Abstract
In Chlamydomonas (Chlamydomonas reinhardtii), the VESICLE-INDUCING PROTEIN IN PLASTIDS 1 and 2 (VIPP1 and VIPP2) play roles in the sensing and coping with membrane stress and in thylakoid membrane biogenesis. To gain more insight into these processes, we aimed to identify proteins interacting with VIPP1/2 in the chloroplast and chose proximity labeling (PL) for this purpose. We used the transient interaction between the nucleotide exchange factor CHLOROPLAST GRPE HOMOLOG 1 (CGE1) and the stromal HEAT SHOCK PROTEIN 70B (HSP70B) as test system. While PL with APEX2 and BioID proved to be inefficient, TurboID resulted in substantial biotinylation in vivo. TurboID-mediated PL with VIPP1/2 as baits under ambient and H2O2 stress conditions confirmed known interactions of VIPP1 with VIPP2, HSP70B, and the CHLOROPLAST DNAJ HOMOLOG 2 (CDJ2). Proteins identified in the VIPP1/2 proxiomes can be grouped into proteins involved in the biogenesis of thylakoid membrane complexes and the regulation of photosynthetic electron transport, including PROTON GRADIENT REGULATION 5-LIKE 1 (PGRL1). A third group comprises 11 proteins of unknown function whose genes are upregulated under chloroplast stress conditions. We named them VIPP PROXIMITY LABELING (VPL). In reciprocal experiments, we confirmed VIPP1 in the proxiomes of VPL2 and PGRL1. Our results demonstrate the robustness of TurboID-mediated PL for studying protein interaction networks in the chloroplast of Chlamydomonas and pave the way for analyzing functions of VIPPs in thylakoid biogenesis and stress responses.
TurboID-mediated proximity labeling in the chloroplasts of Chlamydomonas enables the detection of proxiomes under normal and stress conditions.
Introduction
The identification of protein–protein interactions is key for the understanding of protein function. Traditional methods include affinity-purification combined with mass spectrometry (AP-MS) and derivatives like single- or tandem-affinity purification, pull-downs using immobilized bait proteins, co-migration on native gels (complexome profiling), yeast-two-hybrid and its derivatives. The disadvantage of these methods is that protein interactions must persist during cell lysis and unspecific interactions may form after the mixing of compartment contents. Moreover, interactions are studied outside of the native context where important co-factors or scaffolds may be missing. Many of these limitations can be overcome by proximity labeling (PL) (reviewed in Kim and Roux 2016; Mair and Bergmann 2021; Qin et al. 2021; Zhang et al. 2022).
PL is based on the fusion of a bait to an enzyme that produces activated biotin (Roux et al. 2012). Activated biotin binds to amino acid side chains of proteins in the vicinity of the bait, which can be enriched with streptavidin beads and identified by mass spectrometry, thereby detecting stable as well as transient interactors and proteins in close proximity. PL is most commonly realized with two enzymes, the Bifunctional ligase/repressor A (BirA) and the ENHANCED ASCORBATE PEROXIDASE (APEX). Escherichia coli BirA activates biotin by adenylation, consuming ATP. Reactive biotinoyl-5′-AMP is normally transferred to a specific lysine residue of a subunit of the acetyl-CoA carboxylase requiring biotin as cofactor. This specificity is lost in BirA* harboring the R118G mutation such that reactive biotinoyl-AMP is released and proteins in the vicinity of BirA* get biotinylated at exposed primary amines within an estimated range of ∼10 nm (Choi-Rhee et al. 2004; Kim et al. 2014). Adding biotin to human cells expressing a fusion of BirA* with lamin-A in the nuclear lamina allowed the identification of novel interaction partners in vivo and the approach was coined proximity-dependent biotin identification (BioID) (Roux et al. 2012). The disadvantage of BirA* is its slow labeling kinetics, requiring labeling times of 15 to 18 h (Choi-Rhee et al. 2004; Roux et al. 2012). Moreover, BirA* is not very active at temperatures below 37°C (Kim et al. 2016; Zhang et al. 2019; Arora et al. 2020).
APEX is an engineered cytosolic peroxidase from pea or soybean originally destined for electron microscopy studies (Martell et al. 2012). If biotin-phenol and H2O2 are supplied to cells expressing APEX, biotin-phenol is converted into a biotin-phenoxyl radical that attacks electron-rich amino acid side chains of proximal proteins with a labeling radius of <20 nm. This approach was used to map the mitochondrial matrix proteome in human cells (Rhee et al. 2013). APEX was then subjected to directed evolution yielding the much more active APEX2 (Lam et al. 2015). The advantage of APEX2 is the much faster labeling kinetics, requiring labeling times of less than a minute. The disadvantage is that biotin-phenol is less membrane permeable than biotin and that toxic H2O2 must be applied (Hwang and Espenshade 2016; Tan et al. 2020).
The slow labeling kinetics of BirA* has been overcome recently by subjecting BirA* to directed evolution, resulting in TurboID and miniTurboID (Branon et al. 2018). TurboID achieves the same biotin labeling efficiency in 10 min as BirA* in 18 h, has greater activity at ambient temperatures, and a slightly bigger labeling radius of ∼35 nm (May et al. 2020). Disadvantages of TurboID are baseline activity in the presence of endogenous biotin and inability to control enzyme activity by activation as it is possible in the APEX system. Nevertheless, since its publication in 2018, TurboID has been used extensively to map proteomes and to identify protein-interaction networks in a variety of model organisms including mammalian cells (Cho et al. 2020), zebrafish (Xiong et al. 2021), or yeast (Larochelle et al. 2019). There have also been reports on the successful application of TurboID to land plants for the identification of interactors of plant immune receptor N (Zhang et al. 2019), stomatal transcription factor FAMA (Mair et al. 2019), nuclear transport receptor exportin 4 (Xu et al. 2021), nuclear pore complex protein GBPL3 (Tang et al. 2022), and the TPLATE complex (Arora et al. 2020) as well as for the identification of substrates of kinase BRASSINOSTEROID-INSENSITIVE 2 (Kim et al. 2023) and the mapping of the nuclear proteome (Mair et al. 2019).
VESICLE-INDUCING PROTEIN IN PLASTIDS 1 (VIPP1) is a member of the ancient ESCRT-III membrane-remodeling superfamily. Cyanobacterial VIPP1 forms large basket-like assemblies (Gupta et al. 2021; Liu et al. 2021). Inside of the basket, N-terminal amphipathic α-helices (AH) of 24 amino acids length from each monomer align to form large hydrophobic columns, enabling the basket to bind to membranes and to encapsulate a vesicle-like bud. In the chloroplast of Chlamydomonas reinhardtii (Chlamydomonas hereafter), VIPP1 was found in long rods that tubulate membranes and in short rods that form connections between the inner envelope and thylakoids (Gupta et al. 2021). These connections could mediate lipid transfer between the inner envelope and thylakoids, explaining why vipp1 knockout mutants of Arabidopsis (Arabidopsis thaliana) have a severely reduced thylakoid membrane system (Zhang et al. 2012).
The chloroplast HSP70 chaperone system in Chlamydomonas consists of HEAT SHOCK PROTEIN 70B (HSP70B), nucleotide exchange factor CHLOROPLAST GRPE HOMOLOG 1 (CGE1), and at least six J-domain proteins (CDJ1-6) (reviewed in Trösch et al. (2015)). The latter supply HSP70B with specific substrates, which are misfolded proteins in the case of CDJ1 (Willmund et al. 2008) and VIPP1 in the case of CHLOROPLAST DNAJ HOMOLOG 2 (CDJ2) (Liu et al. 2005). HSP70B/CDJ2/CGE1 catalyze the ATP-dependent assembly of VIPP1 monomers/dimers into large assemblies and their disassembly to monomers/dimers in vitro. Large rods were completely disassembled by HSP70B/CDJ2/CGE1 in vitro (Liu et al. 2007).
Several lines of evidence suggest a role of VIPP1 in the biogenesis of thylakoid membrane protein complexes. First, VIPP1 improved protein export via the bacterial and thylakoidal twin-arginine transport pathways (DeLisa et al. 2004; Lo and Theg 2012). Second, in an in vitro reconstituted system to study the co-translational insertion of the D1 protein into thylakoid membranes, VIPP1 stimulated the formation of a D1 insertion intermediate and was found complexed with cpSecY, Alb3, and cpFtsY (Walter et al. 2015). Third, Arabidopsis, Chlamydomonas, and cyanobacterial vipp1 knockdown mutants displayed reduced levels of at least one of the major thylakoid membrane protein complexes (Kroll et al. 2001; Fuhrmann et al. 2009; Nordhues et al. 2012; Zhang et al. 2014, 2016a,b).
Another role of VIPP1 is related to the protection of chloroplast membranes from various stresses. Chlamydomonas vipp1 knockdown mutants or cyanobacterial mutants with mutations in the AH showed swollen thylakoids after exposure to high light (Nordhues et al. 2012; Gupta et al. 2021), and mesophyll cells in Arabidopsis vipp1 knockdown mutants exhibited swollen chloroplasts due to impaired envelope response to hypotonic membrane stress (Zhang et al. 2012). Moreover, VIPP1 overexpression enhanced the recovery of photosynthetic capacity after heat stress, which was proposed to be due to a membrane protective role of VIPP1 (Zhang et al. 2016a,b). And VIPP1 overexpression trans-complemented a chloroplast swelling phenotype in the Arabidopsis ncy1 stay-green mutant proposed to result from oxidative membrane damage (Zhang et al. 2016a,b).
The mechanism by which VIPP1 protects damaged membranes was proposed to be related to its ability to insert the AH into membranes exhibiting stored curvature elastic (SCE) stress. VIPP1 stabilizes stressed membranes by multiple AH insertions, alleviating SCE stress and imparting a scaffold effect that prevents the membrane phase transition into a porous state (McDonald et al. 2015, 2017). An important determinant here is the hydrophobic face of the AH. Chlamydomonas has a paralog of VIPP1 named VIPP2 (Nordhues et al. 2012). VIPP2 has a more hydrophobic AH than VIPP1 and binds more strongly to chloroplast membranes than VIPP1 in cells subjected to H2O2 stress (Theis et al. 2020). VIPP2 is barely expressed under ambient conditions but accumulates strongly under various stress conditions, including high light intensities or elevated cellular H2O2 concentrations (Nordhues et al. 2012; Blaby et al. 2015; Perlaza et al. 2019; Theis et al. 2020), the depletion of the ClpP protease or of thylakoid membrane transporters/integrases (Ramundo et al. 2014; Theis et al. 2020), the addition of nickel ions (Blaby-Haas et al. 2016), the addition of alkylating agents (Fauser et al. 2022), or the inhibition of chloroplast fatty acid synthesis (Heredia-Martínez et al. 2018). In addition to VIPP2, these stresses also result in the accumulation of chloroplast chaperones and proteases including CLPB3, HSP70B, HSP22E/F, and DEG1C, linking chloroplast membrane stress with protein homeostasis. Apparently, misfolded, misassembled, and aggregated proteins in chloroplast membranes cause SCE stress that must be coped with by coordinated action of VIPPs, chaperones, and proteases as part of a chloroplast-specific unfolded protein response (cpUPR) (McDonald et al. 2015; Theis et al. 2020). Accordingly, VIPP1, VIPP2, HSP70B, and HSP22E/F have been found to interact at stressed chloroplast membranes (Theis et al. 2020). The ability of the VIPPs to sense SCE stress could also be the source of a retrograde signal for the cpUPR, which is supported by the finding that the induction of HSP22E/F gene expression upon high light exposure was strongly impaired in the vipp2 mutant (Theis et al. 2020).
The aim of this work was to establish PL in the Chlamydomonas chloroplast based on the transient interaction between CGE1 and HSP70B as test system with the eventual goal to identify the proxiomes of VIPP1 and VIPP2. We show that TurboID fused to CGE1, VIPP1, and VIPP2 resulted in efficient in vivo PL under ambient conditions, heat stress, and oxidative stress. Many known interactions were confirmed, underpinning the strength of PL for revealing protein-interaction networks. We identified CGE2 as a novel co-chaperone of CGE1/HSP70B. In the VIPP1/2 proxiomes generated in three experimental setups, we identified 11 proteins of unknown function whose encoding genes were upregulated under chloroplast stresses; one of which was validated via reciprocal PL. Other VIPP1/2 proxiome proteins play roles in the biogenesis of thylakoid membrane protein complexes and the regulation of photosynthetic electron transport. For the latter, we could verify PROTON GRADIENT REGULATION 5-LIKE 1 (PGRL1), involved in cyclic electron flow, in the VIPP1 proxiome.
Results
PL with APEX2 in Chlamydomonas only works in vitro
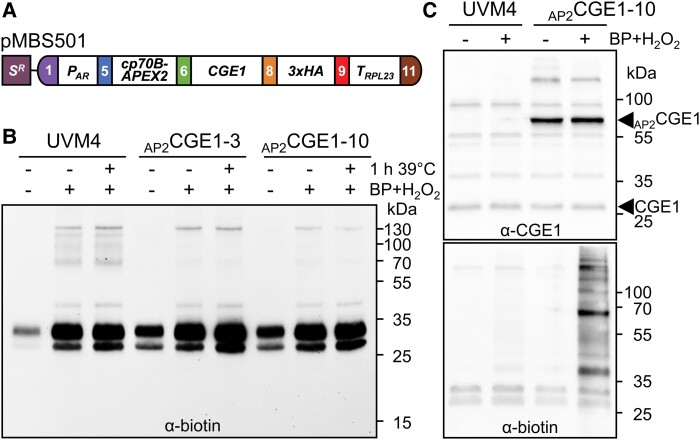
To establish in vivo PL in the chloroplast of Chlamydomonas, we synthesized the sequence encoding engineered soybean ascorbate peroxidase APEX2 (Lam et al. 2015) as a level 0 standard gene part for the Chlamydomonas Modular Cloning (MoClo) kit (Crozet et al. 2018). To enhance gene expression, the sequence was codon-optimized and interrupted by the first Chlamydomonas RBCS2 intron (Baier et al. 2018; Schroda 2019). Moreover, the coding sequence of the chloroplast transit peptide of Chlamydomonas HSP70B (cp70B), including the first HSP70B intron, was added (Drzymalla et al. 1996) (Supplemental Fig. S1). We chose the CGE1 nucleotide exchange factor of chloroplast HSP70B as bait, since it interacts only transiently with HSP70B in the ADP-bound state (Schroda et al. 2001; Schmollinger et al. 2012) and therefore can be regarded as a suitable test system for a transient in vivo interaction. Here, we went for an N-terminal fusion of APEX2 to CGE1, as the N-termini of GrpE-type nucleotide exchange factors represent unstructured regions located proximal to the substrate-binding domain of their Hsp70 partners and could potentially bring APEX2 close to HSP70B substrates (Rosenzweig et al. 2019). We amplified the CGE1 gene without sequences encoding the chloroplast transit peptide from genomic DNA. Parts coding for cp70B-APEX2, CGE1, mCherry (as control), and a 3xHA tag were then assembled with the HSP70A-RBCS2 fusion promoter and the RPL23 terminator into transcription units (level 1) (Crozet et al. 2018). After adding the aadA spectinomycin resistance cassette, resulting level 2 constructs (Fig. 1A; Supplemental Fig. S2) were transformed into the UVM4 expression strain (Neupert et al. 2009). Since CGE1 is an essential gene, complementation of a knock-out mutant was not possible. The APEX2-CGE1 fusion protein was expressed at high frequency and accumulated at higher levels than native CGE1 (Supplemental Fig. S2A). The APEX2-mCherry fusion was also well expressed (Supplemental Fig. S2B).
Figure 1.
Biotin labeling with APEX2. A) Level 2 construct pMBS501 conferring resistance to spectinomycin (SR) and driving the production of CGE1 fused N-terminally to APEX2 (AP2CGE1) and C-terminally to a 3xHA tag. Gene expression is controlled by the HSP70A-RBCS2 promoter (PAR) and the RPL23 terminator (TRPL23). The fusion protein is targeted to the chloroplast via the HSP70B chloroplast transit peptide (cp70B). B) In vivo labeling with biotin-phenol. Chlamydomonas cultures of the recipient strain UVM4 and two AP2CGE1 transformants (Supplemental Fig. S2) were grown at 22°C to mid-log phase. One third of each culture was exposed to 39°C for 1 h. 500 µM biotin-phenol (BP) was added to the stressed and to half of the non-stressed culture for 30 min followed by the addition of 1 mM H2O2 for 1 min. Protein biotinylation in whole-cell proteins was analyzed by SDS-PAGE and immunoblotting using an antibody against biotin. C) In-vitro labeling with biotin-phenol. Chlamydomonas cultures of the recipient strain UVM4 and an AP2CGE1 transformant were grown at 22°C to mid-log phase, harvested, and cells lysed by repeated freeze-thaw cycles. Soluble proteins were left untreated (-) or incubated with 500 µM BP for 30 min followed by the addition of 1 mM H2O2 for 1 min. Proteins were analyzed by SDS-PAGE and immunoblotting using antibodies against CGE1 (top) or biotin (bottom).
