Abstract
PURPOSE
Platinum-based doublets with concurrent and maintenance bevacizumab are standard therapy for ovarian cancer (OC) relapsing after a platinum-free interval (PFI) >6 months. Immunotherapy may be synergistic with bevacizumab and chemotherapy.
PATIENTS AND METHODS
ATALANTE/ENGOT-ov29 (ClinicalTrials.gov identifier: NCT02891824), a placebo-controlled double-blinded randomized phase III trial, enrolled patients with recurrent epithelial OC, one to two previous chemotherapy lines, and PFI >6 months. Eligible patients were randomly assigned 2:1 to atezolizumab (1,200 mg once every 3 weeks or equivalent) or placebo for up to 24 months, combined with bevacizumab and six cycles of chemotherapy doublet, stratified by PFI, PD-L1 status, and chemotherapy regimen. Coprimary end points were investigator-assessed progression-free survival (PFS) in the intention-to-treat (ITT) and PD-L1–positive populations (alpha .025 for each population).
RESULTS
Between September 2016 and October 2019, 614 patients were randomly assigned: 410 to atezolizumab and 204 to placebo. Only 38% had PD-L1–positive tumors. After 3 years' median follow-up, the PFS difference between atezolizumab and placebo did not reach statistical significance in the ITT (hazard ratio [HR], 0.83; 95% CI, 0.69 to 0.99; P = .041; median 13.5 v 11.3 months, respectively) or PD-L1–positive (HR, 0.86; 95% CI, 0.63 to 1.16; P = .30; median 15.2 v 13.1 months, respectively) populations. The immature overall survival (OS) HR was 0.81 (95% CI, 0.65 to 1.01; median 35.5 v 30.6 months with atezolizumab v placebo, respectively). Global health-related quality of life did not differ between treatment arms. Grade ≥3 adverse events (AEs) occurred in 88% of atezolizumab-treated and 87% of placebo-treated patients; grade ≥3 AEs typical of immunotherapy were more common with atezolizumab (13% v 8%, respectively).
CONCLUSION
ATALANTE/ENGOT-ov29 did not meet its coprimary PFS objectives in the ITT or PD-L1–positive populations. OS follow-up continues. Further research on biopsy samples is warranted to decipher the immunologic landscape of late-relapsing OC.
INTRODUCTION
Recent advances in the treatment of gynecologic cancers include antiangiogenic therapy and poly (ADP-ribose) polymerase (PARP) inhibitors in ovarian cancer (OC) and immune checkpoint inhibitors (ICIs) in cervical1,2 and endometrial3 cancers. In OC, ICIs have yet to show significant benefit. The observed additive/synergistic activity between ICIs, antiangiogenic agents, and chemotherapy in lung and renal cancers4-6 prompted evaluation of such combinations in OC. As previous results hinted that immunotherapy might be more effective in less resistant recurrent OC,7 the ATALANTE/ENGOT-ov29 trial evaluated the anti–PD-L1 agent atezolizumab in platinum-sensitive OC (platinum-free interval [PFI] >6 months). In this setting, standard therapy includes a platinum-containing doublet (platinum with gemcitabine,8 paclitaxel,9 or pegylated liposomal doxorubicin [PLD]10) combined with bevacizumab.11-13
CONTEXT
Key Objective
Does incorporation of atezolizumab into a standard regimen of platinum-based chemotherapy with concurrent and maintenance bevacizumab improve outcomes in patients with platinum-sensitive recurrent ovarian cancer (OC)?
Knowledge Generated
To our knowledge, this is the first reported randomized phase III trial evaluating immune checkpoint inhibition in patients with platinum-sensitive OC. Atezolizumab did not improve progression-free survival in the overall population or in the population of patients with PD-L1–positive tumors. Survival follow-up is ongoing.
Relevance (G.F. Fleming)
-
These data show no benefit from the use of atezolizumab in patients with recurrent platinum-sensitive OC, which is in line with multiple negative trials of immune checkpoint inhibition for patients with OC treated in the front-line setting.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD.
Between initiation and primary analysis of ATALANTE/ENGOT-ov29, emerging data reshaped the immunologic treatment landscape. Three phase III trials evaluating various immunotherapy strategies in different OC settings failed to meet their primary objectives, but suggested that PD-L1 positivity may potentially identify patients more likely to benefit from ICIs.7,14,15 Consequently, the ATALANTE/ENGOT-ov29 trial design was adapted to include progression-free survival (PFS) in the PD-L1–positive population as a coprimary objective. We report primary efficacy and safety results.
PATIENTS AND METHODS
ATALANTE/ENGOT-ov29 (ClinicalTrials.gov identifier: NCT02891824) is a European Network for Gynaecological Oncological Trial groups (ENGOT)/Gynecologic Cancer InterGroup double-blind, placebo-controlled, randomized, phase III trial, sponsored by the Groupe d’Investigateurs National des Etudes des Cancers Ovariens et du sein (GINECO). The trial was conducted in accordance with the Declaration of Helsinki and consistent with International Conference on Harmonisation Good Clinical Practice and applicable regulatory requirements. The study was performed according to ENGOT Model A.16 The final trial protocol, informed consent forms, and all other written information/materials provided to patients were approved by each participating country's institutional review board/ethics committee.
Eligible patients had histologically confirmed progressive nonmucinous epithelial OC (including primary peritoneal and/or fallopian tube adenocarcinoma) with first or second relapse after a PFI >6 months. The last chemotherapy had to contain platinum. New anticancer therapy in the 6 months between last platinum and study entry was prohibited (except for maintenance therapy up to 21 days before study entry). Previous ICI was prohibited. Patients had to have normal organ and bone marrow function and an Eastern Cooperative Oncology Group performance status 0/1. All patients provided written informed consent before undergoing any trial-specific procedures or treatment.
Before random assignment, PD-L1 status was determined centrally on a mandatory de novo tumor biopsy obtained within the preceding 2 months. PD-L1–positive status was defined as tumor-infiltrating immune cell (IC) PD-L1 expression on ≥1% of tumor area using the Ventana SP142 immunohistochemistry assay (Ventana Medical Systems, Tucson, AZ), as in previous atezolizumab trials.15,17-19 Investigators selected one of the following platinum-based regimens: carboplatin AUC4 (day 1), gemcitabine 1,000 mg/m2 (days 1, 8), and bevacizumab 15 mg/kg (day 1) once every 3 weeks; carboplatin AUC5, paclitaxel 175 mg/m2, and bevacizumab 15 mg/kg (all day 1, once every 3 weeks); or carboplatin AUC5 (day 1), PLD 30 mg/m2 (day 1), and bevacizumab 10 mg/kg (days 1, 15) once every 4 weeks. After completing six cycles of chemotherapy, all patients received maintenance bevacizumab 15 mg/kg (day 1, once every 3 weeks) until objective radiologic disease progression according to RECIST (version 1.1), unacceptable toxicity, or patient withdrawal. Stratification factors were PFI (6-12 v >12 months), PD-L1 status (negative [IC <1%] v positive v noninformative), and chemotherapy (carboplatin-gemcitabine v carboplatin-PLD v carboplatin-paclitaxel). Eligible patients were randomly assigned 2:1 to receive the selected platinum-based chemotherapy and bevacizumab with either atezolizumab (1,200 mg, day 1, once every 3 weeks) or placebo during chemotherapy and continued with bevacizumab as maintenance therapy.
Investigators assessed tumors by computed tomography or magnetic resonance imaging at screening (<28 days before random assignment), at 12, 24, 48, 72, and 96 weeks, and per local standards thereafter until disease progression per RECIST (version 1.1). Patient-reported outcomes were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire core module (QLQ-C30) and OC-specific module (QLQ-OV28) before starting treatment, before each visit during chemotherapy, once every 12 weeks until subsequent therapy, once every 4 weeks during the first 12 weeks of subsequent therapy, and thereafter once every 12 weeks until second progression, death, or for up to 3 years, whichever occurred first. Adverse events (AEs) were recorded at every cycle, graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
The coprimary outcome measures were investigator-assessed PFS according to RECIST (version 1.1) in the intention-to-treat (ITT) and PD-L1–positive populations. Supportive secondary outcome measures were overall survival (OS), time from random assignment to start of second subsequent therapy or death (TSST), health-related quality of life (HRQoL), patient-reported outcomes, and long-term survival using cure-rate modeling.20 Additional secondary outcome measures included objective response rate (ORR), time to first subsequent therapy (TFST), time to second progression or death (PFS2), PFS and OS in the subgroup with both PD-L1–positive and CD8-positive (≥1% expression) tumors, safety, and tolerability. Exploratory subgroup analyses of PFS according to randomization stratification factors and relevant potential prognostic factors were prespecified.
