Abstract
Contemporary anti-cancer drugs have significantly improved cancer survival, but this has been at the expense of cardiovascular toxicities, including heart disease, thromboembolic disease and hypertension. One of the most common side effects of these drugs is hypertension, especially in patients treated with vascular endothelial growth factor inhibitors, as well as tyrosine kinase inhibitors and proteasome inhibitors. Adjunctive therapy including corticosteroids, calcineurin inhibitors and non-steroidal anti-inflammatories, as well as anti-androgen hormone therapy for prostate cancer may further increase blood pressure in these patients. Cancer therapy-induced hypertension is often dose-limiting, increases cardiovascular mortality in cancer survivors, and is usually reversible after interruption or discontinuation of treatment. Exact molecular mechanisms underlying hypertension are unclear, but recent discoveries indicate an important role for reduced nitric oxide generation, oxidative stress, endothelin-1, prostaglandins, endothelial dysfunction, increased sympathetic outflow, and microvascular rarefaction. In addition, genetic polymorphisms in vascular endothelial growth factor receptors are implicated in vascular endothelial growth factor inhibitor-induced hypertension. Diagnosis, management and follow up of cancer therapy-induced hypertension follow national hypertension guidelines, since evidence-based clinical trials specifically addressing patients who develop hypertension due to cancer therapy, are currently lacking. Rigorous baseline assessment of patients prior to commencing therapy requires particular emphasis on assessing and treating cardiovascular risk factors. Hypertension management follows guidelines for the general population, although special attention should be given to rebound hypotension following termination of cancer therapy. Management of these complex patients requires collaborative care involving oncologists, cardiologists, hypertension specialists, primary care providers and pharmacists to ensure optimal therapeutic effect from cancer treatment while minimizing competing cardiovascular toxicities.
Keywords: cardio-oncology, cardiovascular toxicities, blood pressure, anti-angiogenesis, tyrosine kinase inhibitors
INTRODUCTION
Hypertension is more common in patients on anti-cancer therapy than the general population due to multiple mechanisms, including direct vascular and renal effects of anti-cancer therapy. Hypertension and blood pressure (BP) lability begin at the time of initiation of anti-cancer therapy and continue lifelong, which can result in interruptions in treatment and place patients at increased risk of cardiovascular disease (CVD) and mortality.1 Many contemporary cancer therapies are associated with cardiovascular-toxicity leading to heart disease, thromboembolic disease, and hypertension. Management of hypertension in patients on anti-cancer therapy is largely empiric, with no current trial data supporting specific agents or treatment goals in this distinctive population. This paper provides an overview of the mechanisms and clinical management of anti-cancer therapy-induced hypertension.
EPIDEMIOLOGY
Hypertension and cancer as major causes of global morbidity and mortality
Cancer and CVD are major causes of morbidity and mortality globally.2 Hypertension is one of the main risk factors for the development of CVD, including ischemic heart disease, heart failure, stroke, and kidney disease. The prevalence of hypertension worldwide is increasing, reaching 1.3 billion in adults in 2019.3 Despite availability of effective antihypertensive drugs, BP is inadequately controlled in almost 50% of those known to have hypertension. The current definitions of normal BP, elevated BP, and hypertension, based on the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines as well as other major guidelines are presented in Table 1.4 The incidence of cancer is also increasing. The Global Cancer Observatory estimates that the number of new cancer cases worldwide will increase from 19.3 million in 2020 to >28 million in 2040.5
Table 1:
Definition of hypertension in patients with cancer and the general adult population according to major guidelines
| IC-OS* | Normal SBP ≤130 mmHg DBP ≤80 mmHg |
Treatment threshold CVD or ASCVD risk ≥ 10%: SBP ≥130 mmHg and/or DBP ≥80 mmHg Otherwise: SBP ≥140 mmHg and/or DBP ≥90 mmHg |
Cancer therapy holding threshold SBP ≥180 mmHg and/or DBP ≥110 mmHg |
Exaggerated hypertensive response SBP increase >20 mmHg or mean arterial BP increase >15 mmHg |
Hypertensive emergency response Very high BP elevations associated with acute hypertension-mediated organ damage (heart, brain, kidneys), requiring immediate BP reduction to limit target organ damage |
|
|---|---|---|---|---|---|---|
| NCI CTCAE V5 |
Grade 1 SBP 120–139 mmHg or DBP 80–89 mmHg |
Grade 2 SBP 140–159 mmHg or DBP 90–99 mmHg if previously WNL. Change in baseline medical intervention indicated; recurrent or persistent (≥24 hours); symptomatic increase by >20 mmHg (diastolic) or to >140/90 mmHg; monotherapy indicated or initiate |
Grade 3 SBP ≥160 mmHg or DBP ≥100 mmHg Medical intervention indicated; > 1 drug or more intensive therapy than previously used indicated |
Grade 4 Life-threatening complications (i.e., transient, or permanent neurologic deficit, hypertensive crisis). Urgent intervention indicated |
||
| ACC/AHA 2017 |
Normal SBP <120 mmHg DBP <80 mmHg |
Elevated SBP120–129 mmHg DBP<80 mmHg |
Stage 1 SBP 130–139 mmHg DBP 80–90 mmHg Drug therapy indicated if ASCVD risk>10% |
Stage 2 SBP >140 mmHg DBP >90 mmHg Drug therapy goal BP<130/80 mmHg |
Hypertensive Crisis SBP >180 mmHg DBP >120 mmHg Urgent BP drug therapy initiation |
|
| ESC 2018 |
Optimal SBP<120 mmHg DBP <80 mmHg |
Normal SBP 120–129 mmHg DBP 80–84 mmHg |
High Normal SBP 130–139 mmHg DBP 85–89 mmHg Drug therapy considered if ASCVD risk>10% or established CVD, CKD, or DM |
Grade 1 SBP 140–159 mmHg DBP 90–99 mmHg First drug therapy target <140/90 mmHg, consider <130/80 mmHg if tolerated, but not SBP <120 mmHg. In older >65 years, target SBP 130–140 mmHg, and DBP <80 mmHg, initiate with two-drug combination |
Grade 2 SBP 160–179 mmHg DBP 100–109 mmHg Drug therapy goal as for Grade 1 |
Grade 3 SBP ≥ 180 mmHg DBP ≥110 mmHg Urgent drug therapy goal as for Grade 1 |
| ISH |
Normal SBP <130 mmHg DBP <85 mmHg |
High normal SBP 130–139 mmHg DBP 85–89 mmHg |
Grade 1 SBP 140–159 mmHg DBP 90–99 mmHg Drug therapy indicated if ASCVD risk>10% or established CVD, CKD, DM Target BP reduction by 20/10 mmHg, ideally to <140/90 mmHg. Optimal targets: <65 years: 120–130/70–79mmHg ≥65 years: <140/90 mmHg |
Grade 2 SBP ≥160 mmHg DBP ≥100 mmHg Immediate drug treatment in all patients |
||
ACC, American College of Cardiology; AHA, American Heart Association; ASCVD, atherosclerotic cardiovascular disease; BP, blood pressure; CAD, coronary artery disease; CKD, chronic kidney disease; CTCAE, Common Terminology Criteria for Adverse Events; CV, cardiovascular; CVD, cardiovascular disease; DBP, diastolic BP; DM, diabetes mellitus; ESC, European Society of Cardiology; HTN, hypertension; IC-OS, International Cardio-Oncology Society; ISH, International Society of Hypertension; NCI, National Cancer Institute; SBP systolic BP. BP values are based on office BP measurement.
Definition of hypertension aspect in the cancer patient
Common risk factors of hypertension and cancer
A bidirectional relationship between cancer and hypertension has been proposed. Hypertension is associated with an increased risk of cancer. This is most clearly displayed by the increased risk of renal cell carcinoma in patients with hypertension.6 Additionally, the prevalence of hypertension is higher in patients with cancer and cancer survivors than in the general population.1 A prospective cohort study of >17,000 patients with cancer found that hypertension was the most common comorbidity (38% prevalence).7 The frequent concurrence of cancer and hypertension and the increased cardiovascular risk in patients with cancer are most likely explained by the presence of common risk factors and pathophysiological mechanisms, including smoking, diabetes, chronic kidney disease, physical inactivity, obesity, oxidative stress, and inflammation.8–10 CVD morbidity and mortality are increased in patients with cancer and cancer survivors.11 In addition, many anti-cancer drugs cause BP elevation through numerous mechanisms.
ANTI-CANCER DRUGS THAT CAUSE HYPERTENSION AND POTENTIAL MECHANISMS
Vascular endothelial growth factor (VEGF) signaling pathway inhibitors (VSPIs)
VSPIs exert their anti-cancer effects by inhibition of VEGF-mediated tumor angiogenesis, depriving tumor cells of oxygen and nutrient supply. These agents act via inhibition of VEGF directly or by inhibition of tyrosine kinase receptors (Table 2). As recently reviewed by Camarda et al.,12 they have well-established treatment efficacy in numerous cancers, especially renal, hepatocellular, thyroid, gastrointestinal stromal and others.
Table 2.
Incidence of hypertension induced by different classes of anti-cancer drugs
| Drug Class | Select example drugs | Select malignancies treated | Incidence of hypertension |
|---|---|---|---|
| Vascular endothelial growth factor (VEGF) signaling pathway inhibitors (VSPIs) | Bevacizumab, Sorafenib Sunitinib Nilotinib Pazopanib Dasatinib Regorafenib Cabozantinib Lenvatinib Ponatinib Axitinib Tivozanib Vandetanib Ramucirumab |
Renal, hepatocellular, thyroid, gastrointestinal stromal (GIST) | 20%-90%8 |
| Bruton’s tyrosine kinase inhibitors (TKIs) | Ibrutinib Acalabrutinib |
Chronic lymphocytic leukaemia (CLL), Mantle cell lymphoma | 71%19 |
| Proteasome inhibitors | Carfilzomib Bortezomib |
Multiple myeloma | 32%26 10%26 |
| Platinum-based compounds | Cisplatin Carboplatin Oxaliplatin |
Mesothelioma†, testicular, bladder, gynecological, colorectal, and lung cancers† | 53%30 |
| Alkylating agents | Cyclophosphamide Busulfan Ifosfamide |
Hematologic and solid organ malignancies | 36% in adults10 58% in children10 15% in children34 |
| Calcineurin inhibitors (CNIs) | Tacrolimus Cyclosporin |
After stem cell transplantation | 30%-60%39 |
| Serine/threonine-protein kinase B-Raf/ mitogen-activated extracellular signal-regulated kinase (BRAF/MEK) inhibitors |
Vemurafenib Dabrafenib Encorafenib Trametinib Binimetinib Cobimetinib |
Melanoma, colorectal | 19.5%*18 |
| Rearranged during transfection (RET) kinase inhibitors | Selpercatinib Pralsetinib |
Thyroid, Non-small cell lung cancer | 43%20 21%20 |
| Poly ADP ribose polymerase (PARP) inhibitors | Niraparib | Breast, Ovarian | 19%22 |
| Androgen receptor blockers | Enzalutamide | Metastatic prostate cancer | 11 (5%)42 |
| Androgen synthesis inhibitors | Abiraterone Leuprolide |
Metastatic prostate cancer Prostate cancer |
26 (7%)42 15%8 |
| Aromatase inhibitors | Anastrazole | Breast | 13 %43 |
| Letrozole | Breast | 8 %43 | |
| Exemestane | Breast | 10 %43 | |
| Mammalian target of rapamycin mTOR inhibitors | Everolimus Sirolimus |
Renal cell cancer, breast, pancreatic neuroendocrine tumor (PNET) | 13 % (PNET)8 |
From systematic review including all drugs
Denotes malignancies for which treatment is not approved by the Food and Drug Administration (i.e., currently given off-label)
VSPIs are associated with adverse cardiovascular effects, of which hypertension is the most frequent. They cause an acute increase in BP that is sustained during treatment in the majority of patients. The reported incidence of VSPI-induced hypertension ranges from 20%–90% and varies with VSPI potency and dosage as well as methods and definitions for reporting BP.8 In a meta-analysis of 29,000 patients with cancer, there was a 3.8-fold higher relative risk for hypertension in those treated with a VEGF tyrosine kinase inhibitor (TKI) compared to controls (Table 2).13 VSPI-associated hypertension is reversible and resolves upon discontinuation of the agent, indicating an ‘on-target’ effect. Accordingly, VSPI-induced hypertension has been suggested as a predictor or biomarker of therapeutic efficacy.14
VEGF is a potent vasodilator and its absence is associated with reduced bioavailability of the vasodilator nitric oxide (NO) and increased concentrations of the potent vasoconstrictor, endothelin-1, important in hypertension pathophysiology (Figure 1). VEGF signalling pathway inhibition is also associated with increased generation of reactive oxygen species (ROS), including reactive nitrogen species, H2O2, and O2−, causing vascular oxidative stress.15 Rarefaction (reduced microvascular density) occurs (previously observed in the skin and oral mucosa), with a consequent increase in vascular resistance thought to lead to elevated BP.16 VSPIs are associated with nephrotoxicity, which may also contribute to their prohypertensive effects via impaired natriuresis.8 Current evidence does not suggest a major role for the renin-angiotensin-aldosterone system in the pathophysiology of VSPI-associated hypertension. VSPIs also interfere with other growth factor pathways, including platelet-derived growth factor, fibroblast growth factor, fms-like tyrosine kinase 3 and c-Kit. Inhibition of these pathways evokes further potential prohypertensive mechanisms.8
Figure 1.