We then supplemented cultures of two lines producing APEX2-CGE1 and the UVM4 control strain with 500 µM biotin-phenol and 1 mM H2O2 for 1 min to start APEX2-mediated biotinylation (Lam et al. 2015). However, an antibody against biotin did not detect any protein biotinylation in total protein extracts of APEX2-CGE1-producing strains in addition to endogenously biotinylated proteins also detected in the UVM4 control (Fig. 1B). This was also observed when cells were exposed to 1 h of heat shock at 39°C prior to biotin-phenol and H2O2 addition. Specific biotinylation was not observed in APEX2-CGE1 and APEX2-mCherry production lines even when the preincubation with biotin-phenol was extended up to 24 h or the reaction time with H2O2 was increased up to 1 h (Supplemental Fig. S3). In contrast, when biotin-phenol and H2O2 were added to soluble protein extracts, we detected several biotinylated proteins in an APEX2-CGE1 producing strain that were absent in the UVM4 control, with the most prominent biotinylated protein band migrating at the molecular mass of the APEX2-CGE1 fusion protein (Fig. 1C). We conclude that APEX2-mediated PL only works in vitro, presumably because biotin-phenol is not taken up by intact Chlamydomonas cells.
PL with TurboID in Chlamydomonas works in vivo and can be boosted by biotin addition
Since APEX2 did not support in vivo PL, we turned to the BioID (Roux et al. 2012) and TurboID systems (Branon et al. 2018). Codon-optimized DNA sequences coding for both proteins, interrupted by the 5th HSP70B intron, were synthesized and sequences coding for the HSP70B chloroplast transit peptide, including the first HSP70B intron, were added to generate MoClo level 0 parts for N-terminal fusions. For TurboID, we also generated a level 0 part for C-terminal fusions (Supplemental Fig. S1). Following the design used for APEX2 constructs, we assembled level 2 constructs for the production of BioID-CGE1 and TurboID-CGE1 (N-terminal fusions). Moreover, we assembled constructs for the production of VIPP1, VIPP2, and mCherry with C-terminal TurboID fusions (Fig. 2A). VIPP1 fused C-terminally to GFP fully rescued the lethal phenotype of vipp1 knock-out mutants, suggesting that VIPP function is not impaired by such fusions (Zhang et al. 2012). Chloroplast-targeting of mCherry-TurboID was realized by the CDJ1 chloroplast transit peptide, while for VIPP1 and VIPP2 their native targeting peptides were employed.
Figure 2.
Construction of strains producing bait proteins fused with BioID and TurboID and analysis of their biotinylation patterns. A) Level 2 constructs conferring resistance to spectinomycin (SR) and driving the production of bait proteins fused to BioID and TurboID as described in Fig. 1A. CGE1 is produced with an N-terminal fusion to BioID (BID) and TurboID (TID) and targeted to the chloroplast via an HSP70B chloroplast transit peptide (cp70B). All other baits contain a C-terminal TurboID fusion. mCherry is targeted to the chloroplast via the chloroplast transit peptide of CDJ1 (cpCDJ1), VIPP1 and VIPP2 via their native transit peptides. B) Analysis of fusion protein accumulation and self-biotinylation. Total cell protein extracts corresponding to 2 μg chlorophyll of the UVM4 recipient strain and transformants producing the four baits fused to BioID or TurboID were separated by SDS-PAGE and analyzed by immunoblotting using antibodies against BirA (left panel) or streptavidin coupled with horseradish peroxidase (HRP) (right panel) to capture biotinylated proteins. The home-made BirA antibody also detects plastidic CLPB3 (Supplemental Fig. S6). Asterisks indicate putative chloroplast import precursors. Arrowheads point to proteins whose native biotinylation level decreases upon expression of TurboID fusions. Expected masses for precursor (prec) and mature (mat) proteins are given (Supplemental Table S4). Notice that GrpE proteins generally migrate with larger apparent than calculated masses (Schroda et al. 2001). C) Enhancement of BioID and TurboID in vivo biotinylation activity by externally added biotin. Mid-log phase cultures of the UVM4 recipient strains and transformants producing BioID/TurboID fusions with CGE1, VIPP1, and mCherry were supplied with 500 µM or 1 mM biotin and grown for the indicated times at 22°C. Protein biotinylation was analyzed by immunoblotting of total protein extracts corresponding to 2 µg chlorophyll using streptavidin-HRP.
BioID-CGE1 and TurboID-CGE1 accumulated to roughly the same levels as native CGE1 (Supplemental Figs. S4 and S5A). BioID-CGE1 gave rise to a single protein band in SDS-PAGE, while TurboID-CGE1 gave rise to a double band (Fig. 2B; Supplemental Figs. S4 and S5A). Since the CGE1 gene gives rise to two protein isoforms resulting from alternative splicing that can be distinguished on SDS gels (Schroda et al. 2001; Willmund et al. 2007), it appears likely that the different position of the 5th HSP70B intron in the TurboID CDS versus the BioID CDS favors alternative splicing. In contrast, the larger size difference between the two bands observed for mCherry-TurboID (Fig. 2B; Supplemental Fig. S5B; Supplemental Table S4) suggests inefficient chloroplast targeting via the CDJ1 transit peptide. The single bands detected for VIPP1- and VIPP2-TurboID imply efficient chloroplast targeting with the native transit peptides (Fig. 2B; Supplemental Fig. S6B). VIPP1-TurboID accumulated to much higher levels than VIPP2-TurboID, which was the most weakly expressed of all fusion proteins (Supplemental Fig. S5C). Notice that VIPP2 is barely expressed under non-stress conditions (Nordhues et al. 2012; Theis et al. 2020).
We could detect constitutive in vivo self-biotinylation (or cis-biotinylation, Arora et al. (2020)) without biotin addition for all TurboID fusion proteins but not for BioID-CGE1 despite its high expression levels (Fig. 2B). Interestingly, biotinylation levels of two naturally biotinylated proteins declined in cells expressing TurboID fusion proteins but not in cells expressing BioID-CGE1 (arrowheads in Fig. 2B). The higher the expression levels of the TurboID fusions, the more natural biotinylation was affected. Apparently, TurboID competes with the natural biotinylation machinery in the chloroplast for endogenous biotin. We wondered whether a reduced abundance of naturally biotinylated proteins and the constitutive biotinylation activity of TurboID might affect chloroplast protein homeostasis and cellular fitness. To test this, we exposed lines producing TurboID-CGE1 to 40°C for 24 h and lines producing VIPP1/2-TurboID to high light of 1,000 µE m−2 s−1 for 10 h, allowed them to recover for 16 to 24 h at 22°C, and analyzed levels of cpUPR markers VIPP1, CLPB3, HSP22E/F, HSP70B, and DEG1C (Ramundo et al. 2014; Perlaza et al. 2019). We found no differences in growth behavior or in the abundances of the cpUPR markers between the TurboID lines and the UVM4 control (Supplemental Fig. S7). This suggests that the constitutive biotinylation activity of TurboID has no adverse effects, at least not under the conditions tested here.
Next, we tested whether in vivo biotinylation via BioID and TurboID can be boosted by exogenously added biotin. To this end, we added 500 µM and 1 mM biotin to cultures of the UVM4 control and strains producing BioID- and TurboID fusions with CGE1, VIPP1, and mCherry. As shown in Fig. 2C, with both concentrations of biotin we observed slightly increased cis- and trans-biotinylation already 10 min after biotin addition, which grew dramatically stronger 6 h after biotin addition, while no further biotinylation was observed between 6 and 24 h after biotin addition. Protein biotinylation 1, 6 and 24 h after biotin addition was higher when 1 mM biotin was added than when 500 µM biotin was added. Hence, addition of 1 mM biotin for 1 to 6 h appears optimal for boosting biotinylation of chloroplast proteins. Notice that weak cis-biotinylation of BioID-CGE1 became detectable only when biotin was added for at least 6 h.
We conclude that TurboID fused N- or C-terminally to different bait proteins allowed for efficient in vivo protein biotinylation which can be boosted by the addition of biotin to the cultures. BioID does not promote efficient biotinylation. Naturally occurring biotinylation suffers proportionally to TurboID expression levels.
TurboID-CGE1 allows capturing the interaction with HSP70B without biotin boost and reveals CGE2 as novel co-chaperone
The efficient in vivo biotinylation observed for TurboID-fusion proteins encouraged us to test whether TurboID-mediated PL would allow capturing the transient interaction between CGE1 and HSP70B. To this end, we used a strain producing TurboID-CGE1 and, as controls, two strains producing mCherry-TurboID at different expression levels as well as the UVM4 recipient strain. Cells grown at ambient temperature and heat-stressed at 40°C for 1 h were lysed, and lysates were incubated with streptavidin beads without external biotin addition. We first analyzed proteins in the input and in the streptavidin eluate by immunoblotting using streptavidin-HRP and antibodies against CGE1 and HSP70B, and known HSP70B (co-)chaperones HSP90C (Willmund and Schroda 2005), CDJ1 (Willmund et al. 2008), and HSP22E/F (Rütgers et al. 2017) as well as the known HSP70B substrate VIPP1 (Liu et al. 2005). As shown in Fig. 3A, naturally biotinylated proteins and proteins biotinylated via TurboID were clearly enriched with the streptavidin beads. TurboID-CGE1 was also enriched, as was native CGE1, pointing to cis-biotinylation of the fusion protein and its ability to interact with (and trans-biotinylate) native CGE1. HSP70B and its main J-domain co-chaperone CDJ1 were clearly enriched in the TurboID-CGE1 producing line versus the mCherry-TurboID and UVM4 controls. This enrichment was independent of whether cells were exposed to heat shock prior to lysis. No specific enrichment was found for HSP90C, HSP22E/F, and VIPP1. Rather, HSP22E/F after induction by heat shock appeared to interact unspecifically with the beads.
Figure 3.
TurboID-based proximity labeling without biotin boost using CGE1 as a bait. A) Immunoblot analysis. Cultures of the UVM4 recipient strain, a transformant producing TIDCGE1, and two transformants accumulating different levels of mCherryTID were grown to mid-log phase at 22°C (CL). Half of the cultures was exposed to 40°C for 60 min (HS). Cells were harvested, lysed and biotinylated proteins were affinity-purified with streptavidin beads. Protein extracts before incubation (0.03% of the input) and after elution from the beads (5% of the eluate) were separated by SDS-PAGE and analyzed by immunoblotting with streptavidin-HRP or antibodies against the proteins indicated. The positions of the TurboID-CGE1 fusion protein (TIDCGE1) and of native CGE1 are indicated. One of three biological replicates is shown. B) Volcano plots of the CGE1 proxiomes under ambient (CL) and heat stress (HS) conditions. Streptavidin bead eluates of the samples shown in (A) were analyzed by mass spectrometry. Shown is a comparison between protein abundances of the TIDCGE1 samples and two TIDmCherry lines after subtraction of contaminants and endogenous biotinylated proteins from the UVM4 control strain. Proteins significantly enriched in the TIDCGE1 samples (q < 0.05) are shown as red data points. The CGE1 bait is shown in blue, known CGE1 interaction partners in green.
We next ran LC-MS/MS analyses on streptavidin eluates obtained from three independent experiments for the four strains under non-stress and heat stress conditions and identified a total of 1,169 protein groups (Supplemental Data Set 1). We first filtered for proteins that were identified in at least one strain and one condition in all three replicates. After median normalization, we filtered for proteins significantly enriched (q < 0.05) at least two-fold in the TurboID-CGE1- and mCherry-TurboID-producing lines versus the UVM4 control. This filtering step removes naturally biotinylated proteins and proteins binding unspecifically to the streptavidin beads (Mair et al. 2019) and left 89 and 76 proteins enriched in TurboID-CGE1 versus UVM4 under non-stress and heat stress conditions, respectively (Supplemental Data Set 1). Next, we filtered for proteins that were significantly enriched (q < 0.05) in the TurboID-CGE1 line versus the two mCherry-TurboID lines under each condition. This step removes proteins that get biotinylated because they are abundant and/or have readily accessible amines, and left ten and five proteins enriched in TurboID-CGE1 versus mCherry-TurboID under non-stress and heat stress conditions, respectively (Fig. 3B). CGE1 was enriched 1,997-fold under non-stress conditions and 985-fold after heat stress, whereas other significantly enriched proteins were enriched between two- and 85-fold (Supplemental Data Set 1). HSP70B and CDJ1 were significantly enriched under non-stress conditions, corroborating our immunoblot data (Fig. 3A) and previous findings (Willmund et al. 2008). Under heat stress, HSP70B was significantly enriched as well, while CDJ1 was significantly enriched only when compared with the line producing mCherry-TurboID at higher levels (Supplemental Data Set 1). Since we found CDJ1 enriched under both conditions in immunoblots, our filtering criteria appear to be rather stringent. Surprisingly, we also found mitochondrial HSP70C significantly enriched under non-stress conditions, which points to dual targeting of this chaperone.
CGE2 was significantly enriched under both conditions (Fig. 3B). It received its name based on amino acid sequence motifs characteristic for chloroplast GrpE-type co-chaperones. Because of a lack of EST support and large sequence insertions in the 5′ part of the CGE2 gene, it was not clear whether it gives rise to a functional protein (Schroda 2004; Schroda and Vallon 2009). Structure prediction by alpha-fold suggests a typical GrpE-fold in the C-terminal part of CGE2 but, except for a few alpha-helices, no prediction could be made for the other sequences at the N-terminus (Supplemental Fig. S8A). Nevertheless, we found 29 peptides covering all parts of the sequence (Supplemental Fig. S8B). Since CGE2 had the highest fold-enrichment of all proteins identified in the CGE1 proxiome and contains the typical GrpE-fold for dimerization, it is likely that CGE1 and CGE2 form heterodimers.
ACC1, significantly enriched with TurboID-CGE1 under non-stress conditions, is a biotin-containing enzyme. Its enrichment is likely due to a more pronounced depletion of its natural biotinylation level in the mCherry-TurboID lines expressing TurboID at higher levels than the TurboID-CGE1 line (Fig. 3A). The clearly cytosolic translation initiation factor eIF5B1 just passes the 2-fold-enrichment threshold (2.07-fold) and presumably is a false positive. Other significantly enriched proteins are a putative phytol kinase (Cre08.g381400), a putative zeta-phytoene desaturase (ZPD1, Cre12.g541750) and three more proteins of unknown function (Cre13.g589167, Cre15.g640250, and Cre17.g720450).
We conclude that TurboID without addition of exogenous biotin allows capturing the transient interaction of CGE1 with HSP70B in complex with co-chaperone CDJ1. It also allowed discovering CGE2 as a novel co-chaperone, but it appears suitable only to a limited extent for the discovery of HSP70B substrates.
Three labeling setups with TurboID confirm known interactions of VIPP1 with chloroplast HSP70B and interactions of VIPP1 and VIPP2 at chloroplast membranes under stress
Based on the encouraging results with TurboID-CGE1, we next wanted to identify proteins interacting with VIPP1 and VIPP2 in activities related to thylakoid biogenesis and chloroplast stress. To this end, VIPP1/2-TurboID lines, and UVM4 and mCherry-TurboID lines as controls, were grown under ambient conditions and exposed to 2 mM H2O2 for 4 h to provoke oxidative stress (Blaby et al. 2015; Theis et al. 2020). We chose three labeling protocols: first, biotin labeling in vivo without the addition of exogenous biotin as done for TurboID-CGE1 (Setup 1). Second, biotin labeling in vitro, where 500 µM biotin together with 2.5 mM ATP and an ATP-regenerating system were added to crude membrane extracts for 30 min (Setup 2). The rationale here was that the proximity of proteins in or at membranes should be preserved also in extracted membranes. After 4-h incubation of cells with or without H2O2, membrane extracts were prepared by repeated freeze/thaw cycles. Third, biotin labeling in vivo with the addition of 1 mM biotin to the cultures for 4 h in parallel to H2O2 exposure (Setup 3).
We first analyzed biotinylated proteins in inputs and streptavidin eluates by immunoblotting. For Setup 1 (Fig. 4A), we observed weak cis-biotinylation of the baits, reduced biotinylation of naturally biotinylated proteins that corresponded to the expression level of the baits, and little trans-biotinylation when compared with UVM4. Exogenously added biotin strongly enhanced cis- and trans-biotinylation (Setups 2 and 3, Fig. 4A) and, in Setup 3, also appeared to enhance natural protein biotinylation.
Figure 4.