The assumed median PFS in the control arm of the ITT population was 13 months, on the basis of results from OCEANS,11 GOG-0213,12 and a single-arm study21 (AGO-OVAR2.2113 results were not available at that time). Median PFS of 15 months was anticipated in the PD-L1–positive population.22 The expected accrual period was 40 months. It was anticipated that atezolizumab would increase median PFS by 4.8 months to 17.8 months in the ITT population (corresponding to a hazard ratio [HR] of 0.73). According to Freedman's method with a 2.5% two-tailed type I error and nearly 85% power, 491 events were required in 600 randomly assigned patients (2:1 random assignment) for the primary analysis. It was assumed that 40% of the ITT population would have PD-L1–positive tumors (on the basis of PD-L1 status in the first 300 patients enrolled, before amending the protocol to introduce PFS in the PD-L1–positive population as a coprimary end point), and that median PFS in these patients would increase from 15 to 24 months, corresponding to an HR of 0.62, with atezolizumab. With a 2.5% two-tailed type I error, 186 PFS events were required in 240 patients with PD-L1–positive tumors to provide almost 80% power for the coprimary analysis.
Efficacy was analyzed in the ITT population (all randomly assigned patients analyzed as randomly assigned) and the PD-L1–positive population. As atezolizumab treatment effect was expected to vary over time, the proportional hazards assumption was to be tested before determining the methodology for the coprimary analyses (Appendix 2, online only). PFS was estimated using Kaplan-Meier methodology; median PFS was reported with 95% CIs (Brookmeyer-Crowley method). If the proportional hazard assumption was not violated, PFS was analyzed using a classical Cox model adjusted on stratification factors with a 2.5% two-sided type-I error rate. If either or both coprimary PFS comparisons reached statistical significance (P < .025), the hierarchical statistical design allowed formal testing of OS in the ITT and PD-L1–positive populations. If neither coprimary end point comparison was significant at P < .025, the trial was considered negative. HRQoL was analyzed using linear mixed models with an interaction between treatment arm and time and a random effect on subject. A restricted likelihood maximization estimation method was used and P values were derived from Kenward-Roger approximation. Safety was analyzed in the safety population (all randomly assigned patients receiving at least one study treatment dose) according to the treatment actually received. Statistical analyses used R software (version 3.3).23
Final analysis of TSST, OS, and PFS2, updated PFS, and cure-rate modeling will be performed after deaths in 491 patients in the ITT population and 186 in the PD-L1–positive population.
RESULTS
Patient Population and Treatment Exposure
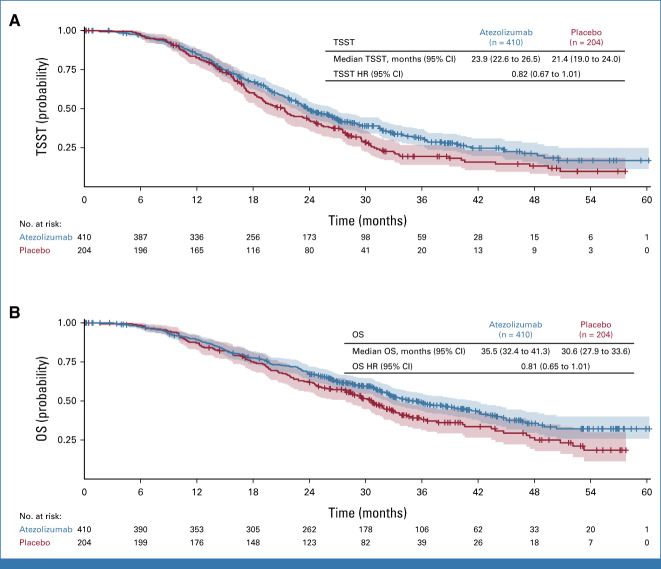
Between September 28, 2016, and October 4, 2019, 614 patients from 74 sites in Europe and Israel were enrolled. Of these, 410 were randomly assigned to atezolizumab-containing therapy and 204 to placebo (Fig 1). Baseline characteristics were generally well balanced (Table 1). Almost two thirds of patients received PLD. Compared with the ITT population, a higher proportion of patients in both treatment arms of the PD-L1–positive population had undergone debulking surgery within 6 months of inclusion, and tumor size was generally smaller. Additionally, within the PD-L1–positive population, there was a slight imbalance in previous lines of chemotherapy.
FIG 1.

CONSORT diagram. ITT, intention-to-treat.
TABLE 1.
Baseline Characteristics
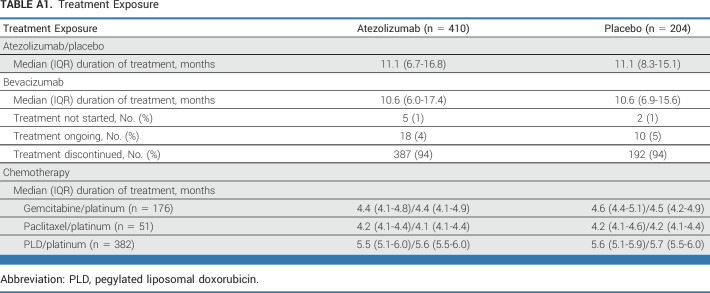
At the primary analysis data cutoff (October 15, 2021), the median duration of follow-up was 36.6 (95% CI, 35.1 to 38.7) months. In both arms, the median duration of study treatment was 11.1 months and the median bevacizumab duration was 10.6 months (Appendix Table A1, online only). The duration of each chemotherapy was balanced between treatment arms, and was slightly longer in the PLD subgroup, reflecting the once every 4 weeks schedule.
Efficacy
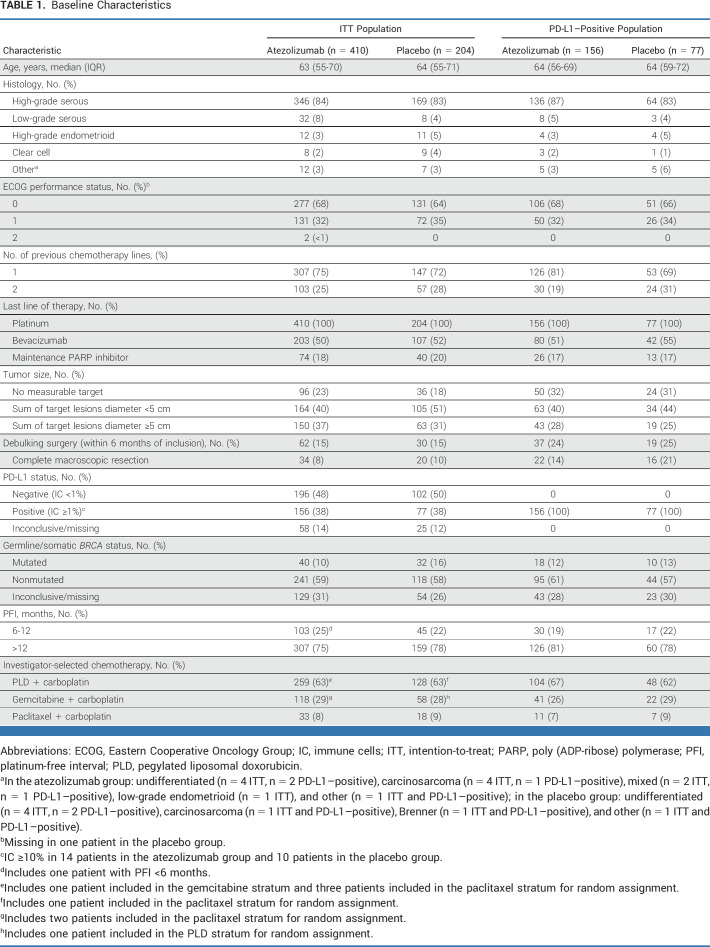
In the ITT population, the PFS HR was 0.83 (95% CI, 0.69 to 0.99; P = .041), which did not meet the coprimary objective (Fig 2A). Median PFS was 13.5 versus 11.3 months with atezolizumab versus placebo, respectively. In the PD-L1–positive population the PFS HR was 0.86 (95% CI, 0.63 to 1.16; P = .30), which did not meet the criteria for statistical significance (Fig 2B). Median PFS was 15.2 versus 13.1 months with atezolizumab versus placebo, respectively.
