New breast MRI protocol finds more cancers with fewer benign biopsies in women with high BPE
Abstract
PURPOSE
To compare breast magnetic resonance imaging (MRI) diagnostic performance using a standard high–spatial resolution protocol versus a simultaneous high–temporal/high–spatial resolution (HTHS) protocol in women with high levels of background parenchymal enhancement (BPE).
MATERIALS AND METHODS
We conducted a retrospective study of contrast-enhanced breast MRIs performed at our institution before and after the introduction of the HTHS protocol. We compared diagnostic performance of the HTHS and standard protocol by comparing cancer detection rate (CDR) and positive predictive value of biopsy (PPV3) among women with high BPE (ie, marked or moderate).
RESULTS
Among women with high BPE, the HTHS protocol demonstrated increased CDR (23.6 per 1,000 patients v 7.9 per 1,000 patients; P = 0. 013) and increased PPV3 (16.0% v 6.3%; P = .021) compared with the standard protocol. This corresponded to a 9.8% (95% CI, 1.29 to 18.3) decrease in the proportion of unnecessary biopsies among high-BPE patients and an additional cancer yield of 15.7 per 1,000 patients (95% CI, 1.3 to 18.3).
CONCLUSION
Among women with high BPE, HTHS MRI improved diagnostic performance, leading to an additional cancer yield of 15.7 cancers per 1,000 women and concomitantly decreasing unnecessary biopsies by 9.8%. A multisite prospective trial is warranted to confirm these findings and to pave the way for more widespread clinical implementation.
INTRODUCTION
Contrast-enhanced breast magnetic resonance imaging (MRI) is the most sensitive diagnostic tool for early detection of breast cancer, outperforming mammography and ultrasound combined.1 Current clinical guidelines therefore recommend annual breast MRI for patients at a >20% lifetime risk of cancer and consideration for screening MRI in women with personal histories of breast cancer and/or dense breast tissue.2 In light of recent evidence,3-5 the European Society of Breast Imaging recently issued a recommendation that women age 50-70 years with extremely dense breasts should be offered screening breast MRI every 2-4 years.6
CONTEXT
Key Objective
Contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive tool for breast cancer detection but can be limited in patients with high levels of background parenchymal enhancement (BPE). A simultaneous high–temporal/high–spatial resolution (HTHS) breast MRI protocol allows radiologists to evaluate the breast at earlier timepoints after contrast injection, before BPE complicates interpretation. In this study, we compare the diagnostic performance of a standard breast protocol versus the HTHS protocol in women with high BPE.
Knowledge Generated
For women with high BPE, the HTHS protocol demonstrated both a higher cancer detection rate (23.6 per 1,000 patients v 7.9 per 1,000 patients) and a higher positive predictive value (16.0% v 6.3%), compared with the standard protocol.
Relevance (A.C. Wolff)
-
New imaging protocols may improve the diagnostic performance of MRI to detect breast cancer in women with high levels of BPE.*
*Relevance section written by JCO Associate Editor Antonio C. Wolff, MD, FACP.
Breast cancers enhance on MRI at a very early stage because of tumor angiogenesis, which is present in lesions as small as 2-3 mm.7 However, the detection of enhancing cancers on breast MRI can be complicated by the presence of background parenchymal enhancement (BPE), which refers to the physiologic/hormonal enhancement of surrounding normal breast parenchyma. BPE may hide underlying cancers and/or mimic the presence of cancer,8 and higher levels of BPE have been associated with higher abnormal interpretation rates and subsequent unnecessary breast biopsies or follow-up recommendations.9 This has prompted many imaging centers to schedule breast MRI examinations during the first 2 weeks of a woman's menstrual cycle when BPE levels are at a minimum, although more recent studies show that menstrual cycle timing does not improve breast MRI cancer detection rates (CDRs) or reduce false positives.10,11
Using dynamic contrast-enhanced (DCE) MRI, radiologists distinguish suspicious lesions from BPE and other benign lesions by a combination of their suspicious morphology and early enhancement pattern (ie, kinetics), according to the American College of Radiology Breast Imaging and Reporting Data System (BI-RADS).12 Historically, MRI technology limitations forced a trade-off between high spatial resolution (to visualize morphology) and high temporal resolution (to characterize kinetics).13 An evidence-based consensus emerged in the radiology community to prioritize spatial resolution, and today, almost all breast centers use a high–spatial/low–temporal resolution protocol (ie, 1 mm2 in-plane spatial resolution and 60-90 seconds temporal resolution).14 Recently developed fast MRI techniques, however, circumvent this trade-off with parallel imaging and strategic information-sharing between temporal timepoints during the image acquisition and reconstruction process.15-20 To date, one reader study has demonstrated the noninferiority of fast MRI,20 but no studies have yet evaluated whether diagnostic performance might improve in certain clinical situations.
A simultaneous high–temporal/high–spatial resolution (HTHS) MRI protocol enables radiologists to use both lesion morphology and lesion kinetics in the detection and classification of breast lesions. Malignant lesions demonstrate increased vascular permeability and enhance much earlier (usually within the first minute) compared with nonmalignant sources of breast enhancement, such as benign lesions and BPE, which enhance at a later timepoint.16-21 The HTHS protocol, therefore, may be used to search for enhancing cancers at an earlier timepoint after contrast injection, before BPE washes in and complicates the interpretation task.
We hypothesized that for patients with high levels of BPE, the HTHS protocol would significantly improve CDRs while concomitantly minimizing unnecessary biopsies. The aim of our study was to evaluate whether a simultaneous HTHS MRI protocol improves diagnostic accuracy in women with high BPE, compared with a standard breast MRI.
MATERIALS AND METHODS
Inclusion and Exclusion of Breast MRI Examinations
In this institutional review board–approved Health Insurance Portability and Accountability Act–compliant retrospective study for which informed consent was waived, all contrast-enhanced breast MRI examinations performed at our institution from January 1, 2016, to December 31, 2016 (the final year when a standard breast MRI protocol was exclusively used), and from January 1, 2019, to December 31, 2019 (the first year that the full HTHS protocol, described below, was implemented), were reviewed. These date ranges were selected to enable comparison between a standard breast MRI protocol and a HTHS protocol. To focus on the screening population, examinations were excluded: (1) if the patient had been recently diagnosed with breast cancer (including all examinations with a BI-RADS 6 assessment) or (2) if the MRI was performed to follow up a finding on a recently performed contrast-enhanced mammogram. Examinations were also excluded if the radiology report lacked a standardized BI-RADS designation (ie, BI-RADS 1-5) or a standardized BPE assessment or if negative examinations lacked at least a 1-year negative follow-up.