TurboID-based proximity labeling using VIPP1 and VIPP2 as baits. A) Immunoblot analysis. Cultures of the UVM4 recipient strain, transformants producing VIPP1TID and VIPP2TID, and two transformants accumulating different levels of mCherryTID were grown to mid-log phase at 22°C (CL). Half of the cultures was exposed to 2 mM H2O2 for 4 h. Before the treatments, cells were supplemented without biotin (Setup 1) or with 1 mM biotin (Setup 3), harvested, and lysed. Alternatively, 500 µM biotin was added for 30 min to membrane fractions following the H2O2 treatment and removed again by passage through PD-10 desalting columns (Setup 2). Biotinylated proteins were affinity-purified with streptavidin beads. Protein extracts before incubation (input) and after elution from the beads (eluate) were separated by SDS-PAGE and analyzed by immunoblotting with streptavidin-HRP. One of three biological replicates each is shown. B) Volcano plots of the VIPP1 and VIPP2 proxiomes resulting from Setups 1 to 3. Streptavidin bead eluates of the samples shown in (A) were analyzed by mass spectrometry. Shown is a comparison between protein abundances of the VIPP1/2TID samples and two TID-mCherry lines after subtraction of contaminants and endogenous biotinylated proteins from the UVM4 control strain. Proteins significantly enriched in the VIPP1/2TID samples (q < 0.05) are shown as red data points. VIPP1/2 baits are shown in blue, known VIPP1/2 interaction partners in green. C) Venn diagram showing proteins of the VIPP1/2 proxiomes identified only under non-stress conditions (CL), only after H2O2 treatment, or under both conditions. Proteins in bold were identified in at least two of the three labeling approaches, proteins in green are known VIPP1/2 interaction partners.
LC-MS/MS analysis of streptavidin eluates performed in biological triplicates resulted in the identification of a total of 897 (Setup 1), 477 (Setup 2), and 1,464 (Setup 3) protein groups (Supplemental Data Sets 2 to 4). These numbers meet the expectation from the immunoblot data that exogenously added biotin boosts protein biotinylation and that protein complexity is lower when focusing on membranes. Accordingly, following the same filtering steps employed for TurboID-CGE1, the largest number of proteins significantly enriched in VIPP1/2-TurboID was for Setup 3 with 10 to 22 proteins, followed by Setup 1 with 4 to 10 proteins and Setup 2 with 1 to 6 proteins (Fig. 4B). VIPP1/2-TurboID baits were enriched between 724- and 3,246-fold (Supplemental Table S1; Supplemental Data Sets 2 to 4). Only for Setup 3, the enrichment for VIPP1-TurboID was markedly lower (140- to 148-fold). This is due to a stronger labeling of VIPP1 in the mCherry-TurboID control lines, presumably because of an enhanced labeling rate in this setup combined with the relatively high abundance of VIPP1 (0.05% of total cell proteins, which corresponds to a less abundant Calvin–Benson-cycle enzyme [Liu et al. 2007; Hammel et al. 2020]). The enrichment for non-bait proteins ranged between 3- and 1,163-fold (Supplemental Table S1).
We first looked whether the LC-MS/MS data can confirm known interactions. VIPP2 was enriched with VIPP1-TurboID only after H2O2 treatment (Fig. 4, B and C; Supplemental Table S1). This was expected, since VIPP2 is barely expressed under ambient conditions and strongly upregulated under stress (Nordhues et al. 2012; Ramundo et al. 2014; Perlaza et al. 2019; Theis et al. 2020). Conversely, VIPP1 was enriched with VIPP2-TurboID under ambient and H2O2 stress conditions. The enrichment of VIPP1 with VIPP2-TurboID and VIPP2 with VIPP1-TurboID in Setup 2 suggests that both proteins form heterooligomeric complexes at membranes under H2O2 stress, corroborating previous AP-MS data (Theis et al. 2020). Other proteins known to interact with VIPP1 are HSP70B and its co-chaperone CDJ2, which control the oligomeric state of VIPP1 (Liu et al. 2007). Both were enriched with VIPP1-TurboID under ambient and H2O2 stress conditions. CDJ2, but not HSP70B, was enriched with VIPP2-TurboID under both conditions. This suggests that the chaperones can control the oligomeric state of both VIPPs under ambient and stress conditions. HSP70B was enriched in Setup 2 under ambient conditions, suggesting an interaction at chloroplast membranes. In Setup 3 under non-stress and H2O2 stress conditions, we found mitochondrial HSP70C to be enriched with VIPP1-TurboID to the same extent as HSP70B, again pointing to dual targeting of HSP70C.
In summary, exogenously added biotin strongly enhanced TurboID-mediated protein biotinylation and biotinylation of naturally biotinylated proteins. In vivo labeling and in vitro labeling on membrane extracts confirms a formation of VIPP1-VIPP2 heterooligomers at membranes under stress and confirms the interaction between VIPPs and the chloroplast HSP70 system.
Genes encoding 17 proteins in the proxiomes of VIPP1 and VIPP2 were upregulated under chloroplast stress
Since we were interested in identifying VIPP1/2 interacting proteins with functions related to chloroplast stress, we wondered whether the genes encoding the 39 proteins significantly enriched with VIPP1/2-TurboID were responsive to conditions provoking chloroplast stress. To elucidate this, we consulted previous RNA-seq studies monitoring genes up-regulated after depletion of ClpP (Ramundo et al. 2014), after addition of chloroplast fatty acid synthesis inhibitor cerulenin (Heredia-Martínez et al. 2018), and after the addition of Ni2+ ions (Blaby-Haas et al. 2016) or H2O2 (Blaby et al. 2015). A total of 17 of the 39 genes were upregulated under at least one of these stresses (Supplemental Table S1). Among these were genes encoding (co-)chaperones HSP70B, HSP70C, CDJ2, and CLPB3. CLPB3 was enriched with VIPP1-TurboID only in Setup 3 and only under H2O2 stress. It functions as a protein disaggregase in the chloroplast and localizes to stromal foci situated next to the thylakoid membrane system (Kreis et al. 2023). Of the remaining 13 genes, only two encoded proteins with a clear functional annotation: CHLP1, which was enriched with VIPP1- and VIPP2-TurboID only under ambient conditions, and LPA3, which was enriched only with VIPP1 under H2O2 stress. CHLP1 is a geranylgeranyl reductase that catalyzes the reduction of geranylgeranyl diphosphate to phytyl diphosphate providing phytol for tocopherol and chlorophyll biosynthesis (Tanaka et al. 1999). LPA3 acts together with LPA2 in the stable assembly of CP43 into the photosystem II core complex (Chi et al. 2012). As no clear functional annotation exists for the proteins encoded by the last 11 genes, we named them VIPP PROXIMITY LABELING (VPL1-11). The most highly enriched among these were VPL1-3, with maximum enrichment factors of 645-, 1163-, and 60-fold, respectively (Supplemental Table S1). They showed the same enrichment pattern with VIPP1- and VIPP2-TurboID, with VPL1 and VPL2 enriched under ambient and H2O2 stress conditions, while VPL3 was enriched only under H2O2 stress (Fig. 4C). The enrichment of VPL2 and VPL3 in Setup 2 suggests that they are close to the VIPPs also at membranes (VPL3 has four predicted transmembrane helices while VPL2 has none). VPL1 and VPL3 are conserved in the green lineage, while VPL2 is conserved only in Chlorophyceae (Supplemental Fig. S9B). Although VPL1 was annotated as an isocitrate lyase, there is no experimental evidence for this function and ICL2 shows only 38% sequence identity with mitochondrial ICL1. No functional annotation exists for VPL2. Alpha-fold predicts VPL2 to contain 4 to 5 extended α-helices with the propensity to form coiled-coils, interrupted by unstructured regions (Supplemental Fig. S9A). VPL3/CPLD50 is annotated as a member of the Fe–S cluster biosynthesis family but experimental evidence is missing. VPL4-10 are enriched with VIPP1-TurboID, VPL4 and VPL7 also with VIPP2-TurboID and VPL11 is only enriched with VIPP2-TurboID. Among these, VPL4, 5, 8, and 10 were enriched only under H2O2 stress. According to InterPro (Blum et al. 2021), VPL5, 6, 8, and 10 have no functional domains, VPL4 and VPL7 are kinases, VPL9 is a chlorophyllase, cleaving off the phytol tail from chlorophyll, and VPL11 is a half-size ABC transporter recently named ABCB4 (Li et al. 2022). Predalgo predicts VPL11/ABCB4 to localize to mitochondria, while VPL1-10 are all predicted to localize to the chloroplast.
In summary, 17 proteins in the VIPP1/2 proxiome are encoded by genes that were upregulated under chloroplast stress conditions. 11 of them lack a clear functional annotation and were named VPL1-11.
Two large groups of proteins in the VIPP1/2 proxiomes are involved in the biogenesis of thylakoid membrane protein complexes and in the regulation of photosynthetic electron flow
Twenty-two more proteins were significantly enriched with VIPP1/2-TurboID whose encoding genes were not induced by the four chloroplast stresses (Supplemental Table S1). Four of them can be grouped into proteins that are involved in various assembly processes and include LPA1 (PS II assembly) (Peng et al. 2006), Y3IP1 (PS I assembly) (Albus et al. 2010), CGL160 (chloroplast ATP synthase assembly) (Fristedt et al. 2015), and GET3B (targeting of tail-anchored proteins to thylakoids) (Anderson et al. 2021). LPA3 and CHLP1 from the chloroplast stress-inducible proteins can also be included here.
A second group comprises proteins involved in the regulation of photosynthetic electron flow, including TIC62, PGRL1, potentially RDP5, and two thioredoxins. TIC62 anchors FNR to the thylakoid membrane (Benz et al. 2009), which influences the speed at which photosynthetic control is induced and therefore plays a role in alleviating high light stress (Rodriguez-Heredia et al. 2022). PGRL1 together with PGR5 is involved in antimycin A-sensitive cyclic electron flow (DalCorso et al. 2008). RDP5 is related to the Ca2+-sensing receptor (CAS) protein and, although it has no predicted transmembrane domain, was significantly enriched with VIPP1-TurboID at membranes (Fig. 4B, Setup 2) (Petroutsos et al. 2011; Wang et al. 2016). Thioredoxin z (TRXz) has been shown to activate Calvin-Benson cycle protein phosphoribulokinase in vitro (Le Moigne et al. 2021). Plant TRXy interacts with 2-Cys peroxiredoxins (Jurado-Flores et al. 2020).
Several significantly enriched proteins cannot be functionally grouped, including KEA1, which is located to the chloroplast envelope and together with KEA2 plays a critical role for the rapid downregulation of stromal pH, especially during light–dark transitions (Aranda Sicilia et al. 2021); AAI1, which catalyzes the second step in branched chain amino acid synthesis (Vallon and Spalding 2009); DLA1, which is the E2 subunit of mitochondrial pyruvate decarboxylase (Bohne et al. 2013); a kinesin-like motor protein (KIL8); a putative plastid lipid-associated protein (PAP); and a putative lipid peroxidase (LOX).
Six proteins significantly enriched with VIPP1/2-TurboID have no clear functional annotation (CGLD38, CGL143, Cre01.g004000, Cre06.g278269, Cre06.g259100, and Cre09.g416850). Biotin-containing enzyme ACC1 was (mildly) enriched with VIPP1-TurboID, presumably due to a more pronounced depletion of its endogenous biotinylation level in the mCherry-TurboID lines, as postulated for the TurboID-CGE1 experiment.
Among the 39 proteins significantly enriched with VIPP1/2-TurboID, 32 were predicted by TargetP (Almagro Armenteros et al. 2019) and/or Predalgo (Tardif et al. 2012) to be targeted to the chloroplast, five were predicted by both programs to be targeted to mitochondria (HSP70C, VPL11/ABCB3, CGL143, DLA1, LOX), and one each to be targeted to cytosol (Cre01.g004000) and secretory pathway (Cre06.g278269) (Supplemental Table S1).
In summary, in addition to the group of proteins associated with chloroplast stress, other proteins in the VIPP1/2 TurboID proxiomes are involved in the biogenesis of thylakoid membrane protein complexes, the control of photosynthetic electron flow, or have individual functions.
Reciprocal VPL2- and PGRL1-TurboID labeling confirms the proximity of both proteins to VIPP1
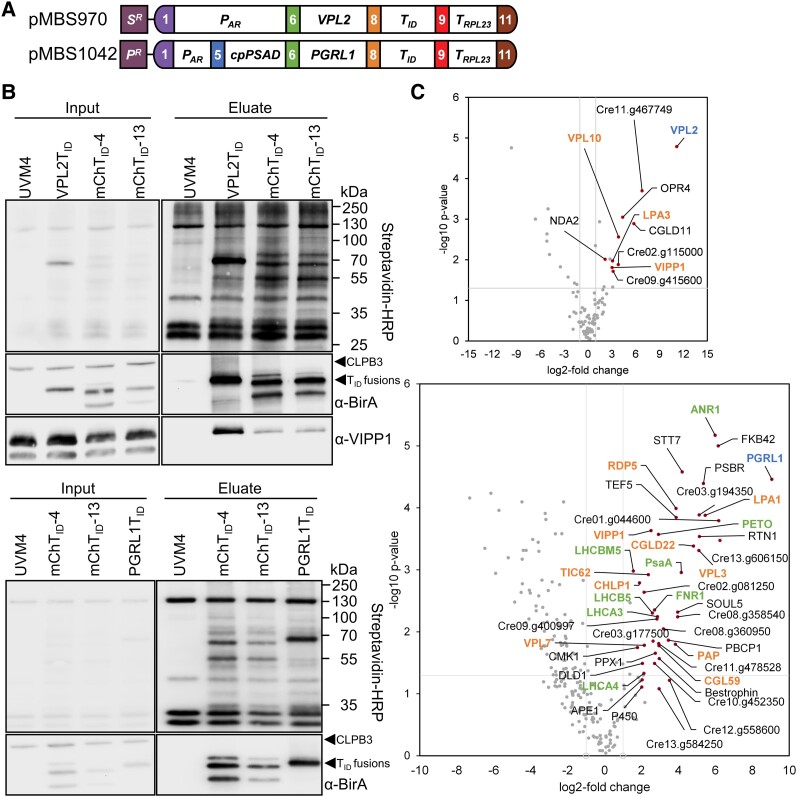
To confirm our results from VIPP1/2-TurboID PL for proteins upregulated upon chloroplast stress and controlling photosynthetic electron flow, we selected VPL2 and PGRL1, respectively, as baits for reciprocal labeling experiments. We synthesized the VPL2 and PGRL1 coding sequences, interrupted by RBCS2 introns, with optimized codon usage (Supplemental Fig. S1). For VPL2, its own chloroplast transit peptide was used, whereas for PGRL1 we used the transit peptide from PSAD in case an N-terminal TurboID fusion would have been necessary. Level 2 constructs were assembled following the design used for APEX2 constructs (Fig. 5A). We identified several transformants accumulating VPL2-TurboID and PGRL1-TurboID in single protein bands (Supplemental Figs. S10 and S11).
Figure 5.
Reciprocal TurboID-based proximity labeling using VPL2 and PGRL1 as baits. A) Level 2 constructs conferring resistance to spectinomycin (SR) or paromomycin (PR) and driving the production of VPL2 or PGRL1 with a C-terminal TurboID fusion as described in Fig. 1A. B) Immunoblot analysis. Cultures of the UVM4 recipient strain, a transformant producing VPL2TID (top panel) or PGRL1TID (bottom panel) and two transformants accumulating different levels of mCherryTID were grown to mid-log phase at 22°C. Cells were supplemented with 1 mM biotin for 4 h, harvested, and lysed. Biotinylated proteins were affinity-purified with streptavidin beads. Protein extracts before incubation with beads (input) and after elution from the beads (eluate) were separated by SDS-PAGE and analyzed by immunoblotting with streptavidin-HRP and antibodies against BirA and VIPP1. One of 3 to 4 biological replicates is shown. C) Volcano plots of the VPL2 (top) and PGRL1 (bottom) proxiomes. Streptavidin bead eluates of the samples shown in (B) were analyzed by mass spectrometry. Shown is a comparison between protein abundances of the VPL2TID and PGRL1TID samples and two TID-mCherry lines after subtraction of contaminants and endogenously biotinylated proteins from the UVM4 control strain. Proteins significantly enriched in the TID sample (q < 0.05) are shown as red data points. The VPL2 and PGRL1 baits are shown in blue, known interaction partners are shown in green, and proteins found before in the VIPP1/2 proxiomes in orange.