FIG 2.
PFS: (A) ITT population (N = 614); (B) PD-L1–positive population (n = 233). PFS was defined as the interval between random assignment and the date of first objective radiologic disease progression (investigator-assessed per RECIST version 1.1) or death. Sensitivity analyses in the per-protocol population showed consistent results (ITT per-protocol population: HR, 0.82; 95% CI, 0.69 to 0.99; P = .037; median 13.6 months with atezolizumab v 11.3 months with placebo). Shaded area for each line represents the 95% CI. HR, hazard ratio; ITT, intention-to-treat; PFS, progression-free survival.
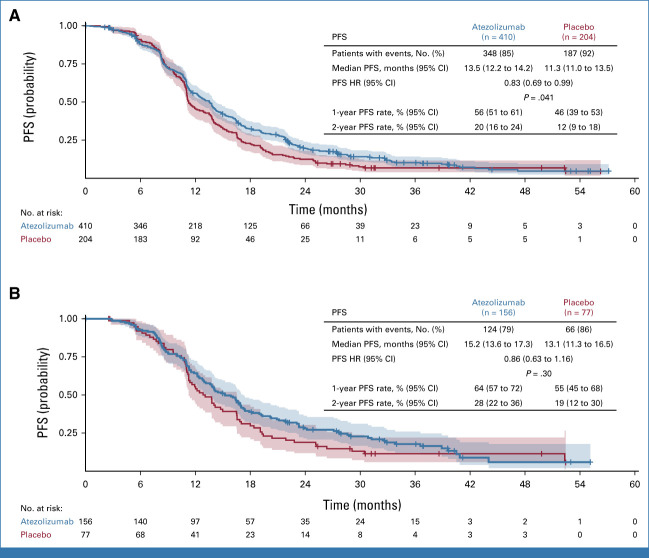
Subgroup analysis of PFS showed no significant interaction with atezolizumab treatment in any of the subgroups evaluated, including the prespecified subgroup of patients with both PD-L1–positive and CD8-positive tumors according to baseline biopsy (Fig 3). There was no evidence of an enhanced atezolizumab treatment effect in the PLD cohort compared with other regimens.
FIG 3.
Subgroup analysis of PFS (intention-to-treat population). Subgroup analyses according to randomization stratification factors categorized patients for PFI, PD-L1 status, and chemotherapy cohort as recorded in the electronic case report form. CA-125, cancer antigen-125; HR, hazard ratio; PFS, progression-free survival; PLD, pegylated liposomal doxorubicin; SLD, sum of largest diameter.
As neither coprimary end point reached the threshold for statistical significance, the remaining end points were not formally tested. In the ITT population, the ORR was 62% with atezolizumab versus 66% with placebo; in the PD-L1–positive population, ORRs were 62% versus 61%, respectively.
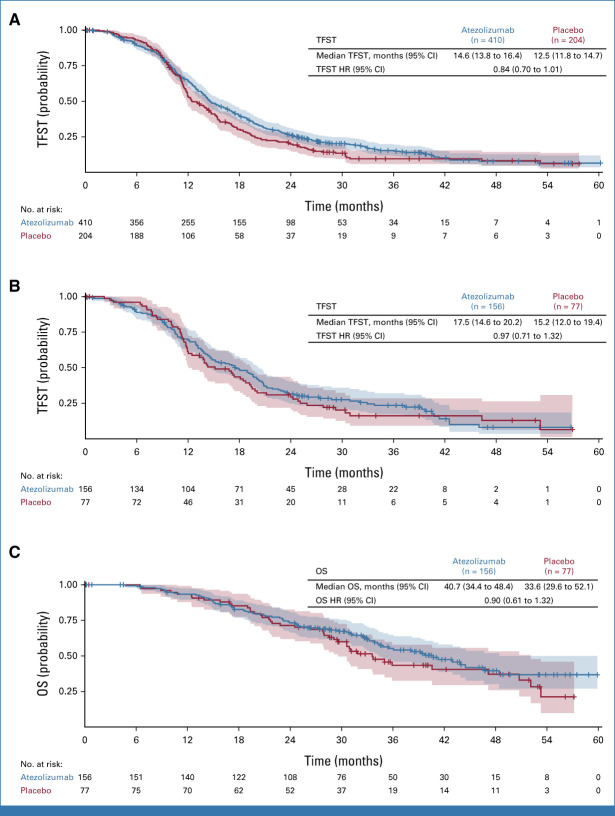
TFST numerically favored atezolizumab in the ITT population (HR, 0.84; 95% CI, 0.70 to 1.01; median 14.6 months with atezolizumab v 12.5 months with placebo) but there was negligible difference in the PD-L1–positive population (HR, 0.97; 95% CI, 0.71 to 1.32; median, 17.5 v 15.2 months, respectively; Appendix Fig A1).
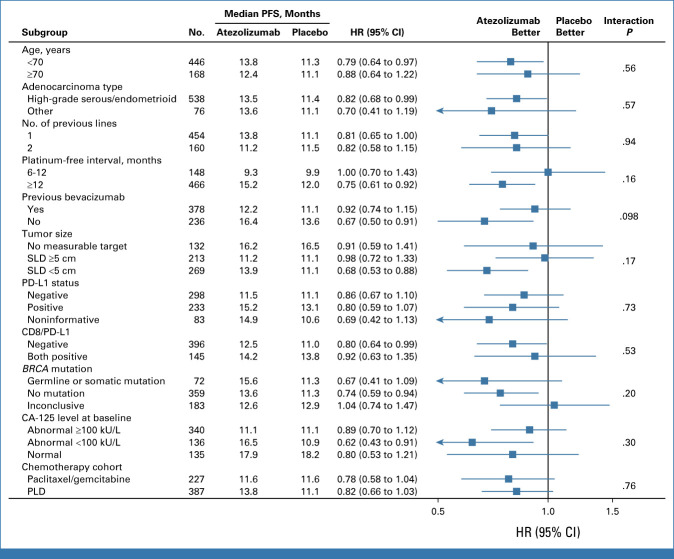
TSST, which overlaps to some extent with PFS2, showed a numerical trend favoring atezolizumab-containing therapy in the ITT population (Fig 4A). The TSST HR was 0.82 (95% CI, 0.67 to 1.01), with median values of 23.9 versus 21.4 months in the atezolizumab versus placebo arms, respectively. The separation of the Kaplan-Meier curves over time is illustrated by the increasing absolute difference in the proportions of patients without TSST events (85% v 83%, respectively, at 1 year; 49% v 43%, respectively, at 2 years; 31% v 19%, respectively, at 3 years).
FIG 4.
Secondary end points. (A) Time to second subsequent therapy in the ITT population. (B) OS (interim analysis) in the ITT population. Shaded area for each line represents the 95% CI. HR, hazard ratio; ITT, intention-to-treat; OS, overall survival; TSST, time from random assignment to start of second subsequent therapy or death.
At the data cutoff for the primary analysis, OS data were immature (333 of 491 events required for final OS analysis). However, there was a numerical trend favoring atezolizumab in the ITT population (Fig 4B). Median OS was 35.5 versus 30.6 months with atezolizumab versus placebo, respectively. The OS HR was 0.81 (95% CI, 0.65 to 1.01), and the Kaplan-Meier curves showed increasing separation over time, as indicated by the OS rates at 1 year (89% with atezolizumab v 87% with placebo), 2 years (67% v 62%, respectively), and 3 years (49% v 38%, respectively). The proportion of events was lower in the PD-L1–positive than in the ITT population, consistent with the better prognosis associated with PD-L1 positivity. The OS HR was 0.90 (95% CI, 0.61 to 1.32). Median OS was 40.7 (95% CI, 34.4 to 48.4) months versus 33.6 (95% CI, 29.6 to 52.1) months with atezolizumab versus placebo, respectively (Appendix Fig A1C).
