentage of patients due to the outgrowth of mutant virus. This problem is particularly acute in protease inhibitor-experienced patients, because mutations selected by one inhibitor may compromise the ability of a second regimen to adequately suppress viral replication (19). Since the rate of resistance to protease inhibitors is inversely related to the minimum (trough) levels of drug in the plasma of patients (20), the maintenance of concentrations in plasma far in excess of the antiviral potency ex vivo, i.e., in the presence of HS, is essential for a durable response. All currently available protease inhibitors either are highly bound to serum proteins (thus reducing ex vivo potency) and/or are rapidly metabolized and eliminated (thus resulting in inadequate trough levels between doses). In this report, we describe ABT-378, a protease inhibitor with high potency in the presence of HS and high levels in plasma following coadministration with small amounts of ritonavir. Taken together, these attributes suggest that ABT-378 may represent an improvement over current drugs for the treatment of HIV infection.
The Ki of ABT-378 toward wild-type HIV protease was significantly (ca. 10-fold) lower than that of ritonavir. This high binding affinity may be a consequence of a strong hydrogen-bonding interaction between the cyclic urea of ABT-378 and either Asp 30 or Asp 29 of the protease active site, analogous to that observed for the P2 Asn side chain of saquinavir (13). The difference in affinity described above was reflected in the increased anti-HIV activity of ABT-378 compared to that of ritonavir, particularly in the presence of serum. Whereas the EC50 of ritonavir was modulated by 18-fold in the presence of 50% HS, the activity of ABT-378 was affected by only 6-fold. Consequently, the potency of ABT-378 ex vivo was 10-fold greater than that of ritonavir. The effect of 50% serum on the activity of ABT-378 is primarily a consequence of binding to a1-acid glycoprotein, whereas the potency of ritonavir is affected by both a1-acid glycoprotein and albumin (21). Although the free fraction of ABT-378 decreases by about twofold with 100% HS compared with that with 50% HS (13a), the further attenuation of antiviral potency is likely to be less than twofold because of the nonlinear response observed with increasing amounts of HS (21).
The Ki of ABT-378 for V82A, V82F, and V82T HIV proteases differed by less than fourfold from the Ki for the wild-type protease. In contrast, the Ki of ritonavir for the mutant proteases was 12- to 52-fold higher than that for the wild-type enzyme. The maintenance of nearly full activity against Val 82 mutants is consistent with the modeled structure of ABT-378 in the HIV protease active site (Fig. 1), which shows a clear difference in the overall surface area of contact between the inhibitor and the side chain of Val 82 compared to that of ritonavir. Indeed, in vitro selection with ABT-378 produced mutations at positions 84, 46, and 10 but not at position 82 (2). Although the anti-HIV activity of ABT-378 declined by 6- to 13-fold against ritonavir-resistant HIV with multiple mutations, the potency of ABT-378 against these resistant viruses remained similar to the activity of ritonavir against wild-type HIV. Clinical studies have established that ritonavir is highly suppressive of wild-type HIV in vivo, even when it is used as monotherapy (4, 17). These results suggest that therapy producing plasma ABT-378 levels equal to or greater than those currently achieved with ritonavir (8) would be highly suppressive of not only wild-type HIV but also ritonavir-resistant HIV.
In order to identify a new protease inhibitor with a pharmacokinetic profile superior to those of existing agents, we elected to evaluate the levels of potential candidates in plasma after oral coadministration with ritonavir (10). In rats and dogs, plasma ABT-378 concentrations were elevated by ritonavir to significantly higher levels than those observed after coadministration of ritonavir with other protease inhibitors (10). The unique sensitivity of ABT-378 to enhancement by ritonavir is consistent with its extremely high rate of in vitro metabolism in the absence of ritonavir (15) and the lower IC50 of ritonavir for inhibition of ABT-378 metabolism in rat liver microsomes compared to those of other protease inhibitors. Consequently, even low concentrations of ritonavir produced a significant effect on the levels of ABT-378 in rats. This observation was paralleled in humans, in whom a low (50-mg) codose of ritonavir with 400 mg of ABT-378 produced a 77-fold enhancement of the AUC of ABT-378 from 0 to 24 h. Although inhibition of CYP3A by low-dose ritonavir is expected to produce drug-drug interactions with other agents as well, we anticipate that these interactions will be significantly less than those observed with ritonavir given at 600 mg twice a day (the recommended therapeutic dosage). Indeed, with this dosage, the Cmax of ritonavir was only 0.2 μg/ml (<2% of the maximum levels observed when the drug was given at 600 mg twice a day). In contrast, the mean concentration of ABT-378 after 12 h in humans was 3 μM, ca. 30-fold over the EC50 and >15-fold over the EC90 against wild-type HIV in the presence of 50% HS. Since high, sustained levels in plasma have been associated with a delayed emergence of resistance (20), these results support the investigation of a novel treatment regimen for HIV infection containing ABT-378 with small amounts of ritonavir that are present solely for the enhancement of concentrations in plasma. The efficacy of this regimen is under investigation in phase II clinical trials.