Biotin labeling was done according to experimental Setup 3 (addition of 1 mM biotin for 4 h to the cultures) under ambient conditions. Immunoblot analysis with streptavidin-HRP revealed strong cis-biotinylation of VPL2-TurboID and PGRL1-TurboID and successful enrichment of TurboID fusion proteins and trans-biotinylated proteins with the streptavidin beads (Fig. 5B). Again, the biotin boost rescued reduced biotinylation of naturally biotinylated proteins. Immunoblot analysis with the VIPP1 antibody revealed strong enrichment of VIPP1 with VPL2-TurboID. Less VIPP1 was enriched with mCherry-TurboID and none when TurboID was absent (UVM4 control). LC-MS/MS analysis on streptavidin eluates obtained from three independent experiments for VPL2-TurboID and four independent experiments for PGRL1-TurboID resulted in the identification of a total of 831 and 1,047 protein groups, respectively (Supplemental Data Sets 5 and 6).
Following the same filtering steps employed for TurboID-CGE1, we found a 2,298-fold enrichment of VPL2 and an enrichment of non-bait proteins ranging between 4.5- and 112-fold (Supplemental Data Set 5). Nine proteins were significantly enriched with VPL2-TurboID (Fig. 5C). All were soluble proteins predicted by TargetP and/or Predalgo to be targeted to the chloroplast. Enriched proteins included VIPP1, VPL10 and LPA3, which had been detected in the VIPP1/2 proxiome (Fig. 6). Other significantly enriched proteins included NADH:plastoquinone reductase NDA2, which is involved in the antimycin A insensitive pathway for cyclic electron flow and in chlororespiration (Jans et al. 2008); CGLD11, which is involved in F1 assembly during the biogenesis of chloroplast ATP synthase in Arabidopsis (Grahl et al. 2016); octatricopeptide repeat protein OPR4; and two more proteins of unknown function.
Figure 6.
Protein interaction network. Interactions are based on the proxiomes of VIPP1/2 (blue), VPL2 (red), and PGRL1 (purple) determined in this study and on interactions reported previously by Wang et al. (2022) (orange) and Iwai et al. (2010) (green). From the PL datasets, only proteins identified in at least one other data set are shown. From the Wang et al. dataset, only high-confidence interactions were included. ++ indicates several more LHC proteins found in the CEF supercomplex by Iwai et al. (2010). The hierarchy of coloring is blue > purple = red > orange = green.
PGRL1 was 533-fold enriched and the enrichment of non-bait proteins ranged from 3- to 76-fold (Supplemental Data Set 6). Forty-three proteins were significantly enriched with PGRL1-TurboID (Fig. 5C), of which 57% harbor transmembrane helices. Thirty-five were predicted to be targeted to the chloroplast, seven to the cytosol, and two to mitochondria. The enriched proteins included VIPP1, VPL3, VPL7, LPA1, CGL59, CGLD22, CHLP1, PAP, TIC62, and RDP5, which had been detected in the VIPP1/2 proxiome (Figs. 5 and 6). Eight enriched proteins had been identified in complex with PGRL1 in previous studies, including ANR1, PETO, FNR1, PsaA, LHCA3, LHCA4, LHCB5, and LHCBM5 (Iwai et al. 2010; Terashima et al. 2012; Takahashi et al. 2016) (Figs. 5 and 6). Other proteins in the PGRL1 proxiome with known functions in controlling photosynthetic electron flow include: state transition kinase STT7 and phosphatase PBCP1 (Bellafiore et al. 2005; Cariti et al. 2020); TEF5/PSB33/LIL8, which is involved in balancing energy transfer between the photosystems (Kato et al. 2017); APE1, which promotes PS II heterogeneity to reduce PS II overexcitation in high light (Chazaux et al. 2020); and PSBR, which is required for the stable binding of LHCSR3 to PS II-LHC II supercomplexes (Xue et al. 2015). The remaining 20 enriched proteins have other or unknown functions.
We conclude that reciprocal TurboID with VPL2 and PGRL1, both present in the VIPP1/2 proxiomes, resulted in significant enrichment of VIPP1. Two and ten proteins from the VIPP1/2 proxiomes were also enriched in the VPL2 and PGRL1 proxiomes, respectively, thus further confirming the VIPP1/2 TurboID data.
Using PL data to decipher the chloroplast proteome
PL not only allows identifying the proxiome of a particular bait, but also provides information on the composition of a compartment's proteome (Rhee et al. 2013; Kim et al. 2014; Mair et al. 2019). To get this information, we combined all proteins that were significantly enriched in the streptavidin eluates on extracts of transformants expressing our TurboID-tagged baits against the eluates from the UVM4 control strain. Here, we filter out contaminants and natively biotinylated proteins and should retain only proteins that were biotinylated by TurboID activity in the chloroplast. Given the incomplete chloroplast-targeting of mCherry-TurboID, as judged from the accumulation of a protein band presumably derived from the precursor protein, the mCherry data were omitted and only those with CGE1, VIPP1, VIPP2, VPL2, and PGRL1 as baits considered. This resulted in a total of 308 proteins (Supplemental Data Set 7). Of these, 232 (75%) were predicted to localize to the chloroplast based mainly on Predalgo predictions (Tardif et al. 2012) and manual curation (Westrich et al. 2021). 42 proteins were predicted to be mitochondrial, 30 to be cytosolic, and four to be secreted.
Discussion
APEX2 and BioID are not suitable for PL in the Chlamydomonas chloroplast
Here, we report on the development of PL for studying protein interaction networks in the Chlamydomonas chloroplast. We have applied the three most commonly used PL systems based on APEX2 (Lam et al. 2015), BioID (Roux et al. 2012), and TurboID (Branon et al. 2018) with a total of six stromal and membrane-peripheral/integral baits (CGE1, VIPP1, VIPP2, VPL2, PGRL1, and mCherry as control). APEX2 fused to CGE1 did not result in protein biotinylation after biotin-phenol addition to Chlamydomonas cell cultures even if longer incubation times with biotin-phenol or H2O2 were employed (Fig. 1B; Supplemental Fig. S3). Presumably, Chlamydomonas cells are not permeable for biotin-phenol. The failure of APEX2-mediated PL was also reported in a parallel study establishing PL in Chlamydomonas (Lau et al. 2023). Cell permeability problems have been reported also in some mammalian cell types and tissues, in thicker Drosophila tissues, or in fission yeast. While labeling could eventually be achieved in mammalian cells by increasing biotin-phenol concentrations from 0.5 to 2.5 mM and higher (Tan et al. 2020), this was not sufficient in fission yeast, where cell permeabilization by increased osmolarity was required (Hwang and Espenshade 2016). In Drosophila, small amounts of detergent were required to increase permeability (Mannix et al. 2019). Since PL in chloroplasts requires biotin-phenol to traverse three membranes, we did not pursue APEX2-mediated PL further, albeit we could demonstrate rapid labeling in soluble cell extracts (Fig. 1C).
Cis-biotinylation of BioID fused to CGE1 was only clearly detectable after 24 h of labeling time and was more pronounced when 1 mM biotin was used compared with 500 µM (Fig. 2C). Compared to TurboID fusions, trans-biotinylation was hardly detectable (Fig. 2C). Long labeling times of 22 to 48 h and biotin concentrations ranging between 50 µM and 2 mM also were required in studies employing BioID fusions in cytoplasm/nucleus or at plasma membranes of Nicotiana benthamiana, tomato (Solanum lycopersicum), rice (Oryza sativa), and Arabidopsis cells but in these studies BioID-mediated protein biotinylation was much higher than in Chlamydomonas (Lin et al. 2017; Conlan et al. 2018; Khan et al. 2018; Das et al. 2019; Mair et al. 2019; Zhang et al. 2019; Arora et al. 2020). This suggests that BioID is at most of limited use for PL in the chloroplast of Chlamydomonas.
Differences and commonalities of TurboID-mediated PL in Chlamydomonas and land plants
In contrast to APEX2 and BioID, TurboID fused to any of the six baits resulted in efficient protein biotinylation in the Chlamydomonas chloroplast. We observed clearly detectable cis- and trans-biotinylation even in the absence of exogenous biotin (Figs. 2, B and C, 3A and 4A; Supplemental Fig. S7B). However, the addition of biotin to the cultures strongly enhanced protein biotinylation even under H2O2 stress conditions, and we found biotin concentrations of 1 mM and labeling times of 1 to 6 h to be optimal (Figs. 2C and 4A). Background biotinylation in the absence of biotin was observed also for TurboID fusions with three Calvin–Benson-cycle enzymes in Chlamydomonas in a parallel study (Lau et al. 2023). In that study, similar labeling times and biotin concentrations as used by us were found to be optimal even in another strain background, demonstrating the reproducibility of TurboID-mediated PL in the chloroplast of Chlamydomonas.
TurboID applications to identify protein–protein interaction networks in Arabidopsis and N. benthamiana used only 50 to 200 µM of exogenously added biotin but similar labeling times of 0.5 to 12 h, although strong protein biotinylation was observed already after as little as 15 min (Mair et al. 2019; Zhang et al. 2019; Xu et al. 2021; Tang et al. 2022; Wurzinger et al. 2022; Kim et al. 2023). While in these studies no effects on naturally biotinylated proteins were reported, we observed strong effects in Chlamydomonas with loss of natural protein biotinylation that corresponded to TurboID expression levels (Figs. 2, B and C, 3A and 4A; Supplemental Fig. S7B). Hence, the high activity of TurboID effectively competes with natural biotinylation in the chloroplast. Unexpectedly, we observed no negative effects on growth and no increased accumulation of cpUPR marker proteins in TurboID-expressing lines compared to the wild type even if cells were subjected to 24 h heat stress or 10 h of high light (Supplemental Fig. S7). Nevertheless, the reduced natural biotinylation should be kept in mind when using TurboID. This is also because a greater reduction in natural biotinylation in control cells compared with bait cells can lead to an enrichment of naturally biotinylated proteins in bait cells, as was observed with ACC1 (Figs. 3B and 4B). Importantly, natural biotinylation could be rescued to some extent by the addition of 1 mM exogenous biotin for up to 6 h, which also abolished ACC1 enrichment (Figs. 2C, 4A and 5B). The expression of TurboID-baits should not be too high to avoid too much interference with natural biotinylation. We estimate fusions of TurboID with CGE1 and VIPP1 to account for 0.01% to 0.05% of total cell proteins, based on the accumulation of the proteins to levels similar to the native proteins (Liu et al. 2007). Notice, however, that effective biotinylation was observed with VIPP2-TurboID, which was produced at much lower levels than TurboID-CGE1 and VIPP1-TurboID (Fig. 2B).
In land plants, a desalting step was essential to remove excess biotin from protein extracts prior to incubation with streptavidin beads (Mair et al. 2019; Zhang et al. 2019; Arora et al. 2020; Xu et al. 2021; Tang et al. 2022; Wurzinger et al. 2022; Kim et al. 2023). This desalting step was required for Chlamydomonas only if biotin was added to crude membrane extracts. If added to the cell culture, the routine cell harvesting protocol including one washing step removes excess biotin sufficiently.
Boosting protein biotinylation by adding biotin improves the power of TurboID to reveal proximal proteins
In the three experimental setups used for VIPP1/2-TurboID (Fig. 4; Supplemental Data Sets 2 to 4), the number of identified biotinylated proteins was highest in Setup 3 (1,464 proteins), followed by Setup 1 (897 proteins) and Setup 2 (477 proteins). The 1.6-fold increase in the number of proteins identified in Setup 3 compared with Setup 1 suggests that the addition of biotin to the cultures greatly increases TurboID-mediated specific and background biotinylation. The number of proteins identified could be even higher if a mass spectrometer with an ion trap (C-trap, trapped ion mobility spectrometry) had been used; we used a TripleTOF instrument without an ion trap. In a first filtering step comparing the proteins identified in the TurboID lines with the wild type, about 90% of the proteins were removed. These are naturally biotinylated proteins and contaminants that bind to the streptavidin beads. In a second filtering step comparing the proteins identified in the bait lines with the mCherry controls, another up to 90% of the proteins were removed. These are proteins that are biotinylated because they are abundantly present in the same compartment as the bait and/or expose readily accessible primary amines (Mair et al. 2019; Zhang et al. 2019; Mair and Bergmann 2021).
Even after the two filtering steps, the number of significantly enriched proteins in Setup 3 was 1.6- to 2.5-fold higher than in Setup 1. Thus, the larger number of biotinylated proteins in Setup 3 compared to Setup 1 also resulted in a proportionally larger number of significantly enriched proteins. More candidates improve statistical power, as shown by the enrichment of TurboID itself in Setup 1 and 2, but not in Setup 3 (Fig. 4B). This is consistent with previous results where most FAMA transcription factor interaction candidates were identified by TurboID-mediated PL after 3 h of labeling time compared with 0.5 h of labeling time (Mair et al. 2019). Nevertheless, experimental Setup 1 allowed the identification of known CGE1 partner proteins HSP70B and CDJ1 and of the obvious new partner CGE2 (Fig. 3B). Setup 1 also allowed the identification of the highly enriched proteins VPL1, VPL2, and RDP5 in the VIPP1/2 proxiomes (Fig. 4; Supplemental Table S1). However, Setup 1 only allowed probing of the known interaction between VIPP1 and VIPP2, but not the known interaction of VIPP1 with HSP70B and CDJ2, which were probed in Setup 3 (Fig. 4). Hence, consistent with the findings of Mair et al. (2019), Setup 3 appears to give a more comprehensive picture on a protein's proxiome than Setup 1.
The strong enrichment of biotinylated VIPP1/2-TurboID and even mCherry-TurboID in Setup 2 suggests that at least cis-biotinylation could be enhanced in crude membrane extracts (Fig. 4A). However, because no proteins were enriched in Setup 2 in addition to those found in Setups 1 and 3, this in vitro PL setup has no additional benefit compared with the in vivo PL setups. It is also problematic here that mCherry-TurboID is no good control because it is present in membrane fractions only as a contaminant from soluble proteins, whereas VIPP1/2-TurboID are truly membrane-associated.
Not all interactions are probed by TurboID-mediated PL
Despite the ability of PL in Setup 3 to probe known interactions of VIPP1 with VIPP2, HSP70B, and CDJ2, other known interactions of VIPP1 with CGE1, HSP90C, and HSP22E/F (Liu et al. 2005; Heide et al. 2009; Theis et al. 2020) were not probed. Moreover, we did not detect subunits of the cytochrome b6/f complex in the PGRL1 proxiome, which were detected in a PGRL1-containing CEF supercomplex (Iwai et al. 2010). In that complex, also more PS I subunits and more LHCs were found, while our PGRL1 proxiome only contained PsaA and four LHCs (Fig. 5C). Also, in a parallel study by Lau et al. (2023), not all expected pyrenoid proteins were identified by TurboID-mediated PL in Chlamydomonas. And in previous TurboID applications in land plants, not all known interactors of the bait proteins used were found either (Zhang et al. 2019; Arora et al. 2020).
General reasons why proteins proximal to a bait are not mapped by PL include that primary amines are masked by steric hindrance or conformation, that the distance between bait and proximal protein is beyond the labeling radius of activated biotin (e.g. in multiprotein complexes), that peptide abundance of a proximal protein is below the detection limit of the mass spectrometer, and that there are discrepancies between the time windows of labeling and specific interactions taking place. One specific reason for the failure to map known interactions in our study is that we used two mCherry TurboID controls with different abundance levels. This could be too stringent and lead to false-negative results. For example CDJ1, a known component of the HSP70B-CDJ1-CGE1 complex, was significantly enriched with TurboID-CGE1 against one control after heat stress, but not against two controls (Supplemental Data Set 1). Using only the first filtering step against the UVM4 control leads to the presence of known substrate VIPP1 and co-chaperone HSP90C of the HSP70B-CGE1 complex in the CGE1 proxiome (Supplemental Data Set 7). In addition, several more candidates of interest such as FtsH-like ATPases/metalloproteases are found in the proxiomes of VIPP1/2 and CGE1. However, performing only the filtering step against the UVM4 control will include proteins in the proxiomes that are biotinylated because they are abundantly present in the stroma and/or expose readily accessible primary amines and thus represent false positives. Knowing the basal biotinylation rates and absolute abundance of each protein in a compartment could be a better control and will be the subject of future work.
The mentioned limitations should also be kept in mind when PL is used for the mapping of compartment-specific proteomes, i.e. deduced from the enrichment of proteins with TurboID compared to the wild-type control (Supplemental Data Set 7). There will be a bias against proteins of low abundance, proteins that lack or have a low accessibility of primary amines such as proteins deeply buried in protein complexes or in membranes, and proteins in confined subcompartments (Qin et al. 2021).
Can the degree of enrichment be used as a measure of proximity?