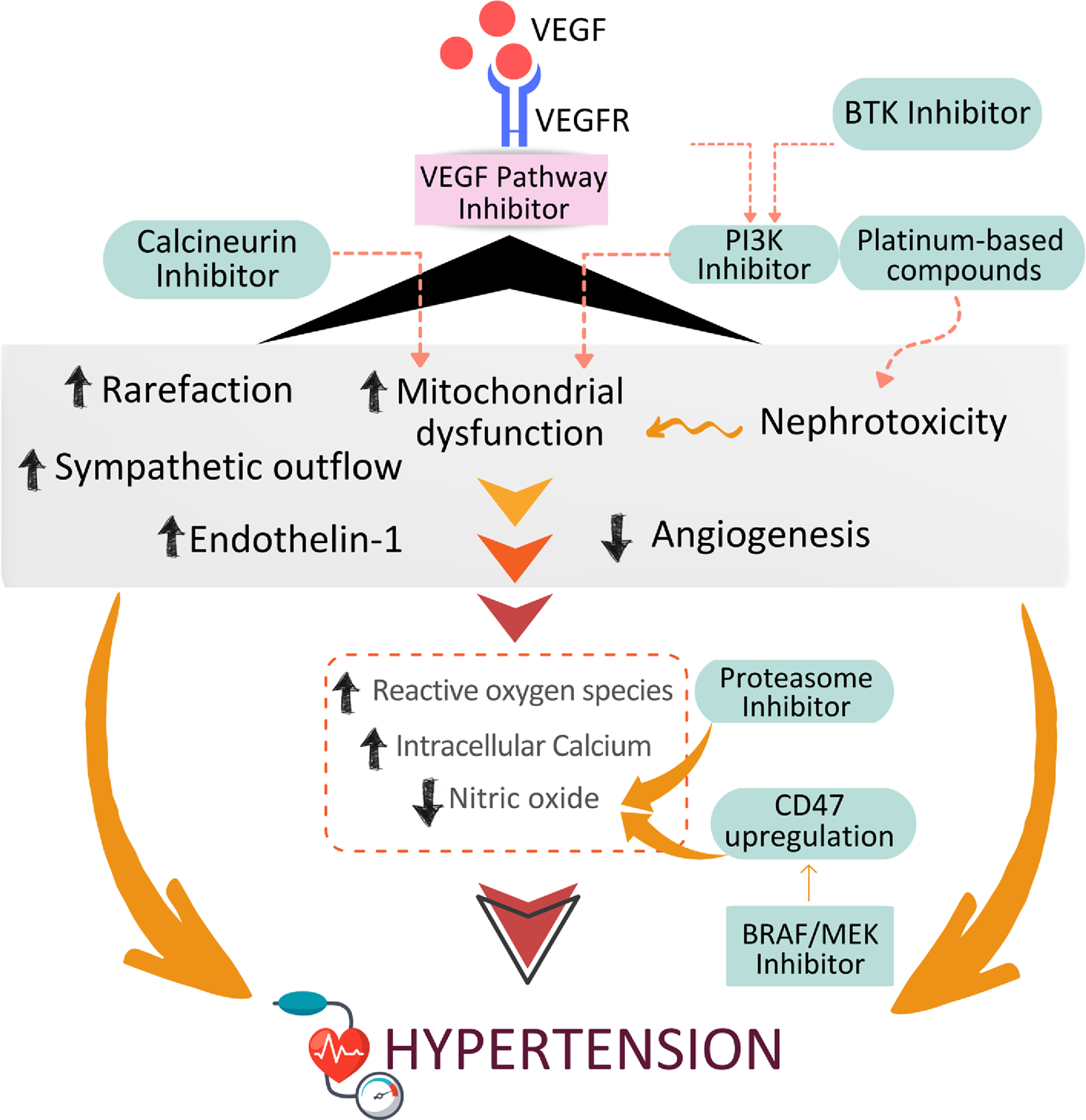
Putative mechanisms whereby major classes of anti-cancer therapies cause hypertension. VEGF, Vascular epidemanl growth factor; VEGFR, VEGF receptor; BTK, Brutons tyrosine kinase; PI3K, Phosphoinositide 3-kinases; CD47, Cluster of Differentiation 47; MEK, mitogen-actvated protein kinase kinase.
Rapidly accelerated fibrosarcoma B-type (BRAF) and mitogen-activated kinase kinase (MEK) inhibitors
BRAF/MEK inhibitors are used in the treatment of BRAF-mutant melanoma and BRAF mutant colorectal cancer.17 These agents are frequently prescribed in combination. Approximately 60% of patients with melanoma harbor a BRAF gene mutation with subsequent dysregulation of the Raf-MEK-extracellular signal-regulated kinases signaling pathway (Table 2). These pathways are also necessary for normal vascular and cardiac physiology.
Hypertension is the most common adverse cardiovascular event reported with BRAF/MEK inhibitors.17 A meta-analysis including 5 randomized clinical trials (RCTs) reported an increased risk of systemic hypertension, which occurred in 19.5% of patients treated with combined BRAF/MEK inhibitors and 14% of those treated with BRAF inhibitor monotherapy.18 BRAF/MEK inhibitor-associated hypertension may be a consequence of reduced NO bioavailability. Inhibition of BRAF and MEK is associated with upregulation of expression of Cluster of Differentiation 47 in melanoma cells in vitro. Cluster of Differentiation 47 inhibits NO/cyclic guanosine monophosphate signaling via thrombospondin-1, leading to a reduction in NO bioavailability with consequent vasoconstriction and hypertension.18
Bruton’s tyrosine kinase inhibitors
Bruton’s TKIs are used in the treatment of chronic lymphocytic leukemia and mantle cell lymphoma. Ibrutinib has variable prohypertensive effects. In a study of 562 patients, treatment with ibrutinib was associated with new-onset hypertension in 71% of patients with normal BP at baseline, and with worsening of preexisting hypertension in 83%.19 A meta-analysis including 2,580 participants revealed a 2.8-fold increased risk for hypertension in patients treated with ibrutinib.19 Given that both hypertension and Bruton’s TKIs increase the risk of atrial fibrillation, the prohypertensive effect of these drugs is of added significance.19 Patients are frequently treated with Bruton’s TKIs for many years and therefore the life-time risk of exposure to the prohypertensive effects is especially relevant. Mechanisms underpinning Bruton’s TKI-associated hypertension are unclear, but a decrease in heat shock protein 70 signaling and inhibition of phosphatidylinositol 3-kinase-dependent NO production may be important.8
Rearranged during transfection receptor (RET)-TKIs
RET-TKIs are indicated for the treatment of non-small cell lung cancer and thyroid cancer with an activating mutation in the RET proto-oncogene. In a study of 162 patients with thyroid cancer treated with selpercatinib, 43% developed hypertension and 21% had BP >160/100 mmHg.20 In the ARROW RCT examining pralsetinib in non-small cell lung cancer, 21% of those treated with pralsetinib developed hypertension.21 Mechanisms underlying the prohypertensive effects of RET-TKIs are unknown.
Poly adenosine diphosphate ribose polymerase inhibitors
Poly adenosine diphosphate ribose polymerase inhibitors are used in the treatment of breast and ovarian cancer with BReast CAncer (BRCA) mutation. In this drug class, only niraparib has been associated with prohypertensive effects. In the NOVA RCT of niraparib in recurrent ovarian cancer, 19% patients treated with niraparib developed hypertension, versus 5% of patients receiving placebo.22 In the recent NORA RCT (niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose), hypertension occurred in 11% of patients treated with niraparib, compared with only 1% of patients receiving placebo.23, 24 When niraparib was given in combination with the VSPI bevacizumab for ovarian cancer, hypertension occurred in 56% of patients.23, 24 The mechanisms underlying niraparib-induced hypertension remain unclear.8
Proteasome inhibitors
Proteasome inhibitors (bortezomib, carfilzomib and ixazomib) have become the cornerstone of therapy for multiple myeloma. Cardiovascular adverse events occur with all proteasome inhibitors, but are mostly associated with carfilzomib.25 In the carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR) trial, hypertension developed in 32% of patients receiving carfilzomib compared to 10% receiving bortezomib.26 In a prospective study of 70 patients with multiple myeloma treated with carfilzomib, 33% developed adverse cardiovascular events, 91% of whom had uncontrolled hypertension.27 Hypertension often reverses after discontinuation of these agents.
Proteasome inhibitors mediate oxidative stress by increasing ROS production and suppressing antioxidant pathways. By binding to the 20S proteolytic core of the proteasome and thereby inhibiting its catalytic activity, proteasome inhibitors lead to an intracellular accumulation of aggregated proteins that can be toxic to malignant cells.28 This also has adverse cardiovascular effects, including endothelial dysfunction and reduced NO bioavailability.28 Pre-existing vascular risk factors and anti-cancer therapies associated with oxidative stress make the vasculature more vulnerable to these adverse effects.29
Platinum-based compounds
Platinum-based compounds (cisplatin, carboplatin, oxaliplatin) are used to treat testicular, bladder, gynecological, breast, colorectal and lung cancers, as well as mesothelioma. Their anti-cancer effects result from platinum uptake to DNA with consequent apoptotic cell death. Hypertension is associated with exposure to platinum-based compounds but tends to be a late effect, which can occur many years after treatment of the index cancer. Platinum-based chemotherapy is associated with excellent long-term survival for patients with testicular cancer, the most common cancer in young men. In a study of testicular cancer survivors with a median follow-up of 11 years, hypertension was observed in 53% of those treated with cisplatin and was 2.3 times more frequent than in healthy controls.30
Cisplatin is detected in the circulation even after 13 years post-drug exposure and this may be responsible for chronic endothelial injury and dysfunction. Increased circulating platinum concentrations are associated with an increased risk of hypertension.31 Proximal tubular renal cells are the major site for cisplatin-induced renal injury, and accumulation of cisplatin in the kidney can reach levels five times higher than the serum concentration.32 Cisplatin nephrotoxicity is dose-dependent and has been attributed to cellular mitochondrial damage, oxidative stress, apoptosis, and decreased NO bioavailability.33
Alkylating agents
Alkylating antineoplastic agents such as busulfan, ifosfamide and cyclophosphamide are used in the treatment of hematologic malignancies and solid organ malignancies. These are classical chemotherapeutic agents that have been previously linked to hypertension; however, concomitant use of glucocorticoids may be a confounding factor in these observations.