REFERENCES
- 1.Cameron D W, Heath-Chiozzi M, Danner S, Cohen C, Krabcik S, Maurath C, Sun E, Henry D, Rode R, Potoff A, Leonard J. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351:543–549. doi: 10.1016/s0140-6736(97)04161-5. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo A, Stewart K D, Sham H L, Norbeck D W, Kohlbrenner W E, Leonard J M, Kempf D J, Molla A. In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J Virol. 1998;72:7532–7541. doi: 10.1128/jvi.72.9.7532-7541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clumeck N. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada. Washington, D.C: American Society for Microbiology; 1997. Clinical benefit of saquinavir (SQV) plus zalcitabine (ddC) plus zidovudine (ZDV) in untreated/minimally treated HIV-infected patients, abstr. LB-4. [Google Scholar]
- 4.Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, Gil Aguado A, Garcia de Lomas J, Delgado R, Borleffs J C C, Hsu A, Valdes J, Boucher C A B, Cooper D A. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1528–1533. doi: 10.1056/NEJM199512073332303. [DOI] [PubMed] [Google Scholar]
- 5.Greco W R, Hakala M T. Evaluation of methods for estimating the dissociation constant of tight binding inhibitors. J Biol Chem. 1979;254:12104–12109. [PubMed] [Google Scholar]
- 6.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Feinberg J E, Balfour H H, Dayton L R, Chodakewitz J A, Fischl M A. A controlled trial of 2 nucleoside analogs plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch M S, D’Aquila R T. Therapy for human immunodeficiency virus infection. N Engl J Med. 1993;328:1686–1695. doi: 10.1056/NEJM199306103282307. [DOI] [PubMed] [Google Scholar]
- 8.Hsu A, Granneman R, Witt G, Locke C, Denissen J, Molla A, Valdes J, Smith J, Erdman K, Lyons N, Niu P, Decourt J-P, Fourtillan J-B, Girault J, Leonard J M. Multiple-dose pharmacokinetics of ritonavir in human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 1997;41:898–905. doi: 10.1128/aac.41.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempf D, Marsh K C, Denissen J F, McDonald E, Vasavanonda S, Flentge C A, Green B E, Fino L, Park C H, Kong X-P, Wideburg N E, Saldivar A, Ruiz L, Kati W M, Sham H L, Robins T, Stewart K D, Hsu A, Plattner J J, Leonard J M, Norbeck D W. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci USA. 1995;92:2484–2488. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempf D J, Marsh K C, Kumar G, Rodrigues A D, Denissen J F, McDonald E, Kukulka M J, Hsu A, Granneman G R, Baroldi P A, Sun E, Pizzuti D, Plattner J J, Norbeck D W, Leonard J M. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–660. doi: 10.1128/aac.41.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempf D J, Marsh K C, Paul D A, Knigge M F, Norbeck D W, Kohlbrenner W E, Codacovi L, Vasavanonda S, Bryant P, Wang X C, Wideburg N E, Clement J J, Plattner J J, Erickson J. Antiviral and pharmacokinetic properties of C2 symmetric inhibitors of the human immunodeficiency virus type 1 protease. Antimicrob Agents Chemother. 1991;35:2209–2214. doi: 10.1128/aac.35.11.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempf D J, Sham H L, Marsh K C, Flentge C A, Betebenner D, Green B E, McDonald E, Vasavanonda S, Saldivar A, Wideburg N E, Kati W M, Ruiz L, Zhao C, Fino L, Patterson J, Molla A, Plattner J J, Norbeck D W. Discovery of ritonavir, a potent inhibitor of HIV protease with high oral bioavailability and clinical efficacy. J Med Chem. 1998;41:602–617. doi: 10.1021/jm970636+. [DOI] [PubMed] [Google Scholar]
- 13.Kröhn A, Redshaw S, Ritchie J C, Graves B J, Hatada M H. Novel binding mode of highly potent HIV-proteinase inhibitors incorporating the (R)-hydroxyethylamine isostere. J Med Chem. 1991;34:3340–3342. doi: 10.1021/jm00115a028. [DOI] [PubMed] [Google Scholar]
- 13a.Kumar, G. Unpublished data.