The degree of enrichment of a protein in our PL experiments results from the ratio of its biotinylation in bait-TurboID cells (numerator) to mCherry-TurboID cells (denominator). The extent to which a protein is biotinylated depends on its abundance, the accessibility of primary amines, its localization, and its proximity to TurboID. An abundant protein with accessible primary amines distributed throughout the target compartment is likely to be biotinylated in mCherry-TurboID cells, contributing to a high denominator value. Even if it is enhanced biotinylated in bait-TurboID cells due to its proximity to the bait, the degree of enrichment could be moderate. In contrast, a low abundant protein with low accessibility of primary amines may not be biotinylated at all in mCherry-TurboID cells. Here, Perseus imputes a very low value for the missing value leading to a very low denominator value. Thus, even if this protein is biotinylated as much as the abundant one due to its proximity to the bait (same numerator value), it could have a much higher enrichment level. Thus, high enrichment may be due to a protein's proximity to a bait, but it may also be due to its low abundance or low accessibility of primary amines. Finally, consider that a high baseline level of biotinylation prior to biotin and stress application reduces the fold change induced by stress.
Proximity to TurboID can result from interaction with the bait, but also from mere co-localization in a limited subcompartment, such as phase-separated condensates. If a protein is exclusively localized in a limited subcompartment, it may not be biotinylated in mCherry-TurboID cells, and few biotinylations by activated biotin generated by TurboID in this subcompartment may result in a very high enrichment value. In this case, strong enrichment can be achieved without close spatial proximity.
What could be learned from the proxiomes obtained?
With TurboID we were able to probe many known interactions, including those of CGE1 with HSP70B and CDJ1 (Fig. 3B) (Willmund et al. 2008), and those of VIPP1 with VIPP2, HSP70B, and CDJ2 (Fig. 4B) (Liu et al. 2005; Theis et al. 2020). PL confirms the interaction of VIPP1 with VIPP2 at chloroplast membranes under oxidative stress shown previously by AP-MS (Theis et al. 2020). CLPB3 in the VIPP1 proxiome under oxidative stress conditions supports the idea that CLPB3 may aid in the removal of protein aggregates from thylakoid membranes, as proposed previously based on localization data (Kreis et al. 2023). CDJ2 in the VIPP2 proxiome suggests that the assembly state of VIPP2, like that of VIPP1, is controlled by the HSP70B/CDJ2/CGE1 chaperone system (Liu et al. 2007) (notice that VIPP2 forms rods like VIPP1 [Theis et al. 2020]). CGE2, found in the CGE1 proxiome (Fig. 3B), very likely is a novel co-chaperone of CGE1, as it contains a GrpE fold for dimerization (Supplemental Fig. S8), signature sequences of chloroplast GrpEs (Schroda et al. 2001), and is predicted to be targeted to the chloroplast (Schroda and Vallon 2009). VPL2 in the proxiome of VIPP1 could be confirmed in a reciprocal PL experiment, and LPA3 and VPL10 were in the proxiomes of both, VIPP1/2 and VPL2 (Figs. 4B, 5C and 6).
Surprisingly, we found mitochondrial HSP70C in the proxiomes of CGE1 and VIPP1 (Figs. 3B and 4B). In fact, HSP70C was previously found to co-precipitate with VIPP1 (Liu et al. 2005). This interaction was considered to be nonspecific and a consequence of the mixing of compartment contents during cell lysis before immunoprecipitation. Given the presence of HSP70C in the CGE1 and VIPP1 proxiomes, we must consider that HSP70C may be dually targeted to mitochondria and chloroplasts. A recent report on the localization of more than 1,000 candidate chloroplast proteins by fluorescent tagging has shown that dual targeting is a common phenomenon in Chlamydomonas (Wang et al. 2022). Dual targeting might therefore explain why in our PL-based chloroplast proteome ∼23% of the proteins are predicted to localize to mitochondria or cytosol (Supplemental Data Set 7).
In addition to probing known or expected protein interaction networks, PL has revealed several new candidate proteins with the potential to provide fresh insights into the function of VIPPs in the chloroplast. Such proteins have been difficult to find by conventional AP-MS based methods (Jouhet and Gray 2009; Lo and Theg 2012; Bryan et al. 2014). One group in the VIPP1/2 proxiomes comprises 13 proteins whose genes have been found previously to be upregulated under chloroplast stress conditions. Eleven of them lack a clear functional annotation and we named them VPL1-11. We speculate that these proteins may play a role in coping with chloroplast membrane stress and mediating retrograde signaling for the cpUPR. A second group of proteins has reported roles in the biogenesis of PS II (LPA1, LPA3), PS I (Y3IP1), and ATP synthase (CGL160, CGLD11), the targeting of tail-anchored proteins (GET3B), and the synthesis of phytol (CHLP1). A role of VIPP1 in supporting these biogenesis processes would account for the reduced levels of major thylakoid membrane protein complexes in Arabidopsis, Chlamydomonas, and cyanobacterial vipp1 knockdown mutants (Kroll et al. 2001; Fuhrmann et al. 2009; Nordhues et al. 2012; Zhang et al. 2014, 2016a,b). A third group of proteins plays roles in photosynthetic electron flow, including PGRL1, NAD2, TIC62, TRXy, TRXz, and potentially RDP5. Impaired functioning of these processes could account for the deregulation of high light-induced LHCSR3 gene expression in Chlamydomonas vipp1 knockdown and vipp2 knockout lines (Nordhues et al. 2012; Theis et al. 2020).
While the involvement of VIPP1 in so many functions would explain the pleiotropic phenotypes observed in vipp1 mutants, how can VIPP1 be involved in so many functions? We previously proposed the idea that VIPP1 might be able to organize domains in chloroplast membranes that resemble eisosomes found in fungal plasma membranes (Rütgers and Schroda 2013; Theis and Schroda 2016; Theis et al. 2019a,b, 2020). Local membrane bending and enrichment of specific lipid species at such domains may be required for the optimal functioning of various processes taking place at membranes (Foderaro et al. 2017).
In conclusion, TurboID-mediated PL has enabled the probing of known and new protein interaction networks in the nucleus, cytoplasm and at the plasma membrane of land plants with high sensitivity and specificity (Mair et al. 2019; Zhang et al. 2019; Arora et al. 2020; Xu et al. 2021; Tang et al. 2022; Kim et al. 2023). Our work and that of two parallel studies by Lau et al. (2023) and Wurzinger et al. (2022) add Chlamydomonas as another plant model and the chloroplast as another compartment amenable to the great power of TurboID-mediated PL. The availability of TurboID as a standard component of the Chlamydomonas MoClo toolkit, allowing assembly with any bait in a single cloning step, will greatly facilitate PL in the community.
Materials and methods
Strains and culture conditions
Chlamydomonas (Chlamydomonas reinhardtii) UVM4 cells (Neupert et al. 2009) were grown in Tris-Acetate-Phosphate (TAP) medium (Kropat et al. 2011) on a rotatory shaker at a constant light intensity of ∼40 μmol photons m−2 s−1 provided by MASTER LEDtube HF 1200 mm UO 16W830 T8 and 16W840 T8 (Philips). For heat stress experiments, exponentially growing cells were harvested by centrifugation at 3,500 × g for 2 min at 25°C, resuspended in TAP medium prewarmed to 40°C, and incubated in a 40°C water bath under agitation and constant illumination at ∼40 μmol photons m−2 s−1 for 1 h. For H2O2 treatments, exponentially growing cells were incubated with 2 mM H2O2 (Sigma-Aldrich) for 4 h. Transformation was performed with the glass beads method (Kindle 1990) as described previously (Hammel et al. 2020), with constructs linearized by EcoRV. Transformants were selected on TAP medium containing 100 µg mL−1 spectinomycin or 100 µg mL−1 paromomycin. Cell densities were determined using a Z2 Coulter Counter (Beckman Coulter) or photometrically by optical density measurements at 750 nm (OD750).
Cloning of coding sequences for baits, APEX2, BioID, and TurboID
Bait genes for level 0—The CGE1 gene containing all seven introns and eight exons (with exon 1 lacking sequences encoding the chloroplast transit peptide) was amplified by PCR from genomic DNA. Genomic DNA of Chlamydomonas strain CC-4533 was extracted as described previously (Theis et al. 2020). Four fragments were amplified to remove endogenous BsaI and BbsI restriction sites by silent mutations (primers used and product sizes are listed in Supplemental Table S2). Each fragment was flanked with BbsI restriction sites, generating unique overhangs upon BbsI digestion such that they could be directionally assembled into the pAGM1287 vector (Weber et al. 2011) during the restriction-ligation reaction (5 h at 37°C, 5 min at 50°C, and 10 min at 80°C), yielding pMBS589. The VIPP2 gene, including all nine introns and the sequences encoding the chloroplast transit peptide, was previously assembled in the same way (Theis et al. 2020). The VIPP1 gene, including all nine introns and the sequences encoding the chloroplast transit peptide, was synthesized as described previously (Gupta et al. 2021). The 354-amino acids VPL2 protein (Cre07.g333150), including its chloroplast transit peptide, was reverse translated using the most-preferred Chlamydomonas codons. To enhance gene expression (Baier et al. 2018; Schroda 2019), the first two Chlamydomonas RBCS2 introns were inserted with the flanking sites AG/intron/GC. The sequence was split into four fragments flanked by BbsI recognition sites giving rise to distinct overhangs, synthesized (Integrated DNA Technologies), and assembled into the pAGM1287 vector in a restriction-ligation reaction, yielding pMBS969. Likewise, the 292-amino acids PGRL1 protein (Cre07.g340200), lacking its chloroplast transit peptide, was reverse translated using the most-preferred Chlamydomonas codons. Chlamydomonas RBCS2 intron 1 was inserted twice, and intron 2 once with the flanking sites AG/intron/GC. The sequence was split into two fragments flanked by BbsI recognition sites giving rise to distinct overhangs, synthesized, and assembled into the pAGM1287 vector in a restriction-ligation reaction, yielding pMBS1045. A vector with the coding sequence for mCherry, containing the first RBCS2 intron, was produced previously (pCM0-067) (Crozet et al. 2018). All constructs represent level 0 parts for the B3/4 position according to the Modular Cloning (MoClo) syntax for plant genes (Weber et al. 2011; Patron et al. 2015).
APEX2, BioID, and TurboID for level 0—The APEX2 amino acid sequence encoded by vector pcDNA3 APEX2-NES (Rhee et al. 2013; Lam et al. 2015), including a GS-linker and a FLAG-tag (DYKDDDDK) at the N-terminus, and the LQLPPLERLTLD nuclear export signal at the C-terminus, was reverse translated using the most-preferred Chlamydomonas codons. The first RBCS2 intron was inserted (AG/intron/GG) and the sequence was synthesized with BbsI restriction sites at the 5′- and 3′-termini (producing GACT and AATG overhangs) and cloned into pBS SK+ by GeneCust (Luxembourg), yielding pMBS977. To target APEX2 to the chloroplast, sequences encoding the HSP70B chloroplast transit peptide containing the first HSP70B intron, were amplified by PCR on pMBS639 (Niemeyer et al. 2021) (Supplemental Table S2). The resulting 249-bp PCR product, pMBS977, and destination vector pAGM1276 (Weber et al. 2011) were subjected to a restriction-ligation reaction with BbsI, resulting in level 0 vector pMBS454, placing cp70B-APEX2 into the B2 position. For C-terminal APEX2 fusions, pMBS527 was used as a template for PCR to amplify the APEX2 gene with flanking BbsI restriction sites producing TTCG and GCTT overhangs (Supplemental Table S2). The PCR product and pAGM1301 were subjected to a restriction-ligation reaction with BbsI, resulting in level 0 vector pMBS527, placing APEX2 into the B5 position.
The BioID (BirA*) amino acid sequence (Choi-Rhee et al. 2004; Roux et al. 2012) with a C-terminal GGGGS-linker was reverse translated using the most-preferred Chlamydomonas codons and equipped with the transit peptide sequence of HSP70B containing the first HSP70B intron. The fifth HSP70B intron was inserted into the BioID coding sequence (AG/intron/GG). Synthesis and cloning into the XbaI-XhoI site of pBS SK+ was done by GeneCust (Luxembourg), yielding pMBS976. Since the original gene design was not compatible with the MoClo syntax, we amplified three fragments by PCR to remove two BsaI sites from the first HSP70B intron and the BirA* coding sequence and to introduce flanking BbsI restriction sites giving rise to CCAT and AATG overhangs (primers used and product sizes are listed in Supplemental Table S2). PCR products and destination vector pAGM1276 were subjected to a restriction-ligation reaction with BbsI, resulting in level 0 vector pMBS197.
For C-terminal TurboID fusions, the TurboID amino acid sequence (Branon et al. 2018), equipped with an N-terminal SGGGG-linker, was reverse translated using the most-preferred Chlamydomonas codons and the fifth HSP70B intron (AG/intron/GC) was inserted. The sequence was synthesized with flanking BsaI restriction sites (producing TTCG and GCTT overhangs) and cloned into the pUC57 vector by BioCat (Heidelberg, Germany), yielding level 0 construct pMBS512 with TurboID in the B5 position. To target TurboID to the chloroplast, the TurboID protein, equipped with a C-terminal GGGGS-linker, was reverse translated using the most-preferred Chlamydomonas codons and the fifth HSP70B intron (AG/intron/GC) was inserted. The sequence was synthesized with flanking BbsI restriction sites (producing TCAG and AATG overhangs) and cloned into the pUC57 vector by BioCat (Heidelberg, Germany), yielding pMBS513. Sequences encoding the HSP70B chloroplast transit peptide and the first HSP70B intron were amplified by PCR on pMBS639 (Supplemental Table S2). The resulting 245-bp PCR product, pMBS513, and destination vector pAGM1276 were subjected to a restriction-ligation reaction with BbsI, resulting in level 0 vector pMBS515, placing cp70B-TurboID into the B2 position. All PCRs were done with KAPA HiFi PCR Kit (KapaBiosystems) following the manufacturer's instructions. Correct cloning of all level 0 constructs made was verified by Sanger sequencing. Level 0 constructs for all baits and biotin activases are shown in Supplemental Fig. S1.
Level 1 and 2 constructs—The newly constructed level 0 parts were complemented with level 0 parts (pCM) from the Chlamydomonas MoClo toolkit (Crozet et al. 2018) to fill the respective positions in level 1 modules as follows: A1-B1—pCM0-015 (HSP70A-RBCS2 promoter + 5′ UTR); A1-B2—pCM0-020 (HSP70A-RBCS2 promoter + 5′ UTR); B2—pMBS454 (chloroplast targeted APEX2), pMBS197 (chloroplast targeted BioID), pMBS515 (chloroplast targeted TurboID), pCM0-052 (PSAD chloroplast transit peptide), and pMBS640 (CDJ1 chloroplast transit peptide, Niemeyer et al. (2021)); B3/4—pMBS375 (CGE1), pMBS478 (VIPP1, Gupta et al. (2021)), pMBS277 (VIPP2, Theis et al. (2020)) or pCM0-067 (mCherry); B5—pCM0-100 (3xHA), pCM0-101 (MultiStop) or pMBS512 (TurboID-C); B6—pCM0-119 (RPL23 3′ UTR). The respective level 0 parts and destination vector pICH47742 (Weber et al. 2011) were combined with BsaI and T4 DNA ligase and directionally assembled into the seven level 1 modules shown in Supplemental Table S3. The level 1 modules were then combined with pCM1-01 (level 1 module with the aadA gene conferring resistance to spectinomycin flanked by the PSAD promoter and terminator) from the Chlamydomonas MoClo kit, with plasmid pICH41744 containing the proper end-linker, and with destination vector pAGM4673 (Weber et al. 2011), digested with BbsI, and ligated to yield eight of the ten level 2 devices displayed in Supplemental Table S3. For the VPL2 (pMBS970) and PGRL1 (pMBS1042) constructs, level 0 parts were directly assembled into level 2 destination vector pMBS807 and pMBS808 already containing the aadA and aphVIII resistance cassettes, respectively (Niemeyer and Schroda 2022). All newly generated level 0 and level 2 plasmids (Supplemental Table S3) can be ordered from the Chlamydomonas Research Center (https://www.chlamycollection.org/).
Cloning, expression, and purification of recombinant BirA
The BirA coding region was amplified by colony PCR from TOP10F’ cells (Invitrogen) (Supplemental Table S2). The 981-bp PCR product was digested with BamHI and HindIII and cloned into BamHI-HindIII-digested pETDuet-1 vector (Novagen), giving pMS977. BirA was expressed with an N-terminal hexa-histidine (6xHis) tag in E. coli Rosetta cells (DE3, Novagen) after inducing expression with 1 mM IPTG for 16 h at 20°C and purified by cobalt-nitrilotriacetic acid affinity chromatography according to the manufacturer's instructions (G-Biosciences), including a washing step with 5 mM Mg-ATP. Eluted BirA was gel filtrated using an Enrich SEC650 column. The purity of the recombinant protein was analyzed by Coomassie brilliant blue staining (Roth) after separating it on a 12% (w/v) SDS-polyacrylamide gel. Fractions containing BirA were pooled and concentrated in Amicon Ultra-4 Centrifugal Filter Units (Ultracel-3K, Merck Millipore Ltd), with a subsequent buffer exchange to 6 M Urea, 50 mM NaCl, 20 mM Tris-HCl, pH 7.5. The protein concentration was determined by NanoDrop 2000 (ThermoFischer Scientific) based on the molar extinction coefficient and molecular weight of 6xHis-tagged BirA. 1 mg of purified BirA protein was used for the raising of an antiserum in rabbits according to a 3-month standard immunization protocol (Bioscience, bj-diagnostik, Göttingen).