Busulfan is mainly used as part of a pre-transplant conditioning regimen for both pediatric and adult patients with hematological malignancies. Hypertension has been noted in up to 36% of adults treated with busulfan and in up to 58% of children.10 The prevalence of hypertension after ifosfamide was reported to be 15% at 5 years of follow-up in a childhood cancer survivorship study.34
In cyclophosphamide-treated patients with breast cancer, reduced VEGF concentrations have been demonstrated with associated endothelial dysfunction. These processes can explain, in part, the development of hypertension in patients on cyclophosphamide.35 In addition, direct vascular toxicity and nephrotoxicity may contribute to the prohypertensive effects of these agents.36
Calcineurin inhibitors (CNIs)
CNIs such as tacrolimus and cyclosporin are often administered concurrently with other cancer agents, mainly after hematopoietic stem cell transplantation to prevent or treat graft versus host disease.37 CNIs contribute to the development of hypertension or worsening BP control in patients with a history of hypertension.38 In patients undergoing bone marrow transplantation, there was a 30–60% increase in the rate of hypertension diagnoses after cyclosporin became the mainstay of treatment.39 CNIs cause widespread vasoconstriction, with activation of the renin-angiotensin-aldosterone system, oxidative stress, an increase in endothelin-1 generation and sympathetic nervous system activity.40 In addition, they inhibit NO synthesis and NO-mediated vasodilation.41
Mammalian target of rapamycin (mTOR) inhibitors
Inhibitors of mTOR, including everolimus and sirolimus, are third-line treatment options for renal cell carcinoma. In a RCT including patients with metastatic renal cell carcinoma, everolimus was associated with a 10% incidence of hypertension.8 In vitro studies in predominantly tumor cell lines show decreased VEGF secretion upon mTOR inhibition. Thus, inhibition of mTOR may have prohypertensive effects similar to VSPIs.41 The combination of everolimus with the VSPI lenvatinib was associated with hypertension in 41% of patients and high-grade hypertension in 14%, higher than either medication alone.41
Endocrine therapy (anti-androgens/aromatase inhibitors)
Survival for patients with metastatic prostate cancer is improved by androgen deprivation therapy. These agents block the trophic effects of androgens on prostate cancer cells. Gonadotropin-releasing hormone agonists directly inhibit androgen production and signaling and are associated with potentially adverse cardiovascular effects including elevation of serum lipid levels, decreased insulin sensitivity and obesity. Abiraterone, an inhibitor of androgen synthesis and enzalutamide, an androgen receptor antagonist, are both associated with hypertension.8, 42 Abiraterone inhibits testosterone production via inhibition of the cytochrome P450 enzyme with consequent accumulation of mineralocorticoid precursors. This effect contributes to its prohypertensive effects.42 Mechanisms underlying enzalutamide-induced hypertension are unclear.
The cardiovascular toxic effects of abiraterone and enzalutamide were evaluated in a meta-analysis of 8660 patients with prostate cancer. All-grade and high-grade hypertension were observed more frequently in the abiraterone group (26% and 7%, respectively) compared with placebo (15% and 4%, respectively). Similar results were reported with enzalutamide (11% and 5%, respectively) compared with placebo (4% and 2%, respectively).42 These rates of hypertension occurred irrespective of corticosteroid use, which is often administered concomitantly.8, 42
Aromatase inhibitors including nonsteroidal (anastrazole and letrozole) and steroidal (exemestane) formulations, reduce breast cancer-related mortality in post-menopausal women with estrogen positive breast cancer.43, 44 The association between aromatase inhibitors and cardiovascular risk is controversial with some studies identifying increased risk of hypertension and cardiovascular-related mortality.8, 43
Adjunctive Therapies Used in Cancer Management
In addition to the BP effects associated with the specific anti-cancer drugs outlined above, many other agents prescribed for patients with cancer can contribute to unwanted BP effects. Corticosteroids are a frequent component of contemporary cancer treatment regimens and often used in supportive care management. Corticosteroids can provoke a substantial rise in BP, in isolation, and in the context of other prohypertensive therapies. Similarly, exogenous erythropoietin and non-steroidal anti-inflammatory drugs frequently cause BP elevation.8
DIAGNOSIS AND MANAGEMENT OF ANTI-CANCER THERAPY-INDUCED HYPERTENSION
Diagnosis of hypertension in anti-cancer therapy-induced hypertension.
Accurate measurement of BP is crucial for hypertension diagnosis and management and special attention should be given to optimal control of anxiety and pain in patients with cancer. In general, patients should be seated quietly for 3–5 minutes before any measurement is taken, the patient’s bladder should be empty, and they should have abstained from exercise and consumption of caffeine or smoking for 30 minutes.4 The legs should touch the floor and not be crossed and the back should be well supported. The arm used for BP measurement should be in a resting position at the level of the heart. A calibrated and validated automated office BP device and a proper cuff size should be used.10 At least 2–3 BP readings should be obtained and averaged. Elevated BP readings should be verified on at least 1 more occasion prior to a diagnosis of hypertension.4, 45 24-hour ambulatory BP monitoring is recommended for confirmation after initial readings of an elevated BP but is not always feasible. Home BP monitoring (self-monitoring of BP) with a validated device should be used in situations where frequent BP measurements over longer time periods are required, e.g. during treatment initiation or dose changes in patients receiving VSPIs wherein worsening hypertension often occurs in days and can progress to hypertensive emergency.46, 47 Baseline measurement before the initiation of prohypertensive anti-cancer therapies is mandatory, as some patients may present with a notable rise in BP while on these agents requiring prompt initiation or escalation of antihypertensive therapy.
Blood Pressure targets
Patients with cancer are frequently underrepresented or excluded from major cardiovascular or hypertension RCTs. RCTs are needed to define BP targets either in the context of specific anti-cancer therapies, or in patients with cancer in general. Defining BP goals for treatment and diagnosis in patients with cancer therefore is an important unmet need. The definition of hypertension varies between guidelines (Table 1). Based on the AHA/ACC guidelines, BP is categorized as normal, elevated, stage 1, or stage 2 hypertension. Normal BP is defined as <120/<80 mm Hg; elevated BP as 120–129/<80 mm Hg; hypertension stage 1 as systolic BP 130–139 or diastolic BP 80–89 mm Hg, and hypertension stage 2 as ≥140 or ≥90 mm Hg.4 For patients with atherosclerotic CVD 10-year risk ≥10%, or comorbidities such as chronic kidney disease and diabetes mellitus, treatment should be initiated in stage 1 hypertension. For others, treatment should be initiated in stage 2. The goal BP is <130/80 mmHg for all indivduals. To date there are no specific hypertension guidelines for patients with cancer, although the International Cardio-Oncology Society recently published a consensus statement on the definition of hypertension in cancer, representing a multidisciplinary effort.47 The treatment threshold is defined as in the AHA/ACC recommendations, and treatment goals match the treatment threshold. The threshold for recommending withholding anti-cancer therapy is systolic BP ≥180 mmHg or diastolic BP ≥110 mmHg, which is similar to the hypertensive crisis definition in the AHA/ACC guidelines (≥180 mmHg systolic and ≥120 mmHg diastolic).
Management of hypertension in anti-cancer therapy-induced hypertension
Antihypertensive Medications
Among patients with hypertension, antihypertensive therapy should be optimized prior to initiation of anti-cancer therapy, preferably with multi-disciplinary input from the cardio-oncology care team (Figure 2). Patients should be counseled on the potential need to escalate antihypertensive treatment quickly if potentially pro-hypertensive anti-cancer therapy is scheduled. Insufficient evidence exists supporting a specific antihypertensive medication strategy specific to patients with anti-cancer therapy-induced hypertension. Therefore, antihypertensive management should reflect current guidelines for the general population.4, 48 First-line therapy should include an angiotensin receptor blocker or angiotensin-converting enzyme inhibitor, dihydropyridine calcium channel blocker, or thiazide or thiazide-like diuretic.48 Selection of antihypertensive therapy should otherwise be informed by individual patient risk factors. For example, patients with proteinuria should be treated with an angiotensin receptor blocker or angiotensin-converting enzyme inhibitor45, 48; those at high risk of volume depletion should avoid use of diuretics.10 As with the general population, mineralocorticoid receptor antagonists should be the initial agent of choice for the treatment of resistant hypertension, unless there is a contraindication such as hyperkalemia.4, 49 Beta-blockers should not be used as first-line antihypertensive therapy, and should be reserved for individuals with a specific indication for their use (e.g., atrial fibrillation, recent myocardial infarction, heart failure with reduced ejection fraction), who are already optimized on maximum tolerated doses of first-line antihypertensive agents, or with contraindications to first-line antihypertensive agents.4, 45 Non-dihydropyridine calcium channel blockers (e.g., diltiazem, verapamil) should be used with caution in this patient population, as these antihypertensive agents are susceptible to interactions with several anti-cancer therapies that are metabolized by P-glycoprotein and cytochrome P450 3A4.50
Figure 2.
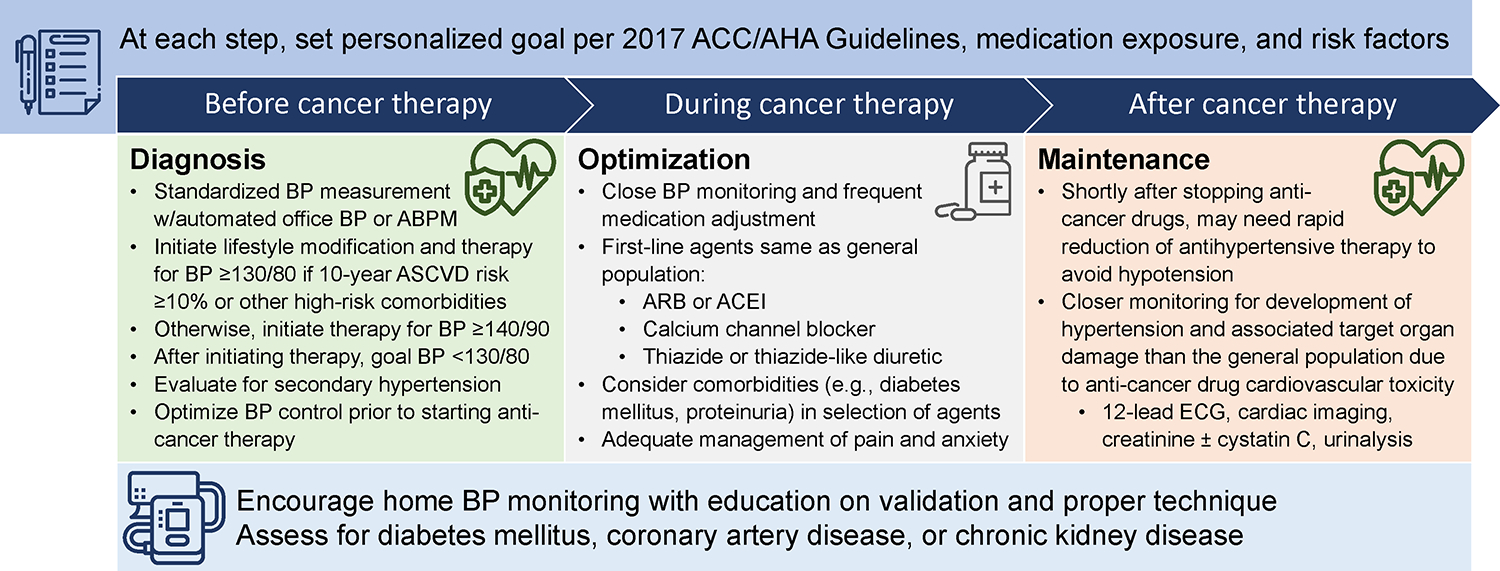
Practical suggestions for the diagnosis and management of hypertension before, during and after commencing anti-cancer therapy.