Breast MRI Protocol
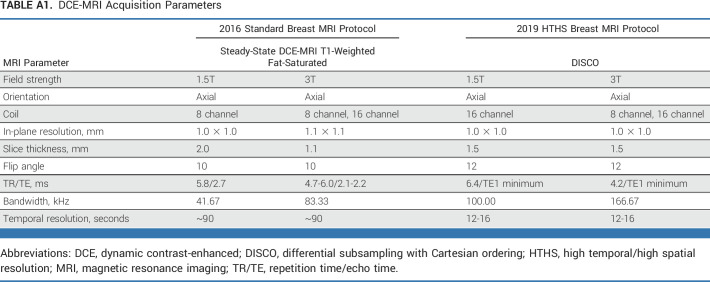
All breast DCE MRI examinations were performed on a 1.5T or 3.0T system (Discovery 750, GE Healthcare, Chicago, IL) using a dedicated 8- or 16-channel breast coil and a gadolinium-based contrast agent. In 2016, all breast MRIs at our institution were acquired using a standard high-resolution protocol, including three postcontrast phases, each lasting 90 seconds, and with a spatial resolution of ≤1.1 mm3. In 2019, all breast MRIs were acquired using the HTHS protocol (ie, General Electric differential subsampling with Cartesian ordering [DISCO]), with a fast temporal footprint of 12-16 seconds and a spatial resolution of ≤1.0 × 1.0 × 1.5 mm3. HTHS image reconstruction generates 12-15 postcontrast phases, allowing the interpreting radiologist to retrospectively select the ideal postcontrast phase at the time of diagnostic interpretation. Additional imaging protocol details are summarized in Appendix Table A1 (online only). Breast MRIs from 2016 and 2019 were interpreted by the same group of radiologists from the breast imaging service at our institution, who were trained in HTHS breast MRI interpretation before the start of the 2019 study period.
Data Collection
For each examination, BPE status was determined from the text of the radiology report, using automated extraction (ie, marked, moderate, mild, minimal). For each examination, the BI-RADS assessment assigned by the radiologist at the time of image interpretation was used to classify the examinations as positive (ie, BI-RADS 4, 5) or negative (ie, BI-RADS 1, 2, or 3). For positive examinations, the reference standard was based on a combination of histopathology results, extracted from the pathology report text, and our hospital's tumor registry database.
For negative examinations, the reference standard was determined by the presence of at least a 12-month negative follow-up (ie, no positive imaging study or entry in our hospital's tumor registry within 1 year and a last date of service that was at least 1 year after the breast MRI date). Examinations with high-risk lesions were classified as negative; however, if a high-risk lesion was upgraded to in situ or invasive disease at surgical excision, the examination was reclassified as positive.
Statistical Analysis
The primary objective of this study was to compare the CDR and the positive predictive value of biopsy (PPV3) between the HTHS and standard protocol in patients with high BPE. These measures were used to derive the additional cancer yield (ie, the difference in cancer detection between the two protocols) and the decrease in unnecessary biopsies with the HTHS protocol (ie, 1–PPV3). Secondary performance metrics, such as sensitivity, specificity, interval cancer rate, and negative predictive value, were also calculated. All performance metrics were calculated with 95% CIs with continuity correction.
The CDR and PPV3 for baseline MRI and nonbaseline MRI subgroups were also calculated as part of a secondary analysis. MRIs were designated as nonbaseline if a previous contrast-enhanced breast MRI was available at the time of interpretation (including outside previous MRIs). PPV3 was defined as the proportion of breast biopsies performed that yield malignant results.12 Interval cancers were defined as a diagnosis of cancer that occurred <11 months after a negative breast MRI examination. The unnecessary biopsy rate was calculated as the proportion of biopsies yielding nonmalignant pathology (ie, 1–PPV3).
The primary hypothesis that CDR and PPV3 would increase in high-BPE patients with the HTHS protocol versus the standard protocol was tested using a one-tailed two-proportion Z-test with continuity correction. A post hoc Bonferroni adjustment accounted for the two primary objectives of the study, with 0.0125 considered statistically significant.
Patient demographics, examination characteristics, and cancer breakdown were compared using a two-tailed two-proportion Z-test. The proportion of examinations assigned to each BI-RADS category were also calculated and compared.
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC) and MATLAB 2017b (MathWorks, Natick, MA).
RESULTS
Demographics and Patient Examination Information
A total of 9,966 contrast-enhanced breast DCE MRI examinations were performed at our institution in 2016 and in 2019. To focus on the screening population, we excluded 2,351 examinations from patients with recently diagnosed breast cancer or who had a recent finding on contrast-enhanced mammogram that prompted the MRI study. Additional examinations were also excluded because of the lack of follow-up or the lack of standardized reporting (see Fig 1 for the study flowchart). This left 6,702 examinations in 6,384 patients (mean age 52 years ± 12) for inclusion in the study (3,210 from 2016 and 3,492 from 2019). According to the radiology reports, high BPE was present in 22% (1,481-6,702) of these examinations. The 1,481 high-BPE examinations in 1,414 patients (mean age 46 years ± 11) were included in the main analysis (761 from 2016 and 720 from 2019).
FIG 1.
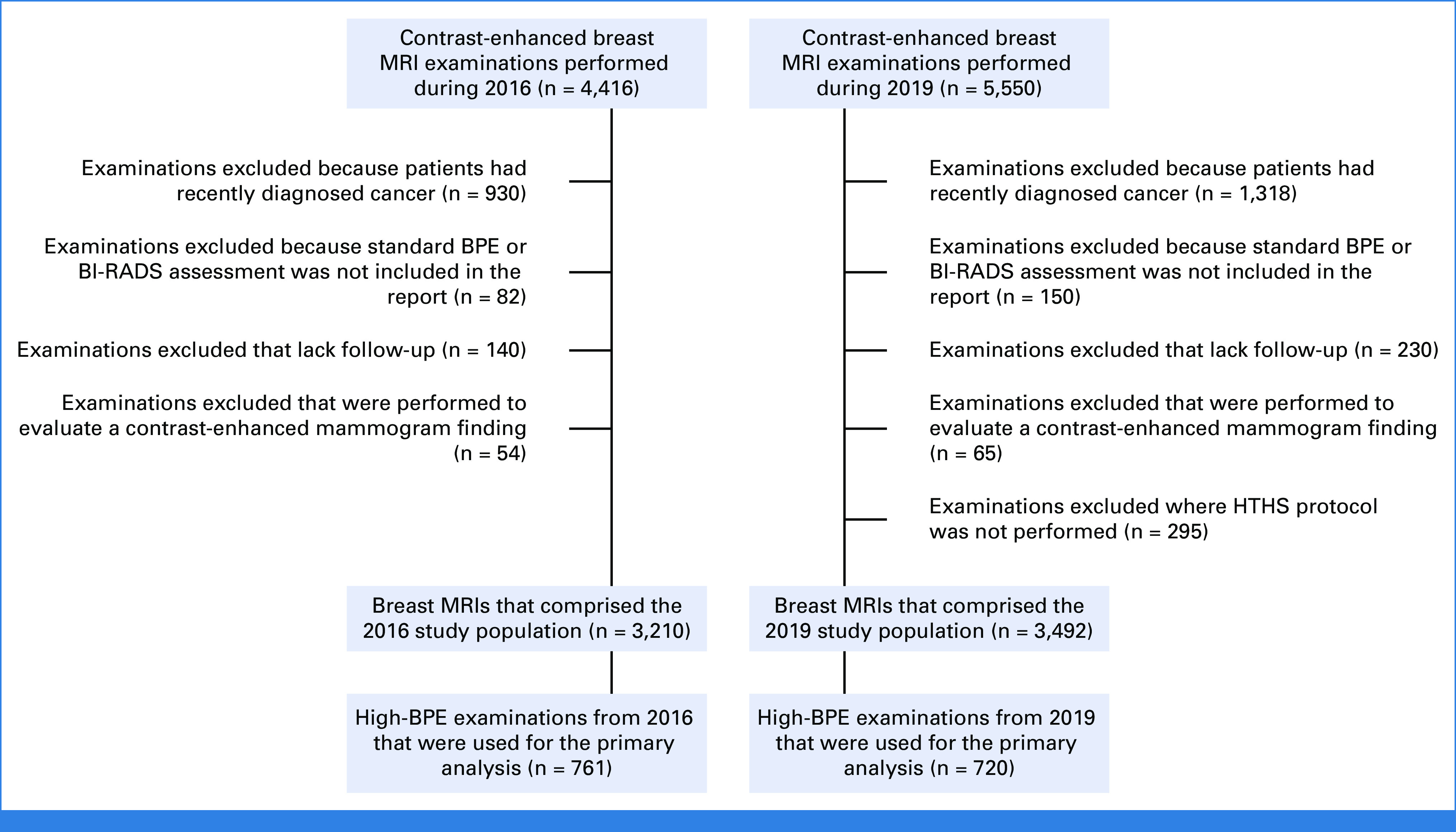
Study flowchart. BI-RADS, Breast Imaging and Reporting Data System; BPE, background parenchymal enhancement; HTHS, high temporal/high spatial resolution; MRI, magnetic resonance imaging.