HRQoL
At baseline, there was no difference between treatment arms in QLQ-C30 global health score. Mean change from baseline in global health score showed no significant impact of treatment on HRQoL over time in either treatment arm, and there was no difference in mean change over time between treatment arms (P = .074; Appendix Fig A2).
Safety
The frequency of grade ≥3 AEs was similar in the atezolizumab and placebo groups (88% v 87%, respectively); treatment-related grade ≥3 AEs occurred in 33% versus 35%, respectively. AEs were fatal in 14 (3%) atezolizumab-treated patients and five (2%) placebo-treated patients. Of these, five were considered to be treatment-related: cardiac arrest, peritonitis, and acute myeloid leukemia with atezolizumab, and pulmonary embolism and bowel perforation with placebo.
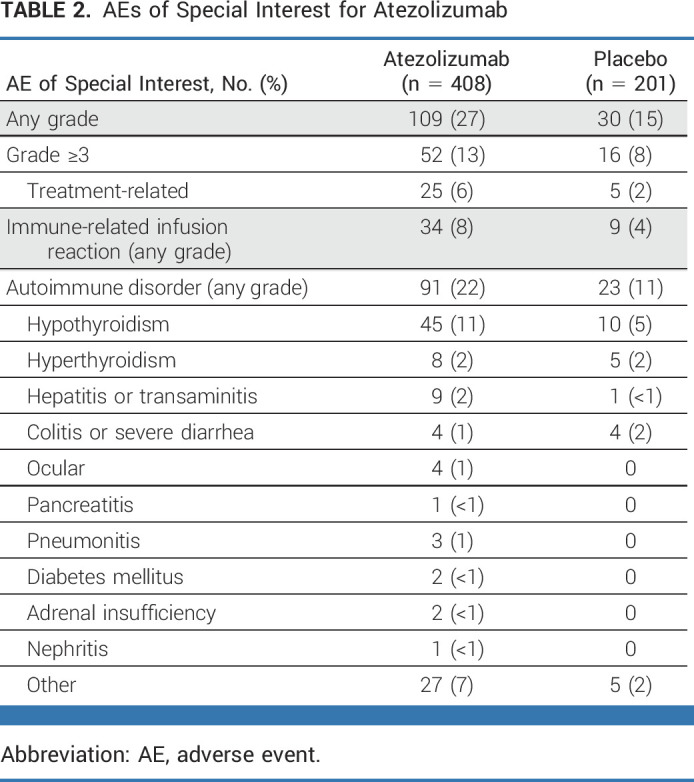
AEs of special interest for atezolizumab were more common with atezolizumab than with placebo (all grades: 27% v 15%, respectively; grade ≥3: 13% v 8%, respectively; Table 2). Immune-related infusion reactions (8% v 4%, respectively) and autoimmune disorders (22% v 11%, respectively) were twice as common with atezolizumab than with placebo, the latter driven by hypothyroidism (11% v 5%, respectively).
TABLE 2.
AEs of Special Interest for Atezolizumab
A higher proportion of patients discontinued atezolizumab than placebo because of AEs (25% v 18%, respectively); bevacizumab was discontinued for AEs in 34% versus 27%, respectively, and similar proportions of patients in both arms discontinued chemotherapy because of AEs (15% v 15%, respectively). Similar proportions of patients temporarily interrupted atezolizumab or placebo because of AEs (65% v 63%, respectively).
DISCUSSION
To our knowledge, ATALANTE/ENGOT-ov29 is the first randomized phase III trial reporting outcomes in patients receiving an ICI with standard therapy for platinum-sensitive OC. The trial did not meet its coprimary objective in either the ITT or the PD-L1–positive population. Safety was consistent with previous atezolizumab trials and the individual profiles of each drug and their combination.
In the control arm, median PFS durations of 11.3 months in the ITT population (11.1 months in the PLD subgroup) and 13.1 months in the PD-L1–positive population were slightly shorter than assumed when designing the trial, but broadly consistent with recent phase III trials evaluating these regimens.13,24 Only 38% of patients had PD-L1–positive tumors, lower than the 60% prevalence observed in IMagyn050 using the same assay but in the neoadjuvant/frontline setting.15 A potential explanation is the influence of disease evolution and/or exposure to antineoplastic therapies on tumor immunogenicity in platinum-sensitive recurrent OC compared with untreated newly diagnosed OC.25 In JAVELIN Ovarian 200, the PD-L1 prevalence was 57%, albeit in platinum-resistant/refractory disease using a different assay and scoring method.7
In both JAVELIN Ovarian 200 and IMagyn050, the effect of ICI was more pronounced in the PD-L1–positive than in the ITT population.7,15 Consequently, the ATALANTE/ENGOT-ov29 protocol was amended during the trial to include PFS in the PD-L1–positive population as a coprimary end point, assuming that the larger-than-expected population of patients with PD-L1–negative disease was less likely to benefit from atezolizumab (on the basis of findings from IMagyn05015 and atezolizumab trials in other tumor types17,26,27). However, in ATALANTE/ENGOT-ov29, there was no clear signal that patients with PD-L1–positive tumors derived greater benefit from atezolizumab. Plausibly, IC ≥1% may not represent the optimal cutoff for PD-L1 positivity in OC, as suggested by exploratory analyses of IMagyn050 in patients with IC ≥5%.15 A higher cutoff (as used in urothelial carcinoma) may be more appropriate to define PD-L1 positivity in OC and analyses exploring a higher cutoff may be of interest. Additionally, exploration of PD-L1 expression on tumor cells (v ICs) is justified, as done in IMAGYN05015 and JAVELIN Ovarian 200.7 However, only 66 patients (11%) in ATALANTE/ENGOT-ov29 had PD-L1 expression of IC ≥5% and 24 patients (4%) had IC ≥10%, thus sample sizes may be too small for meaningful interpretation.
The trial also tested treatment effect in the prespecified subgroup of patients with PD-L1–positive CD8-positive tumors. It was hypothesized that PD-L1 inhibition would be ineffectual if there were no cytotoxic lymphocytes within the tumor. In JAVELIN Ovarian 200, positive PD-L1 and CD8 T-cell tumor status appeared to define a subgroup of patients deriving both PFS and OS benefit from the addition of avelumab to single-agent PLD.7 However, these findings were not replicated with atezolizumab in ATALANTE/ENGOT-ov29: there was no pronounced benefit from ICIs in patients with PD-L1–positive ‘hot’ tumors and the anticipated unleashing of CD8 against the tumor was not observed, although interpretation is limited by the small sample size of this subgroup.
Although PFS differences did not reach statistical significance, preliminary OS results showed an encouraging signal and warrant further analyses with longer follow-up. In other tumor types, the effect of immunotherapy on ORR and PFS has been modest but the impact on OS and late PFS has been greater.28,29 Thus, a potential criticism of the trial is that OS was not a coprimary objective, although it was planned for hierarchical testing if either PFS coprimary objective was met. As in previous OC trials of immunotherapy, any treatment effect was not immediately apparent, emerging later and with a greater impact on the tail of the curve. Final OS results are anticipated in late 2024 and will provide important information on long-term effects in patients potentially deriving sustained benefit from ICIs.
Additional possible criticisms include the heterogeneity of the enrolled population (including patients with low-grade serous, high-grade endometrioid, and clear-cell carcinoma). However, when the trial was designed, it was unclear whether ICIs may be effective in histologic subtypes beyond high-grade serous OC. Subgroup analyses within IMagyn050, although in few patients, suggest a similar or enhanced atezolizumab effect in non–high-grade serous subtypes.15 Furthermore, PD-1 blockade demonstrated promising activity in heavily pretreated clear-cell carcinoma in the PEACOCC trial,30 and the BOUQUET trial (ClinicalTrials.gov identifier: NCT04931342) is evaluating atezolizumab in rare histologies. Similarly, some may criticize enrollment of an all-comer population without selecting patients according to molecular subtype. However, without evidence of a biomarker that reliably identifies patients deriving greater benefit from atezolizumab, it is impossible to design a trial in a biomarker-selected population. When the trial was designed, PD-L1 status seemed the most promising marker and our trial aimed, but failed, to validate these findings.