Lifestyle Modifications
Patients should be counseled on important lifestyle modifications that can help to improve BP control, including limiting sodium intake (with consideration for risk of hypovolemia), non-steroidal anti-inflammatory drug use, caffeine use, and alcohol use and increasing physical activity and potassium intake.4, 45, 49 When evaluating patients for rising BP during anti-cancer therapy, providers should address the potential role of inadequate pain control and concomitant therapies that may exacerbate hypertension, such as erythropoietin stimulating agents and corticosteroids. Evaluation of co-morbidities that may increase BP (e.g., obstructive sleep apnea) is also important.
Long-term management of cancer drug-induced hypertension in cancer survivors
After completing anti-cancer therapy with agents known to increase BP, patients may need to reduce antihypertensive treatment to avoid rebound hypotension and ischemic events. In patients treated with anti-cancer therapy requiring significant escalation of antihypertensive therapy at the onset, reduction in antihypertensive therapy may need to occur within days of cessation of treatment, and daily home BP monitoring may be necessary. Over the long-term, the prevalence of hypertension in cancer survivors is higher than in the general population.1 Therefore, cancer survivors are likely to benefit from closer monitoring for the development of hypertension than the general population, including a combination of in-office and home BP monitoring. Similar to patients undergoing active anti-cancer therapy, there is insufficient evidence to support a specific or targeted approach to managing hypertension in cancer survivors. Therefore, these patients should be treated based on current best evidence for the general population.4, 45, 49
KEY GAPS AND UNMET NEEDS
Gaps in knowledge remain in our understanding of the epidemiology of concomitant hypertension and cancer, especially regarding common risk factors. While these conditions are both more prevalent with aging, age alone does not seem to be the link between hypertension and cancer. Contributing to the clinical burden of co-morbidity, many anti-cancer drugs themselves cause hypertension especially VSPIs, BRAF/MEK inhibitors, BTK inhibitors, RET-TKIs, some poly adenosine diphosphate ribose polymerase inhibitors, and mTOR inhibitors. Pro-hypertensive effects may be direct or indirect but underlying mechanisms are elusive. There are major gaps in the understanding of how these anti-cancer drugs cause hypertension. In addition, the relative contributions of direct vascular and renal toxicity to hypertension induced by alkylating agents, the role of decreased VEGF secretion as a potential mediator of hypertension induced by the Inhibition of mTOR, and the association between aromatase inhibitors and increased cardiovascular risk including hypertension, need to be elucidated. Whether anti-cancer therapy-induced hypertension is truly a predictor of drug efficacy awaits further clarification in large prospective cancer trials. Key gaps also include the timing and frequency of BP monitoring and cardiovascular risk assessment in patients with cancer during, and after therapy, and the relationship of concurrent cardiovascular risk factors with anti-cancer therapy-induced hypertension and outcomes. Evidence-based trials to treat anti-cancer drug-induced hypertension are lacking and accordingly it remains unclear as to which antihypertensive drugs should be used.
FUTURE DIRECTIONS
Future directions for research on hypertension in cancer include epidemiological topics on common risk factors and mechanisms of hypertension and cancer, optimal strategies for BP monitoring during and after anti-cancer therapy, and the diagnosis and management of anti-cancer drug-induced hypertension. Future research will need to address molecular mechanisms underlying anti-cancer therapy-induced hypertension to better understand measures needed to minimize cardiovascular toxicity and hypertension, while at the same time optimizing cancer survival. The bidirectional relationship between cancer and hypertension, pathophysiology of heart failure with preserved ejection fraction resulting from anti-cancer therapy, risk of lifetime exposure to Bruton’s TKIs for prolonged periods, extent of lifetime risk from cisplatin exposure, potential cardiovascular protective effect of Janise kinase inhibitors possibly through their ant-inflammatory effects are all topics for further exploration. The optimal timing and frequency of BP monitoring in patients with cancer and cancer survivors on drugs that induce hypertension, particularly at initiation of and changes in cancer medication regimen, must be evaluated. The optimal BP targets in individuals with cancer will also need to be determined, especially since it is unclear whether the final BP is more important for end organ effects than the magnitude of initial BP increase from baseline. Use of algorithms for monitoring vital signs, laboratory values, and ECG’s with certain frequency for particular drugs should be incorporated into treatment plans for patients on anti-cancer therapy. BP management when cancer medication therapies are stopped (completed or discontinued) will need to be streamlined, especially since there may be rebound hypotension. RCTs are needed to investigate the “best antihypertensives” for individuals with cancer treated with various cancer medications to optimize BP and limit end organ damage. Of major importance sex and racial/ethnic differences, differential therapeutic responses, as well as disparities regarding management, outcomes, and adherence in patients with hypertension and cancer, will need to be carefully evaluated.
CLINICAL PEARLS.
Many anti-cancer drugs have cardiovascular toxicities including hypertension.
Cancer therapy-induced hypertension, especially by VSPIs and proteasome inhibitors, is often reversible after discontinuation of these agents.
Many anti-cancer drugs may worsen BP control in patients with existing hypertension.
Hypertension control is important prior to, during, and following completion of cancer treatment.
At least weekly BP monitoring is suggested for the first 4–8 weeks on cancer drugs that increase BP and upon discontinuing these drugs, given distinct risk of BP lability; holding parameters and atypical dosing of antihypertensive medications may be necessary.
Home BP monitoring should be encouraged.
Cancer survivors are at increased risk of hypertension-associated complications such as atrial fibrillation, heart failure, and chronic kidney disease, necessitating a multidisciplinary approach for optimal management.
ACKNOWLEDGMENTS
The following support is acknowledged: JBC, National Institutes of Health (NIH) (K23-HL133843, R01-HL153646, R01-HL157108, U01-HL160277, U01-TR003734, R01-DK123104, U24-DK060990, R01-AG074989), AHA Bugher Award; SAB, National Center for Advancing Translational Sciences, NIH (UL1TR001436, KL2TR001438); NNL, British Heart Foundation Centre (BHF) (RE/18/6/34217); SMH, NIH (K08 DK118120); GYO, University of Alberta Hospital Foundation, Canadian Institute of Health Research, Heart and Stroke Foundation, Canada Research Chair; NJB, NIH (HL145293, DK117875); RMT, BHF (RE/18/6/34217, CH/12/4/297620), Leducq foundation, Phil Gold Chair-McGill University.
Footnotes
CONFLICTS
JBC, SMH, DCHvD have no conflicts. RMT provided expert opinion to Novartis. NJB serves on the Scientific Advisory Board for Alnylam Pharmaceuticals and as a consultant to Pharvaris and ESTAR Bio. GYO has received support from Sanofi-Genzyme, Takeda and Bristol Myers Squibb. NNL has received funding from Roche Diagnostics, Astra Zeneca and Boehringer Ingelheim and speaker’s fees/advisory board fees from Roche, Pharmacosmos, Astra Zeneca and Novartis. SD has served on advisory boards of Novartis and Astra Zeneca and has received honoraria from Novartis, Pfizer and Astra Zeneca.
REFERENCES