- 13b.Kumar, G., et al. Unpublished results.
- 14.Kumar G N, Rodrigues A D, Buko A M, Denissen J F. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–431. [PubMed] [Google Scholar]
- 15.Kumar, G. N., V. Jayanti, R. Lee, D. Whittern, J. Uchic, S. Thomas, P. Johnson, B. Grabowski, H. Sham, D. Betebenner, D. Kempf, and J. F. Denissen. In vitro metabolism of the HIV-1 protease inhibitor ABT-378: species comparison and metabolite identification. Drug Metab. Dispos., in press. [PubMed]
- 16.Lal R, Hsu A, Chen P, Dennis S, El-Shourbagy T, Locke C, Lam W, Japour A, Leonard J, Granneman G R, Sun E. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Single dose pharmacokinetics of ABT-378 in combination with ritonavir, abstr. I-194; p. 279. [Google Scholar]
- 17.Markowitz M, Saag M, Powderly W G, Hurley A M, Hsu A, Valdes J M, Henry D, Sattler F, La Marca A, Leonard J M, Ho D D. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 17a.Marsh, K., et al. Unpublished results.
- 18.Matayoshi E D, Wang G T, Krafft G A, Erickson J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science. 1990;247:954–958. doi: 10.1126/science.2106161. [DOI] [PubMed] [Google Scholar]
- 19.Molla, A., G. R. Granneman, E. Sun, and D. Kempf. Antivir. Res., in press. [DOI] [PubMed]
- 20.Molla A, Korneyeva M, Gao Q, Vasavanonda S, Schipper P J, Mo H-M, Markowitz M, Chernyavskiy T, Niu P, Lyons N, Hsu A, Granneman G R, Ho D D, Boucher C A B, Leonard J M, Norbeck D W, Kempf D J. Ordered accumulation of mutations in HIV protease confers resistance to ritonavir. Nat Med. 1996;2:760–766. doi: 10.1038/nm0796-760. [DOI] [PubMed] [Google Scholar]
- 21.Molla, A., S. Vasavanonda, J. Denissen, G. Kumar, B. Grabowski, H. Sham, D. Norbeck, W. Kohlbrenner, J. Plattner, D. Kempf, and J. Leonard. Human serum attenuates the activity of protease inhibitors toward wild type and mutant human immunodeficiency virus. Virology, in press. [DOI] [PubMed]
- 21a.Park, C. Unpublished results.
- 22.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 23.Sham H L, Zhao C, Stewart K D, Betebenner D A, Lin S, Park C H, Kong X-P, Rosenbrook J, Herrin W T, Madigan D, Vasavanonda S, Lyons N, Molla A, Saldivar A, Marsh K C, McDonald E, Wideburg N E, Denissen J F, Robins T, Kempf D J, Plattner J J, Norbeck D W. A novel, picomolar inhibitor of human immunodeficiency virus type 1 protease. J Med Chem. 1996;39:392–397. doi: 10.1021/jm9507183. [DOI] [PubMed] [Google Scholar]
- 24.Stein D S, Fish D G, Bilello J A, Preston S L, Martineau G L, Drusano G L. A 24-week open-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir) AIDS. 1996;10:485–492. doi: 10.1097/00002030-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Tong L, Pav S, Mui S, Lamarre D, Yoakim C, Beaulieu P, Anderson P C. Crystal structures of HIV-2 protease in complex with inhibitors containing the hydroxyethylamine dipeptide isostere. Structure. 1995;3:33–40. doi: 10.1016/s0969-2126(01)00133-2. [DOI] [PubMed] [Google Scholar]