A strength of the trial is the rigorous collection of de novo tumor samples, providing a rich resource for translational research. To our best knowledge, this is the first report of PD-L1 status from de novo biopsies in platinum-sensitive OC. An extensive biomarker program aims to deepen understanding of the immunologic landscape of platinum-sensitive disease. This may provide clues for new research avenues, perhaps generating hypotheses for new targets and approaches as alternatives to ICIs. This fifth negative trial of PD-(L)1 inhibition in epithelial OC7,14,15,31 may dampen our enthusiasm for inhibiting this pathway in OC using the strategies evaluated to date, but ongoing trials evaluating ICIs (including agents targeting other pathways) with PARP inhibitors and in different treatment settings and combinations may yet elucidate a role. It remains to be seen whether blockade of PD-1 rather than PD-L1 yields greater efficacy in OC. The MK-7339-001/KEYLYNK-001/ENGOT-ov43/GOG-3036 (ClinicalTrials.gov identifier: NCT03740165) and ATHENA-COMBO (ClinicalTrials.gov identifier: NCT03522246) trials have completed recruitment.
ACKNOWLEDGMENT
The authors thank all patients and their families, the study investigators (listed in the Appendix 1), the staff from the participating study groups (Ophélie Baconnet, Christine Montoto-Grillot, Yassine Omri, Espérance Nzeymana, Sébastien Armanet, Bénédicte Votan [ARCAGY-GINECO], Anja Krueger [AGO Study Group], Angela Riha [AGO-Austria], Eve Peeraer [BGOG], Ivana Nohova [CEEGOG], and Ana Levin [GEICO]), Amandine Pommier (Euraxi Pharma), the statisticians (Bernard Asselain and Monica Turinici), the staff from the ARCAGY-GINECO Translational Research Centre at Institut Curie (Alexandre Degnieau and Eloïse Glais), members of the Independent Data Monitoring Committee (Cristiana Sessa, James Paul, and Diane Provencher), and F. Hoffmann-La Roche for funding the trial. The authors also acknowledge Jennifer Kelly, MA (Medi-Kelsey Ltd, Ashbourne, United Kingdom), for medical writing assistance, funded by ARCAGY-GINECO.
APPENDIX 1. ATALANTE/ENGOT-ov29 INVESTIGATORS
J.-E. Kurtz, S. Abadie-Lacourtoisie, C. Abdeddaim, J. Alexandre, P. Augereau, D. Avenin, M. Azemar, N. Baba-Hamed, O. Bally, J. Barriere, F. Bazan, D. Berton, N. Bonichon-Lamichhane, N. Bonnin, E. Boughalem, R. Boustany Grenier, P.-E. Brachet, F. Brocard, E. Bultot-Boissier, M.A. Cappiello-Bataller, H. Castanie, L. Chaigneau, C. Chakiba, D. Chocteau-Bouju, P. Combe, A. Comte, E. Coquan, C. Costan, P. Cottu, L. Crouzet, H. Cure, J. Dauba, H. Dawood, L. De Cock, T. De La Motte Rouge, C. Debelleix, C. Delbaldo, M. Demarchi, M. Deslandres, R. Despax, V. D’Hondt, A.F. Dillies-Legrain, A. Donnadieu, C. Dubot, J.-M. Extra, M. Fabbro, C. Falandry, F. Fiteni, A. Floquet, P. Follana, J.S. Frenel, G. Freyer, D. Garbay-Decoopman, C. Gavoille, V. Girre, L. Gladieff, F. Goldwasser, A. Gratet, J. Grenier, A.-C. Hardy-Bessard, P.-E. Heudel, F. Joly, A. Jouinot, E. Kalbacher, M.-C. Kaminsky, L. Lancry-Lecomte, R. Largillier, F. Le Du, A. Leary, C. Lebreton, C. Lefeuvre-Plesse, A. Lesoin, T. L’Haridon, J. Long, A. Lortholary, J.-P. Lotz, L. Mansi, J. Martin-Babau, M. Martinez, J. Medioni, E. Meriaux, J. Meunier, L. Moïse, J.-F. Moulin, M.-A. Mouret-Reynier, M. Mousseau, P. Pautier, J. Peron, C. Perrin, T. Petit, D. Petran, F. Priou, M. Provansal, N. Raban, I. Ray-Coquard, P. Regnault De La Mothe, C. Riedl, M. Rodrigues, C. Roemer-Becuwe, R. Sabatier, F. Savinelli, C. Sebbag, F. Selle, P. Soulie, D. Spaeth, R. Sverdlin, M. Timar-David, O. Tredan, P. Tresca, V. Trillet-Lenoir, T. Valentin, Y.A. Vano, B. You (GINECO, France), F. Heitz, H. Bronger, P. Buderath, N. De Gregorio, T. Fehm, E.-M. Grischke, M. Gropp-Meier, A. Hartkopf, C. Jackisch, M. Koegel, A. Mueller, T.-W. Park-Simon, I. Runnebaum, F. Schochter, B. Schmalfeldt, B. Schnappauf, J. Sehouli, F. Trillsch, P. Wimberger (AGO Study Group, Germany), A. Oaknin, J.D. Alarcon, S. Alonso, A. Alonso Herrero, P. Barretina, A. Casado, C. Churruca, I. Fernandez, L. Gaba, J. Garcia-Donas, S. Gonzalez, G. Marquina, J. Martinez-García, A. Redondo, D. Vicente (GEICO, Spain), C. Marth, S. Aust, E. Petru, A. Reinthaller, C. Schauer (AGO-Austria, Austria), D. Cibula (CEEGOG, Czech Republic), I. Vergote, S. Altintas, H. Denys, E. Van Nieuwenhuysen (BGOG, Belgium), O. Rosengarten (ISGO, Israel).
APPENDIX 2. SUPPLEMENTARY MATERIALS
Testing Proportional Hazard Assumptions
Progression-free survival (PFS) distributions were compared between the two study arms using a Cox model including a potential time-dependent treatment effect and adjusted on the stratification factors platinum-free interval, PD-L1 expression, and investigator-selected chemotherapy.
where Z is the treatment arm (0 v 1), b1 the non–time-dependent coefficient, and b2 the time-dependent coefficient associated with a potential modification of the treatment effect with time. To study proportional hazard assumptions, we first tested the following null hypothesis: H0: b2 = 0, using a likelihood ratio test. In the intention-to-treat and PD-L1–positive populations, the P values of the likelihood ratio tests were .78 and .44, respectively, suggesting there is no time dependency. These results were also confirmed by the Harrel test (P = .58 and .77, respectively), confirming that the proportional hazard assumption was not violated. Diagnosis of proportional hazard assumptions was also assessed graphically with Schoenfeld's residuals. No major deviation to proportional hazard assumptions was detected (Appendix Fig A3). Therefore, the primary analysis of PFS used an adjusted Cox model. Likewise, in the PD-L1–positive population, there was no violation of the proportional hazard assumption and, therefore, PFS was analyzed using an adjusted Cox model.
TABLE A1.
Treatment Exposure
FIG A1.
(A) TFST in the intention-to-treat population. (B) TFST in the PD-L1–positive population. (C) OS in the PD-L1–positive population (interim analysis). HR, hazard ratio; OS, overall survival; TFST, time to first subsequent therapy.
FIG A2.
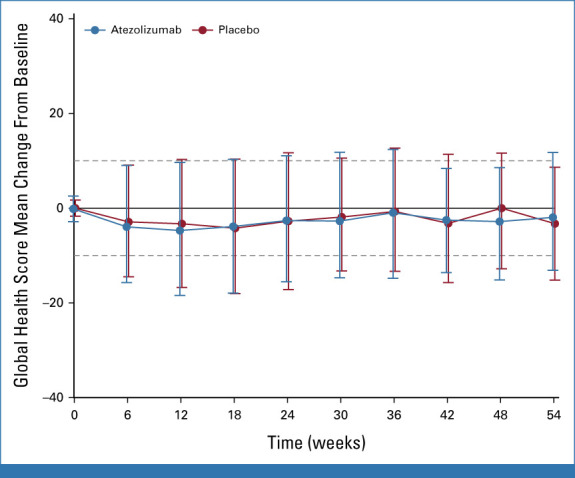
Mean change from baseline in HRQoL. Dashed lines represent the 10% threshold considered to be a clinically relevant change. Error bars represent 95% CIs. HRQoL was scored according to EORTC guidelines, described by mean and standard deviations, and compared between treatment arm using a linear mixed model. EORTC, European Organisation for Research and Treatment of Cancer; HRQoL, health-related quality of life.