- 1.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, Mertens AC, Border W, Durand JB, Robison LL and Meacham LR. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, Abdollahi M, Abdollahpour I, Abolhassani H, Aboyans V, Abrams EM, Abreu LG, Abrigo MRM, Abu-Raddad LJ, Abushouk AI, Acebedo A, Ackerman IN, Adabi M, Adamu AA, Adebayo OM, Adekanmbi V, Adelson JD, Adetokunboh OO, Adham D, Afshari M, Afshin A, Agardh EE, Agarwal G, Agesa KM, Aghaali M, Aghamir SMK, Agrawal A, Ahmad T, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmadpour E, Akalu TY, Akinyemi RO, Akinyemiju T, Akombi B, Al-Aly Z, Alam K, Alam N, Alam S, Alam T, Alanzi TM, Albertson SB, Alcalde-Rabanal JE, Alema NM, Ali M, Ali S, Alicandro G, Alijanzadeh M, Alinia C, Alipour V, Aljunid SM, Alla F, Allebeck P, Almasi-Hashiani A, Alonso J, Al-Raddadi RM, Altirkawi KA, Alvis-Guzman N, Alvis-Zakzuk NJ, Amini S, Amini-Rarani M, Aminorroaya A, Amiri F, Amit AML, Amugsi DA, Amul GGH, Anderlini D, Andrei CL, Andrei T, Anjomshoa M, Ansari F, Ansari I, Ansari-Moghaddam A, Antonio CAT, Antony CM, Antriyandarti E, Anvari D, Anwer R, Arabloo J, Arab-Zozani M, Aravkin AY, Ariani F, Ärnlöv J, Aryal KK, Arzani A, Asadi-Aliabadi M, Asadi-Pooya AA, Asghari B, Ashbaugh C, Atnafu DD, Atre SR, Ausloos F, Ausloos M, Ayala Quintanilla BP, Ayano G, Ayanore MA, Aynalem YA, Azari S, Azarian G, Azene ZN, Babaee E, Badawi A, Bagherzadeh M, Bakhshaei MH, Bakhtiari A, Balakrishnan S, Balalla S, Balassyano S, Banach M, Banik PC, Bannick MS, Bante AB, Baraki AG, Barboza MA, Barker-Collo SL, Barthelemy CM, Barua L, Barzegar A, Basu S, Baune BT, Bayati M, Bazmandegan G, Bedi N, Beghi E, Béjot Y, Bello AK, Bender RG, Bennett DA, Bennitt FB, Bensenor IM, Benziger CP, Berhe K, Bernabe E, Bertolacci GJ, Bhageerathy R, Bhala N, Bhandari D, Bhardwaj P, Bhattacharyya K, Bhutta ZA, Bibi S, Biehl MH, Bikbov B, Bin Sayeed MS, Biondi A, Birihane BM, Bisanzio D, Bisignano C, Biswas RK, Bohlouli S, Bohluli M, Bolla SRR, Boloor A, Boon-Dooley AS, Borges G, Borzì AM, Bourne R, Brady OJ, Brauer M, Brayne C, Breitborde NJK, Brenner H, Briant PS, Briggs AM, Briko NI, Britton GB, Bryazka D, Buchbinder R, Bumgarner BR, Busse R, Butt ZA, Caetano dos Santos FL, Cámera LLAA, Campos-Nonato IR, Car J, Cárdenas R, Carreras G, Carrero JJ, Carvalho F, Castaldelli-Maia JM, Castañeda-Orjuela CA, Castelpietra G, Castle CD, Castro F, Catalá-López F, Causey K, Cederroth CR, Cercy KM, Cerin E, Chandan JS, Chang AR, Charlson FJ, Chattu VK, Chaturvedi S, Chimed-Ochir O, Chin KL, Cho DY, Christensen H, Chu D-T, Chung MT, Cicuttini FM, Ciobanu LG, Cirillo M, Collins EL, Compton K, Conti S, Cortesi PA, Costa VM, Cousin E, Cowden RG, Cowie BC, Cromwell EA, Cross DH, Crowe CS, Cruz JA, Cunningham M, Dahlawi SMA, Damiani G, Dandona L, Dandona R, Darwesh AM, Daryani A, Das JK, Das Gupta R, das Neves J, Dávila-Cervantes CA, Davletov K, De Leo D, Dean FE, DeCleene NK, Deen A, Degenhardt L, Dellavalle RP, Demeke FM, Demsie DG, Denova-Gutiérrez E, Dereje ND, Dervenis N, Desai R, Desalew A, Dessie GA, Dharmaratne SD, Dhungana GP, Dianatinasab M, Diaz D, Dibaji Forooshani ZS, Dingels ZV, Dirac MA, Djalalinia S, Do HT, Dokova K, Dorostkar F, Doshi CP, Doshmangir L, Douiri A, Doxey MC, Driscoll TR, Dunachie SJ, Duncan BB, Duraes AR, Eagan AW, Ebrahimi Kalan M, Edvardsson D, Ehrlich JR, El Nahas N, El Sayed I, El Tantawi M, Elbarazi I, Elgendy IY, Elhabashy HR, El-Jaafary SI, Elyazar IRF, Emamian MH, Emmons-Bell S, Erskine HE, Eshrati B, Eskandarieh S, Esmaeilnejad S, Esmaeilzadeh F, Esteghamati A, Estep K, Etemadi A, Etisso AE, Farahmand M, Faraj A, Fareed M, Faridnia R, Farinha CSeS, Farioli A, Faro A, Faruque M, Farzadfar F, Fattahi N, Fazlzadeh M, Feigin VL, Feldman R, Fereshtehnejad S-M, Fernandes E, Ferrari AJ, Ferreira ML, Filip I, Fischer F, Fisher JL, Fitzgerald R, Flohr C, Flor LS, Foigt NA, Folayan MO, Force LM, Fornari C, Foroutan M, Fox JT, Freitas M, Fu W, Fukumoto T, Furtado JM, Gad MM, Gakidou E, Galles NC, Gallus S, Gamkrelidze A, Garcia-Basteiro AL, Gardner WM, Geberemariyam BS, Gebrehiwot AM, Gebremedhin KB, Gebreslassie AAAA, Gershberg Hayoon A, Gething PW, Ghadimi M, Ghadiri K, Ghafourifard M, Ghajar A, Ghamari F, Ghashghaee A, Ghiasvand H, Ghith N, Gholamian A, Gilani SA, Gill PS, Gitimoghaddam M, Giussani G, Goli S, Gomez RS, Gopalani SV, Gorini G, Gorman TM, Gottlich HC, Goudarzi H, Goulart AC, Goulart BNG, Grada A, Grivna M, Grosso G, Gubari MIM, Gugnani HC, Guimaraes ALS, Guimarães RA, Guled RA, Guo G, Guo Y, Gupta R, Haagsma JA, Haddock B, Hafezi-Nejad N, Hafiz A, Hagins H, Haile LM, Hall BJ, Halvaei I, Hamadeh RR, Hamagharib Abdullah K, Hamilton EB, Han C, Han H, Hankey GJ, Haro JM, Harvey JD, Hasaballah AI, Hasanzadeh A, Hashemian M, Hassanipour S, Hassankhani H, Havmoeller RJ, Hay RJ, Hay SI, Hayat K, Heidari B, Heidari G, Heidari-Soureshjani R, Hendrie D, Henrikson HJ, Henry NJ, Herteliu C, Heydarpour F, Hird TR, Hoek HW, Hole MK, Holla R, Hoogar P, Hosgood HD, Hosseinzadeh M, Hostiuc M, Hostiuc S, Househ M, Hoy DG, Hsairi M, Hsieh VC-r, Hu G, Huda TM, Hugo FN, Huynh CK, Hwang B-F, Iannucci VC, Ibitoye SE, Ikuta KS, Ilesanmi OS, Ilic IM, Ilic MD, Inbaraj LR, Ippolito H, Irvani SSN, Islam MM, Islam M, Islam SMS, Islami F, Iso H, Ivers RQ, Iwu CCD, Iyamu IO, Jaafari J, Jacobsen KH, Jadidi-Niaragh F, Jafari H, Jafarinia M, Jahagirdar D, Jahani MA, Jahanmehr N, Jakovljevic M, Jalali A, Jalilian F, James SL, Janjani H, Janodia MD, Jayatilleke AU, Jeemon P, Jenabi E, Jha RP, Jha V, Ji JS, Jia P, John O, John-Akinola YO, Johnson CO, Johnson SC, Jonas JB, Joo T, Joshi A, Jozwiak JJ, Jürisson M, Kabir A, Kabir Z, Kalani H, Kalani R, Kalankesh LR, Kalhor R, Kamiab Z, Kanchan T, Karami Matin B, Karch A, Karim MA, Karimi SE, Kassa GM, Kassebaum NJ, Katikireddi SV, Kawakami N, Kayode GA, Keddie SH, Keller C, Kereselidze M, Khafaie MA, Khalid N, Khan M, Khatab K, Khater MM, Khatib MN, Khayamzadeh M, Khodayari MT, Khundkar R, Kianipour N, Kieling C, Kim D, Kim Y-E, Kim YJ, Kimokoti RW, Kisa A, Kisa S, Kissimova-Skarbek K, Kivimäki M, Kneib CJ, Knudsen AKS, Kocarnik JM, Kolola T, Kopec JA, Kosen S, Koul PA, Koyanagi A, Kravchenko MA, Krishan K, Krohn KJ, Kuate Defo B, Kucuk Bicer B, Kumar GA, Kumar M, Kumar P, Kumar V, Kumaresh G, Kurmi OP, Kusuma D, Kyu HH, La Vecchia C, Lacey B, Lal DK, Lalloo R, Lam JO, Lami FH, Landires I, Lang JJ, Lansingh VC, Larson SL, Larsson AO, Lasrado S, Lassi ZS, Lau KM-M, Lavados PM, Lazarus JV, Ledesma JR, Lee PH, Lee SWH, LeGrand KE, Leigh J, Leonardi M, Lescinsky H, Leung J, Levi M, Lewington S, Li S, Lim L-L, Lin C, Lin R-T, Linehan C, Linn S, Liu H-C, Liu S, Liu Z, Looker KJ, Lopez AD, Lopukhov PD, Lorkowski S, Lotufo PA, Lucas TCD, Lugo A, Lunevicius R, Lyons RA, Ma J, MacLachlan JH, Maddison ER, Maddison R, Madotto F, Mahasha PW, Mai HT, Majeed A, Maled V, Maleki S, Malekzadeh R, Malta DC, Mamun AA, Manafi A, Manafi N, Manguerra H, Mansouri B, Mansournia MA, Mantilla Herrera AM, Maravilla JC, Marks A, Martins-Melo FR, Martopullo I, Masoumi SZ, Massano J, Massenburg BB, Mathur MR, Maulik PK, McAlinden C, McGrath JJ, McKee M, Mehndiratta MM, Mehri F, Mehta KM, Meitei WB, Memiah PTN, Mendoza W, Menezes RG, Mengesha EW, Mengesha MB, Mereke A, Meretoja A, Meretoja TJ, Mestrovic T, Miazgowski B, Miazgowski T, Michalek IM, Mihretie KM, Miller TR, Mills EJ, Mirica A, Mirrakhimov EM, Mirzaei H, Mirzaei M, Mirzaei-Alavijeh M, Misganaw AT, Mithra P, Moazen B, Moghadaszadeh M, Mohamadi E, Mohammad DK, Mohammad Y, Mohammad Gholi Mezerji N, Mohammadian-Hafshejani A, Mohammadifard N, Mohammadpourhodki R, Mohammed S, Mokdad AH, Molokhia M, Momen NC, Monasta L, Mondello S, Mooney MD, Moosazadeh M, Moradi G, Moradi M, Moradi-Lakeh M, Moradzadeh R, Moraga P, Morales L, Morawska L, Moreno Velásquez I, Morgado-da-Costa J, Morrison SD, Mosser JF, Mouodi S, Mousavi SM, Mousavi Khaneghah A, Mueller UO, Munro SB, Muriithi MK, Musa KI, Muthupandian S, Naderi M, Nagarajan AJ, Nagel G, Naghshtabrizi B, Nair S, Nandi AK, Nangia V, Nansseu JR, Nayak VC, Nazari J, Negoi I, Negoi RI, Netsere HBN, Ngunjiri JW, Nguyen CT, Nguyen J, Nguyen M, Nguyen M, Nichols E, Nigatu D, Nigatu YT, Nikbakhsh R, Nixon MR, Nnaji CA, Nomura S, Norrving B, Noubiap JJ, Nowak C, Nunez-Samudio V, Oţoiu A, Oancea B, Odell CM, Ogbo FA, Oh I-H, Okunga EW, Oladnabi M, Olagunju AT, Olusanya BO, Olusanya JO, Oluwasanu MM, Omar Bali A, Omer MO, Ong KL, Onwujekwe OE, Orji AU, Orpana HM, Ortiz A, Ostroff SM, Otstavnov N, Otstavnov SS, Øverland S, Owolabi MO, P A M, Padubidri JR, Pakhare AP, Palladino R, Pana A, Panda-Jonas S, Pandey A, Park E-K, Parmar PGK, Pasupula DK, Patel SK, Paternina-Caicedo AJ, Pathak A, Pathak M, Patten SB, Patton GC, Paudel D, Pazoki Toroudi H, Peden AE, Pennini A, Pepito VCF, Peprah EK, Pereira A, Pereira DM, Perico N, Pham HQ, Phillips MR, Pigott DM, Pilgrim T, Pilz TM, Pirsaheb M, Plana-Ripoll O, Plass D, Pokhrel KN, Polibin RV, Polinder S, Polkinghorne KR, Postma MJ, Pourjafar H, Pourmalek F, Pourmirza Kalhori R, Pourshams A, Poznańska A, Prada SI, Prakash V, Pribadi DRA, Pupillo E, Quazi Syed Z, Rabiee M, Rabiee N, Radfar A, Rafiee A, Rafiei A, Raggi A, Rahimi-Movaghar A, Rahman MA, Rajabpour-Sanati A, Rajati F, Ramezanzadeh K, Ranabhat CL, Rao PC, Rao SJ, Rasella D, Rastogi P, Rathi P, Rawaf DL, Rawaf S, Rawal L, Razo C, Redford SB, Reiner RC, Reinig N, Reitsma MB, Remuzzi G, Renjith V, Renzaho AMN, Resnikoff S, Rezaei N, Rezai Ms, Rezapour A, Rhinehart P-A, Riahi SM, Ribeiro ALP, Ribeiro DC, Ribeiro D, Rickard J, Roberts NLS, Roberts S, Robinson SR, Roever L, Rolfe S, Ronfani L, Roshandel G, Roth GA, Rubagotti E, Rumisha SF, Sabour S, Sachdev PS, Saddik B, Sadeghi E, Sadeghi M, Saeidi S, Safi S, Safiri S, Sagar R, Sahebkar A, Sahraian MA, Sajadi SM, Salahshoor MR, Salamati P, Salehi Zahabi S, Salem H, Salem MRR, Salimzadeh H, Salomon JA, Salz I, Samad Z, Samy AM, Sanabria J, Santomauro DF, Santos IS, Santos JV, Santric-Milicevic MM, Saraswathy SYI, Sarmiento-Suárez R, Sarrafzadegan N, Sartorius B, Sarveazad A, Sathian B, Sathish T, Sattin D, Sbarra AN, Schaeffer LE, Schiavolin S, Schmidt MI, Schutte AE, Schwebel DC, Schwendicke F, Senbeta AM, Senthilkumaran S, Sepanlou SG, Shackelford KA, Shadid J, Shahabi S, Shaheen AA, Shaikh MA, Shalash AS, Shams-Beyranvand M, Shamsizadeh M, Shannawaz M, Sharafi K, Sharara F, Sheena BS, Sheikhtaheri A, Shetty RS, Shibuya K, Shiferaw WS, Shigematsu M, Shin JI, Shiri R, Shirkoohi R, Shrime MG, Shuval K, Siabani