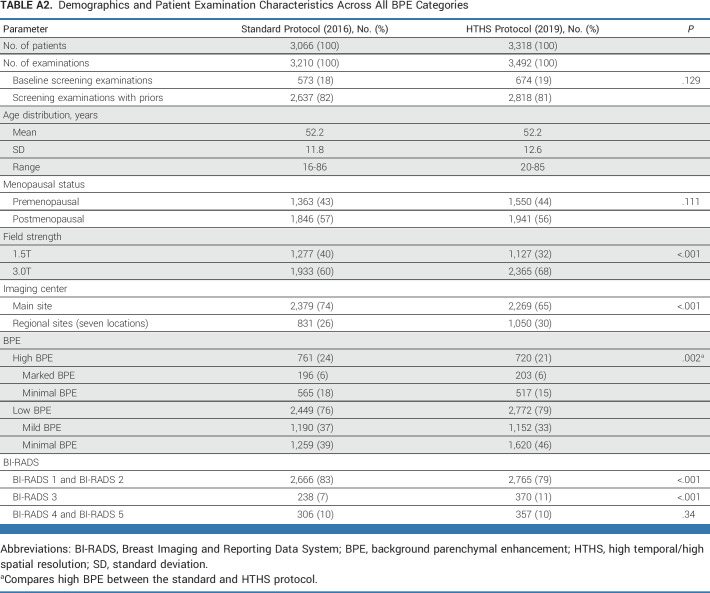
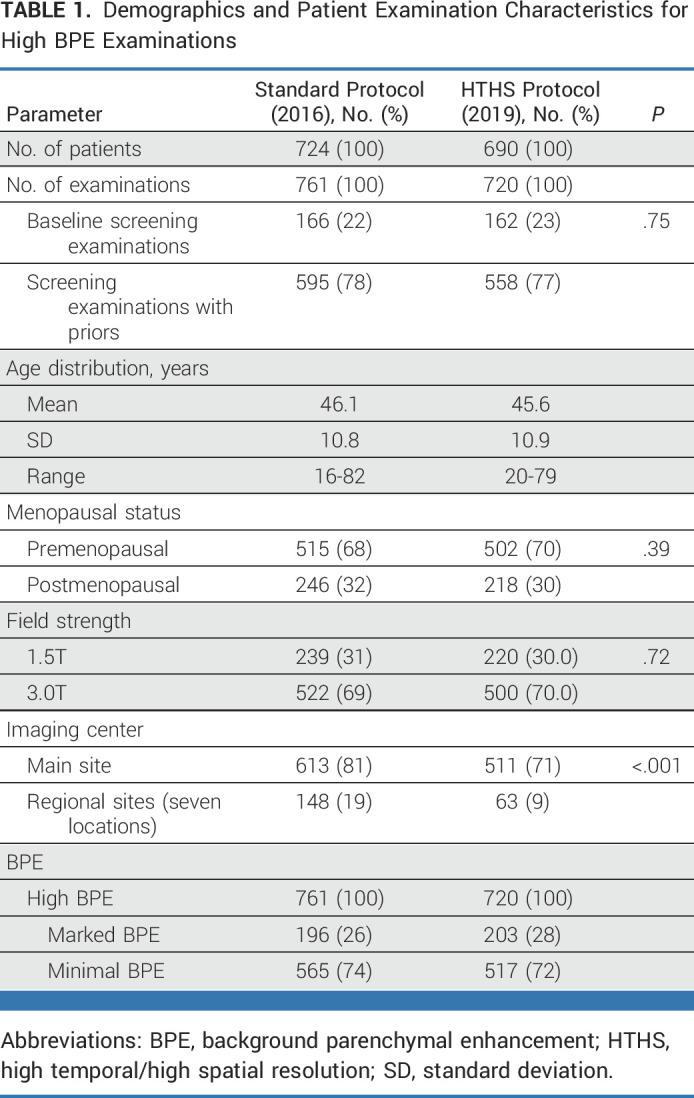
Across high BPE examinations, 22% (328-1,481) were baseline MRI examinations, including 22% (166-761) of 2016 examinations and 23% (162-720) of 2019 examinations (P = .75). In 2016, 69% (522-761) of examinations were performed using a 3.0T scanner, whereas in 2019, 70% (500-720) of examinations were performed using 3.0T (P = .72). In 2016, 81% (613-761) of examinations were performed at the main imaging site, which decreased to 71% (511-720) in 2019 (P < .001). See Table 1 for additional demographics and patient examination information for high-BPE cases (see Appendix Table A2 for the corresponding table across all BPE categories).
TABLE 1.