Protein analyses
Protein extractions, SDS-PAGE, semi-dry blotting and immunodetections were carried out as described previously (Liu et al. 2005; Schulz-Raffelt et al. 2007). Sample amounts loaded were based on protein determination as described by Bradford (1976) or based on chlorophyll concentrations (Porra et al. 1989). Antisera used were against BirA (this work), CDJ1 (Willmund et al. 2008), CGE1 (Schroda et al. 2001), CLPB3 (Kreis et al. 2023), DEG1C (Theis et al. 2019a,b), the HA epitope (Sigma-Aldrich H3663), HSP22E/F (Rütgers et al. 2017), HSP70B (Schroda et al. 1999), HSP90C (Willmund and Schroda 2005), mCherry (Crozet et al. 2018), PRPL1 (Ries et al. 2017), and VIPP1 (Liu et al. 2005). Anti-rabbit-HRP (Sigma-Aldrich) and anti-mouse-HRP (Santa Cruz Biotechnology sc-2031) were used as secondary antibodies. For the detection of biotinylated proteins, proteins were separated on a 12% SDS-PAGE gel and transferred to a nitrocellulose membrane, stained with Ponceau S (1 min in 0.1% w/v Ponceau S in 5% acetic acid/water), and blocked with “biotin blocking buffer” (3% w/v BSA and 0.1% Tween-20 in PBS) at 22°C for 30 min. The blots were immersed in streptavidin-HRP (1:20,000 dilution, Abcam ab7403) or anti-Biotin-HRP (1:40,000, Sigma-Aldrich A0185) in biotin blocking buffer at 22°C for 60 min, then rinsed 3 times with PBS-T for 5 min. Detections were performed via enhanced chemiluminescence (ECL) and the FUSION-FX7 Advanceimaging system (PEQLAB) or ECL ChemoStar V90D (INTAS Science Imaging).
In vivo labeling with biotin-phenol
Cells were preincubated with biotin-phenol for time periods of 10 min up to 24 h at 22°C. From a 100 mM biotin-phenol stock in dimethyl sulfoxide (DMSO), biotin-phenol was diluted directly into cell cultures to a final concentration of 500 µM. Labeling was started by the addition of H2O2 to a final concentration of 1 mM and allowed to proceed for time periods of 1 min up to 1 h. Labeling was stopped by transferring cells on ice and washing with fresh, ice-cold TAP medium.
In vitro labeling with biotin-phenol
10 mL of exponentially growing cells were harvested by centrifugation for 3 min at 4,000 × g and 22°C, resuspended in 1 mL KMH buffer (20 mM HEPES-KOH pH 7.2, 10 mM KCl, 1 mM MgCl2, 154 mM NaCl, 1× cOmplete™, EDTA-free protease inhibitor cocktail [Roche]). Cells were lysed by 3 freezing and thawing cycles. Soluble proteins were prepared by centrifugation at 20,000 × g for 30 min at 4°C. Biotin-phenol was then added from a 500 mM stock in DMSO to a final concentration of 500 µM. After a preincubation period of 30 min at 22°C, 1 mM H2O2 was added for 1 min. Labeling was stopped by transferring the proteins on ice.
In vivo biotin-labeling without biotin addition and streptavidin affinity purification
Five hundred milliliters of exponentially growing cells were harvested by centrifugation for 2 min at 4,000 × g and 22°C and resuspended in 15 mL RIPA buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 2.5 mM EDTA, 1 mM DTT, 1× protease inhibitor cocktail [Roche], 1 mM phenylmethylsulfonyl fluoride [PMSF]). Cells were snap-frozen in liquid nitrogen and stored at -80°C prior to cell lysis. Cell samples were vortexed, sonicated (4 × 10 s, minimal output, 7 cycles with 1-min breaks on ice) and centrifuged at 14,000 × g and 4°C for 30 min. Precleared whole-cell lysates were next applied to streptavidin agarose resin (200 µL, Thermo-Fischer), and incubated overnight at 4°C on a SB2 rotator (Stuart). The resin was then washed at least twice in 10 mL RIPA buffer, once in 1 mL 1 M KCl, once in 1 mL 0.1 M Na2CO3 at 4°C. After transfer to a fresh tube at 22°C, the resin was washed once in urea buffer (2 M urea in 10 mM Tris-HCl, pH 8.0), twice with RIPA buffer, followed by a further transfer to a fresh tube. Proteins were eluted by boiling the resin for 10 min at 98°C in elution buffer (125 mM Tris pH 7.5, 4% SDS, 20 mM DTT, 2 mM biotin, 0.05% bromphenol blue) and subjected to SDS-PAGE for immunoblotting as well as to mass spectrometry analysis.
In vivo biotin-labeling with biotin addition and streptavidin affinity purification
Biotin was added from a 100 mM stock in DMSO to a final concentration of 1 mM into a 250-mL culture of exponentially growing cells for 4 h. If indicated, H2O2 treatment was done in parallel. Labeling was stopped by transferring the cells to ice and washing once with ice-cold fresh TAP medium to remove the biotin. Cells were harvested by centrifugation for 2 min at 4,000 × g at 4°C, resuspended in 6 mL RIPA buffer, snap-frozen in liquid nitrogen and stored at -80°C prior to cell lysis. The cells were thawed on ice, vortexed, sonicated (4 × 10 s, minimal output, 6 × cycles with 30 s breaks on ice), and centrifuged at 20,000 × g for 20 min at 4°C. Precleared cell lysates were applied to streptavidin agarose resin (100 µl, Thermo), and incubated overnight at 4°C on a rotator. Washing and elution were performed as described above.
In vitro biotin-labeling on isolated membrane proteins and streptavidin affinity purification
500 mL of exponentially growing cells were harvested by centrifugation for 2 min at 4,000 × g at 22°C, resuspended in 5 mL KMH buffer, snap-frozen in liquid nitrogen, and stored at -80°C prior to cell lysis. The cells were broken by three freeze/thaw cycles, distributed to 2 ml tubes to separate soluble and membrane fractions by centrifugation at 10,000 × g for 30 min and 4°C. The soluble fraction was discarded. Membranes were homogenized using a potter in 1.5 ml KMH buffer supplemented with 1 mM PMSF. Biotin was added to the lysate from a 500 mM biotin stock dissolved in H2O to a final concentration of 500 µM and an ATP regeneration system was added (2.5 mM ATP, 80 mM phosphocreatine disodium salt hydrate, 0.125 µg/µl creatine phosphokinase from bovine heart). Labeling was stopped after 30 min by transferring the cells to ice and the addition of extraction reagents (0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 2.5 mM EDTA, 1 mM DTT and 1 × protease inhibitor cocktail [Roche]). The samples were sonicated (4 × 30 s, minimal output, 6 cycles with 1.5-min breaks on ice), centrifuged at 20,000 × g for 20 min, followed by the removal of biotin by desalting on PD10 columns (GE Healthcare). The desalted lysates were then applied to streptavidin agarose resin (100 µl, Thermo), and incubated overnight at 4°C on a rotator. Further processing was done as described for in vivo-labeling above.
MS sample preparation and mass spectrometry
For in-gel digestion, biotinylated proteins were precipitated overnight at -20°C after adding ice-cold acetone to a final volume of 80%. Precipitated proteins were pelleted by centrifugation for 20 min at 25,000 × g and 4°C. After washing with 80% acetone, the pelleted proteins were air-dried, resuspended in Laemmli buffer (Laemmli 1970) and allowed to just migrate into the separating gel of a 10% SDS-polyacrylamide gel and stained with colloidal Coomassie G (Candiano et al. 2004). Entire protein lanes were excised in 2 to 3 bands to separate streptavidin, cut in cubes and destained (40% methanol, 7% acetic acid). Alkylation of cysteines and tryptic digest over night at 37°C was performed as described earlier (Veyel et al. 2014). Hydrophilic peptides were extracted from the gel pieces with 10% acetonitrile and 2% formic acid for 20 min and afterwards all other tryptic peptides were extracted with 60% acetonitrile, 1% formic acid. Samples were then desalted according to Rappsilber et al. (2007). Mass spectrometry on a LC-MS/MS system (Eksigent nanoLC 425 coupled to a TripleTOF 6600, ABSciex) was performed basically as described previously (Hammel et al. 2018). For peptide separation, a HPLC flow rate of 4 μl/min was used and gradients (buffer A 2% acetonitrile, 0.1% formic acid; buffer B 90% acetonitrile, 0.1% formic acid) ramped within 48 min from 2% to 35% buffer B, then to 50% buffer B within 5 min and finishing with wash and equilibration steps. MS1 spectra (350 to 1,250 m/z) were recorded for 250 ms and 35 MS/MS scans (110 to 1,600 m/z) were triggered in high sensitivity mode with a dwell time of 60 ms resulting in a total cycle time of 2,400 ms. Analysed precursors were excluded for 10 s, singly charged precursors or precursors with a response below 150 cps were excluded completely from MS/MS analysis.
Evaluation of MS data
The analysis of MS runs was performed using MaxQuant version 1.6.0.1 (Cox and Mann 2008). Library generation for peptide spectrum matching was based on Chlamydomonas reinhardtii genome release 5.5 (Merchant et al. 2007) including chloroplast and mitochondrial proteins as well as TurboID and mCherry. Oxidation of methionine and acetylation of the N-terminus were considered as peptide modifications. Maximal missed cleavages were set to 3 and peptide length to 6 amino acids, the maximal mass to 6,000 Da. Thresholds for peptide spectrum matching and protein identification were set by a false discovery rate (FDR) of 0.01. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD038515 (Perez-Riverol et al. 2019).
MS data analysis—identification of enriched proteins
Filtering and statistical analysis were done with Perseus (version 2.0.3.0.) (Tyanova et al. 2016). The “proteinGroups.txt” output file from MaxQuant was imported into Perseus using the intensity values as main category. The data matrix was filtered to remove proteins marked as “only identified by site”, “reverse”, and “potential contaminant”. Intensity values were log2 transformed and proteins were removed that were not identified/quantified in all three replicates of at least one strain under one condition. Normalization was achieved by subtracting the median using columns as matrix access. Next, missing values were imputed for statistical analysis using the “Replace missing values from normal distribution” function with the following settings: width = 0.3, down shift = 1.8, and mode = total matrix. To identify proteins enriched in TID-expressing samples versus the WT control, unpaired two-tailed Student's t-tests were performed comparing the TIDCGE1, VIPP1TID, VIPP2TID, PGRL1TID, VPL2TID, or mCherryTID with the corresponding WT samples at each time point. The integrated modified permutation-based FDR was used for multiple sample correction with an FDR of 0.05, an S0 of 1, and 250 randomizations to determine the cutoff. Significantly enriched proteins were kept. To further identify proteins that are significantly enriched in TIDCGE1, VIPP1TID, VIPP2TID, PGRL1TID, and VPL2TID versus mCherryTID, corresponding t-tests were performed on the reduced datasets using the same parameters as for the first t-tests. Finally, to determine proteins that were enriched in at least one of the two treatments (CL or HS, CL or H2O2) compared to mCherryTID, the cutoff parameters were set to a q < 0.05 and minimal fold change log2 ≥ 1. Scatterplots were made in Excel using t-test results exported from Perseus.
Accession numbers
All accession numbers of the genes/proteins described in this study can be found in the Supplemental Data Sets and in Supplemental Table S1.
Supplementary Material
Contributor Information
Elena Kreis, Molekulare Biotechnologie & Systembiologie, RPTU Kaiserslautern-Landau, Paul-Ehrlich Straße 23, D-67663 Kaiserslautern, Germany.
Katharina König, Molekulare Biotechnologie & Systembiologie, RPTU Kaiserslautern-Landau, Paul-Ehrlich Straße 23, D-67663 Kaiserslautern, Germany.
Melissa Misir, Molekulare Biotechnologie & Systembiologie, RPTU Kaiserslautern-Landau, Paul-Ehrlich Straße 23, D-67663 Kaiserslautern, Germany.
Justus Niemeyer, Molekulare Biotechnologie & Systembiologie, RPTU Kaiserslautern-Landau, Paul-Ehrlich Straße 23, D-67663 Kaiserslautern, Germany.
Frederik Sommer, Molekulare Biotechnologie & Systembiologie, RPTU Kaiserslautern-Landau, Paul-Ehrlich Straße 23, D-67663 Kaiserslautern, Germany.
Michael Schroda, Molekulare Biotechnologie & Systembiologie, RPTU Kaiserslautern-Landau, Paul-Ehrlich Straße 23, D-67663 Kaiserslautern, Germany.
Author contributions
E.K., K.K., M.M., and J.N. generated all constructs and performed all experiments. F.S performed the mass spectrometry analyses. E.K. established the data evaluation. M.S. conceived and supervised the project and wrote the article with contributions from all authors.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Level 0 constructs for baits and biotin activators used in this study.
Supplemental Figure S2. Screening for transformants accumulating CGE1 and mCherry with N-terminal fusions to APEX2.
Supplemental Figure S3. Dependency of APEX2 activity on biotin-phenol preincubation time and labeling reaction time.
Supplemental Figure S4. Screening for transformants accumulating CGE1 N-terminally fused to BioID.
Supplemental Figure S5. Screening for transformants accumulating TurboID fusions to VIPP1, VIPP2, CGE1, and mCherry.
Supplemental Figure S6. Production of recombinant BirA and characterization of the antiserum raised against it.
Supplemental Figure S7. Impact of TurboID mediated biotinylation on cell fitness.
Supplemental Figure S8. Comparison of structures and sequences of CGE2 and CGE1.
Supplemental Figure S9. Structures and alignments of putative VPL2 orthologs.
Supplemental Figure S10. Screening of transformants accumulating VPL2 with a C-terminal TurboID fusion.
Supplemental Figure S11. Screening of transformants accumulating PGRL1 with a C-terminal TurboID fusion.
Supplemental Table S1. List of proteins in the VIPP1/2 proxiomes and upregulation of their encoding genes by chloroplast stresses in previous studies.
Supplemental Table S2. Primers used for cloning.
Supplemental Table S3. MoClo constructs employed and generated.
Supplemental Table S4. Predicted molecular masses of fusion proteins.
Supplemental Data Set 1. Proteins significantly enriched after in vivo biotinylation in TurboID-CGE1 vs. WT and in TurboID-CGE1 vs. mCherry-TurboID.
Supplemental Data Set 2. Proteins significantly enriched after in vivo biotinylation in VIPPs-TurboID vs. WT and in VIPPs-TurboID vs. mCherry-TurboID.
Supplemental Data Set 3. Proteins significantly enriched after boosted in vitro biotinylation on isolated membranes in VIPPs-TurboID vs. WT and in VIPPs-TurboID vs. mCherry-TurboID.
Supplemental Data Set 4. Proteins significantly enriched after boosted in vivo biotinylation in VIPPs-TurboID vs. WT and in VIPPs-TurboID vs. mCherry-TurboID.
Supplemental Data Set 5. Proteins significantly enriched after boosted in vivo biotinylation in VPL2-TurboID vs. WT and in VPL2-TurboID vs. mCherry-TurboID.
Supplemental Data Set 6. Proteins significantly enriched after boosted in vivo biotinylation in PGRL1-TurboID vs. WT and in PGRL1-TurboID vs. mCherry-TurboID.
Supplemental Data Set 7. Cellular localization of proteins identified in the TurboID-mediated VIPP1/2, VPL2, PGRL1, and CGE1 proxiomes.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft [SFB/TRR175, project C02; SPP1927, project Schr 617/11-1].
Data availability
All data supporting the findings of this study are available from the corresponding author upon request. Processed data are available in the supplementary materials.