S, Sigfusdottir ID, Sigurvinsdottir R, Silva JP, Simpson KE, Singh A, Singh JA, Skiadaresi E, Skou STS, Skryabin VY, Sobngwi E, Sokhan A, Soltani S, Sorensen RJD, Soriano JB, Sorrie MB, Soyiri IN, Sreeramareddy CT, Stanaway JD, Stark BA, Ştefan SC, Stein C, Steiner C, Steiner TJ, Stokes MA, Stovner LJ, Stubbs JL, Sudaryanto A, Sufiyan MaB, Sulo G, Sultan I, Sykes BL, Sylte DO, Szócska M, Tabarés-Seisdedos R, Tabb KM, Tadakamadla SK, Taherkhani A, Tajdini M, Takahashi K, Taveira N, Teagle WL, Teame H, Tehrani-Banihashemi A, Teklehaimanot BF, Terrason S, Tessema ZT, Thankappan KR, Thomson AM, Tohidinik HR, Tonelli M, Topor-Madry R, Torre AE, Touvier M, Tovani-Palone MRR, Tran BX, Travillian R, Troeger CE, Truelsen TC, Tsai AC, Tsatsakis A, Tudor Car L, Tyrovolas S, Uddin R, Ullah S, Undurraga EA, Unnikrishnan B, Vacante M, Vakilian A, Valdez PR, Varughese S, Vasankari TJ, Vasseghian Y, Venketasubramanian N, Violante FS, Vlassov V, Vollset SE, Vongpradith A, Vukovic A, Vukovic R, Waheed Y, Walters MK, Wang J, Wang Y, Wang Y-P, Ward JL, Watson A, Wei J, Weintraub RG, Weiss DJ, Weiss J, Westerman R, Whisnant JL, Whiteford HA, Wiangkham T, Wiens KE, Wijeratne T, Wilner LB, Wilson S, Wojtyniak B, Wolfe CDA, Wool EE, Wu A-M, Wulf Hanson S, Wunrow HY, Xu G, Xu R, Yadgir S, Yahyazadeh Jabbari SH, Yamagishi K, Yaminfirooz M, Yano Y, Yaya S, Yazdi-Feyzabadi V, Yearwood JA, Yeheyis TY, Yeshitila YG, Yip P, Yonemoto N, Yoon S-J, Yoosefi Lebni J, Younis MZ, Younker TP, Yousefi Z, Yousefifard M, Yousefinezhadi T, Yousuf AY, Yu C, Yusefzadeh H, Zahirian Moghadam T, Zaki L, Zaman SB, Zamani M, Zamanian M, Zandian H, Zangeneh A, Zastrozhin MS, Zewdie KA, Zhang Y, Zhang Z-J, Zhao JT, Zhao Y, Zheng P, Zhou M, Ziapour A, Zimsen SRM, Naghavi M and Murray CJL. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B, Singleton RK, Sophiea MK, Iurilli MLC, Lhoste VPF, Cowan MJ, Savin S, Woodward M, Balanova Y, Cifkova R, Damasceno A, Elliott P, Farzadfar F, He J, Ikeda N, Kengne AP, Khang Y-H, Kim HC, Laxmaiah A, Lin H-H, Margozzini Maira P, Miranda JJ, Neuhauser H, Sundström J, Varghese C, Widyahening IS, Zdrojewski T, Abarca-Gómez L, Abdeen ZA, Abdul Rahim HF, Abu-Rmeileh NM, Acosta-Cazares B, Adams RJ, Aekplakorn W, Afsana K, Afzal S, Agdeppa IA, Aghazadeh-Attari J, Aguilar-Salinas CA, Agyemang C, Ahmad NA, Ahmadi A, Ahmadi N, Ahmadi N, Ahmadizar F, Ahmed SH, Ahrens W, Ajlouni K, Al-Raddadi R, Alarouj M, AlBuhairan F, AlDhukair S, Ali MM, Alkandari A, Aa Alkerwi, Allin K, Aly E, Amarapurkar DN, Amougou N, Amouyel P, Andersen LB, Anderssen SA, Anjana RM, Ansari-Moghaddam A, Ansong D, Aounallah-Skhiri H, Araújo J, Ariansen I, Aris T, Arku RE, Arlappa N, Aryal KK, Aspelund T, Assah FK, Assunção MCF, Auvinen J, Avdićová M, Azevedo A, Azimi-Nezhad M, Azizi F, Azmin M, Babu BV, Bahijri S, Balakrishna N, Bamoshmoosh M, Banach M, Banadinović M, Bandosz P, Banegas JR, Baran J, Barbagallo CM, Barceló A, Barkat A, Barreto M, Barros AJD, Barros MVG, Bartosiewicz A, Basit A, Bastos JLD, Bata I, Batieha AM, Batyrbek A, Baur LA, Beaglehole R, Belavendra A, Ben Romdhane H, Benet M, Benson LS, Berkinbayev S, Bernabe-Ortiz A, Bernotiene G, Bettiol H, Bezerra J, Bhagyalaxmi A, Bhargava SK, Bia D, Biasch K, Bika Lele EC, Bikbov MM, Bista B, Bjerregaard P, Bjertness E, Bjertness MB, Björkelund C, Bloch KV, Blokstra A, Bo S, Bobak M, Boeing H, Boggia JG, Boissonnet CP, Bojesen SE, Bongard V, Bonilla-Vargas A, Bopp M, Borghs H, Bovet P, Boyer CB, Braeckman L, Brajkovich I, Branca F, Breckenkamp J, Brenner H, Brewster LM, Briceño Y, Brito M, Bruno G, Bueno-de-Mesquita HB, Bueno G, Bugge A, Burns C, Bursztyn M, Cabrera de León A, Cacciottolo J, Cameron C, Can G, Cândido APC, Capanzana MV, Čapková N, Capuano E, Capuano V, Cardoso VC, Carlsson AC, Carvalho J, Casanueva FF, Censi L, Cervantes-Loaiza M, Chadjigeorgiou CA, Chamukuttan S, Chan AW, Chan Q, Chaturvedi HK, Chaturvedi N, Chee ML, Chen C-J, Chen F, Chen H, Chen S, Chen Z, Cheng C-Y, Cheraghian B, Cherkaoui Dekkaki I, Chetrit A, Chien K-L, Chiolero A, Chiou S-T, Chirita-Emandi A, Chirlaque M-D, Cho B, Christensen K, Christofaro DG, Chudek J, Cinteza E, Claessens F, Clarke J, Clays E, Cohen E, Concin H, Cooper C, Coppinger TC, Costanzo S, Cottel D, Cowell C, Craig CL, Crampin AC, Crujeiras AB, Cruz JJ, Csilla S, Cui L, Cureau FV, Cuschieri S, D’Arrigo G, d’Orsi E, Dallongeville J, Dankner R, Dantoft TM, Dauchet L, Davletov K, De Backer G, De Bacquer D, De Curtis A, de Gaetano G, De Henauw S, de Oliveira PD, De Ridder D, De Smedt D, Deepa M, Deev AD, DeGennaro V Jr, Delisle H, Demarest S, Dennison E, Deschamps V, Dhimal M, Di Castelnuovo AF, Dias-da-Costa JS, Diaz A, Dickerson TT, Dika Z, Djalalinia S, Do HTP, Dobson AJ, Donfrancesco C, Donoso SP, Döring A, Dorobantu M, Dörr M, Doua K, Dragano N, Drygas W, Duante CA, Duboz P, Duda RB, Dulskiene V, Dushpanova A, Džakula A, Dzerve V, Dziankowska-Zaborszczyk E, Eddie R, Eftekhar E, Eggertsen R, Eghtesad S, Eiben G, Ekelund U, El-Khateeb M, El Ati J, Eldemire-Shearer D, Eliasen M, Elosua R, Erasmus RT, Erbel R, Erem C, Eriksen L, Eriksson JG, Escobedo-de la Peña J, Eslami S, Esmaeili A, Evans A, Faeh D, Fakhretdinova AA, Fall CH, Faramarzi E, Farjam M, Fattahi MR, Fawwad A, Felix-Redondo FJ, Felix SB, Ferguson TS, Fernandes RA, Fernández-Bergés D, Ferrante D, Ferrao T, Ferrari M, Ferrario MM, Ferreccio C, Ferreira HS, Ferrer E, Ferrieres J, Figueiró TH, Fink G, Fischer K, Foo LH, Forsner M, Fouad HM, Francis DK, Franco MdC, Frikke-Schmidt R, Frontera G, Fuchs FD, Fuchs SC, Fujita Y, Fumihiko M, Furdela V, Furer A, Furusawa T, Gaciong Z, Galbarczyk A, Galenkamp H, Galvano F, Gao J, Gao P, Garcia-de-la-Hera M, Garcia P, Gareta D, Garnett SP, Gaspoz J-M, Gasull M, Gazzinelli A, Gehring U, Geleijnse JM, George R, Ghanbari A, Ghasemi E, Gheorghe-Fronea O-F, Ghimire A, Gialluisi A, Giampaoli S, Gieger C, Gill TK, Giovannelli J, Gironella G, Giwercman A, Gkiouras K, Goldberg M, Goldsmith RA, Gomez LF, Gomula A, Gonçalves H, Gonçalves M, Gonçalves Cordeiro da Silva B, Gonzalez-Chica DA, Gonzalez-Gross M, González-Rivas JP, González-Villalpando C, González-Villalpando M-E, Gonzalez AR, Gorbea MB, Gottrand F, Graff-Iversen S, Grafnetter D, Grajda A, Grammatikopoulou MG, Gregor RD, Grodzicki T, Grosso G, Gruden G, Gu D, Guan OP, Gudmundsson EF, Gudnason V, Guerrero R, Guessous I, Guimaraes AL, Gulliford MC, Gunnlaugsdottir J, Gunter MJ, Gupta PC, Gupta R, Gureje O, Gurzkowska B, Gutierrez L, Gutzwiller F, Ha S, Hadaegh F, Haghshenas R, Hakimi H, Halkjær J, Hambleton IR, Hamzeh B, Hange D, Hanif AAM, Hantunen S, Hao J, Hardman CM, Hari Kumar R, Hashemi-Shahri SM, Hata J, Haugsgjerd T, Hayes AJ, He Y, Heier M, Hendriks ME, Henrique RdS, Henriques A, Hernandez Cadena L, Herqutanto, Herrala S, Heshmat R, Hill AG, Ho SY, Ho SC, Hobbs M, Holdsworth M, Homayounfar R, Horasan Dinc G, Horimoto ARVR, Hormiga CM, Horta BL, Houti L, Howitt C, Htay TT, Htet AS, Htike MMT, Hu Y, Huerta JM, Huhtaniemi IT, Huiart L, Huisman M, Husseini AS, Huybrechts I, Hwalla N, Iacoviello L, Iannone AG, Ibrahim MM, Ibrahim Wong N, Ikram MA, Iotova V, Irazola VE, Ishida T, Isiguzo GC, Islam M, Islam SMS, Iwasaki M, Jackson RT, Jacobs JM, Jaddou HY, Jafar T, James K, Jamrozik K, Janszky I, Janus E, Jarvelin M-R, Jasienska G, Jelaković A, Jelaković B, Jennings G, Jha AK, Jiang CQ, Jimenez RO, Jöckel K-H, Joffres M, Johansson M, Jokelainen JJ, Jonas JB, Jørgensen T, Joshi P, Joukar F, Jóżwiak J, Juolevi A, Jurak G, Jureša V, Kaaks R, Kafatos A, Kajantie EO, Kalmatayeva Z, Kalpourtzi N, Kalter-Leibovici O, Kampmann FB, Kannan S, Karaglani E, Kårhus LL, Karki KB, Katibeh M, Katz J, Kauhanen J, Kaur P, Kavousi M, Kazakbaeva GM, Keil U, Keinan Boker L, Keinänen-Kiukaanniemi S, Kelishadi R, Kemper HCG, Keramati M, Kerimkulova A, Kersting M, Key T, Khader YS, Khalili D, Khaw K-T, Kheiri B, Kheradmand M, Khosravi A, Kiechl-Kohlendorfer U, Kiechl S, Killewo J, Kim DW, Kim J, Klakk H, Klimek M, Klumbiene J, Knoflach M, Kolle E, Kolsteren P, Kontto JP, Korpelainen R, Korrovits P, Kos J, Koskinen S, Kouda K, Kowlessur S, Koziel S, Kratenova J, Kriaucioniene V, Kristensen PL, Krokstad S, Kromhout D, Kruger HS, Kubinova R, Kuciene R, Kujala UM, Kulaga Z, Kumar RK, Kurjata P, Kusuma YS, Kutsenko V, Kuulasmaa K, Kyobutungi C, Laatikainen T, Lachat C, Laid Y, Lam TH, Landrove O, Lanska V, Lappas G, Larijani B, Latt TS, Le Coroller G, Le Nguyen Bao K, Le TD, Lee J, Lee J, Lehmann N, Lehtimäki T, Lemogoum D, Levitt NS, Li Y, Lilly CL, Lim W-Y, Lima-Costa MF, Lin X, Lin Y-T, Lind L, Lingam V, Linneberg A, Lissner L, Litwin M, Lo W-C, Loit H-M, Lopez-Garcia E, Lopez T, Lotufo PA, Lozano JE, Lukačević Lovrenčić I, Lukrafka JL, Luksiene D, Lundqvist A, Lundqvist R, Lunet N, Lustigová M, Luszczki E, Ma G, Ma J, Machado-Coelho GLL, Machado-Rodrigues AM, Macia E, Macieira LM, Madar AA, Maggi S, Magliano DJ, Magriplis E, Mahasampath G, Maire B, Majer M, Makdisse M, Malekzadeh F, Malekzadeh R, Malhotra R, Mallikharjuna Rao K, Malyutina SK, Maniego LV, Manios Y, Mann JI, Mansour-Ghanaei F, Manzato E, Marcil A, Mårild SB, Marinović Glavić M, Marques-Vidal P, Marques LP, Marrugat J, Martorell R, Mascarenhas LP, Matasin M, Mathiesen EB, Mathur P, Matijasevich A, Matlosz P, Matsha