Demographics and Patient Examination Characteristics for High BPE Examinations
Cancer Yield With the HTHS Versus Standard Protocol
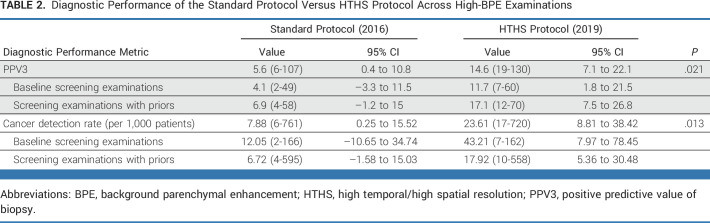
For patients with high BPE, CDR was increased for the HTHS protocol compared with the standard protocol (23.6-1,000 [17-720] v 7.9 [6-761]; P = .021). This translates into an additional cancer yield of 15.7 cancers per 1,000 women (95% CI, 3.0 to 28.5). Table 2 and Figure 2 provide additional details. In Figure 3, two cancer case examples demonstrate how the early postcontrast phases of the HTHS protocol improved the visualization of suspicious lesions in the context of marked BPE.
TABLE 2.
Diagnostic Performance of the Standard Protocol Versus HTHS Protocol Across High-BPE Examinations
FIG 2.
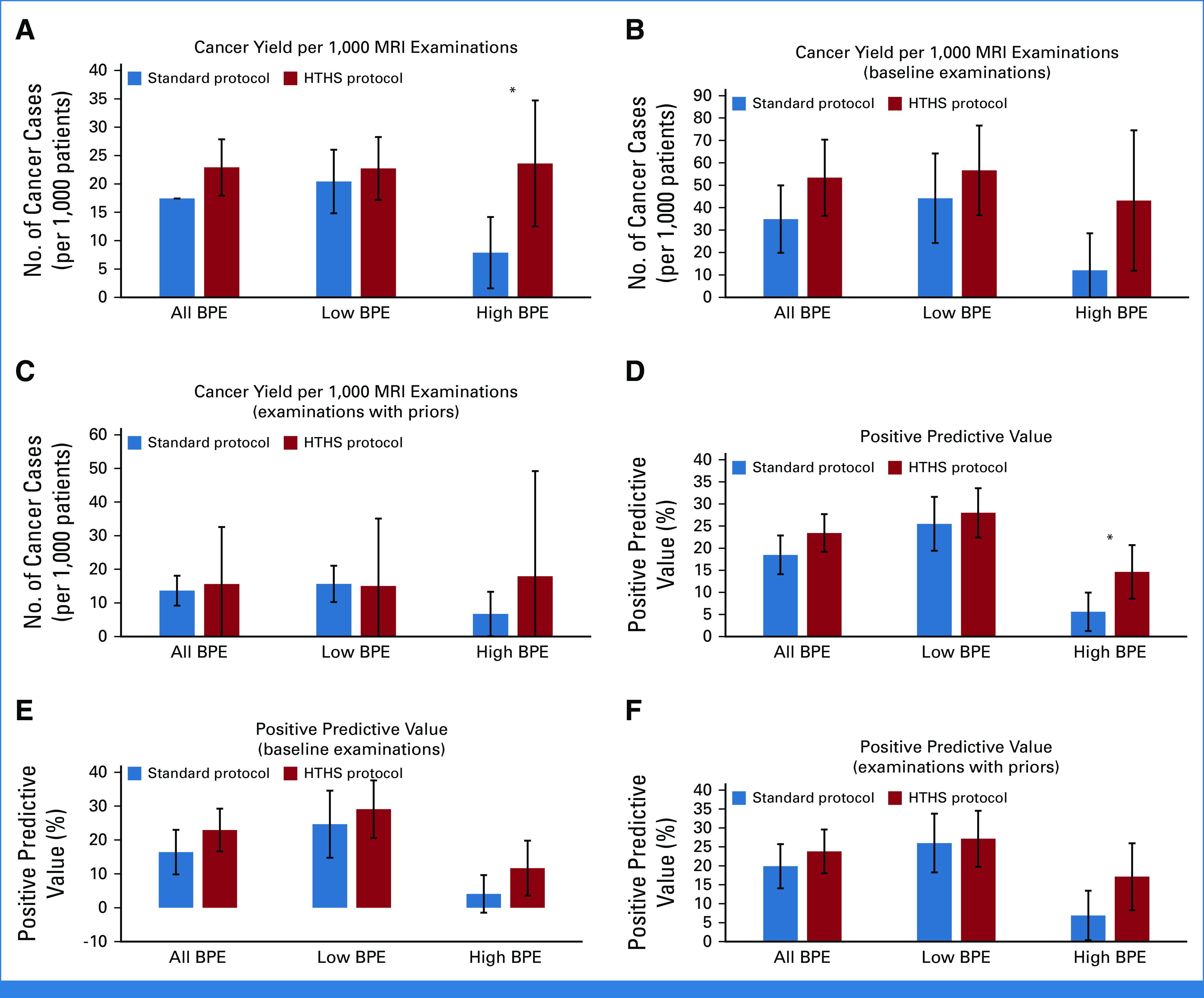
Breast MRI diagnostic performance with the HTHS protocol versus the standard breast MRI protocol. For patients with high BPE, (A) the CDR is higher with HTHS compared with the standard protocol. CDR subgroup analysis was performed for (B) baseline and (C) nonbaseline examinations. For high-BPE patients, the PPV3 is also increased with HTHS compared with (D) the standard protocol. PPV3 subgroup analysis was also performed for (E) baseline and (F) nonbaseline examinations. * indicates statistical significance. BPE, background parenchymal enhancement; CDR, cancer detection rate; HTHS, high temporal/high spatial resolution; MRI, magnetic resonance imaging; PPV3, positive predictive value of biopsy.
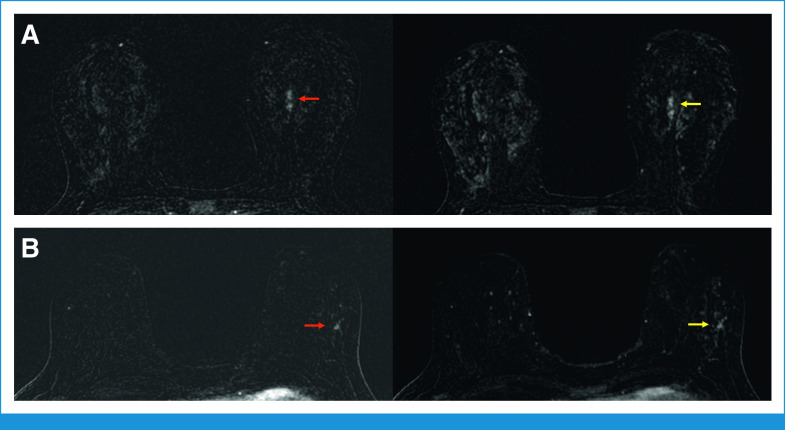
FIG 3.