References
- Albus CA, Ruf S, Schöttler MA, Lein W, Kehr J, Bock R. Y3IP1, a nucleus-encoded thylakoid protein, cooperates with the plastid-encoded Ycf3 protein in photosystem I assembly of tobacco and Arabidopsis. Plant Cell. 2010:22(8):2838–2855. 10.1105/tpc.110.073908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almagro Armenteros JJ, Salvatore M, Emanuelsson O, Winther O, von Heijne G, Elofsson A, Nielsen H. Detecting sequence signals in targeting peptides using deep learning. Life Sci Alliance. 2019:2(5):e201900429. 10.26508/lsa.201900429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Satyanarayan MB, Wessendorf RL, Lu Y, Fernandez DE. A homolog of GuidedEntry of tail-anchored proteins3 functions in membrane-specific protein targeting in chloroplasts of Arabidopsis. Plant Cell. 2021:33(8):2812–2833. 10.1093/plcell/koab145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda Sicilia MN, Romero ME S, Rosales MP R, Venema K. Plastidial transporters KEA1 and KEA2 at the inner envelope membrane adjust stromal pH in the dark. New Phytol. 2021:229(4):2080–2090. 10.1111/nph.17042 [DOI] [PubMed] [Google Scholar]
- Arora D, Abel NB, Liu C, Van Damme P, Yperman K, Eeckhout D, Vu LD, Wang J, Tornkvist A, Impens F, et al. Establishment of proximity-dependent biotinylation approaches in different plant model systems. Plant Cell. 2020:32(11):3388–3407. 10.1105/tpc.20.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baier T, Wichmann J, Kruse O, Lauersen KJ. Intron-containing algal transgenes mediate efficient recombinant gene expression in the green microalga Chlamydomonas reinhardtii. Nucleic Acids Res. 2018:46(13):6909–6919. 10.1093/nar/gky532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD. State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature. 2005:433(7028):892–895. 10.1038/nature03286 [DOI] [PubMed] [Google Scholar]
- Benz JP, Stengel A, Lintala M, Lee YH, Weber A, Philippar K, Gügel IL, Kaieda S, Ikegami T, Mulo P, et al. Arabidopsis Tic62 and ferredoxin-NADP(H) oxidoreductase form light-regulated complexes that are integrated into the chloroplast redox poise. Plant Cell. 2009:21(12):3965–3983. 10.1105/tpc.109.069815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaby-Haas CE, Castruita M, Fitz-Gibbon ST, Kropat J, Merchant SS. Ni Induces the CRR1-dependent regulon revealing overlap and distinction between hypoxia and Cu deficiency responses in Chlamydomonas reinhardtii. Metallomics. 2016:8(7):679–691. 10.1039/C6MT00063K [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaby IK, Blaby-Haas CE, Perez-Perez ME, Schmollinger S, Fitz-Gibbon S, Lemaire SD, Merchant SS. Genome-wide analysis on Chlamydomonas reinhardtii reveals the impact of hydrogen peroxide on protein stress responses and overlap with other stress transcriptomes. Plant J. 2015:84(5):974–988. 10.1111/tpj.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Chang HY, Chuguransky S, Grego T, Kandasaamy S, Mitchell A, Nuka G, Paysan-Lafosse T, Qureshi M, Raj S, et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021:49(D1):D344–D354. 10.1093/nar/gkaa977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne AV, Schwarz C, Schottkowski M, Lidschreiber M, Piotrowski M, Zerges W, Nickelsen J. Reciprocal regulation of protein synthesis and carbon metabolism for thylakoid membrane biogenesis. PLoS Biol. 2013:11(2):e1001482. 10.1371/journal.pbio.1001482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976:72(1–2):248–254. 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, Ting AY. Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol. 2018:36(9):880–887. 10.1038/nbt.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan SJ, Burroughs NJ, Shevela D, Yu J, Rupprecht E, Liu LN, Mastroianni G, Xue Q, Llorente-Garcia I, Leake MC, et al. Localisation and interactions of the Vipp1 protein in cyanobacteria. Mol Microbiol. 2014:94(5):1179–1195. 10.1111/mmi.12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 2004:25(9):1327–1333. 10.1002/elps.200305844 [DOI] [PubMed] [Google Scholar]
- Cariti F, Chazaux M, Lefebvre-Legendre L, Longoni P, Ghysels B, Johnson X, Goldschmidt-Clermont M. Regulation of light harvesting in Chlamydomonas reinhardtii two protein phosphatases are involved in state transitions. Plant Physiol. 2020:183(4):1749–1764. 10.1104/pp.20.00384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaux M, Caffarri S, Da Graça J, Cuiné S, Floriani M, Brzezowski P, Peltier G, Genty B, Alric J, Johnson X. ACCLIMATION OF PHOTOSYNTHESIS TO THE ENVIRONMENT 1 regulates photosystem II supercomplex dynamics in response to light in Chlamydomonas reinhardtii. bioRxiv 2020.2002.2026.966580. 10.1101/2020.02.26.966580, 29 February 2020, preprint: not peer reviewed. [DOI]
- Chi W, Ma J, Zhang L. Regulatory factors for the assembly of thylakoid membrane protein complexes. Philos Trans R Soc Lond B Biol Sci. 2012:367(1608):3420–3429. 10.1098/rstb.2012.0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KF, Branon TC, Udeshi ND, Myers SA, Carr SA, Ting AY. Proximity labeling in mammalian cells with TurboID and split-TurboID. Nat Protoc. 2020:15(12):3971–3999. 10.1038/s41596-020-0399-0 [DOI] [PubMed] [Google Scholar]
- Choi-Rhee E, Schulman H, Cronan JE. Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci. 2004:13(11):3043–3050. 10.1110/ps.04911804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan B, Stoll T, Gorman JJ, Saur I, Rathjen JP. Development of a rapid in planta BioID system as a probe for plasma membrane-associated immunity proteins. Front Plant Sci. 2018:9:1882. 10.3389/fpls.2018.01882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M. Maxquant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008:26(12):1367–1372. 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- Crozet P, Navarro FJ, Willmund F, Mehrshahi P, Bakowski K, Lauersen KJ, Perez-Perez ME, Auroy P, Gorchs Rovira A, Sauret-Gueto S, et al. Birth of a photosynthetic chassis: a MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas reinhardtii. ACS Synth Biol. 2018:7(9):2074–2086. 10.1021/acssynbio.8b00251 [DOI] [PubMed] [Google Scholar]
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell. 2008:132(2):273–285. 10.1016/j.cell.2007.12.028 [DOI] [PubMed] [Google Scholar]
- Das PP, Macharia MW, Lin Q, Wong SM. In planta proximity-dependent biotin identification (BioID) identifies a TMV replication co-chaperone NbSGT1 in the vicinity of 126kDa replicase. J Proteomics. 2019:204:103402. 10.1016/j.jprot.2019.103402 [DOI] [PubMed] [Google Scholar]
- DeLisa MP, Lee P, Palmer T, Georgiou G. Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J Bacteriol. 2004:186(2):366–373. 10.1128/JB.186.2.366-373.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzymalla C, Schroda M, Beck CF. Light-inducible gene HSP70B encodes a chloroplast-localized heat shock protein in Chlamydomonas reinhardtii. Plant Mol Biol. 1996:31(6):1185–1194. 10.1007/BF00040835 [DOI] [PubMed] [Google Scholar]
- Fauser F, Vilarrasa-Blasi J, Onishi M, Ramundo S, Patena W, Millican M, Osaki J, Philp C, Nemeth M, Salomé PA, et al. Systematic characterization of gene function in the photosynthetic alga Chlamydomonas reinhardtii. Nat Genet. 2022:54(5):705–714. 10.1038/s41588-022-01052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foderaro JE, Douglas LM, Konopka JB. MCC/Eisosomes regulate cell wall synthesis and stress responses in fungi. J Fungi (Basel). 2017:3(4):61. 10.3390/jof3040061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R, Martins NF, Strenkert D, Clarke CA, Suchoszek M, Thiele W, Schottler MA, Merchant SS. The thylakoid membrane protein CGL160 supports CF1CF0 ATP synthase accumulation in Arabidopsis thaliana. PLoS One. 2015:10(4):e0121658. 10.1371/journal.pone.0121658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann E, Gathmann S, Rupprecht E, Golecki J, Schneider D. Thylakoid membrane reduction afects the photosystem stoichiometry in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2009:149(2):735–744. 10.1104/pp.108.132373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahl S, Reiter B, Gügel IL, Vamvaka E, Gandini C, Jahns P, Soll J, Leister D, Rühle T. The Arabidopsis protein CGLD11 is required for chloroplast ATP synthase accumulation. Mol Plant. 2016:9(6):885–899. 10.1016/j.molp.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Gupta TK, Klumpe S, Gries K, Heinz S, Wietrzynski W, Ohnishi N, Niemeyer J, Spaniol B, Schaffer M, Rast A, et al. Structural basis for VIPP1 oligomerization and maintenance of thylakoid membrane integrity. Cell. 2021:184(14):3643–3659.e23. 10.1016/j.cell.2021.05.011 [DOI] [PubMed] [Google Scholar]
- Hammel A, Sommer F, Zimmer D, Stitt M, Muhlhaus T, Schroda M. Overexpression of sedoheptulose-1,7-bisphosphatase enhances photosynthesis in Chlamydomonas reinhardtii and has no effect on the abundance of other Calvin-Benson Cycle enzymes. Front Plant Sci. 2020:11:868. 10.3389/fpls.2020.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel A, Zimmer D, Sommer F, Mühlhaus T, Schroda M. Absolute quantification of major photosynthetic protein complexes in Chlamydomonas reinhardtii using quantification concatamers (QconCATs). Front Plant Sci. 2018:9:1265. 10.3389/fpls.2018.01265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide H, Nordhues A, Drepper F, Nick S, Schulz-Raffelt M, Haehnel W, Schroda M. Application of quantitative immunoprecipitation combined with knockdown and cross-linking to Chlamydomonas reveals the presence of vesicle-inducing protein in plastids 1 in a common complex with chloroplast HSP90C. Proteomics. 2009:9(11):3079–3089. 10.1002/pmic.200800872 [DOI] [PubMed] [Google Scholar]
- Heredia-Martínez LG, Andrés-Garrido A, Martínez-Force E, Pérez-Pérez ME, Crespo JL. Chloroplast damage induced by the inhibition of fatty acid synthesis triggers autophagy in Chlamydomonas. Plant Physiol. 2018:178(3):1112–1129. 10.1104/pp.18.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Espenshade PJ. Proximity-dependent biotin labelling in yeast using the engineered ascorbate peroxidase APEX2. Biochem J. 2016:473(16):2463–2469. 10.1042/BCJ20160106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J. Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature. 2010:464(7292):1210–1213. 10.1038/nature08885 [DOI] [PubMed] [Google Scholar]
- Jans F, Mignolet E, Houyoux PA, Cardol P, Ghysels B, Cuine S, Cournac L, Peltier G, Remacle C, Franck F. A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc Natl Acad Sci U S A. 2008:105(51):20546–20551. 10.1073/pnas.0806896105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouhet J, Gray JC. Interaction of actin and the chloroplast protein import apparatus. J Biol Chem. 2009:284(28):19132–19141. 10.1074/jbc.M109.012831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado-Flores A, Delgado-Requerey V, Gálvez-Ramírez A, Puerto-Galán L, Pérez-Ruiz JM, Cejudo FJ. Exploring the functional relationship between y-type thioredoxins and 2-cys peroxiredoxins in Arabidopsis chloroplasts. Antioxidants. 2020:9(11):1072. 10.3390/antiox9111072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Yokono M, Akimoto S, Takabayashi A, Tanaka A, Tanaka R. Deficiency of the stroma-lamellar protein LIL8/PSB33 affects energy transfer around PSI in Arabidopsis. Plant Cell Physiol. 2017:58(11):2026–2039. 10.1093/pcp/pcx124 [DOI] [PubMed] [Google Scholar]
- Khan M, Youn J-Y, Gingras A-C, Subramaniam R, Desveaux D. In planta proximity dependent biotin identification (BioID). Sci Rep. 2018:8(1):9212. 10.1038/s41598-018-27500-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Birendra KC, Zhu W, Motamedchaboki K, Doye V, Roux KJ. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc Natl Acad Sci U S A. 2014:111(24):E2453–E2461. 10.1073/pnas.1406459111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Jensen SC, Noble KA, Kc B, Roux KH, Motamedchaboki K, Roux KJ. An improved smaller biotin ligase for BioID proximity labeling. Mol Biol Cell. 2016:27(8):1188–1196. 10.1091/mbc.E15-12-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-W, Park CH, Hsu C-C, Kim Y-W, Ko Y-W, Zhang Z, Zhu J-Y, Hsiao Y-C, Branon T, Kaasik K, et al. Mapping the signaling network of BIN2 kinase using TurboID-mediated biotin labeling and phosphoproteomics. Plant Cell. 2023:35(3):975–993. 10.1093/plcell/koad013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Roux KJ. Filling the void: proximity-based labeling of proteins in living cells. Trends Cell Biol. 2016:26(11):804–817. 10.1016/j.tcb.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle KL. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1990:87(3):1228–1232. 10.1073/pnas.87.3.1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis E, Niemeyer J, Merz M, Scheuring D, Schroda M. CLPB3 Is required for the removal of chloroplast protein aggregates and for thermotolerance in Chlamydomonas. J Exp Bot. 2023:erad109. 10.1093/jxb/erad109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll D, Meierhoff K, Bechtold N, Kinoshita M, Westphal S, Vothknecht UC, Soll J, Westhoff P. VIPP1, A nuclear gene of Arabidopsis thaliana essential for thylakoid membrane formation. Proc Natl Acad Sci U S A. 2001:98(7):4238–4242. 10.1073/pnas.061500998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Hong-Hermesdorf A, Casero D, Ent P, Castruita M, Pellegrini M, Merchant SS, Malasarn D. A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J. 2011:66(5):770–780. 10.1111/j.1365-313X.2011.04537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970:227(5259):680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lam SS, Martell JD, Kamer KJ, Deerinck TJ, Ellisman MH, Mootha VK, Ting AY. Directed evolution of APEX2 for electron microscopy and proximity labeling. Nat Methods. 2015:12(1):51–54. 10.1038/nmeth.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle M, Bergeron D, Arcand B, Bachand F. Proximity-dependent biotinylation mediated by TurboID to identify protein–protein interaction networks in yeast. J Cell Sci. 2019:132(11):jcs232249. 10.1242/jcs.232249 [DOI] [PubMed] [Google Scholar]
- Lau CS, Dowle A, Thomas G, Girr P, Mackinder LCM. A phase-separated CO2-fixing pyrenoid proteome determined by TurboID in Chlamydomonas reinhardtii. Plant Cell. 2023:koad131. 10.1093/plcell/koad131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moigne T, Gurrieri L, Crozet P, Marchand CH, Zaffagnini M, Sparla F, Lemaire SD, Henri J. Crystal structure of chloroplastic thioredoxin z defines a type-specific target recognition. Plant J. 2021:107(2):434–447. 10.1111/tpj.15300 [DOI] [PubMed] [Google Scholar]
- Li X, Li X, Yang X, Lan C, Huang Y, Jia B. Identification and characterization of ATP-binding cassette transporters in Chlamydomonas reinhardtii. Mar Drugs. 2022:20(10):603. 10.3390/md20100603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Zhou Z, Luo W, Fang M, Li M, Li H. Screening of proximal and interacting proteins in rice protoplasts by proximity-dependent biotinylation. Front Plant Sci. 2017:8:749. 10.3389/fpls.2017.00749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Tassinari M, Souza DP, Naskar S, Noel JK, Bohuszewicz O, Buck M, Williams TA, Baum B, Low HH. Bacterial Vipp1 and PspA are members of the ancient ESCRT-III membrane-remodeling superfamily. Cell. 2021:184(14):3660–3673e3618. 10.1016/j.cell.2021.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Willmund F, Golecki JR, Cacace S, Hess B, Markert C, Schroda M. The chloroplast HSP70B-CDJ2-CGE1 chaperones catalyse assembly and disassembly of VIPP1 oligomers in Chlamydomonas. Plant J. 2007:50(2):265–277. 10.1111/j.1365-313X.2007.03047.x [DOI] [PubMed] [Google Scholar]
- Liu C, Willmund F, Whitelegge JP, Hawat S, Knapp B, Lodha M, Schroda M. J-domain protein CDJ2 and HSP70B are a plastidic chaperone pair that interacts with vesicle-inducing protein in plastids 1. Mol Biol Cell. 2005:16(3):1165–1177. 10.1091/mbc.e04-08-0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SM, Theg SM. Role of vesicle-inducing protein in plastids 1 in cpTat transport at the thylakoid. Plant J. 2012:71(4):656–668. 10.1111/j.1365-313X.2012.05020.x [DOI] [PubMed] [Google Scholar]