FIG A3.
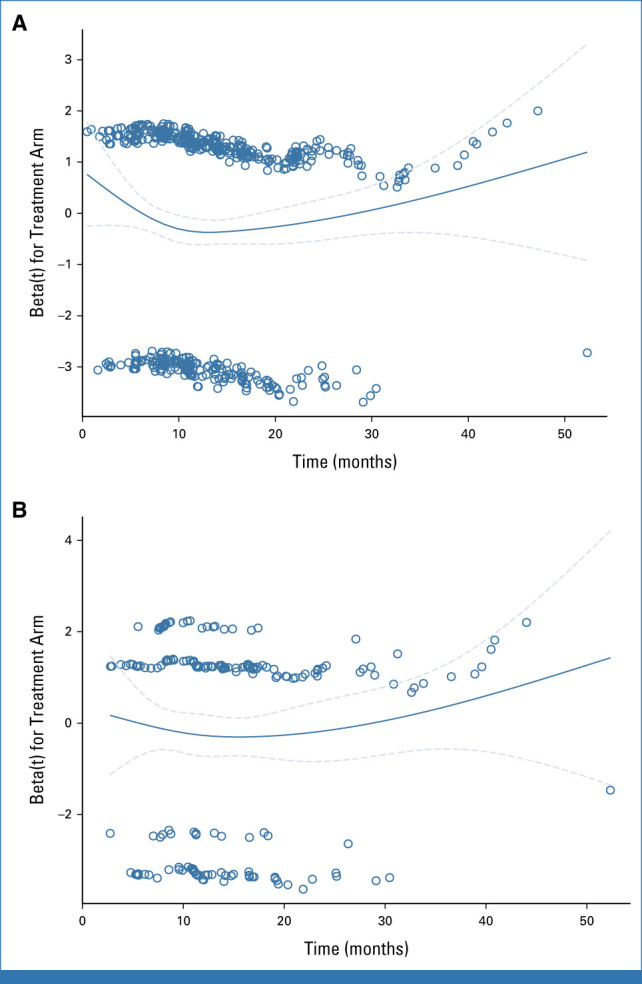
Schoenfeld's residuals for progression-free survival. (A) Intention-to-treat population. (B) PD-L1–positive population. The solid lines represent a smoothed estimate of beta. The dashed lines represent 95% CIs of this estimate.
Jean-Emmanuel Kurtz
Employment: MSD (I)
Honoraria: GlaxoSmithKline, Clovis Oncology
Consulting or Advisory Role: Tesaro, AstraZeneca, Clovis Oncology, Dragonfly Therapeutics
Travel, Accommodations, Expenses: Roche, PharmaMar, Tesaro
Eric Pujade-Lauraine
Employment: ARCAGY-GINECO
Honoraria: AstraZeneca, GlaxoSmithKline
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Incyte, Agenus
Research Funding: AstraZeneca (Inst)
Other Relationship: ARCAGY-GINECO
Ana Oaknin
Consulting or Advisory Role: AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana, GSK, Deciphera, Agenus, Corcept Therapeutics, Eisai, Roche, Merck Sharp & Dohme, Novocure, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, Seagen, OneXerna Therapeutics, Inc, Regeneron, Exelixis
Research Funding: AbbVie (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), Eisai (Inst), Roche (Inst), Regeneron (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Immunogen (Inst), Medimmune (Inst), Merck Sharp & Dohme (Inst), Tesaro (Inst), Amgen (Inst), Millennium Pharmaceuticals Inc (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Katharina Leitner
Travel, Accommodations, Expenses: Seagen, GlaxoSmithKline
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche, GlaxoSmithKline, Novocure, Akeso Biopharma, MSD, Mersana
Hannelore Denys
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), AstraZeneca (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), GlaxoSmithKline (Inst), MSD (Inst), Seagen (Inst), Gilead Sciences (Inst)
Research Funding: Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), Teva (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Manuel Rodrigues
Honoraria: Immunocore
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: Daiichi Sankyo/AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Nikolaus de Gregorio
Consulting or Advisory Role: MSD, Roche, Eisai Germany, AstraZeneca, GlaxoSmithKline, Myriad Genetics, Novartis, Clovis Oncology, Gilead Sciences
Travel, Accommodations, Expenses: AstraZeneca
Jeronimo Martinez Garcia
Consulting or Advisory Role: AstraZeneca Spain, GlaxoSmithKline
Travel, Accommodations, Expenses: PharmaMar, Roche, MSD Oncology
Edgar Petru
Honoraria: Roche, AstraZeneca, Daiichi Sankyo/AstraZeneca
Consulting or Advisory Role: Roche, Daiichi Sankyo/AstraZeneca
Travel, Accommodations, Expenses: Roche, AstraZeneca/Daiichi Sankyo
Ignace Vergote
Consulting or Advisory Role: AstraZeneca, Elevar Therapeutics, Genmab, Immunogen, Jazz Pharmaceuticals, Mersana, MSD, Novocure, Sotio, Verastem, Zentalis, Roche, Agenus, Eisai, Novartis, Seagen, Akeso Biopharma, Bristol Myers Squibb, Deciphera, Exelixis, GlaxoSmithKline, Karyopharm Therapeutics, Oncoinvent, OncXerna Therapeutics, Regeneron, Sanofi
Research Funding: Roche (Inst), Amgen (Inst), Oncoinvent (Inst)
Travel, Accommodations, Expenses: Karyopharm Therapeutics, Genmab, Novocure
Patricia Pautier
Consulting or Advisory Role: MSD Oncology (Inst), AstraZeneca (Inst), Onxeo (Inst), PharmaMar (Inst), MSD vaccines (Inst), Seagen
Research Funding: PharmaMar (Inst), Onxeo (Inst)
Expert Testimony: Onxeo (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: AstraZeneca, MSD Oncology, GlaxoSmithKline, OSE Immunotherapeutics, PharmaMar, Amgen
Other Relationship: PharmaMar
Barbara Schmalfeldt
Honoraria: Roche/Genentech, AstraZeneca, GlaxoSmithKline, Eisai, Clovis Oncology
Consulting or Advisory Role: Roche/Genentech, GlaxoSmithKline, MSD Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, GlaxoSmithKline, Eisai Germany
Research Funding: MSD Oncology, GlaxoSmithKline, AstraZeneca, Roche Pharma AG
Travel, Accommodations, Expenses: GlaxoSmithKline, Roche Pharma AG
Lydia Gaba
Consulting or Advisory Role: GlaxoSmithKline, MSD Oncology, AstraZeneca Spain, Clovis Oncology, PharmaMar
Travel, Accommodations, Expenses: GlaxoSmithKline/Tesaro, MSD/AstraZeneca
Stephan Polterauer
Honoraria: AstraZeneca, MSD, GlaxoSmithKline, Sandoz
Consulting or Advisory Role: AstraZeneca, MSD, GlaxoSmithKline, Eisai
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, GlaxoSmithKline
Jalid Sehouli
Honoraria: AstraZeneca, Eisai, Clovis Oncology, Olympus Medical Systems, Johnson & Johnson, PharmaMar, Pfizer, Teva, Tesaro, MSD Oncology, GlaxoSmithKline, Bayer
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, PharmaMar, Merck, Pfizer, Tesaro, MSD Oncology, Lilly, Novocure, Johnson & Johnson, Roche, Ingress Health, Riemser, Sobi, GlaxoSmithKline, Novartis, Alkermes
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Merck (Inst), Bayer (Inst), PharmaMar (Inst), Pfizer (Inst), Tesaro (Inst), MSD Oncology (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche Pharma AG, Tesaro, MSD Oncology, Olympus
Cristina Churruca
Consulting or Advisory Role: GlaxoSmithKline
Speakers' Bureau: PharmaMar
Travel, Accommodations, Expenses: GSK, MSD
Frédéric Selle
Honoraria: AstraZeneca, Clovis Oncology, MSD, GlaxoSmithKline/Tesaro, Sandoz
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline/Tesaro, MSD
Speakers' Bureau: AstraZeneca, MSD, GlaxoSmithKline/Tesaro
Travel, Accommodations, Expenses: Roche, AstraZeneca, MSD, GlaxoSmithKline/Tesaro
Florence Joly
Consulting or Advisory Role: AstraZeneca, Janssen, Ipsen, Pfizer, MSD Oncology, Bristol Myers Squibb, GlaxoSmithKline, Astellas Pharma, Clovis Oncology, Amgen, Seagen, Bayer, 3A Pharmaceuticals & Diagnostics, Eisai
Travel, Accommodations, Expenses: Janssen, AstraZeneca, Ipsen, GlaxoSmithKline, BMS, Eisai, MSD Oncology
Véronique D'Hondt
Travel, Accommodations, Expenses: GlaxoSmithKline, Lilly, Pfizer
Coriolan Lebreton
Honoraria: Eisai, Clovis Oncology, MSD Oncology, GlaxoSmithKline
Travel, Accommodations, Expenses: GlaxoSmithKline
Pierre-Etienne Heudel
Stock and Other Ownership Interests: GeodAIsics
Honoraria: Pfizer, Novartis (Inst), Seagen, Pierre Fabre, Amgen, AstraZeneca (Inst), Roche, Mylan, Gilead Sciences
Consulting or Advisory Role: Novartis (Inst), Seagen, Lilly
Research Funding: Fresenius (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Roche, Lilly
Florian Heitz
Honoraria: Roche, AstraZeneca, Tesaro/GSK
Consulting or Advisory Role: Roche, AstraZeneca, GlaxoSmithKline, Novocure, PharmaMar
Research Funding: AstraZeneca (Inst), Amedes (Inst)
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology Congress 2022, Paris, France, September 9-13, 2022.