The HTHS protocol facilitates cancer detection in patients with marked BPE. (A) Forty-three-year-old woman with a left breast focal nonmass enhancement in the central left breast. The nonmass enhancement is well appreciated on a T1-weighted fat-saturated subtraction image the third postcontrast phase of the HTHS protocol (red arrow), acquired 36 seconds after contrast injection. The lesion becomes obscured by marked BPE on the seventh postcontrast phase of the HTHS protocol (yellow arrow), acquired 84 seconds after contrast injection. Pathology yielded HR+/HER2– high-grade invasive lobular carcinoma. (B) Forty-nine-year-old women with focal nonmass enhancement in the outer left breast, which is well appreciated on a T1-weighted fat saturated subtraction image during the third postcontrast phase of the HTHS protocol (red arrow), acquired 36 seconds after contrast injection. The lesion is obscured by marked BPE on the seventh postcontrast phase of the HTHS protocol (yellow arrow), acquired 84 seconds after contrast injection. Pathology yielded HR+ high-grade ductal carcinoma in situ. BPE, background parenchymal enhancement; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; HTHS, high temporal/high spatial resolution.
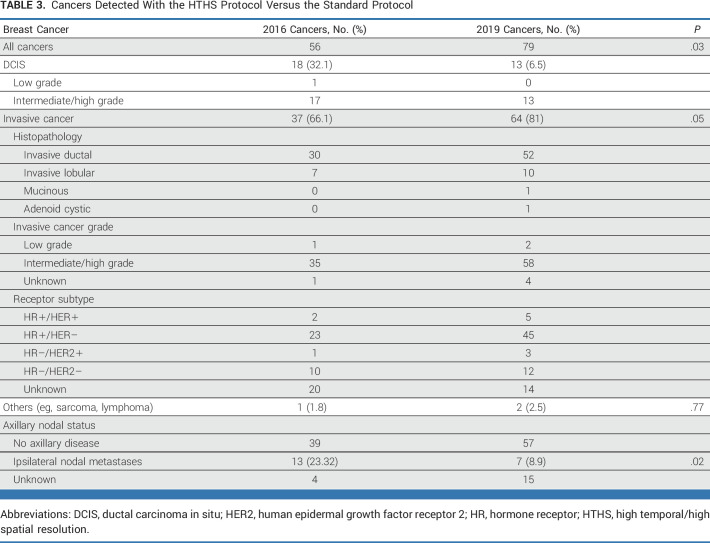
Across all patients, of the 56 cancers diagnosed with the standard protocol, 18 (32%) were ductal carcinoma in situ (DCIS), 30 (54%) were invasive ductal carcinoma (IDC), and 7 (13%) were invasive lobular carcinoma (ILC). Of the 79 cancers diagnosed with the HTHS protocol, 13 (16%) were DCIS, 52 (66%) were IDC, and 10 (13%) were ILC. Axillary nodal metastases were present in 13 (23%) of the standard protocol cases and in 7 (9%) of the HTHS protocol cases. See Table 3 for additional pathology details.
TABLE 3.
Cancers Detected With the HTHS Protocol Versus the Standard Protocol
Unnecessary Biopsies With the HTHS Versus Standard Protocol
For patients with high BPE, PPV3 was increased for the HTHS protocol compared with the standard protocol (16.0% [17-106] v 6.3% [6-96]; P = .014). As such, there was a 9.8% decrease in unnecessary biopsies (ie, the proportion of biopsies yielding nonmalignant results) with the HTHS protocol compared with the new protocol (95% CI, 1.3 to 18.3; Table 2).
Interval Cancers
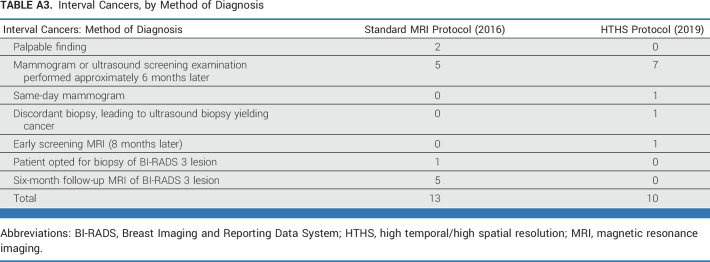
Across high-BPE patients, the interval cancer rate was not significantly different between the two protocols: 7 per 1,000 (5 per 720) for the HTHS protocol and 5 per 1,000 (4 per 761) for the standard protocol. Most interval cancers presented as calcifications on a screening mammogram performed 6 months after a negative breast MRI (Appendix Table A3).
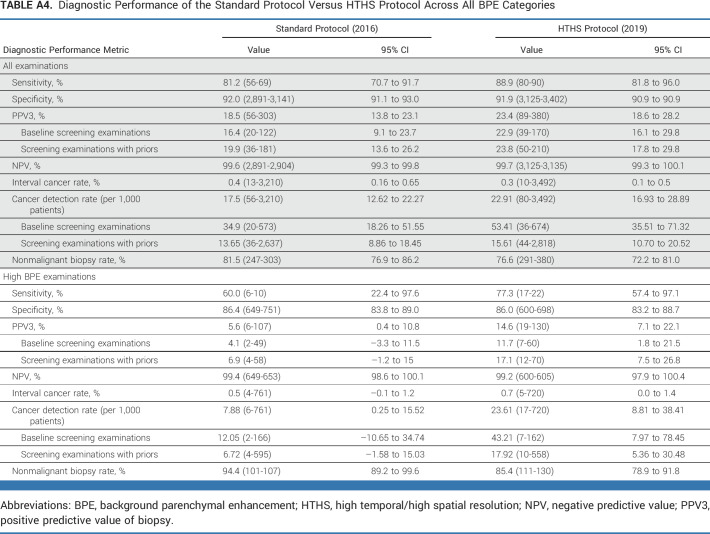
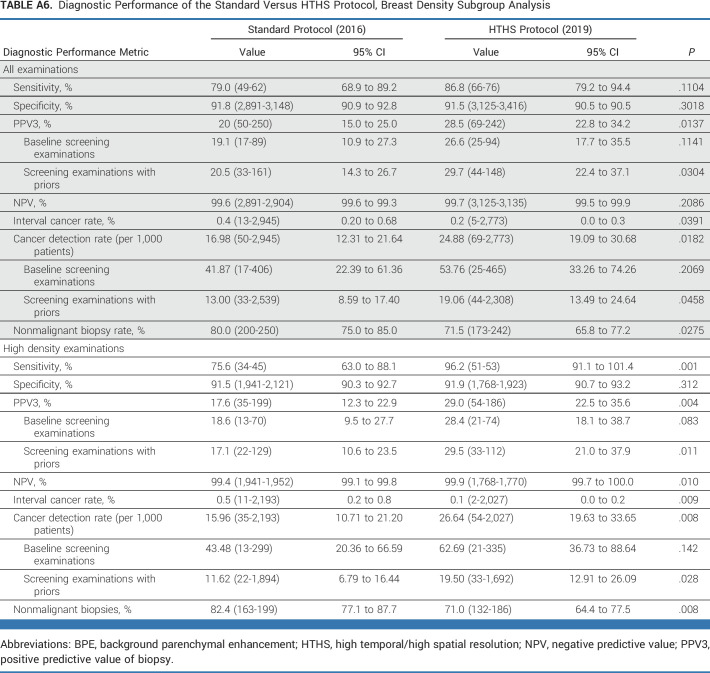
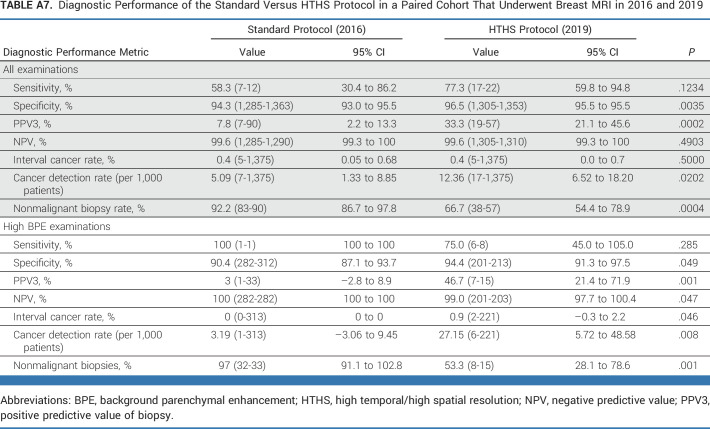
Supplementary analysis was also performed for all patients across all BPE categories (ie, low BPE and high BPE examinations) for sensitivity, specificity, PPV3, negative predictive value, and CDR (Appendix Table A4). Subgroup analysis was performed for the main imaging site alone (Appendix Table A5), stratified by mammographic breast density categories (Appendix Table A6), and for a paired cohort that underwent breast MRI in 2016 and 2019 (Appendix Table A7).