- Mair A, Bergmann DC. Advances in enzyme-mediated proximity labeling and its potential for plant research. Plant Physiol. 2021:188(2):756–768. 10.1093/plphys/kiab479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair A, Xu S-L, Branon TC, Ting AY, Bergmann DC. Proximity labeling of protein complexes and cell-type-specific organellar proteomes in Arabidopsis enabled by TurboID. Elife. 2019:8:e47864. 10.7554/eLife.47864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix KM, Starble RM, Kaufman RS, Cooley L. Proximity labeling reveals novel interactomes in live Drosophila tissue. Development. 2019:146(14):dev176644. 10.1242/dev.176644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell JD, Deerinck TJ, Sancak Y, Poulos TL, Mootha VK, Sosinsky GE, Ellisman MH, Ting AY. Engineered ascorbate peroxidase as a genetically encoded reporter for electron microscopy. Nat Biotechnol. 2012:30(11):1143–1148. 10.1038/nbt.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May DG, Scott KL, Campos AR, Roux KJ. Comparative application of BioID and TurboID for protein-proximity biotinylation. Cells. 2020:9(5):1070. 10.3390/cells9051070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Jovanovic G, Ces O, Buck M. Membrane stored curvature elastic stress modulates recruitment of maintenance proteins PspA and Vipp1. MBio. 2015:6(5):e01188-01115. 10.1128/mBio.01188-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Jovanovic G, Wallace BA, Ces O, Buck M. Structure and function of PspA and Vipp1 N-terminal peptides: insights into the membrane stress sensing and mitigation. Biochim Biophys Acta. 2017:1859(1):28–39. 10.1016/j.bbamem.2016.10.018 [DOI] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007:318(5848):245–250. 10.1126/science.1143609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert J, Karcher D, Bock R. Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J. 2009:57(6):1140–1150. 10.1111/j.1365-313X.2008.03746.x [DOI] [PubMed] [Google Scholar]
- Niemeyer J, Scheuring D, Oestreicher J, Morgan B, Schroda M. Real-time monitoring of subcellular H2O2 distribution in Chlamydomonas reinhardtii. Plant Cell. 2021:33(9):2935–2949. 10.1093/plcell/koab176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer J, Schroda M. New destination vectors facilitate modular cloning for Chlamydomonas. Curr Genet. 2022:68(3–4):531–536. 10.1007/s00294-022-01239-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhues A, Schöttler MA, Unger AK, Geimer S, Schönfelder S, Schmollinger S, Rütgers M, Finazzi G, Soppa B, Sommer F, et al. Evidence for a role of VIPP1 in the structural organization of the photosynthetic apparatus in Chlamydomonas. Plant Cell. 2012:24(2):637–659. 10.1105/tpc.111.092692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron NJ, Orzaez D, Marillonnet S, Warzecha H, Matthewman C, Youles M, Raitskin O, Leveau A, Farre G, Rogers C, et al. Standards for plant synthetic biology: a common syntax for exchange of DNA parts. New Phytol. 2015:208(1):13–19. 10.1111/nph.13532 [DOI] [PubMed] [Google Scholar]
- Peng L, Ma J, Chi W, Guo J, Zhu S, Lu Q, Lu C, Zhang L. LOW PSII ACCUMULATION1 is involved in efficient assembly of photosystem II in Arabidopsis thaliana. Plant Cell. 2006:18(4):955–969. 10.1105/tpc.105.037689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, Inuganti A, Griss J, Mayer G, Eisenacher M, et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019:47(D1):D442–D450. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlaza K, Toutkoushian H, Boone M, Lam M, Iwai M, Jonikas MC, Walter P, Ramundo S. The Mars1 kinase confers photoprotection through signaling in the chloroplast unfolded protein response. Elife. 2019:8:e49577. 10.7554/eLife.49577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroutsos D, Busch A, Janssen I, Trompelt K, Bergner SV, Weinl S, Holtkamp M, Karst U, Kudla J, Hippler M. The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii. Plant Cell. 2011:23(8):2950–2963. 10.1105/tpc.111.087973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta. 1989:975(3):384–394. 10.1016/S0005-2728(89)80347-0 [DOI] [Google Scholar]
- Qin W, Cho KF, Cavanagh PE, Ting AY. Deciphering molecular interactions by proximity labeling. Nat Methods. 2021:18(2):133–143. 10.1038/s41592-020-01010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramundo S, Casero D, Mühlhaus T, Hemme D, Sommer F, Crevecoeur M, Rahire M, Schroda M, Rusch J, Goodenough U, et al. Conditional depletion of the Chlamydomonas chloroplast ClpP protease activates nuclear genes involved in autophagy and plastid protein quality control. Plant Cell. 2014:26(5):2201–2222. 10.1105/tpc.114.124842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007:2(8):1896–1906. 10.1038/nprot.2007.261 [DOI] [PubMed] [Google Scholar]
- Rhee HW, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, Ting AY. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013:339(6125):1328–1331. 10.1126/science.1230593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries F, Carius Y, Rohr M, Gries K, Keller S, Lancaster CRD, Willmund F (2017) Structural and molecular comparison of bacterial and eukaryotic trigger factors. Sci Rep. 7(1): 10680. 10.1038/s41598-017-10625-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Heredia M, Saccon F, Wilson S, Finazzi G, Ruban AV, Hanke GT. Protection of photosystem I during sudden light stress depends on ferredoxin:NADP(H) reductase abundance and interactions. Plant Physiol. 2022:188(2):1028–1042. 10.1093/plphys/kiab550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig R, Nillegoda NB, Mayer MP, Bukau B. The Hsp70 chaperone network. Nat Rev Mol Cell Biol. 2019:20(11):665–680. 10.1038/s41580-019-0133-3 [DOI] [PubMed] [Google Scholar]
- Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012:196(6):801–810. 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgers M, Muranaka LS, Mühlhaus T, Sommer F, Thoms S, Schurig J, Willmund F, Schulz-Raffelt M, Schroda M. Substrates of the chloroplast small heat shock proteins 22E/F point to thermolability as a regulative switch for heat acclimation in Chlamydomonas reinhardtii. Plant Mol Biol. 2017:95(6):579–591. 10.1007/s11103-017-0672-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgers M, Schroda M. A role of VIPP1 as a dynamic structure within thylakoid centers as sites of photosystem biogenesis? Plant Signal Behav. 2013:8(11):e27037. 10.4161/psb.27037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmollinger S, Strenkert D, Offeddu V, Nordhues A, Sommer F, Schroda M. A protocol for the identification of protein–protein interactions based on 15N metabolic labeling, immunoprecipitation, quantitative mass spectrometry and affinity modulation. J Vis Exp. 2012:67:4083. 10.3791/4083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda M. The Chlamydomonas genome reveals its secrets: chaperone genes and the potential roles of their gene products in the chloroplast. Photosynth Res. 2004:82(3):221–240. 10.1007/s11120-004-2216-y [DOI] [PubMed] [Google Scholar]
- Schroda M. Good news for nuclear transgene expression in Chlamydomonas. Cells. 2019:8(12):1534. 10.3390/cells8121534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda M, Vallon O. Chaperones and proteases. In: The Chlamydomonas sourcebook. 2nd ed. San Diego (CA): Elsevier/Academic Press, pp. 671–729; 2009 [Google Scholar]
- Schroda M, Vallon O, Whitelegge JP, Beck CF, Wollman FA. The chloroplastic GrpE homolog of Chlamydomonas: two isoforms generated by differential splicing. Plant Cell. 2001:13(12):2823–2839. 10.1105/tpc.010202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroda M, Vallon O, Wollman FA, Beck CF. A chloroplast-targeted heat shock protein 70 (HSP70) contributes to the photoprotection and repair of photosystem II during and after photoinhibition. Plant Cell. 1999:11(6):1165–1178. 10.1105/tpc.11.6.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz-Raffelt M, Lodha M, Schroda M. Heat shock factor 1 is a key regulator of the stress response in Chlamydomonas. Plant J. 2007:52(2):286–295. 10.1111/j.1365-313X.2007.03228.x [DOI] [PubMed] [Google Scholar]
- Takahashi H, Schmollinger S, Lee JH, Schroda M, Rappaport F, Wollman FA, Vallon O. PETO Interacts with other effectors of cyclic electron flow in Chlamydomonas. Mol Plant. 2016:9(4):558–568. 10.1016/j.molp.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Tan B, Peng S, Yatim S, Gunaratne J, Hunziker W, Ludwig A. An optimized protocol for proximity biotinylation in confluent epithelial cell cultures using the peroxidase APEX2. STAR Protoc. 2020:1(2):100074. 10.1016/j.xpro.2020.100074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Oster U, Kruse E, Rudiger W, Grimm B. Reduced activity of geranylgeranyl reductase leads to loss of chlorophyll and tocopherol and to partially geranylgeranylated chlorophyll in transgenic tobacco plants expressing antisense RNA for geranylgeranyl reductase. Plant Physiol. 1999:120(3):695–704. 10.1104/pp.120.3.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Ho MI, Kang B-H, Gu Y. GBPL3 Localizes to the nuclear pore complex and functionally connects the nuclear basket with the nucleoskeleton in plants. PLoS Biol. 2022:20(10):e3001831. 10.1371/journal.pbio.3001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif M, Atteia A, Specht M, Cogne G, Rolland N, Brugière S, Hippler M, Ferro M, Bruley C, Peltier G, et al. Predalgo: a new subcellular localization prediction tool dedicated to green algae. Mol Biol Evol. 2012:29(12):3625–3639. 10.1093/molbev/mss178 [DOI] [PubMed] [Google Scholar]
- Terashima M, Petroutsos D, Hudig M, Tolstygina I, Trompelt K, Gabelein P, Fufezan C, Kudla J, Weinl S, Finazzi G, et al. Calcium-dependent regulation of cyclic photosynthetic electron transfer by a CAS, ANR1, and PGRL1 complex. Proc Natl Acad Sci U S A. 2012:109(43):17717–17722. 10.1073/pnas.1207118109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis J, Gupta TK, Klingler J, Wan W, Albert S, Keller S, Engel BD, Schroda M. VIPP1 Rods engulf membranes containing phosphatidylinositol phosphates. Sci Rep. 2019a:9(1):8725. 10.1038/s41598-019-44259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis J, Lang J, Spaniol B, Ferte S, Niemeyer J, Sommer F, Zimmer D, Venn B, Mehr SF, Muhlhaus T, et al. The Chlamydomonas deg1c mutant accumulates proteins involved in high light acclimation. Plant Physiol. 2019b:181(4):1480–1497. 10.1104/pp.19.01052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis J, Niemeyer J, Schmollinger S, Ries F, Rutgers M, Gupta TK, Sommer F, Muranaka LS, Venn B, Schulz-Raffelt M, et al. VIPP2 Interacts with VIPP1 and HSP22E/F at chloroplast membranes and modulates a retrograde signal for HSP22E/F gene expression. Plant Cell Environ. 2020:43(5):1212–1229. 10.1111/pce.13732 [DOI] [PubMed] [Google Scholar]
- Theis J, Schroda M. Revisiting the photosystem II repair cycle. Plant Signal Behav. 2016:11(9):e1218587. 10.1080/15592324.2016.1218587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trösch R, Mühlhaus T, Schroda M, Willmund F. ATP-dependent molecular chaperones in plastids–more complex than expected. Biochim Biophys Acta. 2015:1847(9):872–888. 10.1016/j.bbabio.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, Mann M, Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016:13(9):731–740. 10.1038/nmeth.3901 [DOI] [PubMed] [Google Scholar]
- Vallon O, Spalding M. Amino acid metabolism. In: The Chlamydomonas sourcebook. 2nd ed. San Diego (CA): Elsevier/Academic Press, pp. 115–158. 2009 [Google Scholar]
- Veyel D, Sommer F, Muranaka LS, Rütgers M, Lemaire SD, Schroda M. In vitro characterization of bacterial and chloroplast Hsp70 systems reveals an evolutionary optimization of the co-chaperones for their Hsp70 partner. Biochem J. 2014:460(1):13–24. 10.1042/BJ20140001 [DOI] [PubMed] [Google Scholar]
- Walter B, Hristou A, Nowaczyk MM, Schunemann D. In vitro reconstitution of co-translational D1 insertion reveals a role of the cpSec-Alb3 translocase and Vipp1 in photosystem II biogenesis. Biochem J. 2015:468(2):315–324. 10.1042/BJ20141425 [DOI] [PubMed] [Google Scholar]
- Wang L, Patena W, Van Baalen KA, Xie Y, Singer ER, Gavrilenko S, Warren-Williams M, Han L, Harrigan HR, Chen V. et al. A chloroplast protein atlas reveals novel structures and spatial organization of biosynthetic pathways. bioRxiv: 2022.2005.2031.493820. 10.1101/2022.05.31.493820, 31 May 2022, preprint: not peer reviewed. [DOI] [PubMed]
- Wang L, Yamano T, Takane S, Niikawa Y, Toyokawa C, Ozawa SI, Tokutsu R, Takahashi Y, Minagawa J, Kanesaki Y, et al. Chloroplast-mediated regulation of CO2-concentrating mechanism by Ca2+-binding protein CAS in the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2016:113(44):12586–12591. 10.1073/pnas.1606519113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S. A modular cloning system for standardized assembly of multigene constructs. PLoS One. 2011:6(2):e16765. 10.1371/journal.pone.0016765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich LD, Gotsmann VL, Herkt C, Ries F, Kazek T, Trosch R, Armbruster L, Muhlenbeck JS, Ramundo S, Nickelsen J, et al. The versatile interactome of chloroplast ribosomes revealed by affinity purification mass spectrometry. Nucleic Acids Res. 2021:49(1):400–415. 10.1093/nar/gkaa1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmund F, Dorn KV, Schulz-Raffelt M, Schroda M. The chloroplast DnaJ homolog CDJ1 of Chlamydomonas reinhardtii is part of a multichaperone complex containing HSP70B, CGE1, and HSP90C. Plant Physiol. 2008:148(4):2070–2082. 10.1104/pp.108.127944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmund F, Mühlhaus T, Wojciechowska M, Schroda M. The NH2-terminal domain of the chloroplast GrpE homolog CGE1 is required for dimerization and cochaperone function in vivo. J Biol Chem. 2007:282(15):11317–11328. 10.1074/jbc.M608854200 [DOI] [PubMed] [Google Scholar]
- Willmund F, Schroda M. HEAT SHOCK PROTEIN 90C is a bona fide Hsp90 that interacts with plastidic HSP70B in Chlamydomonas reinhardtii. Plant Physiol. 2005:138(4):2310–2322. 10.1104/pp.105.063578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurzinger B, Stael S, Leonardelli M, Perolo C, Melzer M, Chaturvedi P, Afjehi-Sadat L, Weckwerth W, Teige M. Proximity labelling allows to study novel factors in chloroplast development. bioRxiv. 10.1101/2022.12.08.519630, 10 December 2022, preprint: not peer reviewed. [DOI]
- Xiong Z, Lo HP, McMahon K-A, Martel N, Jones A, Hill MM, Parton RG, Hall TE. In vivo proteomic mapping through GFP-directed proximity-dependent biotin labelling in zebrafish. Elife. 2021:10:e64631. 10.7554/eLife.64631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Jia M, Li X, Tang Y, Jiang K, Bao J, Gu Y. Exportin-4 coordinates nuclear shuttling of TOPLESS family transcription corepressors to regulate plant immunity. Plant Cell. 2021:33(3):697–713. 10.1093/plcell/koaa047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Tokutsu R, Bergner SV, Scholz M, Minagawa J, Hippler M. Photosystem II subunit R is required for efficient binding of light-harvesting complex stress-related protein 3 to Photosystem II-light-harvesting supercomplexes in Chlamydomonas reinhardtii. Plant Physiol. 2015:167(4):1566–1578. 10.1104/pp.15.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kato Y, Otters S, Vothknecht UC, Sakamoto W. Essential role of VIPP1 in chloroplast envelope maintenance in Arabidopsis. Plant Cell. 2012:24(9):3695–3707. 10.1105/tpc.112.103606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kondo H, Kamikubo H, Kataoka M, Sakamoto W. VIPP1 Has a disordered C-terminal tail necessary for protecting photosynthetic membranes against stress. Plant Physiol. 2016a:171(3):1983–1995. 10.1104/pp.16.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kusaba M, Tanaka A, Sakamoto W. Protection of chloroplast membranes by VIPP1 rescues aberrant seedling development in Arabidopsis nyc1 mutant. Front Plant Sci. 2016b:7:533. 10.3389/fpls.2016.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Li Y, Huang T, Li Z. Potential application of TurboID-based proximity labeling in studying the protein interaction network in plant response to abiotic stress. Front Plant Sci. 2022:13:974598. 10.3389/fpls.2022.974598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Shen G, Li Z, Golbeck JH, Bryant DA. Vipp1 is essential for the biogenesis of photosystem I but not thylakoid membranes in Synechococcus sp. PCC 7002. J Biol Chem. 2014:289(23):15904–15914. 10.1074/jbc.M114.555631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Song G, Lal NK, Nagalakshmi U, Li Y, Zheng W, Huang PJ, Branon TC, Ting AY, Walley JW, et al. TurboID-based proximity labeling reveals that UBR7 is a regulator of N NLR immune receptor-mediated immunity. Nat Commun. 2019:10(1):3252. 10.1038/s41467-019-11202-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available from the corresponding author upon request. Processed data are available in the supplementary materials.