TE, Mavrogianni C, Mbanya JCN, Mc Donald Posso AJ, McFarlane SR, McGarvey ST, McLachlan S, McLean RM, McLean SB, McNulty BA, Mediene Benchekor S, Medzioniene J, Mehdipour P, Mehlig K, Mehrparvar AH, Meirhaeghe A, Meisinger C, Mendoza Montano C, Menezes AMB, Menon GR, Mereke A, Meshram II, Metspalu A, Meyer HE, Mi J, Michels N, Mikkel K, Milkowska K, Miller JC, Minderico CS, Mini GK, Mirjalili MR, Mirrakhimov E, Mišigoj-Duraković M, Modesti PA, Moghaddam SS, Mohajer B, Mohamed MK, Mohamed SF, Mohammad K, Mohammadi MR, Mohammadi Z, Mohammadifard N, Mohammadpourhodki R, Mohan V, Mohanna S, Mohd Yusoff MF, Mohebbi I, Mohebi F, Moitry M, Møllehave LT, Molnár D, Momenan A, Mondo CK, Monterrubio-Flores E, Monyeki KDK, Moon JS, Moosazadeh M, Moreira LB, Morejon A, Moreno LA, Morgan K, Moschonis G, Mossakowska M, Mostafa A, Mostafavi S-A, Mota J, Motlagh ME, Motta J, Moura-dos-Santos MA, Mridha MK, Msyamboza KP, Mu TT, Muhihi AJ, Muiesan ML, Müller-Nurasyid M, Murphy N, Mursu J, Musa KI, Musić Milanović S, Musil V, Mustafa N, Nabipour I, Naderimagham S, Nagel G, Naidu BM, Najafi F, Nakamura H, Námešná J, Nang EEK, Nangia VB, Narake S, Ndiaye NC, Neal WA, Nejatizadeh A, Nenko I, Neovius M, Nguyen CT, Nguyen ND, Nguyen QV, Nguyen QN, Nieto-Martínez RE, Niiranen TJ, Nikitin YP, Ninomiya T, Nishtar S, Njelekela MA, Noale M, Noboa OA, Noorbala AA, Norat T, Nordendahl M, Nordestgaard BG, Noto D, Nowak-Szczepanska N, Nsour MA, Nunes B, O’Neill TW, O’Reilly D, Ochimana C, Oda E, Odili AN, Oh K, Ohara K, Ohtsuka R, Olié V, Olinto MTA, Oliveira IO, Omar MA, Onat A, Ong SK, Ono LM, Ordunez P, Ornelas R, Ortiz PJ, Osmond C, Ostojic SM, Ostovar A, Otero JA, Overvad K, Owusu-Dabo E, Paccaud FM, Padez C, Pahomova E, Paiva KMd, Pająk A, Palli D, Palmieri L, Pan W-H, Panda-Jonas S, Panza F, Paoli M, Papandreou D, Park S-W, Park S, Parnell WR, Parsaeian M, Pasquet P, Patel ND, Pavlyshyn H, Pećin I, Pednekar MS, Pedro JM, Peer N, Peixoto SV, Peltonen M, Pereira AC, Peres KGDA, Peres MA, Peters A, Petkeviciene J, Peykari N, Pham ST, Pichardo RN, Pigeot I, Pikhart H, Pilav A, Pilotto L, Pitakaka F, Piwonska A, Pizarro An, Plans-Rubió P, Polašek O, Porta M, Poudyal A, Pourfarzi F, Pourshams A, Poustchi H, Pradeepa R, Price AJ, Price JF, Providencia R, Puhakka SE, Puiu M, Punab M, Qasrawi RF, Qorbani M, Queiroz D, Quoc Bao T, Radić I, Radisauskas R, Rahimikazerooni S, Rahman M, Raitakari O, Raj M, Rakhimova EM, Ramachandra Rao S, Ramachandran A, Ramos E, Rampal L, Rampal S, Rangel Reina DA, Rarra V, Rech CR, Redon J, Reganit PFM, Regecová V, Revilla L, Rezaianzadeh A, Ribeiro R, Riboli E, Richter A, Rigo F, Rinke de Wit TF, Ritti-Dias RM, Robitaille C, Rodríguez-Artalejo F, Rodriguez-Perez MdC, Rodríguez-Villamizar LA, Roggenbuck U, Rojas-Martinez R, Romaguera D, Romeo EL, Rosengren A, Roy JGR, Rubinstein A, Ruidavets J-B, Ruiz-Betancourt BS, Ruiz-Castell M, Rusakova IA, Russo P, Rutkowski M, Sabanayagam C, Sabbaghi H, Sachdev HS, Sadjadi A, Safarpour AR, Safi S, Safiri S, Saidi O, Sakarya S, Saki N, Salanave B, Salazar Martinez E, Salmerón D, Salomaa V, Salonen JT, Salvetti M, Sánchez-Abanto J, Sans S, Santos DA, Santos IS, Santos LC, Santos MP, Santos R, Saramies JL, Sardinha LB, Sarganas G, Sarrafzadegan N, Sathish T, Saum K-U, Savva S, Sawada N, Sbaraini M, Scazufca M, Schaan BD, Schargrodsky H, Schipf S, Schmidt CO, Schnohr P, Schöttker B, Schramm S, Schultsz C, Schutte AE, Sebert S, Sein AA, Sen A, Senbanjo IO, Sepanlou SG, Servais J, Shalnova SA, Shamah-Levy T, Shamshirgaran M, Shanthirani CS, Sharafkhah M, Sharma SK, Shaw JE, Shayanrad A, Shayesteh AA, Shi Z, Shibuya K, Shimizu-Furusawa H, Shin DW, Shirani M, Shiri R, Shrestha N, Si-Ramlee K, Siani A, Siantar R, Sibai AM, Silva CRdM, Silva DAS, Simon M, Simons J, Simons LA, Sjöström M, Slowikowska-Hilczer J, Slusarczyk P, Smeeth L, So H-K, Soares FC, Sobngwi E, Söderberg S, Soemantri A, Sofat R, Solfrizzi V, Somi MH, Sonestedt E, Song Y, Sørensen TIA, Sørgjerd EP, Sorić M, Sossa Jérome C, Soumaré A, Sparboe-Nilsen B, Sparrenberger K, Staessen JA, Starc G, Stavreski B, Steene-Johannessen J, Stehle P, Stein AD, Stergiou GS, Stessman J, Stieber J, Stöckl D, Stocks T, Stokwiszewski J, Stronks K, Strufaldi MW, Suka M, Sun C-A, Sung Y-T, Suriyawongpaisal P, Sy RG, Syddall HE, Sylva RC, Szklo M, Tai ES, Tammesoo M-L, Tamosiunas A, Tan EJ, Tang X, Tanser F, Tao Y, Tarawneh MR, Tarqui-Mamani CB, Taylor A, Taylor J, Tebar WR, Tell GS, Tello T, Tham YC, Thankappan KR, Theobald H, Theodoridis X, Thijs L, Thinggaard M, Thomas N, Thorand B, Thuesen BH, Timmermans EJ, Tjandrarini DH, Tjonneland A, Toft U, Tolonen HK, Tolstrup JS, Topbas M, Topór-Madry R, Tormo MJ, Tornaritis MJ, Torrent M, Torres-Collado L, Touloumi G, Traissac P, Triantafyllou A, Trichopoulos D, Trichopoulou A, Trinh OTH, Trivedi A, Tshepo L, Tsugane S, Tuliakova AM, Tulloch-Reid MK, Tullu F, Tuomainen T-P, Tuomilehto J, Turley ML, Twig G, Tynelius P, Tzourio C, Ueda P, Ugel E, Ulmer H, Uusitalo HMT, Valdivia G, Valvi D, van Dam RM, van den Born B-J, Van der Heyden J, van der Schouw YT, Van Herck K, Van Minh H, Van Schoor NM, van Valkengoed IGM, van Zutphen EM, Vanderschueren D, Vanuzzo D, Varbo A, Vasan SK, Vega T, Veidebaum T, Velasquez-Melendez G, Veronesi G, Verschuren WMM, Verstraeten R, Victora CG, Viet L, Villalpando S, Vineis P, Vioque J, Virtanen JK, Visvikis-Siest S, Viswanathan B, Vlasoff T, Vollenweider P, Voutilainen A, Wade AN, Walton J, Wambiya EOA, Wan Bebakar WM, Wan Mohamud WN, Wanderley Júnior RdS, Wang M-D, Wang N, Wang Q, Wang X, Wang YX, Wang Y-W, Wannamethee SG, Wareham N, Wei W, Weres A, Werner B, Whincup PH, Widhalm K, Wiecek A, Wilks RJ, Willeit J, Willeit P, Williams EA, Wilsgaard T, Wojtyniak B, Wong-McClure RA, Wong A, Wong TY, Woo J, Wu FC, Wu S, Wyszynska J, Xu H, Xu L, Yaacob NA, Yan W, Yang L, Yang X, Yang Y, Yasuharu T, Ye X, Yiallouros PK, Yoosefi M, Yoshihara A, You S-L, Younger-Coleman NO, Yusoff AF, Zainuddin AA, Zakavi SR, Zamani F, Zambon S, Zampelas A, Zapata ME, Zaw KK, Zejglicova K, Zeljkovic Vrkic T, Zeng Y, Zhang L, Zhang Z-Y, Zhao D, Zhao M-H, Zhen S, Zheng Y, Zholdin B, Zhu D, Zins M, Zitt E, Zocalo Y, Zoghlami N, Zuñiga Cisneros J and Ezzati M. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet. 2021;398:957–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr,., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr,., Spencer CC, Stafford RS, Taler, Thomas RJ, Williams KA Sr., Williamson JDand Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 5.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 6.Seretis A, Cividini S, Markozannes G, Tseretopoulou X, Lopez DS, Ntzani EE and Tsilidis KK. Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep. 2019;9:8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccirillo JF, Tierney RM, Costas I, Grove L and Spitznagel EL Jr,. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. [DOI] [PubMed] [Google Scholar]
- 8.van Dorst DCH, Dobbin SJH, Neves KB, Herrmann J, Herrmann SM, Versmissen J, Mathijssen RHJ, Danser AHJ and Lang NN. Hypertension and Prohypertensive Antineoplastic Therapies in Cancer Patients. Circ Res. 2021;128:1040–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koene RJ, Prizment AE, Blaes A and Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JB, Geara AS, Hogan JJ and Townsend RR. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol. 2019;1:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP and Zaorsky NG. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40:3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camarda N, Travers R, Yang VK, London C and Jaffe IZ. VEGF Receptor Inhibitor-Induced Hypertension: Emerging Mechanisms and Clinical Implications. Curr Oncol Rep. 2022;24:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Totzeck M, Mincu RI, Mrotzek S, Schadendorf D and Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: A meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25:482–494. [DOI] [PubMed] [Google Scholar]
- 14.Osumi H, Shinozaki E, Ooki A, Wakatsuki T, Kamiimabeppu D, Sato T, Nakayama I, Ogura M, Takahari D, Chin K and Yamaguchi K. Early hypertension and neutropenia are predictors of treatment efficacy in metastatic colorectal cancer patients administered FOLFIRI and vascular endothelial growth factor inhibitors as second-line chemotherapy. Cancer Med. 2021;10:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neves KB, Rios FJ, van der Mey L, Alves-Lopes R, Cameron AC, Volpe M, Montezano AC, Savoia C and Touyz RM. VEGFR (Vascular Endothelial Growth Factor Receptor) Inhibition Induces Cardiovascular Damage via Redox-Sensitive Processes. Hypertension. 2018;71:638–647. [DOI] [PubMed] [Google Scholar]
- 16.van der Veldt AA, de Boer MP, Boven E, Eringa EC, van den Eertwegh AJ, van Hinsbergh VW, Smulders YM and Serne EH. Reduction in skin microvascular density and changes in vessel morphology in patients treated with sunitinib. Anticancer Drugs. 2010;21:439–46. [DOI] [PubMed] [Google Scholar]