BI-RADS Classification for HTHS Versus Standard Protocols
Across all patients, the percentage of suspicious examinations (ie, BI-RADS 4 and BI-RADS 5) was not significantly different between the HTHS and standard protocol (10.2% [357-3,492] v 9.5% [306-3,210]; P = .34). The HTHS protocol, however, had more BI-RADS 3 examinations (11% [370-3,492] v 7% [238-3,210]; P < .0001) and fewer negative/benign examinations (ie, BI-RADS 1 or BI-RADS 2) compared with the standard protocol (79% [2,765-3,492] v 83% [2,666-3,210]; P < .001; Appendix Table A2).
DISCUSSION
Our results show that screening breast MRI using a HTHS protocol increases the CDR, increases PPV, and reduces unnecessary biopsies for patients with high levels of BPE. Specifically, the HTHS protocol led to an increased cancer yield of 15.7 per 1,000 patients (95% CI, 3.0 to 28.5) and a decrease in unnecessary biopsies of 9.8% (95% CI, 1.3 to 18.3). While the proportion of examinations that assigned a suspicious assessment (ie, BI-RADS 4 or BI-RADS 5) was similar between the HTHS and standard breast MRI protocol across all patients, HTHS examinations were more likely to be assigned a BI-RADS 3 assessment and less likely to be negative/benign assessments (ie, BI-RADS 1, BI-RADS 2).
Breast DCE MRI is a highly sensitive tool for cancer detection and yields impressive CDRs. However, our study demonstrates that breast MRI using a standard protocol does not perform uniformly well across all patients. Breast MRI is particularly challenging in 20%-25% of patients who have high BPE. BPE decreases cancer visibility by reducing the contrast-to-noise ratio between enhancing cancers and surrounding fibroglandular tissue, making high BPE a potential pitfall of breast MRI interpretation.8,9,22-25 Previous work has established that high BPE is associated with higher abnormal MRI interpretation rates, higher biopsy rates, and lower specificity,8,9,23,26 and we have also found that high BPE is associated with lower CDR and lower PPV3.37 These results are particularly troubling given that BPE is a breast cancer risk factor27-29; there are more cancers to be found in patients with high BPE, and so CDRs in high-BPE patients should be higher, not lower, than those in low-BPE patients.
To improve diagnostic performance in high-BPE patients, breast MRI acquisition may be tailored to exploit the pathophysiologic differences between cancer and BPE. Invasive cancers demonstrate rapid initial enhancement during the first 60 seconds postinjection, whereas BPE demonstrates a slow initial phase, peaking only around 210 seconds.29 The standard low–temporal resolution breast MRI protocol, therefore, fails to capture the imaging sweet spot where cancers enhance before BPE. Recently developed ultrafast MRI protocols can achieve temporal footprints of 4-10 seconds and have been shown to improve lesion detectability compared with standard breast MRI,30,31 but they come at the cost of a slightly decreased spatial resolution (eg, 1.0 × 0.9 × 2.5 mm).18-20,32 Our HTHS protocol, by contrast, generates a slightly longer temporal footprint (12-15 seconds) than an ultrafast protocol, which permits us to maintain the same high spatial resolution as a standard breast MRI protocol (eg, 1.0 × 1.0 × 1.5 mm). The HTHS protocol resolves the spatial resolution concern that has made the radiology community hesitant to adopt an ultrafast approach. In our protocol, 12-16 HTHS phases are generated, allowing the interpreting radiologist to retrospectively select an ideal postcontrast phase for diagnostic interpretation—where the cancer enhances, but the BPE has not yet washed in. This sweet spot will vary by patient and by lesion and can be empirically determined at the time of image interpretation. Although the HTHS protocol generates many more images than the standard protocol, the radiologists need not look at all of them. Instead, they may toggle to the ideal postcontrast phase for each examination, enabling an efficient and personalized approach to image interpretation.
The HTHS technique was introduced by Saranathan et al15 and has demonstrated comparable image quality and accuracy with standard breast MRI in preliminary studies.33-35 In patients with high BPE, fast MRI techniques have been found to improve lesion conspicuity.30,31,36 Now, to our knowledge, for the first time, our study shows that high–spatial/high–temporal resolution breast MRI not only improves lesion visualization but also improves cancer detection and decreases unnecessary biopsies in patients with high BPE. The apparent trade-off is that the HTHS protocol leads to increases in BI-RADS 3 examinations (ie, short-term follow-up recommendations) and decreases in BI-RADS 1 and BI-RADS 2 (negative/benign examinations), whereas the percentage of suspicious examinations warranting biopsy (ie, BI-RADS 4 and BI-RADS 5) was unchanged. The increased number of BI-RADS 3 examinations with the HTHS protocol could be due to the lack of HTHS priors for comparison. Another consideration is that radiologists were less experienced at HTHS interpretation and therefore more cautious in interpretation. It is likely that this is a transient effect that will resolve with HTHS priors available and a reader learning curve; however, more work is needed to evaluate the diagnostic accuracy of the HTHS protocol over time. We also plan to evaluate how the HTHS protocol affects radiologist interpretation time. The HTHS protocol can be implemented on any MRI scanner; the major vendors all have this sequence available (eg, General Electric has DISCO, and Siemens has time-resolved angiography with interleaved stochastic trajectories [TWIST]).