SUPPORT
Supported by F. Hoffmann-La Roche, Ltd (no grant number applicable). The study was performed by Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens (GINECO), the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group, Grupo Español de Investigación en Cáncer de Ovario (GEICO), AGO-Austria, Central and Eastern European Gynecologic Oncology Group (CEEGOG), Belgium and Luxembourg Gynaecological Oncology Group (BGOG), and Israeli Society of Gynecologic Oncology (ISGO). The sponsor (GINECO, represented by the first author) was responsible for writing the report with the support of a medical writer. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
CLINICAL TRIAL INFORMATION
NCT02891824 (ATALANTE/ENGOT-ov29)
DATA SHARING STATEMENT
Currently, no mechanism is in place to allow sharing of individual deidentified patient data. Requests sent to ARCAGY-GINECO will be considered on a case-by-case basis.
AUTHOR CONTRIBUTIONS
Conception and design: Jean-Emmanuel Kurtz, Eric Pujade-Lauraine
Administrative support: Eric Pujade-Lauraine
Provision of study materials or patients: Jean-Emmanuel Kurtz, Eric Pujade-Lauraine, Ana Oaknin, Katharina Leitner, David Cibula, Hannelore Denys, Ora Rosengarten, Manuel Rodrigues, Nikolaus de Gregorio, Jeronimo Martinez García, Edgar Petru, Roman Kocián, Ignace Vergote, Patricia Pautier, Barbara Schmalfeldt, Lydia Gaba, Stephan Polterauer, Marie-Ange Mouret Reynier, Jalid Sehouli, Cristina Churruca, Frédéric Selle, Florence Joly, Véronique D'Hondt, Émilie Bultot-Boissier, Coriolan Lebreton, Jean-Pierre Lotz, Rémy Largillier, Pierre-Etienne Heudel, Florian Heitz
Collection and assembly of data: All authors
Data analysis and interpretation: Lisa Belin, Jean-Emmanuel Kurtz, Eric Pujade-Lauraine
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Atezolizumab Combined With Bevacizumab and Platinum-Based Therapy for Platinum-Sensitive Ovarian Cancer: Placebo-Controlled Randomized Phase III ATALANTE/ENGOT-ov29 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jean-Emmanuel Kurtz
Employment: MSD (I)
Honoraria: GlaxoSmithKline, Clovis Oncology
Consulting or Advisory Role: Tesaro, AstraZeneca, Clovis Oncology, Dragonfly Therapeutics
Travel, Accommodations, Expenses: Roche, PharmaMar, Tesaro
Eric Pujade-Lauraine
Employment: ARCAGY-GINECO
Honoraria: AstraZeneca, GlaxoSmithKline
Consulting or Advisory Role: AstraZeneca, Roche, Merck, Incyte, Agenus
Research Funding: AstraZeneca (Inst)
Other Relationship: ARCAGY-GINECO
Ana Oaknin
Consulting or Advisory Role: AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab, Mersana, GSK, Deciphera, Agenus, Corcept Therapeutics, Eisai, Roche, Merck Sharp & Dohme, Novocure, Shattuck Labs, Sutro Biopharma, ITeos Therapeutics, Seagen, OneXerna Therapeutics, Inc, Regeneron, Exelixis
Research Funding: AbbVie (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Aprea Therapeutics (Inst), Clovis Oncology Inc (Inst), Eisai (Inst), Roche (Inst), Regeneron (Inst), Bristol Myers Squibb International Corporation (BMS) (Inst), Immunogen (Inst), Medimmune (Inst), Merck Sharp & Dohme (Inst), Tesaro (Inst), Amgen (Inst), Millennium Pharmaceuticals Inc (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Katharina Leitner
Travel, Accommodations, Expenses: Seagen, GlaxoSmithKline
David Cibula
Consulting or Advisory Role: AstraZeneca, Sotio, Roche, GlaxoSmithKline, Novocure, Akeso Biopharma, MSD, Mersana
Hannelore Denys
Consulting or Advisory Role: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), AstraZeneca (Inst), Lilly (Inst), Novartis (Inst), Amgen (Inst), GlaxoSmithKline (Inst), MSD (Inst), Seagen (Inst), Gilead Sciences (Inst)
Research Funding: Gilead Sciences (Inst)
Travel, Accommodations, Expenses: Pfizer (Inst), Roche (Inst), PharmaMar (Inst), Teva (Inst), AstraZeneca (Inst), Gilead Sciences (Inst)
Manuel Rodrigues
Honoraria: Immunocore
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline
Research Funding: Daiichi Sankyo/AstraZeneca
Travel, Accommodations, Expenses: AstraZeneca
Nikolaus de Gregorio
Consulting or Advisory Role: MSD, Roche, Eisai Germany, AstraZeneca, GlaxoSmithKline, Myriad Genetics, Novartis, Clovis Oncology, Gilead Sciences
Travel, Accommodations, Expenses: AstraZeneca
Jeronimo Martinez Garcia
Consulting or Advisory Role: AstraZeneca Spain, GlaxoSmithKline
Travel, Accommodations, Expenses: PharmaMar, Roche, MSD Oncology
Edgar Petru
Honoraria: Roche, AstraZeneca, Daiichi Sankyo/AstraZeneca
Consulting or Advisory Role: Roche, Daiichi Sankyo/AstraZeneca
Travel, Accommodations, Expenses: Roche, AstraZeneca/Daiichi Sankyo
Ignace Vergote
Consulting or Advisory Role: AstraZeneca, Elevar Therapeutics, Genmab, Immunogen, Jazz Pharmaceuticals, Mersana, MSD, Novocure, Sotio, Verastem, Zentalis, Roche, Agenus, Eisai, Novartis, Seagen, Akeso Biopharma, Bristol Myers Squibb, Deciphera, Exelixis, GlaxoSmithKline, Karyopharm Therapeutics, Oncoinvent, OncXerna Therapeutics, Regeneron, Sanofi
Research Funding: Roche (Inst), Amgen (Inst), Oncoinvent (Inst)
Travel, Accommodations, Expenses: Karyopharm Therapeutics, Genmab, Novocure
Patricia Pautier
Consulting or Advisory Role: MSD Oncology (Inst), AstraZeneca (Inst), Onxeo (Inst), PharmaMar (Inst), MSD vaccines (Inst), Seagen
Research Funding: PharmaMar (Inst), Onxeo (Inst)
Expert Testimony: Onxeo (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: AstraZeneca, MSD Oncology, GlaxoSmithKline, OSE Immunotherapeutics, PharmaMar, Amgen
Other Relationship: PharmaMar
Barbara Schmalfeldt
Honoraria: Roche/Genentech, AstraZeneca, GlaxoSmithKline, Eisai, Clovis Oncology
Consulting or Advisory Role: Roche/Genentech, GlaxoSmithKline, MSD Oncology, AstraZeneca
Speakers' Bureau: Roche/Genentech, AstraZeneca, GlaxoSmithKline, Eisai Germany
Research Funding: MSD Oncology, GlaxoSmithKline, AstraZeneca, Roche Pharma AG
Travel, Accommodations, Expenses: GlaxoSmithKline, Roche Pharma AG
Lydia Gaba
Consulting or Advisory Role: GlaxoSmithKline, MSD Oncology, AstraZeneca Spain, Clovis Oncology, PharmaMar
Travel, Accommodations, Expenses: GlaxoSmithKline/Tesaro, MSD/AstraZeneca
Stephan Polterauer
Honoraria: AstraZeneca, MSD, GlaxoSmithKline, Sandoz
Consulting or Advisory Role: AstraZeneca, MSD, GlaxoSmithKline, Eisai
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, GlaxoSmithKline
Jalid Sehouli
Honoraria: AstraZeneca, Eisai, Clovis Oncology, Olympus Medical Systems, Johnson & Johnson, PharmaMar, Pfizer, Teva, Tesaro, MSD Oncology, GlaxoSmithKline, Bayer
Consulting or Advisory Role: AstraZeneca, Clovis Oncology, PharmaMar, Merck, Pfizer, Tesaro, MSD Oncology, Lilly, Novocure, Johnson & Johnson, Roche, Ingress Health, Riemser, Sobi, GlaxoSmithKline, Novartis, Alkermes
Research Funding: AstraZeneca (Inst), Clovis Oncology (Inst), Merck (Inst), Bayer (Inst), PharmaMar (Inst), Pfizer (Inst), Tesaro (Inst), MSD Oncology (Inst), Roche (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche Pharma AG, Tesaro, MSD Oncology, Olympus
Cristina Churruca
Consulting or Advisory Role: GlaxoSmithKline