- 17.Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T and Totzeck M. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open. 2019;2:e198890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickerson T, Wiczer T, Waller A, Philippon J, Porter K, Haddad D, Guha A, Rogers KA, Bhat S, Byrd JC, Woyach JA, Awan F and Addison D. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood. 2019;134:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caldeira D, Alves D, Costa J, Ferreira JJ and Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS One. 2019;14:e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, Worden F, Brose M, Patel J, Leboulleux S, Godbert Y, Barlesi F, Morris JC, Owonikoko TK, Tan DSW, Gautschi O, Weiss J, de la Fouchardiere C, Burkard ME, Laskin J, Taylor MH, Kroiss M, Medioni J, Goldman JW, Bauer TM, Levy B, Zhu VW, Lakhani N, Moreno V, Ebata K, Nguyen M, Heirich D, Zhu EY, Huang X, Yang L, Kherani J, Rothenberg SM, Drilon A, Subbiah V, Shah MH and Cabanillas ME. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. The New England journal of medicine. 2020;383:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gainor JF, Curigliano G, Kim D-W, Lee DH, Besse B, Baik CS, Doebele RC, Cassier PA, Lopes G, Tan DSW, Garralda E, Paz-Ares LG, Cho BC, Gadgeel SM, Thomas M, Liu SV, Taylor MH, Mansfield AS, Zhu VW, Clifford C, Zhang H, Palmer M, Green J, Turner CD and Subbiah V. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. The Lancet Oncology. 2021;22:959–969. [DOI] [PubMed] [Google Scholar]
- 22.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Madry R, Christensen RD, Berek JS, Dorum A, Tinker AV, du Bois A, Gonzalez-Martin A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA and Investigators E-ON. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. The New England journal of medicine. 2016;375:2154–2164. [DOI] [PubMed] [Google Scholar]
- 23.Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, Wu LY, Liu ZL, Gao YN, Wang DB, Lou G, Yang HY, Zhou Q, Kong BH, Huang Y, Chen LP, Li GL, An RF, Wang K, Zhang Y, Yan XJ, Lu X, Lu WG, Hao M, Wang L, Cui H, Chen QH, Abulizi G, Huang XH, Tian XF, Wen H, Zhang C, Hou JM and Mirza MR. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled phase III trial(). Ann Oncol. 2021;32:512–521. [DOI] [PubMed] [Google Scholar]
- 24.Ison G, Howie LJ, Amiri-Kordestani L, Zhang L, Tang S, Sridhara R, Pierre V, Charlab R, Ramamoorthy A, Song P, Li F, Yu J, Manheng W, Palmby TR, Ghosh S, Horne HN, Lee EY, Philip R, Dave K, Chen XH, Kelly SL, Janoria KG, Banerjee A, Eradiri O, Dinin J, Goldberg KB, Pierce WF, Ibrahim A, Kluetz PG, Blumenthal GM, Beaver JA and Pazdur R. FDA Approval Summary: Niraparib for the Maintenance Treatment of Patients with Recurrent Ovarian Cancer in Response to Platinum-Based Chemotherapy. Clin Cancer Res. 2018;24:4066–4071. [DOI] [PubMed] [Google Scholar]
- 25.Kubiczkova L, Pour L, Sedlarikova L, Hajek R and Sevcikova S. Proteasome inhibitors - molecular basis and current perspectives in multiple myeloma. J Cell Mol Med. 2014;18:947–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dimopoulos MA, Goldschmidt H, Niesvizky R, Joshua D, Chng W-J, Oriol A, Orlowski RZ, Ludwig H, Facon T, Hajek R, Weisel K, Hungria V, Minuk L, Feng S, Zahlten-Kumeli A, Kimball AS and Moreau P. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. The Lancet Oncology. 2017;18:1327–1337. [DOI] [PubMed] [Google Scholar]
- 27.Bruno G, Bringhen S, Maffei I, Iannaccone A, Crea T, Ravera A, Astarita A, Vallelonga F, Salvini M, Gay F, Veglio F and Milan A. Cardiovascular Organ Damage and Blood Pressure Levels Predict Adverse Events in Multiple Myeloma Patients Undergoing Carfilzomib Therapy. Cancers (Basel). 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Q and Xia Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem. 2006;281:21652–21659. [DOI] [PubMed] [Google Scholar]
- 29.Gavazzoni M, Vizzardi E, Gorga E, Bonadei I, Rossi L, Belotti A, Rossi G, Ribolla R, Metra M and Raddino R. Mechanism of cardiovascular toxicity by proteasome inhibitors: New paradigm derived from clinical and pre-clinical evidence. Eur J Pharmacol. 2018;828:80–88. [DOI] [PubMed] [Google Scholar]
- 30.Sagstuen H, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA, Wilsgaard T and Bremnes RM. Blood pressure and body mass index in long-term survivors of testicular cancer. J Clin Oncol. 2005;23:4980–90. [DOI] [PubMed] [Google Scholar]
- 31.Boer H, Proost JH, Nuver J, Bunskoek S, Gietema JQ, Geubels BM, Altena R, Zwart N, Oosting SF, Vonk JM, Lefrandt JD, Uges DR, Meijer C, de Vries EG and Gietema JA. Long-term exposure to circulating platinum is associated with late effects of treatment in testicular cancer survivors. Ann Oncol. 2015;26:2305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.dos Santos NA, Carvalho Rodrigues MA, Martins NM and dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–50. [DOI] [PubMed] [Google Scholar]
- 33.Choi YM, Kim HK, Shim W, Anwar MA, Kwon JW, Kwon HK, Kim HJ, Jeong H, Kim HM, Hwang D, Kim HS and Choi S. Mechanism of Cisplatin-Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS One. 2015;10:e0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knijnenburg SL, Jaspers MW, van der Pal HJ, Schouten-van Meeteren AY, Bouts AH, Lieverst JA, Bokenkamp A, Koning CC, Oldenburger F, Wilde JC, van Leeuwen FE, Caron HN and Kremer LC. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin J Am Soc Nephrol. 2012;7:1416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C, Orlando L, Cinieri S, de BF, Viale G and Goldhirsch A. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. [DOI] [PubMed] [Google Scholar]
- 36.Kooijmans EC, Bokenkamp A, Tjahjadi NS, Tettero JM, van Dulmen-den Broeder E, van der Pal HJ and Veening MA. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2019;3:CD008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton BK, Rybicki L, Dean R, Majhail NS, Haddad H, Abounader D, Hanna R, Sobecks R, Duong H, Hill BT, Copelan E, Bolwell B and Kalaycio M. Cyclosporine in combination with mycophenolate mofetil versus methotrexate for graft versus host disease prevention in myeloablative HLA-identical sibling donor allogeneic hematopoietic cell transplantation. Am J Hematol. 2015;90:144–8. [DOI] [PubMed] [Google Scholar]
- 38.Tonia T, Mettler A, Robert N, Schwarzer G, Seidenfeld J, Weingart O, Hyde C, Engert A and Bohlius J. Erythropoietin or darbepoetin for patients with cancer. Cochrane Database Syst Rev. 2012;12:CD003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Textor SC, Canzanello VJ, Taler SJ, Wilson DJ, Schwartz LL, Augustine JE, Raymer JM, Romero JC, Wiesner RH, Krom RAF and Burnett JC. Cyclosporine-Induced Hypertension After Transplantation. Mayo Clinic Proceedings. 1994;69:1182–1193. [DOI] [PubMed] [Google Scholar]
- 40.Hoskova L, Malek I, Kopkan L and Kautzner J. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension. Physiol Res. 2017;66:167–180. [DOI] [PubMed] [Google Scholar]
- 41.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, Jassem J, Zolnierek J, Maroto JP, Mellado B, Melichar B, Tomasek J, Kremer A, Kim H-J, Wood K, Dutcus C and Larkin J. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. The Lancet Oncology. 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 42.Iacovelli R, Ciccarese C, Bria E, Romano M, Fantinel E, Bimbatti D, Muraglia A, Porcaro AB, Siracusano S, Brunelli M, Mazzarotto R, Artibani W and Tortora G. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clin Genitourin Cancer. 2018;16:e645–e653. [DOI] [PubMed] [Google Scholar]
- 43.Khosrow-Khavar F, Filion KB, Bouganim N, Suissa S and Azoulay L. Aromatase Inhibitors and the Risk of Cardiovascular Outcomes in Women With Breast Cancer: A Population-Based Cohort Study. Circulation. 2020;141:549–559. [DOI] [PubMed] [Google Scholar]
- 44.Baselga J, Campone M, Piccart M, Burris HA 3rd,, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D and Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. The New England journal of medicine. 2012;366:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I and Group ESCSD. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 46.Touyz RM, Herrmann SMS and Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12:409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, Lopez-Fernandez T, Fradley MG, Ganatra S, Curigliano G, Mitchell JD, Minotti G, Lang NN, Liu JE, Neilan TG, Nohria A, O’Quinn R, Pusic I, Porter C, Reynolds KL, Ruddy KJ, Thavendiranathan P and Valent P. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43:280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X-Y, Sun J-F and Hu S-Q. The renin-angiotensin system blockers as adjunctive therapy for cancer: a meta-analysis of survival outcome. Eur Rev Med Pharmacol Sci. 2017;21:1375–1383. [PubMed] [Google Scholar]
- 49.Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM and Group ESCSD. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 50.Beavers CJ, Rodgers JE, Bagnola AJ, Beckie TM, Campia U, Di Palo KE, Okwuosa TM, Przespolewski ER, Dent S, American Heart Association Clinical Pharmacology C, Cardio-Oncology Committee of the Council on Clinical C, Council on G, Precision M and the Council on Peripheral Vascular D. Cardio-Oncology Drug Interactions: A Scientific Statement From the American Heart Association. Circulation. 2022;145:e811–e838. [DOI] [PubMed] [Google Scholar]