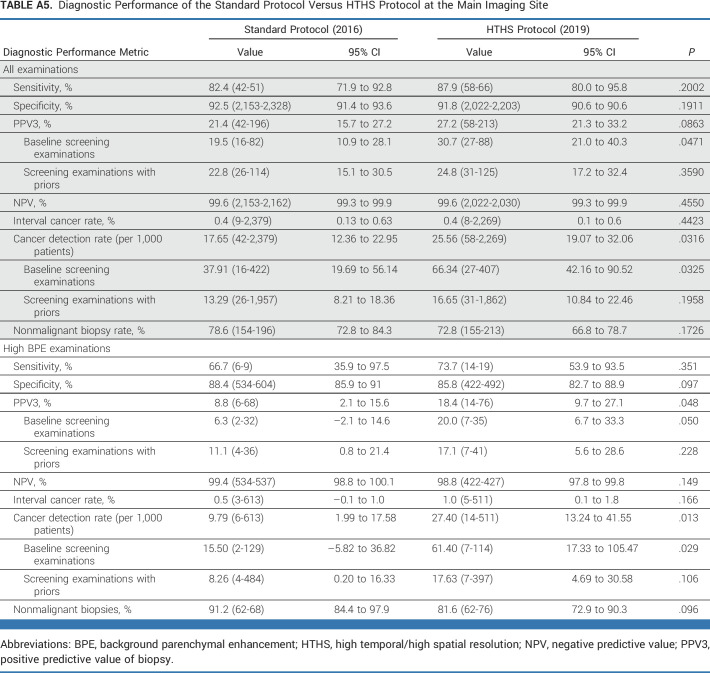
This retrospective study compared standard breast MRIs from 2016 with HTHS MRIs in 2019. Breast MRI examinations from 2017 and 2018 were not included in this study as our MRI protocol underwent flux during the 2017-2018 period and the purpose of the study was to compare the standard DCE-MRI protocol with the full HTHS protocol. Our study was limited to breast MRIs from a single institution although we diversified the caseload by including screening breast MRI examinations performed not only at our main hospital but also at several of our regional imaging sites across three US states. The percentage of breast MRIs performed at the main imaging site versus the regional imaging sites decreased from 2016 to 2019, introducing a potential confounding factor. However, subgroup analysis of the main imaging site data alone demonstrated almost identical results as the multisite results (ie, additional cancer yield of 17.6 per 1,000 patients and a 9.6% decrease in unnecessary biopsies—Appendix Table A3). Finally, the strikingly high HTHS protocol detection rate among high-BPE patients (23.6 v 7.9 cancers per 1,000 patients) may be due in part to it being a prevalence screen (ie, the first time these patients underwent HTHS protocol). In future work, we will investigate whether the high CDR persists during subsequent rounds of screening. Further evaluation with larger numbers of patients and from multiple institutions is prudent to confirm the experience of our single large tertiary care cancer center.
Today, HTHS breast MRI protocols have been implemented at a few major academic medical centers, but they are not routinely used in academic or private practices. To both maximize cancer detection and minimize unwarranted biopsies, our work suggests that HTHS holds promise to be the new breast MRI protocol of choice.
In conclusion, in patients with high BPE, the HTHS protocol demonstrates improved diagnostic performance compared with a standard breast DCE MRI protocol, detecting an additional 15.7 cancers per 1,000 patients, while concomitantly decreasing unnecessary biopsies by 9.8%. Our study suggests that an HTHS breast MRI protocol may improve cancer screening for women with high BPE. A prospective multicenter validation trial is now warranted to confirm these results and to pave the way for more widespread clinical implementation.
ACKNOWLEDGMENT
The authors thank Joanne Chin, MFA, ELS, for editing the manuscript.
APPENDIX
TABLE A1.
DCE-MRI Acquisition Parameters
TABLE A2.
Demographics and Patient Examination Characteristics Across All BPE Categories
TABLE A3.
Interval Cancers, by Method of Diagnosis
TABLE A4.
Diagnostic Performance of the Standard Protocol Versus HTHS Protocol Across All BPE Categories
TABLE A5.
Diagnostic Performance of the Standard Protocol Versus HTHS Protocol at the Main Imaging Site
TABLE A6.
Diagnostic Performance of the Standard Versus HTHS Protocol, Breast Density Subgroup Analysis
TABLE A7.
Diagnostic Performance of the Standard Versus HTHS Protocol in a Paired Cohort That Underwent Breast MRI in 2016 and 2019
Janice S. Sung
Honoraria: Bayer, Bracco Diagnostics, Northwest Imaging Forums
Speakers' Bureau: Guerbet
Research Funding: GE Healthcare (Inst)
Christopher Comstock
Consulting or Advisory Role: Guerbet
Speakers' Bureau: Bracco Diagnostics, Bayer, Northwest Imaging Forums
Kimberly Feigin
Leadership: HSG Clinical Research
Consulting or Advisory Role: Covera Health, Fujifilm, Asklepios BioPharmaceutical, Aspen Pharma, PTC Therapeutics, Annexon Biosciences
Research Funding: Prilenia
Katja Pinker
Consulting or Advisory Role: Merantix Healthcare, AURA Health Technologies GmbH, Genentech (Inst)
Speakers' Bureau: Bayer HealthCare Pharmaceuticals, Olea Medical, Medscape
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented as an oral presentation at the Annual Scientific Meeting of the International Society of Magnetic Resonance in Medicine (ISMRM), London, United Kingdom, May 9, 2022.