Speakers' Bureau: PharmaMar
Travel, Accommodations, Expenses: GSK, MSD
Frédéric Selle
Honoraria: AstraZeneca, Clovis Oncology, MSD, GlaxoSmithKline/Tesaro, Sandoz
Consulting or Advisory Role: AstraZeneca, GlaxoSmithKline/Tesaro, MSD
Speakers' Bureau: AstraZeneca, MSD, GlaxoSmithKline/Tesaro
Travel, Accommodations, Expenses: Roche, AstraZeneca, MSD, GlaxoSmithKline/Tesaro
Florence Joly
Consulting or Advisory Role: AstraZeneca, Janssen, Ipsen, Pfizer, MSD Oncology, Bristol Myers Squibb, GlaxoSmithKline, Astellas Pharma, Clovis Oncology, Amgen, Seagen, Bayer, 3A Pharmaceuticals & Diagnostics, Eisai
Travel, Accommodations, Expenses: Janssen, AstraZeneca, Ipsen, GlaxoSmithKline, BMS, Eisai, MSD Oncology
Véronique D'Hondt
Travel, Accommodations, Expenses: GlaxoSmithKline, Lilly, Pfizer
Coriolan Lebreton
Honoraria: Eisai, Clovis Oncology, MSD Oncology, GlaxoSmithKline
Travel, Accommodations, Expenses: GlaxoSmithKline
Pierre-Etienne Heudel
Stock and Other Ownership Interests: GeodAIsics
Honoraria: Pfizer, Novartis (Inst), Seagen, Pierre Fabre, Amgen, AstraZeneca (Inst), Roche, Mylan, Gilead Sciences
Consulting or Advisory Role: Novartis (Inst), Seagen, Lilly
Research Funding: Fresenius (Inst)
Travel, Accommodations, Expenses: Pfizer, Novartis, Roche, Lilly
Florian Heitz
Honoraria: Roche, AstraZeneca, Tesaro/GSK
Consulting or Advisory Role: Roche, AstraZeneca, GlaxoSmithKline, Novocure, PharmaMar
Research Funding: AstraZeneca (Inst), Amedes (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Tewari KS, Monk BJ, Vergote I, et al. : Survival with cemiplimab in recurrent cervical cancer. N Engl J Med 386:544-555, 2022 [DOI] [PubMed] [Google Scholar]
- 2.Colombo N, Dubot C, Lorusso D, et al. : Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 385:1856-1867, 2021 [DOI] [PubMed] [Google Scholar]
- 3.Makker V, Colombo N, Casado Herráez A, et al. : Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386:437-448, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Socinski MA, Jotte RM, Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Plimack ER, Stus V, et al. : Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Powles T, Atkins MB, et al. : Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 393:2404-2415, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E, Fujiwara K, Ledermann JA, et al. : Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): An open-label, three-arm, randomised, phase 3 study. Lancet Oncol 22:1034-1046, 2021 [DOI] [PubMed] [Google Scholar]
- 8.Pfisterer J, Plante M, Vergote I, et al. : Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol 24:4699-4707, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Parmar MK, Ledermann JA, Colombo N, et al. : Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: The ICON4/AGO-OVAR-2.2 trial. Lancet 361:2099-2106, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Pujade-Lauraine E, Wagner U, Aavall-Lundqvist E, et al. : Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J Clin Oncol 28:3323-3329, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Aghajanian C, Blank SV, Goff BA, et al. : OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30:2039-2045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coleman RL, Brady MF, Herzog TJ, et al. : Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 18:779-791, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfisterer J, Shannon CM, Baumann K, et al. : Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol 21:699-709, 2020 [DOI] [PubMed] [Google Scholar]
- 14.Monk BJ, Colombo N, Oza AM, et al. : Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): An open-label, randomised, phase 3 trial. Lancet Oncol 22:1275-1289, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Moore KN, Bookman M, Sehouli J, et al. : Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: Placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol 39:1842-1855, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergote I, Pujade-Lauraine E, Pignata S, et al. : European Network of Gynaecological Oncological Trial Groups' requirements for trials between academic groups and pharmaceutical companies. Int J Gynecol Cancer 20:476-478, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Schmid P, Adams S, Rugo HS, et al. : Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Mittendorf EA, Zhang H, Barrios CH, et al. : Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 396:1090-1100, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Miles D, Gligorov J, André F, et al. : Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 32:994-1004, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Yin G, Ibrahim JG: Cure rate models: A unified approach. Can J Stat 33:559-570, 2005 [Google Scholar]
- 21.del Carmen MG, Micha J, Small L, et al. : A phase II clinical trial of pegylated liposomal doxorubicin and carboplatin plus bevacizumab in patients with platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecol Oncol 126:369-374, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Webb JR, Milne K, Kroeger DR, et al. : PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol 141:293-302, 2016 [DOI] [PubMed] [Google Scholar]
- 23.R Core Team : R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2016 [Google Scholar]
- 24.Pignata S, Lorusso D, Joly F, et al. : Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: A randomised, phase 3 trial. Lancet Oncol 22:267-276, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Khairallah AS, Genestie C, Auguste A, et al. : Impact of neoadjuvant chemotherapy on the immune microenvironment in advanced epithelial ovarian cancer: Prognostic and therapeutic implications. Int J Cancer 143:8-15, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Felip E, Altorki N, Zhou C, et al. : Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 398:1344-1357, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Herbst RS, Giaccone G, de Marinis F, et al. : Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 383:1328-1339, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Ye J, Ji X, Dennis PA, et al. : Relationship between progression-free survival, objective response rate, and overall survival in clinical trials of PD-1/PD-L1 immune checkpoint blockade: A meta-analysis. Clin Pharmacol Ther 108:1274-1288, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Kristeleit R, Clamp A, Gourley C, et al. : Efficacy of pembrolizumab monotherapy (PM) for advanced clear cell gynaecological cancer (CCGC): Phase II PEACOCC Trial. Ann Oncol 33(Suppl 7):S783, 2022 [Google Scholar]
- 31.Hamanishi J, Takeshima N, Katsumata N, et al. : Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: Open-label, randomized trial in Japan (NINJA). J Clin Oncol 39:3671-3681, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Currently, no mechanism is in place to allow sharing of individual deidentified patient data. Requests sent to ARCAGY-GINECO will be considered on a case-by-case basis.