SUPPORT
Supported in part by National Cancer Institute Cancer Center Core Grant P30 CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: Sarah Eskreis-Winkler, Janice S. Sung, Ragni Jindal, Christopher Comstock, Kimberly Feigin, Katja Pinker
Collection and assembly of data: Sarah Eskreis-Winkler, Linden Dixon, Natasha Monga, Ragni Jindal, Amber Simmons, Sunitha Thakur, Elizabeth Sutton, Katja Pinker
Data analysis and interpretation: Sarah Eskreis-Winkler, Janice S. Sung, Linden Dixon, Ragni Jindal, Sunitha Thakur, Varadan Sevilimedu, Christopher Comstock, Katja Pinker
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
High–Temporal/High–Spatial Resolution Breast Magnetic Resonance Imaging Improves Diagnostic Accuracy Compared With Standard Breast Magnetic Resonance Imaging in Patients With High Background Parenchymal Enhancement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Janice S. Sung
Honoraria: Bayer, Bracco Diagnostics, Northwest Imaging Forums
Speakers' Bureau: Guerbet
Research Funding: GE Healthcare (Inst)
Christopher Comstock
Consulting or Advisory Role: Guerbet
Speakers' Bureau: Bracco Diagnostics, Bayer, Northwest Imaging Forums
Kimberly Feigin
Leadership: HSG Clinical Research
Consulting or Advisory Role: Covera Health, Fujifilm, Asklepios BioPharmaceutical, Aspen Pharma, PTC Therapeutics, Annexon Biosciences
Research Funding: Prilenia
Katja Pinker
Consulting or Advisory Role: Merantix Healthcare, AURA Health Technologies GmbH, Genentech (Inst)
Speakers' Bureau: Bayer HealthCare Pharmaceuticals, Olea Medical, Medscape
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kuhl C, Weigel S, Schrading S, et al. : Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: The EVA trial. J Clin Oncol 28:1450-1457, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Monticciolo DL, Newell MS, Moy L, et al. : Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR. J Am Coll Radiol 15:408-414, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Veenhuizen SGA, de Lange SV, Bakker MF, et al. : Supplemental breast MRI for women with extremely dense breasts: Results of the second screening round of the DENSE trial. Radiology 299:278-286, 2021 [DOI] [PubMed] [Google Scholar]
- 4.Bakker MF, de Lange SV, Pijnappel RM, et al. : Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 381:2091-2102, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Comstock CE, Gatsonis C, Newstead GM, et al. : Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 323:746-756, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann RM, Athanasiou A, Baltzer PAT, et al. : Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur Radiol 32:4036-4045, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillen F, Griffioen AW: Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev 26:489-502, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray KM, Kerlikowske K, Lobach IV, et al. : Effect of background parenchymal enhancement on breast MR imaging interpretive performance in community-based practices. Radiology 286:822-829, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeMartini WB, Liu F, Peacock S, et al. : Background parenchymal enhancement on breast MRI: Impact on diagnostic performance. AJR Am J Roentgenol 198:W373-W380, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Dontchos BN, Rahbar H, Partridge SC, et al. : Influence of menstrual cycle timing on screening breast MRI background parenchymal enhancement and diagnostic performance in premenopausal women. J Breast Imaging 1:205-211, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CH, Bryce Y, Zheng J, et al. : Outcome of screening MRI in premenopausal women as a function of the week of the menstrual cycle. AJR Am J Roentgenol 214:1175-1181, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Morris EA, Comstock C, Lee CH, et al. : ACR BI-RADS ® magnetic resonance imaging, in ACR BI-RADS ® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology, 2013 [Google Scholar]
- 13.Kuhl CK, Schild HH, Morakkabati N: Dynamic bilateral contrast-enhanced MR imaging of the breast: Trade-off between spatial and temporal resolution. Radiology 236:789-800, 2005 [DOI] [PubMed] [Google Scholar]
- 14.ACR practice parameter for the performance of contrast enhanced MRI of the breast. http://www.acr.org/∼/media/2a0eb28eb59041e2825179afb72ef624.pdf
- 15.Saranathan M, Rettmann DW, Hargreaves BA, et al. : Variable spatiotemporal resolution three-dimensional Dixon sequence for rapid dynamic contrast-enhanced breast MRI. J Magn Reson Imaging 40:1392-1399, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onishi N, Sadinski M, Gibbs P, et al. : Differentiation between subcentimeter carcinomas and benign lesions using kinetic parameters derived from ultrafast dynamic contrast-enhanced breast MRI. Eur Radiol 30:756-766, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onishi N, Sadinski M, Hughes MC, et al. : Ultrafast dynamic contrast-enhanced breast MRI may generate prognostic imaging markers of breast cancer. Breast Cancer Res 22:58, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mann RM, Mus RD, van Zelst J, et al. : A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: High-resolution ultrafast dynamic imaging. Invest Radiol 49:579-585, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Mus RD, Borelli C, Bult P, et al. : Time to enhancement derived from ultrafast breast MRI as a novel parameter to discriminate benign from malignant breast lesions. Eur J Radiol 89:90-96, 2017 [DOI] [PubMed] [Google Scholar]
- 20.van Zelst JCM, Vreemann S, Witt HJ, et al. : Multireader study on the diagnostic accuracy of ultrafast breast magnetic resonance imaging for breast cancer screening. Invest Radiol 53:579-586, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Veltman J, Stoutjesdijk M, Mann R, et al. : Contrast-enhanced magnetic resonance imaging of the breast: The value of pharmacokinetic parameters derived from fast dynamic imaging during initial enhancement in classifying lesions. Eur Radiol 18:1123-1133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giess CS, Yeh ED, Raza S, et al. : Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 34:234-247, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Hambly NM, Liberman L, Dershaw DD, et al. : Background parenchymal enhancement on baseline screening breast MRI: Impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol 196:218-224, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Vreemann S, Gubern-Merida A, Lardenoije S, et al. : The frequency of missed breast cancers in women participating in a high-risk MRI screening program. Breast Cancer Res Treat 169:323-331, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watt GP, Sung J, Morris EA, et al. : Association of breast cancer with MRI background parenchymal enhancement: The IMAGINE case-control study. Breast Cancer Res 22:138, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sippo DA, Rutledge GM, Mercaldo SF, et al. : Impact of background parenchymal enhancement on diagnostic performance in screening breast MRI. Acad Radiol 27:663-671, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Dontchos BN, Rahbar H, Partridge SC, et al. : Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 276:371-380, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King V, Brooks JD, Bernstein JL, et al. : Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 260:50-60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao GJ, Henze Bancroft LC, Strigel RM, et al. : Background parenchymal enhancement on breast MRI: A comprehensive review. J Magn Reson Imaging 51:43-61, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honda M, Kataoka M, Iima M, et al. : Background parenchymal enhancement and its effect on lesion detectability in ultrafast dynamic contrast-enhanced MRI. Eur J Radiol 129:108984, 2020 [DOI] [PubMed] [Google Scholar]
- 31.Pineda FD, Medved M, Wang S, et al. : Ultrafast bilateral DCE-MRI of the breast with conventional Fourier sampling: Preliminary evaluation of semi-quantitative analysis. Acad Radiol 23:1137-1144, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vreemann S, Rodriguez-Ruiz A, Nickel D, et al. : Compressed sensing for breast MRI: Resolving the trade-off between spatial and temporal resolution. Invest Radiol 52:574-582, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Pinker K, Grabner G, Bogner W, et al. : A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: Initial results. Invest Radiol 44:553-558, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Morrison CK, Henze Bancroft LC, DeMartini WB, et al. : Novel high spatiotemporal resolution versus standard-of-care dynamic contrast-enhanced breast MRI: Comparison of image quality. Invest Radiol 52:198-205, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benkert T, Block KT, Heller S, et al. : Comprehensive dynamic contrast-enhanced 3D magnetic resonance imaging of the breast with fat/water separation and high spatiotemporal resolution using radial sampling, compressed sensing, and parallel imaging. Invest Radiol 52:583-589, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomida T, Urikura A, Uematsu T, et al. : Contrast enhancement in breast cancer and background mammary-gland tissue during the super-early phase of dynamic breast magnetic resonance imaging. Acad Radiol 24:1380-1386, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Eskreis-Winkler S, Sung J, Sevilimedu V, et al. : Breast MRI cancer detection rates decreased in high background parenchymal enhancement (BPE) exams. Presented at Radiological Society of North America 2022 Scientific Assembly and Annual Meeting, Chicago, IL, November 27-December 1, 2022 (abstr) [Google Scholar]